Abstract
Background and objectives: During Carnival, groups of ≥60 drummers go drumming with their hands and marching for periods of 2 to 4 h. The objective of this study was to determine the frequency and type of urinary abnormalities after candombe drumming and to evaluate possible pathogenic mechanisms.
Design, setting, participants, & measurements: For analysis of pathogenic mechanisms, a group of individuals were prospectively evaluated before and after candombe drumming.
Methods: Candombe drummers were recruited in January 2006, 1 wk before prolonged drumming. After clinical evaluation, urine and blood samples were obtained before and immediately after drumming.
Results: Forty-five healthy individuals (four women and 41 men), median age 31 yr (14 to 56), were evaluated. Predrumming urine and plasma samples were obtained for 30 individuals. Nineteen (42%) of 45 had a previous history of rust urine emission temporally related with candombe drumming. After drumming, 18 of 26 showed urine abnormalities; six of 26 showed rust urine, eight of 26 had microhematuria, and seven of 26 had proteinuria >1 g/L. The candombe drummers who showed rust urine after heavy drumming presented significantly higher levels of lactate dehydrogenase and total bilirubin when compared with those without urine abnormalities. Haptoglobin was significantly lower in the rust urine group. Fragmented red cells were observed in the blood smear of individuals with rust urine. Rust urine after drumming was associated with previous episodes of rust urine and glucosuria.
Conclusions: Taken together, these data confirm that rust urine is caused by extracorpuscular hemolysis.
Since 1899, when Williams (1) reported the effects of violent and prolonged exercise on the heart, several urinary abnormalities related to exercise have been described. The most common of these abnormalities is hematuria. The dysmorphic appearance of the red cells associated with the presence of red cell casts indicates the glomerular source for this common form of exercise hematuria (2,3); however, a number of genitourinary tract diseases may also cause gross or microscopic hematuria in some physically active patients (4,5). Rust urine (RU) has been noticed after heavy drumming. In 1974, Furie and Penn (6) reported a patient who developed pigmenturia after Conga drumming. Although they reported a second patient who developed transient nonoliguric renal failure, drumming is not usually considered in the literature as a cause of acute kidney injury (AKI).
Candombe is a drum-based musical form of Uruguay. Groups of ≥60 drummers go drumming with their hands and marching for periods of 2 to 4 h. During the main carnival event, called “las llamadas,” which takes place in summer, >40 groups march drumming for hours. After drumming, some individuals reported passing RU.
Between February 1999 and February 2005, we assisted five young adult patients who were admitted to the emergency department with a history of red-brown urine temporally related to candombe drumming. Immediately after intensive drumming, they reported passing red-brown urine followed by oliguria, nausea and vomiting, back pain, and weakness. All of them denied abuse substance consumption. All of them showed severe AKI and were treated with intensive parenteral rehydration and acid-base correction. Two patients required hemodialysis. All of the patients recovered renal function (7,8). Our aim was to determine the frequency and type of urinary abnormalities after candombe drumming and to evaluate possible pathogenic mechanisms.
Materials and Methods
In January 2006, previous to the main drumming event, also known as “las llamadas,” 45 volunteers were recruited. After informed consent, a clinical evaluation was performed, asking about previous episodes of RU, use of nonsteroidal anti-inflammatory drugs, and drug and alcohol consumption. Samples were obtained before and after drumming for 30 individuals; 15 individuals were evaluated only after drumming. Weight and BP were registered.
Hematuria was confirmed by dipstick examination and urine microscopy. Proteinuria was defined as >300 mg/L and microhematuria as three or more red cells per high-resolution field.
We used enzymatic methods for blood glucose, urea, uric acid, cholesterol, triglycerides, glutamic oxaloacetic transaminase, glutamic pyruvic transaminase, γ-glutamyltransferase, alkaline phosphatase, creatine kinase (CK), and lactate dehydrogenase (LDH). We measured albumin with bromocresol green; total proteins with the Biuret method; creatinine with the Jaffe method; total and direct bilirubin by the method of Jendrassik, and for CK isoenzyme MB immunologic inhibition. All of the reagent controls and calibrations were supplied by Roche (Basel, Switzerland). Haptoglobin determination was performed with reagents from (Dade Behring, Newark, NJ). Hemoglobinuria was determined by the ammonium sulfate method.
Phosphorus, creatinine, urea, uric acid, creatine phosphokinase (CPK), total bilirubin, LDH, myoglobin, and haptoglobin, were analyzed with multianalyzer Roche Hitachi 911 (Indianapolis, IN). Sodium, potassium, and calcium were analyzed with electrolyte analyzer 983-S (Roche Diagnostics). Hemogram and free hemoglobin were determined with hematologic counter Cell Dyn 1700 (Abbott, IL) and plasmatic bicarbonate and lactic acid with ABL 735 Radiometer (Copenhagen, Denmark). We also performed a blood smear looking for fragmented red cells and hemoglobin electrophoresis. We performed urine dipstick analyses (Multistix-Clinitek 500; Bayer, Leverkusen, Germany) and urinary sodium and creatinine, qualitative myoglobinuria, hemoglobinuria, and urinary sediment analyses.
Those who showed RU were evaluated in the next 48 h with urinalysis and renal function. All of them were contacted for follow-up. The study protocol was approved by the Ethics Committee of the Hospital of Clinics, Universidad de la República.
Statistical Analysis
Statistic analyses, with t test for independent variables, were performed. We considered significance at P < 0.05. Association of variables was evaluated by χ2.
Results
Samples from 45 individuals were obtained after drumming session (four women and 41 men); 11 were of black race, and the median age was 31 yr (range 14 to 56). Previous episodes of RU after drumming were reported by 19 (42%) of 45. Nine of 45 admitted drug consumption, and eight of 45 reported nonsteroidal anti-inflammatory drug consumption. Previous history of hypertension was reported by six of 45, and BP >139/89 was registered in the previous control in four of 30.
For the 30 individuals from whom the samples were obtained before drumming, mean creatinine was 0.95 ± 0.33 mg/dl. In one case, creatinine was 2.48 mg/dl. He reported intense hand drumming 5 d before the samples were obtained.1 Predrumming urine analyses were normal except for one case that was positive for proteins.
Postdrumming Evaluation
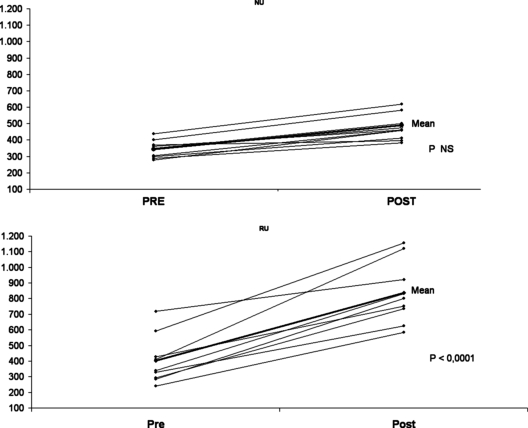
Results before and after drumming are shown in Table 1. Significant changes were demonstrated in creatinine, phosphorus, uric acid, total and indirect bilirubin, LDH, glutamic oxaloacetic transaminase, CK MB, and lactic acid. CK >235 UI/L (maximum level) was present in 27 (60%) of 45; LDH >285 UI/L in 44 (98%) of 45, and bilirubin >1.3 mg/dl in nine (20%) of 45 individuals. Urine abnormalities were present in 29 (64%) of 45, RU was present in nine (20%; Figure 1), microhematuria and/or proteinuria (M/PU) was present in 20 (44%), and normal urine was present in 16 (35%).
Table 1.
Laboratory data from 30 individuals before and after drumminga
| Parameter | Before
|
After
|
P | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Na (mEq/L) | 143.70 | 5.45 | 141.92 | 21.47 | NS |
| K (mEq/L) | 3.88 | 0.29 | 3.80 | 0.86 | NS |
| Urea (mg/dl) | 33.27 | 9.69 | 36.06 | 9.96 | NS |
| Cr (mg/dl) | 0.95 | 0.33 | 1.13 | 0.30 | <0.0500 |
| Ca (mg/dl) | 9.74 | 1.12 | 9.76 | 1.69 | NS |
| P (mg/dl) | 3.31 | 0.67 | 3.75 | 0.80 | <0.0500 |
| Uric acid (mg/dl) | 5.48 | 1.03 | 6.80 | 1.36 | <0.0500 |
| Total bilirubin (mg/dl) | 0.49 | 0.25 | 1.00 | 0.49 | <0.0001 |
| Direct bilirubin (mg/dl) | 0.11 | 0.07 | 0.13 | 0.09 | NS |
| Indirect bilirubin (mg/dl) | 0.39 | 0.19 | 0.87 | 0.47 | <0.0001 |
| Proteins (g/L) | 7.16 | 0.51 | 7.55 | 1.20 | NS |
| Albumin (g/L) | 4.23 | 0.37 | 4.42 | 0.70 | NS |
| ALP (U/L) | 196.17 | 135.70 | 196.52 | 125.38 | NS |
| GGT (U/L) | 28.07 | 18.37 | 30.91 | 21.14 | NS |
| GOT (U/L) | 20.87 | 5.84 | 29.60 | 10.37 | <0.0001 |
| GPT (U/L) | 14.13 | 7.26 | 17.69 | 10.58 | NS |
| LDH (U/L) | 368.47 | 112.69 | 562.57 | 193.02 | <0.0001 |
| CK (U/L) | 223.73 | 180.35 | 316.32 | 231.48 | NS |
| CK-MB (U/L) | 10.47 | 6.25 | 16.13 | 7.39 | <0.0010 |
| Plasmatic HCO3 (mEq/L) | 23.69 | 1.54 | 22.32 | 3.50 | NS |
| Lactate (U/L) | 1.76 | 0.89 | 2.28 | 0.71 | 0.0150 |
T test for paired samples, P < 0.05. ALP, alkaline phosphatase; Ca, calcium; CK-MB, creatine kinase isoenzyme MB; Cr, creatinine; GGT, γ-glutamyltransferase; GOT, glutamic oxaloacetic transaminase; GPT, glutamic pyruvic transaminase; K, potassium; LDH, lactate dehydrogenase; Na, sodium; P, phosphorus.
Figure 1.
Lactate dehydrogenase variation from normal and rusty urine groups.
Hemoglobin in urine was present in all of the patients with RU but only in two of 20 of the M/PU group. Myoglobin in urine was present only in one of the nine individuals with RU. Individuals with RU showed proteinuria (9.25 ± 11.1 g/L) significantly higher than in the group with pathologic urine (1.17 ± 1.34 g/L; P < 0.05).
Glucosuria with normal glycemia was present in five individuals, four of whom also showed RU. Glucosuria was associated with RU (χ2, P < 0.05)
Evidences of Extracorpuscular Hemolysis
In Table 2 we compared the group with RU (n = 9), the M/PU group (n = 20), and the group with normal urine (NU; n = 16). Values for total bilirubin, indirect bilirubin, LDH, and CPK MB were significantly higher in the group with RU when compared with M/PU or NU. Haptoglobin levels were <25.5 mg/dl in seven of nine individuals with RU, whereas only one individual from the 16 with NU showed haptoglobin <25.5 mg/dl (χ2, P < 0.05). When we compared the individuals with pre- and postdrumming samples (n = 30), there was a significant increase in LDH and total and indirect bilirubin (P < 0.05).
Table 2.
Laboratory data from groups with normal, pathologic, and rusty urinea
| Parameter | RU (n = 9)
|
M/PU (n = 20)
|
NU (n = 16)
|
P
|
||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | RU versus NU | RU versus M/PU | |
| Plasmatic creatinine (mg/dl) | 1.22 | 0.15 | 1.11 | 0.37 | 1.16 | 0.17 | NS | NS |
| Uric acid (mg/dl) | 6.44 | 2.00 | 6.67 | 1.12 | 7.08 | 1.20 | NS | NS |
| Total bilirubin (mg/dl) | 1.57 | 0.59 | 0.83 | 0.33 | 0.95 | 0.44 | 0.0070 | 0.0001 |
| Indirect bilirubin (mg/dl) | 1.40 | 0.60 | 0.72 | 0.30 | 0.83 | 0.42 | 0.0100 | 0.0010 |
| Albumin (g/L) | 4.53 | 0.23 | 4.48 | 0.26 | 4.54 | 0.43 | NS | NS |
| LDH (U/L) | 836.11 | 198.97 | 530.80 | 137.49 | 498.69 | 116.69 | 0.0001 | 0.0001 |
| CPK (U/L) | 428.89 | 368.76 | 285.50 | 147.09 | 294.81 | 222.59 | NS | NS |
| CPK MB (U/L) | 22.11 | 6.35 | 14.25 | 6.49 | 15.75 | 8.06 | NS | 0.0050 |
| Myoglobin (U/L) | 153.10 | 84.79 | 140.75 | 89.45 | 131.61 | 123.30 | NS | NS |
| Haptoglobin (mg/dl) | 31.27 | 16.97 | 68.30 | 37.47 | 63.39 | 42.32 | 0.0410 | 0.0090 |
| Hemoglobin (g/dl) | 14.50 | 1.03 | 14.34 | 1.25 | 14.74 | 0.82 | NS | NS |
| Hematocrit (%) | 42.61 | 2.95 | 42.13 | 3.57 | 43.14 | 2.32 | NS | NS |
| Free hemoglobin (g/dl) | 0.19 | 0.03 | 0.18 | 0.08 | 0.18 | 0.07 | NS | NS |
| Urine density | 1022.20 | 6.67 | 1027.50 | 5.00 | 1025.60 | 7.72 | NS | NS |
| Proteinuria (g/L) | 9.25 | 11.09 | 1.17 | 1.34 | 0.00 | 0.00 | 0.0300 | |
| Plasmatic HCO3 (mEq/L) | 22.72 | 1.29 | 22.78 | 1.67 | 22.44 | 1.30 | NS | NS |
| Lactate | 2.58 | 0.77 | 2.38 | 0.73 | 2.50 | 0.56 | NS | NS |
T test between groups P < 0.05. CPK, creatine phosphokinase; M/PU; microhematuria and/or proteinuria; NU, normal urine; RU, rusty urine.
We compared changes in the various groups before and after drumming (Table 3). Total bilirubin, indirect bilirubin, and LDH increased more in the group with RU, compared with the other two groups. Change in creatinine was significantly greater in the group with RU compared with NU. We found only fragmented red cells in blood smears of individuals with RU.
Table 3.
Variation of laboratory data before and after drumming from normal, pathologic, and rusty urine groupsa
| Parameter | RU (n = 9)
|
M/PU (n = 11)
|
NU (n = 10)
|
P
|
||||
|---|---|---|---|---|---|---|---|---|
| Median | SD | Median | SD | Median | SD | RU versus NU | RU versus M/PU | |
| Δ creatinine | 0.31 | 0.15 | 0.17 | 0.13 | 0.13 | 0.16 | 0.0200 | NS |
| Δ total bilirubin | 0.90 | 0.45 | 0.36 | 0.17 | 0.44 | 0.20 | 0.0090 | 0.0020 |
| Δ indirect bilirubin | 0.89 | 0.49 | 0.34 | 0.18 | 0.39 | 0.17 | 0.0070 | 0.0030 |
| Δ LDH | 432.56 | 155.79 | 176.55 | 93.03 | 131.30 | 50.27 | 0.0001 | 0.0001 |
| Δ CPK | 153.89 | 163.52 | 80.64 | 108.48 | 50.20 | 109.33 | NS | NS |
| Δ albumin | 0.20 | 0.39 | 0.38 | 0.36 | 0.32 | 0.55 | NS | NS |
T test between groups, P < 0.05.
Conditions Associated with RU
Previous episodes of RU was the only condition associated with a new episode (χ2, P < 0.05). There was no relation with weight loss during drumming or consumption of social drugs. Nevertheless, nine of the 11 black individuals presented pathologic urine after drumming, although only three of them presented RU. Hemoglobin electrophoresis was normal in all of the individuals with RU.
Follow-up of Individuals with RU
All of the individuals who presented RU were controlled by telephone call. All of them reported that urine had cleared. Normal urine samples were collected 48 to 72 h after drumming in six cases.
Discussion
Candombe drumming is growing as a cultural expression in Uruguay, and hand drumming is very common all over the world. More than 2200 individuals participate in the carnival event of “las llamadas” every year as drummers. Passing RU after drumming is cause for concern in candombe drummers. Frequently, they are evaluated 2 or 3 d after drumming, and they usually show normal urine.
In this study, from 30 individuals who were preparing themselves for the parade (with a frequency of drumming of more than once per week), only one showed an altered urine examination, although, 42% reported previous episodes of RU. In our study 20% of the individuals showed RU after drumming. Recently, we reported five individuals who presented to the emergency department of different hospitals in Uruguay, with severe AKI after drumming (7).
The group with RU after “las llamadas” showed higher levels of LDH and total and indirect bilirubin, when compared with the NU group.
Haptoglobin is a plasma protein that binds free hemoglobin that is released from erythrocytes and disappears from plasma during hemolysis. In seven of the nine studied individuals, haptoglobin after execution was below the detection level of the technique; in the other two cases, the value of haptoglobin was lower after drumming.
When the myoglobin and CPK levels were compared, there were no significant differences between both groups, supporting the concept that RU is a consequence of the extracorpuscular hemolysis and not of rhabdomyolysis. Myoglobinuria was present only in one individual. Although muscular damage was not the cause of RU, it should be evaluated in this setting.
CPK MB levels were significantly higher in the RU group when compared with M/PU group after drumming. There is evidence in the literature about exercise-induced cardiac troponin T and CPK MB release. It has been suggested that minor myocardial damage can occur after prolonged and intense exercise (9–11).
We cannot rule out that the increased levels of CPK MB in drummers with RU can be due to minor cardiac damage. That only the RU group showed CPK MB elevation suggests that test interference with hemolysis can be present in these individuals as described by Delahunty and Foreback (12).
Proteinuria in the group with RU was significantly higher (9.25 ± 11.09 g/L) than in the group with pathologic urine (1.17 ± 1.34 g/L). Even though proteinuria may be due to hemoglobin and/or myoglobin excretion in urine, the magnitude of proteinuria is too high to be explained only by this mechanism.
Albuminuria and urine B2 microglobulin excretion have been described after exercise. The mechanisms postulated for glomerular proteinuria are changes in glomerular hemodynamics and in basal glomerular membrane permeability (13–15). Proteinuria disappeared after drumming in all of the individuals with RU.
After drumming, there was a significant increase of plasma creatinine levels in the individuals with RU. Because the individuals were drumming for 2 to 4 h and blood samples were obtained immediately after drumming, we cannot assume that creatinine increased as a consequence of a renal impairment; we instead suggest that it can be related to an increase in creatinine muscular release.
The individuals with RU had fragmented red cells in the blood smear. We were not able to detect free hemoglobin in this group, which is probably related to the lack of sensitivity of the used technique.
In 1974, Furie and Penn (6) described a case of a young man who after conga drumming presented red-brown urine. The patient performed conga drumming under controlled conditions during his hospitalization. After a performance, the patient passed black urine that was subsequently analyzed. Serum obtained at that time showed an LDH level of 460 IU/L, haptoglobin of 16 mg/100 ml, and CPK of 140 IU/L. No evidence of renal impairment was detected in this case.
Levy et al. (16) examined the erythrocyte membrane proteins, glycoproteins, and urinary polypeptides in a patient who exhibited intermittent pigmenturia associated with conga drumming. Significant excretion of hemoglobin, albumin, and probably erythrocyte carbonic anhydrase but not myoglobin occurred during the acute phase of the conga drumming–induced pigmenturia. This usually ceased within 24 to 48 h. They found no evidence of aberrant erythrocyte membrane components by electrophoresis with either protein staining or a range of 125I-labeled lectins used for detection (16).
Only one of our patients presented urine myoglobin. Heme pigment–associated acute renal failure (ARF) is usually related to rhabdomyolysis (17). Three mechanisms are believed to contribute to the development of myoglobinuric ARF: Renal vasoconstriction, heme-mediated tubular cell injury, and intratubular cast formation. Although the cases reported showed an increase in plasma CPK levels, it was far below the levels usually observed in patients with ARF related to rhabdomyolysis (17). Urinary myoglobin causes a typical reddish-brown (port wine–like) color that can also be related with hemoglobinuria. Hemoglobin is structurally and functionally related to myoglobin. Although the molecular weight of hemoglobin (64,600 Da) is much higher than that of myoglobin (18,800 Da), hemoglobin is still able to cross the glomerular barrier and induce ARF by similar mechanisms. In patients with hemoglobinuria but not in patients with myoglobinuria, the plasma will be discolored as well. The urinary benzidine dipstick does not differentiate among myoglobin, hemoglobin, and red blood cells (17).
The presence of glucosuria associated with RU suggests tubular proximal damage. Tubular damage has been described related to hemoglobin toxicity in proximal tubular cells (18). Early markers of renal dysfunction in patients with sickle cell/thalassemia include markers of proximal tubular damage (19). Glucosuria has been reported in patients with paroxysmal nocturnal hemoglobinuria and related to recurrent hemoglobinuria episodes (20).
Candombe drumming is growing as a cultural expression in Uruguay, and hand drumming is very common all over the world. Seventy percent of individuals had urinary anomalies after drumming, with RU present in 20% of the cases. RU is related to extracorpuscular hemolysis as a result of the multiple manual traumas.
There is similarity between this phenomenon and other conditions that are associated with microvascular injury, such as exercise-induced hemoglobinuria. Prevalence of running-related hematuria is approximately 20 to 25%. Macroscopic hematuria was found mainly in long-distance runners (>10 km) and seems usually to be asymptomatic. It is usually most pronounced in the first urine void after exercise, normalizes often within 72 h after running, and seems to be independent of the exercise intensity (21,22). The pathogenesis of running-related hematuria is complex. There is a wide body of literature reporting red cell hemolysis as occurring after various forms of exercise. Trauma associated with footstrike is thought to be the major cause of hemolysis after running (23).
Related to hydration status and its influence in the presence of RU and functional impairment, we found in our previous report that the individuals with RU presented significantly lower natriuria levels after drumming when compared with individuals with NU (58 ± 32 versus 132 ± 56 mEq/L), suggesting that sodium retention mechanisms were taking place. Our recommendation after this report was that drummers should increase water intake before and during the parade (7).
It needs to be addressed whether repetitive episodes of RU may produce chronic tubular interstitial damage. Chronic renal damage has been described in patients with repetitive episodes of hemoglobinuria related to paroxysmal nocturnal hemoglobinuria (20,24) and sickle cell disease (25).
Passing RU after drumming should not be underestimated, because some individuals may develop AKI. We suggest that renal function be evaluated and a search for hemolysis and muscle damage be conducted in these individuals. We highly recommend intense hydration and avoidance of toxic and analgesic substance consumption before and during drumming sessions.
The only condition associated with RU was a previous history of RU after drumming. We looked for red cell abnormalities in the RU group, but hemoglobin electrophoresis was normal in these individuals. Recent reports suggested that exercise-induced hemolysis is caused by plasma membrane red cell protein modifications during exercise (26).
One of the individuals showed an elevated creatinine in the predrumming sample; it was not detected previously because all of the samples were analyzed simultaneously after drumming. This individual recovered normal renal function during the follow-up.
A public information campaign in Uruguay encourages the drummers to increase hydration and to avoid the use of toxic substances. We think that the use of a strong glove for drumming can be beneficial to diminish the incidence of RU after drumming.
To our knowledge this is the first report that systematically confirms that RU in drummers is due to extracorpuscular hemolysis, supporting Furie's initial case report (6). This study also underlines the need for clinical follow-up of these patients. Chronic consequences of repetitive episodes of RU should be addressed.
Disclosures
None.
Acknowledgments
Some data from this article were presented as an oral communication at the annual meeting of the American Society of Nephrology; November 8 through 13, 2005; Philadelphia, PA (J Am Soc Nephrol 16: 45A, 2005).
We acknowledge G. Sire, N. Bula, A. Langeneker, R. Bassetti, V. Da Costa, P. Da Costa, and M. Lopez; NGO-Afro Alternative: S. Baldriz, B. Romero, and R. Vazquez, who collaborated at the laboratory; Municipality of Montevideo (IMM), Division of Health and Tourism: F. Gonzalez y Anzalone; IMM, Service of Light Network Connection; and IMM, Communal Center no. 20.
Published online ahead of print. Publication date available at www.cjasn.org.
Footnotes
This patient reported hand drumming 5 d before; afterward, he experienced during 48 h muscle pain and nausea. There was no variation in creatinine before and after drumming. He had no previous history of renal disease, and 1 mo after the episode, his creatinine was 0.88 mg/dl, confirming that he developed postdrumming acute renal injury.
References
- 1.Williams H, Arnold HD: The effects of violent and prolonged muscular exercise upon the heart. Phila Med J 3: 1233–1239, 1899 [PMC free article] [PubMed] [Google Scholar]
- 2.Fassett RG, Owen JE, Fairley J, Birch DF, Fairley KF: Urinary red-cell morphology during exercise. BMJ (Clin Res Ed) 285: 1455–1457, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerth J, Ott U, Funfstuck R, Bartsch R, Keil E, Schubert K, Hubscher J, Scheucht S, Stein G: The effects of prolonged physical exercise on renal function, electrolyte balance and muscle cell breakdown. Clin Nephrol 57: 425–431, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Algazy KM: Spinner's hematuria. N Engl J Med 346: 1676, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Mueller EJ, Thompson IM: Bladder carcinoma presenting as exercise-induced hematuria. Postgrad Med 84: 173–176, 1988 [DOI] [PubMed] [Google Scholar]
- 6.Furie B, Penn AS: Pigmenturia from conga drumming: Hemoglobinuria and myoglobinuria. Ann Intern Med 80: 727–729, 1974 [DOI] [PubMed] [Google Scholar]
- 7.Tobal D, Olascoaga A, Sans A, Fernanadez C, LarreBorges P, Moreira A, Gonzalez Martinez F, Noboa O: Pigmenturia and acute renal failure after candombe drumming. Rev Med Urug 22: 299–304, 2006 [Google Scholar]
- 8.Noboa O, Tobai D, Olascoaga A, Sans A, Fernandez C, Larre Borges P, Gonzalez Martinez F: Pigmenturia and acute renal failure after candombe drumming. J Am Soc Nephrol 16: 45A, 2005 [Google Scholar]
- 9.Apple FS, Rogers MA, Sherman WM, Costill DL, Hagerman FC, Ivy JL: Profile of creatine kinase isoenzymes in skeletal muscles of marathon runners. Clin Chem 30: 413–416, 1984 [PubMed] [Google Scholar]
- 10.Shave R, George KP, Atkinson G, Hart E, Middleton N, Whyte G, Gaze D, Collinson PO: Exercise-induced cardiac troponin T release: A meta-analysis. Med Sci Sports Exerc 39: 2099–2106, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Konig D, Schumacher YO, Heinrich L, Schmid A, Berg A, Dickhuth HH: Myocardial stress after competitive exercise in professional road cyclists. Med Sci Sports Exerc 35: 1679–1683, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Delahunty TJ, Foreback CC: Automated creatine kinase-MB estimation by immuno-inhibition: A clinical evaluation. Clin Chem 26: 568–572, 1980 [PubMed] [Google Scholar]
- 13.Senturk UK, Kuru O, Kocer G, Gunduz F: Biphasic pattern of exercise-induced proteinuria in sedentary and trained men. Nephron Physiol 105: 22–32, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Poortmans JR, Brauman H, Staroukine M, Verniory A, Decaestecker C, Leclercq R: Indirect evidence of glomerular/tubular mixed-type postexercise proteinuria in healthy humans. Am J Physiol 254: F277–F283, 1988 [DOI] [PubMed] [Google Scholar]
- 15.Poortmans JR: Postexercise proteinuria in humans: Facts and mechanisms. JAMA 253: 236–240, 1985 [PubMed] [Google Scholar]
- 16.Levy RD, Khaleeli AA, Griffiths BL, Edwards YH: A study of erythrocyte membrane proteins and urinary polypeptides in conga drumming haemoglobinuria. Clin Sci (Lond) 69: 105–108, 1985 [DOI] [PubMed] [Google Scholar]
- 17.Vanholder R, Sever MS, Erek E, Lameire N: Rhabdomyolysis. J Am Soc Nephrol 11: 1553–1561, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Zager RA, Gamelin LM: Pathogenetic mechanisms in experimental hemoglobinuric acute renal failure. Am J Physiol 256: F446–F455, 1989 [DOI] [PubMed] [Google Scholar]
- 19.Voskaridou E, Terpos E, Michail S, Hantzi E, Anagnostopoulos A, Margeli A, Simirloglou D, Loukopoulos D, Papassotiriou I: Early markers of renal dysfunction in patients with sickle cell/beta-thalassemia. Kidney Int 69: 2037–2042, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Saito T, Furyama T, Onodera S, Saito H, Shioji R: Renal impairment in patients with paroxysmal nocturnal hemoglobinuria. Tohoku J Exp Med 116: 267–275, 1975 [DOI] [PubMed] [Google Scholar]
- 21.Siegel AJ, Hennekens CH, Solomon HS, Van Boeckel B: Exercise-related hematuria: Findings in a group of marathon runners. JAMA 241: 391–392, 1979 [DOI] [PubMed] [Google Scholar]
- 22.Whisnant JD: Exercise-related hematuria. JAMA 242: 1610, 1979 [PubMed] [Google Scholar]
- 23.Telford RD, Sly GJ, Hahn AG, Cunningham RB, Bryant C, Smith JA: Footstrike is the major cause of hemolysis during running. J Appl Physiol 94: 38–42, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Zachee P, Henckens M, Van Damme B, Boogaerts MA, Rigauts H, Verberckmoes RK: Chronic renal failure due to renal hemosiderosis in a patient with paroxysmal nocturnal hemoglobinuria. Clin Nephrol 39: 28–31, 1993 [PubMed] [Google Scholar]
- 25.Guasch A, Navarrete J, Nass K, Zayas CF: Glomerular involvement in adults with sickle cell hemoglobinopathies: Prevalence and clinical correlates of progressive renal failure. J Am Soc Nephrol 17: 2228–2235, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Yusof A, Leithauser RM, Roth HJ, Finkernagel H, Wilson MT, Beneke R: Exercise-induced hemolysis is caused by protein modification and most evident during the early phase of an ultraendurance race. J Appl Physiol 102: 582–586, 2007 [DOI] [PubMed] [Google Scholar]