Abstract
Background and objectives: Recent studies show high prevalence of suboptimal 25-hydroxyvitamin D levels in chronic kidney disease patients. This study sought to test the hypothesis that the prevalence of 25-hydroxyvitamin D deficiency is significantly higher in chronic kidney disease patients and, in diabetic nephropathy, low serum 25-hydroxyvitamin D is associated with abnormal serum parathyroid hormone, bone mineral density, and coronary artery calcification.
Design, setting, participants, & measurements: Study A used data from the Third National Health and Nutrition Examination Survey. Study B was a post hoc analysis of an observational study of coronary artery calcification in non–dialysis-dependent diabetic nephropathy.
Results: In study A, the adjusted odds for 25-hydroxyvitamin D deficiency were 32% higher in chronic kidney disease patients. This higher prevalence of 25-hydroxyvitamin D deficiency, however, could not be explained by differences in total vitamin D intakes. The consequences of suboptimal 25-hydroxyvitamin D levels were analyzed in 146 patients with diabetic nephropathy. The significant, inverse relationship between serum 25-hydroxyvitamin D and parathyroid hormone levels was attenuated to a nonsignificant level on multivariate adjustment. There was a significant, inverse relationship between bone mineral density and coronary artery calcification scores; neither was independently associated with serum 25-hydroxyvitamin D. The serum 25-hydroxyvitamin D levels declined modestly in 72 patients studied after 12.4 ± 0.4 mo.
Conclusions: 25-Hydroxyvitamin D deficiency is more common in chronic kidney disease, but this higher prevalence is unlikely to be a result of lower vitamin D intakes. The consequences of suboptimal 25-hydroxyvitamin D levels remain to be definitively elucidated.
Vitamin D is important for maintaining bone health and muscle function (1). Serum 25 hydroxyvitamin D (25OHD) levels are a sensitive marker of the total body vitamin D stores. Epidemiologic and interventional studies suggest that serum 25OHD levels of at least 30 ng/ml are probably necessary to ensure optimal bone health and muscle function (1,2). Using this threshold, a large proportion of elderly, nursing home residents, dark-skinned people, and individuals who require hospitalization are either vitamin D insufficient (15 to 30 ng/ml) or deficient (<15 ng/ml) (1,3,4). In the absence of data from individuals with chronic kidney disease (CKD), the Kidney Disease Outcomes Quality Initiative (KDOQI) workgroup extrapolated these data to make opinion-based recommendations to maintain serum 25OHD levels >30 ng/ml in patients with stage 3 or 4 CKD (5). Numerous studies have demonstrated that the vast majority of non–dialysis-dependent patients with CKD have suboptimal 25OHD levels (6–9); however, none of these studies included a sufficiently large sample of control subjects to determine whether CKD is independently associated with a higher prevalence of suboptimal 25OHD levels. Moreover, secondary hyperparathyroidism and reduced bone mineral density (BMD)—the most common consequences of suboptimal 25OHD levels in the general population—are often present in patients with CKD. There is a paucity of data evaluating the role of 25OHD in the reduced BMD in CKD.
We undertook this study to test the hypothesis that CKD is an independent predictor of 25OHD deficiency in a random sample of community-dwelling individuals of the United States using the Third National Health and Nutrition Survey (NHANES III; study A); this may, in part, be accounted for by lower daily vitamin D intakes. Furthermore, in a cohort of patients with diabetes and diabetic nephropathy (DN), we sought to determine the effects of suboptimal 25OHD levels on bone health (elevated serum parathyroid hormone (PTH) levels and reduced BMD) and coronary artery calcification (CAC) scores (study B).
Materials and Methods
Study A
NHANES III was a study conducted between 1988 and 1994 (10). Study participants were selected using a complex, multistage, stratified, and clustered probability sampling of the noninstitutionalized population of the United States. The study included a deliberate oversampling of black, Mexican American, and elderly individuals (11). The cohort for these analyses constituted individuals who were ≥18 yr of age and had had measurements of serum creatinine and 25OHD and urine albumin and creatinine.
Definition of Variables and Laboratory Measurements
Estimated GFR (eGFR), using the abbreviated Modification in Diet in Renal Disease (MDRD) equation, and urine albumin/creatinine ratio were used to determine CKD (12). The creatinine measurements in the NHANES III study were calibrated to the MDRD laboratory by subtracting 0.23 mg/dl, as described previously (13). A patient was identified as having CKD when (1) eGFR was <60 ml/min per 1.73 m2 or (2) eGFR was ≥60 but urine albumin/creatinine was >17 mg/g in men and >25 mg/g in women (12).
Serum 25OHD levels were measured using RIA (DiaSorin, Stillwater, MN). The total daily oral intake of vitamin D was estimated as a sum of that derived from diet and supplements. Dietary intakes were estimated using a single 24-h recall and the dietary nutrient intakes were calculated using the US Department of Agriculture's Survey Nutrient Database. Study participants were asked about their use of vitamin and mineral supplements during the preceding month, and this was used to calculate the average daily intake. The daily 25OHD intake in the supplements taken by an individual was estimated by linking these data with the SUPLCONC file.
Race/ethnicity was self-described by study participants. Hypertension was defined as present when the patient was taking antihypertensive medications or the measured BP was >140/90 mmHg. Diabetes was defined as a history of diabetes, being treated with medications for diabetes, or a fasting blood glucose >126 mg/dl. Education was used as a dichotomous variable (<12 or ≥12 completed years of schooling). Patients were classified as having any health insurance or none. The effect of income was assessed by determining whether the participant's household income was <200% or ≥200% of the federal poverty level.
Non-HDL cholesterol was calculated as a difference between the total and HDL cholesterol concentrations. Serum concentrations of calcium and phosphorus were measured using a Hitachi model 737 multichannel analyzer (Boehringer Mannheim Diagnostics, Indianapolis, IN). Serum albumin was measured using bromocresol purple and C-reactive protein (CRP) by latex-enhanced nephelometry. Total serum calcium was corrected for serum albumin.
Study B
The data from non–dialysis-dependent individuals who had type 2 diabetes and DN and were enrolled in observational studies that evaluated CAC were used for this analysis. DN was deemed to be present when a patient had type 2 diabetes (age of onset >30 yr and treatment with oral medications for at least 6 mo), overt proteinuria (urine protein/creatinine ratio >0.5), and met one of the following criteria: (1) renal biopsy evidence for DN or (2) diabetes duration ≥5 yr and diabetic retinopathy or (3) diabetes duration ≥10 yr. Other data on some of these patients were published previously (14,15). A report that included the results of serum 25OHD was sent to the patient with a recommendation to share the information with his or her physician. Of the 146 patients, 100 were enrolled in a longitudinal cohort study and were eligible for re-evaluation either 12 mo after the baseline visit or within 3 mo of first dialysis treatment, whichever was earlier.
Definition of Risk Factors
All eligible patients who consented to participate in the study came in fasting to the outpatient General Clinical Research Center at Los Angeles Biomedical Research Institute. None of the patients enrolled in the study had been prescribed phosphate binders, vitamin D or its analogs, or bisphosphonates by their treating physicians at the time of the study visit. Identical study procedures were followed for the baseline and follow-up visits. A fasting blood sample was drawn for serum chemistry, including the measurement of creatinine, albumin, lipid panel, calcium, and phosphorus. Glycosylated hemoglobin (HbA1c) was measured using HPLC and serum high-sensitivity CRP using an immunoassay. Intact PTH concentrations were measured using immunochemiluminometric assay (reference range 10 to 65 pg/ml), and 25OHD levels were measured in Quest Diagnostics laboratory using liquid chromatography, tandem mass spectroscopy (analytic sensitivity 4 ng/ml).
Method for calculation of eGFR and definition of hypertension were identical to those used in study A. Dyslipidemia was defined as present when the LDL cholesterol was >130 mg/dl or HDL cholesterol was <40 mg/dl or treatment with lipid-lowering medications.
CAC and BMD
All patients underwent electron beam computed tomography with a C-100 or C-150 scanner (Imatron, South San Francisco, CA) on the day of the study visit. The thickness of the sections was 3 mm, and, in total, 40 sections were obtained starting at the level of the carina and proceeding to the level of the diaphragm. Images were electrocardiographically triggered at 60 to 80% of the R-R interval. CAC was defined as a plaque of at least three consecutive pixels (area 1.03 mm2) with attenuation of ≥130 HU. Quantitative calcium scores were calculated according to the method described by Agatston et al. (16).
BMD was estimated using quantitative computed tomography (QCT), and images were acquired at the same time as the assessment of the coronary arteries (17). A standard bone density calibration phantom was placed under the lumbar vertebra, and a single QCT scan of the L1, L2, and L3 lumbar vertebrae was acquired. The reader identified the three lumbar vertebrae by using anatomic landmarks and highlighted the trabecular bone with the software. The software then measured bone density and averaged over the image slices.
Statistical Analyses
Data are expressed as mean ± SEM. The significance of difference between continuous variables was tested using unpaired t test or one-way ANOVA or Mann-Whitney rank sum test and for categorical variables using χ2 or Fisher exact test as appropriate. Correlations were tested using Spearman correlation coefficient.
For study A, all analyses were performed using sample weights that account for unequal probability of selection, nonresponse, and planned oversampling of elderly, black, and Mexican American individuals. Estimates were made using the recommended SAS-callable SUDAAN software (version 8; Research Triangle Park, NC). Multivariate logistic regression analyses were used to determine whether CKD was an independent predictor for 25OHD levels <30 or <15 ng/ml. Two different models were tested: In the first model, the data were adjusted for known determinants of serum 25OHD levels and socioeconomic variables. In the second model, additional adjustments were made for other patient characteristics.
For study B, multivariate analyses were performed using linear regression analyses. Age, gender, race, and quarter of year when the patients were studied were forced into each model; additional variables with P < 0.10 on univariate analyses were eligible for stepwise selection. The additional variables that were eligible for stepwise selection as independent predictors of serum 25OHD were eGFR, serum albumin, albumin-to-creatinine ratio, and body mass index (BMI). Serum calcium, phosphorus, and PTH levels were not considered for inclusion in this analysis. For ascertainment of the independent predictors for log-transformed serum PTH, the additional variables that were eligible for stepwise selection were diabetes duration, eGFR, serum calcium, CRP, 25OHD, HbA1c, and BMI; for predictors of BMD, diabetes duration, serum calcium, CRP, HbA1c, and 25OHD levels. Finally, serum 25OHD level was the only additional variable to enter the stepwise selection to determine the predictors of log-transformed CAC scores. The goodness of fit for all models was tested by inspecting normal P-P plot of regression-standardized residuals. A limited number of interactions were tested. P < 0.05 was considered significant for all analyses.
Results
Study A
Of the 19,618 participants who were ≥18 yr of age, 3662 were excluded because they did not have enough information to ascertain the presence of CKD, and 25OHD measurements were unavailable for 128 patients. Thus, 15,828 patients constituted the study cohort (Table 1).
Table 1.
Demographic, clinical, dietary, and laboratory characteristics of adults enrolled in the NHANES III, based on the presence and stage of CKDa
| Characteristic | Non-CKD | CKD
|
P | |||
|---|---|---|---|---|---|---|
| Stage 1 | Stage 2 | Stage 3 | Stage 4 or 5 | |||
| Prevalence (%) | 86.2 | 6.2 | 3.5 | 3.8 | 0.2 | |
| Age (yr) | 41.1 ± 0.4 | 43.7 ± 0.8 | 59.5 ± 0.9 | 70.2 ± 0.8 | 70.7 ± 1.8 | <0.0001 |
| Male gender (%) | 48.4 | 44.6 | 53.0 | 38.7 | 39.6 | 0.0070 |
| Race/ethnicity (%) | ||||||
| white | 76.0 | 64.8 | 80.9 | 86.8 | 66.7 | |
| black | 10.6 | 17.5 | 10.7 | 7.5 | 23.1 | <0.0001 |
| Mexican American | 5.5 | 7.3 | 3.0 | 1.4 | 2.8 | |
| other | 7.9 | 10.4 | 5.4 | 4.3 | 7.4 | |
| Serum creatinine (mg/dl) | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.2 ± 0.0 | 1.5 ± 0.0 | 4.0 ± 0.4 | <0.0001 |
| eGFR (ml/min per 1.73 m2) | 103.7 ± 0.6 | 115.2 ± 1.0 | 76.8 ± 0.4 | 50.8 ± 0.3 | 21.1 ± 1.3 | <0.0001 |
| Vitamin D levels (ng/ml; %) | ||||||
| >30 | 45.9 | 33.4 | 39.5 | 36.5 | 17.0 | |
| 15 to 30 | 45.7 | 52.5 | 51.4 | 52.8 | 55.8 | <0.0001 |
| <15 | 8.4 | 14.1 | 9.1 | 10.7 | 27.2 | |
| Taking vitamin D supplements (%) | 27.9 | 23.2 | 25.6 | 27.1 | 17.1 | 0.0800 |
| Total vitamin D intake (diet + supplements; IU/d) | 309.1 ± 7.3 | 282.0 ± 13.4 | 324.5 ± 22.3 | 319.6 ± 19.2 | 288.7 ± 57.9 | 0.3000 |
| Current smokers (%) | 29.2 | 32.6 | 23.2 | 13.7 | 19.6 | <0.0001 |
| Hypertension (%) | 17.6 | 33.3 | 59.3 | 72.7 | 74.4 | <0.0001 |
| BMI (kg/m2) | 26.3 ± 0.1 | 27.2 ± 0.3 | 28.2 ± 0.5 | 27.7 ± 0.2 | 26.7 ± 1.1 | <0.0001 |
| Diabetes (%) | 4.6 | 18.6 | 28.3 | 21.7 | 29.8 | <0.0001 |
| Non-HDL cholesterol ≥160 (mg/dl, % | 38.7 | 42.9 | 57.4 | 68.4 | 50.2 | <0.0001 |
| Serum CRP (mg/dl; %) | ||||||
| undetectable | 74.1 | 60.6 | 58.7 | 50.8 | 35.1 | |
| 0.30 to 0.99 | 19.8 | 24.1 | 27.0 | 30.8 | 37.4 | <0.0001 |
| ≥1 | 6.1 | 15.2 | 14.3 | 18.4 | 27.4 | |
| Serum albumin (g/dl) | 4.2 ± 0.0 | 4.2 ± 0.0 | 4.1 ± 0.0 | 4.0 ± 0.0 | 3.8 ± 0.1 | <0.0001 |
| Corrected serum calcium (mg/dl) | 9.2 ± 0.0 | 9.3 ± 0.0 | 9.4 ± 0.1 | 9.5 ± 0.0 | 9.4 ± 0.1 | <0.0001 |
| Serum phosphorus (mg/dl) | 3.5 ± 0.0 | 3.4 ± 0.0 | 3.4 ± 0.0 | 3.5 ± 0.0 | 4.1 ± 0.1 | <0.0001 |
| No medical insurance (%) | 13.6 | 14.7 | 7.6 | 2.5 | 0.1 | <0.0001 |
| Education <12 yr (%) | 19.7 | 27.9 | 32.8 | 43.8 | 49.4 | <0.0001 |
| Income <200% of federal poverty level (%) | 32.6 | 42.0 | 36.8 | 45.9 | 55.1 | <0.0001 |
BMI, body mass index; CKD, chronic kidney disease; CRP, C-reactive protein; eGFR, estimated GFR; NHANES III, Third National Health and Nutrition Examination Survey.
Patients with CKD were substantially less likely to have optimal 25OHD levels (>30 ng/ml) and had a higher prevalence of vitamin D deficiency; however, there was no significant difference in the total vitamin D intake between patients with and without CKD (Table 1). Controlling for variables that previously were described to be associated with 25OHD (age, gender, race/ethnicity, BMI, month of year), as well as for socioeconomic variables (medical insurance, educational level, family income) and total daily vitamin D intake, patients with CKD were 32% more likely to have vitamin D deficiency (Table 2, analysis 1). The higher odds for vitamin D deficiency among patients with CKD persisted after further, more extensive multivariate adjustments (Table 2, analysis 2).
Table 2.
Adjusted odds for 25OHD insufficiency (15 to 30 ng/ml) and deficiency (<15 ng/ml) among the participants of the NHANES III with CKDa
| Parameter | CKD (OR [95% CI]) |
|---|---|
| Analysis 1b | |
| 25OHD 15 to 30 ng/ml | 1.13 (0.92 to 1.39) |
| 25OHD <15 ng/ml | 1.32 (1.03 to 1.72) |
| Analysis 2c | |
| 25OHD 15 to 30 ng/ml | 1.15 (0.92 to 1.44) |
| 25OHD <15 ng/ml | 1.39 (1.09 to 1.77) |
25OHD, 25-hydroxyvitamin D.
Adjusted for age, gender, race/ethnicity, BMI, vitamin D intake (diet and supplements), medical insurance, educational level, family income, and month of the year (n = 12,620).
Adjusted for age, gender, race/ethnicity, BMI, vitamin D intake (diet and supplements), medical insurance, educational level, family income, month of the year, current smoking, hypertension, diabetes, non-HDL cholesterol, serum CRP, and serum albumin (n = 12,475).
Study B
The distribution of CKD stages among the 146 patients with DN was as follows: Stage 1, 11%; stage 2, 32%; stage 3, 43%; stage 4, 10%; and stage 5, 4%. Nephrotic-range proteinuria was present in 55 patients. Mean eGFR was 55.4 ± 2. ml/min per 1.73 m2. The characteristics of the study patients are summarized in Table 3. More than 80% of patients had suboptimal 25OHD levels: Deficiency 38% and insufficiency 45% (Table 3). Using multivariate linear regression analyses, younger age, black race, female gender, lower serum albumin, and higher albumin-to-creatinine ratio were independently predictive of lower 25OHD levels.
Table 3.
Characteristics of patients with diabetic nephropathy enrolled in study Ba
| Characteristic | Overall | Deficiency (<15 ng/ml) | Insufficiency (15 to 30 ng/ml) | Optimal (>30 ng/ml) | P |
|---|---|---|---|---|---|
| n | 146 | 55 | 66 | 25 | |
| Age (yr) | 57.0 ± 0.6 | 54.0 ± 0.9 | 57.5 ± 0.8 | 62.5 ± 1.8 | <0.001 |
| Male gender (%) | 51 | 40 | 59 | 52 | 0.110 |
| Race/ethnicity (%) | |||||
| Latino | 77 | 66 | 82 | 88 | 0.002 |
| black | 10 | 24 | 3 | 0 | |
| white | 13 | 11 | 15 | 12 | |
| Serum creatinine (mg/dl)b | 1.3 (1.0, 1.8) | 1.6 (1.1, 2.1) | 1.3 (1.0, 1.8) | 1.0 (0.8, 1.5) | 0.003 |
| eGFR (ml/min per 1.73 m2) | 55.4 ± 2.1 | 47.9 ± 23.8 | 57.3 ± 2.9 | 66.8 ± 5.1 | 0.004 |
| Urine albumin-to-creatinine ratio (mg/mg)b | 1.99 (0.79, 3.93) | 3.02 (0.85, 4.31) | 2.05 (0.97, 3.77) | 0.91 (0.58, 2.21) | 0.020 |
| Diabetes duration (yr) | 15.3 ± 0.5 | 16.7 ± 0.9 | 14.3 ± 0.7 | 15.2 ± 1.2 | 0.110 |
| Current smokers (%) | 13 | 9 | 15 | 16 | 0.550 |
| Hypertension (%) | 95 | 96 | 91 | 100 | 0.180 |
| Dyslipidemia (%) | 84 | 82 | 86 | 84 | 0.790 |
| BMI (kg/m2) | 31.2 ± 0.6 | 32.7 ± 1.0 | 30.7 ± 1.0 | 29.2 ± 1.1 | 0.110 |
| Serum CRPb | 0.4 (0.1, 0.8) | 0.5 (0.1, 0.9) | 0.4 (0.1, 0.9) | 0.2 (0.1, 0.5) | 0.200 |
| HbA1c (%) | 8.6 ± 0.2 | 8.3 ± 0.4 | 8.7 ± 0.3 | 9.1 ± 0.4 | 0.350 |
| Serum albumin (g/dl) | 3.2 ± 0.1 | 3.1 ± 0.1 | 3.3 ± 0.1 | 3.5 ± 0.1 | 0.002 |
| Corrected serum calcium (mg/dl) | 9.8 ± 0.0 | 9.7 ± 0.1 | 9.8 ± 0.1 | 9.8 ± 0.1 | 0.630 |
| Serum phosphorus (mg/dl) | 4.3 ± 0.1 | 4.4 ± 0.1 | 4.3 ± 0.1 | 4.2 ± 0.1 | 0.310 |
| Serum PTH (pg/ml)b | 51 (32, 79) | 61 (40, 112) | 45 (31, 67) | 40 (22, 55) | <0.001 |
| BMD (mg/cm3) | 130.2 ± 4.3 | 143.4 ± 6.2 | 127.0 ± 7.0 | 108.3 ± 8.3 | 0.020 |
| Prevalence of T score <−1.0 (%) | 64 | 56 | 66 | 79 | |
| Coronary artery calcification | |||||
| Prevalence (score >10) (%) | 75 | 58 | 83 | 88 | 0.002 |
| scoreb | 149 (9, 488) | 85 (0, 325) | 200 (25, 637) | 196 (59, 370) | 0.040 |
BMD, bone mineral density; HbA1c, glycosylated hemoglobin; PTH, parathyroid hormone.
Median (25th, 75th percentiles).
Relationships Of 25OHD Levels to Relevant Surrogate Markers at Baseline.
There was a significant, inverse relationship between serum PTH and vitamin D levels on univariate analyses (r = −0.33, P < 0.001). On multivariate analyses, black race, female gender, and lower eGFR were independent predictors of higher serum PTH levels. Serum 25OHD was not predictive of serum PTH whether entered either as a continuous or a categorical variable.
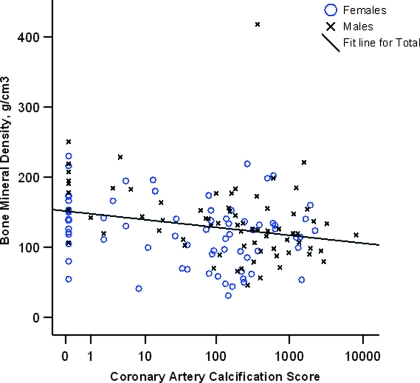
There was an inverse relationship between BMD, by QCT, and CAC score, ascertained by electron beam computed tomography (Figure 1). In the study group, 64% of patients had T score for BMD <−1.0 (Table 3). Three quarters of the study cohort had CAC score >10 with a median score of 149 (interquartile range 9 to 488); the scores for the vast majority of patients were significantly higher than those for age- and gender-matched control subjects from the general population. On multivariate analyses, increasing age and nonblack race were the only independent predictors of low BMD; serum 25OHD was not predictive. Increasing age, male gender, and non-Hispanic white race were independently associated with higher CAC scores on multivariate analyses; serum 25OHD did not enter the final model.
Figure 1.
Scatter plot showing an inverse relationship between coronary artery calcification score and bone mineral density in non–dialysis-dependent patients with chronic kidney disease (Spearman correlation coefficient −0.26, P = 0.002).
Change in 25OHD Levels over Time.
Of the 100 participants who were eligible for follow-up evaluation (88 Latino, 12 non-Hispanic white), 72 underwent repeat testing after a mean of 12.4 ± 0.4 mo. The reasons for inability to reevaluate the 28 patients were as follows: Death, 11; moved out of greater Los Angeles area, 7; lost to follow-up, 3; withdrew consent, 2; and inability to keep return appointment, 5. The serum PTH among patients who did not complete the follow-up was significantly lower than that for those who were reevaluated; there were no other significant differences between any patients with and without complete follow-up. The characteristics of the study patients who underwent serial evaluation are summarized in Table 4. Only one of these 72 patients had received 25OHD supplementation between the two assessments. During the period of follow-up, a statistically significant decrease in serum 25OHD levels occurred (mean −1.7 ± 1.1 ng/ml; Table 4). Neither the baseline characteristics nor the change in eGFR over time was associated with the change in 25OHD levels. Similarly, neither the baseline serum 25OHD levels nor the change in levels over time predicted the change in serum PTH.
Table 4.
Characteristics of patients who had diabetic nephropathy and underwent follow-up evaluationa
| Characteristic | Overall | Deficiency (<15 ng/ml) | Insufficiency (15 to 30 ng/ml) | Optimal (>30 ng/ml) | P |
|---|---|---|---|---|---|
| Number | 72 | 24 | 33 | 15 | |
| Age (yr) | 57.5 ± 0.9 | 53.8 ± 1.3 | 57.5 ± 1.2 | 63.3 ± 2.0 | <0.001 |
| Male gender (%) | 43 | 29 | 49 | 53 | 0.230 |
| Race/ethnicity (%) | |||||
| Latino | 89 | 96 | 82 | 93 | 0.210 |
| white | 11 | 4 | 18 | 7 | |
| Diabetes duration (yr) | 15.4 ± 0.7 | 16.0 ± 1.2 | 14.2 ± 1.0 | 16.8 ± 1.7 | 0.310 |
| Current smokers (%) | 10 | 4 | 18 | 0 | 0.080 |
| Hypertension (%) | 94 | 96 | 91 | 100 | 0.420 |
| Dyslipidemia (%) | 88 | 88 | 94 | 73 | 0.140 |
| Baseline BMI (kg/m2) | 31.1 ± 0.9 | 33.6 ± 1.7 | 30.7 ± 1.4 | 27.7 ± 1.4 | 0.060 |
| Baseline serum CRPa | 0.4 (0.1, 0.6) | 0.4 (0.2, 0.5) | 0.5 (0.1, 0.7) | 0.2 (0.1, 0.6) | 0.280 |
| Baseline HbA1c (%) | 8.9 ± 0.3 | 8.5 ± 0.5 | 8.9 ± 0.4 | 9.7 ± 0.7 | 0.320 |
| Baseline serum albumin (g/dl) | 3.2 ± 0.1 | 2.8 ± 0.1 | 3.3 ± 0.1 | 3.5 ± 0.1 | <0.001 |
| Baseline serum creatinine (mg/dl)a | 1.3 (1.0, 1.7) | 1.5 (1.1, 1.8) | 1.3 (1.0, 1.8) | 1.0 (0.8, 1.3) | 0.090 |
| eGFR (ml/min per 1.73 m2) | |||||
| baseline | 53 (37, 75) | 44 (35, 64) | 52 (37, 74) | 75 (47, 80) | 0.060 |
| follow-up | 44 (27, 58) | 33 (16, 53) | 39 (27, 58) | 59 (43, 80) | 0.010 |
| change | −11 (−21, −4) | −16 (−27, −4) | −12 (−16, −5) | −8 (−14, −2) | 0.560 |
| Urine albumin-to-creatinine ratio (mg/mg)a | |||||
| baseline | 2.1 (1.0, 4.1) | 4.1 (1.8, 5.1) | 2.0 (1.1, 3.7) | 1.4 (0.6, 2.5) | 0.006 |
| follow-up | 2.3 (0.7, 4.8) | 3.7 (1.9, 6.9) | 1.6 (0.6, 4.1) | 0.9 (0.5, 3.2) | 0.008 |
| Corrected serum calcium (mg/dl) | |||||
| baseline | 9.8 ± 0.0 | 9.7 ± 0.1 | 9.8 ± 0.1 | 9.8 ± 0.1 | 0.950 |
| follow-up | 9.7 ± 0.1 | 9.7 ± 0.1 | 9.6 ± 0.1 | 9.8 ± 0.1 | 0.140 |
| Serum phosphorus (mg/dl) | |||||
| baseline | 4.3 ± 0.1 | 4.3 ± 0.1 | 4.4 ± 0.1 | 4.3 ± 0.1 | 0.510 |
| follow-up | 4.4 ± 0.1 | 4.5 ± 0.3 | 4.4 ± 0.1 | 4.2 ± 0.2 | 0.670 |
| Serum PTH (pg/ml)a | |||||
| baseline | 53 (35, 87) | 70 (46, 109) | 45 (34, 67) | 47 (32, 71) | 0.060 |
| follow-up | 61 (36, 96) | 71 (51, 112) | 49 (28, 91) | 52 (28, 72) | 0.040 |
| Serum 25OHD levels (ng/ml) | |||||
| baseline | 20.9 ± 1.2 | 9.7 ± 0.6 | 22.6 ± 0.7 | 35.5 ± 1.0 | <0.001 |
| follow-up | 19.3 ± 1.1 | 12.2 ± 1.2 | 20.3 ± 1.4 | 28.3 ± 3.5 | <0.001 |
| change | −1.7 ± 1.1 | 2.5 ± 1.4 | −2.3 ± 1.4 | −7.1 ± 3.6 | 0.008 |
| Vitamin D category on follow-up (n [%]) | |||||
| deficient | 26 (36) | 17 (71) | 7 (21) | 2 (13) | <0.001 |
| insufficient | 34 (47) | 6 (25) | 22 (67) | 6 (40) | |
| optimal | 12 (17) | 1 (4) | 4 (12) | 7 (47) |
Median {25th, 75th percentiles).
Discussion
This study addresses some important issues related to hypovitaminosis D in CKD. First, in a nationally representative cohort, patient with CKD were more likely to have 25OHD deficiency that could not be explained by differences in total daily vitamin D intakes. Second, our data suggest that urinary protein losses may be more important than loss of GFR in inducing vitamin D depletion. Finally, we were unable to demonstrate any relationship between 25OHD levels and bone health or CAC scores in patients with diabetes and CKD.
It is widely agreed that maintaining serum 25OHD levels within the reference range provided by clinical laboratories is often insufficient to maintain optimal health (1,2). Serum levels of >30 ng/ml are generally considered to be necessary to maintain bone health. Even though it has previously been posited that patients with CKD are more likely to have 25OHD deficiency, to our knowledge, the only study to have demonstrated this evaluated vitamin D levels in a geographically restricted population of patients who were sick enough to be admitted to a hospital (4,5). Herein, using a random sample of the noninstitutionalized population of the United States, we demonstrate the independent relationship between CKD and 25OHD deficiency after multiple adjustments. The KDOQI workgroup considered many potential causes for the possibly higher likelihood of lower 25OHD levels in CKD, including a lower vitamin D intake (5). Using a comprehensive assessment of intakes from both diet and supplements, we demonstrate a remarkable similarity between the total, daily vitamin D ingestion in individuals with and without CKD. Thus, the higher prevalence of 25OHD deficiency in CKD is unlikely to be a result of lower vitamin D intakes. This study does not allow us to determine the causes that contribute to lower 25OHD levels in CKD but may reflect increased urinary losses and/or reduced gastrointestinal absorption; however, these need to be determined in future studies.
In a relatively large sample of patients with albuminuria and diabetes, we then sought to identify the determinants and consequences of suboptimal 25OHD levels in CKD. In this subgroup of patients with CKD, urinary protein losses were a more important predictor than the eGFR. Nephrotic syndrome has long been recognized as an important risk factor for 25OHD deficiency osteomalacia (18,19). Consistent with the recent analyses using the NHANES III data, our study demonstrates that the graded relationship between serum 25OHD levels and proteinuria is present even at subnephrotic levels (20).
In contrast, in our DN cohort, neither was eGFR an independent risk factor in the cross-sectional analyses nor was there any relationship between the change in eGFR and 25OHD levels in the longitudinal analyses. Most of the patients with diabetes have albuminuria, and glomerular diseases account for an additional one quarter of new patients with ESRD; thus, the higher prevalence of 25OHD deficiency may in part be related to urinary losses in many patients with CKD. In this cohort of diabetic CKD, we also evaluated the relationship between serum 25OHD to two measures of bone health (serum PTH and BMD) and one measure of cardiovascular disease (CAC). In the general population, PTH levels decrease with increasing serum 25OHD, and many studies suggested that serum 25OHD level of approximately 30 ng/ml is the inflection point beyond which no further decrease in serum PTH is observed (1). Moreover, vitamin D supplementation lowers serum PTH in the general population (21). Recent cell culture studies have demonstrated that 1α-hydroxylase activity is present in bovine parathyroid cells, and these cells produce 1,25 dihydroxyvitamin D therein; this, in turn, acts locally to suppress PTH synthesis and secretion (22). This autocrine effect of vitamin D raises the possibility that circulating 25OHD may play a more important role than previously recognized in regulating serum PTH levels in CKD. Consistent with this, many studies have demonstrated an inverse relationship between serum PTH and 25OHD levels; the strength of the relationship on univariate analyses in our study cohort is similar to what has been demonstrated in other studies (6–8,23); however, previously published studies either reported only univariate analyses or performed only limited multivariate adjustment. For example, the multivariate analyses in the study by LaClair et al. (7) did not include race; black individuals have a higher prevalence of 25OHD deficiency and hyperparathyroidism, and, thus, race may be an important confounder. In our study, we were unable to demonstrate any significant relationship between serum PTH and 25OHD levels on multivariate adjustment, leading us to conclude that suboptimal 25OHD may contribute only modestly to the hyperparathyroidism in CKD. This notion is further supported by three uncontrolled studies in which vitamin D supplementation led to statistically but not clinically significant decreases in serum PTH in patients with stage 3 CKD and no change in patients with stage 4 CKD (9,24,25). The magnitude of benefit of 25OHD supplementation on lowering serum PTH levels needs to be tested in randomized, controlled trials.
In the general population, there is a graded relationship between BMD and the risk for fracture. Numerous studies in the past have demonstrated a high prevalence of reduced BMD in individuals with CKD (reviewed in reference [15]). In this study, we assessed BMD using QCT, a technique that has several advantages over dual-energy x-ray absorptiometry. QCT allows a distinction to be made between cortical and trabecular bone; herein, we measured only the trabecular BMD. Furthermore, using QCT ensures that BMD is not inadvertently overestimated by the presence of aortic calcium. To our knowledge, this is the first study to use QCT in non–dialysis-dependent patients with CKD and confirms the high prevalence of osteopenia in this population. The value of reduced BMD in predicting the future risk for fractures in non–dialysis-dependent patients with CKD, however, has not been consistently demonstrated (26). The pathophysiologic basis for the reduction in BMD in CKD is likely multifactorial (27). In this study of diabetic CKD, we were unable to demonstrate any relationship between serum 25OHD and BMD. For a better understanding of the pathophysiologic basis for the reduced BMD that frequently is seen in patients with CKD, it is probably necessary to include bone biopsies along with such noninvasive measures such as QCT.
Finally, we attempted to ascertain whether BMD and serum 25OHD levels were associated with CAC burden. Studies in both the general population and dialysis patients have demonstrated an inverse relationship between BMD and the severity of CAC, likely as a result of shared pathophysiology (28). In this study, we extend this finding to non–dialysis-dependent patients with DN. This inverse relationship is probably a result of biologic processes that reduce bone mass on one hand and induce vascular calcification on the other. Cell culture studies suggested that oxidized LDL cholesterol and leptin are two candidates that may explain this shared biology: CKD is associated with increased oxidative stress and hyperleptinemia (29,30). In addition to this inverse relationship between bone health and CAC, the rationale for this analysis included a recent study from our group wherein we reported that low 25OHD levels co-varied with increasing prevalence and/or severity of traditional risk factors for cardiovascular disease (31). Furthermore, serum 25OHD levels seemed to be inversely associated with insulin resistance and microalbuminuria (20,32). Finally, an inverse relationship was previously demonstrated between serum 1,25 dihydroxyvitamin D and CAC score in the general population (33,34); however, we were unable to demonstrate any relationship between serum 25OHD levels and cardiovascular disease burden in patients with diabetes and CKD.
There are several limitations of our study. In the NHANES III study, data were collected only at one point in time, and a significant proportion of patients did not have sufficient information to determine the presence of CKD. The dietary intake of vitamin D was ascertained by a 24-h diet recall, with its inherent limitation of recall bias and that estimation of intake is limited to a single 24-h period. The study of the consequences of 25OHD deficiency was limited to patients with albuminuria and diabetes and mild to moderate CKD, because serum PTH levels were not measured in the NHANES III cohort. Even though the absence of relationship between serum 25OHD and PTH levels was confirmed on longitudinal analyses, follow-up evaluation was not completed on some of the patients. The vast majority of the patients in study B were Latino; care should be exercised in extrapolating these findings to other racial/ethnic groups. The evaluation of the relationship between serum 25OHD and BMD and CAC was limited to cross-sectional analyses. The stronger effects of diabetes in inducing cardiovascular disease may have masked the association between vitamin D deficiency and CAC. Finally, we were not able to examine potential differences in vitamin D receptor polymorphisms, which may modify the cellular affects of low 25OHD and/or the ratio of generated PTH fragments that could have important health implications.
Conclusions
25OHD deficiency is more common in CKD, but this higher prevalence is not explained by differences in total daily intake of vitamin D. Suboptimal 25OHD levels are very common in patients with albuminuria and diabetes; however, the consequence of the suboptimal levels remains to be definitively elucidated.
Disclosures
R.M. has received grant support from Shire, Genzyme, and Amgen and serves as a consultant to Shire and Novartis. K.N. has received research support from Abbott Laboratories and honoraria from Abbott Laboratories, Merck, and Monarch pharmaceuticals.
Acknowledgments
R.M. is supported by grant RR18298 from the National Institutes of Health (NIH). Additional support for this work was provided by Harbor-UCLA General Clinical Research Center grant M01-RR00425 from the NIH. Support for this work was also provided by NIH grants RR011145 (D.K. and K.N.), RR019234 (D.K. and K.N.), RR014616 (K.N.), and MD000182 (K.N.).
Part of this work was presented in the form of a poster at the 39th annual meeting of the American Society of Nephrology; November 14 through 19, 2006; San Diego, CA.
Published online ahead of print. Publication date available at www.cjasn.org.
Access to UptoDate on-line is available for additional clinical information at http://www.cjasn.org/
References
- 1.Holick MF: Vitamin D deficiency. N Engl J Med 357: 266–281, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B: Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr 84: 18–28, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Zadshir A, Tareen N, Pan D, Norris K, Martins D: The prevalence of hypovitaminosis D among US adults: Data from the NHANES III. Ethn Dis 15: S5–97–S5–101, 2005 [PubMed] [Google Scholar]
- 4.Thomas MK, Lloyd-Jones DM, Thadhani RI, Shaw AC, Deraska DJ, Kitch BT, Vamvakas EC, Dick IM, Prince RL, Finkelstein JS: Hypovitaminosis D in medical inpatients. N Engl J Med 338: 777–783, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Kidney Disease Outcome Quality Initiative: Clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42: S1–S201, 2003 [PubMed] [Google Scholar]
- 6.Gonzalez EA, Sachdeva A, Oliver DA, Martin KJ: Vitamin D insufficiency and deficiency in chronic kidney disease: A single center observational study. Am J Nephrol 24: 503–510, 2004 [DOI] [PubMed] [Google Scholar]
- 7.LaClair RE, Hellman RN, Karp SL, Kraus M, Ofner S, Li Q, Graves KL, Moe SM: Prevalence of calcidiol deficiency in CKD: A cross-sectional study across latitudes in the United States. Am J Kidney Dis 45: 1026–1033, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, Andress DL: Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: Results of the study to evaluate early kidney disease. Kidney Int 71: 31–38, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Zisman AL, Hristova M, Ho LT, Sprague SM: Impact of ergocalciferol treatment of vitamin D deficiency on serum parathyroid hormone concentrations in chronic kidney disease. Am J Nephrol 27: 36–43, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–1994. Series 1: Programs and collection procedures. Vital Health Stat 1 1–407, 1994 [PubMed]
- 11.Ezzati TM, Massey JT, Wakesberg J, Chu A, Maurer KR: Sample design: Third National Health and Nutrition Examination Survey. Vital Health Stat 2 113: 1–35, 1992 [PubMed] [Google Scholar]
- 12.National Kidney Foundation: K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification and stratification. Am J Kidney Dis 39: S1–S266, 2002 [PubMed] [Google Scholar]
- 13.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS: Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis 41: 1–12, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Mehrotra R, Budoff MJ, Christenson P, Ipp E, Takasu J, Gupta A, Norris K, Adler SA: Determinants of coronary artery calcification in diabetics with and without nephropathy. Kidney Int 66: 2022–2031, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Mehrotra R: Disordered mineral metabolism and vascular calcification in nondialyzed chronic kidney disease. J Ren Nutr 16: 100–118, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R: Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 15: 827–832, 1990 [DOI] [PubMed] [Google Scholar]
- 17.Wong M, Papa A, Lang T, Hodis HN, Labree L, Detrano R: Validation of thoracic quantitative computed tomography as a method to measure bone mineral density. Calcif Tissue Int 76: 7–10, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Eastwood JB, Stamp TC, Harris E, de Wardener HE: Vitamin-D deficiency in the osteomalacia of chronic renal failure. Lancet 4: 1209–1211, 1976 [DOI] [PubMed] [Google Scholar]
- 19.Mason RS, Lissner D, Wilkinson M, Posen S: Vitamin D metabolites and their relationship to azotaemic osteodystrophy. Clin Endocrinol (Oxf) 13: 375–385, 1980 [DOI] [PubMed] [Google Scholar]
- 20.de Boer IH, Ioannou GN, Kestenbaum B, Brunzell JD, Weiss NS: 25-Hydroxyvitamin D levels and albuminuria in the Third National Health and Nutrition Examination Survey (NHANES III). Am J Kidney Dis 50: 69–77, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Chapuy MC, Arlot ME, Duboeuf F, Brun J, Crouzet B, Arnaud S, Delmas PD, Meunier PJ: Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med 327: 1637–1642, 1992 [DOI] [PubMed] [Google Scholar]
- 22.Ritter CS, Armbrecht HJ, Slatopolsky E, Brown AJ: 25-Hydroxyvitamin D(3) suppresses PTH synthesis and secretion by bovine parathyroid cells. Kidney Int 70: 654–659, 2006 [DOI] [PubMed] [Google Scholar]
- 23.St John A, Thomas MB, Davies CP, Mullan B, Dick I, Hutchison B, van der Schaff A, Prince RL: Determinants of intact parathyroid hormone and free 1,25-dihydroxyvitamin D levels in mild and moderate renal failure. Nephron 61: 422–427, 1992 [DOI] [PubMed] [Google Scholar]
- 24.DeVille J, Thorp ML, Tobin L, Gray E, Johnson ES, Smith DH: Effect of ergocalciferol supplementation on serum parathyroid hormone and serum 25-hydroxyvitamin D in chronic kidney disease. Nephrology (Carlton) 11: 555–559, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Al-Aly Z, Qazi RA, Gonzalez EA, Zeringue A, Martin KJ: Changes in serum 25-hydroxyvitamin D and plasma intact PTH levels following treatment with ergocalciferol in patients with CKD. Am J Kidney Dis 50: 59–68, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Ensrud KE, Lui LY, Taylor BC, Ishani A, Shlipak MG, Stone KL, Cauley JA, Jamal SA, Antoniucci DM, Cummings SR: Renal function and risk of hip and vertebral fractures in older women. Arch Intern Med 167: 133–139, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Sprague SM: Renal function and risk of hip and vertebral fractures in older women: Is it always osteoporosis? Arch Intern Med 167: 115–116, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Braun J, Oldendorf M, Moshage W, Heidler R, Zeitler E, Luft FC: Electron beam computed tomography in the evaluation of cardiac calcification in chronic dialysis patients. Am J Kidney Dis 27: 394–401, 1996 [DOI] [PubMed] [Google Scholar]
- 29.Parhami F, Morrow AD, Balucan J, Leitinger N, Watson AD, Tintut Y, Berliner JA, Demer LL: Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation: A possible explanation for the paradox of arterial calcification in osteoporotic patients. Arterioscler Thromb Vasc Biol 17: 680–687, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Parhami F, Tintut Y, Ballard A, Fogelman AM, Demer LL: Leptin enhances the calcification of vascular cells: Artery wall as a target of leptin. Circ Res 88: 954–960, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Martins D, Wolf M, Pan D, Zadshir A, Tareen N, Thadhani R, Felsenfeld A, Levine B, Mehrotra R, Norris K: Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: Data from the Third National Health and Nutrition Examination Survey. Arch Intern Med 167: 1159–1165, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Chonchol M, Scragg R: 25-Hydroxyvitamin D, insulin resistance, and kidney function in the Third National Health and Nutrition Examination Survey. Kidney Int 71: 134–139, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Watson KE, Abrolat ML, Malone L, L, Hoeg JM, Doherty T, Detrano R, Demer LL: Active serum vitamin D levels are inversely correlated with coronary calcification. Circulation 96: 1755–1760, 1997 [DOI] [PubMed] [Google Scholar]
- 34.Doherty TM, Tang W, Dascalos S, Watson KE, Demer LL, Shavelle RM, Detrano RC: Ethnic origin and serum levels of 1alpha,25-dihydroxyvitamin D3 are independent predictors of coronary calcium mass measured by electron-beam computed tomography. Circulation 96: 1477–1481, 1997 [DOI] [PubMed] [Google Scholar]