Abstract
Background and objectives: Albuminuria is regarded a sensitive measure of progression of glomerular disease. This study was undertaken in patients who had systemic lupus erythematosus glomerulonephritis (n = 57) and were followed in the Ohio SLE Study to determine whether measuring albuminuria offered clinical advantages over that of total proteinuria.
Design, setting, participants, & measurements: Twenty-four-hour urine collections (n = 127) were obtained at baseline and annually for measurement of microalbumin, total protein, and creatinine.
Results: There was a strong linear relationship between microalbumin-creatinine and protein-creatinine ratios over the entire range of protein-creatinine ratios; however, in the protein-creatinine ratio range 0.0 to 0.3, as the protein-creatinine ratio increased, the microalbumin-protein ratio increased much more than the protein-creatinine ratio. Also, the greater the protein-creatinine ratio, the greater was the evidence for nonselective proteinuria (protein-creatinine ratio − microalbumin-creatinine ratio).
Conclusions: For the diagnosis of proteinuria renal flare, measuring albuminuria offers no advantage over measuring total proteinuria because changes in protein-creatinine and microalbumin-creatinine ratios are highly correlated over the designated ranges for systemic lupus erythematosus glomerulonephritis proteinuric flares. In those with normal-range proteinuria, subsequent changes in microalbumin-protein ratio might be a better forecaster of renal flare than changes in protein-creatinine or microalbumin-creatinine ratio. High protein-creatinine ratios are associated with evidence of nonselective proteinuria, which may increase the nephrotoxicity of proteinuria. Thus, using high-threshold criteria for systemic lupus erythematosus flare (allowing greater proteinuria increase before flare is declared) may expose the kidney to greater nephrotoxicity than using the low-threshold criteria for systemic lupus erythematosus flare.
Glomerular injury usually induces an increase in glomerular permeability to macromolecules, resulting in increased urinary excretion of plasma proteins. Under conditions of severe glomerular injury, albumin (molecular weight approximately 69 kD) is the most abundant protein excreted in the urine, generally accounting for much more than 50% of the urinary proteins (1). Thus, albuminuria is the hallmark of glomerular proteinuria; however, under conditions of mild glomerular injury, albumin usually comprises much less than 50% of urinary proteins (1). The low rate of albuminuria compared with that of total proteinuria (albumin + nonalbumin proteinuria) in mild glomerular injury is thought to be the result, at least in part, of the greater capacity of the renal tubules to absorb filtered albumin compared with that of larger proteins such as IgG (molecular weight approximately 150 kD) (2–4). Hereafter, “total proteinuria” is referred to as proteinuria.
The prime example of using albuminuria to monitor progression of early glomerular injury is in diabetic glomerulosclerosis, where increases in albuminuria are indicative of progression of diabetic glomerulosclerosis, even when the proteinuria rate is within the normal range (e.g., <200 mg/24 h) (5–8). For measurement of low-level changes in albuminuria, immunoassays have been developed to detect urine albumin in concentrations <1 mg/dl. These are referred to as “microalbumin” assays (1). Normal 24-h urine albumin excretion by these assays is <30 mg albumin/g creatinine (8). Albuminuria rates of 30 to 300 mg/g creatinine are referred to as “microalbuminuria.” Albuminuria rates beyond that range are referred to as “macroalbuminuria” (8). When the macroalbuminuria range is reached in diabetic nephropathy, albumin becomes the dominant urinary protein and proteinuria parallels albuminuria. At that point, the advantage of measuring albuminuria over proteinuria is generally lost (7).
It is well established that in chronic kidney disease (CKD), albuminuria and proteinuria are highly correlated (7–9), particularly when 24-h proteinuria exceeds 500 mg (7); however, in systemic lupus erythematosus glomerulonephritis (SLE GN), the relationship between proteinuria and albuminuria has not been rigorously examined (10–12). It is plausible that albuminuria–proteinuria relationships are different in SLE GN compared with that of other forms of CKD. Mechanisms that could account for such differences include the following: (1) The microalbuminuria assay does not detect intact albumin that has been modified in vivo. The latter process is particularly common in patients with diabetes and CKD (13). The extent to which albumin is modified in SLE is unknown. (2) Albumin that undergoes glomerular filtration in CKD is extensively absorbed by the renal tubules. The fraction of albumin that is not absorbed undergoes extensive degradation, apparently by proximal tubular lysosomes, with excretion in urine as low molecular weight peptides <10 kD. These peptides are not measured by the immunoassay for microalbuminuria or by the usual clinical measures of proteinuria such as pyrogallol red or Coomassie blue (4,13,14). In experimental models of GN, renal tubular degradation of albumin does not occur (13,14). Thus, intact albumin could be overrepresented in SLE GN urine compared with that of CKD urine. (3) There is evidence from experimental models that some conditions of moderate albuminuria may be entirely the result of failure of renal tubular retrieval of albumin that normally is filtered by the normal glomerulus (15,16). Renal tubular albumin retrieval could differ between SLE and other CKD conditions. (4) Hypoalbuminuria independent of urine protein loss commonly occurs in SLE. The apparent mechanism is inflammation-induced albumin catabolism (17). This mechanism could influence albuminuria–proteinuria relationship in SLE GN compared with that of other causes of CKD in which inflammation is not a prominent feature. This study explores the relationship between albuminuria and proteinuria in patients with SLE GN across a wide range of proteinuria, including threshold ranges that commonly are used for identifying SLE proteinuric flares.
Materials and Methods
This study involved 57 patients who had SLE GN and were screened for or followed in the Ohio SLE Study (OSS) (18), a research protocol approved by the Ohio State University institutional review board and that required informed written consent for participation and is in adherence to the Declaration of Helsinki. Each patient underwent kidney biopsy. This showed 5% with World Health Organization (WHO) class II, 19% with class III, 44% with class IV, and 32% with class V. For those who were followed in the OSS, 24-h testing for protein and creatinine was done bimonthly (18). Annually, testing for microalbuminuria was included in the 24-h testing regimen.
For assessment of the diagnostic usefulness of albuminuria compared with proteinuria in assessing SLE proteinuric flare, regression of albumin (A)-creatinine (C) ration on protein (P)-C ratio was examined in relationship to the previously published criteria for SLE GN proteinuric flare. These criteria can be organized into three general categories, as follows: Low-threshold criteria for the diagnosis of proteinuric SLE flare are those reported by the OSS (18), the LJP 394 Study (19), and the British Isle Lupus Assessment Group (BILAG) Category B renal flare (20). In the cited studies, the minimum increase in proteinuria that qualifies as a proteinuric renal flare are OSS (24-h urine P-C ratio increasing from <0.5 to ≥1.0) (18), LJP 394 (24-h proteinuria increasing by 800 mg along with corroborative changes in urine sediment [19]), and BILAG category B (24-h proteinuria of >1.0 g, increasing by >50 but <100% [20]). The intermediate threshold criterion is that of BILAG category A renal flare (proteinuria increasing from <0.2 to >1.0 g/24 h) (21). The high threshold criteria are those reported by Moroni et al. (22), Ioannidis et al. (23), Illei et al. (24), Mosca et al. (25), and Mok et al. (26). In those studies, the minimum increase in proteinuria that qualified as an SLE proteinuric flare was, in a patient with 24-h proteinuria <2.0 g, an increase in 24-h proteinuria of ≥2.0 g/24 h.
In the OSS, proteinuria and albuminuria rates are expressed as the P-C ratio or the A-C ratio of intended 24-h urine collection. This was done to adjust for inaccuracies in the collection of the intended 24-h urine collections (27,28). To convert the 24-h protein excretion rates of the other cited studies (18–26) to 24-h P-C ratios, we divided the 24-h proteinuria values used for each of the flare criteria by 1.3. The latter value is the mean 24-h urine creatinine excretion in the OSS patients with SLE GN based on 645 consecutive intended 24-h urine collections (28).
Analytic Studies
All analyses were performed in the laboratories of the Ohio State University Medical Center. Urine protein was measured by an automated pyrogallol red method (29), coefficient of variation (CV) 3.3% at control level 71.6 mg/dl. Urine creatinine was measured by an automated picric acid method CV 2.8%, control level 79.0 mg/dl. Microalbuminuria was measured by immunoassay CV 0.7%, control level 9.4 to 13.5 mg/dl.
Statistical Analysis
All mean values are ± 1 SD. The relationship between albuminuria or nonselective proteinuria and total proteinuria was examined by linear regression.
Results
The baseline clinical characteristics of the 57 patients were mean age of 34.4 ± 10.8 yr; 91% female; 64% European American, 33% African American, and 3% other races; mean serum creatinine of 1.11 ± 0.6 mg/dl; mean 24-h urine P-C ratio 1.51 ± 2.36; mean C3 98 ± 39 mg/dl; mean C4 17 ± 12 mg/dl. At baseline, therapy included prednisone in 52 (91%) of 57, an immunosuppressive (mycophenolate, azathioprine, or cyclophosphamide) in 50 (88%) of 57, angiotensin-converting enzyme inhibitor (ACEI) or angiotensin receptor blocker in 31 (54%) of 57. All of the patients with abnormal proteinuria (P-C ratio >0.2) were receiving either an ACEI or an angiotensin receptor blocker. Other therapies that can influence proteinuria (30) either were not used (e.g., nonsteroidal anti-inflammatory drugs) or were used in too few patients to influence this study. Specifically, statins were received by eight patients, nondihydropyridine calcium channel blocker by one patient, and dihydropyridine calcium channel blocker by five patients. A total of 127 baseline and annual 24-h urine collections were tested for protein, microalbumin, and creatinine. The median number of 24-h urine collections tested per patient was 2.0.
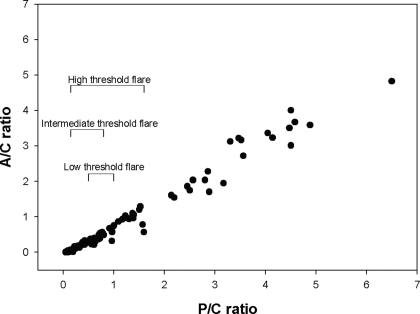
Figure 1 shows the relationship between the P-C ratio and A-C ratio for each of the 24-h urine collections. As shown, P-C and A-C were linearly related and highly correlated (r = 0.99, slope = 0.79, P < 0.0001). Shown in relationship to the regression of A-C on P-C is the minimum increase in proteinuria required to diagnose SLE proteinuric flare by the low- (18,19), intermediate- (19,20), and high-threshold criteria (22–26).
Figure 1.
Relationship between protein (P)-creatinine (C) and albumin (A)-C ratios in the 24-h urine collections (n = 127) of the patients with systemic lupus erythematosus glomerulonephritis (SLE GN; n = 57) in this study. Also shown are representative minimum P-C ratio changes for a proteinuric renal flare according to whether low-, intermediate-, or high-threshold criteria are used. The flare criteria have been adjusted so that they correspond to changes in P-C ratio (see the Materials and Methods). As shown, P-C and A-C ratios are highly correlated over the entire range of the P-C ratios (slope = 0.79, r = 0.99, P < 0.0001).
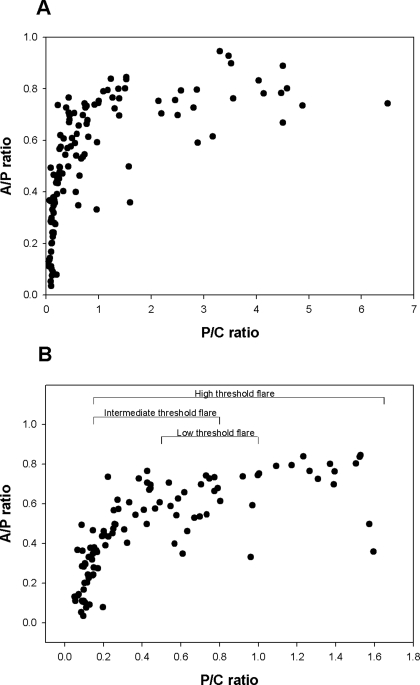
To explore further the informative value of albuminuria measurements, we examined the relationship between 24-h urine P-C ratio and the corresponding A-P ratio (the proportion of urine protein that is immunoassayable albumin). This relationship is shown in Figure 2 over the entire range of 24-h urine P-C ratios (Figure 2A) and over the range of P-C ratios <1.8 (Figure 2B). The data in Figure 2 indicate that as proteinuria increases from normal to higher levels, the A-P ratio increases more rapidly than the P-C ratio, until a P-C ratio of approximately 0.3 is reached. Thereafter, the A-P ratio plateaus.
Figure 2.
Relationship between P-C ratio and the proportion of urine protein that is albumin (A-P). (A) These relationships across the entire range of P-C ratios of this study. (B) These relationships over the subset of patients with low-level proteinuria (24-h urine P-C ratios ≤1.8). As shown, the proportion of total proteinuria that is albumin rises sharply until the P-C ratio is approximately 0.3. Thereafter, the A-P ratio tends to plateau.
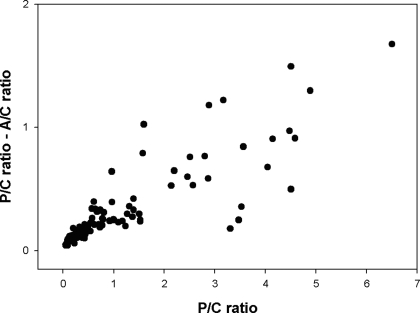
There is considerable evidence that proteinuria itself is nephrotoxic, particularly nonselective proteinuria (reviewed in references [30,31]). In this study, we estimated the magnitude of nonselective proteinuria as P-C ratio − A-C ratio. This parameter is shown in relationship to P-C ratio in Figure 3. As shown, the greater the P-C ratio, the greater is the magnitude of A-C ratio − P-C ratio (r = 0.86, slope = 0.21, P < 0.0001). There was no relationship between the patients' renal biopsy WHO classification (determined at study baseline) and the proteinuria relationships described (determined at baseline, during OSS follow-up [up to 4 yr]), or both).
Figure 3.
Relationship between P-C ratio and (P-C ratio − A-C ratio), which is nonalbuminuria proteinuria (r = 0.86, slope = 0.21, P < 0.0001).
Discussion
This study is the first to examine in detail the relationship between albuminuria and proteinuria in SLE GN. The comparison of albuminuria with proteinuria was based on annual testing and without regard to the patient's proteinuria status. Thus, this analysis is not a longitudinal assessment; however, we suggest that it is a rigorous cross-sectional assessment of the relationship between albuminuria and proteinuria in SLE GN because each segment of the regression of A-C on P-C ratio is represented by multiple patients (the median measure per patient is 2.0), and the principal therapies that most influence proteinuria (ACEI, angiotensin receptor blocker) were uniformly used in the patients with abnormal proteinuria. Thus, we suggest that the shape of the cross-sectional relationships shown in Figures 1, 2, and 3 are representative of the expected longitudinal relationships of these parameters.
We found no correlation between the albuminuria/proteinuria relationships of this study and the study patients' kidney biopsy WHO classification; however, this issue was not rigorously tested by the study protocol because kidney biopsy generally was done at or before entry into the OSS, and most of the proteinuric events reported in this study occurred ≥1 yr after study entry. Because WHO class changes with therapy, we cannot be sure of the patient's WHO classification at the time of the proteinuric flare. Nevertheless, because of the relatively good coherence of the albuminuria–proteinuria relationships among the individuals of this study, it seems unlikely that, at least for the WHO classifications III, IV, and V, there are important differences in albuminuria–proteinuria relationships according to WHO class.
This work does not determine whether the albuminuria–proteinuria relationships differ between those tested during SLE proteinuric flare compared with those tested during chronic proteinuric states of SLE GN. To test that question rigorously would require a testing protocol that was activated in relationship to proteinuric flare; however, the protocol of this study involved testing proteinuria at regular intervals, rather than in response to proteinuric flare. Nevertheless, virtually all of the data describing albuminuria–proteinuria relationships under conditions of heavy proteinuria (e.g., P-C ratio >1.0) represent SLE flare conditions. The reason is that virtually all of the patients in the OSS who experienced proteinuric flare were treated and achieved complete or near-complete remission of their proteinuric flare. As a consequence, this study is largely describing the albuminuria–proteinuria relationships under conditions of SLE proteinuric flare.
This work shows that over the entire range of P-C ratios of this study (0.0 to 6.5), A-C and P-C ratios are linearly related and highly correlated. Specifically, albuminuria is nearly a constant proportion of proteinuria over the range of P-C ratios that define the low- (18,19), intermediate- (19,20), and high- threshold criteria (22–26) for proteinuric flares. Thus, there seems to be no advantage in measuring albuminuria rather than proteinuria to diagnose SLE proteinuric flare; however, measuring albuminuria might have an advantage in forecasting SLE proteinuric flare. This is suggested by the finding that albuminuria as a proportion of proteinuria (A-P ratio) rises rapidly over the P-C range of 0.0 to 0.3. Thus, compared with P-C ratio, monitoring trends in A-P ratio might be more sensitive in identifying patients who have SLE GN and are at increased risk for proteinuric flare. To test this hypothesis would require study of a large cohort of patients who have SLE GN and have developed complete remission (24-h urine P-C ratio <0.3) and then are followed longitudinally for measurement of both P-C and A-P ratios to determine which measure better predicts renal flare. Although simple in concept, such a determination is well beyond the scope of this work.
The finding that the A-P ratio is more sensitive to changes in low-level proteinuria than the P-C ratio, whereas the A-C ratio is not, seems contradictory; however, comparing A-C with P-C ratio (Figure 1) is a comparison of the absolute difference between albuminuria and proteinuria. At normal proteinuria rates (P-C ratios <0.3), the absolute differences between albuminuria and proteinuria are very small and for this reason are not evident in Figure 1. By contrast, at P-C ratio <0.3, the relative changes in albuminuria to proteinuria (A-P ratio) are very large and therefore are evident when comparing A-P with P-C ratio (Figure 2). Thus, for those with baseline low-level proteinuria, monitoring change in A-P ratio should make it easier to detect GN progression than measuring change in A-C or P-C ratio.
Although A-P ratio is more sensitive to change than P-C ratio when P-C ratio is in the normal range, we suggest that it cannot be assumed that trends in A-P ratio would be more informative than trends in P-C ratio in forecasting SLE flare. The basis for this interpretation is that, in the OSS patients with GN and low-level proteinuria, it is common to observe fluctuations in the bimonthly 24-h urine P-C ratios (unpublished observations). These low-level fluctuations in 24-h P-C ratio could represent subthreshold proteinuric flares that resolve spontaneously. Virtually all of the OSS patients were receiving prednisone, immunosuppressive therapy, or both. This therapy could be sufficient to suppress a mild GN flare. Thus, having a more sensitive method to detect “mini flares” (e.g., by monitoring A-P ratio) might not be clinically important.
Consistent with the suggestion that spontaneous resolution of mild SLE GN flares in those who are on maintenance therapy are our previous observations (32). We found that patients who had SLE GN and low-level proteinuria and experienced mild nephritic flares (recurrence of red cell or mixed red cell leukocyte casts) usually experienced remission without an increase in immunosuppressive or steroid therapy (32). Furthermore, it is plausible that the level of glomerular injury that carries a high risk for progression to proteinuric flare is one that induces nonselective proteinuria (both albumin and the larger molecular weight proteins are excreted in increased amounts in the urine). Under that scenario, A-P ratio would have decreased sensitivity to predict SLE flare because albuminuria and proteinuria would change in parallel.
A further reservation regarding the routine use of microalbuminuria to monitor SLE GN status is the substantially greater cost of the microalbumin assay compared with the proteinuria assay (30). In light of all of these concerns, we suggest that monitoring SLE GN by routine microalbuminuria assay is not warranted at this time.
This study also shows that, in general, nonalbumin proteinuria (P-C ratio − A-C ratio) increases progressively with increasing P-C ratio. This is consistent with the notion that in SLE GN, the greater the P-C ratio, the greater is the amount of nonselective proteinuria. The rationale for using nonalbumin proteinuria as a surrogate for nonselective proteinuria is as follows: In patients with overt glomerulopathy, (1) increasing proteinuria is associated with increasing albuminuria (Figure 1). (2) As albuminuria increases, also increased is the excretion of both high molecular weight proteins (e.g., IgG, α2 macroglobulin, and IgM) and low molecular weight proteins (e.g., β2 microglobulin) (33–35). Thus, nonalbumin proteinuria (P-C ratio − A-C ratio) increases. Because the nonalbumin proteinuria fraction contains the high molecular weight proteins, it follows that increased nonalbumin proteinuria is evidence of increased nonselective proteinuria. Also, the pyrogallol method underestimates IgG by approximately one third (9). Thus, the increase in nonselective proteinuria suggested by Figure 3 likely is even greater than that shown by the term (P-C ratio − A-C ratio).
Nonselective proteinuria may be more nephrotoxic than selective proteinuria (30,31). Thus, the use of high-threshold criteria for SLE proteinuric flare (22–26) may expose the kidney to greater nephrotoxicity than the use of the low-threshold criteria for SLE flare (18–20). On this basis, it could be argued that, compared with the routine use of low-threshold criteria, the routine use of high-threshold criteria for proteinuric flare before triggering an increase in therapy will delay therapy and might increase the risk for irreversible kidney damage. Conversely, using the low-threshold criteria to trigger an increase in therapy might expose the patient to more steroid and immunosuppressive therapy than is needed to control SLE GN. No randomized trials have compared the risks and benefits of treating patients with SLE GN by the low-, medium-, or high-threshold criteria. Our data suggest, however, that this is an important issue that should be addressed in future studies.
Conclusions
For the diagnosis of proteinuria renal flare, measuring albuminuria offers no advantage over measuring total proteinuria because changes in P-C and A-C ratio are highly correlated over the designated ranges for SLE GN proteinuric flares; however, in those with normal-range proteinuria, subsequent changes in A-P ratio might be a better forecaster of renal flare than changes in P-C or A-C ratio. High P-C ratios are associated with evidence of nonselective proteinuria, which may increase the nephrotoxicity of proteinuria. Thus, using high-threshold criteria for SLE flare (allowing greater proteinuria increase before flare is declared) may expose the kidney to greater nephrotoxicity than using the low-threshold criteria for SLE flare.
Disclosures
None.
Acknowledgments
Supported in part by National Institutes of Health grants PO1 DK55546, UO1 DK48621, and MO1 RR00034.
This work was published in abstract form (J Am Soc Nephrol 18: 566A, 2007).
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Johnson AM: Amino acids, peptides, and proteins. In: Tietz Textbook of Clinical Chemistry and Molecular Diagnostics, Vol. 1, 4th Ed., edited by Burtis CA, Ashwood ER, Bruns DE, St. Louis, Elsevier Saunders, 2006, pp 533–554 [Google Scholar]
- 2.Birn H, Christensen EI: Renal albumin absorption in physiology and pathology. Kidney Int 69: 440–449, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Gudehithlu KP, Pegoraro AA, Dunea G, Arruda JA, Singh AK: Degradation of albumin by the renal proximal tubule cells and the subsequent fate of its fragments. Kidney Int 65: 2113–2122, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Russo LM, Bakris GL, Comper WD: Renal handling of albumin: A critical review of basic concepts and perspective. Am J Kidney Dis 39: 899–919, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Mogensen CE: Microalbuminuria as a predictor of clinical diabetic nephropathy. Kidney Int 31: 673–689, 1987 [DOI] [PubMed] [Google Scholar]
- 6.Chavers BM, Bilous RW, Ellis EN, Steffes MW, Mauer SM: Glomerular lesions and urinary albumin excretion in type I diabetes without overt proteinuria. N Engl J Med 320: 966–970, 1989 [DOI] [PubMed] [Google Scholar]
- 7.KDOQI: Clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification: Guideline 5. Assessment of proteinuria. Am J Kidney Dis 39: S93–S102, 2002 [PubMed] [Google Scholar]
- 8.KDOQI: Clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis 49: S42–S61, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Agarwal R: On the nature of proteinuria with acute renal injury in patients with chronic kidney disease. Am J Physiol Renal Physiol 288: F265–F271, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Cottiero RA, Madaio MP, Levey AS: Glomerular filtration rate and urinary albumin excretion rate in systemic lupus erythematosus. Nephron 69: 140–146, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Batlle-Gualda E, Martinez AC, Guerra RA, Pascual E: Urinary albumin excretion in patients with systemic lupus erythematosus without renal disease. Ann Rheum Dis 56: 386–389, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valente de Almeida R, Rocha de Carvalho JG, de Azevedo VF, Mulinari RA, Ioshhi SO, da Rosa Utiyama S, Nisihara R: Microalbuminuria and renal morphology in the evaluation of subclinical lupus nephritis. Clin Nephrol 52: 218–229, 1999 [PubMed] [Google Scholar]
- 13.Comper WD, Osicka TM, Clark M, MacIsaac RJ, Jerums G: Earlier detection of microalbuminuria in diabetic patients using a new urinary albumin assay. Kidney Int 65: 1850–1855, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Strong KJ, Osicka TM, Comper WD: Urinary-peptide excretion by patients with and volunteers without diabetes. J Lab Clin Med 145: 239–246, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Gekle M: Renal albumin handling: A look at the dark side of the filter. Kidney Int 71: 479–481, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Russo LM, Sandoval RM, McKee M, Osicka TM, Collins AB, Brown D, Molitoris BA, Comper WD: The normal kidney filters nephrotic levels of albumin retrieved by proximal tubule cells: Retrieval is disrupted in nephrotic states. Kidney Int 71: 504–513, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Niwa Y, Iio A, Niwa G, Sakane T, Tsunematsu T, Kanoh T: Serum albumin metabolism in rheumatic diseases: Relationship to corticosteroids and peptic ulcer. J Clin Lab Immunol 31: 11–16, 1990 [PubMed] [Google Scholar]
- 18.Rovin BH, Song H, Birmingham DJ, Hebert LA, Yu CY, Nagaraja HN: Urine chemokines as biomarkers of human SLE activity. J Am Soc Nephrol 16: 467–473, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Alarcon-Segovia D, Tumlin JA, Furie RA, McKay JD, Cardiel MH, Strand V, Bagin RG, Linnik MD, Hepburn B: LJP 394 for the prevention of renal flare in patients with systemic lupus erythematosus: Results from a randomized, double-blind, placebo-controlled study. Arthritis Rheum 48: 442–454, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Yee CS, Farewell V, Isenberg DA, Prabu A, Sokoll K, Teh LS, Rahman A, Bruce IN, Griffiths B, Akil M, McHugh N, D'Cruz D, Khamashta MA, Bowman S, Maddison P, Zoma A, Allen E, Gordon C: Revised British Isles Lupus Assessment Group 2004 index: A reliable tool for assessment of systemic lupus erythematosus activity. Arthritis Rheum 54: 3300–3305, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Isenberg DA, Rahman A, Allen E, Farewell V, Akil M, Bruce IN, D'Cruz D, Griffiths B, Khamashta M, Maddison P, McHugh N, Snaith M, Teh LS, Yee CS, Zoma A, Gordon C: BILAG 2004. Development and initial validation of an updated version of the British Isles Lupus Assessment Group's disease activity index for patients with systemic lupus erythematosus. Rheumatology (Oxford) 44: 902–906, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Moroni G, Quaglini S, Maccario M, Banfi G, Ponticelli C: “Nephritic flares” are predictors of bad long-term renal outcome in lupus nephritis. Kidney Int 50: 2047–2053, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Ioannidis JP, Boki KA, Katsorida ME, Drosos AA, Skopouli FN, Boletis JN, Moutsopoulos HM: Remission, relapse, and re-remission of proliferative lupus nephritis treated with cyclophosphamide. Kidney Int 57: 258–264, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Illei GG, Takada K, Parkin D, Austin HA, Crane M, Yarboro CH, Vaughan EM, Kuroiwa T, Danning CL, Pando J, Steinberg AD, Gourley MF, Klippel JH, Balow JE, Boumpas DT: Renal flares are common in patients with severe proliferative lupus nephritis treated with pulse immunosuppressive therapy: Long-term followup of a cohort of 145 patients participating in randomized controlled studies. Arthritis Rheum 46: 995–1002, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Mosca M, Bencivelli W, Neri R, Pasquariello A, Batini V, Puccini R, Tavoni A, Bombardieri S: Renal flares in 91 SLE patients with diffuse proliferative glomerulonephritis. Kidney Int 61: 1502–1509, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Mok CC, Ying KY, Tang S, Leung CY, Lee KW, Ng WL, Wong RW, Lau CS: Predictors and outcome of renal flares after successful cyclophosphamide treatment for diffuse proliferative lupus glomerulonephritis. Arthritis Rheum 50: 2559–2568, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Shidham G, Hebert LA: Timed urine collections are not needed to measure urine protein excretion in clinical practice. Am J Kidney Dis 47: 8–14, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Birmingham DJ, Rovin BH, Shidham G, Nagaraja HN, Zou X, Bissell M, Yu CY, Hebert LA: Spot urine protein/creatinine ratios are unreliable estimates of 24 h proteinuria in most systemic lupus erythematosus nephritis flares. Kidney Int 72: 865–870, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Watanabe N, Kamei S, Ohkubo A, Yamanaka M, Ohsawa S, Makino K, Tokuda K: Urinary protein as measured with a pyrogallol red-molybdate complex, manually and in a Hitachi 726 automated analyzer. Clin Chem 32: 1551–1554, 1986 [PubMed] [Google Scholar]
- 30.Wilmer WA, Rovin BH, Hebert CJ, Rao SV, Kumor K, Hebert LA: Management of glomerular proteinuria: A commentary. J Am Soc Nephrol 14: 3217–3232, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Abbate M, Zoja C, Remuzzi G: How does proteinuria cause progressive renal damage? J Am Soc Nephrol 17: 2974–2984, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Hebert LA, Dillon JJ, Middendorf DF, Lewis EJ, Peter JB: Relationship between appearance of urinary red blood cell/white blood cell casts and the onset of renal relapse in systemic lupus erythematosus. Am J Kidney Dis 26: 432–438, 1995 [DOI] [PubMed] [Google Scholar]
- 33.Reichert LJ, Koene RA, Wetzels JF: Urinary IgG excretion as a prognostic factor in idiopathic membranous nephropathy. Clin Nephrol 48: 79–84, 1997 [PubMed] [Google Scholar]
- 34.Tencer J, Torffvit O, Thysell H, Rippe B, Grubb A: Proteinuria selectivity index based upon alpha 2-macroglobulin or IgM is superior to the IgG based index in differentiating glomerular diseases. Technical note. Kidney Int 54: 2098–2105, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Bazzi C, Petrini C, Rizza V, Arrigo G, D'Amico G: A modern approach to selectivity of proteinuria and tubulointerstitial damage in nephrotic syndrome. Kidney Int 58: 1732–1741, 2000 [DOI] [PubMed] [Google Scholar]