Abstract
Kidneys damaged by ischemia have the potential to regenerate through a mechanism involving intrarenal induction of protective factors, including bone morphogenetic protein-7 (BMP7). Epigenetic changes, such as alterations in histone modifications, have also been shown to play a role in various pathologic conditions, but their involvement in ischemic injury and regeneration remains unknown. This study investigated whether changes in histone acetylation, regulated by histone acetyltransferase and histone deacetylase (HDAC), are induced by renal ischemia and involved in the regenerative response. Ischemia/reperfusion of the mouse kidney induced a transient decrease in histone acetylation in proximal tubular cells, likely as a result of a decrease in histone acetyltransferase activity as suggested by experiments with energy-depleted renal epithelial cells in culture. During recovery after transient energy depletion in epithelial cells, the HDAC isozyme HDAC5 was selectively downregulated in parallel with the return of acetylated histone. Knockdown of HDAC5 by RNAi significantly increased histone acetylation and BMP7 expression. BMP7 induction and HDAC5 downregulation in the recovery phase were also observed in proximal tubular cells in vivo after transient ischemia. These data indicate that ischemia induces dynamic epigenetic changes involving HDAC5 downregulation, which contributes to histone re-acetylation and BMP7 induction in the recovery phase. This highlights HDAC5 as a modulator of the regenerative response after ischemia and suggests HDAC5 inhibition may be a therapeutic strategy to enhance BMP7 expression.
Epigenetic mechanisms including histone acetylation play a key role in organ development and cellular homeostasis by regulating gene expressions in a tissue- and developmental stage–specific manner.1–3 Increase in histone acetylation, the levels of which are determined by the balance between the activities of histone acetyltransferase (HAT) and histone deacetylase (HDAC), generally stimulates gene transcription by relaxing the chromatin structure.1,2 Recent reports indicated that changes in the histone acetylation level are also correlated with several pathologic conditions, including cancer,1,4 cardiac hypertrophy,5 chronic obstructive pulmonary disease,6 denervation,7 and recovery of learning and memory after neuronal loss.8
Ischemic organ damage is a major cause of morbidity and mortality in industrialized countries. Ischemia and subsequent reperfusion induce a variety of metabolic changes in the affected organs, including a decrease in the ATP levels, production of reactive oxygen species, and alterations in the intracellular ion and pH homeostasis.9–11 In organs damaged by ischemia, including the heart,12,13 brain,14,15 and kidney,11,16 proteins that are involved in organogenesis during embryonic development have been shown to be re-induced. One of the unique features of the kidney, as compared with those of the heart and brain, is that it can regenerate almost completely after transient ischemia, making it an ideal organ system to study the mechanisms underlying regeneration after ischemia. Recent evidence indicated that re-induction of proteins involved in nephrogenesis and proliferation of resident tubular epithelial cells play major roles in kidney regeneration after ischemic injury.17,18 Proteins such as leukemia inhibitory factor (LIF) and bone morphogenetic protein-7 (BMP7), which play pivotal roles in nephron formation during kidney development, have been shown to be induced and to contribute critically to the proliferation and repair of the tubular cells after ischemia.11,19,20 In addition, administration of BMP7 has been shown to exert beneficial effects in various models of renal injury.21–23 The elucidation of mechanisms underlying the induction of protective factors after ischemic insult is of great potential importance from the viewpoint of development of therapy aimed at organ repair after injury. Given the critical role of epigenetic mechanisms in the induction and silencing of developmental genes,2,3 we reasoned that chromatin remodeling may somehow be modulated by ischemia. In this regard, whether chromatin remodeling plays some role in ischemia and the subsequent regenerative processes remains largely unknown. To examine the possible involvement of epigenetic mechanisms in the tissue response to ischemia, we focused on the alterations in the histone acetylation level and HDAC expression. We used the renal ischemia/reperfusion model for in vivo evaluation and also used an in vitro model of ischemia,19,24–26 in which transient energy depletion is achieved by inhibiting mitochondrial oxidative phosphorylation.
RESULTS
Reversible Decrease of Histone Acetylation in the Ischemic Kidney
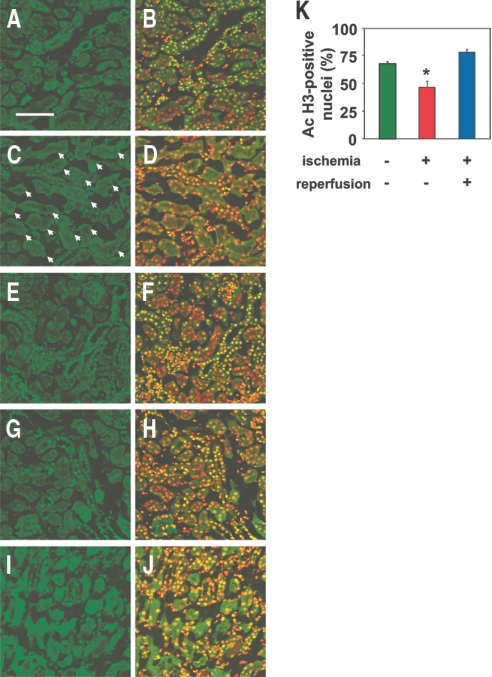
Ischemia of the kidney by clamping of the renal pedicle for 40 min induced a marked decrease in the staining for histone acetylation in the proximal tubular cells of the outer medulla (Figure 1, A through D), the region of the kidney where ischemic injury is most prominent as a result of the low blood flow and high energy requirements even under normal conditions.11 In contrast, the changes in the histone acetylation level were less pronounced in the cortex (Figure 1, E through H). Cell counting analysis revealed that the percentage of cells positive for acetylated histone was significantly reduced in the outer medulla of the kidneys subjected to ischemia, as compared with that in the kidneys not subjected to ischemia (Figure 1K). Immunohistochemical analysis of the kidneys obtained 24 h after reperfusion revealed the restoration of histone acetylation in the tissue, although some proximal tubular cells in the outer medulla still remained negative for histone acetylation staining (Figure 1, I and J). The percentage of cells positive for acetylated histone in the outer medulla 24 h after reperfusion recovered to levels that were not significantly different from those in the kidneys without ischemia (Figure 1K).
Figure 1.
Reversible decrease in histone acetylation after ischemia. (A through H) Immunohistochemical staining of the renal outer medulla (A through D) and cortex (E through H) from nonischemic (A, B, E, and F) and ischemic (C, D, G, and H) kidneys with acetylated histone (green) immediately after 40 min of ischemia. Localization of nuclei in the same sections A, C, E, and G is shown by co-staining with TOPRO-3 (red) in B, D, F, and H, respectively. Bar = 100 μm. The proximal tubules stained negative for acetylated histone are shown by arrows in C. (I and J) Immunohistochemical staining of the renal outer medulla of the ischemic kidney with acetylated histone (green) 24 h after ischemia/reperfusion. Localization of nuclei in the same section I is shown by co-staining with TOPRO-3 (red) in J. Results are from representative sections of four mice in each group. (K) Quantitative analysis of nuclei positive for acetylated histone in the outer medulla of kidneys is shown. Nonischemic and ischemic kidneys obtained from the mice immediately after 40 min of ischemia and ischemic kidneys 24 h after ischemia/reperfusion were analyzed. Data are mean percentage of total nuclei ± SEM (n = 4). *P < 0.05 versus values without ischemia.
Histone Remodeling after Transient Energy Depletion in Kidney Epithelial Cells
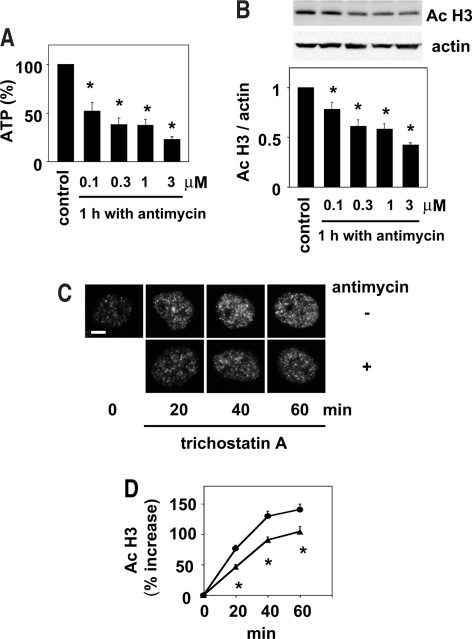
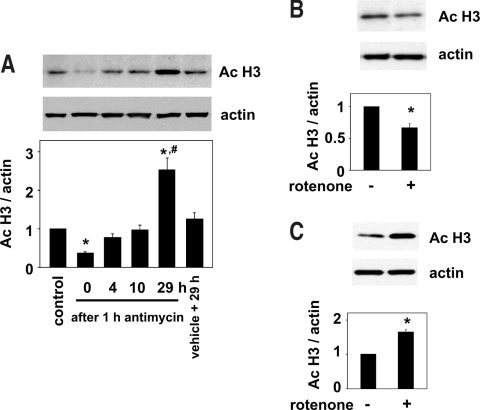
To investigate in further detail the epigenetic changes caused by ischemia, we next used a cell culture model of ischemia.19,24–26 The ATP contents in normal kidney epithelial cells, NRK 52E cells, were decreased after treatment with antimycin A, an inhibitor of mitochondrial oxidative phosphorylation that induces energy depletion, in a concentration-dependent manner (Figure 2A). In parallel with the decrease in the cellular ATP levels, histone acetylation, as determined by Western blot analysis, was also decreased by antimycin A (Figure 2B). To examine the mechanism involved in the decline of histone acetylation after energy depletion induced by antimycin A, we next investigated the in situ HAT activity. The in situ HAT activity, determined by monitoring the increase in histone acetylation associated with inhibition of HDAC by the addition of trichostatin A, an inhibitor of HDAC, was significantly decreased by antimycin A in the renal tubular cells (Figure 2, C and D). We next examined the changes in the histone acetylation in the recovery phase after transient energy depletion. Consistent with the results published previously,19 the ATP contents were stored to the levels not significantly different from those measured just before the incubation with antimycin A (98.5 ± 3.0% of the value obtained just before incubation with antimycin A; n = 5; P > 0.05) at 4 h after washout of antimycin A. In parallel with the recovery of the ATP levels, the histone acetylation levels also recovered to baseline levels by 4 h after the washout of antimycin A (Figure 3A). After this recovery to the baseline, a significant increase in histone acetylation was observed at 29 h after the transient energy depletion (Figure 3A), although ATP levels were indistinguishable in the two groups at this late time point (data not shown). Consistent with the results obtained using antimycin A, the levels of histone acetylation were significantly decreased by treatment with rotenone (Figure 3B), another inhibitor of mitochondrial oxidative phosphorylation, and significantly increased by 29 h after washout of the reagent (Figure 3C). These observations suggest that the cellular energy levels dynamically regulate the histone acetylation level under ischemic conditions.
Figure 2.
Energy depletion reduces histone acetylation via decreased in situ HAT activity in the renal tubular cells. (A) NRK 52E cells were incubated with or without (control) various concentrations of antimycin A (0.1 to 3 μM) for 1 h, and the cellular ATP levels were determined. Values normalized to the levels in the control are means ± SEM (n = 5). *P < 0.05 versus control values. (B) NRK 52E cells were incubated with or without (control) various concentrations of antimycin A (0.1 to 3 μM) for 1 h, and Western blot analysis of acetylated histone was performed. Representative films of Western blot analysis for acetylated histone and actin are shown in the top panels. The results of densitometric analysis are shown in the bottom panel. Data are means ± SEM (n = 4). *P < 0.05 versus control values. (C) Representative immunofluorscent images of NRK 52E cell nuclei stained with acetylated histone 0, 20, 40, or 60 min after the addition of trichostatin A (300 nM), the HDAC inhibitor, in the presence or absence of antimycin A (1 μM). Bar = 3 μm. (D) The signal intensity of acetylated histone from at least 40 cells for each condition described in C was obtained by laser confocal microscopy. Increases in the signals from the baseline (time 0) with (▴) or without (•) antimycin A were calculated and presented as means ± SEM (n = 40). The signals at the baseline were defined as 100%. *P < 0.05 versus values without antimycin A at each time point.
Figure 3.
Time-dependent changes in histone acetylation after transient energy depletion. (A) After 1 h of treatment with antimycin A (1 μM), NRK 52E cells were incubated in the maintenance medium for the recovery of ATP. After various incubation periods up to 29 h, the cells were harvested and Western blot analysis was performed. Control denotes cells harvested before incubation with antimycin A. Cells harvested 29 h after incubation in the maintenance medium after treatment with vehicle instead of antimycin A for 1 h were also analyzed (vehicle + 29h). Representative films of Western blot analysis for acetylated histone and actin are shown in the top panels. The results of densitometric analysis are shown in the bottom panel. Data are means ± SEM (n = 3). *P < 0.05 versus control values; #P < 0.05 versus values with vehicle + 29h. (B) NRK 52E cells were incubated with or without rotenone (10 μM) for 1 h, and Western blot analysis was performed. Representative films of Western blot analysis for acetylated histone and actin are shown in the top panels. The results of densitometric analysis are shown in the bottom panel. Data are means ± SEM (n = 6). *P < 0.05 versus values without rotenone. (C) After 1 h of treatment with or without rotenone (10 μM), NRK 52E cells were incubated in the maintenance medium for 29 h, and Western blot analysis was performed. Representative films of Western blot analysis for acetylated histone and actin are shown in the top panels. The results of densitometric analysis are shown in the bottom panel. Data are means ± SEM (n = 3). *P < 0.05 versus values without rotenone.
Downregulation of HDAC5 after Transient Energy Depletion Induces Histone Re-acetylation and BMP7 Expression
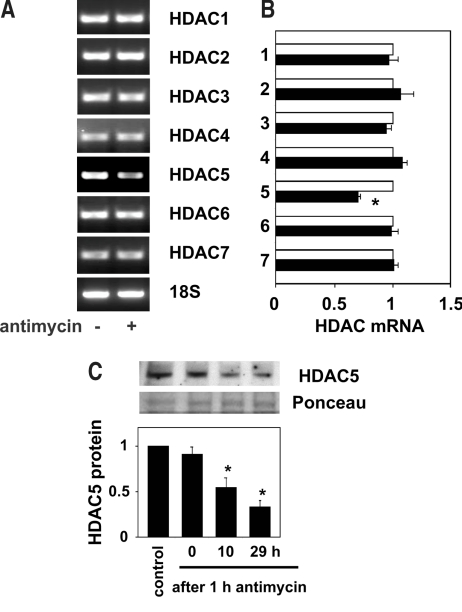
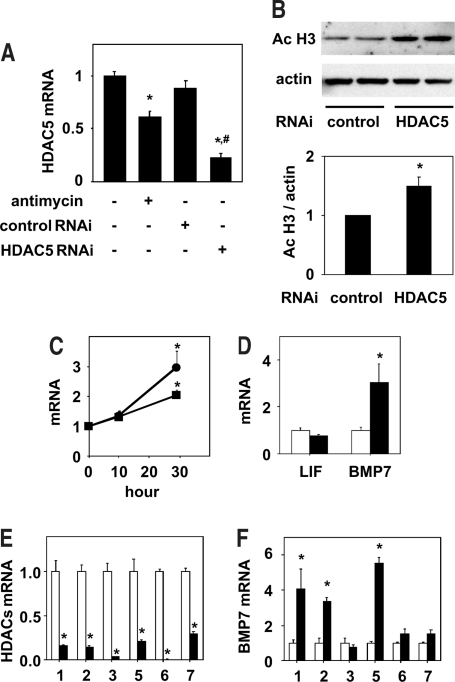
Because rebound increase in histone acetylation was observed after transient energy depletion, we reasoned that some mechanisms that actively increase histone acetylation may have been triggered during the recovery phase. To gain insight into the underlying mechanisms, we next examined the mRNA levels of the HDAC isozymes in the renal tubular cells. Reverse transcriptase–PCR (RT-PCR) analysis revealed that HDAC5 mRNA levels were significantly decreased, whereas those of HDAC 1, 2, 3, 4, 6, and 7 remained unaltered after transient cellular energy depletion (Figures 4, A and B, and 5A). The decline in the HDAC5 mRNA level was accompanied by a time-dependent decrease in the nuclear HDAC5 protein level (Figure 4C).
Figure 4.
Downregulation of HDAC5 after transient energy depletion. (A and B) After 1 h of incubation with or without antimycin A (1 μM), NRK 52E cells were incubated in the maintenance medium for 29 h, and the HDAC mRNA and 18S ribosomal RNA levels were analyzed by semiquantitative RT-PCR. Representative agarose gels are shown in A. Values obtained by densitometric analysis of RT-PCR for HDAC with (▪) or without (□) antimycin A treatment were normalized to those for 18S and expressed as relative values to those obtained without antimycin A in B. Data are means ± SEM (n = 4 to 6). *P < 0.05 versus values without antimycin A. (C) Nuclear HDAC5 protein levels were determined by Western blot analysis in NRK 52E cells before exposure to antimycin A (control), after treatment with antimycin A for 1 h, and after 10 and 29 h of incubation in the maintenance medium after 1 h of exposure to antimycin A. Representative films of Western blot analysis for HDAC5 and Ponceau S staining of the membrane before Western blotting are shown in the top panels. The results of densitometric analysis are shown in the bottom panel. Data are means ± SEM (n = 4). *P < 0.05 versus control values.
Figure 5.
HDAC5 knockdown increases histone acetylation and BMP7 mRNA expression. (A) After 1 h of treatment with or without antimycin A (1 μM), NRK 52E cells were incubated in the maintenance medium for 29 h, and the HDAC5 mRNA levels were analyzed by real-time RT-PCR. Cells for the knockdown experiments were treated with control or HDAC5 Stealth RNAi for 24 h before the 1-h treatment with PBS-based medium and the subsequent incubation in maintenance medium for 29 h. Data are means ± SEM (n = 7 to 8). *P < 0.05 versus values without antimycin A or Stealth RNAi; #P < 0.05 versus values with control Stealth RNAi. (B) The levels of acetylated histone in NRK 52E cells treated with control or HDAC5 Stealth RNAi as described in A were determined by Western blot analysis. Representative films of Western blot analysis for acetylated histone and actin are shown in the top panels. The results of densitometric analysis are shown in the bottom panel. Data are means ± SEM (n = 4). *P < 0.05 versus values with control RNAi. (C) Time-dependent increase in LIF and BMP7 mRNA after transient energy depletion. The levels of LIF (▪) and BMP7 (•) mRNA in NRK 52E cells treated with antimycin A as described in A and incubated in the maintenance medium for 10 and 29 h were determined by real time RT-PCR analysis. Data are means ± SEM (n = 4). Time 0 denotes a group of cells before exposure to antimycin A. *P < 0.05 versus values without antimycin A. (D) The levels of LIF and BMP7 mRNA in NRK 52E cells treated with control (□) or HDAC5 (▪) Stealth RNAi as described in A were determined by real time RT-PCR analysis. Data are means ± SEM (n = 8 to 9). *P < 0.05 versus values with control RNAi. (E and F) NRK 52E cells were incubated with control (□) or HDAC isozyme (▪) Stealth RNAi for 48 h. For HDAC5 knockdown, second HDAC5 RNAi was used. The mRNA levels of each HDAC isozyme (E) and BMP7 (F) were determined by real-time RT-PCR. Data are means ± SEM (n = 4). *P < 0.05 versus control values.
To investigate whether the decrease in HDAC5 played a significant role in the increase in histone acetylation after transient energy depletion, we knocked down HDAC5 expression by using RNA interference (RNAi). HDAC5 mRNA levels were markedly reduced after transfection of HDAC5 Stealth RNAi but not by that of control Stealth RNAi (Figure 5A). The levels of histone acetylation were also significantly increased in the cells treated with HDAC5 Stealth RNAi as compared with those in the cells treated with control RNAi (Figure 5B). On the basis of the finding that the activity of HDAC5 regulates the histone acetylation level, we next investigated the possibility that HDAC5 downregulation may induce alterations in the expressions of LIF and BMP7, which are known to be induced in the renal tubules in the recovery phase after ischemia.19,20 Consistent with previous reports,19,20 the mRNA levels of LIF and BMP7 both were increased after transient energy depletion (Figure 5C). Moreover, the mRNA level of BMP7 was significantly increased in the cells treated with HDAC5 Stealth RNAi as compared with that in the cells treated with control RNAi, whereas that of LIF was unchanged (Figure 5D). These results indicate that downregulation of HDAC5 induces expression of BMP7 but not of LIF after transient energy depletion.
HDAC1, 2, and 5 Regulate BMP7 Expression
Experiments using another HDAC5 RNAi provided further evidence that HDAC5 indeed regulates the expression of BMP7 (Figure 5, E and F). To explore whether the effect of HDAC inhibition on BMP7 expression is specific for HDAC5, we examined the effects of RNAi for other HDAC, whose expressions were relatively high in renal epithelial cells as judged from RT-PCR. Knockdown of HDAC1 and 2 markedly increased the BMP7 mRNA level, whereas HDAC3, 6, and 7 RNAi had no significant effect (Figure 5, E and F). These results indicate that, in addition to HDAC5, HDAC1 and 2 can act as regulators of BMP7 expression.
Induction of BMP7 and Downregulation of HDAC5 in the Renal Proximal Tubular Cells after Ischemia
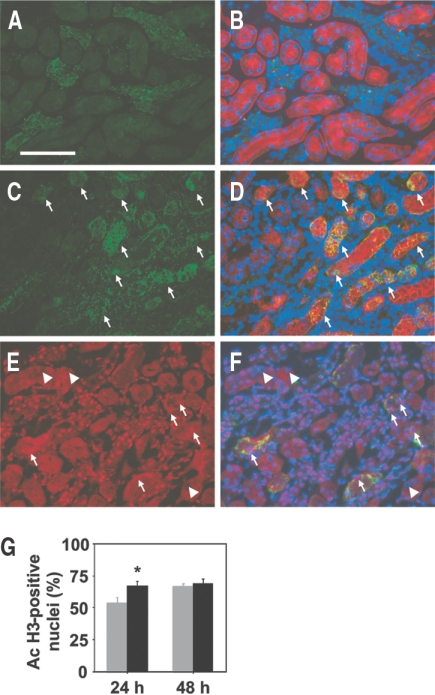
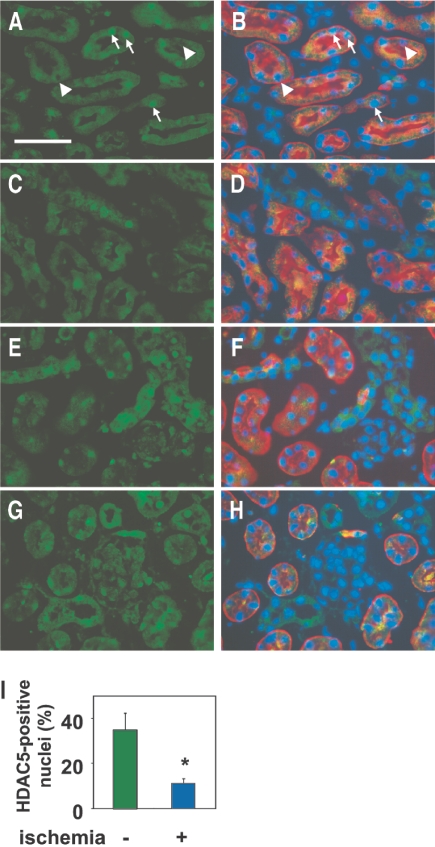
To examine the relationship between BMP7 and HDAC5 in vivo, we investigated the expression of these molecules in the recovery phase after renal ischemia by immunohistochemical analysis. BMP7 has been reported to be expressed in the adult kidney but not in uninjured proximal tubular cells.27,28 After ischemia, strong BMP7 induction has been noted in the proximal tubular cells, which reaches the peak at 48 h after reperfusion.20 Consistent with these previous findings, BMP7 expression was confined to the nonproximal tubular epithelial cells in the kidneys not subjected to ischemia (Figure 6, A and B). In contrast, marked induction of BMP7 was observed in the proximal tubular cells of the outer medulla at 48 h after ischemia/reperfusion (Figure 6, C and D). We compared the histone acetylation level between the proximal tubular cells with and without BMP7 expression in the early phase (24 h) and the peak (48 h) of BMP7 induction. The percentage of cells positive for acetylated histone in cells with BMP7 induction was significantly increased as compared with that in cells without BMP7 expression at 24 h (Figure 6, E through G); however, the percentage of cells positive for acetylated histone became similar in cells with and without BMP7 induction at 48 h. These results suggest that the histone acetylation level may recover earlier in cells in which BMP7 is induced than in those without BMP7 induction. Although HDAC5 has been shown to be expressed in the kidney,29,30 the intrarenal distribution of HDAC5 has not been reported; therefore, we first determined the expression pattern of HDAC5 in the nonischemic kidneys. Nuclear HDAC5 expression was detected in the glomerular cells and epithelial cells in the cortex and medulla, including the proximal tubular cells, in the kidneys not subjected to ischemia, although not all nuclei were positive for HDAC5 (Figure 7, A, B, E, and F). Because both histone remodeling and BMP7 induction were observed mainly in the proximal tubular cells of the outer medulla, we focused on the expression of HDAC5 in these cells after ischemia. The number of cells positive for nuclear HDAC5 was markedly decreased in the proximal tubular cells of the outer medulla in the kidneys subjected to ischemia, whereas the HDAC5 expression was relatively well maintained in the cortex (Figure 7, C, D, G, and H). Cell-counting analysis revealed that the percentage of cells positive for nuclear HDAC5 was significantly reduced in the proximal tubular cells of the outer medulla in the kidneys subjected to ischemia, as compared with that in the kidneys not subjected to ischemia (Figure 7I).
Figure 6.
Induction of BMP7 and recovery of acetylated histone in the proximal tubular cells of the outer medulla in the recovery phase after ischemia. Immunohistochemical staining of the renal outer medulla from nonischemic (A and B) and ischemic (C and D) kidneys for BMP7 (green) at 48 h after ischemia/reperfusion. Localization of the proximal tubules and nuclei of the same sections A and C is shown by co-staining with Lotus tetragonolobus (red), a marker of proximal tubular cells, and DAPI (blue) in B and D, respectively. The proximal tubules with BMP7 induction after ischemia/reperfusion are indicated by arrows in C and D. Bar = 100 μm. Immunohistochemical staining of the renal outer medulla from ischemic (E and F) kidneys for acetylated histone (red) at 24 h after ischemia/reperfusion. Localization of nuclei (TOPRO-3, blue) and BMP7-positive tubules (green) in the same section E is shown by co-staining in F. Results are from representative sections of four mice in each group. Nuclei positive for acetylated histone (arrows) appear purple in F, whereas those negative for acetylated histone (arrowheads) remain blue in F. (G) Quantitative analysis of nuclei positive for acetylated histone in the proximal tubular cells with (▪) and without (□) BMP7 induction is shown. The proximal tubular cells in the outer medulla of ischemic kidneys obtained from the mice 24 and 48 h after ischemia/reperfusion were analyzed. Data are mean percentage of total nuclei of the proximal tubular cells with or without BMP7 induction ± SEM (n = 4). *P < 0.05 versus values without BMP7 induction.
Figure 7.
Decreased nuclear HDAC5 expression in the recovery phase after ischemia. Immunohistochemical staining of the renal outer medulla (A through D) and cortex (E through H) from nonischemic (A, B, E, and F) and ischemic (C, D, G, and H) kidneys for HDAC5 (green) at 48 h after ischemia/reperfusion. Localization of the proximal tubules and nuclei of the same sections A, C, E, and G is shown by co-staining with Lotus tetragonolobus (red) and DAPI (blue) in B, D, F, and H, respectively. Nuclei positive for HDAC5 appear light blue (arrows), whereas those negative for HDAC5 appear dark blue (arrowheads), as shown in B. Most of nuclei of the proximal tubular cells are negative for HDAC5 in D. Bar = 50 μm. Results are from representative sections of four mice in each group. (I) Quantitative analysis of HDAC5-positive nuclei of the proximal tubules in the outer medulla from kidneys with and without ischemia is shown. Data are mean percentage of total nuclei of the proximal tubular cells ± SEM (n = 4). *P < 0.05 versus values without ischemia.
DISCUSSION
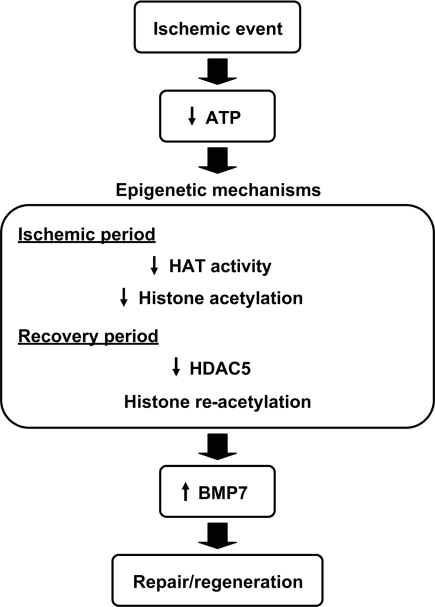
This study demonstrates that ischemia induces histone remodeling, which may be involved in the subsequent regenerative response (Figure 8). In the in vitro model of ischemia, we showed that cellular energy conditions can regulate the levels of histone acetylation, an important determinant of the epigenetic status that regulates gene transcription, in response to ischemia. Alterations in the histone acetylation level with the sensing of the cellular energy conditions and the related changes in gene transcription may represent a cellular adaptation mechanism to ischemia. The decrease in the histone acetylation in response to energy depletion may be attributable to a decrease in HAT activity. The observation of the reduced in situ HAT activity under the energy-depleted condition is consistent with a previous report of decrease in the HAT activity associated with ATP depletion in a purified, recombinant assay system31 and indicates that this is also the case in living cells.
Figure 8.
Model showing the proposed role of epigenetic mechanisms in ischemia and the subsequent response in the kidney. In response to ischemia, the levels of acetylated histone decrease in the proximal tubular cells, probably as a result of the decreased in situ HAT activity. After reperfusion, the levels of acetylated histone recover in part through HDAC5 downregulation. Decreased levels of HDAC5 contribute to the induction of BMP-7 in the proximal tubules, which may be involved in the regeneration of renal tubules.
In the recovery phase after transient energy depletion, the histone acetylation level returned to the baseline in parallel with the ATP content. Among the HDAC isozymes, mRNA and nuclear protein levels of HDAC5 were found to decrease in parallel with the recovery of histone acetylation. Class II HDAC, to which HDAC5 belongs, are known to be expressed in a tissue-specific manner, whereas class I and III HDAC are distributed ubiquitously.1,5 HDAC5 has been shown to be present in organs including the heart, brain, skeletal muscle, lung, liver, and kidney.29,30 Although the critical role of HDAC5 in the heart under stress has been well characterized,5 the regulation and functions of HDAC5 after ischemia have not been reported. Because HDAC5 knockdown by RNAi increased the histone acetylation level, decrease in HDAC5 is likely to be involved in the recovery of histone acetylation after transient energy depletion. Consistent with the data obtained in vitro, HDAC5 downregulation was indeed observed in the proximal tubular cells of the outer medulla in the recovery phase after ischemia in vivo.
After ischemic injury of the kidney, developmental factors are known to reappear and to contribute to the regeneration of the organ.11 BMP7 is known as a factor that critically contributes to the development of the kidney. Although BMP7 is present in the precursor cells of the nephron, its expression is repressed in the proximal tubular cells after maturation of the kidney.27,28,32 BMP7 has been shown to be strongly induced in the proximal tubular cells in the recovery phase after ischemia.20 Our findings are consistent with these reports and also suggest the involvement of epigenetic mechanisms in the reappearance of BMP7 in the proximal tubular cells after ischemia/reperfusion. This notion is lent support by the finding of BMP7 induction in the proximal tubular cells of the medulla, where the histone remodeling and concurrent HDAC5 downregulation were most prominently observed. The results of HDAC5 knockdown experiments using RNAi provided direct evidence of BMP7 expression induced by HDAC5 downregulation; however, involvement of other factors in BMP7 induction in vivo, including changes in the chromatin structure of the BMP7 promoter region, remains to be clarified.
The results obtained in this study are consistent with our previous report that trichostatin A, an inhibitor of classes I and II HDAC, induced BMP7 mRNA expression in human renal tubular cells.33 Several HDAC inhibitors have begun to be used clinically in the treatment of malignant tumors.1,34 The therapeutic potential of HDAC inhibitors in other conditions refractory to the usual treatments has also been described, including motor neuron disease35 and dementia8; therefore, the functions and regulation of HDAC need to be urgently delineated. Information on each HDAC isozyme should be clarified individually given that class I and II HDAC can even exert opposing effects on cardiac hypertrophy.5,36 In this regard, this study reveals that, among the isozymes, HDAC5 selectively functions as a switch molecule in response to energy depletion. In addition, the ability to regulate BMP7 expression was found to be shared by HDAC1, HDAC2, and HDAC5 in renal epithelial cells. The regenerative effects of BMP7 administration have been demonstrated in various models of renal injury.21–23 In addition to the favorable effect of direct BMP7 administration, enhancement of endogenous BMP signaling has been shown to ameliorate renal injury.37,38 Taken together, this study indicates that HDAC5 downregulation may activate a regenerative process after ischemia by inducing BMP7 and also suggests the potential of HDAC5 inhibition as a novel therapeutic strategy for enhancing BMP7 expression aimed at repair of the injured kidney.
This study demonstrates that transient ischemia induces epigenetic changes characterized by a decrease in histone acetylation probably as a result of decreased in situ HAT activity in the ischemic period and subsequent downregulation of HDAC5 that contributes, at least in part, to histone re-acetylation and BMP7 induction in the recovery period. These observations reveal a novel function of HDAC5 as a sensor that transmits the cellular energy conditions to chromatin and also suggest HDAC5 inhibition as a potential therapeutic strategy for enhancing BMP7 expression aimed at regeneration of the injured kidney.
CONCISE METHODS
Ischemia/Reperfusion Injury
Ischemia/reperfusion injury was induced in 7- or 8-wk-old female C57BL6 mice (18 to 22 g; Tokyo Laboratory Animal Center, Tokyo, Japan). Mice were anesthetized with intraperitoneal pentobarbital (50 mg/kg) and placed on a heating pad to maintain the core body temperature at 37°C. The left kidneys were exposed through the flank, and the renal pedicles were clamped with vascular clamps (Roboz Surgical Instrument Co., Gaithersburg, MD) for 40 min. The kidneys from one group were then perfused with saline and removed as samples for analysis of the effects of ischemia without reperfusion. Other mice were allowed to recover, and the kidneys were harvested after 24 or 48 h under anesthesia with pentobarbital. The right kidneys from the same mice were used as samples for examination of kidneys not subjected to ischemia. Animal care and treatment complied with the standards described in the Guidelines for the Care and Use of Laboratory Animals of the University of Tokyo.
Cell Culture and Transient Energy Depletion
The normal rat kidney epithelium-derived cell line, NRK 52E, was obtained from the American Type Culture Collection and grown in DMEM with 5% FBS in a humidified 5% CO2/95% air environment at 37°C. An established cell culture model for ischemia was used to induce transient ATP depletion in the NRK 52E cells.19,24–26 In brief, a confluent monolayer of NRK 52E cells, which had been incubated in the maintenance medium (DMEM with 1% FBS) for 1 d, was incubated in PBS with 1.5 mM CaCl2, 2 mM MgCl2, and 1 μM antimycin A (Sigma, St. Louis, MO) for 60 min. During the recovery phase after the 1-h injury period, the cells were replenished with ATP by incubation in the maintenance medium. After various incubation periods up to 29 h, the cells were harvested and analyzed. Cellular ATP levels were determined with a luciferase-based assay kit (Sigma).
Immunohistochemistry
For immunohistochemical staining of acetylated histone H3, BMP7, and HDAC5 in the renal tissue, coronal sections of the renal tissue were immersion-fixed in 4% buffered paraformaldehyde for 12 h; washed with 10, 15, and 20% sucrose in PBS for 4 h each time; embedded in OCT compound; and snap-frozen in liquid nitrogen. Frozen sections, 6 μm in thickness, were stained with rabbit anti-acetylated histone H3 (Lys9) polyclonal antibody (Upstate Biotechnology, Lake Placid, NY; dilution 1:300), goat anti-BMP7 polyclonal antibody (Santa Cruz Biotechnologies, Santa Cruz, CA; dilution 1:50), and rabbit anti-HDAC5 polyclonal antibody (Santa Cruz Biotechnologies; dilution 1:25) as the respective primary antibodies. Alexa Fluor 488 goat anti-rabbit IgG (Molecular Probes, Eugene, OR) and Alexa 488 donkey anti-goat IgG (Molecular Probes) were used as the secondary antibody at a dilution of 1:200. Sections stained for acetylated histone were analyzed by confocal laser-scanning microscopy (Leica DMIR/E2 TCS SL; Leica, Wetzlar, Germany) equipped with a 40 × 0.55 NA objective after nuclear staining with TOPRO-3 (Molecular Probes; dilution 1:500). To determine the percentage of cells positive for acetylated histone, we analyzed three different fields in the outer medulla of each kidney, each containing at least 200 cells. Sections stained for BMP7 and HDAC5 were then incubated with the proximal tubular cell marker,39 biotin-conjugated lectin from Lotus tetragonolobus (Vector Laboratories, Burlingame, CA; dilution 1:100), and then with 20 μg/ml UltraAvidin conjugated with Texas Red (Leinco Technologies, St. Louis, MO). Slides were mounted with Vectashield anti-fade mounting medium with DAPI (Vector Laboratories) and viewed under an Eclipse E600 epifluorescence microscope equipped with 20 × 0.5 NA and 40 × 0.75 NA objectives. Images were obtained with a digital camera (DXM 1200C; Nikon, Tokyo, Japan). To compare the percentage of cells positive for acetylated histone between cells with and without BMP7 induction, we analyzed at least 10 proximal tubules with or without BMP7 induction in the outer medulla of each kidney. For determination of the percentage of cells positive for nuclear HDAC5, four different fields in the outer medulla of each kidney, each containing at least 50 proximal tubular cells, were analyzed. For determination of the expression of acetylated histone H3 in the NRK52E cells, the cells were fixed in ice-cold methanol and stained with a rabbit polyclonal antibody against acetyl histone H3 (Lys9; Upstate Biotechnology; dilution 1:300) as the primary antibody. After incubation with Alexa Fluor 488 goat anti-rabbit IgG (Molecular Probes) as the secondary antibody at a dilution of 1:200, the cells were analyzed by confocal laser-scanning microscopy (Leica DMIR/E2 TCS SL; Leica) with a 40 × 0.55 NA objective and a 63 × 1.4 NA oil-immersion objective. Nuclei were stained with TOPRO-3. Negative controls for all of the immunohistochemical stainings were obtained by substitution of the primary antibody with PBS. Image processing was performed using Photoshop 7.0 (Adobe, San Jose, CA). Adjustment of brightness or contrast was used in some cases but without obscuring, eliminating, or misrepresenting information. Overlay images were generated using PenguinMate (version 1.00.7; Pixera Corp., Los Gatos, CA).
Western Blotting
NRK52E cells were lysed with Laemmli sample buffer. After determination of the protein concentration of the lysates with a protein assay kit (Biorad, Richmond, CA), equal amounts of protein (10 μg) were immunoblotted with rabbit anti-acetyl histone H3 (Lys9) polyclonal antibody (Upstate Biotechnology; dilution 1:10000) and analyzed by standard SDS-PAGE and Western blot analysis according to a previously described method.40 Values obtained by densitometric analysis of Western blots for acetylated histone were normalized to those for actin and expressed as relative values to those of control. To measure the nuclear HDAC5 protein levels, NRK52E cells were washed with ice-cold phosphate buffer saline, and the nuclear protein was extracted using the Nuclear/Cytosol Fraction Kit (BioVision Research Products, Mountain View, CA) in accordance with the manufacturer's instructions. After determination of the protein concentration with a protein assay kit (Biorad), equal amounts of protein (8 μg) were immunoblotted with a rabbit anti-HDAC5 polyclonal antibody (Epigentek, Brooklyn, NY; dilution 1:500) and analyzed by standard SDS-PAGE and Western blot analysis. In experiments to determine nuclear HDAC5 protein levels, protein loading was verified by Ponceau S staining (Sigma) of the nitrocellulose membranes. Values obtained by densitometric analysis of Western blots for HDAC5 were normalized to those for 45 kD of protein detected by Ponceau S staining and expressed as relative values to those of control.
In Situ HAT Activity
In situ HAT activity was determined according to a previously published method.41 After inhibition of HDAC activity by the addition of trichostatin A (300 nM), an HDAC inhibitor, NRK 52E cells were incubated in PBS with 1.5 mM CaCl2 and 2 mM MgCl2 with or without antimycin A for 0, 20, 40, or 60 min and fixed with ice-cold methanol. Time-dependent increase in the acetylation level of histone after the addition of trichostatin was considered to represent the HAT activity in situ.41 Images of the cells stained for acetylated histone H3 were obtained according to the methods described previously. The signal intensity of at least 40 cells in each condition was quantified with TCS SL (Leica). Laser and microscope settings were maintained constant for the entire image series. Increases in the signals from the baseline (time 0) were calculated and plotted.
Analysis of the mRNA Levels
Total RNA was extracted from NRK 52E cells with the RNA extraction kit RNeasy Mini (QIAGEN K.K., Tokyo, Japan). For semiquantitative RT-PCR analysis, the cDNA product was synthesized and amplified by PCR using a commercial kit (QIAGEN OneStep RT-PCR Kit). PCR primers were designed using the software Primer Express (Applied Biosystems, Foster City, CA) with published sequence data from the National Center for Biotechnology Information database. The primer sequences used were as follows: HDAC1 sense 5′-TTGCGTTCTATTCGCCCAGA-3′ and antisense 5′-CCAGGATGGCCAAGACGATAT-3′, with an expected product size of 258 bp (GenBank accession no. XM_576595); HDAC2 sense 5′-ACTTGCCGTTGCTGATGCTT-3′ and antisense 5′-TTGAACACCAGGCGCATGT-3′, with an expected product size of 265 bp (XM_342149); HDAC3 sense 5′-ACATGTGCCGCTTCCATTCT-3′ and antisense 5′-GCCTCAAACTTCTTGGCATGA-3′, with an expected product size of 252 bp (NM_053448); HDAC4 sense 5′-ATGAGGCACAGCTGCATGAAC-3′ and antisense 5′-GGCCAGAGCCTTCTTCTTGTT-3′, with an expected product size of 250 bp (XM_343629); HDAC5 sense 5′-GCAGGAGAGCTCAAGAATGGA-3′ and antisense 5′-AAGTTCCCATTGTCGTAGCGA-3′, with an expected product size of 250 bp (XM_213469); HDAC6 sense 5′-TGATGTTGGTTCACAGCCTGG-3′ and antisense 5′-ACCCATCCATAAGACTGCGCT-3′, with an expected product size of 251 bp (XM_228753); HDAC7 sense 5′-CAGCCGCCTCAAACTGGATAA-3′ and antisense 5′-AGCAAAAGCCCATGGCTGTAG-3′, with an expected product size of 259 bp (XM_345868). The primers of 18S ribosomal RNA (QuantumRNA 18S Internal Standards) were obtained from Ambion (Austin, TX). After 30 min of incubation at 50°C for the reverse transcriptase reaction, the PCR was initiated by 15 min of incubation at 95°C, followed by 23 (for 18S), 25 (for HDAC1, 2, 3, 5, 6, and 7), or 27 (for HDAC4) cycles of 30 s at 94°C, 1 min at 56°C, and 1 min at 72°C. The resultant reaction products were analyzed by agarose gel electrophoresis (2% agarose). The linear range of PCR cycles was first determined for each gene, and all subsequent RT-PCR experiments were carried out using a cycle number within the exponential phase.
For real-time RT-PCR analysis, the cDNA product was generated using the high-capacity cDNA archive kit (Applied Biosystems). The expression levels of HDAC5 and LIF were then analyzed with the ABI 7500 sequence detection system using the TaqMan Universal PCR Master Mix (Applied Biosystems). For detection of BMP7, SYBR Green PCR Master Mix (Applied Biosystems) was used. The primers and probes of rat HDAC5, LIF, and 18S ribosomal RNA were obtained from Applied Biosystems. The primer sequences designed using the software Primer Express for BMP7 and HDAC isozymes were as follows: BMP7 sense 5′-TCATGAGCTTCGTCAACCTAGTG-3′ and antisense 5′-CGGATGTAGTCCTTATAGATCCTGAAC-3′ (XM_001053727); HDAC1 sense 5′-AATTTGCTGCTCAACTATGGTCTCT-3′ and antisense 5′-TGATGTAGTCGTCGCTGTGGTACT-3′; HDAC2 sense 5′-CCATGGCGTACAGTCAAGGA-3′ and antisense 5′-AATAATTCCCGATATCACCGTCATA-3′; HDAC3 sense 5′-CTGCAGTGTGGCGCTGACT-3′ and antisense 5′-ACATTTCGGACGGTGTAACCA-3′; HDAC6 sense 5′-AGACCCCAAGGGAGAGATGTCT-3′ and antisense 5′-TGAGCCACTGACTCCCTTA-3′; HDAC7 sense 5′-CTGCCAAGCCCAGTGAGAA-3′ and antisense 5′-TGTTCCAGGCCATCATTCG-3′. The thermal cycling parameters were 95°C for 10 min for AmpliTaq Gold activation, followed by 40 cycles of 15 s at 95°C for denaturation and 1 min at 60°C for annealing/extension. Values were normalized to the levels of 18S rRNA and expressed as relative values to those obtained with control.
RNAi for Knockdown of HDAC5
Subconfluent NRK 52E cells were transfected with 33 nM HDAC5 Stealth RNAi (Invitrogen Japan KK, Tokyo, Japan) or Stealth RNAi Negative Control Duplex (medium GC Duplex; Invitrogen) using Lipofectamine RNAiMAX (Invitrogen), in accordance with the manufacturer's instructions. The sequences of the HDAC5 Stealth RNAi were as follows: 5′-GGAGGAGUCCAGUGCUGGUUACAAA-3′ (sense) and 5′-UUUGUAACCAGCACUGGACUCCUCC-3′ (antisense). After 24 h of incubation, cells were treated with PBS containing 1.5 mM CaCl2 and 2 mM MgCl2 for 1 h and further incubated in DMEM with 1% FBS for 29 h. For determination the effects of each HDAC isozyme knockdown on BMP7 expression, cells were incubated with Stealth RNAi Negative Control Duplex or each HDAC Stealth RNAi for 48 h before mRNA analysis. The sequences used were as follows: HDAC1 sense 5′-CGGCAUUGAUGAUGAGUCCUAUGAA-3′ and antisense 5′-UUCAUAGGACUCAUCAUCAAUGCCG-3′; HDAC2 sense 5′-CCUAACUGUCAAAGGUCACGCUAAA-3′ and antisense 5′-UUUAGCGUGACCUUUGACAGUUAGG-3′; HDAC3 sense 5′-GGUCAUGACUGUGUCCUUCCACAAA-3′ and antisense 5′-UUUGUGGAAGGACACAGUCAUGACC-3′; HDAC5 sense 5′-GAAGGUUCUACAGAGAGCGAGAGCA-3′ and antisense 5′-UGCUCUCGCUCUCUGUAGAACCUUC-3′ (designated as second HDAC5 RNAi); HDAC6 sense 5′-AGGUGUSCUGCAGUCGCUAUGUCAA-3′ and antisense 5′-UUGACAUAGCGACUGCAGUACACCU-3′; HDAC7 sense 5′-GAGCUGCAGUCAGUCCACUCGAAA-3′ and antisense 5′-UUUCAGAGUGGACUGACUGCAGCUC-3′.
Statistical Analyses
All data are expressed as means ± SEM. Multiple parametric comparisons were performed by ANOVA, followed by Fisher protected least significant difference test. Comparisons between two groups were performed by the t test. P < 0.05 was considered statistically significant.
DISCLOSURES
None.
Acknowledgments
This work was supported by Mochida Pharmaceutical Co. Ltd.; Takeda Science Foundation; and a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science.
We thank Dr. Hideki Uchimura for performing immunohistochemical staining.
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Bolden JE, Peart MJ, Johnstone RW: Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov 5: 769–784, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Bernstein BE, Meissner A, Lander ES: The mammalian epigenome. Cell 128: 669–681, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Reik W: Stability and flexibility of epigenetic gene regulation in mammalian development. Nature 447: 425–432, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Seligson DB, Horvath S, Shi T, Yu H, Tze S, Grunstein M, Kurdistani SK: Global histone modification patterns predict risk of prostate cancer recurrence. Nature 435: 1262–1266, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Backs J, Olson EN: Control of cardiac growth by histone acetylation/deacetylation. Circ Res 98: 15–24, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Ito K, Ito M, Elliott WM, Cosio B, Caramori G, Kon OM, Barczyk A, Hayashi S, Adcock IM, Hogg JC, Barnes PJ: Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N Engl J Med 352: 1967–1976, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Mejat A, Ramond F, Bassel-Duby R, Khochbin S, Olson EN, Schaeffer L: Histone deacetylase 9 couples neuronal activity to muscle chromatin acetylation and gene expression. Nat Neurosci 8: 313–321, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH: Recovery of learning and memory is associated with chromatin remodelling. Nature 447: 178–182, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Giaccia AJ, Simon MC, Johnson R: The biology of hypoxia: The role of oxygen sensing in development, normal function, and disease. Genes Dev 18: 2183–2194, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giordano FJ: Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest 115: 500–508, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devarajan P: Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol 17: 1503–1520, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Dispersyn GD, Mesotten L, Meuris B, Maes A, Mortelmans L, Flameng W, Ramaekers F, Borgers M: Dissociation of cardiomyocyte apoptosis and dedifferentiation in infarct border zones. Eur Heart J 23: 849–857, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Heusch G, Schulz R, Rahimtoola SH: Myocardial hibernation: A delicate balance. Am J Physiol Heart Circ Physiol 288: H984–H999, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Sasaki T, Iwata S, Okano HJ, Urasaki Y, Hamada J, Tanaka H, Dang NH, Okano H, Morimoto C: Nedd9 protein, a Cas-L homologue, is upregulated after transient global ischemia in rats: Possible involvement of Nedd9 in the differentiation of neurons after ischemia. Stroke 36: 2457–2462, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Liu XS, Zhang ZG, Zhang RL, Gregg S, Morris DC, Wang Y, Chopp M: Stroke induces gene profile changes associated with neurogenesis and angiogenesis in adult subventricular zone progenitor cells. J Cereb Blood Flow Metab 27: 564–574, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Bonventre JV: Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J Am Soc Nephrol 14[Suppl 1]: S55–S61, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Lin F, Moran A, Igarashi P: Intrarenal cells, not bone marrow-derived cells, are the major source for regeneration in postischemic kidney. J Clin Invest 115: 1756–1764, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duffield JS, Park KM, Hsiao LL, Kelley VR, Scadden DT, Ichimura T, Bonventre JV: Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J Clin Invest 115: 1743–1755, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshino J, Monkawa T, Tsuji M, Hayashi M, Saruta T: Leukemia inhibitory factor is involved in tubular regeneration after experimental acute renal failure. J Am Soc Nephrol 14: 3090–3101, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Villanueva S, Cespedes C, Vio CP: Ischemic acute renal failure induces the expression of a wide range of nephrogenic proteins. Am J Physiol Regul Integr Comp Physiol 290: R861–R870, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Vukicevic S, Basic V, Rogic D, Basic N, Shih MS, Shepard A, Jin D, Dattatreyamurty B, Jones W, Dorai H, Ryan S, Griffiths D, Maliakal J, Jelic M, Pastorcic M, Stavljenic A, Sampath TK: Osteogenic protein-1 (bone morphogenetic protein-7) reduces severity of injury after ischemic acute renal failure in rat. J Clin Invest 102: 202–214, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morrissey J, Hruska K, Guo G, Wang S, Chen Q, Klahr S: Bone morphogenetic protein-7 improves renal fibrosis and accelerates the return of renal function. J Am Soc Nephrol 13[Suppl 1]: S14–S21, 2002 [PubMed] [Google Scholar]
- 23.Zeisberg M, Hanai J, Sugimoto H, Mammoto T, Charytan D, Strutz F, Kalluri R: BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med 9: 964–968, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Kuznetsov G, Bush KT, Zhang PL, Nigam SK: Perturbations in maturation of secretory proteins and their association with endoplasmic reticulum chaperones in a cell culture model for epithelial ischemia. Proc Natl Acad Sci U S A 93: 8584–8589, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly KJ, Plotkin Z, Dagher PC: Guanosine supplementation reduces apoptosis and protects renal function in the setting of ischemic injury. J Clin Invest 108: 1291–1298, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cybulsky AV, Takano T, Papillon J, Bijian K: Role of the endoplasmic reticulum unfolded protein response in glomerular epithelial cell injury. J Biol Chem 280: 24396–24403, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Gould SE, Day M, Jones SS, Dorai H: BMP-7 regulates chemokine, cytokine, and hemodynamic gene expression in proximal tubule cells. Kidney Int 61: 51–60, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Wetzel P, Haag J, Campean V, Goldschmeding R, Atalla A, Amann K, Aigner T: Bone morphogenetic protein-7 expression and activity in the human adult normal kidney is predominantly localized to the distal nephron. Kidney Int 70: 717–723, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Grozinger CM, Hassig CA, Schreiber SL: Three proteins define a class of human histone deacetylases related to yeast Hda1p. Proc Natl Acad Sci U S A 96: 4868–4873, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang S, McKinsey TA, Zhang CL, Richardson JA, Hill JA, Olson EN: Histone deacetylases 5 and 9 govern responsiveness of the heart to a subset of stress signals and play redundant roles in heart development. Mol Cell Biol 24: 8467–8476, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dilworth FJ, Fromental-Ramain C, Yamamoto K, Chambon P: ATP-driven chromatin remodeling activity and histone acetyltransferases act sequentially during transactivation by RAR/RXR In vitro. Mol Cell 6: 1049–1058, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Vukicevic S, Kopp JB, Luyten FP, Sampath TK: Induction of nephrogenic mesenchyme by osteogenic protein 1 (bone morphogenetic protein 7). Proc Natl Acad Sci U S A 93: 9021–9026, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshikawa M, Hishikawa K, Marumo T, Fujita T: Inhibition of histone deacetylase activity suppresses epithelial-to-mesenchymal transition induced by TGF-beta1 in human renal epithelial cells. J Am Soc Nephrol 18: 58–65, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Garber K: HDAC inhibitors overcome first hurdle. Nat Biotechnol 25: 17–19, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Avila AM, Burnett BG, Taye AA, Gabanella F, Knight MA, Hartenstein P, Cizman Z, Di Prospero NA, Pellizzoni L, Fischbeck KH, Sumner CJ: Trichostatin A increases SMN expression and survival in a mouse model of spinal muscular atrophy. J Clin Invest 117: 659–671, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trivedi CM, Luo Y, Yin Z, Zhang M, Zhu W, Wang T, Floss T, Goettlicher M, Noppinger PR, Wurst W, Ferrari VA, Abrams CS, Gruber PJ, Epstein JA: Hdac2 regulates the cardiac hypertrophic response by modulating Gsk3beta activity. Nat Med 13: 324–331, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Lin J, Patel SR, Cheng X, Cho EA, Levitan I, Ullenbruch M, Phan SH, Park JM, Dressler GR: Kielin/chordin-like protein, a novel enhancer of BMP signaling, attenuates renal fibrotic disease. Nat Med 11: 387–393, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Yanagita M, Okuda T, Endo S, Tanaka M, Takahashi K, Sugiyama F, Kunita S, Takahashi S, Fukatsu A, Yanagisawa M, Kita T, Sakurai T: Uterine sensitization-associated gene-1 (USAG-1), a novel BMP antagonist expressed in the kidney, accelerates tubular injury. J Clin Invest 116: 70–79, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang J, Shultz RW, Mars WM, Wegner RE, Li Y, Dai C, Nejak K, Liu Y: Disruption of tissue-type plasminogen activator gene in mice reduces renal interstitial fibrosis in obstructive nephropathy. J Clin Invest 110: 1525–1538, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marumo T, Uchimura H, Hayashi M, Hishikawa K, Fujita T: Aldosterone impairs bone marrow-derived progenitor cell formation. Hypertension 48: 490–496, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Kruhlak MJ, Hendzel MJ, Fischle W, Bertos NR, Hameed S, Yang XJ, Verdin E, Bazett-Jones DP: Regulation of global acetylation in mitosis through loss of histone acetyltransferases and deacetylases from chromatin. J Biol Chem 276: 38307–38319, 2001 [DOI] [PubMed] [Google Scholar]