Abstract
It is unknown whether treatment for osteoporosis with raloxifene is safe or effective in those with chronic kidney disease (CKD). With data from a multicenter, randomized, placebo-controlled trial of 7705 postmenopausal women with osteoporosis, the effect of raloxifene on rate of change of bone mineral density (BMD), incidence of fractures, and adverse events by stage of CKD was examined over 3 yr. Baseline serum creatinine values were available for 7316 women, and these values were used to assign a category of creatinine clearance (CrCl) using the Cockcroft-Gault formula (CrCl <45, 45 to 59, and ≥60 ml/min). BMD was measured at baseline and annually by dual x-ray absorptiometry. Within the placebo group, lower baseline CrCl was associated with a trend for higher annual losses of BMD at the femoral neck; however, within the raloxifene group, lower baseline CrCl was associated with greater increases in femoral neck BMD. This interaction between category of CrCl and treatment assignment was significant for rate of change of BMD at the hip. Irrespective of kidney function, raloxifene treatment was associated with a greater increase in spine BMD, a reduction in vertebral fractures, and no effect on nonvertebral fractures compared with placebo. Within each category of kidney function, adverse events were similar between the raloxifene and placebo groups. In conclusion, raloxifene increases BMD at both the hip and the spine and reduces the risk for vertebral fractures among individuals with CKD. The effect of raloxifene on hip BMD is greater among those with mild to moderate CKD.
The prevalence of low bone mineral density (BMD) and osteoporosis increases with greater severity of chronic kidney disease (CKD).1–5 CKD may have an increased risk for osteoporosis for several reasons, including shared risk factors for both conditions such as advanced age and female gender. Alternatively, CKD may lead to metabolic abnormalities that accelerate bone loss, such as chronic metabolic acidosis, hypogonadism, hyperparathyroidism, and abnormalities with vitamin D metabolism.6 Despite the increased burden of osteoporosis among those with CKD, the majority of randomized trials evaluating the efficacy of pharmacologic agents in preventing fractures in postmenopausal women with osteoporosis have excluded women with serum cre-atinine levels >2.0 mg/dl.7–14 Because therapies for osteoporosis have not typically included those with CKD, it remains uncertain whether the beneficial effects of osteoporosis therapy, specifically raloxifene, extend to those with kidney disease and whether these therapies are safe for those with CKD. This uncertainty is reflected in the recent review article on osteoporosis in CKD, which emphasized the unknown impact of osteoporosis therapies on BMD in those with CKD and their uncertain safety profile.15
Raloxifene hydrochloride is a nonsteroidal benzothiophene that binds to estrogen receptors and has been demonstrated to increase BMD and reduce the risk for vertebral fractures in postmenopausal women with osteoporosis; however, the effects of raloxifene among women with osteoporosis and CKD are uncertain. Using data from the Multiple Outcomes of Raloxifene Evaluation (MORE) trial, we examined the efficacy and safety of raloxifene therapy in postmenopausal women with osteoporosis in risk subgroups defined at baseline by presence and severity of CKD.
RESULTS
Baseline characteristics of the 7316 women by category of creatinine clearance (CrCl) are shown in Table 1. Among the cohort, 1480 (20%) had a CrCl of <45 ml/min, 3493 (48%) had a CrCl of 45 to 59 ml/min, and 2343 (32%) had CrCl ≥60 ml/min. Among the women with a CrCl <45 ml/min, only 55 had a CrCl <30 ml/min (lowest CrCl 20.0 ml/min). There were several differences in baseline characteristics across categories of CrCl. Women with lower CrCl were more likely to be older and more years postmenopausal; to have a lower body mass index (BMI); to have a lower baseline BMD at the femoral neck, trochanter, and lumbar spine; and to have prevalent fractures. They also were less likely to have had a hysterectomy and had higher concentrations of 25-hydroxy vitamin D (P < 0.001 for all comparisons, except hysterectomy P = 0.006; Table 1). These trends in baseline data were similar across levels of renal function when individuals were categorized by Modification of Diet in Renal Disease (MDRD) estimated GFR (eGFR) with the following significant difference: BMI was more similar across categories of eGFR (26.2, 25.4, and 25.0 for eGFR categories <45, 40 to 59, and ≥60 ml/min per 1.73 m2. Findings were similar when analyses using CrCl or eGFR were limited to the 5675 with baseline and repeat BMD measurement at 36 mo.
Table 1.
Baseline characteristics by CrCl category
| Characteristic | Category of CrCl (ml/min)
|
Pa | ||
|---|---|---|---|---|
| <45 (n = 1480) | 45 to 59 (n = 3493) | ≥60 (n = 2343) | ||
| CrCl (ml/min; median [min, max]) | 40.6 (20.0, 44.9) | 52.4 (45.0, 59.9) | 67.4 (60.0, 124.8) | |
| Age (yr; mean ± SD) | 71.7 ± 5.3 | 67.0 ± 6.2 | 62.2 ± 6.7 | <0.001 |
| Years since menopause (mean ± SD) | 23.6 ± 7.5 | 19.0 ± 7.8 | 15.1 ± 7.9 | <0.001 |
| BMI (kg/m2; mean ± SD) | 22.7 ± 3.2 | 24.6 ± 3.2 | 27.8 ± 4.1 | <0.001 |
| BMD (g/cm2; mean ± SD) | ||||
| femoral neck | 0.59 ± 0.08 | 0.62 ± 0.07 | 0.65 ± 0.07 | <0.001 |
| lumbar spine | 0.79 ± 0.14 | 0.81 ± 0.13 | 0.83 ± 0.13 | <0.001 |
| trochanter | 0.52 ± 0.08 | 0.55 ± 0.08 | 0.58 ± 0.08 | <0.001 |
| White (n [%]) | 1427 (96.4) | 3382 (96.8) | 2268 (96.8) | 0.747 |
| Prevalent vertebral fracture (n [%]) | ||||
| 0 | 818 (55.4) | 2202 (63.3) | 1544 (66.2) | <0.001 |
| 1 | 310 (21.0) | 694 (19.9) | 420 (18.0) | |
| ≥2 | 350 (23.7) | 584 (16.8) | 369 (15.8) | |
| Previously received estrogen therapy (n [%]) | 420 (28.4) | 998 (28.7) | 706 (30.2) | 0.365 |
| Previously had hysterectomy (n [%]) | 297 (20.1) | 773 (22.1) | 572 (24.4) | 0.006 |
| Current smoker (n [%]) | 224 (15.4) | 599 (17.4) | 423 (18.2) | 0.078 |
| Walks for exercise (n [%])b | 196 (67.6) | 404 (68.2) | 218 (68.6) | 0.946 |
| Diabetic (n [%])c | 48 (3.3) | 119 (3.4) | 111 (4.8) | 0.015 |
| PTH (pmol/L; mean ± SD)d | 3.8 ± 1.9 | 3.6 ± 1.6 | 3.8 ± 1.7 | <0.001 |
| 25-Hydroxy vitamin D (nmol/L; mean ± SD) | 74.3 ± 32.3 | 72.1 ± 31.3 | 65.6 ± 28.8 | <0.001 |
P values for age, BMI, and BMD are from an ANOVA; years since menopause, PTH, and 25-hydroxy vitamin D from a Kruskal-Wallis test because of skewness; categorical data from a χ 2 test.
Walks for exercise was asked only of a subset of n = 1201. These are English-speaking women at megasites in the United States.
Defined as those reporting a preexisting condition, fasting blood glucose ≥7.0 mmol/L, or on hypoglycemic medication.
To convert PTH to pg/ml, divide by 0.11.
As anticipated given the randomized trial design, baseline characteristics were similar across treatment groups within each category of CrCl (Table 2) with the following exceptions: Among women with CrCl <45 ml/min, those assigned raloxifene had slightly lower parathyroid hormone (PTH) levels compared with those assigned placebo (P = 0.012). Also, among women with a baseline CrCl ≥60 ml/min, those assigned to raloxifene therapy were more likely to have had a prevalent vertebral fracture (P = 0.002), diabetes (P = 0.016), and higher PTH concentrations (P = 0.018) at baseline compared with those assigned to placebo (Table 2).
Table 2.
Baseline characteristics by CrCl category and treatment
| Characteristic | Category of CrCl (ml/min)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| <45
|
45 to 59
|
≥60
|
|||||||
| Placebo (n = 510) | Raloxifene (n = 970) | P* | Placebo (n = 1170) | Raloxifene (n = 2323) | P* | Placebo (n = 771) | Raloxifene (n = 1572) | P* | |
| CrCl (ml/min; median [min, max]) | 40.5 (20.0, 44.9) | 40.7 (21.7, 44.9) | 0.662 | 52.5 (45.0, 59.9) | 52.4 (45.0, 59.9) | 0.454 | 66.9 (60.0, 124.8) | 67.8 (60.0, 120.5) | 0.145 |
| Age (yr; mean ± SD) | 71.7 ± 5.3 | 71.7 ± 5.3 | 0.874 | 67.3 ± 6.1 | 66.9 ± 6.2 | 0.058 | 62.1 ± 6.7 | 62.3 ± 6.8 | 0.539 |
| Years since menopause (mean ± SD) | 23.7 ± 7.6 | 23.5 ± 7.5 | 0.929 | 19.4 ± 7.8 | 18.8 ± 7.9 | 0.126 | 14.8 ± 7.9 | 15.2 ± 7.9 | 0.338 |
| BMI (kg/m2; mean ± SD) | 22.7 ± 3.3 | 22.7 ± 3.2 | 0.605 | 24.7 ± 3.2 | 24.6 ± 3.2 | 0.276 | 27.8 ± 4.1 | 27.9 ± 4.1 | 0.805 |
| BMD (g/cm2; mean ± SD) | |||||||||
| femoral neck | 0.59 ± 0.08 | 0.59 ± 0.08 | 0.833 | 0.62 ± 0.08 | 0.62 ± 0.07 | 0.751 | 0.64 ± 0.07 | 0.65 ± 0.07 | 0.556 |
| lumbar spine | 0.79 ± 0.14 | 0.79 ± 0.14 | 0.842 | 0.81 ± 0.14 | 0.81 ± 0.13 | 0.406 | 0.83 ± 0.13 | 0.83 ± 0.13 | 0.851 |
| trochanter | 0.52 ± 0.08 | 0.52 ± 0.08 | 0.682 | 0.55 ± 0.08 | 0.55 ± 0.08 | 0.940 | 0.58 ± 0.08 | 0.58 ± 0.08 | 0.686 |
| White (n [%]) | 488 (95.7) | 939 (96.8) | 0.271 | 1130 (96.6) | 2252 (96.9) | 0.564 | 751 (97.4) | 1517 (96.5) | 0.242 |
| Prevalent vertebral fracture (n [%]) | |||||||||
| 0 | 297 (58.4) | 521 (53.8) | 0.236 | 706 (60.2) | 1496 (64.6) | 0.058 | 545 (71.0) | 999 (63.8) | 0.002 |
| 1 | 101 (19.8) | 209 (21.6) | 241 (20.7) | 453 (19.6) | 112 (14.6) | 308 (19.7) | |||
| ≥2 | 111 (21.8) | 239 (24.7) | 216 (18.6) | 368 (15.9) | 111 (14.5) | 258 (16.5) | |||
| Previously received estrogen therapy (n [%]) | 135 (26.5) | 285 (29.4) | 0.229 | 330 (28.3) | 668 (28.9) | 0.722 | 241 (31.3) | 465 (29.7) | 0.422 |
| Previously had hysterectomy (n [%]) | 105 (20.6) | 192 (19.8) | 0.717 | 255 (21.8) | 518 (22.3) | 0.735 | 195 (25.3) | 377 (24.0) | 0.488 |
| Current smoker (n [%]) | 78 (15.5) | 146 (15.3) | 0.932 | 197 (17.1) | 402 (17.5) | 0.757 | 137 (17.9) | 286 (18.4) | 0.793 |
| Walks for exercise (n [%])b | 67 (65.1) | 129 (68.8) | 0.535 | 140 (70.0) | 264 (67.4) | 0.512 | 72 (71.3) | 146 (67.3) | 0.474 |
| Diabetic (n [%])c | 19 (3.7) | 29 (3.0) | 0.456 | 45 (3.9) | 74 (3.2) | 0.302 | 25 (3.3) | 86 (5.5) | 0.016 |
| PTH (pmol/L; mean ± SD)d | 3.9 ± 1.9 | 3.7 ± 2.0 | 0.012 | 3.6 ± 1.5 | 3.6 ± 1.6 | 0.563 | 3.7 ± 1.5 | 3.8 ± 1.7 | 0.018 |
| 25-Hydroxy vitamin D (nmol/L; mean ± SD) | 72.6 ± 30.9 | 75.2 ± 32.9 | 0.179 | 72.2 ± 30.0 | 72.0 ± 32.0 | 0.518 | 66.3 ± 28.4 | 65.2 ± 29.0 | 0.253 |
a P values for age, BMI, and BMD are from an ANOVA; years since menopause, PTH, and 25-hydroxy vitamin D from a Kruskal-Wallis test because of skewness; categorical data from a χ2 test.
Walks for exercise was asked only of a subset of n = 1201. These are English-speaking women at megasites in the United States.
Defined as those reporting a preexisting condition, fasting blood glucose ≥7.0 mmol/L, or on hypoglycemic medication.
To convert PTH to pg/ml, divide by 0.11.
For all analyses, the results when using the 60 and 120 m/d doses of raloxifene were similar to those using a pooled raloxifene treatment group. Thus, all findings presented are for the pooled raloxifene treatment group.
Bone Mineral Density
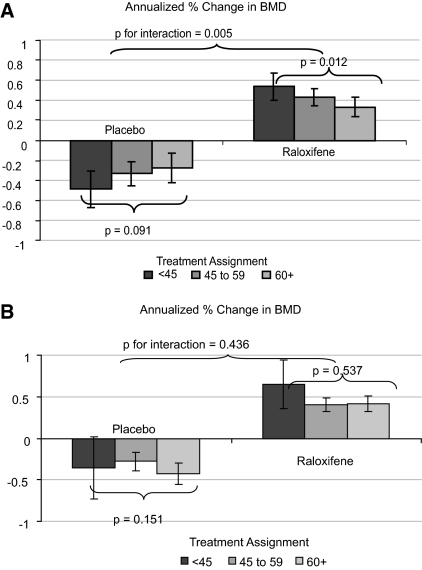
Of the 7316 women in our analysis cohort, 5675 (78%) completed technically adequate BMD measurements at baseline and 3 yr. There was evidence of an interaction between baseline category of CrCl and treatment assignment for prediction of rate of change in BMD at the hip (P = 0.005 for interaction term at the femoral neck and 0.01 at the trochanter). Consequently, all additional analyses performed for change in BMD at either the femoral neck or the trochanter were stratified by treatment assignment. Figure 1A shows the 3-yr annualized percentage change in femoral neck BMD from baseline by category of CrCl and treatment assignment. Within the placebo group, lower CrCl seemed to be associated with higher annual losses of BMD at the femoral neck, but the trend did not reach significance (P = 0.09 for trend); however, within the raloxifene group, the trend was in the opposite direction, in that the effect of raloxifene in increasing femoral neck BMD was most pronounced among women with lower CrCl (P = 0.01 for trend). Compared with placebo, the effect of raloxifene on increasing femoral neck BMD was 1.0% per year among women with a CrCl <45 ml/min, 0.7% per year among women with a CrCl of 45 to 59 ml/min, and 0.6% per year among women with a CrCl ≥60 ml/min (P < 0.001 for all comparisons between pooled raloxifene treatment group and placebo group). Substitution of trochanteric BMD for femoral neck BMD or the absolute rate of change in BMD for percentage change in BMD did not alter the results. When using the MDRD eGFR in place of the Cockcroft-Gault CrCl, there was no evidence of an interaction between category of kidney function and annualized percentage change in BMD at either the femoral neck (P = 0.44 for interaction) or the trochanter (P = 0.35 for interaction; Figure 1B); however, the overall effect on hip BMD was similar, with women in the pooled raloxifene treatment group gaining hip BMD over 3 yr, whereas those in the placebo group experienced losses in hip BMD, irrespective of category of eGFR.
Figure 1.
(A) Annualized percentage change in BMD at the femoral neck by baseline CrCl category (n = 5533; placebo n = 1825; raloxifene n = 3708). (B) Annualized percentage change in BMD at the femoral neck by baseline MDRD GFR category (n = 5533; placebo n = 1825; raloxifene n = 3708).
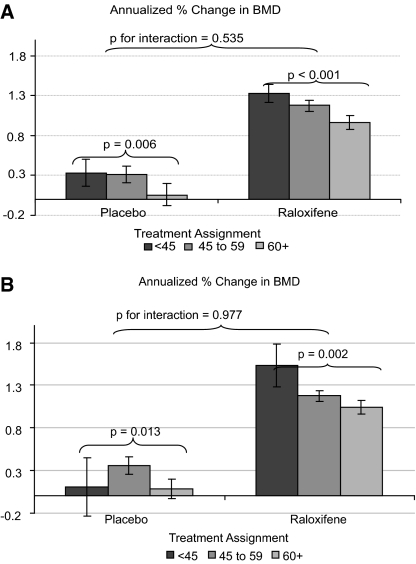
Unlike at the femoral neck, the effect of raloxifene on rate of change in spine BMD did not differ by category of CrCl at baseline (P = 0.54 for interaction). In general, lower CrCl was associated with greater increases in spine BMD irrespective of treatment assignment (P = 0.006 for trend within placebo group and P < 0.001 within pooled raloxifene group; Figure 2A). Results were similar when eGFR was substituted in place of the CrCl (P = 0.98 for interaction; Figure 2B).
Figure 2.
(A) Annualized percentage change in BMD at the lumbar spine by baseline CrCl category (n = 5596; placebo n = 1837; raloxifene n = 3759). (B) Annualized percentage change in BMD at the lumbar spine by baseline MDRD GFR category.
Fractures
Use of raloxifene was associated with an overall decrease in the incidence of vertebral fractures (odds ratio [OR] 0.57; 95% confidence interval [CI] 0.47 to 0.69). There was no evidence of an interaction between treatment assignment and category of baseline kidney function for the prediction of risk for incident vertebral fractures (P = 0.39 for interaction CrCl*treatment assignment [Table 3] and P = 0.93 for interaction eGFR*treatment assignment). Raloxifene therapy was not associated with any benefit compared with placebo in the overall cohort in reducing the risk for any nonvertebral fracture (OR 0.94; 95% CI 0.80 to 1.11). The lack of effect of raloxifene in reducing risk for nonvertebral fracture was consistent within subgroups defined by level of kidney function (using CrCl; Table 3). When individuals were categorized using the MDRD eGFR, there was some evidence that the effectiveness of raloxifene in reducing nonvertebral fracture differed by category of kidney function (P = 0.0503 for interaction eGFR*treatment assignment; Table 3). Raloxifene seemed to lower the risk for nonvertebral fractures among women with an eGFR >60 ml/min per 1.73 m2 and increase the risk among women with eGFR <45 ml/min per 1.73 m2; however, there were few events among women with an eGFR <45 ml/min per 1.73 m2 (n = 32), and 95% CI around the point estimate of effect were wide (0.61 to 2.62).
Table 3.
Effect of Treatment on Incident Fracture by Overall Cohort and Baseline Creatinine Clearance and GFR categories
| Fracture Type | Rate of Fracture (n/N [%]) | Treatment Effect | P for Interaction between Treatment and CrCl/eGFR Category |
|---|---|---|---|
| Incident vertebral fracture | OR (95% CI) | ||
| overall cohort | 479/6472 (7.4) | 0.57 (0.47 to 0.69) | |
| category of CrCl (ml/min) | |||
| <45 | 143/1280 (11.2) | 0.78 (0.54 to 1.11) | 0.386 |
| 45 to 59 | 223/3100 (7.2) | 0.45 (0.34 to 0.59) | |
| ≥60 | 113/2092 (5.4) | 0.64 (0.43 to 0.94) | |
| category of eGFR (ml/min) | |||
| <45 | 25/282 (8.9) | 0.74 (0.32 to 1.68) | 0.933 |
| 45 to 59 | 265/3497 (7.6) | 0.54 (0.42 to 0.69) | |
| ≥60 | 189/2693 (7.0) | 0.60 (0.44 to 0.80) | |
| Incident nonvertebral fracture | Relative Hazard (95% CI) | ||
| overall cohort | 646/7316 (8.8) | 0.94 (0.80 to 1.11) | |
| category of CrCl (ml/min) | |||
| <45 | 143/1480 (9.7) | 0.84 (0.60 to 1.17) | 0.753 |
| 45 to 59 | 298/3493 (8.5) | 1.02 (0.80 to 1.30) | |
| ≥60 | 205/2343 (8.8) | 0.92 (0.69 to 1.23) | |
| category of eGFR (ml/min) | |||
| <45 | 32/343 (9.3) | 1.26 (0.61 to 2.62) | 0.050 |
| 45 to 59 | 335/3921 (8.5) | 1.07 (0.85 to 1.35) | |
| ≥60 | 279/3052 (9.1) | 0.80 (0.62 to 1.00) |
Adverse Events
Women with reduced kidney function were more likely to experience one or more serious adverse effects when categorized using either CrCl or eGFR (Table 4). Women with more advanced kidney disease were also more likely to discontinue the study permanently as a result of an adverse event (11.3, 10.1, and 8.3% for CrCl and 15.5, 9.7, and 9.2% for eGFR categories of <45, 45 to 59, and ≥60 ml/min, respectively); however, within each category of CrCl or eGFR, rates of adverse events by treatment assignment were not different in either the number of serious adverse events or the number of women who discontinued the study (P > 0.1 for interaction between kidney function and treatment assignment for all adverse event categories; Table 4).
Table 4.
Effect of treatment on adverse events by overall cohort and baseline kidney function categorya
| Adverse Event | Rate of Adverse Event (n/N [%]) | Treatment Effect (OR [95% CI]) | P for Interaction between Treatment and Kidney Function Category |
|---|---|---|---|
| Had a serious adverse event | |||
| overall cohort | 1728/7316 (23.6) | 0.89 (0.79 to 1.00) | |
| category of CrCl (ml/min) | |||
| <45 | 436/1480 (29.5) | 0.90 (0.71 to 1.13) | 0.726 |
| 45 to 59 | 786/3493 (22.5) | 0.86 (0.73 to 1.02) | |
| ≥60 | 506/2343 (21.6) | 0.94 (0.76 to 1.16) | |
| category of eGFR (ml/min) | |||
| <45 | 117/343 (34.1) | 0.78 (0.49 to 1.23) | 0.388 |
| 45 to 59 | 928/3921 (23.7) | 0.87 (0.75 to 1.02) | |
| ≥60 | 683/3052 (22.4) | 0.94 (0.79 to 1.12) | |
| Discontinued because of an adverse event | |||
| overall cohort | 716/7316 (9.8) | 1.18 (1.00 to 1.39) | |
| category of CrCl (ml/min) | |||
| <45 | 167/1480 (11.3) | 1.02 (0.72 to 1.43) | 0.423 |
| 45 to 59 | 354/3493 (10.1) | 1.24 (0.97 to 1.57) | |
| ≥60 | 195/2343 (8.3) | 1.24 (0.90 to 1.71) | |
| category of eGFR (ml/min) | |||
| <45 | 53/343 (15.5) | 0.61 (0.34 to 1.09) | 0.337 |
| 45 to 59 | 382/3921 (9.7) | 1.32 (1.04 to 1.67) | |
| ≥60 | 281/3052 (9.2) | 1.18 (0.90 to 1.53) | |
| Discontinued because of an adverse event or death | |||
| overall cohort | 776/7316 (10.6) | 1.15 (0.98 to 1.35) | |
| category of CrCl (ml/min) | |||
| <45 | 191/1480 (12.9) | 1.02 (0.74 to 1.41) | 0.610 |
| 45 to 59 | 379/3493 (10.9) | 1.23 (0.97 to 1.55) | |
| ≥60 | 206/2343 (8.8) | 1.15 (0.85 to 1.57) | |
| category of eGFR (ml/min) | |||
| <45 | 61/343 (17.8) | 0.65 (0.37 to 1.13) | 0.460 |
| 45 to 59 | 410/3921 (10.5) | 1.30 (1.03 to 1.63) | |
| ≥60 | 305/3052 (10.0) | 1.13 (0.88 to 1.45) |
Serious adverse event is defined as an adverse event that resulted in death, hospitalization, cancer, permanent disability, or threat to life; women were counted only once, even when they had more than one serious adverse event.
DISCUSSION
In this post hoc analysis of data from the MORE study, a randomized trial of the efficacy and safety of raloxifene treatment in postmenopausal women with osteoporosis, we found that the effect of raloxifene on rates of spinal bone loss, risk for fractures, and rates of adverse events was consistent across risk subgroups defined by baseline CrCl or eGFR. We also observed that the effect of raloxifene on rates of hip bone loss differed by category of renal function as defined by CrCl (but not eGFR), in that the effect of raloxifene in increasing hip BMD was most pronounced in women with the lowest CrCl (stage 3 CKD).
Our results in the placebo group suggest a difference in the association between CKD and the rate of change of BMD at the lumbar spine as compared with that at the hip. Specifically, greater evidence of CKD among women in the placebo group was associated with greater increases in spine BMD; however, more advanced CKD among women in the placebo group was associated with greater rates of decline in BMD at the femoral neck and trochanter. Spinal BMD measures the bone density not only at the spine but also overlying vascular calcification,16 and adults with more advanced CKD have a greater prevalence and rate of increase in vascular calcification compared with those with preserved kidney function.17 Thus, it is likely that the increases in BMD observed at the spine in the placebo group at least partially reflect the effect of ongoing age-related vascular calcification as opposed to true increases in BMD.
We are aware of a few studies that have evaluated the effects of osteoporosis therapy in those with kidney disease. All had more stringent criteria regarding exclusionary levels of serum creatinine at baseline. The first was a pooled analysis of nine clinical trials that examined the safety and efficacy of risedronate, a bisphosphonate, in women with osteoporosis and CKD (n = 9883).18 That study demonstrated that risedronate led to similar increases in BMD and reductions in fractures irrespective of baseline kidney function. In addition, that study suggested that risedronate was safe to use in women with osteoporosis and CKD over the 2- to 3-yr observation period. In all nine studies pooled, individuals were excluded when their baseline serum creatinine was >1.1 times the upper limit of normal. Despite this inclusion criteria, that study included 572 individuals with a CrCl <30 ml/min. A secondary analysis of the Fracture Intervention Trial (FIT), in which women with low BMD were randomly assigned to placebo or alendronate, showed similar results.19 Specifically, use of alendronate was associated with a reduced risk for clinical fractures irrespective of baseline kidney function. Alendronate use was also associated with a significant increase in total hip BMD compared with placebo. This effect was more pronounced in those with a CrCl <45 ml/min (P = 0.04 for interaction between CrCl and treatment assignment). Adverse events were similar irrespective of baseline kidney function; however, the FIT excluded women with a creatinine level >1.6 mg/dl at baseline. Finally, Miller et al.20 recently evaluated the effectiveness of teriparatide [recombinant human PTH (1-34)] among postmenopausal women with osteoporosis by level of kidney function. That study demonstrated that teriparatide increased BMD at the lumbar spine and femoral neck to similar degrees irrespective of baseline kidney function. Also, the reduction in risk for vertebral and nonvertebral fractures was not different by level of baseline kidney function; however, teriparatide was associated with an increased risk for elevated uric acid in those with moderately impaired kidney function (CrCl 30 to 49 ml/min). That study excluded from participation women with a creatinine level >2 mg/dl. We are aware of only one randomized, controlled trial that specifically recruited individuals with CKD at baseline.21 That small, randomized, controlled trial enrolled postmenopausal women on hemodialysis and compared the effect of raloxifene (60 mg/d) with placebo on rate of change in BMD (n = 50). That trial was of relatively short duration (1 yr) and demonstrated an improvement in BMD at the lumbar spine, with no effect at the femoral neck. Our results extend these findings because we demonstrated that raloxifene is effective in increasing hip BMD and decreasing risk for incident vertebral fractures among women with osteoporosis and mild to moderate CKD. In addition, raloxifene is well tolerated by those with mild to moderate CKD, with an adverse event rate and a discontinuation rate similar to those of placebo.
Impaired kidney function seems to be a risk factor for osteoporosis, progressive bone loss, and ultimately hip fractures. CKD may be associated with a higher prevalence of osteoporosis and osteopenia for several reasons. Individuals with CKD are more likely to be older, female, and vitamin D deficient; have increased PTH levels; and have had an earlier onset of menopause. In addition, there is concern that kidney disease itself may be a risk factor for lower BMD.1–5,22 The biologic basis for progressive bone loss among individuals with impaired kidney function has recently been borne out by two prospective cohorts demonstrating that individuals with poorer kidney function, defined as a lower CrCl23 or a higher cystatin C,24 had greater rates of hip bone loss. Increased rates of hip bone loss may potentially increase the risk for hip fracture among individuals with impaired kidney function. Nickolas et al.25 reported an independent correlation between an eGFR <60 ml/min per 1.73 m2 and the prevalence of hip fractures using the cross-sectional National Health and Nutrition Examination and Survey (NHANES). Ensrud et al.26 reported a graded association between level of kidney function and the subsequent risk for hip fracture among older women. Similarly, elevated cystatin C concentrations have been independently associated with risk for hip fracture among women.27
Irrespective of cause, individuals with CKD have a greater prevalence of osteoporosis and are at increased risk for clinical fractures. Despite this increased risk, the majority of studies evaluating osteoporosis therapy have attempted to exclude systematically individuals with CKD. A review group on osteoporosis in CKD concluded that the use of osteoporosis therapies in patients with CKD is highly controversial, given unknown effectiveness of these therapies on BMD and fractures risk.15 In addition, the review expressed concern regarding the unknown safety profile of osteoporosis therapeutic agents when used in those with CKD. This analysis of data from the MORE trial indicates that the effects of raloxifene on spine BMD, fracture risk, and adverse events are similar among women with and without CKD, whereas it provides some evidence that the rate of gain in hip BMD with raloxifene treatment increases with greater severity of CKD as defined by level of CrCl (down to a CrCl of 30 ml/min).
Our study has several limitations. We were unable to use a direct measure of GFR. Instead, we relied on two indirect measures of kidney function, both based on the serum creatinine. Use of the two creatinine-based formulas resulted in substantial differences in the number of individuals categorized with poor kidney function: 1480 with a CrCl <45 and 343 with an eGFR <45. It is unclear which formula best predicts kidney function in this population of postmenopausal women, because neither has been validated in a similar population. The MDRD equation was derived in middle-aged adults without diabetes and with CKD.28 Whereas some studies have suggested that the MDRD estimate of kidney function is more accurate than the Cockcroft-Gault equation in older people,29–31 others have not supported this claim.32 Despite the differences in the numbers of individuals categorized with CKD between the formulas, the overall effect of raloxifene treatment on BMD we observed was similar, irrespective of formula used. In addition to potential bias introduced by the estimating formulas, serum creatinine itself may be a biased marker of kidney function in the elderly. Previous studies suggested that serum creatinine becomes a less reliable marker of kidney function with aging, specifically in elderly women. Age has been associated with a decline in muscle mass and alterations in creatinine metabolism.33 These deficiencies in estimating kidney function may have led to misclassification in the category of kidney function to which individuals were assigned. This misclassification could explain the similar concentrations of PTH and vitamin D observed across categories of both CrCl and eGFR. Alternatively, the preserved PTH and vitamin D concentrations observed may be the result of recruiting healthy individuals for inclusion into the trial, and results obtained in this population may not be generalizable to those with identified kidney disease. Whereas our primary results suggest a greater therapeutic benefit for raloxifene in increasing hip BMD among individuals with more advanced CKD when categorized by CrCl, our results differed when eGFR was substituted for CrCl, in that the effect of raloxifene in increasing hip BMD was consistent across categories of eGFR. Also, we had limited power to determine whether the effect of raloxifene on risk for nonvertebral fracture was consistent across categories of baseline kidney function and to explore further the inconsistency of results when kidney function was expressed using eGFR versus CrCl. Finally, the MORE trial excluded women from participation when their baseline serum creatinine was >2.6 mg/dl. As a result of this exclusion criterion, few women (n = 55) with a CrCl <30 were included. Consequently, it is unknown whether our results are generalizable to postmenopausal women with serum creatinine levels >2.6 mg/dl and/or a CrCl <30 ml/min.
Among postmenopausal women with osteoporosis and mild to moderate CKD (to stage 3), raloxifene therapy increases BMD at both the hip and the spine with a greater effect on hip BMD in those with mild to moderate CKD as defined by CrCl, reduces risk for vertebral fractures, but has no effect on risk for nonvertebral fractures.
CONCISE METHODS
The MORE study was a 3-yr multicenter, randomized, placebo-controlled trial that began enrollment in 1994. The study enrolled 7705 postmenopausal women aged 31 to 80 in 25 countries. The protocol was approved by the human studies review board at each participating center. The primary objective of the MORE trial was to determine whether raloxifene therapy decreased the risk for incident vertebral fractures compared with placebo in postmenopausal women with osteoporosis. Women were randomly assigned to receive one of three treatments: Placebo or 60 or 120 mg/d raloxifene. All women were also given daily supplements of 500 mg of calcium and 400 to 600 IU of vitamin D. Inclusion and exclusion criteria have been previously published.34 Specifically, the MORE trial included women who were at least 2 yr postmenopausal, did not have severe or long-term disabling conditions, and had osteoporosis at baseline defined as low BMD (T score at the femoral neck or lumbar spine below −2.5) or radiographically apparent vertebral fractures. Individuals were excluded when they had a serum creatinine level >2.6 mg/dl (225 mmol/L) at baseline. Of the 7705 women enrolled in the trial, a total of 7316 (95%) women completed baseline serum creatinine measurements and are the subject of this analysis.
Renal Function
Baseline serum creatinine was measured at a central laboratory using a modified Jaffe reaction on Roche (Basel, Switzerland)/Hitachi (Tokyo, Japan) instrumentation (intra-assay coefficient of variation 3.5%). For the primary analysis, kidney function was determined using the Cockcroft-Gault formula, which is an estimate of CrCl: {CG = 0.85 × [140 − age (years)] × weight (kg)/[72 × creatinine (mg/dl)]}. CrCl was categorized as <45, 45 to 59, and ≥60 ml/min, using a modification of the classification system recommended by the National Kidney Foundation.28 Stage 3 CKD is defined as a CrCl between 30 and 60 ml/min. Secondary analyses were performed in which the MDRD formula {eGFR = 186 × [creatinine (mg/dl)−1.154] × [age (years)−0.203] × 0.742 (if female) × 1.21 (if black)} was used to estimate GFR (eGFR) in place of the Cockcroft-Gault CrCl.
Bone Mineral Density
BMD was measured at the spine and the femoral neck at baseline and annually by dual-energy x-ray absorptiometry. A central reading facility provided correction factors to adjust for intersite differences and changes in the performance of densitometers over time.35,36 Of the 7316 women included in this analysis, 5675 (78%) had technically adequate BMD measurement at both baseline and after 3 yr. The rate of change in BMD was expressed as annualized percentage change between the year 3 value and baseline. There were no carry-forward values of BMD from intervening years.
Fractures
Participants in the MORE trial underwent lateral thoracic and lumbar spine films at baseline and 24 and 36 mo. Incident radiographic vertebral fractures were identified using a combination of morphometric and semiquantitative methods, by comparison of lateral spine films taken at baseline and an average of 2.9 yr later.37 A new incident vertebral fracture was defined as a fracture in a vertebra that was not fractured as baseline. Nonvertebral fractures were reported by participants and confirmed either by x-ray reports or from medical charts. Fractures of the skull, face, metacarpals, fingers, or toes were excluded, as were traumatic fractures. The average follow-up time for nonvertebral fractures was 2.6 ± 0.8 yr.
Adverse Events
All women were questioned about adverse events at each visit. Serious adverse events were defined as those that resulted in death, hospitalization, cancer, or permanent disability or were life-threatening.
Other Measures
Demographic data, medical history, and health-related habits were collected by self-report questionnaire at baseline. At each visit, participants’ height and weight were measured. BMI was calculated as weight in kilograms divided by height in meters squared. Information on walking for exercise was asked of a subset of 1201 English-speaking women at the larger sites in the United States. Women were defined as having diabetes when they reported a preexisting condition of diabetes, had a fasting blood glucose of ≥126 mg/dl (7.0 mmol/L), or were on hypoglycemic medications.
Statistical Analysis
Characteristics of participants at the baseline examination across categories of CrCl were compared using χ2 tests of homogeneity for categorical data, analyses of variance for normally distributed continuous data, and Kruskal-Wallis tests for skewed continuous data. In addition, within each category of CrCl, these same tests were used to compare characteristics at the baseline examination by treatment assignment. Using linear regression (least squared means procedure), the average annual percentage rate of change in BMD at the femoral neck, trochanter, and lumbar spine and its 95% CI was calculated according to category of baseline kidney function (CrCl and eGFR) and treatment assignment. Logistic regression was used to determine the effect of raloxifene on the risk for incident vertebral fractures within each category of baseline kidney function. Cox regression was used to determine the effect of raloxifene on the time to a new nonvertebral fracture within each category of baseline kidney function. Rates of serious adverse events and study discontinuation were calculated by category of baseline kidney function and by treatment assignment, and χ2 tests were performed to examine differences between treatment groups within each category of kidney function. Formal tests for interaction were performed to determine whether the effect of raloxifene on each outcome differed by baseline category of kidney function. The presence of an interaction between renal function and treatment effect was evaluated by including a treatment assignment × category of kidney function (ordinal variable with three levels) term in the models. All tests are presented with two-sided P values; P < 0.05 was considered significant. All analyses were performed using an intention-to-treat model and were conducted using SAS 9.1 (SAS Institute, Cary, NC).
A total of 7316 postmenopausal women (95% of the original MORE trial participants) were included in this analysis. Of the 389 women excluded from our analysis, 309 (79%) were from Australia, New Zealand, and Singapore, because these sites did not send serum specimens to the central laboratory for determination of laboratory parameters, including serum creatinine. Compared with the 7316 women included in the analysis, the 389 women excluded were, on average, slightly older (67.3 versus 66.4 yr; P = 0.02); were more likely to have had a hysterectomy (27 versus 22%; P = 0.03); had a lower BMI (24.7 versus 25.3 kg/m2; P = 0.01); had a slightly higher BMD at the femoral neck (0.65 versus 0.62; P < 0.01), lumbar spine (0.83 versus 0.82; P = 0.05), and trochanter (0.58 versus 0.55; P < 0.01); and were less likely to smoke (7.5 versus 17.3%; P ≤ 0.01).
DISCLOSURES
None.
Acknowledgments
This work was previously presented as a poster at the annual meeting of the American Society of Nephrology; November 8 through 13, 2005; Philadelphia, PA.
We acknowledge the tremendous help provided by Kyle Moen in preparing this manuscript.
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Berlyne GM, Ben Ari J, Kushelevsky A, Idelman A, Galinsky D, Hirsch M, Shainkin R, Yagil R, Zlotnik M: The aetiology of senile osteoporosis: Secondary hyperparathyroidism due to renal failure. Q J Med 44: 505–521, 1975 [PubMed] [Google Scholar]
- 2.Buchanan JR, Myers CA, Greer RB III: Effect of declining renal function on bone density in aging women. Calcif Tissue Int 43: 1–6, 1988 [DOI] [PubMed] [Google Scholar]
- 3.Sherman SS, Tobin JD, Hollis BW, Gundberg CM, Roy TA, Plato CC: Biochemical parameters associated with low bone density in healthy men and women. J Bone Miner Res 7: 1123–1130, 1992 [DOI] [PubMed] [Google Scholar]
- 4.Yendt ER, Cohanim M, Jarzylo S, Jones G, Rosenberg G: Bone mass is related to creatinine clearance in normal elderly women. J Bone Miner Res 6: 1043–1050, 1991 [DOI] [PubMed] [Google Scholar]
- 5.Lindberg JS, Moe SM: Osteoporosis in end-state renal disease. Semin Nephrol 19: 115–122, 1999 [PubMed] [Google Scholar]
- 6.Pitts TO, Piraino BH, Mitro R, Chen TC, Segre GV, Greenberg A, Puschett JB: Hyperparathyroidism and 1,25-dihydroxyvitamin D deficiency in mild, moderate, and severe renal failure. J Clin Endocrinol Metab 67: 876–881, 1988 [DOI] [PubMed] [Google Scholar]
- 7.Schnitzer T, Bone HG, Crepaldi G, Adami S, McClung M, Kiel D, Felsenberg D, Recker RR, Tonino RP, Roux C, Pinchera A, Foldes AJ, Greenspan SL, Levine MA, Emkey R, Santora AC, Kaur A, Thompson DE, Yates J, Orloff JJ: Therapeutic equivalence of alendronate 70 mg once-weekly and alendronate 10 mg daily in the treatment of osteoporosis. Alendronate Once-Weekly Study Group. Aging (Milano) 12: 1–12, 2000 [PubMed] [Google Scholar]
- 8.McClung MR, Geusens P, Miller PD, Zippel H, Bensen WG, Roux C, Adami S, Fogelman I, Diamond T, Eastell R, Meunier PJ, Reginster JY: Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med 344: 333–340, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE: Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 348: 1535–1541, 1996 [DOI] [PubMed] [Google Scholar]
- 10.Liberman UA, Weiss SR, Broll J, Minne HW, Quan H, Bell NH, Rodriguez-Portales J, Downs RW Jr, Dequeker J, Favus M: Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med 333: 1437–1443, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Pols HA, Felsenberg D, Hanley DA, Stepan J, Munoz-Torres M, Wilkin TJ, Qin-sheng G, Galich AM, Vandormael K, Yates AJ, Stych B: Multinational, placebo-controlled, randomized trial of the effects of alendronate on bone density and fracture risk in postmenopausal women with low bone mass: Results of the FOSIT study. Fosamax International Trial Study Group. Osteoporos Int 9: 461–468, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH, Mellstrom D, Oefjord ES, Marcinowska-Suchowierska E, Salmi J, Mulder H, Halse J, Sawicki AZ: Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344: 1434–1441, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Miller PD: Treatment of osteoporosis in chronic kidney disease and end-stage renal disease. Curr Osteoporos Rep 3: 5–12, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, Palermo L, Prineas R, Rubin SM, Scott JC, Vogt T, Wallace R, Yates AJ, LaCroix AZ: Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: Results from the Fracture Intervention Trial. JAMA 280: 2077–2082, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Cunningham J, Sprague SM, Cannata-Andia J, Coco M, Cohen-Solal M, Fitzpatrick L, Goltzmann D, Lafage-Proust MH, Leonard M, Ott S, Rodriguez M, Stehman-Breen C, Stern P, Weisinger J: Osteoporosis in chronic kidney disease. Am J Kidney Dis 43: 566–571, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Schulz E, Arfai K, Liu X, Sayre J, Gilsanz V: Aortic calcification and the risk of osteoporosis and fractures. J Clin Endocrinol Metab 89: 4246–4253, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Goodman WG, London G: Vascular calcification in chronic kidney disease. Am J Kidney Dis 43: 572–579, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Miller PD, Roux C, Boonen S, Barton IP, Dunlap LE, Burgio DE: Safety and efficacy of risedronate in patients with age-related reduced renal function as estimated by the Cockcroft and Gault method: A pooled analysis of nine clinical trials. J Bone Miner Res 20: 2105–2115, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Jamal SA, Bauer DC, Ensrud KE, Cauley JA, Hochberg M, Ishani A, Cummings SR: Alendronate treatment in women with normal to severely impaired renal function: An analysis of the Fracture Intervention Trial. J Bone Miner Res 22: 503–508, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Miller PD, Schwartz EN, Chen P, Misurski DA: Teriparatide in postmenopausal women with osteoporosis and mild or moderate renal impairment. Osteoporos Int 18: 59–68, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Hernandez E, Valera R, Alonzo E, Bajares-Lilue M, Carlini R, Capriles F, Martinis R, Bellorin-Font E, Weisinger JR: Effects of raloxifene on bone metabolism and serum lipids in postmenopausal women on chronic hemodialysis. Kidney Int 63: 2269–2274, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Bianchi ML, Colantonio G, Montesano A, Trevisan C, Ortolani S, Rossi R, Buccianti G: Bone mass status in different degrees of chronic renal failure. Bone 13: 225–228, 1992 [DOI] [PubMed] [Google Scholar]
- 27.Jassal SK, von Muhlen D, Barrett-Connor E: Measures of renal function BMD, bone loss, and osteoporotic fracture in older adults: The Rancho Bernardo Study. J Bone Miner Res 22: 203–210, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fried LF, Shlipak MG, Stehman-Breen C, Mittalhenkle A, Seliger S, Sarnak M, Robbins J, Siscovick D, Harris TB, Newman AB, Cauley JA: Kidney function predicts the rate of bone loss in older individuals: The Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci 61: 743–748, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Nickolas TL, McMahon DJ, Shane E: Relationship between moderate to severe kidney disease and hip fracture in the United States. J Am Soc Nephrol 17: 3223–3232, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Ensrud KE, Lui LY, Taylor BC, Ishani A, Shlipak MG, Stone KL, Cauley JA, Jamal SA, Antoniucci DM, Cummings SR: Renal function and risk of hip and vertebral fractures in older women. Arch Intern Med 167: 133–139, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Fried LF, Biggs ML, Shlipak MG, Seliger S, Kestenbaum B, Stehman-Breen C, Sarnak M, Siscovick D, Harris T, Cauley J, Newman AB, Robbins J: Association of kidney function with incident hip fracture in older adults. J Am Soc Nephrol 18: 282–286, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, De Zeeuw D, Hostetter TH, Lameire N, Eknoyan G: Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 67: 2089–2100, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Verhave JC, Fesler P, Ribstein J, du Cailar G, Mimran A: Estimation of renal function in subjects with normal serum creatinine levels: Influence of age and body mass index. Am J Kidney Dis 46: 233–241, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Cirillo M, Anastasio P, De Santo NG: Relationship of gender, age, and body mass index to errors in predicted kidney function. Nephrol Dial Transplant 20: 1791–1798, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Froissart M, Rossert J, Jacquot C, Paillard M, Houillier P: Predictive performance of the modification of diet in renal disease and Cockcroft-Gault equations for estimating renal function. J Am Soc Nephrol 16: 763–773, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Lamb EJ, Webb MC, Simpson DE, Coakley AJ, Newman DJ, O'Riordan SE: Estimation of glomerular filtration rate in older patients with chronic renal insufficiency: Is the modification of diet in renal disease formula an improvement? J Am Geriatr Soc 51: 1012–1017, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Davison AM: Renal disease in the elderly. Nephron 80: 6–16, 1998 [DOI] [PubMed] [Google Scholar]
- 38.Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, Christiansen C, Delmas PD, Zanchetta JR, Stakkestad J, Gluer CC, Krueger K, Cohen FJ, Eckert S, Ensrud KE, Avioli LV, Lips P, Cummings SR: Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: Results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA 282: 637–645, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Lu Y, Mathur AK, Blunt BA, Gluer CC, Will AS, Fuerst TP, Jergas MD, Andriano KN, Cummings SR, Genant HK: Dual X-ray absorptiometry quality control: Comparison of visual examination and process-control charts. J Bone Miner Res 11: 626–637, 1996 [DOI] [PubMed] [Google Scholar]
- 40.Lu Y, Ye K, Mathur AK, Hui S, Fuerst TP, Genant HK: Comparative calibration without a gold standard. Stat Med 16: 1889–1905, 1997 [DOI] [PubMed] [Google Scholar]
- 41.Genant HK, Jergas M, Palermo L, Nevitt M, Valentin RS, Black D, Cummings SR: Comparison of semiquantitative visual and quantitative morphometric assessment of prevalent and incident vertebral fractures in osteoporosis. The Study of Osteoporotic Fractures Research Group. J Bone Miner Res 11: 984–996, 1996 [DOI] [PubMed] [Google Scholar]