Abstract
Compared with white individuals, black individuals have a significantly higher risk for death in the general population but seem to have a survival advantage in the ESRD population. Data on the relationship of race to survival in early stages of chronic kidney disease (CKD) are inconsistent. This study evaluated racial differences in mortality among the adult participants of the Third National Health and Nutrition Examination Survey, a population-based survey of community-dwelling individuals. CKD was defined either by an estimated GFR <60 ml/min per 1.73 m2 or by the presence of albuminuria, and this status was determined for 14,611 individuals, 2892 of whom were found to have CKD. Adjusting for age, gender, and race, risk for all-cause mortality among individuals with CKD was more than double that of individuals with normal renal function. In the subgroup with CKD, adjusting for age and gender, black individuals had a significantly higher risk for death, and this risk was modified by age; specifically, black individuals who were younger than 65 yr were 78% more likely to die than white individuals, whereas no significant differences in mortality were observed among individuals who were ≥65 yr of age. Further adjustment for cardiovascular risk factors and CKD stage did not materially change the results, but the hazard ratios were significantly attenuated after adjustment for socioeconomic factors. In conclusion, these data demonstrate racial/ethnic disparities in mortality among individuals with CKD. This higher risk for death in early stages of CKD may explain the apparent survival advantage observed among black individuals who live long enough to reach stage 5 CKD.
In the general population, the cardiovascular and noncardiovascular mortality rates for black individuals are persistently higher than those for white individuals.1–3 In contrast, studies among individuals who have stage 5 chronic kidney disease (CKD) and are undergoing maintenance dialysis have consistently shown that black individuals have a survival advantage compared with white individuals.4,5 Even though some studies in the general population have shown lower mortality rates among Mexican Americans despite a higher prevalence of cardiovascular risk factors and lower socioeconomic status (“Hispanic paradox”), studies with more complete follow-up demonstrated poorer outcomes in this ethnic group when compared with non-Hispanic white individuals.2,6,7 In contrast, Hispanic individuals with stage 5 CKD also seem to have a survival advantage.5,8 Despite various hypotheses, these paradoxic disparities with survival advantages for racial/ethnic minorities undergoing maintenance dialysis have not been satisfactorily explained.
We undertook this study to test the hypothesis that black and Mexican American individuals may have a higher mortality than white individuals at earlier stages of CKD. Thus, the apparent survival advantage during stage 5 CKD may be a result of a higher mortality early during the course of CKD, wherein only the healthiest minority individuals survive long enough for their CKD to progress to require maintenance dialysis.
RESULTS
Patient Characteristics
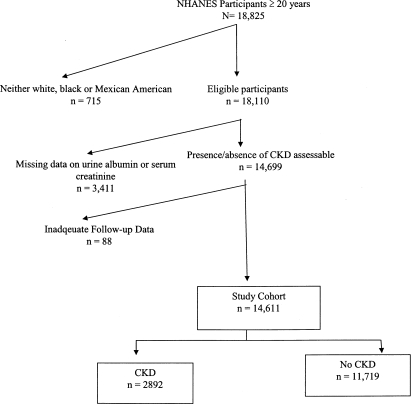
Of the 18,825 study participants who were ≥20 yr of age in the Third National Health and Nutrition Examination Survey (NHANES III), 715 did not identify themselves as white, black or Mexican American, and the data on follow-up interval was inadequate for 88 individuals (Figure 1). An additional 3411 individuals were excluded because they had missing data on urine albumin or serum creatinine. Thus, a total of 14,611 individuals formed the study cohort (Figure 1). Comparing the individuals included in the cohort, those with missing data on urine albumin or serum creatinine were slightly more likely to be black (12.6 versus 11.7%; P = 0.003), to be older (48.6 ± 0.6 versus 44.3 ± 0.5 yr; P < 0.001), and to have <12 yr of education (27.7 versus 23.1%; P = 0.005) and less likely to be obese (19.2 versus 22.8%; P = 0.04).
Figure 1.
Flow diagram illustrating how the cohort was built for analyses for this study from the 18,825 participants who were ≥20 yr of age in the NHANES III.
Of the 14,611 individuals, 2892 had CKD, with a weighted prevalence of 14.1%. Comparisons of the risk factors among the three racial/ethnic groups with CKD are summarized in Table 1. The mean age of white individuals was 7 yr older than black individuals and 12 yr older than Mexican Americans. White individuals had the highest prevalence of hypercholesterolemia and a family history of premature heart disease and were most likely to go to a particular place for health care. Conversely, they were least likely to have diabetes, obesity, low income, or lower educational attainment or be uninsured (Table 1). Black individuals were significantly more likely to be hypertensive and had the highest prevalence of smoking (Table 1). Mexican Americans had the highest prevalence of diabetes, obesity, poverty, having less than a high school education, or being uninsured (Table 1).
Table 1.
Comparison of risk factors among white, black, and Mexican American individuals with CKD
| Parameter | White | Black | Mexican American | P |
|---|---|---|---|---|
| Age (yr; mean ± SD) | 58.4 ± 0.9 | 51.4 ± 0.9 | 46.9 ± 0.9 | <0.0001 |
| Male gender (%) | 44.5 | 44.7 | 48.9 | 0.3200 |
| Hypertension (%) | 52.1 | 60.2 | 40.5 | <0.0001 |
| Total cholesterol ≥200 mg/dl (%) | 63.7 | 57.7 | 54.8 | 0.0010 |
| Diabetes (%) | 21.6 | 24.4 | 29.3 | 0.0200 |
| Obese (%) | 29.9 | 35.6 | 38.3 | 0.0300 |
| Current smoker (%) | 23.4 | 34.1 | 21.1 | 0.0002 |
| Family history of heart disease (%) | 17.7 | 12.4 | 13.5 | 0.0100 |
| Income <200% federal poverty level (%) | 34.5 | 66.6 | 71.9 | <0.0001 |
| Education ≤12 yr (%) | 65.7 | 78.5 | 85.0 | <0.0001 |
| No health insurance (%) | 6.0 | 10.6 | 32.6 | <0.0010 |
| Go to particular place for health care? (%) | 89.4 | 83.7 | 72.0 | <0.0001 |
| Stage of CKD (%) | ||||
| 1 | 36.7 | 58.5 | 71.4 | |
| 2 | 29.4 | 22.3 | 18.9 | |
| 3 | 32.4 | 16.4 | 8.8 | <0.0001 |
| 4 | 1.3 | 1.0 | 0.5 | |
| 5 | 0.1 | 1.8 | 0.5 |
Outcomes
The mean follow-up until the time to event or last follow-up for the cohort was 8.8 ± 0.2 to 9.0 ± 0.2 yr for individuals without CKD and 7.9 ± 0.2 yr for those with CKD. Among the 14,611 participants, 2171 died: 1127 with CKD and 1104 without CKD. The crude, all-cause mortality among individuals with CKD was 3.6 per 100 person-years compared with 0.6 per 100 person-years among those without CKD. Of these 2171 deaths, 969 (45%) were attributed to cardiovascular causes, 578 among those with CKD and 391 among those without CKD. Cardiovascular mortality among individuals with CKD was 1.8 per 100 patient-years compared with 0.2 per 100 patient-years without CKD. After adjustment for age, race, and gender, CKD was associated with 2.2-fold higher hazard ratio (HR; 95% confidence interval [CI] 1.9 to 2.5) for all-cause and 2.5-fold higher HR (95% CI 2.1 to 3.0) for cardiovascular mortality. There was no significant interaction between race and CKD for either all-cause or cardiovascular mortality.
Among the 1127 participants who had CKD and died on follow-up, 707 were white, 243 were black, and 177 were Mexican American. Only five of these 1127 deaths were attributable to AIDS and three to homicide/suicide. After adjustment for age and gender, using white individuals as the reference group, black individuals with CKD had a substantially elevated risk for all-cause mortality; this risk was modified by age. The risk for all-cause mortality was only slightly attenuated upon further adjustment for traditional risk factors for cardiovascular disease (model 2) or for socioeconomic factors and access to care (model 3). Given the significant interaction of race with age on all-cause mortality, age-stratified analyses were conducted (Table 2). Among individuals who were younger than 65 yr, black individuals had a 78% higher risk for death compared with white individuals (Figure 2A). The higher risk for death in this age group remained significant even after adjustment for stage of CKD and cardiovascular risk factors (HR 1.53; 95% CI 1.01 to 2.31) but was attenuated to a NS level upon adjustment for socioeconomic and access-to-care variables (Table 2). Conversely, there was no significant difference in the risk for death in the older subgroups of black and white individuals (Table 2, Figure 2A). There was a trend toward a higher risk for all-cause mortality among Mexican Americans, with white individuals as a reference group; this trend, however, did not reach statistical significance. A similar trend for an interaction of age with ethnicity was observed among Mexican Americans (Table 2, Figure 2B). Entering the data on cardiovascular risk factors as continuous variables or adding laboratory data (hemoglobin, glycosylated hemoglobin, phosphorus, potassium, albumin, homocysteine, insulin, and C-reactive protein) did not change the HR for death among black or Mexican American individuals.
Table 2.
Summary of age-stratified, multivariate analyses of the association of race/ethnicity with all-cause and cardiovascular mortality
| Parameter | Model 1 (Adjusted for Age and Gender)a | Model 2 (Adjusted for Age, Gender, CKD Stage, and Cardiovascular Risk Factors)b | Model 3 (Adjusted for Age, Gender, CKD Stage, and Socioeconomic Status)c |
|---|---|---|---|
| All-cause mortality (reference white) | |||
| <65 yr | |||
| black | 1.78 (1.14 to 2.78) | 1.53 (1.01 to 2.31) | 1.38 (0.83 to 2.28) |
| Mexican American | 1.35 (0.94 to 1.94) | 1.26 (0.84 to 1.90) | 0.94 (0.57 to 1.53) |
| 65 to 75 yr | |||
| black | 1.12 (0.89 to 1.42) | 1.16 (0.89 to 1.52) | 0.90 (0.66 to 1.22) |
| Mexican American | 1.03 (0.76 to 1.40) | 1.00 (0.67 to 1.49) | 0.80 (0.58 to 1.11) |
| >75 yr | |||
| black | 0.94 (0.76 to 1.17) | 0.95 (0.75 to 1.21) | 0.85 (0.66 to 1.09) |
| Mexican American | 0.72 (0.44 to 1.17) | 0.68 (0.41 to 1.11) | 0.79 (0.47 to 1.30) |
| Cardiovascular mortality | |||
| <65 yr | |||
| black | 2.11 (1.00 to 4.47) | 1.78 (0.77 to 4.14) | 1.55 (0.63 to 3.80) |
| Mexican American | 1.56 (0.81 to 3.01) | 1.87 (0.93 to 3.78) | 1.39 (0.65 to 2.96) |
| 65 to 75 yr | |||
| black | 1.06 (0.73 to 1.53) | 1.14 (0.72 to 1.80) | 0.87 (0.56 to 1.36) |
| Mexican American | 1.04 (0.61 to 1.78) | 1.14 (0.57 to 2.27) | 0.72 (0.45 to 1.17) |
| >75 yr | |||
| black | 0.79 (0.55 to 1.12) | 0.84 (0.57 to 1.24) | 0.72 (0.48 to 1.09) |
| Mexican American | 0.71 (0.47 to 1.07) | 0.65 (0.41 to 1.02) | 0.82 (0.52 to 1.28) |
Age and gender.
Age, gender, hypertension, cholesterol ≥200 mg/dl, obesity, diabetes, current smoking, family history of premature cardiovascular disease, and stage of CKD.
Age, gender, stage of CKD, federal poverty level <200%, education <12 yr, medical insurance, and go to particular place for care.
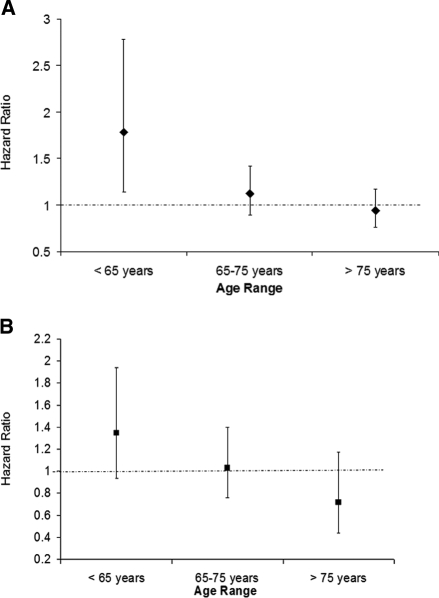
Figure 2.
(A) Effect of age on the differences in outcomes among whites and black individuals with CKD enrolled in the NHANES III. The HR for all-cause mortality, adjusted for age and gender, among black individuals in different age categories were as follows: <65 yr 1.78 (95% CI 1.14 to 2.78); 65 to 75 yr 1.12 (95% CI 0.89 to 1.42) and >75 yr 0.94 (95% CI 0.76 to 1.17). (B) Effect of age on the differences in outcomes among white and Mexican American individuals with CKD enrolled in the NHANES III. The HR for all-cause mortality, adjusted for age and gender, among Mexican Americans in different age categories were as follows: <65 yr 1.35 (95% CI 0.94 to 1.94); 65 to 75 yr 1.03 (95% CI 0.76 to 1.40); and >75 yr 0.72 (95% CI 0.44 to 1.17).
Among the 578 deaths from cardiovascular causes, 384 events occurred among white individuals, 105 among black individuals, and 89 among Mexican Americans. Upon adjustment for age and gender, the risk for cardiovascular death was significantly and substantially higher among black compared with white individuals. The significant interaction of age with race was apparent for cardiovascular mortality as well such that the increased risk for cardiovascular mortality was limited to individuals who were younger than 65 yr (HR 2.11; 95% CI 1.00 to 4.47; Table 2). The same qualitative, albeit NS, trends were observed after adjustment for stage of CKD and cardiovascular risk factors or socioeconomic and access-to-care variables. There was a trend, albeit statistically NS, for a higher risk for cardiovascular death among younger Mexican Americans as well when compared with white individuals (Table 2).
Sensitivity Analyses
Three different sensitivity analyses were performed using modified definitions of CKD. First, only participants with estimated GFR (eGFR) <60 ml/min per 1.73 m2 were defined as having CKD (n = 996). The second analysis was performed to account for the fact that only 63% of the NHANES III participants had persistent microalbuminuria.9 For this analysis, CKD was defined as the presence of any one of the following: (1) Stage 3, 4, or 5 CKD; (2) stage 1 or 2 CKD with overt albuminuria (albumin/creatinine ratio >250 mg/g in men and >355 mg/g in women); and (3) a random selection of 54% of individuals with stage 1 and 73% of individuals with stage 2 CKD with microalbuminuria (total n = 2212).9 Finally, for the third analysis, all 3411 individuals with insufficient information to determine CKD status were considered to have CKD (n = 6207).
The same qualitative trends that were observed in the main cohort were seen in each of the three sensitivity analyses performed (Table 3). Thus, there was a progressive decrease in the HR for all-cause mortality among black compared with white individuals, with increasing age in each of the three sensitivity analyses. The same qualitative trend for a higher risk for death was noted for Mexican Americans.
Table 3.
Summary of the HR for all-cause mortality for black and Mexican American compared with white individuals using three different sensitivity analysesa
| Age (yr) | Black
|
Mexican American
|
||||
|---|---|---|---|---|---|---|
| Sensitivity Analysis 1 | Sensitivity Analysis 2 | Sensitivity Analysis 3 | Sensitivity Analysis 1 | Sensitivity Analysis 2 | Sensitivity Analysis 3 | |
| <65 | 2.11 (0.83 to 5.37) | 1.92 (1.14 to 3.24) | 1.74 (1.28 to 2.39) | 2.20 (0.95 to 5.10) | 1.46 (0.95 to 2.23) | 1.48 (1.07 to 2.03) |
| 65 to 75 | 1.13 (0.68 to 1.88) | 1.14 (0.84 to 1.56) | 1.21 (0.97 to 1.51) | 1.07 (0.61 to 1.87) | 0.96 (0.65 to 1.41) | 0.88 (0.69 to 1.13) |
| >75 | 0.89 (0.65 to 1.21) | 0.95 (0.75 to 1.21) | 1.07 (0.92 to 1.26) | 0.92 (0.59 to 1.42) | 0.74 (0.43 to 1.27) | 0.84 (0.58 to 1.20) |
All models are adjusted for age and gender. Sensitivity 1 = analyses restricted to individuals with stages 3, 4, and 5 CKD (n = 996). Sensitivity 2 = analyses restricted to individuals with stages 3, 4, and 5 CKD and following two groups of individuals with stages 1 and 2 CKD and overt proteinuria and a random selection of 54% of individuals with microalbuminuria and stage 1 CKD and 73% of those with microalbuminuria and stage 2 CKD (n = 2212). Sensitivity 3 = analyses extended to include either individuals categorized as having CKD (based on eGFR and albuminuria) or those with missing values such that their CKD status could not be ascertained (n = 6207).
DISCUSSION
The key finding of this study may have important public health implications. Using a random sample of the population of the United States, black individuals with CKD were found to have a substantially higher risk for all-cause and cardiovascular mortality, a risk modified by age. Similar trends, albeit not statistically significant, were observed among Mexican Americans.
In the general population, racial disparities have been reported with particularly inferior outcomes among black individuals with cardiovascular diseases and cancers.1–3 Disparities in several different outcomes also have been reported in populations with CKD. Thus, black individuals are less likely to receive erythropoietin in the predialysis stage of CKD, more likely to progress to ESRD, less likely to achieve clinical performance measure targets while undergoing maintenance hemodialysis, and less likely to be referred for transplantation and have a lower transplant allograft survival when compared with similar populations of white individuals.10–15 Our study adds to this list of disparities.
Numerous studies have now documented the increased risk for death conferred by the presence of CKD, either by the presence of albuminuria or reduction in GFR16; however, up until recently, racial difference in mortality outcomes among non–dialysis-dependent patients with CKD has received only limited attention. Using pooled analyses of four community-based studies, Weiner et al.17 suggested that there may be a significant interaction between race and CKD. Thus, the presence of CKD conferred a greater increase in risk for the composite end point of death, nonfatal myocardial infarction, and stroke among black than among white individuals. The investigators attributed this interaction to residual confounding, with the differences possibly relating to greater severity of hypertension and/or diabetes in black individuals. Consistent with these findings, CKD has been reported to result in a significantly greater increase in risk for coronary artery disease mortality or incident congestive heart failure among blacks than white individuals.18,19 In our study, death was the only outcome measure studied, and we were unable to demonstrate any interaction between race and CKD.
Our findings are in contrast to those reported among veterans with diabetes, wherein racial and ethnic minorities had a lower risk for death than white individuals, a survival advantage also seen among the subgroup with diabetic nephropathy.20 The differences in outcomes may be a result of better and equal access to care among veterans when compared with the general population.
The modifying effect of age on the racial differences in outcomes has previously been reported in the general population but not among individuals with CKD.21 Thus, the health disparities seem to be greatest in the younger age groups. Similar outcomes among the elderly blacks and white individuals with CKD may reflect either survivor bias (such that only the healthiest black individuals survive into old age) or the benefit of federal/state programs for the elderly, such as Medicare, that provide improved access to care for participating individuals equally, thus minimizing racial disparities. The results of various studies that have examined the racial differences in mortality of elderly with CKD have come to differing conclusions. On the one hand, elderly black individuals who are hospitalized for congestive heart failure or survivors of acute myocardial infarction seem to have a survival advantage over white individuals.22–24 On the other hand, analyses using the Medicare 5% sample standard analytical files demonstrated that black individuals, with or without CKD, had a higher risk for death.25 Unlike previous investigations of the elderly, the cohort in this study was a random selection of noninstitutionalized population of United States, and the baseline assessment of renal function was comprehensive (including serum creatinine and albuminuria) and was done with individuals in a steady state and serum creatinine was calibrated to the Modification of Diet in Renal Disease (MDRD) laboratory measurements.
Our study provides some insight into the reasons for the higher risk for death among younger black individuals with CKD, relative to white individuals. The higher risk for death among younger black compared with white individuals persisted despite multivariate adjustment for cardiovascular risk factors; however, the HR was attenuated to a NS level upon adjustment for socioeconomic and access-to-care variables. These data suggest that factors such as education, poverty, and lower probability of medical insurance may be more important in mediating the high risk for death among younger black individuals than are biologic differences. This, in turn, may help in prioritizing interventions aimed at reducing the disparities among younger black individuals with CKD. Recognition of this disparity among younger black individuals raises two important issues. First, the substantially higher mortality among black individuals with early stage CKD may suggest that the disparity in adverse CKD outcomes is far greater than previously suggested by studies restricted to ESRD progression. Second, the survival advantage of black individuals seen in studies of dialysis patients may be accounted for the higher risk for death seen in this racial group at early stages of CKD such that the black individuals who live long enough to reach stage 5 CKD are healthier than white individuals. This hypothesis is consistent with the recent observations from the Dialysis Outcomes and Practice Patterns Study that reported that minority individuals undergoing maintenance hemodialysis in the United States are healthier than their white counterparts, accounting for the former's survival advantage.5
In recent years, demographic shifts have made Latinos the largest minority group in the United States.26 Unlike the data for black individuals, the comparative data for Latinos are rather limited. Early studies in the general population indicated that despite a higher prevalence of cardiovascular risk factors (obesity and diabetes) and lower socioeconomic status, Latinos had lower all-cause and cardiovascular mortality when compared with non-Hispanic white individuals (“Hispanic paradox”).6 Subsequent data, however, suggested that outcomes among many subgroups of Latinos may actually be worse than that for white individuals.2,7,27 The data on outcomes for Latinos with CKD are far more limited. At least three studies suggested that Latinos, like black individuals, with ESRD may have a survival advantage when compared with white individuals.8,28,29
To our knowledge, this report is the first to describe racial differences in outcomes using a population that included a substantial proportion of non–dialysis-dependent Mexican Americans with CKD. The same qualitative trends, with a higher all-cause and cardiovascular mortality among Mexican Americans, were observed as those for black individuals; however, none of the trends reached statistical significance. This may reflect that there are no significant differences in outcomes of Mexican Americans and white individuals with CKD. Alternatively, this may be a result of relatively smaller sample size and fewer events, particularly cardiovascular, among Mexican Americans. Thus, larger studies with longer follow-up that allow more events to accrue will be needed to determine whether a similar disparity in outcomes exist for Mexican Americans with CKD.
This study has several limitations. First, the sample size and the number of events for some subgroups were small, such as for Mexican Americans. This is also true for the age-stratified analyses and cardiovascular mortality. Second, it is possible that some Mexican Americans may have died in their country of origin and, therefore, their data may not have been captured by a review of the National Death Index (NDI). This may have led us to underestimate the risk for death in this ethnic population (salmon effect). Third, almost one fifth of eligible participants did not have enough data to determine the presence/absence of CKD; however, our sensitivity analyses suggest that this missing information is unlikely to have biased the study results. Fourth, data on risk factors were ascertained only at baseline; therefore, we could not systematically control for differences in severity of risk factors or perform time-dependent analyses. Finally, the baseline evaluation occurred before the benefits of contemporary therapies like blockers of the renin-angiotensin system were established.
This study shows a higher all-cause and cardiovascular mortality for younger black individuals with early-stage CKD. The disparity in all-cause mortality remained after accounting for differences in prevalence of cardiovascular risk factors but was attenuated to NS levels after adjustment for socioeconomic status. Survivor bias, arising from the higher mortality during early stages of CKD, may explain the lower mortality seen among black individuals with ESRD. For determination of whether the noted trends for disparities exist for Mexican Americans with CKD, larger studies with longer follow-ups are needed.
CONCISE METHODS
NHANES III was a study conducted by the National Center for Health Statistics (NCHS) between 1988 and 1994.30 Study participants were selected using a complex, multistage, stratified, and clustered probability sampling of the noninstitutionalized population of the United States, and the baseline data were accrued between 1988 and 1994.31 The study design included a deliberate oversampling of black and Mexican American individuals and the elderly.31
The cohort for this study constituted white, black, and Mexican American individuals who were ≥20 yr of age and had CKD. For the survey, the participants self-identified as belonging to a given racial/ethnic group. eGFR, using the abbreviated MDRD equation, and urine albumin/creatinine ratio were used to determine CKD.32 The creatinine measurements in the NHANES III were calibrated to the MDRD laboratory by subtracting 0.23 mg/dl, as described previously.9 An individual was identified as having CKD when (1) eGFR was <60 ml/min per 1.73 m2 or (2) eGFR was ≥60 but urine albumin/creatinine was >17 mg/g in men and >25 mg/g in women.32
Definition of Variables
Hypertension was defined as present when the individual was taking antihypertensive medications or the measured BP was >140/90 mmHg. Hypercholesterolemia was defined as a total cholesterol ≥200 mg/dl because only one third of the participants had data on LDL cholesterol. Diabetes was defined as a history of diabetes, being treated with medications for diabetes, or having a fasting blood glucose >126 mg/dl. Obesity was defined as a body mass index ≥30 kg/m2. Current smokers were individuals who currently smoked and had consumed at least 100 cigarettes during their lifetime. The National Kidney Foundation's definition was used to designate stages of CKD.32
Education was used as a dichotomous variable (<12 or ≥12 completed years of schooling). Patients were classified as having any health insurance or none. The effect of income was assessed by determining whether the participant's household income was <200 or ≥200% of the federal poverty level. Access to care was defined as whether a participant reported going to a particular facility for care.
Determination of Vital Status
Survival was determined by linking the baseline data to the mortality follow-up file.33 In this file, the MORTSTAT variable indicates the final ascertainment of patients’ vital status by the NCHS, with a participant deemed to be dead when a probabilistic match was made on the NDI screen or on a death certificate or both.33 For the former, the data in the NDI were screened from the time of individuals’ enrollment in the NHANES III until December 31, 2000. To assign accurately the vital status to study participants using the NDI, the NCHS conducted a calibration study to develop the criteria used for the study.33 Cause of death was coded using the International Classification of Diseases, Ninth Revision (ICD-9) up until 1998 and ICD-10 for 1999 and 2000. All deaths before 1999 were recoded by the NCHS into comparable ICD-10 codes; the causes for all deaths of the study participants, using ICD-10 codes, are available in the UCOD_113 variable. The following range of codes for the UCOD_113 variable was used to define cardiovascular deaths: 056 to 063 and 067 to 074. This definition included, among others, causes of death related to hypertensive heart disease, atherosclerosis including coronary and cerebrovascular disease, heart failure, and aortic aneurysms.
Statistical Analysis
All analyses were performed using sample weights that account for unequal probability of selection; nonresponse; and planned oversampling of elderly, black, and Mexican American individuals. Estimates were made using the recommended SAS-callable SUDAAN software (version 8; Research Triangle Park, NC). Continuous variables are expressed as means ± SEM.
All individuals without a probabilistic match were deemed to be alive on December 31, 2000. Cox proportional hazards analysis was used to determine the effect of CKD on survival using data from the entire cohort of 14,611 individuals. All subsequent analyses were restricted to individuals with CKD, and three statistical models were built. Various interaction terms with race were tested in the cohort with CKD; only one (age*race) was statistically significant. This interaction term was included in all subsequent models, and age-stratified analyses were performed (<65, 65 to 75, and >75 yr). Given the significant differences in age between the participants belonging to the various racial/ethnic groups, the primary survival analyses were adjusted for age, race, and gender (model 1). The second model was constructed to test whether the racial/ethnic differences in survival could be accounted for by differences in cardiovascular risk factors and thus included, in addition to demographics (age, race, and gender), traditional cardiovascular risk factors (hypertension, cholesterol ≥200 mg/dl, diabetes, obesity, current smoking, and family history of premature heart disease) and stage of CKD (model 2). The third model was constructed to test whether the racial/ethnic differences in survival could be accounted for by differences in socioeconomic status/access to care and thus included, in addition to demographics, socioeconomic factors (education, insurance, and income), access to care (go to particular place for care), and stage of CKD (model 3). To estimate the racial/ethnic differences in cardiovascular mortality, we performed similar analyses after censoring participants who died of noncardiovascular causes. All of these analyses were repeated on the three data sets created for sensitivity analyses.
DISCLOSURES
None.
Acknowledgments
R.M. is supported by a K23 grant, RR18298, from the National Institutes of Health. Support for this work was also provided by National Institutes of Health grants RR011145 (D.K. and K.N.), RR019234 (D.K. and K.N.), RR014616 (K.N.), and MD000182 (K.N.).
An abstract based on this work was presented at the annual meeting of the American Society of Nephrology; November 14 through 19, 2006; San Diego, CA.
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Levine RS, Foster JE, Fullilove RE, Fullilove MT, Briggs NC, Hull PC, Husaini BA, Hennekens CH: Black-white inequalities in mortality and life expectancy, 1933–1999: Implications for healthy people 2010. Public Health Rep 116: 474–483, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB: State of disparities in cardiovascular health in the United States. Circulation 111: 1233–1241, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Murray CJ, Kulkarni SC, Michaud C, Tomijima N, Bulzacchelli MT, Iandiorio TJ, Ezzati M: Eight Americas: Investigating mortality disparities across races, counties, and race-counties in the United States. PLoS Med 3: 1513–1524, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.United States Renal Data System: Annual Data Report. Bethesda, US Department of Public Health and Human Services, Public Health Service, National Institutes of Health, 2005
- 5.Robinson BM, Joffe MM, Pisoni RL, Port FK, Feldman HI: Revisiting survival differences by race and ethnicity among hemodialysis patients: The Dialysis Outcomes and Practice Patterns Study. J Am Soc Nephrol 17: 2910–2918, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Sorlie PD, Backlund E, Johnson NJ, Rogot E: Mortality by Hispanic status in the United States. JAMA 270: 2464–2468, 1993 [PubMed] [Google Scholar]
- 7.Hunt KJ, Resendez RG, Williams K, Haffner SM, Stern MP, Hazuda HP: All-cause and cardiovascular mortality among Mexican-American and non-Hispanic white older participants in the San Antonio Heart Study: Evidence against the “Hispanic paradox.” Am J Epidemiol 158: 1048–1057, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Frankenfield DL, Rocco MV, Roman SH, McClellan WM: Survival advantage for adult Hispanic hemodialysis patients? Findings from the end-stage renal disease clinical performance measures project. J Am Soc Nephrol 14: 180–186, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS: Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis 41: 1–12, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Hsu CY, Lin F, Vittinghoff E, Shlipak MG: Racial differences in the progression from chronic renal insufficiency to end-stage renal disease in the United States. J Am Soc Nephrol 14: 2902–2907, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Alexander GC, Sehgal AR: Barriers to cadaveric renal transplantation among blacks, women, and the poor. JAMA 280: 1148–1152, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Young CJ, Gaston RS: African Americans and renal transplantation: Disproportionate need, limited access, and impaired outcomes. Am J Med Sci 323: 94–99, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez RA, Sen S, Mehta K, Moody-Ayers S, Bacchetti P, O'Hare AM: Geography matters: Relationships among urban residential segregation, dialysis facilities, and patient outcomes. Ann Intern Med 146: 493–501, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Ward MM: Laboratory abnormalities at the onset of treatment of end-stage renal disease: Are there racial or socioeconomic disparities in care? Arch Intern Med 167: 1083–1091, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Frankenfield DL, Rocco MV, Frederick PR, Pugh J, McClellan WM, Owen WF Jr: Racial/ethnic analysis of selected intermediate outcomes for hemodialysis patients: Results from the 1997 ESRD Core Indicators Project. Am J Kidney Dis 34: 721–730, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, McAlister F, Garg AX: Chronic kidney disease and mortality risk: A systematic review. J Am Soc Nephrol 17: 2034–2047, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, Salem DN, Levey AS, Sarnak MJ: Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: A pooled analysis of community-based studies. J Am Soc Nephrol 15: 1307–1315, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Muntner P, He J, Hamm L, Loria C, Whelton PK: Renal insufficiency and subsequent death resulting from cardiovascular disease in the United States. J Am Soc Nephrol 13: 745–753, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Bibbins-Domingo K, Chertow GM, Fried LF, Odden MC, Newman AB, Kritchevsky SB, Harris TB, Satterfield S, Cummings SR, Shlipak MG: Renal function and heart failure risk in older black and white individuals: The Health, Aging, and Body Composition Study. Arch Intern Med 166: 1396–1402, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Young BA, Maynard C, Boyko EJ: Racial differences in diabetic nephropathy, cardiovascular disease, and mortality in a national population of veterans. Diabetes Care 26: 2392–2399, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Thomas AJ, Eberly LE, Davey Smith G, Neaton JD, Stamler J: Race/ethnicity, income, major risk factors, and cardiovascular disease mortality. Am J Public Health 95: 1417–1423, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McClellan WM, Flanders WD, Langston RD, Jurkovitz C, Presley R: Anemia and renal insufficiency are independent risk factors for death among patients with congestive heart failure admitted to community hospitals: A population-based study. J Am Soc Nephrol 13: 1928–1936, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Smith GL, Shlipak MG, Havranek EP, Masoudi FA, McClellan WM, Foody JM, Rathore SS, Krumholz HM: Race and renal impairment in heart failure: Mortality in blacks versus whites. Circulation 111: 1270–1277, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Newsome BB, McClellan WM, Coffey CS, Allison JJ, Kiefe CI, Warnock DG: Survival advantage of black patients with kidney disease after acute myocardial infarction. Clin J Am Soc Nephrol 1: 993–999, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Xue JL, Eggers PW, Agodoa LY, Foley RN, Collins AJ: Longitudinal study of racial and ethnic differences in developing end-stage renal disease among aged Medicare beneficiaries. J Am Soc Nephrol 18: 1299–1306, 2007 [DOI] [PubMed] [Google Scholar]
- 26.US Census Bureau: US Census 2000. Available at: http://factfinder.census.gov/servlet/DTTable?_bm=y&-geo_id=01000US&-ds_name=PEP_2006_EST&-_lang=en&-state=dt&-mt_name=PEP_2006_EST_G2006_T004_2006&-format=&-CONTEXT=dt. Accessed March 17, 2008
- 27.Goff DC, Nichaman MZ, Chan W, Ramsey DJ, Labarthe DR, Ortiz C: Greater incidence of hospitalized myocardial infarction among Mexican Americans than non-Hispanic whites. The Corpus Christi Heart Project, 1988–1992. Circulation 95: 1433–1440, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Pugh JA, Tuley MR, Basu S: Survival among Mexican-Americans, non-Hispanic whites, and African-Americans with end-stage renal disease: The emergence of a minority pattern of increased incidence and prolonged survival. Am J Kidney Dis 23: 803–807, 1994 [DOI] [PubMed] [Google Scholar]
- 29.Murthy BV, Molony DA, Stack AG: Survival advantage of Hispanic patients initiating dialysis in the United States is modified by race. J Am Soc Nephrol 16: 782–790, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Plan and operation of the Third National health and Nutrition Examination Survey, 1988–1994. Series 1: Programs and Collection Procedures. Vital Health Stat 1. (32): 1–407, 1994 [PubMed]
- 31.Ezzati TM, Massey JT, Wakesberg J, Chu A, Maurer KR: Sample design: Third National Health and Nutrition Examination Survey. Vital Health Stat 2 (113): 1–35, 1992 [PubMed]
- 32.National Kidney Foundation: K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification and stratification. Am J Kidney Dis 39: S1–S266, 2002 [PubMed] [Google Scholar]
- 33.The Third National Health and Nutrition Examination Survey (NHANES III) Linked Mortality File: Matching Methodology, December 2005. Available at: http://www.cdc.gov/nchs/data/datalinkage/matching_methodology_nhanes3_final.pdf. Accessed August 7, 2005