Abstract
Although neurologic sequelae of acute kidney injury (AKI) are well described, the pathogenesis of acute uremic encephalopathy is poorly understood. This study examined the short-term effect of ischemic AKI on inflammatory and functional changes of the brain in mice by inducing bilateral renal ischemia for 60 min and studying the brains 24 h later. Compared with sham mice, mice with AKI had increased neuronal pyknosis and microgliosis in the brain. AKI also led to increased levels of the proinflammatory chemokines keratinocyte-derived chemoattractant and G-CSF in the cerebral cortex and hippocampus and increased expression of glial fibrillary acidic protein in astrocytes in the cortex and corpus callosum. In addition, extravasation of Evans blue dye into the brain suggested that the blood-brain barrier was disrupted in mice with AKI. Because liver failure also leads to encephalopathy, ischemic liver injury was induced in mice with normal renal function; neuronal pyknosis and glial fibrillary acidic protein expression were not increased, suggesting differential effects on the brain depending on the organ injured. For evaluation of the effects of AKI on brain function, locomotor activity was studied using an open field test. Mice subjected to renal ischemia or bilateral nephrectomy had moderate to severe declines in locomotor activity compared with sham-operated mice. These data demonstrate that severe ischemic AKI induces inflammation and functional changes in the brain. Targeting these pathways could reduce morbidity and mortality in critically ill patients with severe AKI.
Mortality during acute kidney injury (AKI) is largely due to extrarenal manifestations.1,2 Central nervous system changes, the signs of which range from decreased mental status to obtundation and seizures, are one of the classic indications to begin dialysis during AKI. Other indications for renal replacement therapy include pulmonary edema, hyperkalemia, pericarditis, and severe acidosis.3 Dialysis improves but does not fully correct central nervous system manifestations or other distant organ effects of renal failure, either acutely or chronically.4 In patients with AKI, the symptoms of encephalopathy are generally more pronounced and progress more rapidly than with chronic kidney disease or ESRD.5,6 Although neurologic sequelae of AKI are well established, the pathogenesis of acute uremic encephalopathy is poorly understood. A multitude of potential uremic toxins, including the nitric oxide synthase modulating guanidino compounds (e.g., creatinine, guanidine, guanidinosuccinic acid, methylguanidine), have been implicated in uremic encephalopathy.7–9 Brain edema and alterations in water transport have also been implicated.10,11 Besides the guanidino compounds, decreased brain energy demand, free amino acid changes, and blood-brain barrier derangement have been shown to be involved in both acute and chronic uremic encephalopathy.12–14 We and others have begun to study mechanisms that underlie distant organ effects of AKI and found a significant inflammatory effect of AKI on the lung15–18 and the heart.19 On the basis of these studies, we hypothesized that AKI could lead to brain inflammation. We also examined brain water content and microvascular permeability and postulated that AKI would lead to acute changes in brain function. We found that AKI led to both soluble and cellular inflammation in the brain, with the hippocampus being a prime target. AKI led to an increase in the brain microvascular protein leakage. We found that severe AKI, as well as bilateral nephrectomy, led to more pronounced behavior changes compared with less severe AKI or the combination of sham AKI with surgery and anesthesia.
RESULTS
Renal and Liver Functions in AKI and Acute Liver Injury Models
The effect of clamping or removing of the kidney or clamping of the liver vasculature in the two different models was confirmed by a significant increase in serum creatinine (sham versus 45 min of ischemia-reperfusion injury [IRI] versus 60 min of IRI versus bilateral nephrectomy: 0.20 ± 0.07 versus 3.23 ± 0.32 versus 2.98 ± 0.29 versus 5.07 ± 0.55 mg/dl; P < 0.0001) or an increase in serum alanine aminotransferase (ALT; sham versus acute liver injury [ALI]: 15.4 ± 1.82 versus 154 ± 64.7 U/L; P = 0.016). Mice with ALI had normal renal function (serum creatinine 0.31 ± 0.06 versus 0.30 ± 0.27 mg/dl; P = 0.916, n = 4 to 5)
Mice with Ischemic AKI Exhibit Increased Numbers of Pyknotic Neurons in the Brain
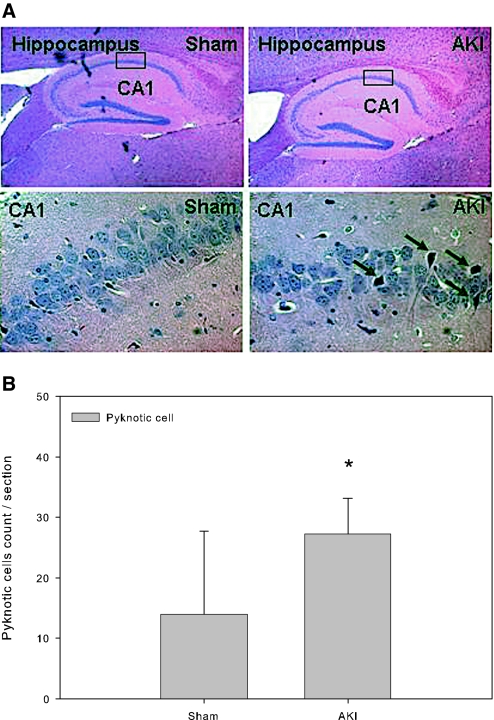
To determine whether AKI resulted in adverse effects on brain neurons, we first examined hematoxylin and eosin (H&E)-stained brain sections from AKI and control mice. We found that, compared with sham control mice, the numbers of pyknotic neuronal cells were significantly increased in the cornu ammonis 1 (CA1) region of the brain hippocampus in mice with AKI (Figure 1). No evidence of pyknotic neurons was evident in adjacent regions of the hippocampus, including the CA3 dentate granule cell layers. No evidence of neuronal damage was found in the cerebral cortex or striatum.
Figure 1.
Pyknotic neurons in the hippocampus of the brain. All mice underwent 60 min of bilateral renal ischemia followed by reperfusion or a sham operation. The brains were harvested at 24 h after surgery and processed for histologic examination by H&E staining. (A) Representative microphotograph of mouse brain hippocampus. The entire hippocampus is shown in the top panel and the high-powered CA1 region is shown in the bottom panel. Arrows indicate pyknotic neuronal cell bodies. (B) Pyknotic neuronal cells count. *P = 0.022 versus sham; n = 6 to 8. Magnifications: ×4 in A, top; ×40 in A, bottom.
Mice with Ischemic AKI Had Increased Numbers of Activated Microglial Cells in the Brain
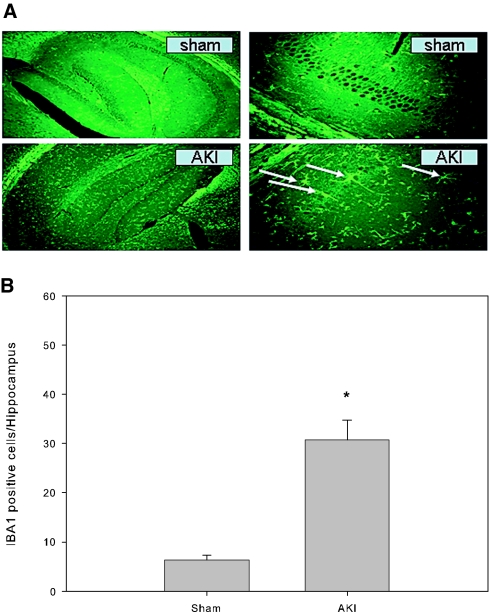
To examine whether mice had inflammatory cell infiltration in the brain after AKI, we stained mouse brain sections for activated microglial cells (brain macrophages) with an antibody against ionized calcium-binding adaptor molecule 1 (Iba1). We found that compared with sham mice, mice with severe ischemic AKI had a significant increase in the number of microglial cells in the hippocampus of the brain (Figure 2).
Figure 2.
Microglial cells in the hippocampus of mouse brain. All mice underwent either 60 min of renal ischemia followed by reperfusion or a sham operation. The brains were harvested at 24 h after surgery and stained with IBA1 antibody for activated microglial cells (macrophage in the brain) by immunofluorescence technique. (A) Representative microphotographs of the hippocampus. Arrows indicate positively stained microglia. (B) IBA1-positive microglial cell count in the hippocampus. *P = 0.001 versus sham; n = 6 to 7. Magnifications: ×4 in A, left; ×40 in A, right.
Mice with AKI Had Increased Serum C-Reactive Protein Concentration and Serum G-CSF
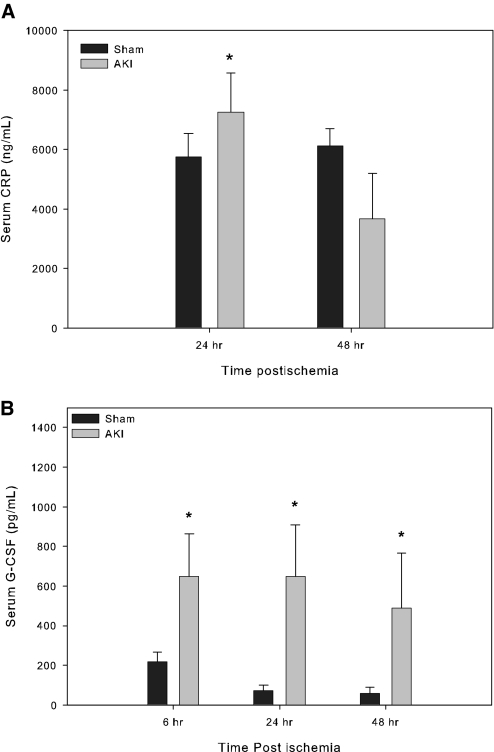
To determine whether systemic inflammation was induced by severe AKI, we measured serum C-reactive protein (CRP) by ELISA and serum G-CSF by Bioplex cytokine/chemokine protein array. We found that after renal ischemia, serum CRP was significantly increased at 24 h and declined at 48 h. The serum G-CSF was significantly increased at 6, 24, and 48 h after surgery when compared with sham-operated mice (Figure 3).
Figure 3.
Serum CRP (A) and serum G-CSF (B). All mice underwent either 60 min of renal ischemia followed by reperfusion or a sham operation. The blood samples were obtained and measured for serum CRP and G-CSF. Compared with sham-operated mice, mice with AKI showed significantly increased serum CRP at 24 h and increased serum G-CSF at 6, 24, and 48 h after surgery. (A) Serum CRP, sham versus AKI, 5759 ± 782 versus 7243 ± 1331 ng/ml (*P = 0.026, n = 7). (B) Serum G-CSF, sham versus AKI, at 6 h: 218.7 ± 49.5 versus 647.2 ± 217.3 ng/ml (*P = 0.022); at 24 h: 76.33 versus 681.9 ng/ml (*P = 0.016); at 48 h: 47.17 versus 409.05 ng/ml (*P = 0.036 by t-test in A and B at 6 h and by Rank sum test in B at 24 or 48 h, n = 3 to 5).
Markers of Inflammation Are Increased in Mouse Brain in Response to AKI
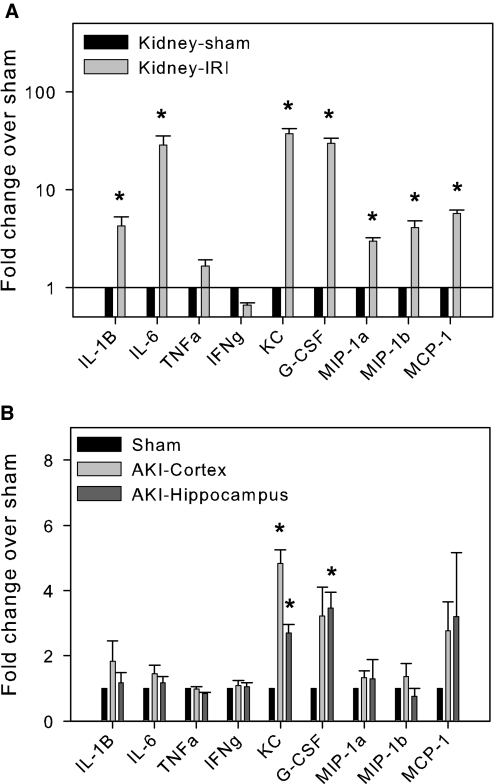
To determine whether AKI results in increased production of cytokines and chemokines in the brain, we used a multiplex protein array to measure proinflammatory mediators in brain and kidney tissue. We found that after AKI, there were increases in levels of kidney IL-1β, IL-6, keratinocyte-derived chemoattractant (KC), G-CSF, MIP-1α, Macrophage Inflammatory Protein (MIP)-1β, and monocyte chemoattractant protein-1 (MCP-1) and a trend toward an increase in TNF-α. Brains from AKI mice had significant increases in KC and G-CSF and a trend toward an increase in MCP-1 in both the cerebral cortex and hippocampus when compared with sham controls (Figure 4).
Figure 4.
Cytokine/chemokine protein array in the kidney (A; *P < 0.03 to 0.002 versus sham) and the brain (B; *P < 0.003 to 0.0008 versus sham). All mice underwent 60 min of bilateral renal ischemia followed by reperfusion or a sham operation and were followed for 24 h. The kidneys and brains were harvested after exsanguinations and processed for cytokine/chemokine protein array. Data are presented as a fold change of each protein from ischemic mice over that from sham-operated mice. Compared with sham mice, KC and G-CSF were significantly increased in both the kidney and the brain (cortex and hippocampus; n = 3).
AKI Causes an Increase in the Level of Glial Fibrillary Acidic Protein in the Brain
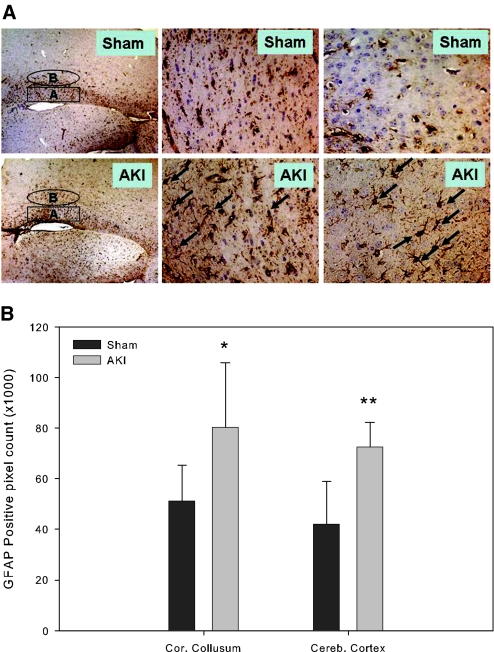
To determine whether the findings of an increase in brain soluble inflammatory mediators was accompanied by cellular signs of inflammation, we performed immunohistochemical staining for glial fibrillary acidic protein (GFAP), a marker for activated glial cells during brain inflammation. Compared with sham mice, mice with AKI had increased GFAP expression in astrocytes in both the cerebral cortex and the corpus callosum (Figure 5A). Densitometric analysis revealed significantly greater amounts of GFAP immunoreactivity in the cerebral cortex and the corpus callosum regions of AKI mice compared with sham mice (Figure 5B).
Figure 5.
GFAP expression in the mouse brain astrocytes. All mice underwent 60 min of bilateral renal ischemia followed by reperfusion or a sham operation. The brains were harvested at 24 h after surgery and stained with antibody against GFAP for activated astrocytes. (A) Microphotographs (circled B in A and D indicates adjacent cerebral cortex, high powered in C and F; squared A in A and D indicates corpus callosum, high powered in B and E. Arrows indicate GFAP positively stained astrocytes. (B) GFAP-positive pixel count, sham versus AKI, cerebral cortex: 42130 ± 16791 versus 72660 ± 9548, P = 0.001; corpus callosum: 51316 ± 14193 versus 80383 ± 25613. (*P = 0.049, **P = 0.001 versus sham; n = 5 to 8). Magnifications: ×4 in A and D; ×40 in B, C, E, and F.
Lack of Brain Neuronal Pyknosis or Expression of GFAP Increased in Mice with Acute Liver Injury
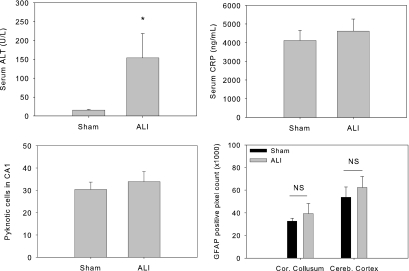
To determine whether the findings of increased brain neuronal pyknosis and expression of GFAP seen in mice with ischemic AKI were kidney specific, we performed an alternative organ ischemia model: Liver ischemia reperfusion injury induced by clamping the portal vasculature (portal vein and hepatic artery) for 45 min followed by reperfusion. Control mice underwent a sham operation. The brains were harvested at 24 h after surgery and processed for neuronal pyknosis and GFAP expression assessment. Whereas there was an increased serum ALT corresponding to the liver IRI, the serum CRP was not significantly increased at 24 h after liver ischemia. No increases in neuronal pyknosis in the hippocampus CA1 region were seen; neither was there increased expression of GFAP in the corpus callosum or the cerebral cortex found in mice with liver injury when compared with control mice (Figure 6).
Figure 6.
Liver function, blood inflammation, and brain changes in mice with ischemic ALI. Mice underwent either 45 min of lobar ischemia followed by reperfusion or a sham operation. The blood was taken at 24 h after surgery and measured for ALT and CRP. The brains were processed for histology and GFAP staining. Mice with liver ischemia had significant increase in serum ALT meanwhile with a comparable serum CRP, brain pyknotic neuronal cell count, and brain GFAP expression when compared with sham operated mice. (*P = 0.016 versus sham; n = 4 to 5). Sham versus ALI, serum CRP: 4116.154 ± 541.709 versus 4625.344 ± 642.728 ng/ml (P = 0.213; n = 5); pyknotic neurons: 30.4 ± 3.3 versus 33.9 ± 4.5 ng/ml (P = 0.157; n = 5 to 9); GFAP-positive pixel analysis: corpus collosum 32619 ± 2715 versus 39379 ± 8890 ng/ml (P = 0.173); cerebral cortex 53833 ± 9071 versus 62383 ± 9802 ng/ml (P = 0.167; n = 5 to 9).
Mice with AKI Exhibit Increased Brain Vascular Permeability
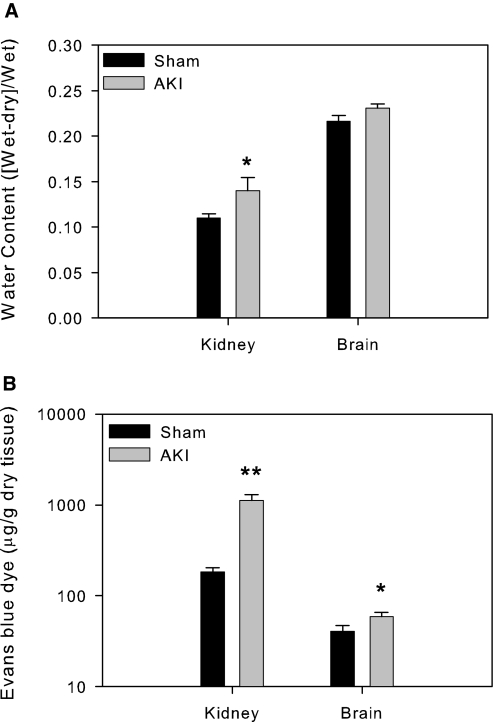
To determine effects of AKI on brain edema and vascular permeability, we harvested brains at 24 h after AKI or sham surgery and processed them for brain water content by weighing mouse brains before and after 3 d of drying at 60°C. Brain vascular permeability was assessed by quantifying Evans blue dye extravasations. Using ischemic kidneys as positive controls, we confirmed that mice with AKI had significantly increased kidney water content and increased vascular permeability when compared with sham-operated mice; however, in the brains from AKI mice, only increased vascular permeability was noted when compared with the sham-operated controls, with no significant change in brain water content (Figure 7).
Figure 7.
Brain water content (A) and Evans blue extravasations (B). All mice underwent either 60 min of bilateral renal ischemia followed by reperfusion or underwent a sham operation. The brains were harvested at 24 h after surgery and accessed for water content or for Evans blue dye extravasations. As a positive control, water content and vascular permeability were increased in the ischemic kidney as expected. There was a significant increase in Evans blue extravasations and a comparable level in water content in the brain from the mice with AKI when compared with sham-operated mice. (A) Brain water content index, sham versus AKI, 0.216 ± 0.007 versus 0.231 ± 0.005, P = 0.10. (B) Brain Evans blue dye extravasations in μg/g tissue, sham versus AKI, 40.7 ± 6.29 versus 58.6 ± 7.13, *P = 0.034, n = 3. In the kidney, **P < 0.001 versus sham, n = 4.
Terminal Deoxynucleotidyl Transferase–Mediated Digoxigenin-Deoxyuridine Nick-End Labeling Staining in the Brain
We performed terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL) staining on the brain sections from mice with AKI or sham-operated mice to ascertain whether neuronal pyknosis is associated with apoptotic changes observed in AKI. We found that compared with the positive control, both AKI and sham mouse brain had minimal TUNEL staining. Caspase 3 immunostaining was also performed with minimal signal in the AKI mouse brain (data not shown).
Mice with AKI Had Reduced Locomotor Activity
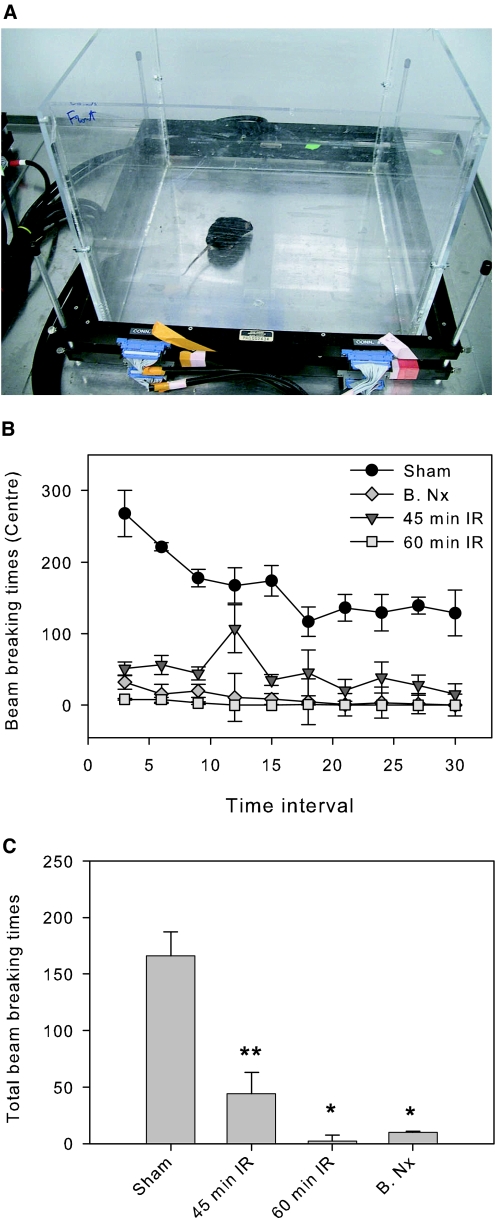
We performed behavioral studies using an open field test to determine whether AKI-induced brain inflammation was associated with functional abnormalities. Mice were subjected to 45 or 60 min of renal ischemia followed by reperfusion or a bilateral nephrectomy. Control mice underwent a sham operation (with full anesthesia, laparotomy, and pedicle dissection). Twenty-four hours after surgery, mice were placed in the activity chambers (Figure 8A) and tested for locomotor activity for 30 min. Mice with 45 min of renal ischemia had a moderate decrease, and mice with 60 min of renal ischemia or bilateral nephrectomy had an extreme decrease in locomotor activity when compared with sham-operated mice (Figure 8B).
Figure 8.
Open field test for locomotor activity. All mice were subjected to 45 or 60 min of bilateral renal ischemia, a bilateral nephrectomy, or a sham operation. Twenty-four hours later, mice underwent a 30-min open field test for locomotive activity in an activity measurement chamber (A). Locomotor activity was assessed by the times breaking electronic beam during the test. Compared with sham-operated mice, locomotion was moderately decreased in mice with 45 min of renal ischemia and was profoundly declined in mice with 60 min of renal ischemia or bilateral nephrectomy (B.Nx). B, average of activity over a 30-min period. C, total activity. *P = 0.01 versus sham; **P < 0.02 versus 60 min of IRI or B.Nx; n = 4 in each group).
DISCUSSION
Although neurologic sequelae of AKI are well established, the underlying mechanisms of acute uremic encephalopathy are poorly understood. We therefore examined the short-term effect of AKI on the brain in an established mouse model of severe renal IRI. We found that mice with ischemic AKI developed marked brain changes evidenced by increased soluble inflammatory proteins KC and G-CSF, increased cellular inflammation with increased GFAP expression in astrocytes, and increased microglial cells. Mice with AKI also had striking cellular abnormalities in the hippocampus, increased vascular permeability in the brain, and behavioral dysfunction with decreased locomotor activity beyond the effects of laparotomy and anesthesia alone.
Most mechanistic work during AKI has focused on intrarenal changes. The increased appreciation that most of the mortality during AKI despite dialysis is from extrarenal organ dysfunction has initiated investigation into the underlying mechanisms. Uremic encephalopathy often occurs with AKI as well as chronic kidney disease, but there have been limited mechanistic studies using new biomedical tools. We initially sought to determine whether an experimental AKI would lead to any measurable short-term changes in the brain. We studied a model of severe AKI to maximize the short-term distant organ effects of AKI. We examined brain histology in mice that underwent 60 min of bilateral renal ischemia followed by 24 h of reperfusion, compared with sham-operated control mice. We found that pyknotic neuronal cells were significantly increased in region CA1 of the hippocampus. The hippocampus plays a major role in learning and memory and is also involved in anxiety and depression.20 CA1 neurons are selectively vulnerable to degeneration in several pathologic conditions, including global cerebral ischemia21,22 and Alzheimer's disease.23 Previous studies in mouse models of prion disorders24and Alzheimer's disease25 both exhibited hippocampal CA1 pathology and inflammation, as well as hypoactivity in the open field test. The damage to CA1 neurons caused by AKI might therefore account, at least in part, for the hypoactivity of the mice.
Previous studies by our team and others have demonstrated that ischemic AKI leads to inflammation in the blood and lung.15–18 We therefore hypothesized that AKI would also lead to brain inflammatory changes and found that AKI did indeed result in significant increases in levels of the chemokines KC and G-CSF in the brain at 24 h after ischemia. These proteins were found to increase in the postischemic kidney; however, many other proinflammatory proteins were increased in the postischemic kidney but were not increased in the brain. The increase in brain KC and G-CSF could either represent brain production of these proinflammatory proteins or represent accumulation of these through an altered blood-brain barrier from a systemic circulation or a kidney source. Our previous study identified an early increased serum level of KC after an ischemic AKI.26 Levels of KC and G-CSF have been shown to increase in the brain in response to injury or infection and are believed to function in the recruitment of neutrophils to the sites of neuronal damage27–29 The chemokines G-CSF and KC-CXC have been implicated in the pathogenesis of stroke.29–31 Further mechanistic studies are needed to determine whether an intervention targeting on KC-CXC or G-CSF could affect the brain cellular and chemical changes.
After finding an increase in specific soluble inflammatory mediators in the brain after AKI, we also sought to determine whether serum CRP was increased. We found that mice with AKI had increased serum CRP at 24 h. We then examined whether there was an inflammatory cell infiltration in the mouse brain after renal ischemia. We stained mouse brain with Iba1 antibody to identify activated microglial cells and brain macrophages and found that the numbers of microglial cells were significantly increased in the hippocampus at 24 h after AKI when compared with sham-operated mice.
Injury to the central nervous system of higher vertebrates, as a result of trauma, disease, genetic disorders, or chemical insult, results in reactive astrocytes termed astrogliosis. Astrogliosis is characterized by rapid synthesis of GFAP and is demonstrated by increase in protein content or by immunostaining with GFAP antibody.32,33 GFAP is the principal intermediate filament in mature astrocytes of the central nervous system. As a member of the cytoskeletal protein family, GFAP is thought to be important in modulating astrocyte motility and shape by providing structural stability to astrocytic processes. We therefore stained brain tissue for GFAP and found that at 24 h after renal ischemia, GFAP expression in astrocytes was significantly increased in both cerebral cortex and corpus callosum regions compared with sham-operated mice. Because the corpus collosum contains axons that transfer information between cerebral hemispheres, AKI may alter such information transfer, although this remains to be established.
It is widely known that patients who initiate dialysis after severe AKI or ESRD can develop disequilibrium syndrome, ranging from mild headaches to frank seizures.34,35 This is believed to reflect the rapid clearance of serum osmoles, although still maintained in the brain cells, resulting in water influx to the brain cells and resulting brain edema.36 We examined whether AKI leads to an increase in brain water and found that AKI did not lead to a significant increase in brain water content. Our findings are consistent with other investigators who have reported that the brain water content during AKI was not increased or even decreased.37,38 Cerebral edema is known to result from increased permeability of the blood-brain barrier and is often associated with inflammatory conditions.39 We therefore examined brain microvascular dysfunction as measured by Evans blue dye extravasations and found that AKI leads to an increase in microvascular permeability in the brain, similar to what is seen in kidney during AKI. Acute uremia may induce these soluble factors that are mechanistically responsible for a leaky blood-brain barrier.
Given that acute liver disease also results in encephalopathy, we used an established liver IRI model40 to determine whether the brain cellular and chemical changes seen in mice with ischemic AKI are organ specific. We used a 45-min liver IRI model because 60 min of liver IRI led to mortality in 60% of mice by 24 h. Unlike what we found in AKI, the serum CRP was not significantly increased at 24 h after liver IRI, despite the rise in serum ALT confirming ALI. When we examined the brains from mice after ischemic liver injury, there was no significant increase in neuronal pyknosis and GFAP expression in the brain at 24 h after liver ischemia. Thus, AKI alters brain in a distinct manner compared with ALI.
Although we found marked biochemical and cellular changes in the brains of AKI mice, it is not known whether AKI can lead to changes in neurologic function in this model. We therefore performed behavior testing with an open field test in mice and sought to determine how different levels of azotemia affected motor activity. An open field test is broadly used to evaluate locomotor activity, exploratory behaviors, and anxiety in mice. We found that 45 min of kidney ischemia followed by 24 h of reperfusion led to a further reduction in activity of mice compared with sham mice. A 60-min ischemia or bilateral nephrectomy led to an even further decrease in locomotor activity in these mice. It is difficult to perform behavior testing in mice soon after surgery; however, the limitations of the severe ischemia model did not allow longer term survival. Future studies with shorter ischemia time will provide the opportunity to evaluate mice further out from the acute surgical procedure. Regarding the mechanism by which motor activity is modulated in uremia, Adachi et al.41 investigated changes in monoamine metabolism in the brain and motor activity in uremic rat and found that uremia could impair motor activity by suppressing rat central dopamine metabolism. Thus, alterations in monoamine metabolism and specifically dopamine may be the mechanism by which uremia alters motor activity. Although it is not possible to evaluate cognitive function in mice during the acute AKI period because of a general depression in activity, it will be of considerable interest to evaluate learning and memory performance by other tests such as a water or Y-shaped maze test and/or an object cognitive test in animals after they have recovered from the acute phase of AKI.
Taken together, this study demonstrates a broad spectrum of major changes in the brain as well as specific regional pathway abnormalities after AKI. Although the brain effects of AKI or ESRD might be different, these data open up a new line of investigation for more detailed studies to explore these mechanisms, including identifying specific pathophysiologic pathways that directly mediate acute uremic encephalopathy.
CONCISE METHODS
Mice
C57BL/6J male mice used in this study were purchased from the Jackson Laboratory (Bar Harbor, ME). All animal study protocols were reviewed and approved by the Institutional Animal Care and Use Committee of Johns Hopkins University, and all experiments were conducted according to National Institutes of Health guidelines. Mice were held under pathogen-free conditions in Johns Hopkins Medical Institutions animal facility with air conditioning and 14/10 h of light/dark cycle. All mice had free access to food and water during the experiments.
AKI Model
Established models of renal IRI42 and bilateral nephrectomy17 were used. Animals were anesthetized by intraperitoneal injection with sodium pentobarbital at 75 mg/kg. An abdominal incision at midline was made, and both renal pedicles were bluntly dissected. For mice assigned to experimental IRI, a nontraumatic microvascular clamp was placed across each renal pedicle for 60 min. During the procedure, animals were kept well hydrated with warm sterile saline, and body temperatures were maintained constantly at 35.5 to 37°C on a heating pad (40°C) until full recovery from anesthesia. After the allotted ischemia time, the clamps were gently removed and the incision was closed in two layers with 4-0 silver suture, and the mice were allowed to recover with free access to food and water. Sham animals underwent the identical procedure and length of time of surgery without placement of the vascular clamps. The mice assigned to bilateral nephrectomy underwent similar procedures except both renal pedicles were ligated with 5-0 silk suture and then the kidneys were removed. The success of the AKI model was confirmed with an increase in serum creatinine concentration along with renal histologic damage at 24 h after ischemia.
Assessment of Renal Function
Blood samples were obtained from mice via tail vein before (0) and at 24 h after renal or liver ischemia, bilateral nephrectomy, or a sham operation. Serum creatinine concentration was measured as a marker of renal function by a Roche Cobas Fara automated system (Roche, Nutley, NJ) using a Creatinine 557 kit (Sigma Diagnostics, St. Louis, MO).
Ischemic ALI Model
An established model of ischemic ALI was used in this study.40 Briefly, mice were anesthetized with isoflurane (Abbott Laboratories, North Chicago, IL) inhalation. The abdomen was shaved and prepped, and a small vertical incision was made, slightly to the right of midline through the skin and peritoneum. The porta-hepatis was exposed, and a pediatric vessel loop was drawn around and tightened to induce total hepatic ischemia for 45 min; the vessel loop was removed, and the portal vein, hepatic artery, and liver were observed for restoration of blood flow (reperfusion). The incision was closed, and the mice were allowed to recover and then were killed at 24 h after ischemia. Serum ALT was measured by IDEXX Laboratories according to the instructions.
Histologic Examination of Mouse Brains
Mice were given an overdose of pentobarbital (100 mg/kg) before being killed. After drawing the blood from Inferior Vena Cava, mice were decapitated and exsanguinated, and then the whole brains were harvested and bi-dissected sagittally into two halves. Half of the brain was fixed with formalin and embedded with paraffin for histologic (hematoxylin and eosin) and immunohistochemical staining. The other half of the brain was further dissected into cortex, hippocampus, striatum, and cerebellum; snap-frozen, and stored in −80°C for cytokine analysis.
Pyknotic Neuronal Cell Count
We examined mouse brain histology under light microscopy (Nikon Eclipse E600) with H&E stained sections. At ×100 magnification (oil immersion), neuronal cells with a condensed and darkly stained cell body and nucleus were considered as pyknotic (Figure 1A, bottom). Pyknotic cells were counted by a neuroscientist (S.C.) who was blinded to the experimental groups in eight ×100 fields in the CA1 region from each section (four sections per mouse) and presented as numbers of pyknotic cells per section (total counts in eight fields per section).
Brain Immunohistochemical Staining
Formalin-fixed and paraffin-embedded sections of brains were deparaffinized, rehydrated with series of alcohols, and blocked with 5 to 10% normal goat serum for 1 h at room temperature. The brain sections were incubated with primary antibodies including a rabbit anti-mouse polyclonal antibody against GFAP at original ready-to-use concentration (Dako North America, Carpinteria, CA), a rabbit anti-mouse IBA1 (Wako Chemicals USA, Richmond, VA) at 1:100 concentration, or a rabbit anti-cleaved caspase-3 (Asp175) antibody (Cell Signaling Technology, Danvers, MA) at 1:100 concentration overnight at 4°C. The following day, sections were washed and incubated with secondary antibody, rabbit ABC reagent (Vectastain-Elite; Vector Laboratories, Burlingame, CA) at 1:200 for GFAP and a goat anti-rabbit Alexa 488 (Invitrogen Corp., Carlsbad, CA) for 30 to 45 min. The immune complex was visualized with diaminobenzidine (DAB) and either counterstained with hematoxylin for GFAP and caspase-3 or visualized with immunofluorescence and counterstained with DAPI (Invitrogen) for IBA1. These immunohistochemistry-stained brain sections were examined using light microscopy or under immunofluorescence microscopy.
Microglial Cell Counts
Iba1-positively stained microglial cells were counted manually using immunofluorescence microscopy at ×20 in the whole hippocampus areas and presented as the numbers of Iba1-positive microglial cells per hippocampus.
Quantification of GFAP Expression
The immune complex was visualized with DAB and counterstained with hematoxylin, and GFAP-positive cells were detected in the presence and absence of distinct staining by using positive pixel-count algorithm that quantifies the amount of a specific stain in a scanner slide image using tissue microarray software (Aperio Technologies, Vista, CA). Briefly, brown color of DAB is specified (range of hues and saturation) and three intensity ranges (weak, positive, and strong). For pixels that satisfy the color specification, the algorithm counts the number and intensity sum in each intensity range, along with three additional quantities: Average intensity, ratio of strong/total number, and average intensity of weak positive pixels. The algorithm has been set for default input parameters when first selected—these inputs have been preconfigured for brown color quantification in the three intensity ranges (220 to 175, 175 to 100, and 100 to 0). Pixels that are stained but do not fall into the positive-color specification are considered negative stained pixels; these pixels are counted as well so that the fraction of positive to total stained pixels is determined. The algorithm is applied to an image by using ImageScope (Aperio Technologies, Inc., Vista, CA). This program allows selection of an image region of analysis, specification of the input parameters, running the algorithm, and viewing/saving the algorithm results. When using the ImageScope program, a pseudocolor markup image is also shown as an algorithm result. The markup image allows the user to confirm that specified inputs are measuring the desired color and intensity ranges. Once a set of algorithm inputs has been confirmed, the settings can be saved in a macrofile for subsequent repeated use.
TUNEL Staining of Brain
Apoptosis of neuronal cells in the brain was examined by TUNEL staining with a mouse In Situ Apoptosis Detection Kit (R&D Systems, Minneapolis, MN) on formalin-fixed and paraffin-embedded sections according to the manufacturer's instructions. A nuclease-pretreated mouse brain section was included as positive control.
Brain Water Content
Water content in the brain from mice that underwent a renal ischemia or a sham operation was assessed by weighing the whole brains before and after 3 d of drying at 60°C. Using the following formula, the brain water content was calculated: Water content = (wet weight − dried weight)/wet weight.
Brain Vascular Permeability
Brain protein leakage through microvasculature during AKI was assessed by extravasations of albumin-bound Evans blue dye from the brain parenchyma.43 Thirty minutes before the mice were killed, 1% of Evans blue dye saline solution (Sigma Chemical Co., St. Louis, MO) was injected via right jugular vein at 2.5 ml/kg body wt while the mice received light ether anesthesia. Immediately after the mice were killed (by an overdose of pentobarbital), the circulation was cleared by transcardiac perfusion with 10 ml of heparinized saline (0.5 ml of heparin in 500 ml of 0.9% NaCl). The flushed brains were removed and got dried at 60°C for 3 d. The dried brains were weighed and homogenized with formamide (1:20, wt/vol), and the homogenates were incubated at 60°C for 18 h (overnight) and then centrifuged for 30 min at 14,000 rpm at 4°C to remove suspended particulate matter. The quantity of extracted Evans blue in the supernatant was determined by measuring absorbance at a dual-wavelength of 620/635 nm and corrected for the extraction volume. A standard curve of Evans blue in blank formamide was used to convert absorbency into micrograms of Evans blue per gram of dried tissue.43
Serum CRP Measurement by ELISA
Blood samples were taken from mice subjected to renal or liver ischemia or from mice that underwent a sham operation at 24 and/or 48 h after surgery, and the serum CRP was measured by a mouse CRP 96-well ELISA kit (Life Diagnostics, West Chester, PA) according to the manufacturer's instructions.
Proinflammatory Mediator Protein Array in the Serum and Brain
For examination of proinflammatory molecules that were generated by ischemia reperfusion injury, protein levels of IL-1β, IL-6, TNF-α, IFN-γ, G-CSF, MIP-1α, MIP-1β, MCP-1, and KC were measured in mouse kidneys and brains and KC/G-CSF in the serum using a Bio-Plex multiple cytokines array technique (Bio-Rad Laboratories, Hercules, CA), previously described in depth,26 according to the manufacturer's instructions. Briefly, snap-frozen kidney tissue was homogenized in a cell lysis buffer, and the homogenates were centrifuged at 12,000 rpm for 15 min at 4°C. Total protein concentration in each supernatant was determined using a Bio-Rad Protein Assay Kit, and the measured protein level in each sample was adjusted to a concentration of 500 μg/ml with cell lysis buffer. Each sample first was incubated with a mixture of all types of microbeads for 90 min at room temperature followed by incubation with biotinylated detection antibodies for 30 min, then with a streptavidin-coupled phycoerythrin for 10 min (room temperature). Finally, the samples were subjected to a flow cytometric system. All acquired data were analyzed using Bio-Plex Manager 3.0 software (Bio-Rad) and corrected for total protein concentration (pg/mg protein).
Open Field Test
Twenty-four hours after renal ischemia, bilateral nephrectomy, or sham surgery, mice were placed in a special chamber (San Diego Instruments, San Diego, CA; Figure 8A) to evaluate their novelty-induced locomotor activity for a 30-min period with an open field test. A typical arena consists of a Plexiglas cage lined with infrared photo beams. A second frame with infrared photo beams is placed above the first one to measure rearing (i.e., vertical activity). Movements including horizontal and vertical activities and the activity in the center area of the chamber or along the walls (thigmotaxis) of the chamber are automatically recorded when a mouse breaks new photo beams. The size of the test arena was 15“ × 15” × 12“. Tests were run at 9 to 11 a.m. and 4 to 6 p.m. for all mice in a pseudorandom manner during the light phase of the dark-light cycle (the lights in the room were off between 8 p.m. and 8 a.m.).
Statistical Analysis
Results from parametric data are expressed as means ± SD and are compared either by an unpaired, two-tailed t test for single comparison between two groups or by repeated measures ANOVA with group and time as independent variables for the locomotor activity analysis. Post hoc tests were run where appropriate to determine significant differences between groups. For data that failed to pass a normality test or an equal variance test, a Mann-Whitney rank sum test was performed as a nonparametric test instead of a t test to verify the difference between two groups is greater than would be expected by chance and is truly statistically significant. Statistical significance of a difference was defined when a P < 0.05.
DISCLOSURES
None.
Acknowledgments
This work was supported by a grant from National Heart, Lung, and Blood Institute PO HL073944 (H.R. and M.L.) and a mini-grant from the National Kidney Foundation in Maryland (M.L.) and by the Intramural Research Program of the National Institute on Aging (S.C., J.L., and M.M.). We thank Dr. Douglas Linfert for careful review of the final manuscript.
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Marconi Revisited: From Kidney to Brain—Two Organ Systems Communicating at Long Distance,” on pages 1253–1255.
REFERENCES
- 1.Rabb H, Chamoun F, Hotchkiss J: Molecular mechanisms underlying combined kidney-lung dysfunction during acute renal failure. Contrib Nephrol 41–52, 2001 [DOI] [PubMed]
- 2.Kelly KJ: Acute renal failure: much more than a kidney disease. Semin Nephrol 26: 105–113, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Star RA: Treatment of acute renal failure. Kidney Int 54: 1817–1831, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Brouns R, De Deyn PP: Neurological complications in renal failure: A review. Clin Neurol Neurosurg 107: 1–16, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Burn DJ, Bates D: Neurology and the kidney. J Neurol Neurosurg Psychiatry 65: 810–821, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Deyn PP, Saxena VK, Abts H, Borggreve F, D'Hooge R, Marescau B, Crols R: Clinical and pathophysiological aspects of neurological complications in renal failure. Acta Neurol Belg 92: 191–206, 1992 [PubMed] [Google Scholar]
- 7.Vanholder R, De Smet R, Glorieux G, Argiles A, Baurmeister U, Brunet P, Clark W, Cohen G, De Deyn PP, Deppisch R, Scamps-Latscha B, Henle T, Jorres A, Lemke HD, Massy ZA, Passlick-Deetjen J, Rodriguez M, Stegmayr B, Stenvinkel P, Tetta C, Wanner C, Zidek W: Review on uremic toxins: Classification, concentration, and interindividual variability. Kidney Int 63: 1934–1943, 2003 [DOI] [PubMed] [Google Scholar]
- 8.D'Hooge R, Van d Vijver G, Van Bogaert PP, Marescau B, Vanholder R, De Deyn PP: Involvement of voltage- and ligand-gated Ca2+ channels in the neuroexcitatory and synergistic effects of putative uremic neurotoxins. Kidney Int 63: 1764–1775, 2003 [DOI] [PubMed] [Google Scholar]
- 9.De Deyn PP, D'Hooge R, Van Bogaert PP, Marescau B: Endogenous guanidino compounds as uremic neurotoxins. Kidney Int Suppl 78: S77–S83, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Arieff AI, Massry SG, Barrientos A, Kleeman CR: Brain water and electrolyte metabolism in uremia: Effects of slow and rapid hemodialysis. Kidney Int 4: 177–187, 1973 [DOI] [PubMed] [Google Scholar]
- 11.Arieff AI, Massry SG: Calcium metabolism of brain in acute renal failure: Effects of uremia, hemodialysis, and parathyroid hormone. J Clin Invest 53: 387–392, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahoney CA, Sarnacki P, Arieff AI: Uremic encephalopathy: Role of brain energy metabolism. Am J Physiol 247: F527–F532, 1984 [DOI] [PubMed] [Google Scholar]
- 13.Kikuchi S, Matsumoto H, Ito M: Free amino acid changes in the cerebral cortex of experimental uremic rat. Neurochem Res 8: 313–318, 1983 [DOI] [PubMed] [Google Scholar]
- 14.Jeppsson B, Freund HR, Gimmon Z, James JH, von Meyenfeldt MF, Fischer JE: Blood-brain barrier derangement in uremic encephalopathy. Surgery 92: 30–35, 1982 [PubMed] [Google Scholar]
- 15.Kramer AA, Postler G, Salhab KF, Mendez C, Carey LC, Rabb H: Renal ischemia/reperfusion leads to macrophage-mediated increase in pulmonary vascular permeability. Kidney Int 55: 2362–2367, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Hoke TS, Douglas IS, Klein CL, He Z, Fang W, Thurman JM, Tao Y, Dursun B, Voelkel NF, Edelstein CL, Faubel S: Acute renal failure after bilateral nephrectomy is associated with cytokine-mediated pulmonary injury. J Am Soc Nephrol 18: 155–164, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Hassoun HT, Grigoryev DN, Lie ML, Liu M, Cheadle C, Tuder RM, Rabb H: Ischemic acute kidney injury induces a distant organ functional and genomic response distinguishable from bilateral nephrectomy. Am J Physiol Renal Physiol 293: F30–F40, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Grigoryev DN, Liu M, Hassoun HT, Cheadle C, Barnes KC, Rabb H: The local and systemic inflammatory transcriptome after acute kidney injury. J Am Soc Nephrol 19: 547–558, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly KJ: Distant effects of experimental renal ischemia/reperfusion injury. J Am Soc Nephrol 14: 1549–1558, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Heldt SA, Stanek L, Chhatwal JP, Ressler KJ: Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry 12: 656–670, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White BC, Grossman LI, O'Neil BJ, DeGracia DJ, Neumar RW, Rafols JA, Krause GS: Global brain ischemia and reperfusion. Ann Emerg Med 27: 588–594, 1996 [DOI] [PubMed] [Google Scholar]
- 22.White BC, Sullivan JM, DeGracia DJ, O'Neil BJ, Neumar RW, Grossman LI, Rafols JA, Krause GS: Brain ischemia and reperfusion: Molecular mechanisms of neuronal injury. J Neurol Sci 179: 1–33, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Giannakopoulos P, Herrmann FR, Bussiere T, Bouras C, Kovari E, Perl DP, Morrison JH, Gold G, Hof PR: Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer's disease. Neurology 60: 1495–1500, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Cunningham C, Deacon RM, Chan K, Boche D, Rawlins JN, Perry VH: Neuropathologically distinct prion strains give rise to similar temporal profiles of behavioral deficits. Neurobiol Dis 18: 258–269, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Nelson RL, Guo Z, Halagappa VM, Pearson M, Gray AJ, Matsuoka Y, Brown M, Martin B, Iyun T, Maudsley S, Clark RF, Mattson MP: Prophylactic treatment with paroxetine ameliorates behavioral deficits and retards the development of amyloid and tau pathologies in 3xTgAD mice. Exp Neurol 205: 166–176, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molls RR, Savransky V, Liu M, Bevans S, Mehta T, Tuder RM, King LS, Rabb H: Keratinocyte-derived chemokine is an early biomarker of ischemic acute kidney injury. Am J Physiol Renal Physiol 290: F1187–F1193, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Kielian T, Barry B, Hickey WF: CXC chemokine receptor-2 ligands are required for neutrophil-mediated host defense in experimental brain abscesses. J Immunol 166: 4634–4643, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Zwijnenburg PJ, Polfliet MM, Florquin S, van den Berg TK, Dijkstra CD, van Deventer SJ, Roord JJ, van der PT, van Furth AM: CXC-chemokines KC and macrophage inflammatory protein-2 (MIP-2) synergistically induce leukocyte recruitment to the central nervous system in rats. Immunol Lett 85: 1–4, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Hasselblatt M, Jeibmann A, Riesmeier B, Maintz D, Schabitz WR: Granulocyte-colony stimulating factor (G-CSF) and G-CSF receptor expression in human ischemic stroke. Acta Neuropathol 113: 45–51, 2007 [DOI] [PubMed] [Google Scholar]
- 30.McColl BW, Rothwell NJ, Allan SM: Systemic inflammatory stimulus potentiates the acute phase and CXC chemokine responses to experimental stroke and exacerbates brain damage via interleukin-1- and neutrophil-dependent mechanisms. J Neurosci 27: 4403–4412, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sehara Y, Hayashi T, Deguchi K, Zhang H, Tsuchiya A, Yamashita T, Lukic V, Nagai M, Kamiya T, Abe K: Decreased focal inflammatory response by G-CSF may improve stroke outcome after transient middle cerebral artery occlusion in rats. J Neurosci Res 85: 2167–2174, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Eng LF, Ghirnikar RS: GFAP and astrogliosis. Brain Pathol 4: 229–237, 1994 [DOI] [PubMed] [Google Scholar]
- 33.Eng LF, Ghirnikar RS, Lee YL: Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000). Neurochem Res 25: 1439–1451, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Hill MB: Dialysis disequilibrium syndrome. Nephrol Nurs J 28: 348–349, 2001 [PubMed] [Google Scholar]
- 35.Tzamaloukas AH, Agaba EI: Neurological manifestations of uraemia and chronic dialysis. Niger J Med 13: 98–105, 2004 [PubMed] [Google Scholar]
- 36.Chen CL, Lai PH, Chou KJ, Lee PT, Chung HM, Fang HC: A preliminary report of brain edema in patients with uremia at first hemodialysis: Evaluation by diffusion-weighted MR imaging. AJNR Am J Neuroradiol 28: 68–71, 2007 [PMC free article] [PubMed] [Google Scholar]
- 37.Silver SM: Cerebral edema after rapid dialysis is not caused by an increase in brain organic osmolytes. J Am Soc Nephrol 6: 1600–1606, 1995 [DOI] [PubMed] [Google Scholar]
- 38.Galons JP, Trouard T, Gmitro AF, Lien YH: Hemodialysis increases apparent diffusion coefficient of brain water in nephrectomized rats measured by isotropic diffusion-weighted magnetic resonance imaging. J Clin Invest 98: 750–755, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hariri RJ: Cerebral edema. Neurosurg Clin N Am 5: 687–706, 1994 [PubMed] [Google Scholar]
- 40.Sakamoto N, Sun Z, Brengman ML, Maemura K, Ozaki M, Bulkley GB, Klein AS: Hepatic reticuloendothelial system dysfunction after ischemia-reperfusion: Role of P-selectin-mediated neutrophil accumulation. Liver Transpl 9: 940–948, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Adachi N, Lei B, Deshpande G, Seyfried FJ, Shimizu I, Nagaro T, Arai T: Uraemia suppresses central dopaminergic metabolism and impairs motor activity in rats. Intensive Care Med 27: 1655–1660, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Rabb H, Ramirez G, Saba SR, Reynolds D, Xu J, Flavell R, Antonia S: Renal ischemic-reperfusion injury in L-selectin-deficient mice. Am J Physiol 271: F408–F413, 1996 [DOI] [PubMed] [Google Scholar]
- 43.Matthew CB, Sils IV, Bastille AM: Tissue-specific extravasation of albumin-bound Evans blue in hypothermic and rewarmed rats. Can J Physiol Pharmacol 80: 233–243, 2002 [DOI] [PubMed] [Google Scholar]