Abstract
Although glucocorticoid (GC)-induced hypertension has commonly been attributed to promiscuous activation of the mineralocorticoid receptor by cortisol, thereby promoting excess reabsorption of sodium and water, numerous lines of evidence indicate that this is not the only or perhaps even the primary mechanism. GC induce a number of effects on vascular smooth muscle (VSM) in vitro that may be pertinent to hypertension, but their contribution in vivo is unknown. To address this question, a mouse model with a tissue-specific knockout (KO) of the GC receptor in the VSM was created and characterized. Similar to control mice, KO mice exhibited normal baseline BP and, interestingly, showed normal circadian variation in BP. When dexamethasone was administered, however, the acute hypertensive response was markedly attenuated in KO mice, and there was a trend toward a decreased chronic hypertensive response. These data suggest that the GC receptor in VSM plays a critical role in the acute hypertensive response to GC in vivo.
Glucocorticoids (GC), whether endogenous, as in Cushing syndrome, or exogenous, via pharmacologic provision, induce hypertension (HTN).1–3 The lack of understanding of the underlying mechanisms of GC-induced HTN leads to empiric and often suboptimal treatment of this common clinical condition. Although traditionally GC have commonly been believed to increase BP via promiscuous activation of the mineralocorticoid receptor (MR) in the kidney,4,5 available data argue that this is not the only or perhaps even the primary mechanism underlying this form of HTN.6–8
In recent years, a wide variety of hypotheses have been suggested to explain how GC may regulate BP. Many of these have focused on vascular effects of GC. There is evidence to confirm the presence of the GC receptor (GR) in vascular smooth muscle (VSM) cells6,9 and in the vascular endothelium (VE).6,9,10 In vitro studies in smooth muscle cells have demonstrated that GC induce an upregulation of angiotensin type 1 receptors8 and alter Na+ and/or Ca2+ influx into the cells.9 Similarly, in the VE, GC trigger a reduction in nitric oxide release and a concomitant reduction in BP and cholinergic-induced vasodilation.11 The contribution of these findings to the chronic elevation of BP induced by GC, however, is uncertain, because GC cause a variety of relevant effects within the body. To date, there has not been a reliable way to assess the role of VSM GR or VE GR in vivo. As a result, there is much that is still poorly understood about vascular responses to GC.
We previously demonstrated that administration of dexamethasone (DEX) in the drinking water induces a rapid and sustained elevation of BP in wild-type (WT) mice.12 Furthermore, we showed that this response is mediated via GR and is substantially independent of MR.12 To investigate the role of VSM in the hemodynamic response to GC, we generated mice in which GR has been specifically removed from VSM using a Cre-loxP approach and assessed basal BP as well as the hemodynamic response of these mice to GC administration.
RESULTS
Specific VSM GR Knockout
Generation of mice with GR knockout (KO) in VSM was achieved in our laboratory through the use of an Sm-22 Cre model, which has been reported to confer approximately 80% excisional efficiency.13 Sm-22 Cre+ GRloxP/loxP and Cre− littermate controls were born in the expected 1:1 ratio. To verify the effectiveness of tissue-specific GR excision, sections of mouse aorta and mouse kidney from Sm-22 Cre+ and Cre− mice were stained with a commercial antibody to GR and counterstained with an antibody to α-smooth muscle actin, a VSM-specific antibody14 (Figure 1A). Control mice showed robust staining of GR in the smooth muscle layers. In contrast, Sm-22 Cre+ GRloxP/loxP mice demonstrated minimal presence of GR in VSM by double staining, indicating that we had achieved a high level of GR excision from the VSM layer. A similar staining pattern was also observed in the renal arteriole (Figure 1B) and the renal and mesenteric arteries (data not shown). To confirm that excision of GR staining was specific to this layer, we also stained these tissues with the endothelial-specific anti-CD31 (Figure 1C). GR staining was preserved in the vascular endothelial layer, confirming that excision of GR from the vascular wall was specific to the smooth muscle layer.
Figure 1.
Specific GR deletion from VSM. (A and B) Representative comparisons of VSM GR in Cre− and Sm-22 Cre+ GRloxP/loxP mice. Four-micrometer sections of mouse aorta (A) and mouse kidney arterioles (B) were stained with a commercial antibody to GR shown in red and an antibody to α-smooth muscle actin, which is specific to the VSM, shown in green. The merged images show clear co-localization of GR in the VSM layer in Cre− mice. In Cre+ mice, this co-localization is almost entirely lost, indicating specific and highly efficient excision of GR from the VSM. (C) Preservation of the VE in Sm-22 Cre+ GRloxP/loxP mice. Higher power view of 4-μm sections of mouse kidney arterioles, which were stained with a commercial antibody to GR (red) and an antibody to CD31 (green). The merged image demonstrates preservation of GR in the endothelial layer in Sm-22 Cre+ mice. Images shown are representative of four animals per group.
No Apparent Role for VSM GR in Basal BP Regulation
After confirming that our mice had high levels of Cre-mediated recombination specifically in VSM of our Sm-22 Cre+ GRloxP/loxP mice, we next studied the clinical phenotype of these mice. Sm-22 Cre+ GRloxP/loxP mice developed normally, attaining the same body weight as Cre− littermates. At 10 wk of age, Cre− males (n = 7) weighed 34.73 ± 2.2 g while Cre+ males (n = 8) weighed 32.60 ± 2.2 g (P = 0.51). Cre− females (n = 11) weighed 24.99 ± 2.1 g while Cre+ females (n = 10) weighed 24.40 ± 1.4 g (P = 0.82).
To determine whether there was a BP phenotype in these mice, radiotelemetric catheters were implanted in male Sm-22 Cre+ GRloxP/loxP mice (n = 7) and gender-matched littermate controls (n = 9), and mice were allowed to recover for 1 wk. Adequacy of recovery was verified by the return of normal diurnal variation of BP for 3 d. There was no difference between the mean arterial pressure (MAP), averaged over 24 h, of Sm-22 Cre+ GRloxP/loxP and control mice at baseline (107.5 ± 2.4 versus 107.8 ± 1.2 mmHg; P = 0.91). There were also no differences between the mean systolic BP (SBP) of Sm-22 Cre+ GRloxP/loxP and control mice averaged over 24 h (123.6 ± 2.9 versus 124.1 ± 1.9 mmHg; P = 0.89) or the mean diastolic BP (DBP) averaged over 24 h (94.9 ± 2.7 versus 90.8 ± 1.1 mmHg; P = 0.16). These data suggest that VSM GR does not play a major role in the basal regulation of BP.
A Delayed BP Response to DEX in Sm-22 Cre GR KO Mice
To determine the effect of the Sm-22 Cre GR KO in a model of GC-induced HTN, we administered DEX 15 mg/L in the drinking water and monitored BP continuously for 1 wk. This dosage was used because previous studies in our laboratory have shown that smaller dosages do not reliably produce BP elevation of >10 mmHg after 2 wk of treatment in these mice, a rise in BP that we believed was necessary for further study (data not shown). On the basis of an average mouse weight of 30 g and an average daily water intake of approximately 2 to 3 ml, we estimate that this dosage provided each mouse with approximately 2 mg/kg per d DEX.
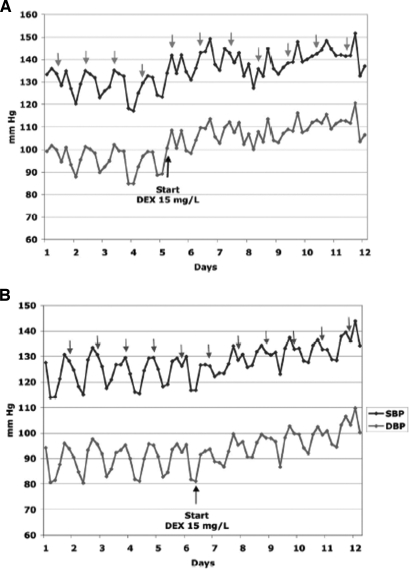
A representative tracing from each group is shown in Figure 2, and cumulative data for each group are shown in Figure 3. As we have noted previously,12 there was a consistent and highly significant rise in BP within hours of the initiation of DEX therapy in control (Cre−) mice. In contrast, the rise in BP was markedly attenuated in Sm-22 Cre+ GRloxP/loxP littermates. On the first day of DEX treatment, MAP, averaged over 24 h, increased by 5.05 ± 1.1 mmHg in control mice compared with a rise of only 1.9 ± 0.9 mmHg in Sm-22 Cre+ mice (P = 0.041).
Figure 2.
Differential BP response to DEX in Sm-22 Cre+ GRloxP/loxP versus Cre− control. (A and B) Representative BP tracing of a control mouse (A) and an Sm-22 Cre+ GRloxP/loxP mouse (B) in response to DEX 15 mg/L in the drinking water. The top line is SBP, and the bottom line is DBP. Each point represents the time-averaged BP over a 4-h period. Arrows indicate the time period from 8 p.m. to midnight, usually the time of peak circadian BP variation in the nocturnal mouse. Both the Cre+ and Cre− mice demonstrate a clear pattern of circadian variation, with the peak BP generally occurring at or near the period of 8 p.m. to midnight. After the initiation of DEX, there is a clear loss of normal diurnal variation in each of these mice; however, whereas BP rises rapidly and substantially in the Cre− mouse, the rise in BP in the Cre+ mouse is attenuated.
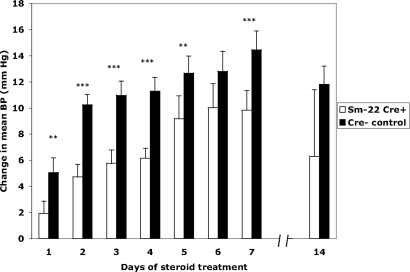
Figure 3.
Attenuated GC-induced acute BP response in Sm-22 Cre+ GRloxP/loxP mice. Controls (n = 9) and Sm-22 Cre+ GRloxP/loxP mice (n = 11) were treated with DEX 15 mg/L in the drinking water for 1 wk, and BP was monitored continuously by catheter. In selected pairs (n = 3), BP monitoring continued for 2 wk. The change in mean arterial BP averaged over 24 h is indicated. **P < 0.05, ***P < 0.001.
This difference remained highly significant for the first week of DEX therapy, except on day 6 of treatment, although there was still a strong trend toward significance (P = 0.16; Figure 3). At the completion of one week of treatment, Sm-22 Cre+ GRloxP/loxP mice attained a MAP rise of 9.8 ± 1.5 mmHg, whereas controls had a MAP rise of 14.5 ± 1.4 mmHg (P = 0.019). Both groups clearly became hypertensive after 1 wk of DEX therapy with MAP of the Sm-22 Cre+ mice increasing from 108.9 ± 2.1 to 118.7 ± 1.7 mmHg (P = 0.006) and MAP of the control mice increasing from 107.2 ± 1.3 to 121.8 ± 1.4 mmHg (P < 0.001).
Guytonian models of BP regulation propose that over a period of days to weeks, renal handling of sodium will compensate for extrarenal stimuli altering BP. To determine whether the difference in BP we observed over 1 wk continued over time, we maintained DEX treatment for a second week in three pairs of mice. BP measured at 14 d showed that Sm-22 Cre+ GRloxP/loxP mice maintained a MAP of 121.0 ± 5.3 mmHg and controls maintained a MAP of 119.2 ± 3.1 mmHg (P = 0.36), suggesting that Sm-22 Cre+ GRloxP/loxP mice likely attain the same rise in BP observed in the controls with continued DEX therapy.
Cardiac GR Does not Account for the Observed BP Phenotype of Sm-22 Cre+ GRloxP/loxP Mice
Previous studies that have used Sm-22 Cre in the generation of transgenic mice have suggested that Sm-22 Cre is expressed at high levels in murine cardiac muscle15; however, in the Sm-22 Cre model we used, only minimal cardiac expression was observed.13 To determine whether GC effects on cardiac muscle could underlie the BP phenotype we observed in our model, we assessed GR staining in the cardiac muscle of Sm-22 Cre+ GRloxP/loxP and control mice and found no significant difference between Cre+ and Cre− littermates (Figure 4A). On Western blot analysis of GR protein from hearts of control and Sm-22 Cre+ GRloxP/loxP mouse pairs, we found that, if anything, heart GR was slightly increased and certainly not decreased in Sm-22 Cre+ GRloxP/loxP mice (Figure 4B), suggesting that there was no significant GR deletion from cardiac myocytes in our KO mice. Consistent with this and previous studies,15,16 we found no differences in heart rate between the two groups either before or after DEX administration (Figure 5).
Figure 4.
Cardiac GR is not decreased in Sm-22 Cre+ GRloxP/loxP mice. (A) Preserved GR staining in the cardiac myocytes of Sm-22 Cre+ GRloxP/loxP mice. Four-micrometer sections of Sm-22 Cre+ GRloxP/loxP and control mouse heart were stained with a commercial antibody to GR. (B) Western blot of control and Sm-22 Cre+ mouse heart homogenates (n = 3/group) probed for GR. Total heart GR is significantly increased in Sm-22 Cre+ mice. C, control; KO, Sm-22 Cre+. **P < 0.05. Images shown are representative of three mice per group.
Figure 5.
No effect of VSM GR KO on the cardiac heart rate response to GC. Mean heart rate is not significantly different in Cre− (n = 11) or Sm-22 Cre+ GRloxP/loxP mice (n = 9) at baseline or during steroid treatment with DEX. GC administration induced a significant fall in heart rate (P < 0.005) that persisted for the duration of GC treatment. There was no significant difference in the heart rate response of the control and Sm-22 Cre+ GRloxP/loxP mice to DEX despite the attenuated BP response in the KO. All mean heart rates were averaged over a 24-h period.
No Significant Difference in Diurnal Variation of BP in Sm-22 Cre+ GRloxP/loxP Mice and Controls
Our data suggest a critical role of VSM GR on the acute hypertensive response to GC. GC have previously been implicated as having a primary role in the diurnal variation of BP.17,18 We questioned whether VSM GR might play an important role in normal circadian variation of BP, and we therefore assessed peak/nadir BP in our Sm-22 Cre+ GRloxP/loxP mice. As seen in Table 1, we observed a similar circadian variation BP pattern in Sm-22 Cre+ GRloxP/loxP and control mice. At baseline, both groups demonstrated a normal circadian variation of BP with nighttime mean BP higher than daytime BP by 12.72 mmHg in the KO animals and 14.31 mmHg in the controls. Consistent with previous reports, DEX induced a dramatic blunting of normal diurnal variation of BP in control mice.17,18 Interestingly, similar effects were noted in the Sm-22 Cre+ GRloxP/loxP mice as well. Both groups showed a similar degree of deranged diurnal variation of BP for the entire time DEX was administered. These data strongly suggest that GR in the VSM does not play any significant role in normal circadian BP variation.
Table 1.
Preserved circadian BP variation in VSM GR KO micea
| Parameter | Sm-22 Cre+ | Control | P |
|---|---|---|---|
| Baseline | 12.72 ± 1.74 | 14.31 ± 2.19 | 0.59 |
| Day 1 | −0.29 ± 2.34 | 1.00 ± 1.91 | 0.67 |
| Day 2 | 3.24 ± 5.73 | 5.30 ± 2.05 | 0.73 |
| Day 3 | 1.13 ± 4.05 | 7.78 ± 1.88 | 0.14 |
| Day 4 | 3.72 ± 4.59 | 9.93 ± 1.82 | 0.21 |
| Day 5 | 6.44 ± 4.25 | 9.57 ± 1.49 | 0.48 |
| Day 6 | 3.55 ± 4.52 | 5.45 ± 1.61 | 0.68 |
| Day 7 | 1.81 ± 3.89 | 9.19 ± 2.39 | 0.10 |
Shown is the mean difference in mean BP (mmHg) between the period of 8 p.m. to midnight and 8 a.m. to noon for Sm-22 Cre+ GRloxP/loxP mice (n = 7) and Cre− controls (n = 8) at baseline and for each day of DEX treatment. VSM GR KO causes no significant effect on circadian BP variation.
Preserved DEX-Induced Natriuresis in Sm-22 Cre+ GRloxP/loxP Mice
Despite—or perhaps owing to—their acute hypertensive effects, GC induce a rapid natriuresis in rodents.19–21 Consistent with these previous observations, we found that our control (Cre−) mice markedly increased renal sodium excretion within 16 h of DEX administration from 0.58 ± 0.07 mmol/mg creatinine before DEX to 0.83 ± 0.09 mmol/mg creatinine after DEX (P = 0.04). We hypothesized that this increased sodium excretion may be due to a pressure natriuresis secondary to the increased BP in these mice; therefore, we assessed electrolyte excretion in our Sm-22 Cre+ GRloxP/loxP mice, in which the acute hypertensive response to DEX is markedly diminished. To our surprise, we found that the Sm-22 Cre+ GRloxP/loxP mice also experienced a significant increase in urine sodium excretion with DEX therapy (Table 2), increasing urine sodium excretion from 0.55 ± 0.09 mmol/mg creatinine before DEX to 1.03 ± 0.18 mmol/mg creatinine after DEX administration (P = 0.03). The difference in sodium excretion between the VSM GR KO and control mice before and after DEX was not statistically significant, suggesting that the DEX-induced natriuresis is not due to the hypertensive effects of GC. Potassium and chloride excretion were also measured. There was a strong trend toward increased urine chloride excretion after DEX administration in both groups, but it did not reach statistical significance. Urine volume was also assessed in this experiment. At baseline, Sm-22 Cre+ GRloxP/loxP and control mice made a similar volume of urine in 16 h (1461 ± 95 versus 1600 ± 197 μl; P = 0.52). After treatment with DEX for 16 h, the urine volume of control mice was essentially unchanged (1566 ± 162 μl; P = 0.90 compared with baseline), whereas the urine volume of the Sm-22 Cre+ GRloxP/loxP mice was significantly increased (2113 ± 218 μl; P = 0.012 compared with baseline).
Table 2.
The acute natriuretic effect of DEX is not affected by VSM GR KOa
| Parameter | Baseline | 16 h after DEX |
|---|---|---|
| Control | ||
| Na+ | 0.58 ± 0.07 | 0.83 ± 0.09 |
| Cl− | 1.03 ± 0.07 | 1.08 ± 0.10 |
| K+ | 1.32 ± 0.10 | 1.22 ± 0.12 |
| urine volume | 1600 ± 197 | 1566 ± 162 |
| Sm-22 Cre+ | ||
| Na+ | 0.55 ± 0.09 | 1.03 ± 0.18 |
| Cl− | 0.90 ± 0.08 | 1.19 ± 0.16 |
| K+ | 1.12 ± 0.12 | 1.20 ± 0.10 |
| urine volume | 1461 ± 95 | 2113 ± 213 |
Urine electrolyte excretion, normalized to creatinine, was measured at baseline and acutely after DEX treatment in Sm-22 Cre+ GRloxP/loxP mice (n = 8) and controls (n = 6; see the Concise Methods section). Control mice develop a significant natriuretic response acutely after DEX administration. This response is unaltered in VSM GR KO mice. Electrolyte values are expressed as mmol/mg creatinine, and urine volume is expressed in μ l.
DISCUSSION
Despite intensive effort, the underlying molecular pathogenesis of GC-induced HTN remains a conundrum. Although promiscuous activation of MR by endogenous steroids has been the purported mechanism of this hypertension,22,23 multiple lines of evidence argue against this being the only—or even the primary—cause. Clinical experience demonstrates that the hypertension induced by steroids occurs much too rapidly to be accounted for solely, if at all, by increased renal sodium reabsorption.1,24 In addition, in animal models, mineralocorticoid antagonists such as spironolactone prevent GC-induced weight gain but do not prevent HTN.25,26 Excess renal salt reabsorption does not seem to be required for GC to induce HTN,1 and DEX and budesonide, synthetic GC with increased affinity for GR and decreased affinity for MR, induce sustained HTN similar to that observed with endogenous GC.27,28 These points argue convincingly that GC have a wide range of hemodynamic effects distinct from their presumed activation of renal MR, thus increasing the understanding of the role of GR in the regulation of BP is of interest.
To understand better the molecular origins of GC-induced HTN, we used emerging technologies in mouse genetics and mouse phenotyping to allow the in vivo study of GC-induced HTN. Here, we studied a mouse model in which GR has been excised specifically from the VSM. Our characterization of this model shows that growth, development, body weight, and baseline HR and BP are similar to controls; however, in response to steroids, the Sm-22 Cre+ GRloxP/loxP mice have an attenuated response to DEX-induced hypertension which is most pronounced in the first 48 h after GC administration.
An important element of this study is the specificity we achieved with the model that we used. Our data indicate that we have achieved high levels of Cre-mediated recombination from the VSM with preservation of GR in the VE. Previous studies assessing vascular reactivity in response to GC have not reliably been able to dissect out the specific roles of endothelium and the smooth muscle. Experiments performed using vascular endothelial KO models have generally used vessels in which the VE is mechanically stripped and hence all functions of the endothelium are lost.29,30 In our model, we demonstrated that GR is intact in the VE, so we believe it unlikely that primary vascular endothelial alterations are playing a role in the phenotype we have identified.
It should be noted that Sm-22 CreTg is highly expressed in a number of tissues with large smooth muscle components, such as the uterus and intestine; however, we believe that effects of GR deletion in these tissues are unlikely to explain the BP phenotype we have observed. Although some Sm-22 CreTg RNA expression has been observed in virtually all tissues studied, histologic evidence indicates this is due principally to the ubiquitous presence of vascular tissue in all organs.13 Although Sm-22 is highly expressed in cardiac tissue, we found the Sm-22 CreTg model that we used did not cause any discernible effect on GR expression in cardiac myocytes. Given the limited or absent expression of Sm-22 CreTg in extravascular tissues, we believe it is highly likely that the BP phenotype observed in our KO mice in response to DEX is primarily, if not wholly, attributable to the loss of GR from VSM.13
In recent years, much attention has been paid to the link between GC and cardiac output and indeed overall heart health; however, there are conflicting results in terms of the reported effects of GC on the cardiovascular system. Bal et al.31 observed reduced systolic function, stable cardiac output, and increased end-diastolic volume with compensatory dilation in neonatal rats treated with GC for prevention of lung disease. In a study of neonatal sheep, administration of DEX was found to increase expression of brainstem AT1 receptor mRNA and in turn to increase responsiveness to angiotensin II (AngII) compared with sheep that received exogenous cortisol. The authors concluded that the two GC had distinct programming actions and that prenatal DEX may lead to increased cardiac responsiveness later in life.32 One postnatal study in adrenalectomized adult rats showed a decrease in mean BP and cardiac output in rats that received only mineralocorticoid replacement compared with controls that received both mineralocorticoid and GC replacement.33 Interestingly, these same rats demonstrated an impairment in urinary concentrating ability with decreased expression of aquaporin-2 and impaired countercurrent multiplication,33 so it is difficult to sort out the effects of GC deficiency versus volume deficiency. Overall, it is unclear whether GC increase or decrease cardiac output; they may have both effects in particular settings. However, it is apparent that there is a complex interplay among GC, AngII and its receptor, and the cardiovascular response.
In addition to an attenuation of the acute BP response to GC, our data demonstrate that the Sm-22 CreTg KO mice ultimately do not achieve the same BP as Cre− littermates after 1 wk of treatment with DEX, although they seem eventually to catch up to the controls after 2 wk of therapy. This finding suggests an additional role of VSM GR in the sustained BP response to GC that will need to be studied further. Guytonian models of BP regulation posit that the sustained hypertensive response to GC requires the active participation of the kidney via alteration in renal sodium reabsorption. This renal response could occur via an alteration of GC-mediated changes in renovascular resistance as a result of the presence of GR in the renal vasculature or perhaps via an altered endocrine or neural stimulation of renal sodium handling. Such a response raises the possibility of differential effects of GC on resistance in different vascular beds.
To begin to understand the effects of GC on the kidney, we assessed acute alterations in urinary electrolyte excretion in our mice in response to GC challenge. We focused on acute alterations because the interpretation of net alterations in chronic sodium balance are difficult to measure and the interpretation of these data requires assumptions about intravascular and extravascular volume that may not be valid. High dosages of GC are known to trigger a rapid natriuresis in rodents, which is thought to be due to the rapid elevation of BP that such dosages induce. To our surprise, GC triggered an acute natriuresis not only in our Cre− mice, in which we observed a robust BP response soon after GC administration, but also in Cre+ mice, in which the hypertensive response to GC is blunted; however, the Cre+ mice also demonstrated a statistically significant increase in urine volume, which suggests the possibility that these mice have a more effective pressure natriuresis than controls and that the presence of GR in the VSM may act as an obstacle to pressure natriuresis in the control state. This hypothesis is consistent with the notion of complex effects of GC on systemic vascular resistance and/or renovascular resistance, and further study will be required to understand this phenomenon better.
Previous work on the role of GR in the regulation of BP primarily relied on in vitro model systems, which can highlight cellular processes relevant to GC effects but cannot be used to understand the physiologic basis of complex integrated phenotypes such a BP. For example, there is literature to support the idea that GC increase vascular reactivity to catecholamines,8 but the mechanism by which VSM responds to GC is unclear. One possible mechanism that several in vitro studies have suggested is that GC may play a role in coupling of G-proteins and α-adrenoceptors in the rat aorta.34,35 Studies in ex vivo afferent arterioles from endothelin-1 transgenic mice demonstrate lower sensitivity to AngII compared with WT that is directly attributable to increased NO bioavailability.36 In addition, studies in the rat show upregulation of the AngII type 1A receptor gene at the level of both mRNA and protein in rat smooth muscle cells in response to DEX7; therefore, we suggest that GC may have direct effects on renovascular resistance, perhaps by preferentially upregulating AngII and ultimately increasing Na and water reabsorption through the actions of aldosterone and ADH. At present, however, we are unable to determine the exact cause for the acute natriuresis observed in the KO group.
Our study is also intriguing with regard to its findings regarding diurnal BP variation. Multiple lines of evidence support an important role of GC in circadian BP rhythms. Humans exhibit diurnal BP variation, with BP peaking soon after the morning cortisol surge, whereas BP peaks in mice and other nocturnal animals soon after the evening GC surge at the time of their awakening. Patients with disordered cortisol secretion, such as those with Cushing syndrome, commonly lose their diurnal BP variation.18 Our data indicate that GR in VSM plays a critical role in the acute response of BP to systemic GC. The absence of a noticeable effect of VSM GR deletion on circadian BP variation in our mice indicates that this acute effect of GC on VSM tone is not necessary for normal circadian BP variation.
In summary, our study demonstrates that VSM GR plays a critical role in mediating the acute and, perhaps to a lesser extent, the chronic BP response to GC. Furthermore, our data suggest that GC may have direct effects on systemic and renovascular resistance, which result in the observed phenotype. Undoubtedly, additional investigation is needed to elucidate the mechanism of this phenomenon.
CONCISE METHODS
Generation of VSM GR KO Mice
WT BL6 mice homozygous for a floxed GR allele, designated GRloxP/loxP, were the gift of the laboratory of Dr. Gunther Schutz37 and were mated with WT males (+/+) possessing Sm-22 Cre. By selective breeding, Sm-22 Cre + GRloxP/loxP homozygotes and Cre− GRloxP/loxP littermate controls were generated. Determination of mouse genotype was by DNA from mouse tail clipping, which was digested by proteinase K and analyzed by standard PCR. The primers for the floxed GR allele had the following sequence: 5′-GGCATGCACATTACTGGCCTTCT-3′ and 5′-CCTTCTCATTCCATGTCAGCATGT-3′. Primers for detection of Cre had the following sequence: 5′-CCGGGCTGCCACGACCAA-3′ and 5′-GGCGCGGCAACACCATTTTT-3′. All experiments were performed according to an Institutional Animal Care and Use Committee–approved protocol at Yale School of Medicine, which is consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Immunofluorescence
Aortas and renal arteries were dissected out and fixed in 4% paraformaldehyde for 4 h at room temperature followed by overnight incubation in PBS at 4°C. Whole kidneys were dissected, mounted in OCT, and flash frozen. Sectioned tissue was fixed by incubation in 100% EtOH at 4°C for 30 min followed by 100% acetone at −20°C for 3 min. Sections were rehydrated by incubation in 1× PBS for 5 min at room temperature. Permeabilization was accomplished by incubation in 0.2% Triton-X in PBS for 10 min at room temperature. Blocking solution consisting of 10% serum and 1% BSA in PBS was applied for 30 to 60 min. Tissue sections were then washed 10× quickly in PBS and then incubated in three fresh washes of PBS for 5 to 7 min. Primary antibody (mouse anti-GR at 1:200 and/or rabbit anti-CD31 at 1:1000 and/or mouse anti–α-smooth muscle actin with FITC conjugate at 1:200) in 2.5% serum/PBS was applied and incubated 1 to 3 h at room temperature. Sections were again washed 10× in PBS followed by incubation in three fresh washes of PBS. Secondary antibody (goat anti-mouse at 1:200 and/or goat anti-rabbit at 1:1000) in 2.5% serum/PBS was applied and incubated for 30 to 60 min at room temperature. Sections were mounted with Vectashield (Vector Laboratories, Burlingame, CA) and allowed to harden overnight at 4°C.
Western Blotting
Whole hearts were dissected from control and Sm-22 Cre + GRloxP/loxP mice, cleared of fat and connective tissue, and washed quickly three times in ice-cold PBS to rinse away blood. Whole-heart homogenates were solubilized in lysis buffer with protease cocktail inhibitors and kept on ice. Proteins (20 to 30 μg) were subjected to SDS-PAGE using 7.5% polyacrylamide gels. Proteins were then transferred from the gel to polyvinylidene difluoride microporous membranes that were used for immunoblotting. After transfer, membranes were stained with Ponceau S solution to confirm complete transfer of proteins to blots and verify consistent protein loading among wells. Membranes were incubated with blocking solution (5% nonfat dry milk and 0.1% Tween in PBS) for 1 h at room temperature to block nonspecific protein binding. Membranes were incubated at 4°C overnight with primary antibody (anti-GR at 1:2000) in blocking solution. After several washes, secondary antibody (goat anti-mouse HRP at 1:2000) was added for 1 h at room temperature. Bound antibody on blots was visualized using an enhanced chemiluminescence system, and relative protein quantities were determined by densitometry.
Telemetric Measurement of BP and Heart Rate
Radiotelemetric BP catheters were implanted in the aortic arch in 8- to 12-wk-old male Sm-22 Cre + GRloxP/loxP mice and Cre− controls as described previously,38 and mice were allowed 1 wk to recover from surgery. Adequate recovery was deemed to have occurred when mice demonstrated normal diurnal variation of BP for 3 consecutive days. Mice were housed individually in a controlled environment with 12-h light and dark cycles and had free access to water and standard mouse diet. Measurement of the following six parameters occurred every 1 min using the DataQuest System (Data Sciences, St. Paul, MN): SBP, DBP, mean BP, pulse pressure, heart rate, and activity level. Measurements were averaged over 4-h periods using an Excel program developed specifically for this purpose. After 3 d of a normal BP pattern, mice were treated with DEX in their drinking water at a concentration of 15 mg/L. On the basis of an average water intake of 2 to 3 ml/d per mouse and an average weight of approximately 30 g/mouse, the ingested dosage of DEX was approximately 2 mg/kg per d for each mouse.
Assessment of Mouse Circadian Rhythm
Mice were housed in facilities with a 12-h light-dark cycle such that 7 a.m. to 7 p.m. was the light cycle and 7 p.m. to 7 a.m. was the dark cycle. Data collected using the DataQuest System software were input into an Excel program that calculated time-averaged SBP, DBP, and MAP for 4-h intervals as follows: 8 p.m. to midnight, midnight to 4 a.m., 4 a.m. to 8 a.m., 8 a.m. to noon, noon to 4 p.m., and 4 p.m. to 8 p.m. An additional function in the program calculated the average change in SBP, DBP, and MAP between each pair of consecutive 4-h periods. All mice studied demonstrated a maximum change in BP from the period of 8 p.m. to midnight to 8 a.m. to noon. This difference was calculated for the MAP of each mouse at baseline and for each day of DEX treatment.
Mouse Urine Collection
Eight- to 12-wk-old male Sm-22 Cre + GRloxP/loxP mice and Cre− controls were housed individually in mouse-specific metabolic cages with free access to water and standard mouse diet. Mice were allowed to acclimate to their new cages for 24 h. On day 2, a 16-h urine collection was started at 5 p.m., shortly before the beginning of the dark cycle. A thin film (50 μl) of mineral oil was used to coat the collection tube and prevent evaporation of urine. On day 3, another 16-h urine collection was started at 5 p.m., and mice were simultaneously treated with DEX 15 mg/L in the drinking water. Cages were cleaned thoroughly in between each urine collection. Urine volumes of <800 μl were excluded from subsequent analysis. Urine electrolytes were determined by the Yale Mouse Phenotyping Core Facility using the COBAS MIRA system (Roche Diagnostics, Nutley, NJ) and mass spectrometry. Each mouse's urine electrolytes were normalized to its own urine creatinine to control for differences in GFR.
Reagents
Dexamethasone phosphate, donkey serum, PBS, and paraformaldehyde were obtained from Sigma Chemicals (St. Louis, MO). Triton-X, acetone, and alcohol were obtained from American Bioanalytical (Natick, MA). Antibody to GR was obtained from Affinity Bioreagents (Golden, CO). Anti-CD31 was courtesy of Dr. Joseph Madri (New Haven, CT). A stock solution of dexamethasone was dissolved in distilled water and prepared fresh before each set of experiments.
Statistical Analysis
Data are expressed as means ± SEM. Statistical differences were determined by paired and unpaired t test or by Wilcoxon signed rank test. P < 0.05 was considered significant.
DISCLOSURES
None.
Acknowledgments
This work was supported by a National Kidney Foundation Postdoctoral Research Fellowship Award to J.E.G. We also acknowledge the following support for the Yale Mouse Metabolic Phenotyping Core: U24 DK59635.
We acknowledge and thank Dr. Gunther Schutz of the Deutsches Krebsforschungszentrum (German Cancer Research Center) for his generous gift of mice with a floxed GR allele.
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Glucocorticoid-Mediated Hypertension: Does the Vascular Smooth Muscle Hold All the Answers?’ on pages 1251–1253.
REFERENCES
- 1.Mangos GJ, Whitworth JA, Williamson PM, Kelly JJ: Glucocorticoids and the kidney. Nephrology (Carlton) 8: 267–273, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Fardet L, Flahault A, Kettaneh A, Tiev KP, Généreau T, Tolédano C, Lebbé C, Cabane J: Corticosteroid-induced clinical adverse events: Frequency, risk factors and patient's opinion. Br J Dermatol 157: 142–148, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Ng MK, Celermajer DS: Glucocorticoid treatment and cardiovascular disease. Heart 90: 829–830, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitworth JA, Coghlan JP, Denton DA, Graham WF, Humphery TJ, Scoggins BA: Comparison of the effects of ‘glucocorticoid’ and ‘mineralocorticoid’ infusions on blood pressure in sheep. Clin Exp Hypertens 1: 649–663, 1979 [DOI] [PubMed] [Google Scholar]
- 5.Mantero F, Boscaro M: Glucocorticoid-dependent hypertension. J Steroid Biochem Mol Biol 43: 409–413, 1992 [DOI] [PubMed] [Google Scholar]
- 6.Provencher PH, Saltis J, Funder JW: Glucocorticoids but not mineralocorticoids modulate endothelin-1 and angiotensin II binding in SHR vascular smooth muscle cells. J Steroid Biochem Mol Biol 52: 219–225, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Uno S, Guo DF, Nakajima M, Ohi H, Imada T, Hiramatsu R, Nakakubo H, Nakamura N, Inagami T: Glucocorticoid induction of rat angiotensin II type 1A receptor gene promoter. Biochem Biophys Res Commun 204: 210–215, 1994 [DOI] [PubMed] [Google Scholar]
- 8.Yang S, Zhang L: Glucocorticoids and vascular reactivity. Curr Vasc Pharmacol 2: 1–12, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Kornel L: The role of vascular steroid receptors in the control of vascular contractility and peripheral vascular resistance. J Steroid Biochem Mol Biol 45: 195–203, 1993 [DOI] [PubMed] [Google Scholar]
- 10.Ray KP, Farrow S, Daly M, Talabot F, Searle N: Induction of the E-selectin promoter by interleukin 1 and tumour necrosis factor alpha, and inhibition by glucocorticoids. Biochem J 328: 707–715, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mangos GJ, Walker BR, Kelly JJ, Lawson JA, Webb DJ, Whitworth JA: Cortisol inhibits cholinergic vasodilation in the human forearm. Am J Hypertens 13: 1155–1160, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Zhang J, Goodwin J, Geller D: Characterization of a Novel Gain of Function Mutation Glucocorticoid Receptor Knock-in Mouse. Presented at The Endocrine Society Meeting; June 24–27, 2006; Boston, MA
- 13.Holtwick R, Gotthardt M, Skryabin B, Steinmetz M, Potthast R, Zetsche B, Hammer RE, Herz J, Kuhn M: Smooth muscle-selective deletion of guanylyl cyclase-A prevents the acute but not chronic effects of ANP on blood pressure. Proc Natl Acad Sci U S A 99: 7142–7147, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petrov L, Laurila H, Hayry P, Vamvakopoulos JE: A mouse model of aortic angioplasty for genomic studies of neointimal hyperplasia. J Vasc Res 42: 292–300, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Lepore JJ, Cheng L, Min Lu M, Mericko PA, Morrisey EE, Parmacek MS: High-efficiency somatic mutagenesis in smooth muscle cells and cardiac myocytes in SM22alpha-Cre transgenic mice. Genesis 41: 179–184, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Zhang JC, Kim S, Helmke BP, Yu WW, Du KL, Lu MM, Strobeck M, Yu Q, Parmacek MS: Analysis of SM22alpha-deficient mice reveals unanticipated insights into smooth muscle cell differentiation and function. Mol Cell Biol 21: 1336–1344, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zelinka T, Strauch B, Pecen L, Widimsky J Jr: Diurnal blood pressure variation in pheochromocytoma, primary aldosteronism and Cushing's syndrome. J Hum Hypertens 18: 107–111, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Zacharieva S, Orbetzova M, Stoynev A, Shigarminova R, Yaneva M, Kalinov K, Nachev E, Elenkova A: Circadian blood pressure profile in patients with Cushing's syndrome before and after treatment. J Endocrinol Invest 27: 924–930, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Muller OG, Parnova RG, Centeno G, Rossier BC, Firsov D, Horisberger JD: Mineralocorticoid effects in the kidney: Correlation between alphaENaC, GILZ, and Sgk-1 mRNA expression and urinary excretion of Na+ and K+. J Am Soc Nephrol 14: 1107–1115, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Marissal-Arvy N, Mormede P: Excretion of electrolytes in Brown Norway and Fischer 344 rats: Effects of adrenalectomy and of mineralocorticoid and glucocorticoid receptor ligands. Exp Physiol 89: 753–765, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Campen TJ, Vaughn DA, Fanestil DD: Mineralo- and glucocorticoid effects on renal excretion of electrolytes. Pflugers Arch 399: 93–101, 1983 [DOI] [PubMed] [Google Scholar]
- 22.Frey FJ, Odermatt A, Frey BM: Glucocorticoid-mediated mineralocorticoid receptor activation and hypertension. Curr Opin Nephrol Hypertens 13: 451–458, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Whitworth JA, Brown MA, Kelly JJ, Williamson PM: Mechanisms of cortisol-induced hypertension in humans. Steroids 60: 76–80, 1995 [DOI] [PubMed] [Google Scholar]
- 24.Wallwork CJ, Parks DA, Schmid-Schonbein GW: Xanthine oxidase activity in the dexamethasone-induced hypertensive rat. Microvasc Res 66: 30–37, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Montrella-Waybill M, Clore JN, Schoolwerth AC, Watlington CO: Evidence that high dose cortisol-induced Na+ retention in man is not mediated by the mineralocorticoid receptor. J Clin Endocrinol Metab 72: 1060–1066, 1991 [DOI] [PubMed] [Google Scholar]
- 26.Williamson PM, Kelly JJ, Whitworth JA: Dose-response relationships and mineralocorticoid activity in cortisol-induced hypertension in humans. J Hypertens Suppl 14: S37–S41, 1996 [PubMed] [Google Scholar]
- 27.Arriza JL, Weinberger C, Cerelli G, Glaser TM, Handelin BL, Housman DE, Evans RM: Cloning of human mineralocorticoid receptor complementary DNA: Structural and functional kinship with the glucocorticoid receptor. Science 237: 268–275, 1987 [DOI] [PubMed] [Google Scholar]
- 28.De Wachter E, Malfroot A, De Schutter I, Vanbesien J, De Schepper J: Inhaled budesonide induced Cushing's syndrome in cystic fibrosis patients, due to drug inhibition of cytochrome P450. J Cyst Fibros 2: 72–75, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Matoba T, Shimokawa H, Nakashima M, Hirakawa Y, Mukai Y, Hirano K, Kanaide H, Takeshita A: Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J Clin Invest 106: 1521–1530, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borges AA, Gomes OM: Effects of midazolam on the contraction and relaxation of segments of thoracic aorta stripped of endothelium and stimulated by adrenaline: Experimental study in rabbits. Mol Cell Biochem 246: 13–17, 2003 [PubMed] [Google Scholar]
- 31.Bal MP, de Vries WB, van der Leij FR, van Oosterhout MF, Berger RM, Baan J, van der Wall EE, van Bel F, Steendijk P: Neonatal glucocorticosteroid treatment causes systolic dysfunction and compensatory dilation in early life: Studies in 4-week-old prepubertal rats. Pediatr Res 58: 46–52, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Dodic M, McAlinden AT, Jefferies AJ, Wintour EM, Cock ML, May CN, Evans RG, Moritz KM: Differential effects of prenatal exposure to dexamethasone or cortisol on circulatory control mechanisms mediated by angiotensin II in the central nervous system of adult sheep. J Physiol 571: 651–660, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen YC, Cadnapaphornchai MA, Summer SN, Falk S, Li C, Wang W, Schrier RW: Molecular mechanisms of impaired urinary concentrating ability in glucocorticoid-deficient rats. J Am Soc Nephrol 16: 2864–2871, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Haigh RM, Jones CT, Milligan G: Glucocorticoids regulate the amount of G proteins in rat aorta. J Mol Endocrinol 5: 185–188, 1990 [DOI] [PubMed] [Google Scholar]
- 35.Haigh RM, Jones CT: Effect of glucocorticoids on alpha 1-adrenergic receptor binding in rat vascular smooth muscle. J Mol Endocrinol 5: 41–48, 1990 [DOI] [PubMed] [Google Scholar]
- 36.Patzak A, Bontscho J, Lai E, Kupsch E, Skalweit A, Richter CM, Zimmermann M, Thöne-Reineke C, Joehren O, Godes M, Steege A, Hocher B: Angiotensin II sensitivity of afferent glomerular arterioles in endothelin-1 transgenic mice. Nephrol Dial Transplant 20: 2681–2689, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Wintermantel TM, Berger S, Greiner EF, Schutz G: Evaluation of steroid receptor function by gene targeting in mice. J Steroid Biochem Mol Biol 93: 107–112, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Plehm R, Barbosa ME, Bader M: Animal models for hypertension/blood pressure recording. Methods Mol Med 129: 115–126, 2006 [DOI] [PubMed] [Google Scholar]