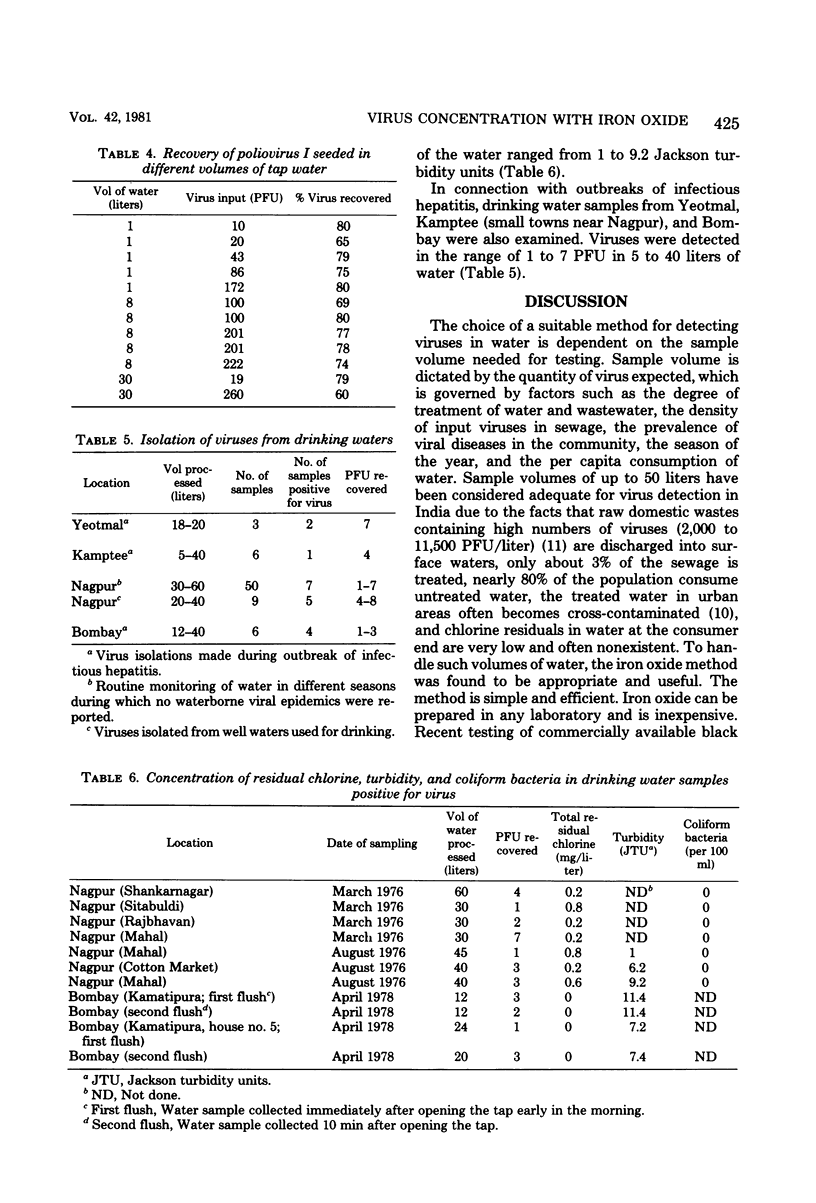
Abstract
Discharge of raw domestic wastes containing human enteric viruses into water courses, consumption of untreated water from canals, streams, and shallow wells in villages, and cross-contamination of water in the distribution system because of intermittent water supply in urban areas continue to cause widespread outbreaks of infectious hepatitis in India. To detect a low number of viruses in 50- to 100-liter samples of water, a method was developed with magnetic iron oxide as the virus adsorbent. Poliovirus-seeded dechlorinated tap water, adjusted to pH 3.0 and 0.0005 M AlCl3, was filtered through a 10-g bed of iron oxide sandwiched between two AP20 prefilter pads held in a 142-mm-diameter, stainless-steel holder. Virus was eluted from iron oxide by recirculating three times a 100-ml volume of 3% beef extract, pH 9.0. The eluate was reconcentrated to 5 ml by adjusting to pH 3, adding 1 g of iron oxide, stirring for 30 min, and eluting the readsorbed virus with 5 ml of beef extract, pH 9.0. Virus recovery varied from 60 to 80%. Using the above method, we took a survey of drinking water at three locations in Nagpur during 1976 and found the presence of virus in 7 of 50 samples. The quantity of virus recovered ranged from 1 to 7 plaque-forming units per 30 to 60 liters. Virus was detected in some samples even with residual chlorine. No coliforms were detected in the virus-positive samples.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bates R. C., Shaffer P. T., Sutherland S. M. Development of poliovirus having increased resistance to chlorine inactivation. Appl Environ Microbiol. 1977 Dec;34(6):849–853. doi: 10.1128/aem.34.6.849-853.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrah S. R., Gerba C. P., Wallis C., Melnick J. L. Concentration of viruses from large volumes of tap water using pleated membrane filters. Appl Environ Microbiol. 1976 Feb;31(2):221–226. doi: 10.1128/aem.31.2.221-226.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrah S. R., Goyal S. M., Gerba C. P., Wallis C., Melnick J. L. Concentration of enteroviruses from estuarine water. Appl Environ Microbiol. 1977 May;33(5):1192–1196. doi: 10.1128/aem.33.5.1192-1196.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenelson E., Fattal B., Hostovesky T. Organic flocculation: an efficient second-step concentration method for the detection of viruses in tap water. Appl Environ Microbiol. 1976 Oct;32(4):638–639. doi: 10.1128/aem.32.4.638-639.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobsey M. D., Gerba C. P., Wallis C., Melnick J. L. Concentration of enteroviruses from large volumes of turbid estuary water. Can J Microbiol. 1977 Jun;23(6):770–778. doi: 10.1139/m77-114. [DOI] [PubMed] [Google Scholar]
- Sobsey M. D., Wallis C., Henderson M., Melnick J. L. Concentration of enteroviruses from large volumes of water. Appl Microbiol. 1973 Oct;26(4):529–534. doi: 10.1128/am.26.4.529-534.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waghmare S. V., Chalapati Rao V., Lakhe S. B. Monkey kidney tissue culture--an approach for improved cell yield. Indian J Exp Biol. 1979 Jun;17(6):597–598. [PubMed] [Google Scholar]
- Wallis C., Henderson M., Melnick J. L. Enterovirus concentration on cellulose membranes. Appl Microbiol. 1972 Mar;23(3):476–480. doi: 10.1128/am.23.3.476-480.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren J., Neal A., Rennels D. Adsorption of myxoviruses on magnetic iron oxides. Proc Soc Exp Biol Med. 1966 Apr;121(4):1250–1253. doi: 10.3181/00379727-121-31020. [DOI] [PubMed] [Google Scholar]


