Abstract
Contrary to most examples of disparities in health outcomes, black patients have improved survival compared with white patients after initiating hemodialysis. Understanding potential explanations for this observation may have important clinical implications for minorities in general. This study tested the hypothesis that greater use of activated vitamin D therapy accounts for the survival advantage observed in black and Hispanic patients on hemodialysis. In a prospective cohort of non-Hispanic white (n = 5110), Hispanic white (n = 979), and black (n = 3214) incident hemodialysis patients, higher parathyroid hormone levels at baseline were the primary determinant of prescribing activated vitamin D therapy. Median parathyroid hormone was highest among black patients, who were most likely to receive activated vitamin D and at the highest dosage. One-year mortality was lower in black and Hispanic patients compared with white patients (16 and 16 versus 23%; P < 0.01), but there was significant interaction between race and ethnicity, activated vitamin D therapy, and survival. In multivariable analyses of patients treated with activated vitamin D, black patients had 16% lower mortality compared with white patients, but the difference was lost when adjusted for vitamin D dosage. In contrast, untreated black patients had 35% higher mortality compared with untreated white patients, an association that persisted in several sensitivity analyses. In conclusion, therapy with activated vitamin D may be one potential explanation for the racial differences in survival among hemodialysis patients. Further studies should determine whether treatment differences based on biologic differences contribute to disparities in other conditions.
Compared with non-Hispanic white individuals, black and Hispanic individuals more frequently develop a variety of chronic diseases, including diabetes and hypertension, and after their onset experience more rapid disease progression, greater end-organ complications, and increased mortality.1–5 Although the quality of health care delivered to racial and ethnic minorities is often inferior to that for non-Hispanic white individuals even after controlling for differences in health insurance, access to care, income, and education,6 the contribution of biologic differences to disparities in clinical outcomes remains controversial and incompletely understood.
Kidney disease rates are increasing and disproportionately affect black and Hispanic individuals.7–10 The increased risk for kidney disease in minorities has been attributed to increased rates of diabetes and hypertension exacerbated by limited access to preventive strategies.10–12 Once patients develop kidney failure, however, racial disparities in health care delivery are at least partially attenuated because universal access to dialysis is mandated in the United States regardless of race, insurance, or socioeconomic status.13,14 Although narrowing disparities in care would be expected to reduce differences in outcomes on dialysis, paradoxically, black and Hispanic individuals demonstrate longer survival on dialysis than non-Hispanic white individuals, a difference that was first noted in the late 1970s but has grown since.15–24
At the initiation of dialysis, black and Hispanic patients tend to have more favorable clinical characteristics22; however, their survival advantage is only partially attenuated after adjustment for these factors, differences in dialysis dosage, or use of erythropoietin and iron.16–24 Intravenous activated vitamin D was introduced in the middle to late 1980s to manage secondary hyperparathyroidism on dialysis, but beyond this role, several observational studies suggested an independent survival benefit associated with its use in dialysis.25–29 Healthy black and Hispanic individuals and those with kidney disease have lower levels of vitamin D and consequently higher levels of parathyroid hormone (PTH) compared with non-Hispanic white individuals.30–34 These differences are exaggerated further once patients reach dialysis,35 suggesting that black and Hispanic individuals would be more likely to be treated with intravenous activated vitamin D. We hypothesized that greater use of activated vitamin D among black and Hispanic patients is one factor that contributes to their survival advantage compared with non-Hispanic white patients. Moreover, we hypothesized that black and Hispanic patients who are not treated with activated vitamin D demonstrate worse survival compared with non-Hispanic white patients, mirroring their worse outcomes in many nondialysis settings.
RESULTS
Baseline Characteristics
Baseline characteristics and laboratory results at the initiation of dialysis are presented in Table 1. Compared with white patients, black patients tended to be younger and heavier, and a higher proportion were women and had hypertension as the assigned cause of renal failure. A smaller proportion had cardiovascular disease, malignancy, or chronic obstructive pulmonary disease. Baseline PTH and creatinine levels were higher for black compared with white patients, whereas calcium, albumin, hemoglobin, and urea reduction ratios were lower. In the subset of patients who had vitamin D levels measured (Table 2), serum 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D were lowest among black patients, who were most likely to be severely vitamin D deficient. For most baseline characteristics, including vitamin D measurements, Hispanic patients had levels that were intermediate between white and black patients.
Table 1.
Baseline characteristics at the initiation of hemodialysis according to race/ethnicitya
| Characteristic | White (n = 5110) | Hispanic (n = 979) | Black (n = 3214) |
|---|---|---|---|
| Demographic characteristics | |||
| age (yr; mean ± SD) | 66.3 ± 14.8 | 61.2 ± 14.6b | 57.8 ± 15.4b,c |
| female (%) | 42 | 44 | 51b,c |
| assigned cause of renal failure (%) | |||
| diabetes | 41 | 64b | 39 |
| hypertension | 32 | 21b | 46b,c |
| glomerulonephritis | 10 | 8 | 9 |
| polycystic kidney disease | 3 | 2 | 1 |
| other | 14 | 5b | 5b |
| systolic BP (mmHg; mean ± SD) | 141 ± 23 | 145 ± 20b | 149 ± 22b,c |
| diastolic BP (mmHg; mean ± SD) | 71 ± 13 | 75 ± 12b | 79 ± 13b,c |
| BMI (kg/m2; mean ± SD) | 27.3 ± 6.9 | 27.3 ± 6.5 | 27.9 ± 7.4b,c |
| vascular access at hemodialysis initiation (%) | |||
| arteriovenous fistula | 25 | 28 | 24 |
| venovenous catheter | 63 | 58 | 59 |
| Comorbid conditions (%) | |||
| coronary artery disease/myocardial infarction | 14 | 7b | 6b |
| congestive heart failure | 15 | 9b | 10b |
| peripheral vascular disease | 8 | 3b | 3b |
| stroke | 4 | 2b | 3c |
| hyperlipidemia | 14 | 10b | 7b,c |
| malignancy | 5 | 1b | 1b |
| chronic obstructive pulmonary disease | 4 | 1b | 1b |
| Baseline laboratory test results | |||
| albumin (g/dl; mean ± SD) | 3.5 ± 0.5 | 3.4 ± 0.6b | 3.4 ± 0.6b |
| creatinine (mg/dl; mean ± SD) | 5.7 ± 2.4 | 6.3 ± 2.4b | 7.3 ± 3.1b,c |
| calcium (mg/dl; mean ± SD) | 8.5 ± 0.8 | 8.3 ± 0.8b | 8.4 ± 0.9b,c |
| phosphorus (mg/dl; mean ± SD) | 4.7 ± 1.6 | 4.8 ± 1.6 | 4.7 ± 1.5 |
| PTH (biointact; pg/ml; mean [IQR])d | 173 (93 to 292) | 192 (113 to 318)b | 279 (161 to 461)b,c |
| alkaline phosphatase (U/L; mean ± SD) | 97 ± 67 | 101 ± 57 | 103 ± 78b |
| hemoglobin (g/L; mean ± SD) | 10.5 ± 1.3 | 10.4 ± 1.3 | 10.1 ± 1.4b,c |
| urea reduction ratio (%; mean ± SD) | 69 ± 11 | 70 ± 11b | 68 ± 11b,c |
BMI, body mass index; IQR, interquartile range.
Significant differences (P < 0.05) versus white.
Significant differences (P < 0.05) between Black and Hispanic.
Reference range on hemodialysis is 75 to 150 pg/ml.53
Table 2.
Vitamin D levels at the initiation of hemodialysis before administration of any exogenous activated vitamin D according to race/ethnicitya
| Parameter | White | Hispanic | Black |
|---|---|---|---|
| 25-Hydroxyvitamin D (ng/ml; mean ± SD) | 23.2 ± 13.7 | 20.7 ± 11.2 | 16.9 ± 10.9b,c |
| ≤10 (%) | 15 | 13 | 30b,c |
| 10 to 30 (%) | 59 | 70 | 58 |
| >30 (%) | 26 | 17 | 12 |
| 1,25-Dihydroxyvitamin D (pg/ml; mean ± SD) | 11.2 ± 9.6 | 10.7 ± 7.3 | 10.2 ± 11.1b |
| ≤10 (%) | 56 | 59 | 61 |
| 10 to 30 (%) | 40 | 41 | 36 |
| >30 (%) | 4 | 0 | 3 |
25-Hydroxyvitamin D levels were available for 653 white, 125 Hispanic, and 372 black patients. 1,25-Dihydroxyvitamin D levels were available for 374 white, 76 Hispanic, and 219 black patients.
Significant differences (P < 0.05) versus White.
Significant differences (P < 0.05) between black and Hispanic.
Crude and Adjusted 1-Yr Mortality
The annualized 1-yr mortality rate in the overall population was 20% (Table 3). Cardiovascular disease accounted for 53% of deaths, and 26% were attributed to infection. There was no significant difference in crude mortality rates between black and Hispanic patients, but each demonstrated a survival advantage compared with white patients (Table 3, Figure 1). The survival advantage of black and Hispanic patients was partially attenuated after adjustment for age and gender but retained statistical significance in the multivariable-adjusted analyses that did not account for activated vitamin D therapy (Table 3).
Table 3.
Mortality rates and crude and adjusted HR for mortality by race/ethnicitya
| Parameter | All Patients (n = 9303) | White (n = 5110) | Hispanic (n = 979) | Black (n = 3214) |
|---|---|---|---|---|
| Deaths (n) | 1432 | 874 | 130 | 428 |
| Absolute mortality rate (%) | 15.4 | 17.1 | 13.3b | 13.3b |
| Annualized mortality rate (%) | 20.0 | 23.8 | 16.0b | 16.1b |
| Crude HR of death (95% CI) | – | Reference | 0.68 (0.57 to 0.82)b | 0.68 (0.61 to 0.77)b |
| Age- and gender-adjusted HR (95% CI) | – | Reference | 0.81 (0.67 to 0.97)b | 0.90 (0.80 to 1.02) |
| Multivariable-adjusted HR (95% CI)c | – | Reference | 0.81 (0.65 to 0.99)b | 0.87 (0.76 to 0.99)b |
| Multivariable and activated vitamin D therapy–adjusted HR (95% CI)c | – | Reference | 0.84 (0.65 to 1.08) | 0.98 (0.83 to 1.16) |
Absolute mortality rate was calculated as the total number of deaths divided by the total number of patients at risk at the outset of the observation period. Annualized mortality rate was the total number of deaths per 100 patient-years of follow-up, censoring for recovery of renal function, kidney transplantation, or loss to follow-up.
Significant differences (P < 0.05) versus white.
Adjusted for age, gender, assigned cause of renal failure, BP, BMI, vascular access at the initiation of hemodialysis, comorbidities, SMR, laboratory tests, and urea reduction ratio.
Figure 1.
Kaplan-Meier survival curves for the first year on hemodialysis according to race/ethnicity (white, n = 5110; Hispanic, n = 979; black, n = 3214). Log-rank tests comparing Hispanic versus white, P < 0.01; black versus white, P < 0.01.
Use of Activated Vitamin D
Among all patients, 77% were treated with activated vitamin D beginning at a median of day 16 (interquartile range 9 to 43 d) after initiating dialysis and continuing for a median duration of 270 d (interquartile range 126 to 348 d), or 77% of the total follow-up period. Compared with white patients, black patients were more likely to be treated, spent a greater proportion of their follow-up time receiving therapy, and received significantly greater dosages (Table 4); vitamin D use among Hispanic patients was intermediate between white and black patients. Although lower calcium levels were associated with subsequent activated vitamin D therapy in univariate analyses, baseline PTH was the strongest independent predictor of therapy (2.4-fold greater likelihood of therapy per 100-pg/ml increase; 95% confidence interval [CI] 2.3 to 2.5). Figure 2 presents the frequency of activated vitamin D use according to race/ethnicity and decile of baseline PTH levels. White patients tended to be concentrated in the lowest deciles of PTH, black patients were skewed toward the upper deciles, and Hispanic patients had intermediate PTH levels. Compared with white patients, black patients were more likely to receive activated vitamin D within each PTH decile: Up to 52% more likely than white patients for PTH 52 to 94 pg/ml (95% CI 34 to 73%), but among patients with baseline PTH levels >206 pg/ml (deciles 6 to 10), the likelihood of therapy was only 4% greater (95% CI 2 to 7%) among black compared with white patients.
Table 4.
Intravenous activated vitamin D therapy during the first year on hemodialysis according to race/ethnicity
| Parameter | White (n = 5110) | Hispanic (n = 979) | Black (n = 3214) |
|---|---|---|---|
| Treatment with activated vitamin D (%) | 71 | 77a | 88 a,b |
| Dialysis day of activated vitamin D initiation (median[IQR]) | 16 (9 to 44) | 21 (12 to 59)a | 16 (9 to 37)b |
| Total activated vitamin D exposure/total follow-up (%) | 57 | 63a | 74 a,b |
| Activated vitamin D dosage (μg/treatment in calcitriol equivalent units; mean ± SD) | 0.6 ± 0.5 | 0.7 ± 0.5a | 1.0 ± 0.7 a,b |
Significant differences (P < 0.05) versus white.
Significant differences (P < 0.05) between black and Hispanic.
Figure 2.
Distribution of baseline PTH levels and the proportion of patients who received activated vitamin D within deciles of baseline PTH by race/ethnicity: (A) White. (B) Hispanic. (C) Black. The bars represent the proportion of patients in each PTH decile. The percentage of patients treated with activated vitamin D in each decile is provided at the top of the corresponding bars.
Race, Ethnicity, Activated Vitamin D, and Survival
In a univariate analysis, therapy with intravenous activated vitamin D was associated with a significant survival advantage compared with no therapy (hazard ratio [HR] 0.48; 95% CI 0.43 to 0.54). There was significant interaction between race/ethnicity, activated vitamin D therapy, and survival in univariate (P < 0.0001) and multivariable-adjusted (P = 0.01) analyses, indicating different relationships between race/ethnicity and survival among treated and untreated patients. Among patients who were treated with activated vitamin D, black and Hispanic patients had significantly better survival compared with white patients (Figure 3A), but among untreated patients, black patients had significantly worse survival compared with white patients (Figure 3B). Multivariable adjustment partially attenuated the survival benefit of the black and Hispanic patients treated with activated vitamin D, but the higher risk for mortality among untreated black patients compared with untreated white patients persisted in age- and gender-adjusted, multivariable-adjusted models and in multivariable models stratified by facility (Table 5). Furthermore, the survival benefit of black patients in the vitamin D–treated stratum was eliminated when adjusted for only age, gender, and dosage of activated vitamin D (HR 1.04; 95% CI 0.90 to 1.21).
Figure 3.
Kaplan-Meier survival curves for the first year on hemodialysis according to race/ethnicity and stratified by activated vitamin D therapy. (A) Patients who were treated with activated vitamin D (white, n = 3567; Hispanic, n = 745; black, n = 2821); log-rank tests comparing Hispanic versus white, P < 0.01; black versus white, P < 0.01. (B) Patients who remained untreated (white, n = 1543; Hispanic, n = 234; black, n = 393); log-rank tests comparing Hispanic versus white, P = 0.8; black versus white, P < 0.01.
Table 5.
Crude and adjusted HR for mortality by race/ethnicity stratified by activated vitamin D therapy
| Parameter | White (HR [95% CI]) | Hispanic (HR [95% CI]) | Black (HR [95% CI]) |
|---|---|---|---|
| Activated vitamin D use | |||
| crude HR of death | Reference | 0.59 (0.46 to 0.76)a | 0.66 (0.57 to 0.76)a |
| age- and gender-adjusted HR of death | Reference | 0.69 (0.53 to 0.88)a | 0.89 (0.77 to 1.03) |
| multivariable-adjusted HR of deathb | Reference | 0.72 (0.55 to 0.94)a | 0.84 (0.71 to 0.98)a |
| multivariable- and facility-adjusted HRb | Reference | 0.65 (0.47 to 0.89)a | 0.73 (0.61 to 0.88)a |
| No activated vitamin D use | |||
| crude HR of death | Reference | 0.98 (0.74 to 1.28) | 1.33 (1.08 to 1.64)a |
| age- and gender-adjusted HR of death | Reference | 1.10 (0.83 to 1.45) | 1.65 (1.33 to 2.04)a |
| multivariable-adjusted HR of deathb | Reference | 0.93 (0.68 to 1.26) | 1.35 (1.07 to 1.69)a |
| multivariable- and facility-adjusted HRb | Reference | 0.96 (0.64 to 1.44) | 1.32 (1.01 to 1.74)a |
Significant differences (P < 0.05) versus white.
Adjusted for age, gender, assigned cause of renal failure, BP, BMI, vascular access at the initiation of hemodialysis, comorbidities, SMR, laboratory tests, and urea reduction ratio.
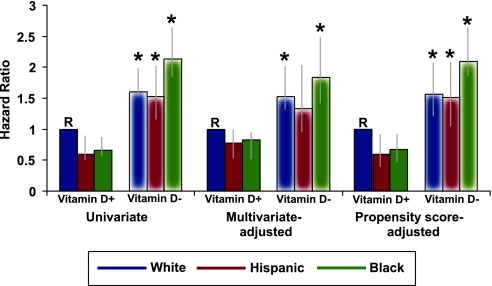
Black patients who were not treated with activated vitamin D were at higher risk for death in univariate, multivariable-adjusted, and propensity score–adjusted analyses of the six race/ethnicity × vitamin D groups (Figure 4). The results were materially unchanged when further adjusted for baseline vitamin D levels (data not shown), although we acknowledge decreased power in the latter analyses. In contrast, there was no effect modification of the relationships between race/ethnicity and survival by iron or erythropoietin therapies; vascular access; age; gender; urea reduction ratio; or levels of albumin, creatinine, hemoglobin, calcium, and PTH (data not shown). Although the magnitude of the survival benefit among black patients varied according to BP, body mass index, and phosphorus levels, in no stratum were black patients at higher risk for mortality as was observed in the group with no vitamin D treatment.
Figure 4.
Risk for death according to race/ethnicity × activated vitamin D therapy groups in unadjusted, multivariable-adjusted, and propensity score–adjusted models. Solid and feathered bars refer to activated vitamin D treated and untreated groups, respectively. *P < 0.05 versus reference group (R).
Black patients were less likely than white patients to receive a kidney transplant (1.5 versus 3.8%; P < 0.01); excluding or censoring those patients did not alter the results. To address further the potential of confounding by indication, we examined the characteristics of patients who were not treated with activated vitamin D during the follow-up period according to race/ethnicity. There was no significant racial difference in the frequency of hospitalization during the 1 yr of follow up (white 1.6 ± 1.9 [median one admission]; black 1.5 ± 1.6 [median one admission]). Although untreated black patients were at a significantly higher risk for mortality compared with white patients, their favorable baseline characteristics, including younger age and fewer comorbidities, suggested that they would have demonstrated lower risk (Table 6). Black patients were 20% (95% CI 11 to 29%) more likely than white patients to be treated at facilities with the highest (upper quartile) standardized mortality rates (SMR). Nevertheless, even after exclusion of these patients, untreated black patients demonstrated higher risk for death compared with untreated white patients (HR 1.51; 95% CI 1.15 to 1.97). Finally, in another sensitivity analysis in which all patients were censored at the time activated vitamin D therapy was begun, black patients remained at a statistically significant higher risk for death compared with white patients (HR 1.22; 95% CI 1.07 to 1.39).
Table 6.
Characteristics of patients who were never treated with activated vitamin D according to race/ethnicity
| Characteristic | White (n = 1543) | Hispanic (n = 234) | Black (n = 393) |
|---|---|---|---|
| Age (yr; mean ± SD) | 67.0 ± 14.9 | 62.9 ± 15.8a | 60.3 ± 16.4a |
| Diabetes as assigned cause of renal failure (%) | 39 | 65a | 37 |
| Systolic BP (mmHg; mean ± SD) | 139 ± 24 | 142 ± 20 | 145 ± 24a |
| Diastolic BP (mmHg; mean ± SD) | 70 ± 13 | 73 ± 11a | 76 ± 14 a,b |
| BMI (kg/m2; mean ± SD) | 26.6 ± 6.7 | 26.2 ± 6.3 | 26.7 ± 8.0 |
| Access at the initiation of hemodialysis (%) | |||
| arteriovenous fistula | 19 | 21 | 13 |
| venovenous catheter | 69 | 68 | 72 |
| Comorbid conditions (%) | |||
| coronary artery disease/myocardial infarction | 14 | 6a | 7a |
| congestive heart failure | 16 | 11a | 12a |
| peripheral vascular disease | 6 | 3a | 4a |
| stroke | 5 | 1a | 4b |
| hyperlipidemia | 12 | 7a | 5 a,b |
| malignancy | 5 | 1a | 3a |
| chronic obstructive pulmonary disease | 4 | 1a | 1a |
| Baseline laboratory test results | |||
| albumin (g/dl; mean ± SD) | 3.4 ± 0.5 | 3.3 ± 0.6a | 3.3 ± 0.6a |
| creatinine (mg/dl; mean ± SD) | 5.4 ± 2.5 | 5.8 ± 2.4a | 6.2 ± 3.0a |
| calcium (mg/dl; mean ± SD) | 8.7 ± 0.8 | 8.5 ± 0.9a | 8.6 ± 0.9 |
| phosphorus (mg/dl; mean ± SD) | 4.5 ± 1.6 | 4.5 ± 1.7 | 4.5 ± 1.7 |
| PTH (biointact, pg/ml; median [IQR])c | 91 (50 to 139) | 89 (59 to 139) | 102 (53 to 166) |
| alkaline phosphatase (U/L; mean ± SD) | 97 ± 71 | 94 ± 43 | 109 ± 107a |
| hemoglobin (g/L; mean ± SD) | 10.4 ± 1.3 | 10.4 ± 1.4 | 10.0 ± 1.4 a,b |
| urea reduction ratio (%; mean ± SD) | 68 ± 11 | 70 ± 11 | 68 ± 11 |
Significant differences (P < 0.05) versus white.
Significant differences (P < 0.05) between black and Hispanic.
Reference range on hemodialysis is 75 to 150 pg/ml.53
DISCUSSION
Upon initiating hemodialysis, black and Hispanic patients had significantly higher PTH levels than white patients and were treated more often and with higher dosages of activated vitamin D, which, in turn, was independently associated with improved survival as in previous observational studies.25–29 Thus, although black and Hispanic patients with kidney disease are at greater risk for developing more severe secondary hyperparathyroidism,34 this disadvantage seems to translate into a potential advantage once dialysis is initiated. This hypothesis is supported by the observation that among patients who were not treated with activated vitamin D, black patients had significantly worse outcomes than white patients, a finding that remained robust to a variety of sensitivity analyses. We believe this is the first report of a population of black dialysis patients who demonstrated significantly worse survival compared with their white counterparts, an observation that mirrors the myriad of clinical situations outside dialysis.
Research of racial and ethnic disparities in health care prominently describe how minorities are less likely to receive specific diagnostic and therapeutic procedures—and thus experience poorer health outcomes—when compared with white individuals, even when controlling for differences in insurance and socioeconomic status. For example, black individuals are referred less frequently than white individuals for cardiac catheterization, coronary artery bypass grafting, renal transplantation, and curative surgical treatment of lung cancer, among others.36–42 To date, most disparities research has focused on differences in the provision of discretionary, often highly skilled, state-of-the-art treatments that are measures of quality of care but do not necessarily have a biologic basis. By limiting the effects of socioeconomic inequity on health care delivery, dialysis represents a unique setting to examine potential biologic bases for disparities in outcomes that could suggest novel strategies to improve minority outcomes outside dialysis. Indeed, the results of this study highlight a clinical situation in which a racial disparity in health outcomes may be explained by biologic mechanisms and in which a significant disparity reverses after accounting for a single common intervention. More important, these results may have important implications for the general population outside dialysis in whom vitamin D deficiency is widespread but especially common and severe among minorities.43
The worldwide epidemic of vitamin D deficiency has led to a resurgence of rickets with its catastrophic musculoskeletal complications44–47; however, given the ubiquitous tissue distribution of the vitamin D receptor, even mild to moderate vitamin D deficiency has been linked to a number of extraskeletal complications that could affect survival. For example, vitamin D deficiency has been linked to insulin resistance; diabetes; hypertension; congestive heart failure; stroke; prostate, colon, and breast cancers; multiple sclerosis; and infections such as tuberculosis.43 Importantly, many of these conditions are more common or more severe among minorities, groups who tend to be more severely vitamin D deficient.43 Furthermore, recent prospective studies of patients from the general population48 and incident dialysis patients49 demonstrated increased cardiovascular events and mortality associated with deficiencies of 25D and 1,25D. Our results should stimulate studies outside dialysis to test whether vitamin D deficiency may represent a modifiable, biologic risk factor that contributes to other health care disparities such as in cardiovascular disease and cancer, where racial differences are also present.50–52
Residual confounding is a potential limitation of all observational studies, and confounding by indication is a particular concern in studies involving therapeutic interventions such as activated vitamin D. We used several strategies to address this limitation, including multivariable analyses that adjusted for calcium, phosphate, PTH, and other characteristics; stratified analyses restricted to patients who were never treated; models that adjusted for individual patients' propensity of receiving treatment; and analyses that censored patients at the time treatment began. A significantly higher risk for death among untreated black patients was detected using each of these approaches. The stratified analyses are especially noteworthy because untreated black patients had otherwise favorable baseline characteristics compared with white patients. Whereas adjusting for baseline characteristics in the overall population partially attenuated the survival benefit among black patients, these factors acted as negative confounders in the analyses of untreated patients in which the significantly increased univariate risk among black patients was magnified with multivariable adjustment. It is interesting that a significant survival benefit persisted in black and Hispanic patients in the stratum that was treated with activated vitamin D. Although this could be due to additional, unknown factors, adjusting for the higher dosage of activated vitamin D administered to black patients essentially eliminated the difference in age- and gender-adjusted models (HR 1.04). This contrasts with most previous studies in which adjustment for a variety of factors diminished the statistical significance between black and white patients but did not completely eliminate the trend toward improved survival among black patients,16–24 lending further support to the hypothesis that differential vitamin D use at least partially explains the racial difference in hemodialysis survival.
Only a randomized, controlled trial could provide definitive evidence of a benefit for treating all vitamin D–deficient patients or those of specific races and ethnicities. Given the known complications of vitamin D deficiency, however, performing such a trial may be a challenge. ESRD would otherwise be the ideal setting for such a trial because of the high “event” rates, the pervasiveness and severity of vitamin D deficiency,49 and the lack of consensus on how best to manage it. However, current national practice guidelines for dialysis advocate vitamin D therapy only for certain PTH levels,53 and the widespread reliance on these guidelines may preclude the possibility of randomly assigning dialysis patients with intact PTH >300 pg/ml to placebo just as fears of adynamic bone disease may limit the feasibility of randomly assigning patients with intact PTH <150 to therapy. Thus, as poignantly highlighted by Himmelfarb,54 opinion-based practice guidelines may actually hinder the development of critically needed randomized trials. Despite these difficulties, our results support the urgent need for randomized studies to determine whether treatment differences based on biologic differences, such as in the vitamin D axis, may contribute to disparities in health outcomes in a variety of clinical settings, including the various stages of chronic kidney disease.
CONCISE METHODS
Accelerated Mortality on Renal Replacement (ArMORR) is a nationally representative prospective cohort study of patients who initiated long-term hemodialysis at US dialysis centers operated by Fresenius Medical Care, North America (FMC, Lexington, MA). Information collected prospectively included patient demographics, comorbidities at the initiation of hemodialysis, laboratory tests, intravenous therapies, and clinical outcomes. Data were entered into a central database by physicians and nurses at the point of care, with rigorous quality assurance/quality control auditing mandated by FMC.26,27 Routine laboratory tests were performed by Spectra East (Rockland, NJ). This study was approved by the institutional review board of the Massachusetts General Hospital, which waived the need for informed consent.
Study Population
Between July 1, 2004, and June 30, 2005, 10,044 incident hemodialysis patients representing 1056 US dialysis units were prospectively enrolled in ArMORR. All incident hemodialysis patients who initiated therapy at a US-based FMC unit were eligible for inclusion in the overall ArMORR cohort. The self-identified racial composition of the cohort was 6115 white and 3235 black; 694 patients were of “other” races and were excluded from this study. The 47 patients (26 white, 21 black) who enrolled in ArMORR after having already spent >30 d on hemodialysis as inpatients were also excluded. Among 1007 patients who reported Hispanic ethnicity, 979 described themselves as white and 28 as black. For this study, the 28 black Hispanic patients were included in the black group, and white Hispanic patients were analyzed separately (hereafter called Hispanic) from non-Hispanic white patients (hereafter called white). The final study population included 9303 patients: 5110 white (55%), 979 Hispanic (11%), and 3214 black (35%). Given the requirement of dialysis providers to record detailed race/ethnicity data for reporting purposes, the potential for misclassification of race and ethnicity was likely small.
Exposures, Outcomes, and Covariates
The primary exposure was race/ethnicity, and the primary outcome was all-cause mortality within the first year after initiating long-term hemodialysis. Death was confirmed by discharge diagnosis reports from the individual dialysis centers. The primary covariate of interest was treatment with intravenous activated vitamin D analyzed as a time-dependent covariate given differential start times. We analyzed mean dosage of activated vitamin D therapy by expressing it in calcitriol equivalent units as mean paricalcitol dosage/455 and mean doxercalciferol dosage/256 calculated from the average dosage over each calendar quarter standardized to the total number of calendar quarters of follow-up. We did not examine the effect of specific vitamin D compounds on outcomes because of limited power for these analyses. Other covariates included age, gender, assigned cause of renal failure (diabetes, hypertension, glomerulonephritis, polycystic kidney disease, or other), BP, body mass index, vascular access at initiation (arteriovenous fistula, graft, or venovenous catheter), urea reduction ratio, facility-specific SMR,26 and comorbidities (coronary artery disease/myocardial infarction, congestive heart failure, peripheral vascular disease, stroke, hyperlipidemia, noncutaneous malignancy, and chronic obstructive pulmonary disease). Comorbidities were ascertained at the initiation of dialysis by the individual patients' practitioners and derived from the initial intake history, physical examination, and medical chart review performed by the dialysis centers. These results differ somewhat from comorbidity rates reported by the US Renal Data System because the latter are ascertained from data collected approximately 90 d after initiation of dialysis and are supplemented by hospital diagnostic codes reported to Medicare but not available to FMC. We analyzed baseline blood levels of albumin, creatinine, calcium, phosphorus, PTH, alkaline phosphatase, hemoglobin, potassium, and bicarbonate. PTH was measured using the Nichols Bio-intact PTH assay that detects amino acids 1 to 84 (target range on hemodialysis 75 to 150 pg/ml53). RIA (DiaSorin, Stillwater, MN) measurements of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels in baseline samples before any therapy with activated vitamin D were available in a nested subset of consecutive patients from a previous study.49
Statistical Analysis
We used one-way ANOVA, Kruskal-Wallis, and χ2 tests to compare demographics, laboratory tests, crude mortality rates, and vitamin D use among the three race/ethnicity groups. When overall significant differences were detected, pairwise differences were tested with the Sidak adjustment for multiple comparisons. Because PTH levels guide activated vitamin D treatment,53 we examined the frequency of treatment across deciles of baseline PTH levels within each race/ethnicity group.
We used Kaplan-Meier curves with log-rank tests to examine survival after initiation of hemodialysis by race/ethnicity groups. Patients were censored when they discontinued dialysis as a result of recovery of renal function (4.4%) or kidney transplantation (2.9%) or were lost to follow-up because they transferred their care to a non-FMC center (12.4%); there were no differences in transfer rates according to race/ethnicity. We used multivariable Cox models to adjust for potential confounding after ensuring that the proportional hazards assumption was not violated. We included covariates in the multivariable models that have been associated with mortality on dialysis in previous studies and those that were significantly different among the race/ethnicity groups in this study. For the multivariable analyses, variables with missing data points were analyzed as categorical predictors with an additional category for missing (<8% missing for any covariate); missing data points were not imputed. Otherwise, continuous variables were analyzed on a continuous scale. We first assessed survival by race/ethnicity without considering activated vitamin D therapy. Next, we formally tested the interaction between race/ethnicity and activated vitamin D therapy where vitamin D therapy was treated as a time-dependent covariate. When significant interaction was detected (P < 0.05) in univariate and multivariable-adjusted analyses, we examined results from models stratified by activated vitamin D therapy (ever versus never treated) and models that incorporated race/ethnicity × activated vitamin D therapy groups. We also tested but did not find an interaction between race/ethnicity and iron and erythropoietin therapies and other clinical factors that may have been associated with dialysis mortality.
We used several approaches to address confounding by indication. We calculated a propensity score of the likelihood of receiving activated vitamin D and adjusted for it in multivariable models. We compared the baseline characteristics and frequency of hospitalization among untreated patients by race/ethnicity. To assess the impact of overall quality of care, we examined models stratified by facility-specific SMR. Finally, we performed an additional survival analysis of all patients who had any untreated period with censoring at the time activated vitamin D was initiated. Analyses were performed using Intercooled Stata 7.0 (Stata Corp., College Station, TX) and two-sided P < 0.05 was considered statistically significant.
DISCLOSURES
M.W. has received research support from Shire and honoraria from Abbott Laboratories, Genzyme, and INEOS; K.N. is an advisor for Abbott Laboratories, Roche, Amgen, and King Pharmaceuticals; and R.T. has received research support from Abbott Laboratories and has received honoraria from Abbott Laboratories and Genzyme.
Acknowledgments
This study was supported by a grant from the American Society of Nephrology-Alaska Kidney Foundation (M.W.); the Center for D-receptor Activation Research (CeDAR) at the Massachusetts General Hospital (www.mghcedar.org); and grants RR017376 (M.W.), DK076116 (M.W.), DK078774 (M.M.), and DK071674 (R.T.) from the National Institutes of Health.
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Cowie CC, Port FK, Wolfe RA, Savage PJ, Moll PP, Hawthorne VM: Disparities in incidence of diabetic end-stage renal disease according to race and type of diabetes. N Engl J Med 321: 1074–1079, 1989 [DOI] [PubMed] [Google Scholar]
- 2.Agodoa L, Norris K, Pugsley D: The disproportionate burden of kidney disease in those who can least afford it. Kidney Int Suppl S1–S3, 2005 [DOI] [PubMed]
- 3.Hsu CY, Lin F, Vittinghoff E, Shlipak MG: Racial differences in the progression from chronic renal insufficiency to end-stage renal disease in the United States. J Am Soc Nephrol 14: 2902–2907, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Xue JL, Eggers PW, Agodoa LY, Foley RN, Collins AJ: Longitudinal study of racial and ethnic differences in developing end-stage renal disease among aged Medicare beneficiaries. J Am Soc Nephrol 18: 1299–1306, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Kalantar-Zadeh K, Kovesdy CP, Derose SF, Horwich TB, Fonarow GC: Racial and survival paradoxes in chronic kidney disease. Nat Clin Pract Nephrol 3: 493–506, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Nelson A: Unequal treatment: Confronting racial and ethnic disparities in health care. J Natl Med Assoc 94: 666–668, 2002 [PMC free article] [PubMed] [Google Scholar]
- 7.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Young CJ, Gaston RS: Renal transplantation in black Americans. N Engl J Med 343: 1545–1552, 2000 [DOI] [PubMed] [Google Scholar]
- 9.US Renal Data System: USRDS 2003 Annual Data Report, Bethesda, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2004
- 10.Agodoa L: Lessons from chronic renal diseases in African Americans: Treatment implications. Ethn Dis 13: S118–S124, 2003 [PubMed] [Google Scholar]
- 11.Kinchen KS, Sadler J, Fink N, Brookmeyer R, Klag MJ, Levey AS, Powe NR: The timing of specialist evaluation in chronic kidney disease and mortality. Ann Intern Med 137: 479–486, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Stamler J: End-stage renal disease in African-American and white men: 16-year MRFIT findings. JAMA 277: 1293–1298, 1997 [PubMed] [Google Scholar]
- 13.Daumit GL, Hermann JA, Coresh J, Powe NR: Use of cardiovascular procedures among black persons and white persons: A 7-year nationwide study in patients with renal disease. Ann Intern Med 130: 173–182, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Young BA, Rudser K, Kestenbaum B, Seliger SL, Andress D, Boyko EJ: Racial and ethnic differences in incident myocardial infarction in end-stage renal disease patients: The USRDS. Kidney Int 69: 1691–1698, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Eggers PW, Connerton R, McMullan M: The Medicare experience with end-stage renal disease: Trends in incidence, prevalence, and survival. Health Care Financ Rev 5: 69–88, 1984 [PMC free article] [PubMed] [Google Scholar]
- 16.Bloembergen WE, Port FK, Mauger EA, Wolfe RA: Causes of death in dialysis patients: Racial and gender differences. J Am Soc Nephrol 5: 1231–1242, 1994 [DOI] [PubMed] [Google Scholar]
- 17.Cowie CC, Port FK, Rust KF, Harris MI: Differences in survival between black and white patients with diabetic end-stage renal disease. Diabetes Care 17: 681–687, 1994 [DOI] [PubMed] [Google Scholar]
- 18.Frankenfield DL, Rocco MV, Roman SH, McClellan WM: Survival advantage for adult Hispanic hemodialysis patients? Findings from the end-stage renal disease clinical performance measures project. J Am Soc Nephrol 14: 180–186, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Morris D, Samore MH, Pappas LM, Ramkumar N, Beddhu S: Nutrition and racial differences in cardiovascular events and survival in elderly dialysis patients. Am J Med 118: 671–675, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Owen WF Jr, Chertow GM, Lazarus JM, Lowrie EG: Dose of hemodialysis and survival: differences by race and sex. JAMA 280: 1764–1768, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Pugh JA, Tuley MR, Basu S: Survival among Mexican-Americans, non-Hispanic whites, and African-Americans with end-stage renal disease: The emergence of a minority pattern of increased incidence and prolonged survival. Am J Kidney Dis 23: 803–807, 1994 [DOI] [PubMed] [Google Scholar]
- 22.Robinson BM, Joffe MM, Pisoni RL, Port FK, Feldman HI: Revisiting survival differences by race and ethnicity among hemodialysis patients: The Dialysis Outcomes and Practice Patterns Study. J Am Soc Nephrol 17: 2910–2918, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Bleyer AJ, Tell GS, Evans GW, Ettinger WH Jr, Burkart JM: Survival of patients undergoing renal replacement therapy in one center with special emphasis on racial differences. Am J Kidney Dis 28: 72–81, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Murthy BV, Molony DA, Stack AG: Survival advantage of Hispanic patients initiating dialysis in the United States is modified by race. J Am Soc Nephrol 16: 782–790, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Kalantar-Zadeh K, Kuwae N, Regidor DL, Kovesdy CP, Kilpatrick RD, Shinaberger CS, McAllister CJ, Budoff MJ, Salusky IB, Kopple JD: Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int 70: 771–780, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Teng M, Wolf M, Lowrie E, Ofsthun N, Lazarus JM, Thadhani R: Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med 349: 446–456, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Teng M, Wolf M, Ofsthun MN, Lazarus JM, Hernán MA, Camargo CA Jr, Thadhani R: Activated injectable vitamin D and hemodialysis survival: A historical cohort study. J Am Soc Nephrol 16: 1115–1125, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Melamed ML, Eustace JA, Plantinga L, Jaar BG, Fink NE, Coresh J, Klag MJ, Powe NR: Changes in serum calcium, phosphate, and PTH and the risk of death in incident dialysis patients: A longitudinal study. Kidney Int 70: 351–357, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Tentori F, Hunt WC, Stidley CA, Rohrscheib MR, Bedrick EJ, Meyer KB, Johnson HK, Zager PG; Medical Directors of Dialysis Clinic Inc.: Mortality risk among hemodialysis patients receiving different vitamin D analogs. Kidney Int 70: 1858–1865, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Bell NH, Greene A, Epstein S, Oexmann MJ, Shaw S, Shary J: Evidence for alteration of the vitamin D-endocrine system in blacks. J Clin Invest 76: 470–473, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dawson-Hughes B: Racial/ethnic considerations in making recommendations for vitamin D for adult and elderly men and women. Am J Clin Nutr 80: 1763S–1766S, 2004 [DOI] [PubMed] [Google Scholar]
- 32.De Boer IH, Gorodetskaya I, Young B, Hsu CY, Chertow GM: The severity of secondary hyperparathyroidism in chronic renal insufficiency is GFR-dependent, race-dependent, and associated with cardiovascular disease. J Am Soc Nephrol 13: 2762–2769, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Harris SS, Soteriades E, Coolidge JA, Mudgal S, Dawson-Hughes B: Vitamin D insufficiency and hyperparathyroidism in a low income, multiracial, elderly population. J Clin Endocrinol Metab 85: 4125–4130, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Martins D, Wolf M, Pan D, Zadshir A, Tareen N, Thadhani R, Felsenfeld A, Levine B, Mehrotra R, Norris K: Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med 167: 1159–1165, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Gupta A, Kallenbach LR, Zasuwa G, Divine GW: Race is a major determinant of secondary hyperparathyroidism in uremic patients. J Am Soc Nephrol 11: 330–334, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Ayanian JZ, Epstein AM: Differences in the use of procedures between women and men hospitalized for coronary heart disease. N Engl J Med 325: 221–225, 1991 [DOI] [PubMed] [Google Scholar]
- 37.Harris DR, Andrews R, Elixhauser A: Racial and gender differences in use of procedures for black and white hospitalized adults. Ethn Dis 7: 91–105, 1997 [PubMed] [Google Scholar]
- 38.Johnson PA, Lee TH, Cook EF, Rouan GW, Goldman L: Effect of race on the presentation and management of patients with acute chest pain. Ann Intern Med 118: 593–601, 1993 [DOI] [PubMed] [Google Scholar]
- 39.Peterson ED, Shaw LK, DeLong ER, Pryor DB, Califf RM, Mark DB: Racial variation in the use of coronary-revascularization procedures: Are the differences real? Do they matter? N Engl J Med 336: 480–486, 1997 [DOI] [PubMed] [Google Scholar]
- 40.Schulman KA, Berlin JA, Harless W, Kerner JF, Sistrunk S, Gersh BJ, Dubé R, Taleghani CK, Burke JE, Williams S, Eisenberg JM, Escarce JJ: The effect of race and sex on physicians' recommendations for cardiac catheterization [erratum appears in N Engl J Med 340: 1130, 1999]. N Engl J Med 340: 618–626, 1999 [DOI] [PubMed] [Google Scholar]
- 41.Ayanian JZ, Cleary PD, Weissman JS, Epstein AM: The effect of patients' preferences on racial differences in access to renal transplantation. N Engl J Med 341: 1661–1669, 1999 [DOI] [PubMed] [Google Scholar]
- 42.Bach PB, Cramer LD, Warren JL, Begg CB: Racial differences in the treatment of early-stage lung cancer. N Engl J Med 341: 1198–1205, 1999 [DOI] [PubMed] [Google Scholar]
- 43.Holick MF: Vitamin D deficiency. N Engl J Med 357: 266–281, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Holick MF: Resurrection of vitamin D deficiency and rickets. J Clin Invest 116: 2062–2072, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomas MK, Lloyd-Jones DM, Thadhani RI, Shaw AC, Deraska DJ, Kitch BT, Vamvakas EC, Dick IM, Prince RL, Finkelstein JS: Hypovitaminosis D in medical inpatients. N Engl J Med 338: 777–783, 1998 [DOI] [PubMed] [Google Scholar]
- 46.van der Wielen RP, Lowik MR, van den Berg H, de Groot LC, Haller J, Moreiras O, van Staveren WA: Serum vitamin D concentrations among elderly people in Europe. Lancet 346: 207–210, 1995 [DOI] [PubMed] [Google Scholar]
- 47.Wharton B, Bishop N: Rickets. Lancet 362: 1389–1400, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Wang T, Pencina M, Booth S, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D'Agostino RB, Wolf M, Vasan RS: Vitamin D deficiency and the risk of cardiovascular disease. Circulation 117: 503–511, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wolf M, Shah A, Gutierrez O, Ankers E, Monroy M, Tamez H, Steele D, Chang Y, Camargo CA Jr, Tonelli M, Thadhani R: Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int 72: 1004–1013, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Robbins AS, Whittemore AS, Thom DH: Differences in socioeconomic status and survival among white and black men with prostate cancer. Am J Epidemiol 151: 409–416, 2000 [DOI] [PubMed] [Google Scholar]
- 51.Howard G, Howard VJ: Ethnic disparities in stroke: The scope of the problem. Ethn Dis 11: 761–768, 2001 [PubMed] [Google Scholar]
- 52.Howard G, Anderson RT, Russell G, Howard VJ, Burke GL: Race, socioeconomic status, and cause-specific mortality. Ann Epidemiol 10: 214–223, 2000 [DOI] [PubMed] [Google Scholar]
- 53.K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42: S1–S201, 2003 [PubMed] [Google Scholar]
- 54.Himmelfarb J: Chronic kidney disease and the public health: Gaps in evidence from interventional trials. JAMA 297: 2630–2633, 2007 [DOI] [PubMed] [Google Scholar]
- 55.Sprague SM, Llach F, Amdahl M, Taccetta C, Batlle D: Paricalcitol versus calcitriol in the treatment of secondary hyperparathyroidism. Kidney Int 63: 1483–1490, 2003 [DOI] [PubMed] [Google Scholar]
- 56.Zisman AL, Ghantous W, Schinleber P, Roberts L, Sprague SM: Inhibition of parathyroid hormone: A dose equivalency study of paricalcitol and doxercalciferol. Am J Nephrol 25: 591–595, 2005 [DOI] [PubMed] [Google Scholar]