Abstract
Racial differences in potassium (K) intake and urinary K excretion may contribute to the higher BP observed in black compared with white individuals. Although black individuals typically consume less dietary K than white individuals, the lower urinary K excretion observed in black individuals may reflect more than differences in intake. In this study, data from the Dietary Approaches to Stop Hypertension (DASH) trial (413 white and black participants) were used to evaluate urinary K excretion in black and white individuals with similar K intake. At screening, mean urinary K excretion was higher in white than black individuals (mean Δ = 645 mg/d for white minus black individuals, adjusted for age, gender, and weight; P < 0.001). After a 3-wk run-in period during which all participants received a low-K control diet, a significant racial difference remained (mean Δ = 201 mg/d, adjusted for age, gender, and caloric intake; P < 0.001). Participants were then randomly assigned to continue the control diet or switch to a high-K diet (either a high fruit/vegetable diet or the DASH diet) for 8 wk. At the end of intervention, the mean difference in urinary K in white compared with black individuals after adjustment for age, gender, and caloric intake was −6 mg/d (P = 0.95) in the control group, 163 mg/d in the fruits/vegetables group (P = 0.39), and 903 mg/d in the DASH group (P < 0.001). Racial differences in urinary K excretion seem to reflect more than intake differences; further studies are needed to understand their potential impact on clinical outcomes.
Potassium (K) intake has been increasingly recognized as an important determinant of BP. Hypertension, one of the most important and modifiable risk factors for the development of chronic kidney disease, cardiovascular disease, and stroke, is more common in black than in white individuals. Multiple studies have shown that increasing K intake (by diet or by supplements) can decrease BP;1–11 the extent of BP reduction from K seems to be greater in black than in white individuals1,3,9 and in those with lower urinary K excretion.2
Black individuals in the United States typically consume less dietary K than do white individuals.1,12–14 The estimated mean daily US intake from 1988 through 1994 for adults 19 to 70 yr of age on the basis of 24-h dietary recalls from the Third National Health and Nutrition Examination Survey (NHANES III) was 3.5 g for white men, 2.8 g for black men, 2.5 g for white women, and 2.1 g for black women.1 In many populations throughout the world, black individuals excrete less urinary K than do white individuals.1,3,14–24 Because urinary K excretion largely reflects K intake, the lower urinary K excretion in black individuals has often been attributed to racial dietary differences; however, racial differences in urinary K excretion may not simply reflect intake differences. Small studies (14 to 59 participants) have documented that urinary K excretion was lower in black than in white individuals at baseline and after fixed-dosage K supplementation.3,16–19 These studies were small and were mostly of short duration (ranging from 4 d to 10 wk). Furthermore, all except one study17 did not control baseline diet. In the absence of controlled feeding, black–white differences in K excretion are difficult to interpret.
We used data from the Dietary Approaches to Stop Hypertension (DASH) randomized, controlled feeding trial2 to determine whether urinary K excretion in black individuals is lower than in white individuals at baseline and after controlled feeding on three diets (a “typical” American diet that has low K content and two diets rich in fruits and vegetables and, therefore, high in K).
RESULTS
Baseline Characteristics
Baseline characteristics are shown in Table 1. By design, there were more black participants (64%) than white participants (36%). With few exceptions, baseline characteristics were similar in black and white participants across randomized dietary treatment groups (control, fruits/vegetables [F/V], and DASH). Of the black participants, 58% were women, whereas 30% of the white participants were women. Systolic and diastolic BP were similar in black and white participants in all treatment groups, likely as a result of the common eligibility criteria. Body weight was similar between white and black women in all diet groups. White men assigned to the F/V group had a lower baseline mean weight (81 kg) than black men in the F/V group (92 kg; P = 0.002). Screening serum K values were available only on a subgroup of participants (n = 36) from the Baltimore center and were similar in white (4.3 ± 0.4 mEq/L) and black participants (4.3 ± 0.3 mEq/L; P = 0.87).
Table 1.
Baseline characteristics
| Characteristic | White (n = 156) | Black (n = 257) | P |
|---|---|---|---|
| Female gender (%) | 33 | 60 | <0.01 |
| Age (yr; mean ± SD) | 46 ± 11 | 44 ± 10 | 0.06 |
| Weight (kg; mean ± SD) | 82 ± 15 | 83 ± 14 | 0.57 |
| Systolic BP (mmHg; mean ± SD) | 131 ± 12 | 131 ± 12 | 0.57 |
| Diastolic BP (mmHg; mean ± SD) | 84 ± 6 | 85 ± 6 | 0.16 |
| Urine Na (mg/d; mean ± SD) | 3843 ± 1623 | 3571 ± 1667 | 0.12 |
Adherence
Objective data (urinary sodium [Na] excretion) and self-reports of dietary intake strongly suggested that black and white participants were similarly compliant with the diets. Twenty-four-hour urine Na and urine creatinine values also suggested similar compliance with urine collection. At the end of run-in, urinary Na excretion was 3230 ± 1055 mg/d in white participants and 3099 ± 1110 mg/d in black participants (after adjustment for age, gender, and caloric intake, the mean difference in white minus black participants was −23 mg/d; P = 0.8). Adjusted 24-h urinary Na excretion was also similar between black and white participants on each diet at the end of intervention (P > 0.24 for each dietary group). Furthermore, as seen in Table 2, the estimated percentage of dietary Na excreted in the urine was similar between black and white participants in each group. Using data derived from daily self-reports and from observations by staff of foods eaten on-site, compliance scores were calculated.25 There was no significant difference in mean compliance scores between black and white participants in any of the groups. Only two participants had urinary creatinine values with a coefficient of variation >80% across the three time points. Two participants had urinary Na values with a coefficient of variation >80% across the two time points of end of run-in and end of intervention (when participants were given a fixed Na intake).
Table 2.
Percentage of dietary K and Na excreted in urine (urinary excretion (mg/day)/dietary intake (mg/day)) * 100
| Visit | Potassium (%; mean ± SD)
|
Sodium (%; mean ± SD)
|
||||
|---|---|---|---|---|---|---|
| White | Black | P | White | Black | P | |
| End of run-in | 74 ± 25 | 67 ± 24 | 0.007 | 91 ± 28 | 92 ± 32 | 0.7 |
| End of intervention | ||||||
| Control diet | 73 ± 27 | 74 ± 24 | 0.838 | 88 ± 34 | 91 ± 31 | 0.6 |
| F/V diet | 60 ± 21 | 53 ± 19 | 0.045 | 83 ± 29 | 88 ± 38 | 0.4 |
| DASH diet | 69 ± 17 | 50 ± 18 | <0.001 | 90 ± 28 | 92 ± 46 | 0.9 |
Effects of Diet on Urinary K Excretion
Screening
As seen in Figure 1, urinary K excretion at screening (baseline) was higher in white (2516 ± 929 mg/d) than in black participants (1791 ± 705 mg/d). After adjustment for age, gender, and weight, the mean difference in urinary K excretion between white and black participants was 645 mg/d (95% confidence interval [CI] 472 to 818; P < 0.001).
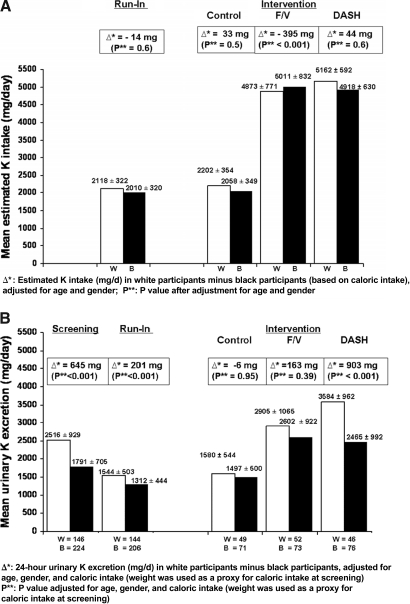
Figure 1.
(A) Estimated K intake (mg/d ± SD) based on estimated caloric intake in white (W; □) and black (B; ▪) participants at the end of run-in and at the end of intervention for each of the three diets. (B) Urinary K excretion (mg/d ± SD) in white (□) and black (▪) participants at baseline (screening), at the end of run-in, and at the end of intervention for each of the three diets.
End of Run-in
Figure 1 also shows that at the end of run-in (when all participants were given the same diet for 3 wk), urinary K excretion was still significantly higher in white (1544 ± 503 mg/d) than in black participants (1312 ± 444 mg/d). After adjustment for age, gender, and caloric intake, the difference in urinary K excretion between white and black participants was 201 mg/d (95% CI 96 to 306; P < 0.001). The mean estimated K intake was 2118 ± 322 mg/d in white participants and 2010 ± 320 mg/d in black participants (after adjustment for age and gender, the mean difference in white minus black participants was −14 mg/d; adjusted P = 0.6). As seen in Table 2, the mean percentage of dietary K excreted in the urine at the end of run-in was 74 ± 25% by white participants and 67 ± 24% by black participants (P = 0.007).
End of Intervention
A significant interaction was found (P < 0.001) between race and diet, suggesting that diet modifies the relationship between race and urinary K excretion. As displayed in Figure 1, at the end of intervention, the estimated K intake in the group fed the DASH diet was 5162 ± 592 mg/d for white participants and 4918 ± 630 mg/d for black participants (after adjustment for age and gender, the mean difference in white minus black participants was 44 mg/d; adjusted P = 0.6). There was a statistically significant difference in urinary K excretion between white (3584 ± 962 mg/d) and black (2465 ± 992 mg/d) participants who were randomly assigned to the DASH diet (adjusted [for age, gender, and caloric intake] difference 903 mg/d; 95% CI 543 to 1264; P < 0.001). This difference was mostly due to the large increase in urinary K excretion in white participants on the DASH diet, which greatly exceeded that of white participants who were fed the F/V diet. Furthermore, as seen in Table 2, white participants on the DASH diet excreted an estimated 69 ± 17% of dietary K in the urine, whereas black participants on the DASH diet excreted approximately 50 ± 18% (P < 0.001).
There was a trend toward a difference in urinary K excretion between white and black participants in the F/V group (2905 ± 1065 mg/d in white participants and 2602 ± 922 mg/d in black participants); the adjusted mean difference in urinary K excretion was 163 mg/d (95% CI −208 to 534; P = 0.39). The estimated percentage of dietary K excreted in the urine was statistically significantly higher in white (60 ± 21%) than in black participants (53 ± 19%; P = 0.045).
For participants who continued on the control (low K) diet for 8 wk, the urinary K excretion was 1580 ± 544 mg/d in white participants and 1497 ± 500 mg/d in black participants. After adjustment for age, gender, and caloric intake, the difference between white and black participants was −6 mg/d (95% CI −197 to 185; P = 0.95). As shown in Table 2, white and black participants excreted (via the urine) a similar fraction of the estimated K intake (73 ± 27 and 74 ± 24%, respectively; P = 0.8). A significant black–white difference in urinary K excretion was seen at the end of run-in when all participants (n = 350) ate the control diet, yet no significant difference was seen at the end of intervention feeding among those who were randomly assigned to the control diet (n = 120).
Sensitivity Analyses
These results are robust in sensitivity analyses, conducted by excluding outliers (of urinary K excretion, urinary Na excretion, urinary creatinine, and urine volume) as well as urine K values from participants with highly variable within-person urinary creatinine or urinary Na excretion. Although there was no evidence of differential noncompliance of diet (based on compliance scores and urinary Na) or of urine collections (based on urinary Na and urinary creatinine values) by race, we adjusted for compliance score and found similar results.
DISCUSSION
In this randomized, controlled feeding study conducted in prehypertensive and hypertensive adults, urinary K excretion was lower in black than in white participants when both groups were fed the same diets. Furthermore, there was a significant diet–race interaction, indicating that diet modified the racial difference in urinary K excretion. Urinary K excretion was significantly lower in black than in white participants at screening, when all participants ate their own diets. At the end of run-in (all participants were fed the low-K control diet for 3 wk), black participants continued to excrete less urinary K than did white participants, even after adjustment for age, gender, and caloric intake. At the end of intervention, after participants were fed one of three diets for 8 wk, urinary K excretion was significantly lower in black compared with white participants for those given the DASH diet. There was also a trend in participants who were fed the F/V diet. There was a statistically significantly higher percentage of dietary K excreted in the urine by white compared with black participants who were fed the F/V and DASH diets.
At the end of run-in, there was a significant racial difference in urinary K excretion; however, this difference was no longer statistically significant at the end of intervention in the participants who were randomly assigned to the control diet. One possibility is that this is due to the reduced sample size (one third of participants were randomly assigned to each dietary group); the minimal detectable difference at 90% power was 310 mg/d. Results were robust in sensitivity analyses that excluded outliers and influential data points.
The estimated percentage of dietary K that participants excreted into their urine ranged between 50 and 74% in black participants and 60 and 74% in white participants. The estimated percentage of dietary Na excreted in urine was not significantly different between black and white participants in each group, suggesting that the difference in K excretion was likely not from differential compliance with dietary intake or urine collections. Our data are consistent with data in the literature regarding variability in urinary Na and K excretion. Urinary Na excretion is believed to more closely reflect intake, with only small amounts excreted in the stool and sweat. Holbrook et al.26 demonstrated that the percentage of dietary K excreted in the urine was lower than the percentage of dietary Na excreted and that individual variation in the percentage of dietary K excreted in the urine was high. The reported range of the percentage of dietary K excreted in the urine, estimated from both self-report and feeding studies, is approximately 63 to 92% of intake.18,26,27 Differences in the fraction of dietary K excreted in the urine between black and white individuals have also been reported. In a study of adolescents and young adults, the estimated percentage of dietary K excreted in the urine was 76% in white individuals and 63% in black individuals on a free-living diet and 81% and 64%, respectively, in white and black participants who received K supplementation.18 The large variability in urinary K excretion and in estimated percentage of dietary K excreted in the urine has not been completely explained; it may be due to day-to-day individual variation or to biologic or methodologic differences.
If urinary K excretion is truly different between black and white individuals for a given K intake, then K excretion via other routes (stool, sweat, and insensible losses) might be higher in black than in white individuals. Racial differences in sweat K have not been well studied; a study of hand sweat of white and black individuals who walked in a desert did not reveal a racial difference in sweat K concentration, but the volume of hand sweat (which has a higher concentration of K than body sweat) was higher, not lower, in white than in black individuals.28 Black–white differences in stool excretion could possibly account for racial differences in urinary K excretion, but this has also not been well studied. In two small studies (one in South Africa and one in the United States), urinary and stool K excretion were both higher in white than in black individuals on uncontrolled diets, but the ratio of fecal to urinary K was higher in black than in white individuals.18,22 The exact mechanism of this is not understood. In our study, fecal and sweat K were not collected.
Alternatively or concomitantly, if black individuals were chronically more deplete in K than white individuals, then total body K would be expected to be higher in white than in black individuals, and urinary K might then be lower in black than in white individuals. Although this has not been well studied, a study of participants on free-living diets showed that total body K was higher in black than white participants until the ninth decade of life.29 Furthermore, although the intracellular fluid can absorb a K load, it is unlikely that the intracellular space would be able to buffer the entire difference in urinary K excretion between black and white individuals. Suh et al.30 suggested that there may be a slower rate of K “disposal” in black than in white individuals as a result of slower skeletal muscle uptake of K, not as a result of differences in baseline diet. Furthermore, there could be genetic differences in renal K handling that could explain racial differences in urinary K excretion. A potential model of increased Na-K-2 chloride (Cl) co-transport activity in the thick ascending limb of Henle to explain both increased salt sensitivity and decreased urinary K excretion in black compared with white individuals was proposed by Aviv et al.3 It was postulated that higher activity of Na-K-2Cl co-transport in black individuals leads to decreased plasma renin activity (either with or without extracellular fluid volume expansion), decreased urinary K excretion, increased Na conservation capacity, and increased glomerular capillary hydraulic pressure, leading to glomerular hyperfiltration and damage to the glomeruli, which could contribute to hypertension.
In our study, diet modified the effect of race on urinary K excretion. There are several possible explanations for this. Dietary factors, such as fiber, can affect K balance by increasing fecal K excretion31 and lowering urinary K excretion. This may explain the relatively low overall urinary K excretion in the DASH and F/V diets. The DASH diet also contains more calcium and protein, slightly more K, and less fat than the F/V diet. It is possible that the effects of fiber or other macronutrients are different in black and in white individuals, resulting in differential urinary K excretion; however, the DASH trial examined the effects of whole dietary patterns as opposed to variations in individual nutrients and dietary components. Hence, it is difficult to attribute differences in K handling to any single nutrient.
Strengths of this study include that it was a relatively large, controlled feeding trial of 11-wk duration. Because intake was closely controlled, we were able to estimate K and Na intake. Previous studies on urinary K supplementation in black versus white individuals were smaller and shorter in duration and did not, for the most part, control total dietary intake. In this study, great care was taken to promote adherence. Compliance scores were high and were similar in both groups, as were objective measures of compliance (e.g., urinary Na). There was no evidence of differential noncompliance. Furthermore, there was a significant improvement in BP in black participants on the DASH diet compared with the control diet, suggesting that these participants were compliant with the diet. Although serum K was not available for most participants, there are no data in the literature to suggest that there is a difference in baseline serum K between black and white individuals30; in addition, serum K does not accurately reflect total body K.
Limitations of the study include the small sample size in the randomly assigned groups, the lack of stool K and sweat K, and a single rather than multiple urine collections at each time point. Although adherence seemed high and similar in both black and white participants, this was an outpatient feeding study; hence, complete dietary compliance cannot be confirmed. Furthermore, this study included just individuals with prehypertension and stage 1 hypertension; whether the results are generalizable to those with normal BP or with more severe hypertension is unknown.
In conclusion, this study documented that the relatively low urine K excretion observed in black compared with white individuals reflects more than just black–white differences in diet. Furthermore, racial differences in urinary K excretion vary by diet. Specifically, in participants on the DASH diet, these differences were quite marked. Additional studies are needed to understand the mechanisms and potential implications of these differences.
CONCISE METHODS
Study Design
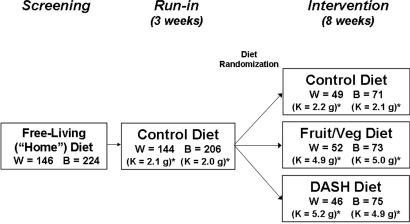
The study design of the DASH trial, a multicenter, randomized, controlled feeding study, has been described in detail elsewhere2,32 and is shown in Figure 2. The goal of the DASH trial was to compare the effects of three diets on BP. The study was approved by institutional review boards at each clinical site. All participants signed informed consent.
Figure 2.
DASH study design. There was a screening period, followed by a 3-wk run-in period. Participants were then randomly assigned to one of three diets for 8 wk. Na content (approximately 3 g/d at the 2100-kcal/d level) and body weight were kept constant. *K content is reported as mean estimated K intake (g) as based on estimated caloric intake. W, white participants; B, black participants.
Participants were 459 adults who were ≥22 yr of age, had a screening systolic BP <160 mmHg and a diastolic BP of 80 to 95 mmHg, and were not on any antihypertensive medications. Screening BP was defined as the average of six random-zero measurements taken during three visits before run-in. The study was designed to include two- thirds minority participants because of the higher prevalence of hypertension in minority populations, particularly black individuals in the United States. Major exclusion criteria were poorly controlled diabetes, hyperlipidemia, a cardiovascular event within the previous 6 mo, renal insufficiency (estimated GFR <60 ml/min per 1.73m2 or serum creatinine >1.5 mg/dl for men and >1.3 mg/dl for women), chronic disease(s) that might interfere with trial participation, pregnancy or lactation, body mass index >35 kg/m2, medications that affect BP (participants were free of antihypertensive medications for at least 2 wk before the first screening visit), alcoholic beverage intake of >14 drinks per week, and an unwillingness to stop taking all vitamin and mineral supplements and magnesium- or calcium-containing antacids.
There were three phases to the trial: Screening, run-in, and intervention. During screening, individuals continued their regular diets. Participants were given specific diets during the run-in and intervention periods. Twenty-four-hour urine samples were collected during screening, end of run-in, and at the end of intervention.
All participants were fed a control diet low in fruits, vegetables, and dairy and high in fat during a 3-wk run-in period. At the end of the run-in period, interested and eligible participants were randomly assigned to one of three diets for 8 more wk (intervention period). Randomization was performed by the coordinating center using computer-generated random allocation assignments, stratified by clinical site and cohort. Participants were randomly assigned to either (1) the control diet; (2) the F/V diet, rich in fruits and vegetables; or 3) the DASH diet, rich in fruits, vegetables, and low-fat dairy, modestly increased in protein, and low in fat. Details of the characteristics of the three diets are given by Karanja et al.33 At the 2100-kcal level of the diet, the control diet provided 1.7 g/d K, the F/V diet provided 4.1 g/d K, and the DASH diet provided 4.4 g/d K.
Because Na intake and weight changes are known to affect BP, Na intake and body weight were maintained at constant levels. Meals at four calorie levels (1600, 2100, 2600, 3100 kcal) were used for each diet and were identical at all of the centers. Within each diet, K and Na intake increased with increasing calorie levels. Na intake was 3 g/d at the 2100-kcal level for all three diets. Participants' weights were measured each weekday. To keep weight stable, energy adjustments were made when weight changed by >2% of baseline weight. Assigned caloric groups were changed when needed, and 100-kcal cookies or muffins (“unit” foods with nutrient contents that corresponded to those of the assigned diets) were added as indicated.
Each weekday, the participants were given lunch or dinner to be eaten at the research site. They were given coolers containing the food for the next 24 h to be eaten off-site. Weekend meals were given to the participants on Fridays and were also eaten off-site. Participants were instructed not to drink more than three servings of designated nonalcoholic beverages per day (containing ≤400 mg/d caffeine) or more than two servings of specific alcohol-containing beverages per day. Two packets of salt (each containing 0.2 g of Na) were given to participants daily for discretionary use. Participants were required to eat all of the study foods given to them and not to eat any other foods. During each day of controlled feeding, participants recorded in a daily diary whether they ate any nonstudy foods or whether they did not eat the required study foods. Adherence was actively promoted throughout the trial and was assessed by daily self-report, by direct observation by clinical staff during on-site meals, and by evaluation of 24-h urinary excretion of Na. Twenty-four-hour urine collections were again obtained at the end of intervention.
Statistical Analysis
Only participants who self-reported to be white or black were included in our analyses. Of the 413 white and black participants who were randomly assigned to one of the three diets, 24-h urinary K values were available for 370 of the participants (90% of white and black participants who were later randomized) at screening, 365 (88%) at run-in, and 380 (92%) at intervention. For the run-in and intervention groups, participants were included in the analyses only when, for that particular time period, they had values for urinary K and for adjustment variables (age, gender, and caloric intake); therefore, 350 participants were included in the run-in analyses, and 367 participants were included in the intervention period. None of the participants at screening were missing values for adjustment variables (age, gender, and body weight).
Statistical analyses were performed using Stata 9.0 (StataCorp, College Station, TX). Independent t-tests were used to evaluate baseline differences between black and white participants. Differences between urinary K excretion in black compared with white participants were tested at three points in the study: During screening, at the end of run-in, and separately by treatment group at the end of intervention. For testing for differences in K excretion in black compared with white participants, multivariate linear regression analyses were performed, adjusting for age, gender, and caloric intake. Because caloric intake was unavailable at screening, we adjusted for body weight as a proxy for caloric intake. Age and gender were adjusted for because of baseline differences in these variables between black and white participants and because these factors may affect urinary K excretion. Caloric intake was adjusted for during the run-in and intervention periods because caloric intake determined the intake of K. Because participants were fed one of three different diets during the intervention period, we tested for a diet–race interaction.
For the screening period, the sample size of 370 (with a ratio of white to black participants of 0.65) provided 90% power to detect a mean black-white difference in urinary K excretion of 302 mg/d. For the run-in period, the sample size of 365 provided 90% power to detect a mean difference in urinary K excretion of 166 mg/d between black and white participants. For the intervention period, the sample sizes provided 90% power to detect a mean black-white difference in urinary K excretion of 310 mg/d (control diet), 583 mg/d (F/V diet), and 667 mg/d (DASH diet).
The consistency of 24-h urinary creatinine excretion within each participant across visits was used as one measure of compliance with urine collection. Because Na intake was kept constant, urinary Na excretion was used as a measure of dietary (and urine collection) compliance. Sensitivity analyses were performed to assess the robustness of the results after the exclusion of outliers and of urine Na and urine creatinine values with a >80% coefficient of variation between visits (between the end of run-in and intervention for urine Na and among all three visits for urinary creatinine) and after adjustment for compliance scores. A coefficient of variation >80% was used on the basis of methods used in the INTERMAP study (Queenie Chan, MSc, Imperial College London, London, United Kingdom, personal communication, July 13, 2006). This sensitivity analysis was also repeated using a coefficient of variation threshold of >50% instead of >80%.
The fraction of dietary K that was excreted in the urine during run-in and intervention was calculated. Meals at four calorie levels, with a set K content for each calorie level, were used for each diet. As discussed, “unit” foods that matched the nutrient profile of the assigned experimental diets were given to the participants when the estimated caloric need did not match one of the four core energy levels. We estimated total caloric intake for the meals (including the “unit” foods) and generated a standard curve from which the level of K intake was estimated for each level of caloric intake. For determination of the percentage of dietary K that was excreted in the urine, the observed urinary K excretion was divided by the estimated K intake. This method was also used to predict the percentage of dietary Na excreted into the urine. P < 0.05 was considered statistically significant for all analyses.
DISCLOSURES
None.
Acknowledgments
This publication was made possible in part by Grant Number 1KL2RR025006-01 (S.T.; Johns Hopkins Clinical Research Scholars Program) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp.
This study was presented in part at the annual meeting of the American Society of Nephrology; November 14 through 19, 2006; San Diego, CA; and was published in part in abstract form (J Am Soc Nephrol 17: 99A, 2006).
Special thanks to the staff and participants of the DASH trial.
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Panel on Dietary Reference Intakes for Electrolytes and Water, Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Institutes of Medicine of the National Academies. Dietary References Intakes for Water, Potassium, Sodium, Chloride, and Sulfate. Washington, DC, The National Academies Press, 2004, pp 186–268
- 2.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N: A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med 336: 1117–1124, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Aviv A, Hollenberg NK, Weder A: Urinary potassium excretion and sodium sensitivity in blacks. Hypertension 43: 707–713, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Chalmers J, Morgan T, Doyle A, Dickson B, Hopper J, Mathews J, Matthews G, Moulds R, Myers J, Nowson C, et al.: Australian National Health and Medical Research Council dietary salt study in mild hypertension. J Hypertens Suppl 4: S629–S637, 1986 [PubMed] [Google Scholar]
- 5.Geleijnse JM, Kok FJ, Grobbee DE: Blood pressure response to changes in sodium and potassium intake: A metaregression analysis of randomised trials. J Hum Hypertens 17: 471–480, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Hajjar IM, Grim CE, George V, Kotchen TA: Impact of diet on blood pressure and age-related changes in blood pressure in the US population: Analysis of NHANES III. Arch Intern Med 161: 589–593, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Intersalt: An international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. Intersalt Cooperative Research Group. BMJ 297: 319–328, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skrabal F, Aubock J, Hortnagl H: Low sodium/high potassium diet for prevention of hypertension: Probable mechanisms of action. Lancet 2: 895–900, 1981 [DOI] [PubMed] [Google Scholar]
- 9.Whelton PK, He J, Cutler JA, Brancati FL, Appel LJ, Follmann D, Klag MJ: Effects of oral potassium on blood pressure: Meta-analysis of randomized controlled clinical trials. JAMA 277: 1624–1632, 1997 [DOI] [PubMed] [Google Scholar]
- 10.Overlack A, Ruppert M, Kolloch R, Gobel B, Kraft K, Diehl J, Schmitt W, Stumpe KO: Divergent hemodynamic and hormonal responses to varying salt intake in normotensive subjects. Hypertension 22: 331–338, 1993 [DOI] [PubMed] [Google Scholar]
- 11.Brancati FL, Appel LJ, Seidler AJ, Whelton PK: Effect of potassium supplementation on blood pressure in African Americans on a low-potassium diet: A randomized, double-blind, placebo-controlled trial. Arch Intern Med 156: 61–67, 1996 [PubMed] [Google Scholar]
- 12.Morris RC Jr, Sebastian A, Forman A, Tanaka M, Schmidlin O: Normotensive salt sensitivity: Effects of race and dietary potassium. Hypertension 33: 18–23, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Gallen IW, Rosa RM, Esparaz DY, Young JB, Robertson GL, Batlle D, Epstein FH, Landsberg L: On the mechanism of the effects of potassium restriction on blood pressure and renal sodium retention. Am J Kidney Dis 31: 19–27, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Grim CE, Luft FC, Miller JZ, Meneely GR, Battarbee HD, Hames CG, Dahl LK: Racial differences in blood pressure in Evans County, Georgia: Relationship to sodium and potassium intake and plasma renin activity. J Chronic Dis 33: 87–94, 1980 [DOI] [PubMed] [Google Scholar]
- 15.Urinary and serum electrolytes in untreated black and white hypertensives. Veterans Administration Cooperative Study Group on Antihypertensive Agents. J Chronic Dis 40: 839–847, 1987 [DOI] [PubMed] [Google Scholar]
- 16.Langford HG, Cushman WC, Hsu H: Chronic effect of KCl on black-white differences in plasma renin activity, aldosterone, and urinary electrolytes. Am J Hypertens 4: 399–403, 1991 [DOI] [PubMed] [Google Scholar]
- 17.Luft FC, Rankin LI, Bloch R, Weyman AE, Willis LR, Murray RH, Grim CE, Weinberger MH: Cardiovascular and humoral responses to extremes of sodium intake in normal black and white men. Circulation 60: 697–706, 1979 [DOI] [PubMed] [Google Scholar]
- 18.Voors AW, Dalferes ER Jr, Frank GC, Aristimuno GG, Berenson GS: Relation between ingested potassium and sodium balance in young blacks and whites. Am J Clin Nutr 37: 583–594, 1983 [DOI] [PubMed] [Google Scholar]
- 19.Wong CM, O'Connor DT, Martinez JA, Kailasam MT, Parmer RJ: Diminished renal kallikrein responses to mineralocorticoid stimulation in African Americans: Determinants of an intermediate phenotype for hypertension. Am J Hypertens 16: 281–289, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Price DA, Fisher ND, Osei SY, Lansang MC, Hollenberg NK: Renal perfusion and function in healthy African Americans. Kidney Int 59: 1037–1043, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Cohen SL, Jhetam D, Da Silva J, Milne FJ, van der Walt A: Sodium and potassium status, plasma renin and aldosterone profiles in normotensive and hypertensive Johannesburg blacks. S Afr Med J 62: 941–944, 1982 [PubMed] [Google Scholar]
- 22.Barlow RJ, Connell MA, Milne FJ: A study of 48-hour faecal and urinary electrolyte excretion in normotensive black and white South African males. J Hypertens 4: 197–200, 1986 [DOI] [PubMed] [Google Scholar]
- 23.Taylor EN, Curhan GC: Differences in 24-hour urine composition between black and white women. J Am Soc Nephrol 18: 654–659, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Jones J, Park JJ, Dowling T, Phares D, Park JY, Brown M: Role of potassium excretion and percent body fat on ethnic differences in plasma aldosterone levels. Ethn Dis 16: S4-10–S4-14, 2006 [PubMed] [Google Scholar]
- 25.Windhauser MM, Evans MA, McCullough ML, Swain JF, Lin PH, Hoben KP, Plaisted CS, Karanja NM, Vollmer WM: Dietary adherence in the Dietary Approaches to Stop Hypertension trial. DASH Collaborative Research Group. J Am Diet Assoc 99: S76–S83, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Holbrook JT, Patterson KY, Bodner JE, Douglas LW, Veillon C, Kelsay JL, Mertz W, Smith JC Jr: Sodium and potassium intake and balance in adults consuming self-selected diets. Am J Clin Nutr 40: 786–793, 1984 [DOI] [PubMed] [Google Scholar]
- 27.Pietinen P: Estimating sodium intake from food consumption data. Ann Nutr Metab 26: 90–99, 1982 [DOI] [PubMed] [Google Scholar]
- 28.Dill DB, Yousef MK, Goldman A, Hillyard SD, Davis TP: Volume and composition of hand sweat of white and black men and women in desert walks. Am J Phys Anthropol 61: 67–73, 1983 [DOI] [PubMed] [Google Scholar]
- 29.He Q, Heo M, Heshka S, Wang J, Pierson RN Jr, Albu J, Wang Z, Heymsfield SB, Gallagher D: Total body potassium differs by sex and race across the adult age span. Am J Clin Nutr 78: 72–77, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Suh A, DeJesus E, Rosner K, Lerma E, Yu W, Young JB, Rosa RM: Racial differences in potassium disposal. Kidney Int 66: 1076–1081, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Cummings JH, Hill MJ, Jenkins DJ, Pearson JR, Wiggins HS: Changes in fecal composition and colonic function due to cereal fiber. Am J Clin Nutr 29: 1468–1473, 1976 [DOI] [PubMed] [Google Scholar]
- 32.Sacks FM, Obarzanek E, Windhauser MM, Svetkey LP, Vollmer WM, McCullough M, Karanja N, Lin PH, Steele P, Proschan MA, et al.: Rationale and design of the Dietary Approaches to Stop Hypertension trial (DASH): A multicenter controlled-feeding study of dietary patterns to lower blood pressure. Ann Epidemiol 5: 108–118, 1995 [DOI] [PubMed] [Google Scholar]
- 33.Karanja NM, Obarzanek E, Lin PH, McCullough ML, Phillips KM, Swain JF, Champagne CM, Hoben KP: Descriptive characteristics of the dietary patterns used in the Dietary Approaches to Stop Hypertension Trial. DASH Collaborative Research Group. J Am Diet Assoc 99: S19–S27, 1999 [DOI] [PubMed] [Google Scholar]