Abstract
New-onset diabetes (NOD) is associated with transplant failure. A few single-center studies have suggested that sirolimus is associated with NOD, but this is not well established. With the use of data from the United States Renal Data System, this study evaluated the association between sirolimus use at the time of transplantation and NOD among 20,124 adult recipients of a first kidney transplant without diabetes. Compared with patients treated with cyclosporine and either mycophenolate mofetil or azathioprine, sirolimus-treated patients were at increased risk for NOD, whether it was used in combination with cyclosporine (adjusted hazard ratio [HR] 1.61; 95% confidence interval [CI] 1.36 to 1.90), tacrolimus (adjusted HR 1.66; 95% CI 1.42 to 1.93), or an antimetabolite (mycophenolate mofetil or azathioprine; adjusted HR 1.36; 95% CI 1.09 to 1.69). Similar results were obtained in a subgroup analysis that included the 16,861 patients who did not have their immunosuppressive regimen changed throughout the first posttransplantation year. In conclusion, sirolimus is independently associated with NOD. Given the negative impact of NOD on posttransplantation outcomes, these findings should be confirmed in prospective studies or in meta-analyses of existing trials that involved sirolimus.
New-onset diabetes (NOD) is an increasingly common posttransplantation complication1,2 that is associated with patient death,3–5 graft loss,6,7 and increased health care expenditures.8 Several risk factors for NOD have been identified: Older age,3,9,10 black race,2,3,10 Hispanic ethnicity,2,10 obesity,2,6 family history of diabetes,10 hepatitis C positivity,2,11 and transplantation of a deceased-donor organ.3,10 In addition, the use of corticosteroids12,13 and calcineurin inhibitors (CNI) has been associated with an increased risk for NOD.2,14–18 The higher risk for NOD in tacrolimus compared with cyclosporine A (CsA)-treated patients identified in observational studies2 was recently confirmed in a randomized, controlled trial.19
Single-center studies have suggested that sirolimus may also be diabetogenic.20,21 There are a number of possible mechanisms by which sirolimus may cause NOD, including impaired insulin-mediated suppression of hepatic glucose production,22 insulin resistance from ectopic triglyceride deposition,23,24 or direct β cell toxicity.25,26 Although multicenter trials using sirolimus failed to demonstrate an association between sirolimus and NOD,27–29 patients in the comparator groups in these studies received corticosteroids and CNI; therefore, an independent association between sirolimus and NOD may not have been evident despite the relatively large number of participants in these trials. We therefore performed this analysis using patients captured in the United States Renal Data System (USRDS) to determine whether there is an association between sirolimus and NOD.
RESULTS
Among the 21,546 adult recipients of a first kidney only transplant who did not have diabetes and had Medicare as their primary payer during the study period, we excluded 1421 patients who were not prescribed a CNI or sirolimus in combination or with mycophenolate mofetil (MMF) or azathioprine (AZA), as described in the Concise Methods section. The 20,124 study patients were less likely to be of white race than excluded patients (68.5 versus 71.6% respectively; P = 0.04). Other demographic variables, including known risk factors for NOD (age, gender, Hispanic ethnicity, cause of ESRD, body mass index, hepatitis C serostatus, deceased-donor source, and corticosteroid use at time of transplantation) were similar between included and excluded patients (data not shown). Study patients were followed for a median of 2.63 yr (quartile 1, quartile 3 = 1.26, 3.00). The majority of patients were prescribed a CNI in combination with MMF/AZA (Table 1). Because of the large sample size, there were a number of statistically significant differences between patients treated with different maintenance immunosuppressive drug combinations in univariate analyses (Table 1).
Table 1.
Patient characteristics and comparison of patients treated with various immunosuppressant medicationsa
| Characteristic | All Patients (n = 20,124) | CSA + MMF/AZA (n = 9095) | TAC + MMF/AZA (n = 8431) | Sir + MMF/AZA (n = 619) | Sir + CSA (n = 800) | Sir + TAC (n = 1179) | P |
|---|---|---|---|---|---|---|---|
| Age (yr; mean [SD]) | 47.0 (14.6) | 47.0 (14.8) | 47.1 (14.4) | 49.0 (14.5) | 45.3 (14.6) | 46.1 (14.1) | <0.0001 |
| Male | 59.4 | 60.9 | 57.0 | 61.2 | 63.5 | 61.3 | <0.0001 |
| Race | |||||||
| white | 68.5 | 72.0 | 65.6 | 68.0 | 70.6 | 60.0 | |
| black | 25.7 | 21.7 | 28.7 | 29.1 | 24.3 | 34.7 | <0.0001 |
| other | 5.8 | 6.3 | 5.7 | 2.9 | 5.1 | 5.3 | |
| Hispanic ethnicity | 12.5 | 11.3 | 12.8 | 12.0 | 19.5 | 15.7 | <0.0001 |
| Acute rejection in first posttransplantation year | 11.8 | 14.9 | 8.9 | 7.6 | 12.2 | 8.6 | <0.0001 |
| Cause of ESRD | |||||||
| glomerulonephritis | 40.0 | 40.4 | 39.6 | 38.1 | 40.9 | 40.0 | 0.0100 |
| hypertension | 26.1 | 25.2 | 26.5 | 28.3 | 29.3 | 27.5 | |
| polycystic | 11.4 | 12.0 | 10.8 | 9.7 | 9.8 | 13.3 | |
| other | 22.5 | 22.4 | 23.1 | 23.9 | 20.1 | 19.4 | |
| Duration of pretransplantation dialysis (yr) | |||||||
| Median (Q1, Q3) | 2.0 (0.8, 3.4) | 1.6 (0.6, 2.8) | 2.4 (0.9, 3.8) | 2.9 (1.4, 4.1) | 1.8 (0.9, 3.1) | 2.7 (1.0, 3.2) | <0.0001 |
| BMI (kg/m2) | |||||||
| <25 | 49.6 | 50.1 | 49.3 | 43.2 | 52.7 | 48.4 | 0.0100 |
| 25 to 30 | 30.3 | 30.6 | 30.3 | 34.1 | 26.4 | 29.7 | |
| >30 | 20.1 | 19.3 | 20.4 | 22.7 | 20.9 | 21.9 | |
| Hepatitis C antibody positive | 4.6 | 3.8 | 5.4 | 6.3 | 3.9 | 5.2 | <0.0001 |
| Comorbid conditions | |||||||
| ischemic heart disease | 4.5 | 4.5 | 4.4 | 5.8 | 4.0 | 3.8 | 0.3600 |
| congestive heart failure | 6.6 | 6.8 | 6.7 | 7.3 | 5.6 | 5.9 | 0.5300 |
| cerebrovascular disease | 1.9 | 1.9 | 1.9 | 2.3 | 2.0 | 1.4 | 0.7500 |
| peripheral vascular disease | 1.8 | 1.8 | 1.9 | 1.5 | 1.0 | 1.1 | 0.1400 |
| Deceased-donor recipient | 64.0 | 61.4 | 65.8 | 74.8 | 64.0 | 64.6 | <0.0001 |
| Preemptive transplant | 9.7 | 10.5 | 9.3 | 7.0 | 9.1 | 8.0 | 0.0010 |
| HLA mismatch | |||||||
| 0 | 5.7 | 6.5 | 5.1 | 5.1 | 4.9 | 4.1 | <0.0001 |
| 1 to 3 | 42.6 | 46.4 | 39.8 | 34.1 | 43.1 | 37.3 | |
| 4 to 6 | 51.8 | 47.1 | 55.1 | 60.8 | 52.0 | 58.6 | |
| Transplantation era | |||||||
| 1995 to 1997 | 12.9 | 23.2 | 4.2 | 0.3 | 13.4 | 0.7 | <0.0001 |
| 1997 to 2000 | 35.9 | 48.4 | 26.5 | 11.0 | 38.6 | 18.0 | |
| 2000 to 2003 | 51.2 | 28.4 | 69.3 | 88.7 | 48.0 | 81.3 | |
| Corticosteroid use at hospital discharge after transplantation | 97.8 | 99.3 | 97.7 | 95.4 | 96.2 | 88.5 | <0.0001 |
Based on maintenance immunosuppressive medications at time of transplantation. Percentages are shown unless otherwise indicated. BMI, body mass index; Q, quartile; Sir, sirolimus; TAC, tacrolimus.
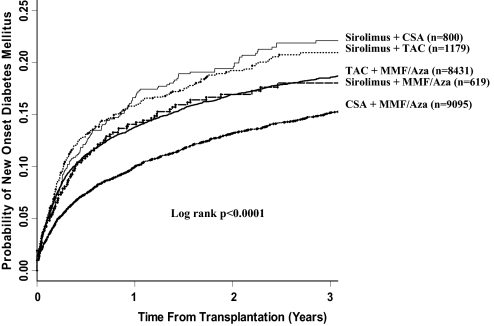
Cumulative Incidence of NOD by Drug Combination
Patients treated with sirolimus in combination with a CNI (either CsA or tacrolimus) had the highest incidence of NOD (Figure 1). The 3-yr cumulative incidence of NOD in patients treated with sirolimus with CsA and with sirolimus with tacrolimus was 21.9 and 21.5%, respectively. Patients treated with tacrolimus and MMF/AZA had the next highest incidence of NOD (cumulative incidence 19.0%). Patient treated with sirolimus and MMF/AZA had a cumulative incidence of NOD of 17.8%. Patients treated with CsA in combination with MMF/AZA had the lowest incidence of NOD (15.6%; overall log rank P < 0.0001).
Figure 1.
Cumulative incidence of NOD within the first 3 yr posttransplantation by drug combination at hospital discharge from transplantation.
Risk for NOD on the Basis of Maintenance Immunosuppressant Drug Combination at the Time of Transplantation
On the basis of the immunosuppressant drug combinations prescribed at the time of transplantation and after adjustment for multiple confounders, patients treated with sirolimus and CsA, sirolimus and tacrolimus, and sirolimus and MMF/AZA all were at increased risk for NOD compared with the reference group of patients treated with CsA and MMF/AZA (Table 2). Of note, these associations were independent of corticosteroid use at the time of hospital discharge after transplantation and acute rejection during the first posttransplantation year. To determine whether the increased risk for NOD in patients treated with the combination of sirolimus and tacrolimus compared with the reference group of patients treated with the combination of CsA and MMF/AZA was simply related to the use of tacrolimus, we repeated the multivariate analysis using patients treated with the combination of tacrolimus and MMF/AZA as the reference group. In this analysis, patients treated with sirolimus and tacrolimus had an increased risk for NOD (hazard ratio [HR] 1.19; 95% confidence interval [CI] 1.02 to 1.37), compared with the reference group of patients treated with the combination of tacrolimus and MMF/AZA, suggesting that sirolimus was associated with an increased risk for NOD independent of any effect of tacrolimus.
Table 2.
Factors associated with NODa
| Factor | HR | 95% CI | P |
|---|---|---|---|
| CSA + MMF/Aza | 1.00 | ||
| TAC + MMF/Aza | 1.40 | 1.29 to 1.52 | <0.0001 |
| Sirolimus + MMF/Aza | 1.36 | 1.09 to 1.69 | <0.0100 |
| Sirolimus + CSA | 1.61 | 1.36 to 1.90 | <0.0001 |
| Sirolimus + TAC | 1.66 | 1.42 to 1.93 | <0.0001 |
| Age (yr) | |||
| 18 to 44 | 0.57 | 0.52 to 0.62 | <0.0001 |
| 45 to 59 | 1.00 | ||
| ≥60 | 1.30 | 1.19 to 1.42 | <0.0001 |
| Female gender | 1.07 | 1.00 to 1.15 | 0.0800 |
| Race | |||
| white | 1.00 | ||
| black | 1.56 | 1.43 to 1.71 | <0.0001 |
| other | 1.20 | 1.02 to 1.42 | 0.0300 |
| Hispanic ethnicity | 1.48 | 1.33 to 1.65 | <0.0001 |
| Acute rejection in first year | |||
| No acute rejection | 1.00 | ||
| Acute rejection in first year | 1.37 | 1.24 to 1.51 | <0.0001 |
| Cause of ESRD | |||
| glomerulonephritis | 1.00 | ||
| hypertension | 1.22 | 1.12 to 1.34 | <0.0001 |
| polycystic disease | 1.02 | 0.90 to 1.16 | 0.7600 |
| other | 1.22 | 1.12 to 1.33 | <0.0001 |
| Duration of dialysis (yr) | |||
| 0 to 1 | 1.00 | ||
| 1 to 3 | 1.04 | 0.99 to 1.09 | 0.1400 |
| >3 | 1.00 | 0.89 to 1.13 | 0.9500 |
| BMI (kg/m2) | |||
| <25 | 1.00 | ||
| 25 to 30 | 1.29 | 1.18 to 1.41 | <0.0001 |
| >30 | 1.65 | 1.50 to 1.82 | <0.0001 |
| Hepatitis C positive | 1.70 | 1.49 to 1.94 | <0.0001 |
| Comorbid conditions | |||
| ischemic heart disease | 1.18 | 1.01 to 1.38 | 0.0400 |
| cerebrovascular disease | 0.91 | 0.71 to 1.17 | 0.4700 |
| peripheral vascular disease | 1.18 | 0.93 to 1.50 | 0.1800 |
| congestive heart failure | 1.22 | 1.07 to 1.37 | <0.0100 |
| Deceased donor | 1.21 | 1.06 to 1.37 | <0.0100 |
| Preemptive transplant | 0.85 | 0.73 to 1.00 | 0.0500 |
| HLA mismatch | |||
| 0 | 1.00 | ||
| 1 to 3 | 1.11 | 0.93 to 1.33 | 0.2400 |
| 4 to 6 | 1.11 | 0.93 to 1.33 | 0.2300 |
| Transplantation era | |||
| 1995 to 1997 | 1.00 | ||
| 1998 to 2000 | 1.23 | 1.08 to 1.39 | <0.0100 |
| 2001 to 2003 | 0.82 | 0.72 to 0.94 | <0.0100 |
| Corticosteroid use at hospital discharge after transplantation | 0.91 | 0.72 to 1.15 | 0.4300 |
Based on maintenance immunosuppressant drug combination at the time of transplantation.
Risk for NOD among Patients Treated with the Same Immunosuppressant Drug Combinations throughout the First Posttransplantation Year
When the Cox multivariate analysis was repeated using only 16,861 (83.8%) patients known to be treated with the same immunosuppressant medications during the first posttransplantation year, patients treated with sirolimus and CsA or with sirolimus and tacrolimus remained at increased risk for NOD compared with the reference group of patients treated with the combination of CsA and MMF/AZA (Table 3). No statistically significant association with NOD was identified in patients treated with the combination of sirolimus and MMF/AZA (only 349 patients could be confirmed to have received the combination of sirolimus and MMF/AZA during the first posttransplantation year). We again considered the possibility that the increased risk for NOD in patients treated with the combination of sirolimus and tacrolimus was simply related to the use of tacrolimus. We therefore repeated the analysis using the patients treated with the combination of tacrolimus and MMF/AZA as the reference group. In this analysis, patients treated with the combination of sirolimus and tacrolimus had an increased risk for NOD (HR 1.25; 95% CI 1.03 to 1.52), suggesting that sirolimus was associated with an increased risk for NOD independent of any effect of tacrolimus.
Table 3.
Factors associated with NOD among 16,861 patients treated with the same immunosuppressant drug combinations throughout the first posttransplantation year
| Factor | HR | 95% CI | P |
|---|---|---|---|
| Drug combination throughout first transplantation year | |||
| CSA + MMF/Aza | 1.00 | ||
| TAC + MMF/Aza | 1.40 | 1.28 to 1.54 | <0.0001 |
| Sirolimus + MMF/Aza | 1.14 | 0.80 to 1.61 | 0.4600 |
| Sirolimus + CSA | 1.78 | 1.44 to 2.21 | <0.0001 |
| Sirolimus + TAC | 1.76 | 1.44 to 2.16 | <0.0001 |
| Age (yr) | |||
| 18 to 44 | 0.56 | 0.51 to 0.62 | <0.0001 |
| 45 to 59 | 1.00 | ||
| ≥60 | 1.31 | 1.18 to 1.45 | <0.0001 |
| Female gender | 1.08 | 0.99 to 1.18 | 0.0600 |
| Race | |||
| white | 1.00 | ||
| black | 1.55 | 1.40 to 1.72 | <0.0001 |
| other | 1.25 | 1.04 to 1.50 | 0.0200 |
| Hispanic ethnicity | 1.48 | 1.31 to 1.67 | <0.0001 |
| Corticosteroid use at transplantation and at 1 yr | |||
| no/no (n = 202, 1.2%) | 1.00 | ||
| yes/no (n = 1197, 7.1%) | 0.98 | 0.61 to 1.56 | 0.9300 |
| no/yes (n = 152, 0.9%) | 2.05 | 1.18 to 3.57 | 0.0100 |
| yes/yes (n = 15,310, 90.8%) | 1.37 | 0.88 to 2.12 | 0.1700 |
| Acute rejection in first year | |||
| no acute rejection | 1.00 | ||
| acute rejection in first year | 1.31 | 1.16 to 1.48 | <0.0001 |
| Cause of ESRD | |||
| glomerulonephritis | 1.00 | ||
| hypertension | 1.25 | 1.12 to 1.38 | <0.0001 |
| polycystic disease | 1.04 | 0.90 to 1.19 | 0.6300 |
| other | 1.18 | 1.05 to 1.31 | <0.01 |
| Duration of dialysis (yr) | |||
| 0 to 1 | 1.00 | ||
| 1 to 3 | 1.03 | 0.97 to 1.09 | 0.3600 |
| >3 | 0.94 | 0.82 to 1.07 | 0.3500 |
| Preemptive transplant | 0.82 | 0.69 to 0.98 | 0.0300 |
| BMI (kg/m2) | |||
| <25 | 1.00 | ||
| 25 to 30 | 1.32 | 1.17 to 1.44 | <0.0001 |
| >30 | 1.66 | 1.49 to 1.85 | <0.0001 |
| Hepatitis C positive | 1.72 | 1.48 to 2.01 | <0.0001 |
| Transplantation era | |||
| 1995 to 1997 | 1.00 | ||
| 1998 to 2000 | 1.25 | 1.09 to 1.44 | 0.3600 |
| 2001 to 2003 | 0.85 | 0.73 to 0.99 | 0.0400 |
| Deceased-donor type | 1.21 | 1.10 to 1.33 | 0.0001 |
| HLA mismatch | |||
| 0 | 1.00 | ||
| 1 to 3 | 1.15 | 0.95 to 1.40 | 0.1600 |
| 4 to 6 | 1.11 | 0.91 to 1.35 | 0.2700 |
| Comorbidity | |||
| ischemic heart disease | 1.13 | 0.94 to 1.35 | 0.2000 |
| cerebrovascular disease | 0.92 | 0.69 to 1.21 | 0.5400 |
| peripheral vascular disease | 1.12 | 0.85 to 1.47 | 0.4300 |
| cardiac failure | 1.20 | 1.04 to 1.39 | 0.0100 |
DISCUSSION
We found that sirolimus-treated patients were at increased risk for NOD. This association was consistent whether sirolimus was used in combination with CsA, tacrolimus, or MMF/AZA. Indeed, because tacrolimus itself is associated with an increased risk for NOD compared with CsA, we considered the possibility that the risk for NOD in patients treated with the combination of tacrolimus and sirolimus, compared with patients treated with the combination of CsA and MMF/AZA, was simply related to the use of tacrolimus. When the multivariate analysis was repeated with patients treated with the combination of tacrolimus and MMF/AZA as the reference group, patients treated with the combination of sirolimus and tacrolimus remained at increased risk for NOD, suggesting that sirolimus itself increases the risk for NOD. The association of sirolimus with NOD was also demonstrated in a restricted analysis involving patients prescribed the same combination of medications during the first posttransplantation year. The associations demonstrated in this observational study should be confirmed by additional prospective studies or meta-analysis of previously completed trials involving sirolimus that reported the incidence of NOD.
To date, only a few clinical studies have suggested that sirolimus and its analogues are associated with hyperglycemia.20,21,30–32 In a randomized trial of 150 renal transplant recipients, the incidence of NOD in patients who received tacrolimus with sirolimus, tacrolimus with MMF, or CsA with sirolimus was 17, 14, and 33%, respectively (P = 0.06), suggesting a possible diabetogenic effect of sirolimus.30,31 In an uncontrolled study, Hricik et al.33 compared clinical outcomes in 56 black patients treated with corticosteroids, sirolimus, and tacrolimus targeted to relatively low tacrolimus trough levels with those in white patients (n = 65) treated with steroids, MMF, and tacrolimus targeted to higher tacrolimus levels. Despite lower tacrolimus levels in the black patients, the incidence of NOD in the black patients was 36 compared with 15% in white patients (P = 0.02). In a retrospective study to examine the incidence of NOD among 86 consecutive renal transplant recipients in a single center between 1997 and 2004, Romagnoli et al.20 reported that patients treated with the combination of sirolimus and CsA had a significantly higher incidence of NOD compared with patients treated with CsA alone. Teutonico et al.21 demonstrated that chronic inhibition of mammalian target of rapamycin (mTOR) caused an increase in peripheral insulin resistance, along with impaired pancreatic β cell response to a glucose load, in a cohort of 26 renal transplant recipients who were converted from treatment with CsA to sirolimus. A pivotal multicenter study using sirolimus did not suggest an increased risk for NOD.28 This may be related to the fact that patients in the comparator group received CsA, which itself is diabetogenic.2,14–18 Using the effect size seen in our analysis, we estimated that enrolment of 1340 patients (670 patients in each treatment group) would be needed to demonstrate an increased risk for NOD in a trial comparing sirolimus with CsA, with α = 0.05 and β = 0.20. Thus, the multicenter trial that enrolled 719 patients28 would have been underpowered to demonstrate an association between sirolimus and NOD.
The mechanisms by which sirolimus may cause NOD are not clearly defined. Sirolimus acts on the mTOR, a serine/threonine kinase that integrates signals from various nutrients and growth factors to regulate protein translation through a variety of downstream effectors.34,35 Overactivation of mTOR downstream from the phosphatidylinositol 3-kinase–AKT pathway modulates insulin signaling by insulin receptor substrates.34,35 Physiologic conditions such as hyperinsulinemia promote serine/threonine phosphorylation of insulin receptor substrate proteins that inhibits their function and promotes their degradation, leading to insulin resistance. Inhibitors of mTOR would therefore be expected to prevent development of insulin resistance through this mechanism. Indeed, sirolimus has been associated with a decreased likelihood of NOD.36 More recently, Di Paolo et al.34 studied 30 patients treated with long-term sirolimus and reported an unexpected impairment of insulin receptor substrate signaling and AKT activation, a finding that could help to explain deterioration of glucose metabolism in sirolimus-treated patients. Other mechanisms that have been proposed for the induction of hyperglycemia by sirolimus include ectopic triglyceride deposition with sirolimus leading to insulin resistance,23,24 impairment of insulin-mediated suppression of hepatic glucose production,22 or a direct toxic effect on pancreatic β cells.25,26
When interpreting the results of this study, readers should consider the inherent limitations of retrospective analyses of administrative data sets including nonrandom assignment of patients to different immunosuppressive medication protocols. Although we adjusted for multiple factors known to be associated with NOD, the associations identified may be confounded by other factors not included in our analysis. We are able to adjust only for the use of corticosteroids at the time of hospital discharge after transplantation and at approximately 1 yr after the date of transplantation. We do not have information regarding the dosage of maintenance corticosteroids used in the various regimens. We included adjustment for the incidence of acute rejection to account for some of the variation in corticosteroid use between patients during the first posttransplantation year in our analyses; however, we did not have information regarding the dosage of corticosteroids used to treat acute rejection. We hypothesize that the association of acute rejection with NOD in our analysis is due to a higher exposure to corticosteroids in patients with acute rejection, but other mechanisms may be responsible. Similarly, we do not have information regarding the dosage of sirolimus and CNI used. The study was limited to patients in the United States who had Medicare as the primary payer, which may limit the applicability of our findings to other patient populations. We defined NOD from Medicare claims data according to previously published and validated methods.2,37–41 It is important to note that this criterion is not the same as the “gold standard” established by the American Diabetes Association and World Health Organization, which requires laboratory results and patient symptoms42,43 that are not available in the USRDS data sets. A consistently high level of accuracy and concordance between cases that were identified by this method and the American Diabetes Association/World Health Organization criteria38,40,41 has been established. Our definition has a sensitivity of 0.75, a specificity of 0.97, and a positive predictive value of 0.88 compared with self-reported diabetes.38
In summary, this study identifies an association of sirolimus with NOD. Given the importance of NOD as a determinant of posttransplantation outcomes and the current use of sirolimus in both pancreas and islet cell transplantation, the findings of our study should be confirmed in further prospective studies or in meta-analyses of existing trials using sirolimus.
CONCISE METHODS
Data Source and Study Population
The data source for the study was the USRDS. The study population included adult patients (≥18 yr) who received a first kidney-only transplant between April 30, 1995, and December 31, 2003. The study population was limited to patients with Medicare as the primary payer to permit ascertainment of NOD from institutional claims data. In addition, patients were included only when they were prescribed one of the following recognized combinations of immunosuppressant medications at the time of transplantation: CsA with MMF or AZA, tacrolimus with MMF or AZA, sirolimus with CsA, sirolimus with tacrolimus, or sirolimus with MMF or AZA. Patients with diabetes before transplantation were excluded from the study. Diabetes before transplantation was identified when diabetes was listed as the cause of ESRD, as a comorbid condition, or when there were any inpatient or outpatient Medicare claims for diabetes in the 12 mo before transplantation (see next section for details of Medicare claims used to identify diabetes).
Definition of NOD and Patient Follow-up
NOD was defined according to previously published and validated methods,2,37–41 based on Medicare claims data. This method required a minimum of one inpatient claim or of two outpatient claims within 1 yr to establish a diagnosis of NOD. Patients were followed from the date of transplantation until death, transplant failure (either dialysis initiation or repeat transplantation), or end of follow-up (December 31, 2004) for the development of NOD. In addition, ascertainment of NOD was limited to the first 3 yr after transplantation, when patients normally retain ESRD Medicare eligibility, to ensure complete ascertainment of NOD from Medicare claims. The specific International Classification of Disease, Ninth Revision, Clinical Modification diagnostic codes used to identify NOD were 250; 250.x (x = 0 to 9), 250.0x, and 250.xy (y = 0 to 3). The date of onset of NOD was assumed to be the date of the earliest Medicare claim.
Descriptive Statistics
Patients were categorized on the basis of the combination of immunosuppressant medications prescribed at the time of transplantation, and group differences were compared with the χ2 test or ANOVA as appropriate.
Association of Sirolimus with NOD
The time to NOD after transplantation was determined with the Kaplan-Meier method among patients prescribed the various combinations of immunosuppressant medications described already, and group differences were compared with the log rank test. A Cox multivariate regression analysis was performed to determine the risk for NOD in patients prescribed the different combinations of immunosuppressant medications. The following variables associated with NOD in univariate analyses were included in the model: Patient age at transplantation, gender, race, ethnicity, acute rejection in the first posttransplantation year, cause of ESRD, duration of dialysis before transplantation, donor source, body mass index, hepatitis C serostatus, transplant year, HLA mismatch, comorbid conditions (ischemic heart disease, cerebrovascular disease, peripheral vascular disease, and cardiac failure), and corticosteroid use. Variables were entered into these models when they met the proportional hazards assumption. The proportional hazards assumption was tested for using log-negative-log plots of the within-group survivor probabilities versus log time. Patients with missing covariate information were coded as “missing” for that covariate and included in the multivariate models. A second Cox multivariate regression model, including only patients who remained on the same immunosuppressant drug combination during the first posttransplantation year, was also performed. All analyses were performed using SAS 9.1 (SAS Institute, Cary, NC) and S-Plus 7.0 (Insightful Corp., Seattle, WA). The study was approved by our local hospital research ethics review board.
DISCLOSURES
None.
Acknowledgments
J.G. is supported by the Michael Smith Foundation for Health Research; O.J. is supported by a grant from the Canadian Institute of Health Research and the Michael Smith Foundation for Health Research.
This work was presented in abstract form at the American Transplant Congress; San Francisco, CA; May 5–9, 2007.
Published online ahead of print. Publication date available at www.jasn.org.
The data reported in this study has been supplied by the USRDS. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US government.
See related editorial, “Diabetes after Transplantation and Sirolimus: What's the Connection?” on pages 1255–1256.
REFERENCES
- 1.Cosio FG, Pesavento TE, Osei K, Henry ML, Ferguson RM: Post-transplant diabetes mellitus: Increasing incidence in renal allograft recipients transplanted in recent years. Kidney Int 59: 732–737, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ: Diabetes mellitus after kidney transplantation in the United States. Am J Transplant 3: 178–185, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Boudreaux JP, McHugh L, Canafax DM, Ascher N, Sutherland DE, Payne W, Simmons RL, Najarian JS, Fryd DS: The impact of cyclosporine and combination immunosuppression on the incidence of posttransplant diabetes in renal allograft recipients. Transplantation 44: 376–381, 1987 [DOI] [PubMed] [Google Scholar]
- 4.Friedman EA, Shyh TP, Beyer MM, Manis T, Butt KM: Posttransplant diabetes in kidney transplant recipients. Am J Nephrol 5: 196–202, 1985 [DOI] [PubMed] [Google Scholar]
- 5.Revanur VK, Jardine AG, Kingsmore DB, Jaques BC, Hamilton DH, Jindal RM: Influence of diabetes mellitus on patient and graft survival in recipients of kidney transplantation. Clin Transplant 15: 89–94, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Miles AM, Sumrani N, Horowitz R, Homel P, Maursky V, Markell MS, Distant DA, Hong JH, Sommer BG, Friedman EA: Diabetes mellitus after renal transplantation: As deleterious as non-transplant-associated diabetes? Transplantation 65: 380–384, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Roth D, Milgrom M, Esquenazi V, Fuller L, Burke G, Miller J: Posttransplant hyperglycemia: Increased incidence in cyclosporine-treated renal allograft recipients. Transplantation 47: 278–281, 1989 [PubMed] [Google Scholar]
- 8.Woodward RS, Schnitzler MA, Baty J, Lowell JA, Lopez-Rocafort L, Haider S, Woodworth TG, Brennan DC: Incidence and cost of new onset diabetes mellitus among U.S. wait-listed and transplanted renal allograft recipients. Am J Transplant 3: 590–598, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Reisaeter AV, Hartmann A: Risk factors and incidence of posttransplant diabetes mellitus. Transplant Proc 33: 8S–18S, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Sumrani NB, Delaney V, Ding ZK, Davis R, Daskalakis P, Friedman EA, Butt KM, Hong JH: Diabetes mellitus after renal transplantation in the cyclosporine era: An analysis of risk factors. Transplantation 51: 343–347, 1991 [DOI] [PubMed] [Google Scholar]
- 11.Bloom RD, Rao V, Weng F, Grossman RA, Cohen D, Mange KC: Association of hepatitis C with posttransplant diabetes in renal transplant patients on tacrolimus. J Am Soc Nephrol 13: 1374–1380, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Arner P, Gunnarsson R, Blomdahl S, Groth CG: Some characteristics of steroid diabetes: a study in renal-transplant recipients receiving high-dose corticosteroid therapy. Diabetes Care 6: 23–25, 1983 [DOI] [PubMed] [Google Scholar]
- 13.David DS, Cheigh JS, Braun DW Jr, Fotino M, Stenzel KH, Rubin AL: HLA-A28 and steroid-induced diabetes in renal transplant patients. JAMA 243: 532–533, 1980 [PubMed] [Google Scholar]
- 14.Drachenberg CB, Klassen DK, Weir MR, Wiland A, Fink JC, Bartlett ST, Cangro CB, Blahut S, Papadimitriou JC: Islet cell damage associated with tacrolimus and cyclosporine: Morphological features in pancreas allograft biopsies and clinical correlation. Transplantation 68: 396–402, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Knoll GA, Bell RC: Tacrolimus versus cyclosporin for immunosuppression in renal transplantation: Meta-analysis of randomised trials. BMJ 318: 1104–1107, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayer AD, Dmitrewski J, Squifflet JP, Besse T, Grabensee B, Klein B, Eigler FW, Heemann U, Pichlmayr R, Behrend M, Vanrenterghem Y, Donck J, van Hooff J, Christiaans M, Morales JM, Andres A, Johnson RW, Short C, Buchholz B, Rehmert N, Land W, Schleibner S, Forsythe JL, Talbot D, Pohanka E, et al.: Multicenter randomized trial comparing tacrolimus (FK506) and cyclosporine in the prevention of renal allograft rejection: A report of the European Tacrolimus Multicenter Renal Study Group. Transplantation 64: 436–443, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Pirsch JD, Miller J, Deierhoi MH, Vincenti F, Filo RS: A comparison of tacrolimus (FK506) and cyclosporine for immunosuppression after cadaveric renal transplantation. FK506 Kidney Transplant Study Group. Transplantation 63: 977–983, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Vincenti F, Jensik SC, Filo RS, Miller J, Pirsch J: A long-term comparison of tacrolimus (FK506) and cyclosporine in kidney transplantation: Evidence for improved allograft survival at five years. Transplantation 73: 775–782, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Vincenti F, Friman S, Scheuermann E, Rostaing L, Jenssen T, Campistol JM, Uchida K, Pescovitz MD, Marchetti P, Tuncer M, Citterio F, Wiecek A, Chadban S, El-Shahawy M, Budde K, Goto N: Results of an international, randomized trial comparing glucose metabolism disorders and outcome with cyclosporine versus tacrolimus. Am J Transplant 7: 1506–1514, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Romagnoli J, Citterio F, Nanni G, Favi E, Tondolo V, Spagnoletti G, Salerno MP, Castagneto M: Incidence of posttransplant diabetes mellitus in kidney transplant recipients immunosuppressed with sirolimus in combination with cyclosporine. Transplant Proc 38: 1034–1036, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Teutonico A, Schena PF, Di Paolo S: Glucose metabolism in renal transplant recipients: Effect of calcineurin inhibitor withdrawal and conversion to sirolimus. J Am Soc Nephrol 16: 3128–3135, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Syed NA, Khandelwal RL: Reciprocal regulation of glycogen phosphorylase and glycogen synthase by insulin involving phosphatidylinositol-3 kinase and protein phosphatase-1 in HepG2 cells. Mol Cell Biochem 211: 123–136, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Lewis GF, Carpentier A, Adeli K, Giacca A: Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev 23: 201–229, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Mittelman SD, Bergman RN: Inhibition of lipolysis causes suppression of endogenous glucose production independent of changes in insulin. Am J Physiol Endocrinol Metab 279: E630–E637, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Bussiere CT, Lakey JR, Shapiro AM, Korbutt GS: The impact of the mTOR inhibitor sirolimus on the proliferation and function of pancreatic islets and ductal cells. Diabetologia 49: 2341–2349, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Kwon G, Marshall CA, Liu H, Pappan KL, Remedi MS, McDaniel ML: Glucose-stimulated DNA synthesis through mammalian target of rapamycin (mTOR) is regulated by KATP channels: Effects on cell cycle progression in rodent islets. J Biol Chem 281: 3261–3267, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Gonwa TA, Hricik DE, Brinker K, Grinyo JM, Schena FP: Improved renal function in sirolimus-treated renal transplant patients after early cyclosporine elimination. Transplantation 74: 1560–1567, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Kahan BD: Efficacy of sirolimus compared with azathioprine for reduction of acute renal allograft rejection: A randomised multicentre study. The Rapamune US Study Group. Lancet 356: 194–202, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Mendez R, Gonwa T, Yang HC, Weinstein S, Jensik S, Steinberg S: A prospective, randomized trial of tacrolimus in combination with sirolimus or mycophenolate mofetil in kidney transplantation: Results at 1 year. Transplantation 80: 303–309, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Ciancio G, Burke GW, Gaynor JJ, Mattiazzi A, Roth D, Kupin W, Nicolas M, Ruiz P, Rosen A, Miller J: A randomized long-term trial of tacrolimus/sirolimus versus tacrolimus/mycophenolate mofetil versus cyclosporine (NEORAL)/sirolimus in renal transplantation. II. Survival, function, and protocol compliance at 1 year. Transplantation 77: 252–258, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Ciancio G, Burke GW, Gaynor JJ, Mattiazzi A, Roth D, Kupin W, Nicolas M, Ruiz P, Rosen A, Miller J: A randomized long-term trial of tacrolimus and sirolimus versus tacrolimus and mycophenolate mofetil versus cyclosporine (NEORAL) and sirolimus in renal transplantation. I. Drug interactions and rejection at one year. Transplantation 77: 244–251, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Sulanc E, Lane JT, Puumala SE, Groggel GC, Wrenshall LE, Stevens RB: New-onset diabetes after kidney transplantation: An application of 2003 International Guidelines. Transplantation 80: 945–952, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Hricik DE, Anton HA, Knauss TC, Rodriguez V, Seaman D, Siegel C, Valente J, Schulak JA: Outcomes of African American kidney transplant recipients treated with sirolimus, tacrolimus, and corticosteroids. Transplantation 74: 189–193, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Di Paolo S, Teutonico A, Leogrande D, Capobianco C, Schena PF: Chronic inhibition of mammalian target of rapamycin signaling downregulates insulin receptor substrates 1 and 2 and AKT activation: A crossroad between cancer and diabetes? J Am Soc Nephrol 17: 2236–2244, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Hay N, Sonenberg N: Upstream and downstream of mTOR. Genes Dev 18: 1926–1945, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Kuypers DR: Benefit-risk assessment of sirolimus in renal transplantation. Drug Saf 28: 153–181, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Burroughs TE, Lentine KL, Takemoto SK, Swindle J, Machnicki G, Hardinger K, Brennan DC, Irish WD, Schnitzler MA: Influence of early posttransplantation prednisone and calcineurin inhibitor dosages on the incidence of new-onset diabetes. Clin J Am Soc Nephrol 2: 517–523, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Hebert PL, Geiss LS, Tierney EF, Engelgau MM, Yawn BP, McBean AM: Identifying persons with diabetes using Medicare claims data. Am J Med Qual 14: 270–277, 1999 [DOI] [PubMed] [Google Scholar]
- 39.McBean AM, Li S, Gilbertson DT, Collins AJ: Differences in diabetes prevalence, incidence, and mortality among the elderly of four racial/ethnic groups: Whites, blacks, Hispanics, and Asians. Diabetes Care 27: 2317–2324, 2004 [DOI] [PubMed] [Google Scholar]
- 40.O'Connor PJ, Rush WA, Pronk NP, Cherney LM: Identifying diabetes mellitus or heart disease among health maintenance organization members: Sensitivity, specificity, predictive value, and cost of survey and database methods. Am J Manag Care 4: 335–342, 1998 [PubMed] [Google Scholar]
- 41.Rector TS, Wickstrom SL, Shah M, Thomas Greeenlee N, Rheault P, Rogowski J, Freedman V, Adams J, Escarce JJ: Specificity and sensitivity of claims-based algorithms for identifying members of Medicare+Choice health plans that have chronic medical conditions. Health Serv Res 39: 1839–1857, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davidson J, Wilkinson A, Dantal J, Dotta F, Haller H, Hernandez D, Kasiske BL, Kiberd B, Krentz A, Legendre C, Marchetti P, Markell M, van der Woude FJ, Wheeler DC: New-onset diabetes after transplantation: 2003 International consensus guidelines. Proceedings of an international expert panel meeting. Barcelona, Spain, 19 February 2003. Transplantation 75: SS3–SS24, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 26[Suppl 1]: S5–S20, 2003 [DOI] [PubMed] [Google Scholar]