Abstract
For poorly understood reasons, patients with end-stage renal disease (ESRD) differ substantially in their response to treatment with recombinant erythropoietin (EPO). Because hypoxia influences many of the biologic pathways involved in erythropoiesis, the altitude at which a patient lives may affect the dose-response relationship of EPO. In this retrospective cohort study, clinical data from 341,737 incident hemodialysis patients registered in the U.S. Renal Data System were combined with elevation data from the U.S. Geological Survey to address this question. Higher altitude was associated with smaller EPO doses and higher hematocrit levels. For example, compared with patients at sea level, patients living above 6000 ft received 19% less EPO (12.9 versus 15.9 thousand units/wk) but had hematocrit levels 1.1 points higher (35.7% versus 34.6%). These associations were found within subgroups defined by sex, race, age, calendar time, cause of ESRD, and dialysis center profit status, and persisted after adjustment for various potential confounding factors. Furthermore, resistance to EPO decreased with elevation. Our results suggest that ESRD patients living at high altitude either increase endogenous EPO production or respond better to endogenous and exogenous EPO.
Patients with chronic kidney disease (CKD), including those with end-stage renal disease (ESRD) produce insufficient amounts of renal erythropoietin to maintain normal hemoglobin levels. The primary treatment for the anemia caused by CKD is human recombinant erythropoietin (EPO). Treatment with EPO has been shown to increase hematocrit levels and reduce the need for blood transfusions in CKD patients with anemia.1–3 However, managing EPO therapy is complicated because of the great within- and between-person variability in the erythropoietic response to EPO.4,5 The within-person variability in response leads to fluctuating hemoglobin levels, which may adversely affect patient outcomes.6–10 An improved understanding of factors that influence EPO responsiveness could lead to greater hemoglobin control and thus safer and more effective anemia management strategies for CKD patients.
The mechanisms underlying EPO response are complex, however, as exogenous EPO and administered iron promote red blood cell production through an intricate cascade of physiologic responses to the tissue hypoxia caused by anemia. In healthy people, lowered oxygen tension in the cell leads to increased production of endogenous EPO and regulation of various pathways involved with iron metabolism.11 In patients with CKD, the production of endogenous EPO decreases with the loss of kidney function; therefore, the biologic response to hypoxia is altered in CKD.
One factor that may illuminate the interrelations of the many factors involved with EPO response is the altitude at which a patient lives. At higher altitudes, patients are exposed to a lower partial pressure of oxygen; thus, altitude can influence tissue hypoxia (similar to anemia) and the array of physiologic responses to hypoxia involved in erythropoiesis. In this study, we sought to determine whether altitude affects either EPO dose requirements or treatment response among a large cohort of ESRD patients on hemodialysis in the United States.
RESULTS
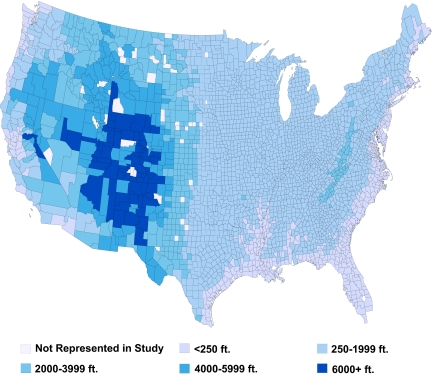
We identified 341,737 ESRD patients who initiated hemodialysis between 1995 and 2004 and who met the study entry requirements. The median elevation of patients in the sample was 407 feet, and 7487 (2.2%) patients lived above 4000 ft. To depict the geographic distribution of elevations inhabited by patients, we mapped the median elevation of patients by county in the lower 48 states (Figure 1). Besides a few counties in the Appalachian Mountains, the median elevation for counties east of the Mississippi was below 2000 ft. The counties with median patient elevations over 6000 ft were found in New Mexico, Arizona, Nevada, Colorado, California, and Wyoming. There were 61 patients in Colorado and California who lived over 9500 ft.
Figure 1.
Map of average county-level elevation of patients in study cohort.
Table 1 presents the characteristics of the sample stratified by elevation group. Race was imbalanced across elevation groups. In the lowest elevation group, 40% of patients were of black race, but this percentage decreased with elevation to only 5% in the over 6000 ft group. In the lowest elevation group, less than 1% of the patients in the study identified themselves as American Indian, but 30% did in the highest elevation group. The percentage of patients in the oldest age group generally decreased with elevation (21% down to 15%). The percentage of patients with hypertension as the reported cause ESRD also decreased with elevation (28% down to 10%), whereas the percentage of patients with diabetes as the reported cause of ESRD increased with elevation (46% up to 61%). Patients in the highest elevation group had slightly worse kidney function at initiation of dialysis than patients in the lowest elevation group (mean glomerular filtration rate = 7.8 versus 8.4) and slightly lower serum albumin levels (3.0 versus 3.2). Compared with patients in the lowest elevation group, fewer patients in the highest elevation group were treated at for-profit dialysis centers (71% versus 77%).
Table 1.
Baseline characteristics of subjects in sample, by elevation group
| <250 ft [N (%) or mean (SE)] | 250 to 1999 ft [N (%) or mean (SE)] | 2000 to 3999 ft [N (%) or mean (SE)] | 4000 to 5999 ft [N (%) or mean (SE)] | ≥6000 ft [N (%) or mean (SE)] | |
|---|---|---|---|---|---|
| N | 140,350 | 184,578 | 9320 | 5934 | 1555 |
| Male gender | 72,024 (51.3%) | 94,595 (51.3%) | 4941 (53.0%) | 3172 (53.5%) | 780 (50.2%) |
| Age, years | |||||
| <18 | 374 (0.3%) | 454 (0.3%) | 30 (0.3%) | 24 (0.4%) | 10 (0.6%) |
| 18–39 | 10,954 (7.8%) | 13,669 (7.4%) | 649 (7.0%) | 496 (8.4%) | 117 (7.5%) |
| 40–59 | 38,423 (27.4%) | 47,259 (25.6%) | 2700 (29.0%) | 1701 (28.7%) | 417 (26.8%) |
| 60–75 | 59,107 (42.1%) | 80,012 (43.4%) | 4115 (44.2%) | 2660(44.8%) | 767 (49.3%) |
| >75 | 29,466 (21.0%) | 40,754 (22.1%) | 1770 (19.0%) | 1032 (17.4%) | 240 (15.4%) |
| Race | |||||
| white | 68,443 (48.8%) | 114,431 (62.0%) | 7475 (80.2%) | 4321 (72.8%) | 926 (59.6%) |
| black | 55,978 (39.9%) | 57,405 (31.1%) | 774 (8.3%) | 416 (7.0%) | 82 (5.3%) |
| American Indian | 714 (0.5%) | 3070 (1.7%) | 676 (7.3%) | 862 (14.5%) | 478 (30.7%) |
| GFR (estimated) | 8.4 (0.01) | 8.8 (0.01) | 8.9 (0.04) | 8.2 (0.05) | 7.8 (0.08) |
| Serum albumin | 3.17 (0.002) | 3.16 (0.002) | 3.10 (0.007) | 3.10 (0.012) | 3.00 (0.023) |
| Cause of ESRD | |||||
| hypertension | 39,104 (27.9%) | 47,970 (26.0%) | 1599 (17.2%) | 767 (12.9%) | 158 (10.2%) |
| diabetes | 64,433 (45.9%) | 86,019 (46.6%) | 5275 (56.6%) | 3298 (55.6%) | 945 (60.8%) |
| glomerulonephritis | 13,625 (9.7%) | 18,575 (10.1%) | 908 (9.7%) | 789 (13.3%) | 184 (11.8%) |
| History of MI | 10,329 (7.4%) | 16,290 (8.8%) | 694 (7.5%) | 510 (8.6%) | 133 (8.6%) |
| History of cancer | 5970 (4.3%) | 9625 (5.2%) | 405 (4.4%) | 228 (3.8%) | 58 (3.7%) |
| History of CHF | 43,706 (31.1%) | 60,574 (32.8%) | 2903 (31.2%) | 1568 (26.4%) | 485 (31.2%) |
| Weight (kg) | 72.4 (0.06) | 75.3 (0.05) | 72.1 (0.23) | 74.6 (0.26) | 70.7 (0.46) |
| Iron administered during index month | 75,801 (54.0%) | 103,101 (55.9%) | 4694 (50.4%) | 2944 (49.6%) | 737 (47.4%) |
| Treated at for-profit center | 107,933 (76.9%) | 134,401 (72.8%) | 7156 (76.8%) | 4055 (68.3%) | 1096 (70.5%) |
CHF, congestive heart failure; GFR, glomerular filtration rate; ESRD, end-stage renal disease; MI, myocardial infarction.
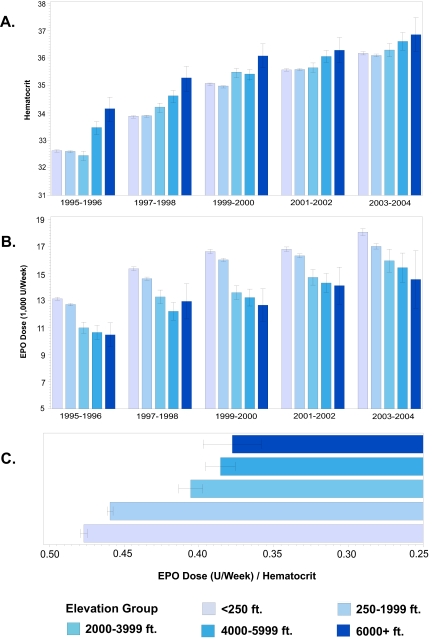
Hematocrit increased with elevation within each time period studied (Figure 2A), and hematocrit levels in the highest elevation group were sharply higher for all time periods. Mean weekly EPO use (U/wk) decreased with elevation within each time period studied (Figure 2B). The trends persisted within subgroups defined by sex, race, age, cause of ESRD, and dialysis profit center status (data not presented). EPO resistance decreased with elevation across the entire study population (Figure 2C).
Figure 2.
(A) Average hematocrit by elevation group and time period. (B) Average EPO dose by elevation group and time period. (C) EPO resistance (EPO dose/hematocrit) by elevation group.
In Table 2, we report average hematocrit, EPO use, and EPO resistance by elevation group and the least-square means from the regression analysis. The least-square means mirror the graphs and reveal decreasing EPO use, increasing hematocrit levels, and decreasing EPO resistance with increasing elevation. Statistical adjustments slightly attenuated the differences between the EPO dose in the highest and lowest elevation groups (Table 2) but had no effect on the difference in hematocrit levels between elevation groups.
Table 2.
Unadjusted and adjusted mean achieved hematocrit, EPO dose (U/week), and EPO resistance by elevation group with 95% confidence intervals
| Elevation Group | Hematocrit (unadjusted) | Hematocrit (adjusteda) | Weekly EPO Dose (1000 U/week) (unadjusted) | Weekly EPO Dose (1000 U/week) (adjusteda) | EPO Resistance (adjusteda) |
|---|---|---|---|---|---|
| <250 ft | 34.6 (34.6–34.6) | 34.3 (34.3–34.4) | 15.9 (15.9–16.0) | 17.0 (16.8–17.3) | 0.52 (0.51–0.52) |
| 250–1999 ft | 34.6 (34.6–34.6) | 34.3 (34.2–34.4) | 15.3 (15.3–15.4) | 16.3 (16.1–16.6) | 0.50 (0.49–0.51) |
| 2000–3999 ft | 34.8 (34.8–34.9) | 34.5 (34.4–34.6) | 13.7 (13.4–14.0) | 15.1 (14.7–15.4) | 0.46 (0.44–0.47) |
| 4000–5999 ft | 35.2 (35.1–35.3) | 34.9 (34.8–35.0) | 13.1 (12.8–13.5) | 14.6 (14.2–15.0) | 0.44 (0.42–0.45) |
| ≥ 6000 ft | 35.7 (35.5–35.9) | 35.4 (35.2–35.7) | 12.9 (12.2–13.6) | 14.7 (14.0–15.4) | 0.44 (0.41–0.46) |
Adjusted for age, sex, race, weight, calendar year, estimated glomerular filtration rate at baseline, cause of ESRD, profit status of treating center, history of myocardial infarction, history of congestive heart failure, history of cancer, and concurrent iron administration. Adjusted means are least-squares means that use the fitted regression to compute a mean for each elevation group standardized to the population distribution of covariates included in the regression model.
In Table 3, we present least-squares means of baseline hematocrit levels, change in hematocrit levels from baseline to the index month, and index EPO dose among patients who did not receive EPO before starting dialysis. This table reveals baseline hematocrit levels increasing with altitude, the change in hematocrit relatively flat across elevation groups, and index EPO doses decreasing with elevation.
Table 3.
Adjusted mean baseline hematocrit, change in hematocrit from baseline, and EPO dose (U/week) by elevation group, among treatment naive patients with 95% confidence intervals
| Elevation Group | Baseline Hematocrit (adjusteda) | Change In Hematocrit (adjusteda) | Weekly EPO Dose (1000 U/week) (adjusteda) |
|---|---|---|---|
| <250 ft | 27.3 (27.2–27.5) | 7.0 (6.9–7.2) | 16.8 (16.6–17.1) |
| 250–1999 ft | 27.4 (27.3–27.5) | 7.0 (6.8–7.1) | 16.0 (15.7–16.3) |
| 2000–3999 ft | 27.5 (27.4–27.7) | 7.0 (6.8–7.2) | 15.0 (14.5–15.4) |
| 4000–5999 ft | 28.0 (27.7–28.2) | 6.8 (6.5–7.0) | 14.4 (13.9–15.0) |
| ≥ 6000 ft | 28.3 (27.9–28.7) | 6.9 (6.3–7.4) | 14.2 (13.2–15.3) |
Change in hematocrit = achieved hematocrit − baseline hematocrit. Adjusted means are least-squares means that use the fitted regression to compute a mean for each elevation group standardized to the population distribution of covariates included in the regression model. The covariates included in the model were age, race, sex, weight, calendar year, estimated glomerular filtration rate at initiation of dialysis, cause of ESRD, profit status of treating center, history of myocardial infarction, history of congestive heart failure, history of cancer, and concurrent iron administration.
In the sensitivity analysis, in which we required that patients have no hospitalizations during the index month, the results were substantively unchanged.
DISCUSSION
In our study of a very large cohort of ESRD patients on maintenance hemodialysis, we found that increasing altitude was associated with modestly lower exogenous EPO use, but with higher achieved hematocrit levels. We also found that EPO resistance decreased monotonically with elevation. These associations existed within subgroups defined by sex, race, age, calendar time, and dialysis center profit status and were not greatly affected by multivariable adjustments for many potential confounding factors. It is known that healthy people living at high altitude have higher average hematocrit levels than people living at sea level; therefore, dialysis providers at high altitude may target higher hematocrit levels in their patients. It is interesting, however, that these higher hematocrit levels are achieved using substantially less EPO.
One possible explanation for our results is that ESRD patients at higher altitudes produce more endogenous EPO than comparable patients living at sea level and thus need less exogenous EPO. This could happen if some renal or extrarenal EPO production capacity is unused at low elevation. Supportive of this hypothesis are reports documenting that ESRD patients increase EPO production in response to acute hypoxic stress, such as pulmonary dysfunction or blood loss.12–14 It is less clear whether EPO levels in ESRD patients would respond to the mild hypoxia induced by moderate increases in elevation. Bosman et al. found that patients with CKD were able to mount a weak EPO response to 5 h of hypobaric hypoxia equivalent to approximately 13,000 ft above sea level.15 A similar study by Quick et al. found no EPO response in ESRD patients exposed to 3 h of hypobaric hypoxia equivalent to approximately 15,000 ft above sea level.16 It is possible, however, that long-term exposure to hypoxia may elicit an EPO response greater than what was observed in short-duration hypobaric experiments.
An alternative explanation for our findings is that patients at higher altitude respond more efficiently to EPO than patients at lower altitudes. This hypothesis has biologic plausibility, as the hypoxia inducible factor (HIF) that regulates EPO expression is known to be involved in the transcription of other proteins participating in erythropoiesis. For example, HIF is known to regulate iron metabolism by controlling expression of transferrin, involved with iron transport, transferrin receptor (TfR), involved with cellular iron uptake, and hepcidin, which affects both intestinal iron absorption and release of iron by macrophages.17–20 Consistent with the hypothesis that EPO response is enhanced at high altitude is a study finding that levels of soluble transferrin receptor (STfR), a measure of TfR expression, were increased under hypobaric hypoxia21 and another study reporting that STfR levels predicted response to EPO therapy among treatment-naïve ESRD patients.22 It has also been reported that pharmacologic stabilization of HIF improves iron utilization.23 If the expression of other proteins involved with erythropoiesis increases with altitude in ESRD, endogenous or exogenous EPO may encounter a more activated system of hypoxia response in patients who live at higher elevations.
Our analysis of patients who did not receive EPO before starting dialysis found that patients at higher elevation start EPO treatment with higher hematocrit levels. This could be caused by either greater endogenous EPO production or increased response to endogenous EPO. However, the observation that patients at high altitude experience a similar increase in hematocrit from baseline to the index month while requiring less exogenous EPO strongly suggests that EPO response is increased at high elevation.
We have conceptualized altitude as a variable that is related to EPO requirements only through its effect on hypoxia-regulated pathways involved with erythropoiesis. However, the distributions of several variables were imbalanced across elevation groups, suggesting that our results could be distorted by unmeasured factors. Racial groups in particular were strongly imbalanced, with most American Indians living at high elevation and most black patients living at sea level. Cause of ESRD, weight, age, and dialysis profit status were also associated with altitude. Nevertheless, when we looked at the associations between elevation and both EPO use and hematocrit levels in subgroups defined by sex, race, age, calendar time, and dialysis profit center status, the same associations were found. Furthermore, multivariable adjustments for an array of clinical and demographic factors did not materially affect our estimates. The robustness of our analysis to restriction and adjustment suggests that our findings are not likely to be the result solely of unmeasured confounding factors.
Further research with more detailed data could help us better understand the biologic processes underlying our findings. For example, measurements of endogenous EPO levels in patients who have not yet begun EPO therapy could indicate whether endogenous EPO production was up-regulated at altitude. More granular longitudinal data on both EPO and iron administration with repeated measures of ferritin and transferrin saturation could be examined to determine whether the interrelations between these factors depended on altitude. Furthermore, data including additional variables associated with EPO response, such as current albumin levels and vascular access type, could help rule out unmeasured confounding as an explanation of these associations.
Given the current concerns about the safety of EPO and persistent questions about optimal hemoglobin targets and dosing algorithms, the results of the present study raise important clinical questions. If patients respond better to EPO at high altitude, it is natural to speculate about whether hemoglobin levels could be normalized at high altitude with a decreased risk of adverse events. Furthermore, the possibility that EPO may be more effective at high altitude raises the question of whether the increased effectiveness could be duplicated at lower elevations. HIF-stabilizing agents, currently under research, mimic the physiologic effects of hypoxia. Future research examining the use of these compounds in combination with EPO therapy may provide useful clinical and scientific information about the process of erythropoiesis and the factors governing EPO treatment response.
CONCISE METHODS
Data
We obtained data from the U.S. Renal Data System (USRDS) and the U.S. Geological Survey (USGS). The USRDS contains detailed data on all patients in Medicare's ESRD program, including information collected at dialysis initiation (reported on the Medical Evidence Form, CMS-2728), describing demographics, primary cause of ESRD, clinical data (e.g., weight), and certain laboratory measurements (e.g., serum albumin and hematocrit levels). In addition, the USRDS contains all Medicare Part A and B claims that include information on diagnoses and procedures recorded for all hospitalizations and outpatient visits. The USRDS also contains claims for total monthly EPO doses, reported with the final hematocrit level recorded during the month.
From USGS data, we obtained a list of 137,061 U.S. cities along with their state and county Federal Information Processing Standards code, and elevation. We also obtained a list of U.S. zip codes with their primary city and the average elevation of the county, as reported by the USGS. We then matched the zip code data to the USGS city elevation data using city name, and the county and state Federal Information Processing Standards code. This allowed us to assign to each zip code the elevation of its primary city. For zip codes that could not be matched to a city in the USGS data, we set the elevation equal to the average elevation in the county. Altitudes for each patient were obtained using the zip code for their primary residence (assessed during the index month) as reported in the USRDS data.
The study investigators obtained Data Use Agreements from the National Institute of Digestive and Diabetes and Kidney Diseases. The Brigham and Women's Hospital Institutional Review Board approved this research.
Patient Selection
From the USRDS standard analytic files, we selected all patients who initiated hemodialysis treatment between January 1, 1995 and December 31, 2004. We excluded all patients who did not survive to 9 mo after entry into the ESRD program. Follow-up began at the start of the 9th month. For each patient, the EPO dose and the hematocrit level were recorded for the first month during follow-up (the index month) in which a patient received EPO and spent less than 5 d in the hospital. Patients were dropped if they switched to peritoneal dialysis, received a transplant before the index month, or reached the 15th month without having received an eligible EPO administration. We converted the total EPO administered during the index month into units per week (U/wk). To compute units per week, we first computed units per day by dividing the total units of EPO administered during the month by the days in the month minus the time the patient spent in hospital during the month (EPO exposure captured in the USRDS is almost entirely exposure from outpatient treatment at a dialysis facility.)
Statistical Analysis
We classified all patients into four groups based on the elevation above sea level of their zip code of residence: <250 ft, 250 to 1999 ft, 2000 to 3999 ft, 4000 to 5999 ft, and >6000 ft. To depict the geographic distribution of the elevations, we computed the median elevation of patients in each county and displayed it on a map. We calculated means and frequencies of patient characteristics by elevation group, and graphed the average hematocrit and EPO dose during the index month across elevation groups. We also explored the associations among altitude, hematocrit, and EPO dose in subgroups defined by sex, race, age, calendar time, cause of ESRD, and dialysis profit center status. We computed a measure of EPO resistance that was defined to be a patient's EPO dose in the index month divided by the hematocrit level reported to Centers for Medicare and Medicaid Services during that month. We plotted EPO resistance and 95% confidence intervals for each elevation group.
To adjust for possible confounding of the association between elevation and achieved hematocrit, EPO dose requirement, and EPO resistance, we fit multivariable linear regression models of hematocrit, EPO dose, EPO resistance that included: age, sex, race, calendar year, weight, primary recorded cause of ESRD (diabetes, hypertension, glomerulonephritis, other), history of cancer, history of myocardial infarction, co-administration of iron, and whether or not the EPO received during the index month was administered at a for-profit dialysis center. These regression models were used to compute a population-averaged (least-squares) mean and 95% confidence interval for each elevation group. This approach uses the fitted regression model to estimate a mean EPO dose and hematocrit value for each elevation group standardized to the distribution of covariates observed in the overall population. In a secondary analysis, we estimated least-squares means of hematocrit levels measured at entry into the ESRD program (baseline hematocrit), change in hematocrit from baseline to the index month, and EPO administered during index month among patients who did not receive EPO before entry into the ESRD program. In a sensitivity analysis, we redefined the index month to be the first month during follow-up in which a patient received EPO and had no hospitalizations.
All statistical analyses were performed in SAS, version 9.1.24
DISCLOSURES
M.A.B. and W.C.W. receive salary support from Amgen for an unrelated research project. B.D.B. is an employee of Amgen. S.S. has consulted with Research Triangle Institute on a project funded by Amgen.
Data reported herein were supplied by the U.S. Renal Data System. Interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the U.S. government.
Acknowledgments
The authors thank Drs. Allan Collins and David Gilbertson at U.S. Renal Data System for helpful comments and Jessica Agnew-Blais for manuscript preparation. M.A.B. was supported by a career development award from the National Institute on Aging (AG-027400).
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, Schwab SJ, Goodkin DA: The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 339: 584–590, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Eschbach JW, Abdulhadi MH, Browne JK, Delano BG, Downing MR, Egrie JC, Evans RW, Friedman EA, Graber SE, Haly R, Korbet S, Krants SB, Lundin AP, Nissenson AR, Ogden DA, Paganini EP, Rader B, Rutsky EA, Stivelman J, Stone WJ, Teschan P, VanStone JC, Van Wyck DB, Zuckerman K, Adamson JW: Recombinant human erythropoietin in anemic patients with end-stage renal disease: results of a phase III multicenter clinical trial. Ann Intern Med 111: 992–1000, 1989 [DOI] [PubMed] [Google Scholar]
- 3.Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, Reddan D; CHOIR Investigators: Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 355: 2085–2098, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Lacson E, Jr., Ofsthun N, Lazarus JM: Effect of variability in anemia management on hemoglobin outcomes in ESRD. Am J Kidney Dis 41: 111–124, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Berns JS, Elzein H, Lynn RI, Fishbane S, Meisels IS, Deoreo PB: Hemoglobin variability in epoetin-treated hemodialysis patients. Kidney Int 64: 1514–1521, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Fishbane S, Berns JS: Hemoglobin cycling in hemodialysis patients treated with recombinant human erythropoietin. Kidney Int 68: 1337–1343, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Ebben JP, Gilbertson DT, Foley RN, Collins AJ: Hemoglobin level variability: associations with comorbidities, intercurrent events, and hospitalizations. Clin J Am Soc Nephrol 1: 1205–1210, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Fishbane S, Berns JS: Evidence and implications of haemoglobin cycling in anaemia management. Nephrol Dial Transplant 22: 2129–2232, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Gilbertson DT, Ebben JP, Foley RN, Weinhandl ED, Bradbury B, Collins AJ: Hemoglobin level variability: associations with mortality. Clin J Am Soc Nephrol 3: 133–138, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang W, Israni RK, Brunelli SM, Joffe MM, Fishbane S, Feldman HI: Hemoglobin variability and mortality in ESRD. J Am Soc Nephrol 18: 3164–3170, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Maxwell P: HIF-1: an oxygen response system with special relevance to the kidney. J Am Soc Nephrol 14: 2712–2722, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Chandra M, Clemons GK, McVicar MI: Relation of serum erythropoietin levels to renal excretory function: evidence for lowered set point for erythropoietin production in chronic renal failure. J Pediatr 113: 1015–1021, 1988 [DOI] [PubMed] [Google Scholar]
- 13.Kato A, Hishida A, Kumagai H, Furuya R, Nakajima T, Honda N: Erythropoietin production in patients with chronic renal failure. Ren Fail 16: 645–651, 1994 [DOI] [PubMed] [Google Scholar]
- 14.Ross RP, McCrea JB, Besarab A: Erythropoietin response to blood loss in hemodialysis patients in blunted but preserved. ASAIO J 40: M880–M885, 1994 [DOI] [PubMed] [Google Scholar]
- 15.Bosman DR, Osborne CA, Marsden JT, Macdougall IC, Gardner WN, Watkins PJ: Erythropoietin response to hypoxia in patients with diabetic autonomic neuropathy and non-diabetic chronic renal failure. Diabet Med 19: 65–69, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Quick J, Eichenberger A, Binswanger U: Stimulation of erythropoietin in renal insufficiency by hypobaric hypoxia. Nephrol Dial Transplant 7: 1002–1006, 1992 [PubMed] [Google Scholar]
- 17.Rolfs A, Kvietikova I, Gassmann M, Wenger RH: Oxygen-regulated transferrin expression is mediated by hypoxia-inducible factor-1. J Biol Chem 272: 20055–20062, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Lok CN, Ponka P: Identification of a hypoxia response element in the transferrin receptor gene. J Biol Chem 274: 24147–24152, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Ganz T: Hepcidin and its role in regulating systemic iron metabolism. Hematology Am Soc Hematol Educ Program 29–35: 507, 2006 [DOI] [PubMed]
- 20.Peyssonnaux C, Zinkernagel AS, Schuepbach RA, Rankin E, Vaulont S, Haase VH, Nizet V, Johnson RS: Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs). J Clin Invest 117: 1926–1932, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robach P, Fulla Y, Westerterp KR, Richalet JP: Comparative response of EPO and soluble transferrin receptor at high altitude. Med Sci Sports Exerc 36: 1493–1498; discussion 1492, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Beguin Y, Clemons GK, Pootrakul P, Fillet G: Quantitative assessment of erythropoiesis and functional classification of anemia based on measurements of serum transferrin receptor and erythropoietin. Blood 81: 1067–1076, 1993 [PubMed] [Google Scholar]
- 23.Klaus S, Arend M, Fourney P, Flippin L, Gervasi D, Guenzler V, Kochendoerfer G, Langsetmo I, Lin A, Lomongsod E, McDaniel D, Meier-Davis S, Seeley T, Spong S, Liu: Induction of erythropoiesis and iron utilization by the HIF prolyl hydroxylase inhibitor FG-4592. [Abstract F-FC050]. American Society of Nephrology 38th Annual Meeting & Scientific Exposition, Philadelphia, 2005
- 24.SAS Institute: SAS computer program, version 9.1. Cary, NC: SAS Institute, 2003