Abstract
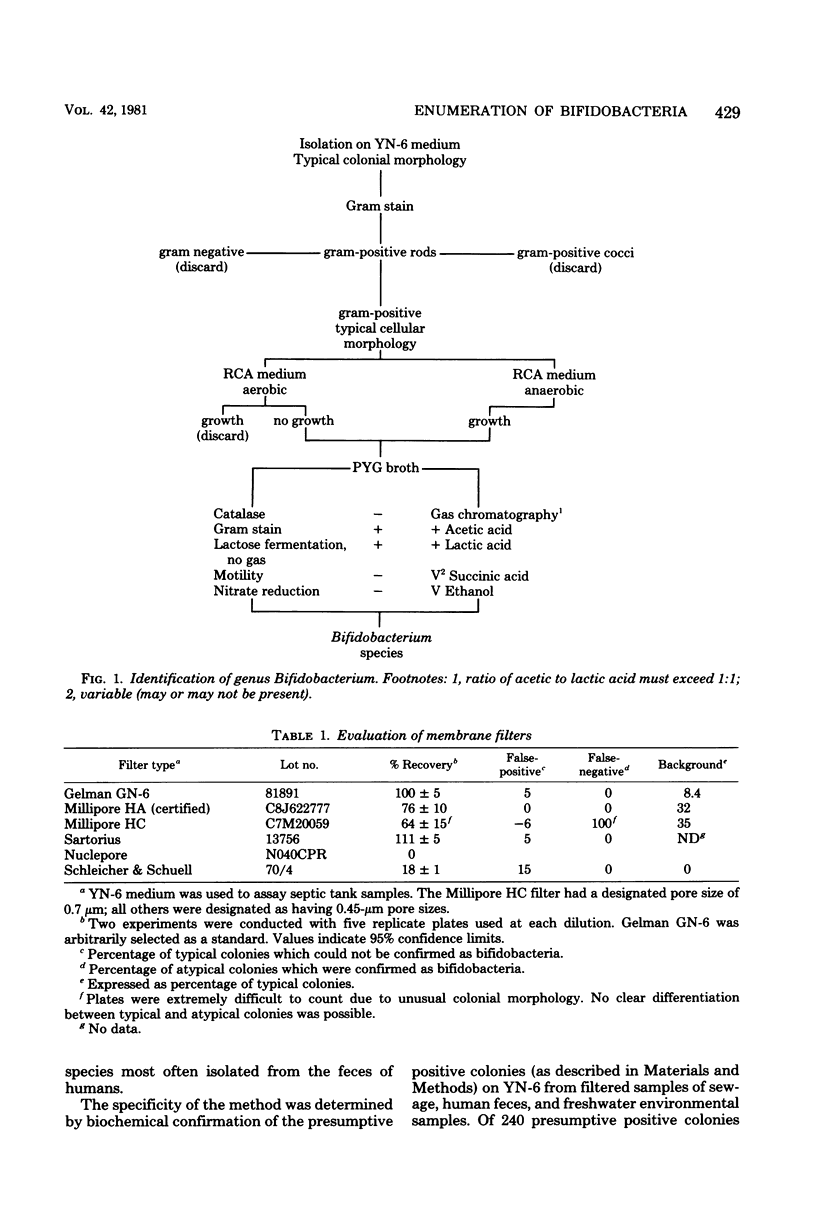
A membrane filter technique has been developed for the enumeration of bifidobacteria in natural aquatic environments. The technique is quantitative, selective, and differential. The medium (YN-6) contains: yeast extract, 2.0 g; agar, 1.5 g; polypeptone peptone, 1.0 g; vitamin-free Casamino Acids, 0.8 g; sodium chloride, 0.32 g; and L-cysteine hydrochloride, 0.003 g; in 100 ml of deionized water. The medium is adjusted to pH 7.0 before autoclaving. Nalidixic acid (80 micrograms/ml), neomycin sulfate (2.5 micrograms/ml), and bromcresol green (300 micrograms/ml) are included as selective and differential agents. After incubation for 48 h at 37 degrees C in an anaerobic environment, Gram-stained smears from green, glistening, smooth entire colonies are examined microscopically for typical bifidobacterial morphology. No significant difference in recoveries was observed when YN-6 was compared with reinforced clostridial agar, using bifidobacteria freshly isolated from feces and raw sewage. Using this technique with aquatic and fecal samples, less than 9% false-positive and 8% false-negative isolates were observed. These results indicated that the medium was able to satisfactorily recover organisms from a variety of situations.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bullen C. L., Tearle P. V. Bifidobacteria in the intestinal tract of infants: an in-vitro study. J Med Microbiol. 1976 Aug;9(3):335–344. doi: 10.1099/00222615-9-3-335. [DOI] [PubMed] [Google Scholar]
- Eisenhart C., Wilson P. W. STATISTICAL METHODS AND CONTROL IN BACTERIOLOGY. Bacteriol Rev. 1943 Jun;7(2):57–137. doi: 10.1128/br.7.2.57-137.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evison L. M., James A. A comparison of the distribution of intestinal bacteria in British and East African water sources. J Appl Bacteriol. 1973 Mar;36(1):109–118. doi: 10.1111/j.1365-2672.1973.tb04078.x. [DOI] [PubMed] [Google Scholar]
- GLICK M. C., SALL T., ZILLIKEN F., MUDD S. Morphological changes of Lactobacillus bifidus var. pennsylvanicus produced by a cell-wall precursor. Biochim Biophys Acta. 1960 Jan 15;37:361–363. doi: 10.1016/0006-3002(60)90251-1. [DOI] [PubMed] [Google Scholar]
- GYLLENBERG H., NIEMELA S., SORMUNEN T. Survival of bifid bacteria in water as compared with that of coliform bacteria and enterococci. Appl Microbiol. 1960 Jan;8:20–22. doi: 10.1128/am.8.1.20-22.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHRAMM M., KLYBAS V., RACKER E. Phosphorolytic cleavage of fructose-6-phosphate by fructose-6-phosphate phosphoketolase from Acetobacter xylinum. J Biol Chem. 1958 Dec;233(6):1283–1288. [PubMed] [Google Scholar]
- de Vries W., Gerbrandy S. J., Stouthamer A. H. Carbohydrate metabolism in Bifidobacterium bifidum. Biochim Biophys Acta. 1967 Apr 25;136(3):415–425. doi: 10.1016/0304-4165(67)90001-3. [DOI] [PubMed] [Google Scholar]


