Synopsis
Sympathetic nervous system activation in heart failure, as indexed by elevated norepinephrine levels, higher muscle sympathetic nerve activity and reduced heart rate variability, is associated with pathologic ventricular remodeling, increased arrhythmias, sudden death, and increased mortality. Recent evidence suggests that HMG-CoA reductase inhibitor (statin) therapy may provide survival benefit in heart failure of both ischemic and non-ischemic etiology, and one potential mechanism of benefit of statins in heart failure is modulation of the autonomic nervous system. Animal models of heart failure demonstrate reduced sympathetic activation and improved sympathovagal balance with statin therapy. Initial human studies have reported mixed results. Ongoing translational studies and outcomes trials will help delineate the potentially beneficial effects of statins on the autonomic nervous system in heart failure.
Keywords: statins, autonomic nervous system, heart failure, sympathetic nervous system
Introduction
Heart failure (HF) is a national public health problem with an overall prevalence in the U.S. of approximately 5 million. Despite advances in our understanding of HF pathophysiology and treatment, one out of five patients newly diagnosed with HF will die within one year. Death is often due to gradual worsening of HF (progressive pump dysfunction), although as many as one-half of HF deaths are sudden1–3. Numerous observational studies suggest that statins have survival benefit in HF, reducing all cause mortality as well as arrhythmic deaths4. Recent evidence suggests that HMG-CoA reductase inhibitors (statins) may have “pleiotrophic” mechanisms, beyond anti-ischemic or lipid-lowering effects, that are beneficial in HF5–9. Statins may improve endothelial function, reverse myocardial remodeling, inhibit inflammatory cytokines, and potentiate nitric oxide (NO) synthesis. Furthermore, modulation of the autonomic nervous system by statins may be an important, potentially beneficial, mechanism of action in HF.
The Autonomic Nervous System in Heart Failure
The processes contributing to the progression of systolic HF are complex and inter-related. At the core of the syndrome is impaired cardiac function, associated with ongoing remodeling, inflammation, neurohormonal activation, and impaired autonomic nervous system (ANS) function. Activation of sympathetic drive plays a pivotal role in the progression of HF. Clinical signs and symptoms of the hyperadrenergic state in some HF patients includes tachycardia, vascular constriction, diaphoresis, and oliguria10. Sympathetic over-activation in HF is characterized by increased levels of circulating norepinephrine as well as increased cardiac and renal norepinephrine spillover11, 12. Both increased neuronal release of norepinephrine and decreased norepinephrine reuptake contribute to the increased cardiac adrenergic drive of HF13.
In addition to elevated systemic and cardiac norepinephrine levels, autonomic imbalance in HF has also been indexed by heart rate variability (HRV) analyses. HRV, using time-domain and frequency-domain indices, is a standardized tool for examining autonomic nervous system activity in various disease states such as hypertension, diabetes, coronary artery disease, as well as myocardial dysfunction. Standard time-domain indices include standard deviation of the normal-normal intervals (SDNN) and the square root of the mean squared differences of successive normal-normal intervals (RMSDD). Standard frequency-domain measurements include low frequency (LF) and high frequency (HF) 14 HRV is decreased in systolic HF, and correlates with extent of left ventricular dysfunction. Similar to studies post-myocardial infarction, HF is characterized by a decrease in time-domain indices of HRV, which correlates with severity of left ventricular dysfunction. The relationship between HF severity frequency-domain indices (spectral components) of HRV is more complex, however; HF severity has been correlated with both increased and decreased LF power.15, 16 17
Muscle sympathetic nerve activity (MSNA, bursts/minute) as quantified by direct sympathetic microneurography at the peroneal nerve, has been validated as a tool to study sympathetic nervous system activation in humans with HF 18–20, as well as in a variety of other disease processes, including hypertension and obesity21. MSNA at rest has consistently been found to be elevated in HF patients when compared to normal controls, and furthermore, life-prolonging therapies for HF patients have also been shown to decrease MSNA 22, 23.
Impairment, or decreased responsivity, of arterial and cardiopulmonary (sympatho-inhibitory) baroreflexes are seen in HF.24 It is now recognized that baroreflex-mediated mechanisms are not necessarily a cause of the increased sympathetic drive of HF, but may be a consequence of it. Additional potential mechanisms of the sympathetic activation of HF include increased sensitivity of the muscle metaboreceptors and/or mechanoreceptors located in skeletal muscle, alteration of the central endogenous nitric oxide mechanism, augmentation of central angiotensin II, stimulation of reactive oxidant species, or HF – associated sleep disordered breathing. Further, norepinephrine levels at the nerve terminal may be augmented by prejunctional facilitation of neurotransmitter release via beta-2 adrenergic or angiotensin AT1 receptors18, 25.
Sympathetic Activation and Prognosis in Heart Failure
Sympatho-excitation in HF is initially a compensatory mechanism, with inotropic and chronotropic responses of the heart serving to maintain cardiac output, blood pressure, and organ perfusion. However, prolonged sympathetic over-activity may lead to depletion of cardiac norepinephrine stores and decreased beta-adrenoreceptor density, impairing compensatory sympathetic-stimulated inotropy, thus leading to depressed cardiac function13, 26, 27. Norepinephrine also has direct, toxic effects on cardiomyocyte viability28 and is a predisposing factor in the development of cardiac arrhythmias29.
Sympathetic nervous system activation is associated with pathologic ventricular remodeling, increased arrhythmias, sudden death, and increased mortality in chronic HF. Supra-normal levels of plasma norephinephrine correlate with progressive HF and sudden death11. Increased cardiac norepinephrine spillover rates also independently predict worse survival in HF30. Cardiac sympathetic nervous system dysfunction, as indexed by reduced cardiac uptake of radiolabelled I-23 metaiodobenzylguanidine (MIBG), also predicts increased mortality in patients with HF and cardiomyopathy31.
Multiple studies have linked HRV abnormalities in HF to prognosis. Depressed HRV predicts hemodynamic compromise, sudden death and death from progressive pump dysfunction32, 33. SDNN has been shown to be strongly predictive of mortality in HF; in the UK-HEART study, SDNN < 50msec was associated with >50% mortality compared to only 5.5% in the group with SDNN > 100msec34. Spectral components of HRV also predict HF prognosis; decreased controlled breathing low frequency power in both derivation and validation samples of an Italian study independently predicted a roughly three-fold increased risk for sudden death33.
Autonomic changes in HF are linked to activation of renin-angiotensin-aldosterone-systems in HF; standard, life-prolonging neurohormonal blockers for HF – including ACEIs, ARBs, beta-blockers, aldosterone antagonists – are all associated with reduced sympathetic activation and improved autonomic balance22, 35–39. Furthermore, improving mechanical function of the ventricles with cardiac resynchronization therapy is also linked to decreased sympathetic nervous system activation as assessed by HRV analysis and sympathetic microneurography. In fact, sympathoinhibition is a characteristic of clinical responders to biventricular pacing39–41.
Statins in Heart Failure
Several observational studies have linked statin therapy in HF to significantly improved survival. The survival benefit associated with statins in HF patients was first reported in an analysis of 551 advanced, systolic HF patients [LVEF 25±7, age 52±13, 77% NYHA III–IV, 45% ischemic etiology] followed at a single university center42. Survival without urgent transplantation at one year was 84% in statin-treated and 70% in non-statin-treated patients (HR 0.45, 95% confidence interval [CI] 0.30 to 0.67). The survival advantage associated with statin therapy was observed in both ischemic and non-ischemic HF subjects. Furthermore, statin therapy was not only associated with decreased all-cause mortality, but was also associated with decreased progressive HF death, and sudden death. Multiple subsequent observational studies in various HF populations have also demonstrated significantly improved survival associated with statin use 43–48.
Observational studies have also demonstrated decreased arrhythmia risk in patients treated with statins. In an analysis of the DEFIbrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) trial, statin use was associated with decreased sudden death and decreased appropriate implantable cardioverter-defibrillator (ICD) shocks in non-ischemic HF patients49 (Figure 1). In patients with coronary artery disease and ICDs, statin therapy has been associated with significantly decreased re-occurrence of ventricular arrhythmias50, 51. Statin use has also been correlated with decreased incidence of atrial fibrillation52. Reduced activation of the sympathetic nervous system may be, in part, responsible for the decreased sudden death and arrhythmias observed with statin therapy.
Figure 1.
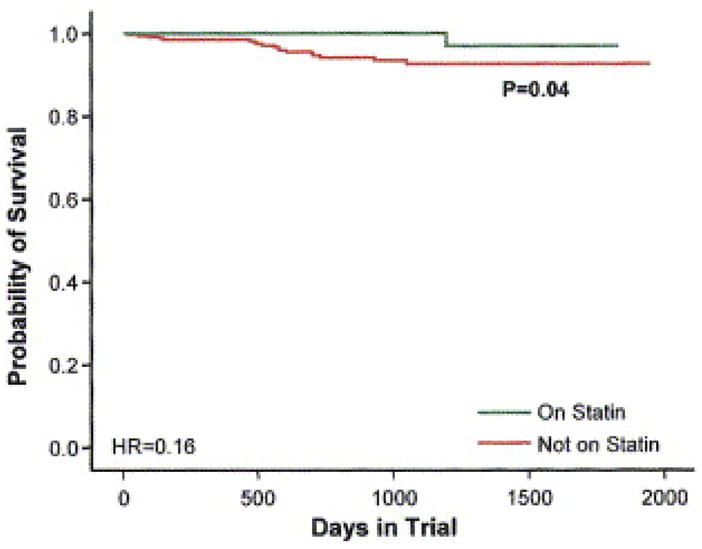
The Kaplan-Meier estimates of arrhythmic sudden death plus resuscitated cardiac arrest among patients treated with statins and those not taking statins in the 458 patients enrolled in the DEFINITE trial. From Goldberger JJ, Subacius H, Schaechter A, et al. Effects of statin therapy on arrhythmic events and survival in patients with nonischemic dilated cardiomyopathy. J Am Coll Cardiol. Sep 19 2006;48(6):1228–1233, with permission.
Statins’ Effect on the Autonomic Nervous System in HF: Animal Studies
Improvement in autonomic nervous system function with statin treatment has been demonstrated in animal models of HF. Pliquett et al. studied autonomic function in rabbits with pacing-induced HF compared to normal control rabbits, focusing on HRV53. Rabbits with HF had significantly reduced HRV, as assessed by SDNN and power spectral analysis compared to non-HF controls. However, HF rabbits fed simvastatin for three weeks had higher HRV than HF rabbits not treated with simvastatin. HRV increased incrementally with simvastatin dose; the HF rabbits fed the highest dose (3mg/kg/day) had HRV similar to non-HF controls, both in terms of SDNN, low frequency power, high frequency power, and total power.
Pliquett et al. also investigated the effects of statin therapy on baroreceptor sensitivity, renal sympathetic nerve activity, and plasma norepinephrine levels in rabbits with pacing-induced HF9. Plasma norepinephrine levels were elevated in HF rabbits, compared to controls; however, norepinephrine levels were significantly lower in HF rabbits who received moderate to high dose simvastatin compared to non-statin treated HF animals. Renal sympathetic nerve activity (RSNA), directly measured by surgically implanted electrodes, also confirmed lower sympathoexcitation in those HF rabbits treated with simvastatin. The statin-treated HF animals had lower resting RSNA, as well as lower RSNA response to smoke inhalation and sodium nitroprusside injection compared to HF animals not on statins (Figure 2). Furthermore, baroreflex responses in terms of heart rate and RSNA were depressed in HF rabbits treated with vehicle but restored to near-normal in HF rabbits treated with 1.5 – 3.0 mg/kg/day of simvastatin. Cholesterol levels were unchanged by simvastatin in both studies, suggesting a cholesterol-independent, or pleiotrophic, effect of statins on autonomic function.
Figure 2.
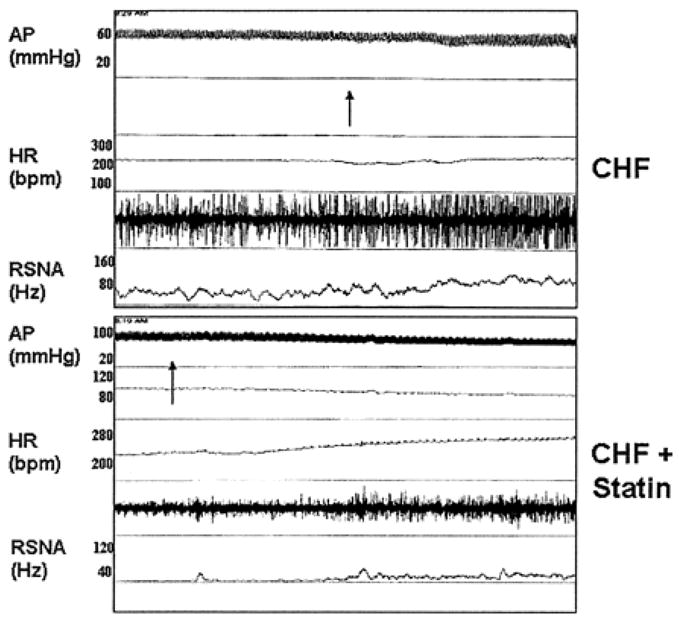
Original recording of arterial pressure (AP), heart rate (HR), and renal sympathetic nerve activity (RSNA) in 1 conscious heart failure animal (top) and 1 conscious heart failure animal treated with simvastatin (bottom) for 3 weeks. At arrows, an injection of SNP was given intravenously. From Pliquett RU, Cornish KG, Peuler JD, Zucker IH. Simvastatin normalizes autonomic neural control in experimental heart failure. Circulation. May 20 2003;107(19):2493–2498, with permission.
Statins’ Effect on the Autonomic Nervous System: Human Studies
Statin-associated improvements in autonomic function have been observed in non-HF disease states such as coronary artery disease and hyperlipidemia. In a study of patients with previous myocardial infarction referred for cardiac catheterization, patients treated with statins had higher SDNN; furthermore, statin use was an independent predictor of higher HRV on multivariate analysis54. In a prospective study of 40 hyperlipidemic subjects with and without coronary artery disease, atorvastatin 20 mg/day for a two year period resulted in significant improvement in time- and frequency- domain indices of HRV, including SDNN, RMSSD, low frequency, and high frequency power compared to controls. LDL level after atorvastatin treatment did not correlate with indices of HRV55. In a third study of 37 subjects with combined hyperlipidemia, both atorvastatin and fenofibrate improved time- and frequency-domain indices of HRV56.
More recently, the effect of statins on autonomic tone in human subjects with HF has been examined. Three studies have investigated the effect of statins on sympathovagal balance as indexed by HRV, with mixed findings. One small, single-arm study of simvastatin 20 mg/day for six weeks in 25 patients with dilated, non-ischemic cardiomyopathy found no treatment-related change in HRV, as indexed by 5 minute sitting total spectral power, respiratory frequency area with deep breathing (parasympathetic stress) or low-frequency area with Valsalva (sympathetic stress)57. Another study of 21 patients with stable, systolic HF (EF < 45%) randomized patients to three months of atorvastatin 40 mg/day vs. placebo. Atorvastatin therapy had no significant effect on time-domain indices of HRV, including SDNN and RMSSD. Atorvastatin therapy impacted frequency domain measures of HRV, with decreased low frequency power and decreased low frequency to high frequency ratio after 3-month treatment period compared to no change seen in controls. The authors concluded that this represented a modest improvement in sympatho-vagal balance, indicating a potential reduction in sympathetic activity.58
The largest study to date evaluated 80 NYHA III, systolic HF patients with hyperlipidemia randomized to atorvastatin 10 mg vs. placebo for three months59. Those randomized to atorvastatin, compared to controls, showed significant increase in the HRV time-domain indices of SDNN and RMSSD, as assessed by five-minute high resolution ECG at baseline and after three months of treatment (Figure 3). Statin therapy was also associated with decreased QT interval variability and decreased corrected QT interval. Cholesterol lowering was not correlated with changes in HRV or repolarization, again suggesting a mechanism of action of statins not associated with lipid-lowering.
Figure 3.
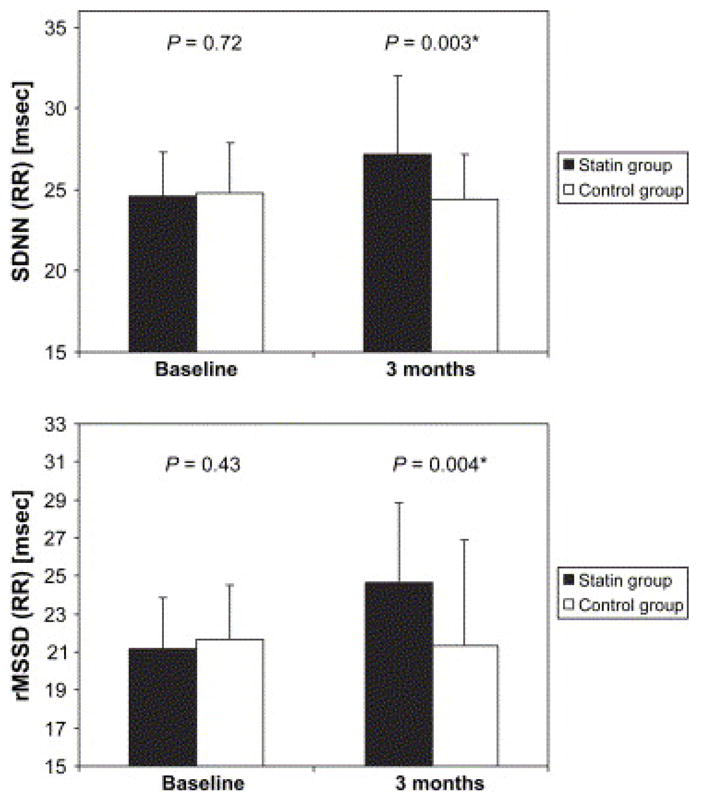
Heart rate variability parameters in the statin and control groups at baseline and after 3 months of the study (the results are presented as mean + SD). From Vrtovec B, Okrajsek R, Golicnik A, Ferjan M, Starc V, Radovancevic B. Atorvastatin Therapy Increases Heart Rate Variability, Decreases QT Variability, and Shortens QTc Interval Duration in Patients With Advanced Chronic Heart Failure. Journal of Cardiac Failure. 2005;11(9):684, with permission.
An additional study evaluated the effects of fluvastatin in 29 ischemic HF patients with hyperlipidemia on heart rate recovery, defined as the difference between the heart rate at peak exercise and the first and third minutes of recovery60. Decreased heart rate recovery after exercise in HF is associated with high mortality, and is thought to represent an abnormal withdrawal of vagal tone61. After 3 months of fluvastatin therapy, heart rate recovery was significantly augmented at both one and three minutes after symptom-limited maximum exercise test. The authors concluded that augmentation of heart rate recovery may have represented lessened sympathetic or increased parasympathetic tone associated with statin therapy.
Preliminary reports demonstrate an effect of statins in lowering MSNA in HF. In a single-arm study of 5 HF patients treated with simvastatin 40 mg QD for one month, resting MSNA was significantly lower post – treatment compared to pre-treatment (65 ± 6 vs. 77 ± 2 bursts/100 beats)62. In another study of 8 HF subjects already on statin therapy, MSNA increased significantly 8 weeks after discontinuation of statin but returned to baseline 4 weeks after statin therapy was restarted. There was no significant effect of statins on plasma norepinephrine levels.63
Potential Mechanisms for Statins’ Modulation of the Autonomic Nervous System
There are several potential mechanisms for the beneficial effects of statins on autonomic nervous system function in HF. Both angiotensin II and nitric oxide have been implicated in the modulation of sympathetic tone by statins in HF. Gao et al. first demonstrated that there is intense free radical stress in the autonomic areas of the brain in the HF state, characterized by upregulation of angiotensin receptors and NADPH oxidase subunits in the rostral ventrolateral medulla as well as NADPH-dependent superoxide anion production64. This group subsequently linked autonomic improvement to an effect of simvastatin on inhibition of central angiotensin II and the superoxide pathway65. In this study of pacing-induced HF in rabbits, the heightened blood pressure and renal sympathetic nerve activity responses to intracerebral angiotensin II injection seen in CHF animals was abolished by simvastatin therapy. Importantly, simvastatin therapy was also seen to decrease mRNA and protein expression of angiotensin receptor and NADPH oxidase subunits and to inhibit production of superoxide (O2−) in the rostral ventrolateral medulla of CHF rabbits.
Investigations in hyperlipidemia have shed light on a potential molecular component for statins’ effects on autonomic function. In a cross-over study of HRV in 30 patients with hyperlipidemia, pravastatin therapy resulted in an increase in HF power, an index of parasympathetic responsiveness. Increased HF power correlated with increased expression of α-subunit of the heterotrimeric G-protein, Gαi2, a molecular component of the parasympathetic signaling component in the heart. Interestingly, simvastatin did not change HRV or Gαi2 expression, which the authors attributed differences in hydrophobicity between the two statins 66.
Conclusions
There is increasing evidence that the non-lipid-lowering, “pleiotrophic” effects of statins may prove beneficial to patients with both ischemic and non-ischemic heart failure. Statins’ ability to decrease sympathetic activation and restore autonomic balance has clearly been demonstrated in animal models of heart failure. Preliminary human studies also suggest a positive effect of statins on sympathovagal balance. Additional investigation is required to further delineate the optimal type of statin, dosage, and course of therapy to improve autonomic function and improve outcomes in heart failure.
Acknowledgments
Dr. Horwich is supported by the National Institutes of Health grant 1K23HL085097. Dr. Middlekauff is supported by the National Institutes of Health grant 1RO1 HL084525-01
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Tamara Horwich, UCLA Medical Center, Los Angeles, CA.
Holly Middlekauff, UCLA Medical Center, Los Angeles, CA.
References
- 1.Jessup M, Brozena S. Heart failure. N Engl J Med. 2003 May 15;348(20):2007–2018. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 2.Cleland JG, Chattopadhyay S, Khand A, Houghton T, Kaye GC. Prevalence and incidence of arrhythmias and sudden death in heart failure. Heart Fail Rev. 2002 Jul;7(3):229–242. doi: 10.1023/a:1020024122726. [DOI] [PubMed] [Google Scholar]
- 3.Heart Disease and Stroke Statistics - 2004 update. Accessed August 21, 2007.
- 4.Khush KK, Waters DD. Effects of Statin Therapy on the Development and Progression of Heart Failure: Mechanisms and Clinical Trials. Journal of Cardiac Failure. 2006;12(8):664. doi: 10.1016/j.cardfail.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Lefer DJ. Statins as potent antiinflammatory drugs. Circulation. 2002 Oct 15;106(16):2041–2042. doi: 10.1161/01.cir.0000033635.42612.88. [DOI] [PubMed] [Google Scholar]
- 6.von Haehling S, Anker SD, Bassenge E. Statins and the role of nitric oxide in chronic heart failure. Heart Fail Rev. 2003 Jan;8(1):99–106. doi: 10.1023/a:1022103222857. [DOI] [PubMed] [Google Scholar]
- 7.Hayashidani S, Tsutsui H, Shiomi T, et al. Fluvastatin, a 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitor, attenuates left ventricular remodeling and failure after experimental myocardial infarction. Circulation. 2002 Feb 19;105(7):868–873. doi: 10.1161/hc0702.104164. [DOI] [PubMed] [Google Scholar]
- 8.Dechend R, Fiebeler A, Park JK, et al. Amelioration of angiotensin II-induced cardiac injury by a 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitor. Circulation. 2001 Jul 31;104(5):576–581. doi: 10.1161/hc3001.092039. [DOI] [PubMed] [Google Scholar]
- 9.Pliquett RU, Cornish KG, Peuler JD, Zucker IH. Simvastatin normalizes autonomic neural control in experimental heart failure. Circulation. 2003 May 20;107(19):2493–2498. doi: 10.1161/01.CIR.0000065606.63163.B9. [DOI] [PubMed] [Google Scholar]
- 10.Tang WH, Francis GS. Neurohormonal upregulation in heart failure. Heart Fail Clin. 2005 Apr;1(1):1–9. doi: 10.1016/j.hfc.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Cohn JN, Levine TB, Olivari MT, et al. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med. 1984 Sep 27;311(13):819–823. doi: 10.1056/NEJM198409273111303. [DOI] [PubMed] [Google Scholar]
- 12.Hasking GJ, Esler MD, Jennings GL, Burton D, Johns JA, Korner PI. Norepinephrine spillover to plasma in patients with congestive heart failure: evidence of increased overall and cardiorenal sympathetic nervous activity. Circulation. 1986 Apr;73(4):615–621. doi: 10.1161/01.cir.73.4.615. [DOI] [PubMed] [Google Scholar]
- 13.Eisenhofer G, Friberg P, Rundqvist B, et al. Cardiac sympathetic nerve function in congestive heart failure. Circulation. 1996 May 1;93(9):1667–1676. doi: 10.1161/01.cir.93.9.1667. [DOI] [PubMed] [Google Scholar]
- 14.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996 Mar 1;93(5):1043–1065. [PubMed] [Google Scholar]
- 15.Ponikowski P, Anker SD, Chua TP, et al. Depressed heart rate variability as an independent predictor of death in chronic congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1997 Jun 15;79(12):1645–1650. doi: 10.1016/s0002-9149(97)00215-4. [DOI] [PubMed] [Google Scholar]
- 16.Szabo BM, van Veldhuisen DJ, van der Veer N, Brouwer J, De Graeff PA, Crijns HJ. Prognostic value of heart rate variability in chronic congestive heart failure secondary to idiopathic or ischemic dilated cardiomyopathy. Am J Cardiol. 1997 Apr 1;79(7):978–980. doi: 10.1016/s0002-9149(97)00026-x. [DOI] [PubMed] [Google Scholar]
- 17.Casolo G, Balli E, Taddei T, Amuhasi J, Gori C. Decreased spontaneous heart rate variability in congestive heart failure. Am J Cardiol. 1989 Nov 15;64(18):1162–1167. doi: 10.1016/0002-9149(89)90871-0. [DOI] [PubMed] [Google Scholar]
- 18.Floras JS. Sympathetic activation in human heart failure: diverse mechanisms, therapeutic opportunities. Acta Physiol Scand. 2003 Mar;177(3):391–398. doi: 10.1046/j.1365-201X.2003.01087.x. [DOI] [PubMed] [Google Scholar]
- 19.Middlekauff HR, Hamilton MA, Stevenson LW, Mark AL. Independent control of skin and muscle sympathetic nerve activity in patients with heart failure. Circulation. 1994 Oct;90(4):1794–1798. doi: 10.1161/01.cir.90.4.1794. [DOI] [PubMed] [Google Scholar]
- 20.Grassi G, Seravalle G, Cattaneo BM, et al. Sympathetic activation and loss of reflex sympathetic control in mild congestive heart failure. Circulation. 1995 Dec 1;92(11):3206–3211. doi: 10.1161/01.cir.92.11.3206. [DOI] [PubMed] [Google Scholar]
- 21.Grassi G, Seravalle G, Quarti-Trevano F, Dell’Oro R, Bolla G, Mancia G. Effects of hypertension and obesity on the sympathetic activation of heart failure patients. Hypertension. 2003 Nov;42(5):873–877. doi: 10.1161/01.HYP.0000098660.26184.63. [DOI] [PubMed] [Google Scholar]
- 22.De Matos LD, Gardenghi G, Rondon MU, et al. Impact of 6 months of therapy with carvedilol on muscle sympathetic nerve activity in heart failure patients. J Card Fail. 2004 Dec;10(6):496–502. doi: 10.1016/j.cardfail.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Hikosaka M, Yuasa F, Yuyama R, et al. Candesartan and arterial baroreflex sensitivity and sympathetic nerve activity in patients with mild heart failure. J Cardiovasc Pharmacol. 2002 Dec;40(6):875–880. doi: 10.1097/00005344-200212000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Creager MA. Baroreceptor reflex function in congestive heart failure. Am J Cardiol. 1992 Jun 4;69(18):10G–15G. 15G–16G. doi: 10.1016/0002-9149(92)91250-8. [DOI] [PubMed] [Google Scholar]
- 25.Zucker IH, Pliquett RU. Novel mechanisms of sympatho-excitation in chronic heart failure. Heart Fail Monit. 2002;3(1):2–7. [PubMed] [Google Scholar]
- 26.Brunner-La Rocca HP, Esler MD, Jennings GL, Kaye DM. Effect of cardiac sympathetic nervous activity on mode of death in congestive heart failure. Eur Heart J. 2001 Jul;22(13):1136–1143. doi: 10.1053/euhj.2000.2407. [DOI] [PubMed] [Google Scholar]
- 27.Fowler MB, Laser JA, Hopkins GL, Minobe W, Bristow MR. Assessment of the beta-adrenergic receptor pathway in the intact failing human heart: progressive receptor down-regulation and subsensitivity to agonist response. Circulation. 1986 Dec;74(6):1290–1302. doi: 10.1161/01.cir.74.6.1290. [DOI] [PubMed] [Google Scholar]
- 28.Mann DL, Kent RL, Parsons B, Cooper Gt. Adrenergic effects on the biology of the adult mammalian cardiocyte. Circulation. 1992 Feb;85(2):790–804. doi: 10.1161/01.cir.85.2.790. [DOI] [PubMed] [Google Scholar]
- 29.Barron HV, Lesh MD. Autonomic Nervous System and Sudden Cardiac Death. Journal of the American College of Cardiology. 1996;27(5):1053. doi: 10.1016/0735-1097(95)00615-X. [DOI] [PubMed] [Google Scholar]
- 30.Kaye DM, Lefkovits J, Jennings GL, Bergin P, Broughton A, Esler MD. Adverse consequences of high sympathetic nervous activity in the failing human heart. J Am Coll Cardiol. 1995 Nov 1;26(5):1257–1263. doi: 10.1016/0735-1097(95)00332-0. [DOI] [PubMed] [Google Scholar]
- 31.Gerson MC, McGuire N, Wagoner LE. Sympathetic nervous system function as measured by I-123 metaiodobenzylguanidine predicts transplant-free survival in heart failure patients with idiopathic dilated cardiomyopathy. Journal of Cardiac Failure. 2003;9(5):384. doi: 10.1054/s1071-9164(03)00134-9. [DOI] [PubMed] [Google Scholar]
- 32.Woo MA, Stevenson WG, Moser DK, Middlekauff HR. Complex heart rate variability and serum norepinephrine levels in patients with advanced heart failure. J Am Coll Cardiol. 1994 Mar 1;23(3):565–569. doi: 10.1016/0735-1097(94)90737-4. [DOI] [PubMed] [Google Scholar]
- 33.La Rovere MT, Pinna GD, Maestri R, et al. Short-term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation. 2003 Feb 4;107(4):565–570. doi: 10.1161/01.cir.0000047275.25795.17. [DOI] [PubMed] [Google Scholar]
- 34.Nolan J, Batin PD, Andrews R, et al. Prospective study of heart rate variability and mortality in chronic heart failure: results of the United Kingdom heart failure evaluation and assessment of risk trial (UK-heart) Circulation. 1998 Oct 13;98(15):1510–1516. doi: 10.1161/01.cir.98.15.1510. [DOI] [PubMed] [Google Scholar]
- 35.Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med. 1996 May 23;334(21):1349–1355. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- 36.Grassi G, Cattaneo BM, Seravalle G, et al. Effects of chronic ACE inhibition on sympathetic nerve traffic and baroreflex control of circulation in heart failure. Circulation. 1997 Aug 19;96(4):1173–1179. doi: 10.1161/01.cir.96.4.1173. [DOI] [PubMed] [Google Scholar]
- 37.Kasama S, Toyama T, Kumakura H, et al. Spironolactone improves cardiac sympathetic nerve activity and symptoms in patients with congestive heart failure. J Nucl Med. 2002 Oct;43(10):1279–1285. [PubMed] [Google Scholar]
- 38.Kasama S, Toyama T, Kumakura H, et al. Effect of spironolactone on cardiac sympathetic nerve activity and left ventricular remodeling in patients with dilated cardiomyopathy. J Am Coll Cardiol. 2003 Feb 19;41(4):574–581. doi: 10.1016/s0735-1097(02)02855-3. [DOI] [PubMed] [Google Scholar]
- 39.Adamson PB, Kleckner KJ, VanHout WL, Srinivasan S, Abraham WT. Cardiac resynchronization therapy improves heart rate variability in patients with symptomatic heart failure. Circulation. 2003 Jul 22;108(3):266–269. doi: 10.1161/01.CIR.0000083368.75831.7A. [DOI] [PubMed] [Google Scholar]
- 40.Hamdan MH, Barbera S, Kowal RC, et al. Effects of resynchronization therapy on sympathetic activity in patients with depressed ejection fraction and intraventricular conduction delay due to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 2002 May 1;89(9):1047–1051. doi: 10.1016/s0002-9149(02)02273-7. [DOI] [PubMed] [Google Scholar]
- 41.Najem B, Unger P, Preumont N, et al. Sympathetic control after cardiac resynchronization therapy: responders versus nonresponders. Am J Physiol Heart Circ Physiol. 2006 December 1, 2006;291(6):H2647–2652. doi: 10.1152/ajpheart.00373.2006. [DOI] [PubMed] [Google Scholar]
- 42.Horwich TB, MacLellan WR, Fonarow GC. Statin therapy is associated with improved survival in ischemic and non-ischemic heart failure. J Am Coll Cardiol. 2004 Feb 18;43(4):642–648. doi: 10.1016/j.jacc.2003.07.049. [DOI] [PubMed] [Google Scholar]
- 43.Mozaffarian D, Nye R, Levy WC. Statin therapy is associated with lower mortality among patients with severe heart failure. Am J Cardiol. 2004 May 1;93(9):1124–1129. doi: 10.1016/j.amjcard.2004.01.039. [DOI] [PubMed] [Google Scholar]
- 44.Ray JG, Gong Y, Sykora K, Tu JV. Statin use and survival outcomes in elderly patients with heart failure. Arch Intern Med. 2005 Jan 10;165(1):62–67. doi: 10.1001/archinte.165.1.62. [DOI] [PubMed] [Google Scholar]
- 45.Go AS, Lee WY, Yang J, Lo JC, Gurwitz JH. Statin Therapy and Risks for Death and Hospitalization in Chronic Heart Failure. JAMA. 2006 November 1, 2006;296(17):2105–2111. doi: 10.1001/jama.296.17.2105. [DOI] [PubMed] [Google Scholar]
- 46.Anker SD, Clark AL, Winkler R, et al. Statin use and survival in patients with chronic heart failure -- results from two observational studies with 5200 patients. International Journal of Cardiology. 2006;112(2):234. doi: 10.1016/j.ijcard.2006.03.057. [DOI] [PubMed] [Google Scholar]
- 47.Dickinson MG, Ip JH, Olshansky B, et al. Statin use was associated with reduced mortality in both ischemic and nonischemic cardiomyopathy and in patients with implantable defibrillators: Mortality data and mechanistic insights from the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) American Heart Journal. 2007;153(4):573. doi: 10.1016/j.ahj.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 48.Fukuta H, Sane DC, Brucks S, Little WC. Statin Therapy May Be Associated With Lower Mortality in Patients With Diastolic Heart Failure: A Preliminary Report. Circulation. 2005 July 19, 2005;112(3):357–363. doi: 10.1161/CIRCULATIONAHA.104.519876. [DOI] [PubMed] [Google Scholar]
- 49.Goldberger JJ, Subacius H, Schaechter A, et al. Effects of Statin Therapy on Arrhythmic Events and Survival in Patients With Nonischemic Dilated Cardiomyopathy. Journal of the American College of Cardiology. 2006;48(6):1228. doi: 10.1016/j.jacc.2006.05.053. [DOI] [PubMed] [Google Scholar]
- 50.De Sutter J, Tavernier R, De Buyzere M, Jordaens L, De Backer G. Lipid lowering drugs and recurrences of life-threatening ventricular arrhythmias in high-risk patients. J Am Coll Cardiol. 2000 Sep;36(3):766–772. doi: 10.1016/s0735-1097(00)00787-7. [DOI] [PubMed] [Google Scholar]
- 51.Mitchell LB, Powell JL, Gillis AM, Kehl V, Hallstrom AP. Are lipid-lowering drugs also antiarrhythmic drugs? An analysis of the Antiarrhythmics versus Implantable Defibrillators (AVID) trial. J Am Coll Cardiol. 2003 Jul 2;42(1):81–87. doi: 10.1016/s0735-1097(03)00498-4. [DOI] [PubMed] [Google Scholar]
- 52.Young-Xu Y, Jabbour S, Goldberg R, et al. Usefulness of statin drugs in protecting against atrial fibrillation in patients with coronary artery disease. Am J Cardiol. 2003 Dec 15;92(12):1379–1383. doi: 10.1016/j.amjcard.2003.08.040. [DOI] [PubMed] [Google Scholar]
- 53.Pliquett RU, Cornish KG, Zucker IH. Statin therapy restores sympathovagal balance in experimental heart failure. J Appl Physiol. 2003 Aug;95(2):700–704. doi: 10.1152/japplphysiol.00265.2003. [DOI] [PubMed] [Google Scholar]
- 54.Riahi S, Christensen JH, Toft E, Skou HA, Schmidt EB. HMG-CoA reductase inhibitors improve heart rate variability in patients with a previous myocardial infarction. Pharmacol Res. 2002 Jun;45(6):479–483. doi: 10.1006/phrs.2002.0988. [DOI] [PubMed] [Google Scholar]
- 55.Pehlivanidis AN, Athyros VG, Demitriadis DS, Papageorgiou AA, Bouloukos VJ, Kontopoulos AG. Heart rate variability after long-term treatment with atorvastatin in hypercholesterolaemic patients with or without coronary artery disease. Atherosclerosis. 2001 Aug;157(2):463–469. doi: 10.1016/s0021-9150(00)00746-2. [DOI] [PubMed] [Google Scholar]
- 56.Melenovsky V, Wichterle D, Simek J, et al. Effect of atorvastatin and fenofibrate on autonomic tone in subjects with combined hyperlipidemia. The American Journal of Cardiology. 2003;92(3):337. doi: 10.1016/s0002-9149(03)00643-x. [DOI] [PubMed] [Google Scholar]
- 57.Gentlesk PJ, Wiley T, Taylor AJ. A prospective evaluation of the effect of simvastatin on heart rate variability in non-ischemic cardiomyopathy. American Heart Journal. 2005;150(3):478. doi: 10.1016/j.ahj.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 58.Hamaad A, Sosin M, Lip GY, Macfadyen RJ. Short-term adjuvant atorvastatin improves frequency domain indices of heart rate variability in stable systolic heart failure. Cardiovasc Drugs Ther. 2005 May;19(3):183–187. doi: 10.1007/s10557-005-2219-8. [DOI] [PubMed] [Google Scholar]
- 59.Vrtovec B, Okrajsek R, Golicnik A, Ferjan M, Starc V, Radovancevic B. Atorvastatin Therapy Increases Heart Rate Variability, Decreases QT Variability, and Shortens QTc Interval Duration in Patients With Advanced Chronic Heart Failure. Journal of Cardiac Failure. 2005;11(9):684. doi: 10.1016/j.cardfail.2005.06.439. [DOI] [PubMed] [Google Scholar]
- 60.Katircibasi MT, Canatar T, Kocum HT, et al. Decreased heart rate recovery in patients with heart failure: effect of fluvastatin therapy. Int Heart J. 2005 Sep;46(5):845–854. doi: 10.1536/ihj.46.845. [DOI] [PubMed] [Google Scholar]
- 61.Nanas S, Anastasiou-Nana M, Dimopoulos S, et al. Early heart rate recovery after exercise predicts mortality in patients with chronic heart failure. International Journal of Cardiology. 2006;110(3):393. doi: 10.1016/j.ijcard.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 62.Fisher JPJC, Ahmed A, et al. The influence of statin therapy on resting sympathetic nerve activity in patients with heart failure. [Accessed September 25, 2007];2007 Experimental Biology Meeting Abstracts. [Google Scholar]
- 63.Gomes MEAW, Lenders JW, et al. The Role of Statins in Reducing Central Sympathetic Outflow in CHF. [Accessed September 25, 2007];American Heart Association Scientific Sessions 2006. [Google Scholar]
- 64.Gao L, Wang W, Li Y-L, et al. Superoxide Mediates Sympathoexcitation in Heart Failure: Roles of Angiotensin II and NAD(P)H Oxidase. Circ Res. 2004 October 29, 2004;95(9):937–944. doi: 10.1161/01.RES.0000146676.04359.64. [DOI] [PubMed] [Google Scholar]
- 65.Gao L, Wang W, Li YL, et al. Simvastatin Therapy Normalizes Sympathetic Neural Control in Experimental Heart Failure. Roles of Angiotensin II Type 1 Receptors and NAD(P)H Oxidase. Circulation. 2005 Sep 12; doi: 10.1161/CIRCULATIONAHA.105.552174. [DOI] [PubMed] [Google Scholar]
- 66.Welzig CM, Shin D-G, Park H-J, Kim Y-J, Saul JP, Galper JB. Lipid Lowering by Pravastatin Increases Parasympathetic Modulation of Heart Rate: G{alpha}i2, a Possible Molecular Marker for Parasympathetic Responsiveness. Circulation. 2003 December 2, 2003;108(22):2743–2746. doi: 10.1161/01.CIR.0000103680.61390.16. [DOI] [PubMed] [Google Scholar]


