Abstract
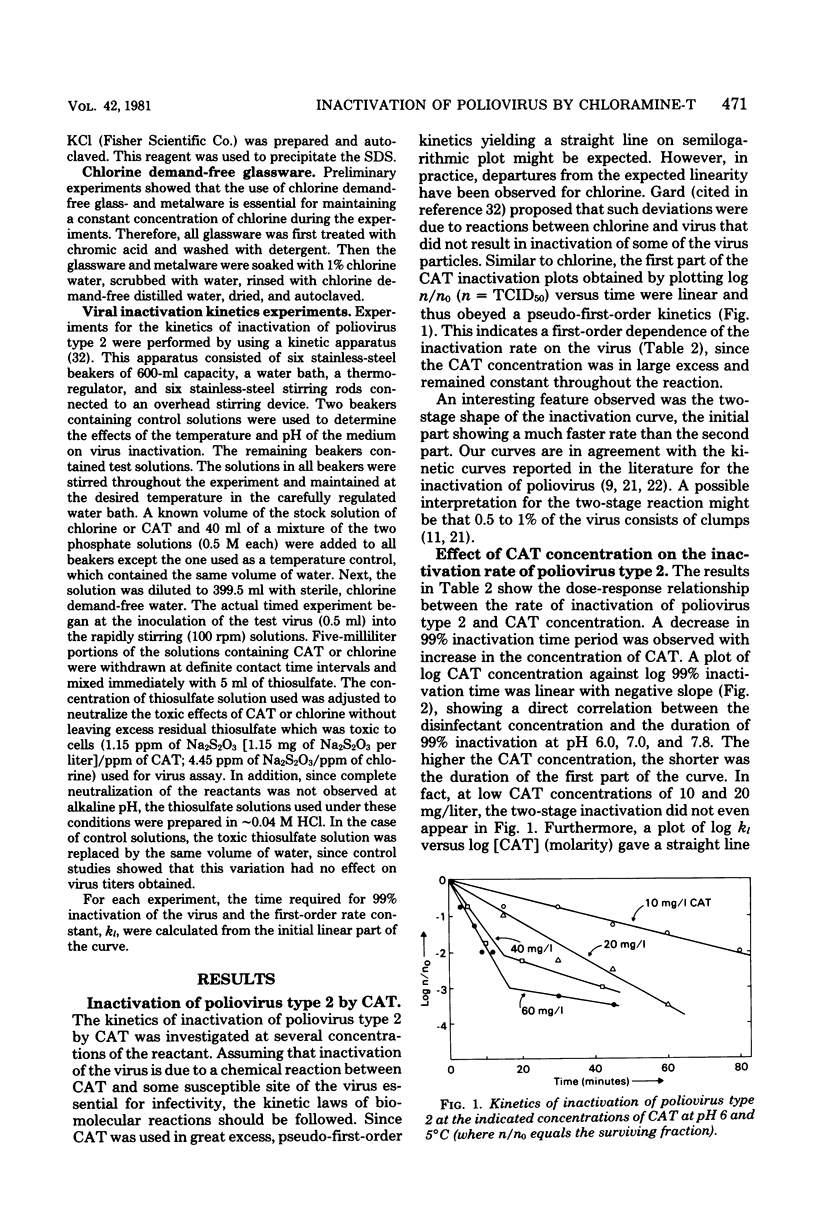
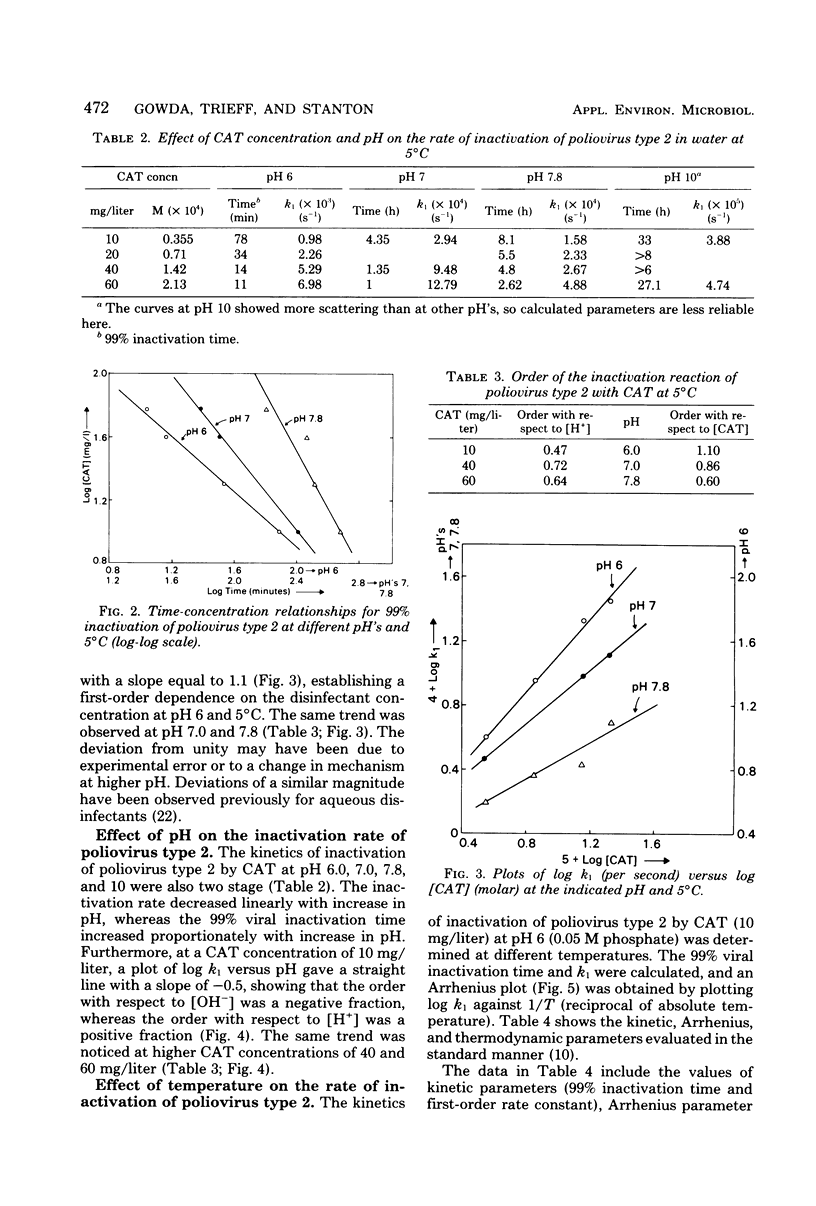
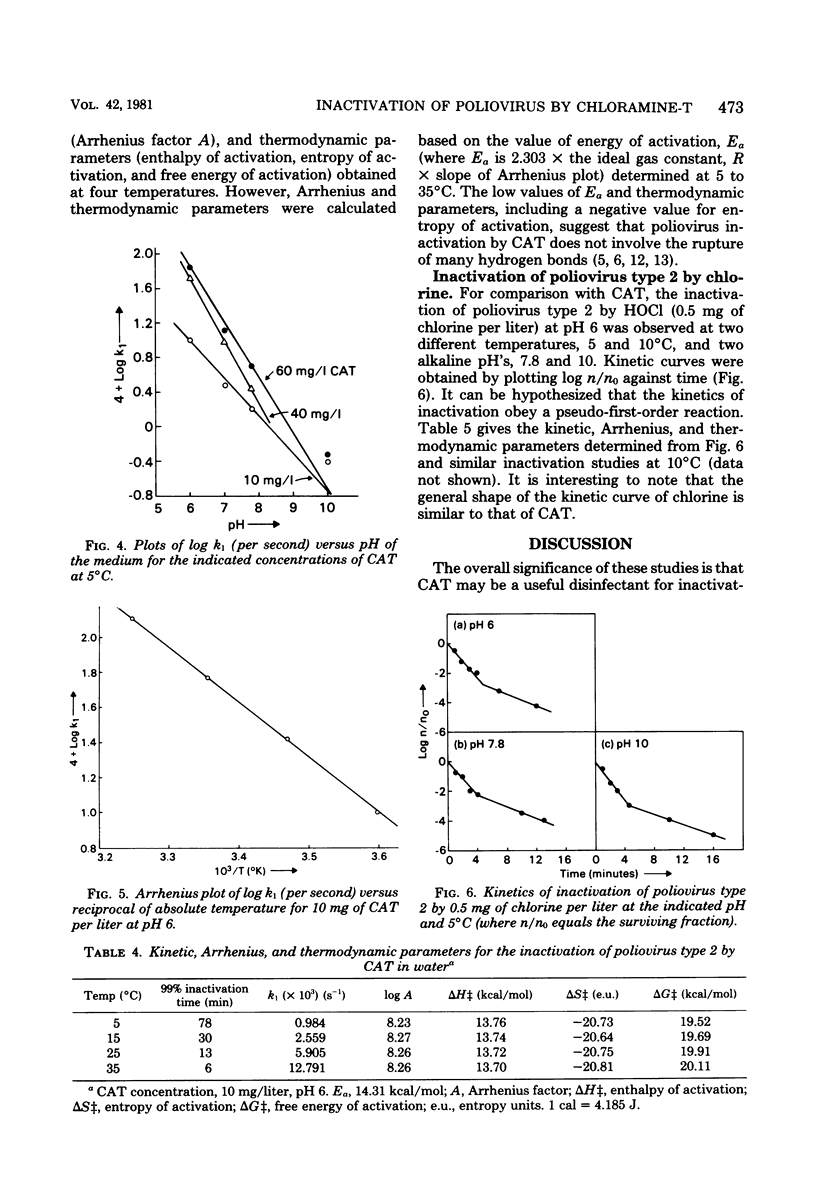
Since concern has recently been expressed about the presence of genotoxic substances due to chlorination of water and wastewater, chloramine-T (CAT) is proposed as an alternative disinfectant to chlorine. The viricidal properties of chlorine and CAT were compared. Kinetics of inactivation of poliovirus type 2 by chlorine and CAT in chlorine demand-free water were investigated by using a kinetic apparatus. Inactivation of the virus by chlorine and CAT occurred in two steps. The initial linear part of the inactivation curve followed a pseudo-first-order reaction with the virus. An obvious dose-response relationship was demonstrated with CAT. The rate of inactivation of the virus by CAT was faster in acid medium than in alkaline medium. Inactivation kinetic studies were performed at different temperatures, and the kinetic, Arrhenius, and thermodynamic parameters were evaluated. The rate of inactivation of poliovirus type 2 by chlorine was faster than that by CAT under identical conditions. A mechanism for the viral inactivation in acid conditions was proposed which led to a rate equation consistent with the experimental results. The results indicate that CAT may be an effective viricide against poliovirus type 2 in an acid medium.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dimmock N. J. Differences between the thermal inactivation of picornaviruses at "high" and "low" temperatures. Virology. 1967 Feb;31(2):338–353. doi: 10.1016/0042-6822(67)90179-1. [DOI] [PubMed] [Google Scholar]
- Floyd R., Johnson J. D., Sharp D. G. Inactivation by bromine of single poliovirus particles in water. Appl Environ Microbiol. 1976 Feb;31(2):298–303. doi: 10.1128/aem.31.2.298-303.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALASSO G. J., SHARP D. G. Virus particle aggregation and the plaque-forming unit. J Immunol. 1962 Mar;88:339–347. [PubMed] [Google Scholar]
- GINOZA W., HOELLE C. J., VESSEY K. B., CARMACK C. MECHANISMS OF INACTIVATION OF SINGLE-STRANDED VIRUS NUCLEIC ACIDS BY HEAT. Nature. 1964 Aug 8;203:606–609. doi: 10.1038/203606a0. [DOI] [PubMed] [Google Scholar]
- GINOZA W. Kinetics of heat inactivation of ribonucleic acid of tobacco mosaic virus. Nature. 1958 Apr 5;181(4614):958–961. doi: 10.1038/181958a0. [DOI] [PubMed] [Google Scholar]
- Gruener N. Mutagenicity of ozonated, recycled water. Bull Environ Contam Toxicol. 1978 Oct;20(4):522–526. doi: 10.1007/BF01683558. [DOI] [PubMed] [Google Scholar]
- Katzenelson E., Koerner G., Biedermann N., Peleg M., Shuval H. I. Measurement of the inactivation kinetics of poliovirus by ozone in a fast-flow mixer. Appl Environ Microbiol. 1979 Apr;37(4):715–718. doi: 10.1128/aem.37.4.715-718.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruoka S., Yamanaka S. Production of mutagenic substances by chlorination of waters. Mutat Res. 1980 Dec;79(4):381–386. doi: 10.1016/0165-1218(80)90163-9. [DOI] [PubMed] [Google Scholar]
- Shih K. L., Lederberg J. Chloramine mutagenesis in Bacillus subtilis. Science. 1976 Jun 11;192(4244):1141–1143. doi: 10.1126/science.818709. [DOI] [PubMed] [Google Scholar]
- Stanton G. J., Langford M. P., Baron S. Effect of interferon, elevated temperature, and cell type on replication of acute hemorrhagic conjunctivitis viruses. Infect Immun. 1977 Nov;18(2):370–376. doi: 10.1128/iai.18.2.370-376.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. S. Carcinogens complicate chlorine question. J Water Pollut Control Fed. 1974 Dec;46(12):2638–2640. [PubMed] [Google Scholar]


