Abstract
The spatial and temporal scales of cardiac organogenesis and pathogenesis make engineering of artificial heart tissue a daunting challenge. The temporal scales range from nanosecond conformational changes responsible for ion channel opening to fibrillation which occurs over seconds and can lead to death. Spatial scales range from nanometre pore sizes in membrane channels and gap junctions to the metre length scale of the whole cardiovascular system in a living patient. Synchrony over these scales requires a hierarchy of control mechanisms that are governed by a single common principle: integration of structure and function. To ensure that the function of ion channels and contraction of muscle cells lead to changes in heart chamber volume, an elegant choreography of metabolic, electrical and mechanical events are executed by protein networks composed of extracellular matrix, transmembrane integrin receptors and cytoskeleton which are functionally connected across all size scales. These structural control networks are mechanoresponsive, and they process mechanical and chemical signals in a massively parallel fashion, while also serving as a bidirectional circuit for information flow. This review explores how these hierarchical structural networks regulate the form and function of living cells and tissues, as well as how microfabrication techniques can be used to probe this structural control mechanism that maintains metabolic supply, electrical activation and mechanical pumping of heart muscle. Through this process, we delineate various design principles that may be useful for engineering artificial heart tissue in the future.
Keywords: myocardial cell, cytoskeleton, integrin, extracellular matrix, mechanotransduction, microfabrication
1. Introduction
The heart is a delicate, yet robust organ, and represents a unique scaling problem. The metabolic costs of maintaining electrical and mechanical synchrony, while simultaneously providing oxygen and chemical nutrients necessary for terminally differentiated myocytes to continually replenish their molecular components, are expensive and lead to the pathological vulnerability of the heart. Moreover, this control scheme works over an incredibly broad range of both spatial and temporal scales. For example, the ATP sensitivity of K+ATP ion channels that are critical for cardiac muscle cell excitation–contraction coupling and only a few nanometres in diameter are regulated by actin microfilaments that can be tens of micrometres in length (Furukawa et al. 1996; Terzic & Kurachi 1996). These cytoskeletal microfilaments are mechanically linked to myosin filaments and organized within the sarcomeres of myofibrils that are responsible for generating mechanical loads during systole (contraction) and bearing loads during diastole (stretching during chamber filling); they also maintain the overall structural integrity of the entire cardiac muscle cell. In addition, these cells must be properly aligned with their neighbours to provide optimal mechanical and electrical coupling as well as contractile function at the tissue level.
This spatial coordination is facilitated by the attachment of many thousands of cells to common extracellular matrix (ECM) scaffolds, such as basement membranes, that join cardiac muscle cells (cardiomyocytes) together to form larger muscle layers that wrap around each other to form the ventricular chambers. These muscular bundles are joined together and to neighbouring neural and vascular tissues, by other interstitial ECM networks, containing collagen fibres and large elastin bundles, that form a connective tissue lattice which gives shape to the whole heart organ and provides it with novel mechanical properties (e.g. elastic recoil). At the organ level, seven orders of spatial magnitude larger than the pore size of an ion channel, mechanical constriction of a single coronary artery can result in an immediate loss of metabolic supply to the ventricular musculature, decreased contractile performance, abnormal propagation of the excitatory action potential wavefront and death due to myocardial infarction or ventricular arrhythmia. Thus, the biochemical, electrical and mechanical functions of the heart are tightly intertwined with biological structures at all size scales. Understanding these relationships between structure and function that spatially and temporally integrate cellular function in the heart is critical for future heart engineering, and this is the focus of this article.
2. Structural determinants of heart function
(a) Cellular populations and three dimensional organization
The heart is composed of contractile cardiac muscle, nerves, blood vessels and connective tissues that must function in an integrated manner. The demographics of the different cell populations that comprise these tissues change considerably during embryonic development and throughout adult life. In the foetus, the numbers of cardiomyocytes and connective tissue cells (fibroblasts) increase approximately in parallel until birth when muscle cell division ceases. However, the fibroblasts continue to proliferate and thus, while cardiac myocytes account for the vast majority of the heart's volume (75%), they represent only about a third of the total cell number in the adult heart (Adler et al. 1981; Manabe et al. 2002). The cardiac fibroblast is the principal source of the heart's interstitial ECM and thus plays an important role in cardiac organogenesis and pathogenesis (e.g. fibrosis; Camelliti et al. 2005). These cells may also contribute to the propagation of the action potential wavefront (Gaudesius et al. 2003; Kohl 2003).
Nerve cells represent another major cell population in the heart. Autonomic (vagal) and sympathetic nerve fibres extend to and through the myocardium. The right vagus nerve primarily innervates the sinoatrial (SA) node, whereas the left vagus innervates the atrioventricular (AV) node; however, there can be significant overlap in their anatomical distribution. Sympathetic efferent nerves are also present throughout the atria (especially in the SA node) and ventricles, including the conduction system of the heart. Thus, while the number of nerve cells in the heart is small when compared with the numbers of myocytes, fibroblasts and vascular cells, the complex neural circuitry of the heart is an important, but extremely difficult, architecture to recapitulate in engineered cardiac tissues.
An equally daunting task in cardiac tissue engineering is to recreate the complex vascular beds that perfuse the myocardium and line the heart's chambers and valves. The metabolic expense of the heart muscle is paid by a dense, branching capillary network with fractal design that runs parallel and immediately adjacent to the cardiac muscle fibres. The maintenance of oxygen tension in the myocardium is a delicate affair, requiring that the vascular network architecture accounts for spatial changes in oxygen tension due to the spacing between red blood cells as they travel through the capillary lumen (Federspiel & Popel 1986; Honig et al. 1989; Hoofd et al. 1994; Rakusan et al. 2001). The inability to build cardiac muscle tissue with a normal vascular network limits the thickness of engineered tissues to the oxygen diffusion limit of approximately 100–200 μm. Vascular smooth muscle wrapping around the coronary arteries, arterioles and the transition to capillaries solely lined by endothelial cells represents another tissue engineering problem, even though the spatial organization of these cells is a simple cylindrical geometry.
The heart's vasculature is also dynamic, as it changes considerably from the womb through the post-partum period, and even in the adult due to changes in lifestyle including exercise and environmental conditions (reviewed by Hudlicka et al. 1992). The result is a constant change in the relative contribution of vascular smooth muscle and capillary endothelial cells to the total cell population of the heart. The ability to assemble an engineered cardiac tissue with a vascular network capable of exhibiting normal growth dynamics has thus far proven to be impossible. In contrast, greater advances have been made in the area of heart valve engineering (Sodian et al. 2000), probably because it does not require construction of these complex vascular networks.
The central problem that must be overcome to successfully engineer functional heart muscle is the challenge of understanding the key design principles that govern the hierarchical organization and complex three-dimensional geometry of the heart from the millimetre to micrometre size scales. The ventricle, for example, is composed of a collection of myocytes organized into distinct layers—each approximately four cell diameters wide—with different orientations separated by different cleavage planes that wrap around the ventricular chamber. The helical wrapping of the laminar structures creates an anisotropic syncytium that endows the muscle with unique electrical and mechanical properties that vary from the base to the apex and throughout its thickness from the endocardium to epicardial surface. Cardiomyocytes are generally 10–15 μm in diameter and up to 100 μm in length, connected end-to-end in the longitudinal direction and side-to-side in the transverse direction. Variations in contractility and excitability, force production and action potential morphology reflect the uniqueness of the spatial organization of this tissue microenvironment.
(b) Extracellular matrix networks
This complex tissue and organ architecture is maintained by extensive three-dimensional ECM networks composed of collagen, elastin bundles and interconnected basement membranes (Caulfield & Borg 1979). ECM in the heart and vascular wall includes fibrous proteins (collagen and elastin), adhesive glycoproteins (e.g. laminin, fibronectin) and proteoglycans. This ECM network orients the myocytes, mechanically couples them to each other and to neighbouring capillaries and nerves, and provides elastic support during ventricular filling (Borg et al. 1981). Similar ECM proteins become organized within relatively rigid fibrous rings that surround each heart valve.
The collagenous connections between adjacent myocytes register sarcomere Z-lines across the cell membranes and thereby ensure equal stretching of contiguous cells while preventing slippage between the cells. In fact, acute disruption of the fibrillar collagen network can decrease myocardial systolic performance without changing myocyte contractility (Baicu et al. 2003). By virtue of maintaining the mechanical continuity between myocytes, the collagen network also supports the electrical connectivity maintained by gap junctional connections between myocytes and the cells of the AV node, bundle branches and Purkinje fibres. When xenogenic ECM scaffolds are used for myocardial repair, they alter the typical scar tissue healing response and instead support development of various tissues, including new capillary blood vessels and functional contractile myocardium (Badylak et al. 2003).
Importantly, the ECM is more than a passive connector; it also provides microstructural cues that regulate cardiac muscle cell morphogenesis, as well as myocardial function. For example, neonatal cardiomyocytes differ in their rate of differentiation into mature contractile cells depending on the type of ECM substrate on which they are cultured (Bick et al. 1998). The ECM regulates the self-assembly of sarcomeres within differentiating myocytes by providing directional cues that guide myofibrillogenesis (Gregorio & Antin, 2000; Russell et al. 2000). Moreover, when cell–ECM interactions are deregulated, they can contribute to heart pathology. Both increased expression of ECM genes and fibrosis are associated with myocardiac dysfunction in failing hearts of spontaneously hypertensive rats (Bing et al. 1997). Changes in the oxygenation of the heart muscle also can impact its function by altering the proportion of different ECM proteins in the tissue (Pelouch 1995). Thus, the physical properties of the tissue microenvironment may be as important as the chemical milieu for the control of heart development.
(c) Mechanical forces as bioregulators
The ECM lattice that physically couples the different cells that comprise heart tissue is critical for integration of structure and function at all size scales, both during development and throughout adult life. However, much of the regulatory information conveyed by ECM is transmitted in the form of physical forces. In the embryo, ECM molecules that are secreted by cells self-assemble into higher order structures, including nanometre-sized cables (collagen fibrils), struts (larger cross-linked collagen bundles), nets (porous sheets of basement membrane collagens) and hydrogels (composed of glycosaminoglycans and filamentous collagens). Besides interacting with each other, all of these molecules also bind to cell surface ECM receptors, known as ‘integrins’ (Ruoslahti 1991), and thereby physically anchor myocytes and surrounding connective tissue (mesenchymal) cells to a common ECM scaffold (i.e. basement membrane). These ECMs provide a critical structural function: even though they are flexible, they are stiffer than the cells, and thus they can balance cell-generated contractile forces and stabilize higher order tissue form.
Local variations in ECM mechanics that alter its ability to resist cell tension, and thereby change the mechanical force balance in the cytoskeleton, appear to drive the local differentials in cell growth and motility that produce specialized tissue patterns, such as budding epithelium and branching capillary networks, in the embryo (Moore et al. 1995). In the developing heart, the mechanical forces of systole and diastole are transmitted to the cells that comprise the organ via the ECM networks that link these cells together. Computational studies suggest that variations in ECM stiffness combined with these cardiac forces may cause the heart tube to bend during the earliest phases of heart morphogenesis (Zamir et al. 2003). Active tension development by neonatal cardiomyocytes is also critical for the maintenance of normal cell shape and oriented myofibrillar architecture (Sharp et al. 1993). At birth, changes in mechanical forces due to alterations in arterial pressure and heart rate associated with birth feedback to alter ECM production (Carver et al. 1993). External mechanical loads (e.g. due to pressure-induced ventricular hypertrophy, mechanical strain) exerted on cardiomyocytes also can induce alterations in gene expression, muscle mass and phenotype, and these effects are mediated by force-dependent changes in signal transduction as well as release of paracrine factors (e.g. angiotensin II; Sadoshima et al. 1992; Sadoshima & Izumo 1993; Kogler et al. 2003). Thus, mechanical forces conveyed by ECM may play a central role in the control of heart form and function.
(d) Mechanotransduction through extracellular matrix, integrins and the cytoskeleton
One of the main ways in which ECM influences cell and tissue development is through its ability to transmit mechanical forces through tissues and focus them on cell surface integrin receptors. Integrins are dimeric transmembrane proteins composed of α and β subunits that bind ECM proteins in the extracellular space and cytoplasmic actin-linker proteins (e.g. vinculin, talin, α-actinin, paxillin and zyxin) inside the cell, thereby mechanically coupling the integrins to the actin cytoskeleton (Wang et al. 1993; Alenghat & Ingber 2002). Cardiac myocytes express various integrin receptor types that bind to collagen I(α3β1), fibronectin (α3β1, α5β1) and laminin (α1β1, α3β1, α7β1; reviewed in Ross & Borg (2001)). When integrins bind ECM proteins, a conformational change causes the receptors to cluster together and activate kinases such as src kinase and focal adhesion kinase (FAK), as well as small GTPases such as Rho, on the cytoplasmic side of the membrane which, in turn, promote assembly of ‘focal adhesion’ anchoring complexes. These specialized adhesion plaques which mechanically anchor the cell to the ECM contain the clustered integrins, associated actin-linker proteins and multiple signal transduction molecules.
Focal adhesions play a central role in mechanotransduction—the process by which cells convert mechanical signals into biochemical responses. Force-dependent changes in shape and conformation of a subset of the load-bearing molecules in these adhesion plaques alter their biochemical activities, and thereby result in stress-dependent remodelling of the focal adhesion as well as associated changes in signal transduction. For example, direct application of shear stresses to integrins using bound magnetic beads in combination with applied magnetic fields can activate cAMP signalling within the focal adhesion and result in force-dependent activation of gene transcription, whereas application of the same stress to other non-integrin transmembrane receptors have no effect (Meyer et al. 2000). Mechanical forces applied to integrins also can be transmitted deep into the cell and nucleus by being channelled over discrete cytoskeletal filament networks (Maniotis et al. 1997; Wang et al. 2001; Hu et al. 2003), and the efficiency of this transmission is sensitive to the level of prestress (isometric tension) in the cytoskeleton (Hu et al. 2003, 2004). Disruption of the continuity of intracellular force transmission, for example, by interfering with the expression of the focal adhesion protein vinculin, can impair cell shape stability (Ezzell et al. 1997) and disrupt normal myofibril architecture in cardiac myocytes (Shiraishi et al. 1997). Forces that are normally transmitted over long distances in the cell based on channelling through the cytoskeleton (Maniotis et al. 1997; Wang et al. 2001; Hu et al. 2003, 2004) can also change biochemical activities at distant sites (e.g. induce calcium influx across the nuclear membrane; Itano et al. 2003).
Cardiac muscle cells appear to have some unique signalling molecules with focal adhesions that are activated by integrin binding, such as melusin which becomes activated following binding of integrin β1 to ECM (Brancaccio et al. 1999). Melusin may be involved in mediating the cardiomyocyte response to pressure overload hypertrophy (Brancaccio et al. 2003) suggesting that these cells may use mechanochemical signalling pathways to enhance their sensitivity to mechanical stresses borne by the ECM. Integrin-mediated attachment to laminin also modulates the β-adrenergic response, affecting excitability, action potential morphology, Ca2+ metabolism, force development and resting tension in cardiac cells (Wang et al. 2000). This represents a unique physical response in which integrins can alter the sensitivity to a soluble mitogen that affects both electrical and mechanical responses.
Integrins provide a potential mechanism for mechanoelectrical coupling among cellular ensembles within heart tissue as well, given that gap junctional communication correlates directly with the presence of β1 integrins in many cell types (Lampe et al. 1998; Matsushita et al. 1999; Ojakian et al. 2001). In fact, integrins mediate the effects of mechanical stress on gap junction function in cardiac muscle cells: up regulation of connexin 43 expression and increases in conduction velocity were observed within 1 h after application of mechanical strain to cardiomyocytes cultured on flexible ECM substrates (Matsushita et al. 1999; Wang et al. 2000; Zhuang et al. 2000). Cytoskeletal actin filaments and gap junction proteins also appear to be directly linked (Larsen et al. 1979; Murray et al. 1997) in a variety of cell types, including cardiac muscle cell, suggesting that real-time control of cell–cell gap junction coupling by mechanical forces may be a heretofore unrecognized mechanism for electrophysiological control.
(e) Control of ion channel function by integrins and mechanical forces
In the past, it was assumed that the cytoskeleton is a downstream target for ion signalling mediated by stress-sensitive ion channels on the cell surface. However, integrins and the mechanical forces they transmit to the cytoskeleton also can influence the nanometre-scale conformational changes that regulate mechanosensitive ion channel currents (Wang et al. 2000; Browe & Baumgarten 2003; Browe & Baumgarten 2004; Cheng et al. 2004). In fact, the cytoskeleton has been shown to regulate the opening kinetics of various stretch-activated ion channels (reviewed in Hu & Sachs (1997)). Interestingly, while much work has focused on how stretch-activated channels differ from volume-activated channels (Morris 1990; Hu & Sachs 1997), recent work suggests that all ion channels whose kinetics are modulated by cytoskeletal proteins have the potential to be regulated by mechanical force (Terzic & Kurachi 1996).
Experimental studies reveal that application of mechanical forces to β1 integrins at one site on a myocyte cell can produce changes in outwardly rectifying Cl− currents at a distant location on the same cell membrane, as measured using the patch clamp technique (Browe & Baumgarten 2003). This finding illustrates how mechanical forces may be propagated through the cytoskeleton to activate signalling pathways at a distance in a living cardiac cell. Transmembrane Ca2+ transport is also influenced by the microtubular network: microtubule disassembly increases the probability of L-type Ca2+ channels to be in the closed state, whereas polymerization of these cytoskeletal filaments increases their open probability and lengthens the mean time the channel is held open. These findings are physiologically relevant because increased microtubule assembly is observed in pressure-overload hypertrophy (Tsutsui et al. 1993). The mechanism by which microtubules regulate ion channels is unclear, but has been hypothesized to be due to restriction of Ca2+-dependent conformational changes in the cytoskeleton (Johnson & Byerly 1994), changes in the concentration of inactivating ions near the channel openings (Galli & DeFelice 1994) or increased direct interaction between microtubules and Ca2+ channels (Galli & DeFelice, 1994). All of these potential mechanisms involve changes in cytoskeletal architecture in the vicinity of the channel. However, increased accumulation of microtubules can also interfere with cell contractility via other mechanisms (e.g. increasing the viscous load on the cardiomyocyte contractile apparatus; Tagawa et al. 1997).
The actin cytoskeleton may also regulate the activities of channels responsible for the depolarization and subsequent repolarization of the cardiomyocyte membrane. Pharmacological stabilization of actin filaments increases the magnitude of the whole cell Ca2+ current, whereas microfilament disruption reduces current strength (Lader et al. 1999). L-type Ca2+ channel kinetics can be similarly regulated by the actin-binding proteins α-actinin and dystrophin (Sadeghi et al. 2002). The inactivation period after the channel opening is reduced in mice lacking dystrophin. Interestingly, a similar effect on channel opening is produced by increasing the number of microtubules in cardiac myocytes (Galli & DeFelice 1994), as is observed in pressure-overload hypertrophy (Parker et al. 2001). This result suggests that the inactivation period of the L-type Ca2+ channel is very sensitive to mechanical stresses borne by the cytoskeleton, a molecular mechanism that may explain altered Ca2+ metabolism during heart failure.
In addition, disruption of actin microfilaments using cytochalasins reduces whole-cell peak Na+ current and slows decay in ventricular cardiac myocytes (Undrovinas et al. 1995). Cytoskeletal disruption causes Na+ channels to exhibit a lower peak open probability, but more persistent activity, indicative of a slowing of the inactivation state (Undrovinas et al. 1995). The metabolic sensitivity of ATP-sensitive K+ channels (KATP) during repolarization is also governed by the actin network: actin filament disruptors thwart the ATP-induced inhibition of KATP currents (Terzic & Kurachi 1996), whereas actin filament stabilizers (e.g. phalloidin) maintain channel activity and partially restore rundown channel activity (Furukawa et al. 1996). This result is important because the KATP channel complex represents a single macromolecular assembly where the mechanical, electrical and metabolic states of the myocyte are intertwined. Moreover, if cytoskeletal filaments transmit forces throughout the cell as indicated by in vitro studies (Maniotis et al. 1997; Wang et al. 2001; Hu et al. 2003, 2004), then mechanical forces transmitted from ECM and integrins over these load-bearing networks may modulate the kinetics of several ion channels simultaneously.
(f) Cellular control through global shape distortion
Although cells may sense physical signals transmitted from ECM locally within their focal adhesions, they integrate these signals with other information relating to the overall structural state of the cell and cytoskeleton before orchestrating a concerted functional response. This can be demonstrated by culturing various cell types (e.g. epithelial, endothelial, muscle, fibroblast) on micrometre-sized ECM islands of defined size, shape and position, surrounded by non-adhesive regions, that can be created with a microfabrication technique, known as microcontact printing (Chen et al. 2000). When cells are cultured on these planar ECM islands, the cells exert traction forces on their ECM adhesions and spread until they reach the perimeter of the island and then they stop (Singhvi et al. 1994; Chen et al. 1997; Parker et al. 2002; Brock et al. 2003). In this manner, cells can be artificially held in any arbitrary shape or size under conditions in which soluble hormones and growth factors (and the local ECM coating density) are held constant (figure 1). In other words, cell shape distortion can be controlled as an independent variable. These studies have revealed that cells can be switched between growth, differentiation and apoptosis solely by varying the degree to which the cell can physically distend and flatten (Singhvi et al. 1994; Chen et al. 1997; Dike et al. 1999). In general, highly spread and flattened cells proliferate, round or retracted cells undergo apoptosis and die, and cells that spread to an intermediate degree differentiate. These effects are independent of the total area of cell–ECM contact (Chen et al. 1997) and integrin signalling can be similar under these different conditions (Yan et al. 2000). The degree to which a cell spreads also feeds back to regulate cell contractility, and this response also can be modulated by altering the mechanical compliance of the ECM substrate on which the cell is cultured (Polte et al. 2004). Recent studies demonstrate similar shape-dependent control of switching between different cell lineages (bone versus fat) in studies with mesenchymal stem cells (McBeath et al. 2004).
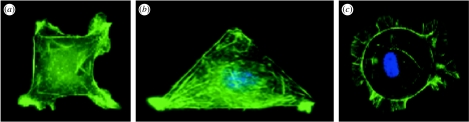
Figure 1.
Engineering cell shape and function. Capillary endothelial cells spread to takes on the shape of (a) square (40×40 μm), (b) triangular (long edge 80 μm) and (c) circular (40 μm diameter) ECM islands coated with fibronectin that were created using a microcontact printing technique (Chen et al. 2000), and stained for actin microfilaments using fluorescent-phalloidin. Cells on (a) square and (b) triangular islands preferentially extend motile processes (lamellipodia) from their corners, whereas cells exhibit no bias on the (c) circular islands (Parker et al. 2002; Brock et al. 2003). Note that lamellipodia preferentially extend from acute angles, rather than from the obtuse angle, on the triangle-shaped island.
Separate studies show that the direction of cell motility—the orientation in which the cell extends new motile processes, such as lamellipodia, filopodia and microspikes—can be controlled by the geometric shape of the cell. Cells cultured on polygonal ECM islands that contain corners (e.g. squares, triangles, pentagons) preferentially extend these new motile processes from these corner regions when stimulated with motility factors, whereas cells cultured on circular islands display no directional bias (figure 1; Parker et al. 2002; Brock et al. 2003). Cells also seem more likely to extend lamellipodia from corners with acute, rather than obtuse, angles (Brock et al. 2003), and studies using more recent methods that permit release of cells from shape-constricted islands confirm that cells actually move (translocate) in the direction in which they extend these new motile process (Jiang et al. 2005). Thus, this information may be useful as design criteria for the development of biomaterials for tissue engineering applications in the future.
(g) Linkage between structural and information processing networks
These studies with microengineered substrates show that cells can be switched between different fates or phenotypes based on the degree to which they physically distort. Yet, when integrin receptors are mechanically stressed on spread versus round cells, intracellular signalling responses (e.g. cAMP production) are activated to a similar degree (Meyer et al. 2000). This means that the spread cell integrates this signal with other cues conveyed by its overall physical state and then decides what phenotype is most appropriate for its local microenvironment. When a cell turns on its differentiation programme, it must also turn off its growth, apoptosis and motility programmes in order to function effectively. The relevance of this robust switching behaviour is supported by the finding that molecular pathways in the cell form a single large connected network (‘giant component’) that spans almost the entire genome (Callaway et al. 2001; Jeong et al. 2001; Marcotte 2001). If virtually all of the genes and proteins of the genome-wide regulatory network are effectively regulated as a single integrated system, then cells must have evolved a mechanism to reliably integrate multiple conflicting signals and respond by selecting one of just a few possible cell fates.
Work from the field of complexity (Kauffman 1969, 1993) suggests that stable ‘attractor’ states will spontaneously emerge in large networks that exhibit a particular class of architecture (level of connectivity between nodes) owing to dynamic constraints imposed by the regulatory interactions among its components. Biological networks exhibit exactly this type of network architecture (Glass & Hill 1998; Fox & Hill 2001; Jeong et al. 2001). This raises the possibility that the robust cell fates that cells express (e.g. growth, differentiation, motility, apoptosis) may similarly represent attractor states in the genome-wide regulatory network (Huang & Ingber 2000). Importantly, recent experimental studies confirm that cell fates represent high-dimensional attractor states in the genome-wide regulatory network of human cells (Huang et al. 2005). This finding implies that cell fate switching may not result from activation of a particular ‘instructive’ pathway or linear series of specific genes. Instead, this process may be more like a ball that can take multiple paths as it rolls over a hill from one valley, or stable cell phenotype, to the next.
The importance of the existence of attractors in the genome-wide regulatory network in the context of the structural hierarchies in cells and tissues that make up the heart is that it can explain how a non-specific stimulus like cell shape distortion can impact the same biochemical machinery responsible for cell fate switches which are controlled by soluble factors that bind to specific transmembrane receptors (Ingber 2003). The key point is that multiple regulatory elements (e.g. genes, signalling proteins) within the genome-wide regulatory network must alter simultaneously in order to produce an attractor switch. The fact that the distortion of the cell and cytoskeleton probably impacts many cytoskeletal-associated signalling molecules at once (Ingber 2003) may explain how mechanical forces and resultant cell shape changes are able to switch cells between different developmentally relevant phenotypes. The importance of these findings in the context of the present discussion is that structure and biochemical regulation are inextricably linked at all size scales in biology. Hence, this relationship should be taken into account when designing future biomaterials for tissue engineering applications.
3. Heart cell engineering
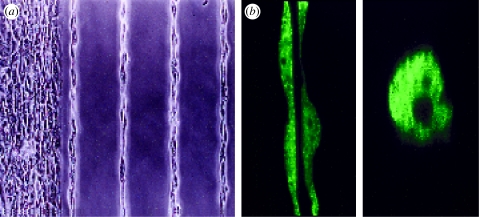
Given the central role that ECM scaffolds, integrins and linked cytoskeletal networks play in both heart development and mechanoelectrical function, any attempt to engineer the specialized architecture of the tissues that comprise the heart will require methods to control cell–ECM interactions. We have made some headway in this area by microfabricating two-dimensional models of the cardiac tissue microenvironment using the microcontact printing technique described previously to pattern different sized and shaped ECM islands on glass coverslips. When the different cell types that comprise the heart (e.g. muscle cells, vascular cells, nerve cells, connective tissue cells) are cultured on micropatterned islands of the ECM protein, the cells spread out and assume the shape of the island as previously described. In addition, novel cellular responses can be induced by varying island geometry. For example, capillary endothelial cells undergo differentiation into hollow capillary tubes with a central lumen when cultured on long thin (10 μm wide) lines of fibronectin (figure 2; Dike et al. 1999). Nerve cells can be cultured on more complex ECM patterns that promote the formation of functioning two-dimensional neural networks (Klein et al. 1999; Leng et al. 2004).
Figure 2.
Control of capillary differentiation using microengineered ECM substrates. (a) Phase contrast and (b) confocal fluorescence microscopic images of capillary endothelial cells forming hollow tubular blood vessels when cultured on fibronectin-coated ECM islands in the form of long thin lines (30 μm wide) that promote only a moderate degree of spreading and support cell–cell contact formation (Dike et al. 1999). A central lumen is visible in both horizontal (left) and vertical (right) cross-sections of the tubes shown in (b).
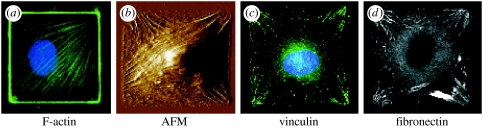
Cells cultured on micropatterned ECM substrates remodel their cytoskeleton differently, depending on the shape of the adhesive island. When capillary endothelial cells are cultured on a square ECM island, actin stress fibres preferentially align along the diagonal axes of the cell (figure 3), and similar diagonal alignment of stress fibres can be detected in fibroblasts using atomic force microscopy (figure 3; Parker et al. 2002). This highly oriented distribution of actin bundles is consistent with the finding that the focal adhesion protein, vinculin, concentrates within the focal adhesion plaques that localized within the corners of the square cells where the actin stress fibres terminated at the cell base (figure 3). The vinculin plaques displayed the same orientation as the stress fibres, aligned so as to bisect the internal angle of the corner. These cells also deposit elongated fibronectin fibrils in a diagonal pattern directly beneath the vinculin-containing focal adhesions (figure 3; Parker et al. 2002; Brock et al. 2003). Fibronectin fibril assembly is tension dependent, and hence this suggested that cells preferentially exert traction forces in these corner regions, and this has been confirmed experimentally (Wang et al. 2002).
Figure 3.
Coordinated reorganization of the cytoskeleton, focal adhesions and ECM within cells on square islands. Oriented distribution of stress fibres (F-actin), vinculin-containing focal adhesions (vinculin) and underlying fibronectin fibrils (fibronectin) in (a) endothelial cells and (b–d) fibroblasts cultured on square fibronectin islands, as detected by fluorescence imaging or atomic force microscopy (AFM). (a) and (b) 50×50 μm islands; (c) and (d) 30×30 μm islands; adapted from Parker et al. (2002).
Importantly, this model system can be used to control myofibrillogenesis in single cardiac muscle cells. The contractile actin cytoskeleton of the cardiomyocyte which is required for the temporal and spatial synchronization of uniform contraction of a muscle cell is characterized by serial alignment of parallel bundles of sarcomeres, known as myofibrils. Several models of myofibrillogenesis have been proposed (Dlugosz et al. 1984; Rhee et al. 1994; Dabiri et al. 1997; Ehler et al. 1999). The Sanger model (Rhee et al. 1994; Dabiri et al. 1997) proposes that premyofibrils containing banded Z-bodies composed of α-actinin and non-muscle myosin IIB form at the edges of spreading cardiac myocytes; the myosin IIB filaments are then exchanged for muscle myosin II filaments and the Z-bands are formed from the fusion of Z-bodies. As the myofibrils increase in width, they align laterally and are characterized by continuous bands of α-actinin. In contrast, Holtzer's model describes these premyofibrils as ‘stress fibre-like structures’ (SFLS) that serve as the scaffold for myofibril assembly (Dlugosz et al. 1984). The fibres contain discrete complexes that form along their length, containing sarcomeric α-actinin, Z-bodies, α-actinin and muscle tropomyosin and the other structural components of the sarcomere. The 2 μm spacing within which sarcomerogenesis occurs is effectively cordoned off by digitization of the Z-line components along an actin fibre stabilized by the tensile load that it bears.
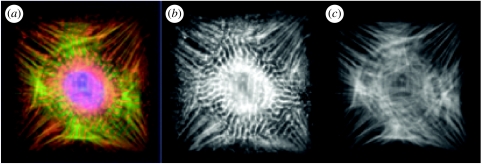
While these models focus on the length scale of a sarcomere, examination of myofibrillogenesis and cardiac tissue assembly at larger length scales using microfabricated model cell systems has yielded unique insight into the functional organization of the myocardium. When single cardiac myocytes are cultured on micropatterned islands, unique myofibrillar patterns form with respect to geometric cues in the ECM. The actin fibres align along the diagonals and sarcomere formation begins in the perinuclear region and proceeds towards the cell periphery, with the actin fibres appearing to act as ‘guides’. Thus, any ECM island that induces a unique actin network will also have a unique myofibrillar pattern. For example, in myocytes cultured on rectangular islands of decreasing aspect ratios (length/width), as the aspect ratio approaches unity (a square), a single axis of contraction is no longer discernable. Specifically, when myocytes are cultured on a square fibronectin island, myofibrillogenesis follows the alignment of actin stress fibres which fall along the diagonals of the square, as observed in non-muscle cells (figure 3). The result is a single myocyte with two axes of contraction, with each one oriented along a diagonal (figure 4). More careful analysis of this process reveals that the newly forming sarcomeric Z-lines register and rotate within the cytoskeleton so that they are always perpendicular to the cell periphery (figure 5). In addition, like in non-muscle cells (Parker et al. 2002; Wang et al. 2002), fibronectin fibrils accumulate in these corner regions, indicating that these areas are also regions where cells focus their contractile forces.
Figure 4.
Geometric control of myofibril architecture in a single cardiomyocyte cultured on a square fibronectin island (50×50 μm). (a) A merged fluorescence microscopic image of the cell after 72 h of culture showing the distribution of sarcomeric α-actinin (red), actin microfilaments (green) and the nucleus (blue). (b) Immunostaining for sarcomeric α-actinin reveals that sarcomerogenesis is initiated in the perinuclear region within the square cell shown on the left. (c) Actin stress fibre bundles that are aligned predominantly along the diagonals of the square in the same cell serve as scaffolds to guide myofibrillogenesis.
Figure 5.
The myofibrillar reorganization observed within a single cardiomyocyte cultured on a square fibronectin island (50×50 μm) correlates with the deposition of new fibronectin fibrils in the ECM beneath the cell. (a) Merged image illustrating the position of the cardiomyocyte nucleus (blue), saromeric α-actinin (red) and fibronectin (green). (b) The same cell stained only for sarcomeric α-actinin showing that myofibrillogenesis results in the self-assembly of sarcomeric Z-lines which register and rotate through the internal angles of the corners of the square myocyte, always perpendicular to the cell periphery. (c) Immunostaining for fibronectin reveals fibronectin fibrils beneath the cell in the corners of the square island. This pattern is indicative suggestive of the pattern of tractional forces exerted by the myocyte on the substrate, which are concentrated in these corner regions.
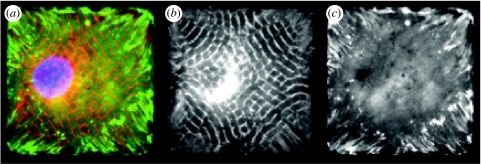
In contrast to cells on square islands, myofibrils self-organize along two different orthogonal axes within cardiomyocytes cultured on ECM islands in the shape of a four-pointed star. Thus, even though the assembly and alignment of sarcomeres begins in the perinuclear region, it is guided by structural cues from the ECM at the furthest points from the nucleus that are able to subdivide and organize the intracellular cytoskeletal structure of the cardiac muscle cell. These studies also illustrate how fibrosis during heart failure may further reduce the efficiency of contractility by misdirecting the alignment of sarcomeres, as well as how cues from the ECM interplay with cellular biochemistry to produce unique structure–function relationships within single cardiac muscle cells.
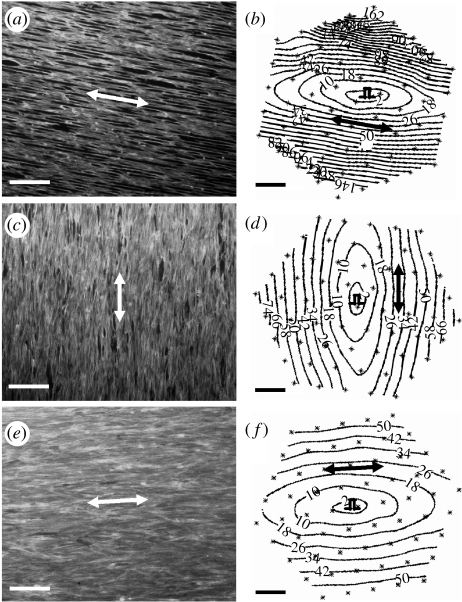
Studies on cardiac myocytes cultured on larger microfabricated ECM islands that support adhesion of multiple cells (and promote cell–cell contact formation) have revealed that higher order features of cardiac organogenesis and pathogenesis can be recapitulated in these microengineered two-dimensional cardiac tissues. For example, when multiple neonatal ventricular rat myocytes are cultured on an array of linear fibronectin islands (12.5–25 μm wide) oriented in parallel, the cells spontaneously assemble into an anisotropic tissue that mimics the structural anisotropy, electrical properties and contractile function of the laminar syncytium of the heart (figure 6). The cardiomyocytes align along the main axis of the fibronectin lines, form sarcomeres whose Z-lines register perpendicular to the long axis of the myocyte, and spontaneously form gap junction connections between the cells, thus establishing electrical connectivity throughout the tissue. The myocytes will beat spontaneously, or can be stimulated electrically to propagate action potential wavefronts with the elliptical shape characteristic of an anisotropic tissue. Moreover, the myocytes contract isometrically in response to electrical activation.
Figure 6.
Anisotropic structure–function relations within a microengineered two-dimensional cardiac tissue. Large numbers of cardiomyocytes were cultured on dishes containing a micropatterned array of linear adhesive islands (12.5–25 μm wide) that were oriented in parallel and coated with fibronectin densities of (a) 0, (c) 1.25 or (e)5 μg ml−1. Cells were stained for F-actin using fluorescent-phalloidin to visualize structural anisotropy. Corresponding isochrones of electrical propagation initiated within these tissues by point stimulation at the tissue centre (pulse symbol) are shown at 8 ms intervals in (b), (d) and (f). Double-headed arrows denote direction of micropatterned fibronectin lines and muscle fibre orientation, and the direction of longitudinal propagation of the action potential. (Reprinted with permission from Bursac et al. (2002)).
This model is unique in that it shows the ability of cardiomyocytes to immediately organize a tissue with respect to directional cues in the ECM. The novel structure of these microengineered tissues induces unique emergent behaviours among the collection of myocytes, including pathogenic behaviours such as reentrant arrhythmias, figure-of-8 reentry patterns and multiple, wandering spiral wavefronts in vitro (K. K. Parker et al. 2004, unpublished observation). These are all lethal arrhythmias that can lead to death in vivo, but owing to the two-dimensional structure of this artificial heart tissue and the fact that it is artificially perfused, their dynamics can be recorded for longer times than is possible in the living organ. Thus, using this microengineered two-dimensional in vitro system, experimentalists can capture key morphogenetic responses that are characteristic of heart development and pathology, and reveal the robustness of these structural and functional hierarchies, even given the reduced dimensionality of this simplified model system.
4. Conclusion and implications for heart tissue engineering
The studies previously described clearly show that the three-dimensional hierarchical structure of the heart is critical for the intricate spatial and temporal control of its function. ECM networks that link cells together within tissues, and tissues together within the whole organ, are central to this control mechanism. The effects of ECM on cell and tissue function reside largely in its mechanical coupling role which leads to coordinated control of cell shape and orientation, electromechanical coupling and spatiotemporal patterns of electrical conduction. This is mediated by ECM–integrin–cytoskeleton coupling at progressively smaller size scales.
In effect, the ECM lattice that physically couples the different cells that comprise heart tissue is a massively parallel network for the analogue communication of mechanical forces. Contractile forces generated by sarcomeres within the cytoskeleton of a cardiomyocyte are transmitted to transmembrane integrin receptors that span the surface membrane and physically connect to the ECM network in the extracellular space. This mechanical communication circuit is bidirectional, in that mechanical forces can be transmitted either out of the myocyte in the form of traction on its ECM adhesions, or into the muscle cell when the force is exerted on the ECM (e.g. due to mechanical distortion of the myocardium caused by fluid filling of heart chamber). Changes in the level of forces exerted on focal adhesions, and hence on the whole cytoskeleton, feedback to alter intracellular signalling and thereby change cellular biochemistry and gene expression. Like paracrine signalling pathways, mechanical communication between adjacent myocytes is greater than that between cells spread at a distance within the heart. However, unlike typical chemical signalling pathways, the speed of signal transmission is much faster and the signals are sustained for substantially longer periods of time.
Mechanical forces that are transmitted over ECM within tissues serve to orient the constituent cells in a consistent manner and to regulate their structural and functional differentiation as indicated, for example, by the myofibrillar architecture of the cardiomyocyte. The finding that the arrangement of these key elements of the contractile apparatus of the cardiomyocyte is sensitive to physical cues in the ECM, including the surface chemistry, topography and mechanics of the substrate, suggests that these are all key features that must be incorporated into any synthetic biomaterials that will be used for heart tissue engineering in the future. Correct ECM molecules or peptide mimetics need to be incorporated into these materials to support adhesion of appropriate cell types; the topography can be altered to promote desired anisotropy, as well as directional motility; and changes in material compliance may be varied in particular regions to produce desired local responses. Moreover, the finding that tensional forces exerted at the whole tissue level can also promote directional sprouting of capillary blood vessels (Korff & Augustin 1999), as well as oriented nerve growth (Bray 1979; Joshi et al. 1985; Condron & Zinn 1997; Lamoureux et al. 2002), suggest that any successful artificial material must be structured properly so as to distribute forces correctly at multiple size scales. Unfortunately, these are challenges that are currently beyond current materials fabrication capabilities used for tissue engineering applications.
Our review of how ECM regulates everything from ion channel pore size to elastic recoil of the ventricle in diastole illustrates other difficulties facing the tissue engineer as the problem moves from a two-dimensional monolayer to a three-dimensional tissue whose dimensions exceed the oxygen diffusion limit. Heterogeneous cell populations and subpopulations, three-dimensional architecture of ECM, and control of vascular and neural network architecture with micrometre precision are all seemingly intractable problems given today's tissue engineering techniques. The ability of cells to interact with their environment and to modify it according to their genetic destinies is another key developmental process that has yet to be fully exploited.
The experimental studies we summarized here show that it is now possible to recapitulate the heart's structural hierarchies in two-dimensional engineered cardiac tissues using microfabrication techniques based on photolithography that allow structure–function relationships to be preserved over the spatial scales ranging from micrometres to centimetres. Obvious limits include the ability to match substrate stiffness and restriction of chemical diffusion to mimic in vivo tissue microenvironment. However, the future of this field depends on lessons learned from cardiac organogenesis and the innate ability of cells, cellular ensembles and large populations of cells to self-organize such that functional higher order structures emerge with scale-appropriate functions. Thus, while heart tissue engineering is commonly presented as a strategy for replacing damaged and diseased myocardium, in vitro assays developed from microengineered heart tissues also may facilitate identification of key molecular pathways that may be exploited therapeutically. In addition, the ability to microengineer cardiac tissues that mimic relevant heart pathologies provides a unique platform for the development of pharmacological therapeutics for arrhythmias and contractile dysfunction. These two pathological conditions are typically divorced in therapeutic regimens, but, as illustrated by this review, may in fact be structurally and functionally interrelated.
Taken together, these observations reaffirm the critical role for ECM scaffolds in tissue development, and thus the importance of engineering synthetic biomaterials for heart tissue engineering that have appropriate structural features. However, design of these features must take into account that these structural networks are always linked to information networks, and thus, it is critical that synthetic scaffolds incorporate more than specific adhesive ligands alone (i.e. as currently dominates the field). Principles uncovered by the microengineering approach described here may help to delineate these critical structural features, and hence lead to identification of important design criteria that may be employed by tissue engineers and nanotechnologists for the development of novel materials and devices for heart repair and reconstruction in the future.
Acknowledgments
This work was supported by grants from NIH and DARPA, and by funding from NSF to Harvard University's Materials Research and Engineering Center.
Footnotes
One contribution of 21 to a Theme Issue ‘Bioengineering the heart’.
References
- Adler C.P, Ringlage W.P, Bohm N. DNA content and cell number in heart and liver of children. Comparable biochemical, cytophotometric and histological investigations. Pathol. Res. Pract. 1981;172:25–41. [Author's transl.] [PubMed] [Google Scholar]
- Alenghat F.J, Ingber D.E. Mechanotransduction: all signals point to cytoskeleton, matrix, and integrins. Sci. STKE. 2002;2002:PE6. doi: 10.1126/stke.2002.119.pe6. [DOI] [PubMed] [Google Scholar]
- Badylak S, Obermiller J, Geddes L, Matheny R. Extracellular matrix for myocardial repair. Heart Surg. Forum. 2003;6:E20–E26. doi: 10.1532/hsf.917. [DOI] [PubMed] [Google Scholar]
- Baicu C.F, Stroud J.D, Livesay V.A, Hapke E, Holder J, Spinale F.G, Zile M.R. Changes in extracellular collagen matrix alter myocardial systolic performance. Am. J. Physiol. Heart Circ. Physiol. 2003;284:H122–H132. doi: 10.1152/ajpheart.00233.2002. [DOI] [PubMed] [Google Scholar]
- Bick R.J, Snuggs M.B, Poindexter B.J, Buja L.M, Van Winkle W.B. Physical, contractile and calcium handling properties of neonatal cardiac myocytes cultured on different matrices. Cell Adhes. Commun. 1998;6:301–310. doi: 10.3109/15419069809010789. [DOI] [PubMed] [Google Scholar]
- Bing O.H, et al. Localization of alpha1(I) collagen mRNA in myocardium from the spontaneously hypertensive rat during the transition from compensated hypertrophy to failure. J. Mol. Cell. Cardiol. 1997;29:2335–2344. doi: 10.1006/jmcc.1997.0465. doi:10.1006/jmcc.1997.0465 [DOI] [PubMed] [Google Scholar]
- Borg T.K, Ranson W.F, Moslehy F.A, Caulfield J.B. Structural basis of ventricular stiffness. Lab. Invest. 1981;44:49–54. [PubMed] [Google Scholar]
- Brancaccio M, et al. Melusin is a new muscle-specific interactor for β1 integrin cytoplasmic domain. J. Biol. Chem. 1999;274:29 282–29 288. doi: 10.1074/jbc.274.41.29282. doi:10.1074/jbc.274.41.29282 [DOI] [PubMed] [Google Scholar]
- Brancaccio M, et al. Melusin, a muscle-specific integrin β1-interacting protein, is required to prevent cardiac failure in response to chronic pressure overload. Nat. Med. 2003;9:68–75. doi: 10.1038/nm805. doi:10.1038/nm805 [DOI] [PubMed] [Google Scholar]
- Bray D. Mechanical tension produced by nerve cells in tissue culture. J. Cell Sci. 1979;37:391–410. doi: 10.1242/jcs.37.1.391. [DOI] [PubMed] [Google Scholar]
- Brock A, Chang E, Ho C.C, LeDuc P, Jiang X, Whitesides G.M, Ingber D.E. Geometric determinants of directional cell motility revealed using microcontact printing. Langmuir. 2003;19:1611–1617. doi: 10.1021/la026394k. doi:10.1021/la026394k [DOI] [PubMed] [Google Scholar]
- Browe D.M, Baumgarten C.M. Stretch of beta 1 integrin activates an outwardly rectifying chloride current via FAK and Src in rabbit ventricular myocytes. J. Gen. Physiol. 2003;122:689–702. doi: 10.1085/jgp.200308899. doi:10.1085/jgp.200308899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browe D.M, Baumgarten C.M. Angiotensin II (AT1) receptors and NADPH oxidase regulate Cl current elicited by β1 integrin stretch in rabbit ventricular myocytes. J. Gen. Physiol. 2004;124:273–287. doi: 10.1085/jgp.200409040. doi:10.1085/jgp.200409040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bursac N, Parker K.K, Iravanian S, Tung L. Cardiomyocyte cultures with controlled macroscopic anisotropy: a model for functional electrophysiological studies of cardiac muscle. Circ. Res. 2002;91:e45–e54. doi: 10.1161/01.res.0000047530.88338.eb. doi:10.1161/01.RES.0000047530.88338.EB [DOI] [PubMed] [Google Scholar]
- Callaway D.S, Hopcroft J.E, Kleinberg J.M, Newman M.E, Strogatz S.H. Are randomly grown graphs really random? Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2001;64:041 902. doi: 10.1103/PhysRevE.64.041902. [DOI] [PubMed] [Google Scholar]
- Camelliti P, Borg T.K, Kohl P. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc. Res. 2005;65:40–51. doi: 10.1016/j.cardiores.2004.08.020. doi:10.1016/j.cardiores.2004.08.020 [DOI] [PubMed] [Google Scholar]
- Carver W, Terracio L, Borg T.K. Expression and accumulation of interstitial collagen in the neonatal rat heart. Anat. Rec. 1993;236:511–520. doi: 10.1002/ar.1092360311. doi:10.1002/ar.1092360311 [DOI] [PubMed] [Google Scholar]
- Caulfield J.B, Borg T.K. The collagen network of the heart. Lab. Invest. 1979;40:364–372. [PubMed] [Google Scholar]
- Chen C.S, Mrksich M, Huang S, Whitesides G.M, Ingber D.E. Geometric control of cell life and death. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. doi:10.1126/science.276.5317.1425 [DOI] [PubMed] [Google Scholar]
- Chen C.S, Ostuni E, Whitesides G.M, Ingber D.E. Using self-assembled monolayers to pattern ECM proteins and cells on substrates. Methods Mol. Biol. 2000;139:209–219. doi: 10.1385/1-59259-063-2:209. [DOI] [PubMed] [Google Scholar]
- Cheng Q, Ross R.S, Walsh K.B. Overexpression of the integrin β1A subunit and the β1A cytoplasmic domain modifies the beta-adrenergic regulation of the cardiac L-type Ca(2+)current. J. Mol. Cell. Cardiol. 2004;36:809–819. doi: 10.1016/j.yjmcc.2004.03.006. doi:10.1016/j.yjmcc.2004.03.006 [DOI] [PubMed] [Google Scholar]
- Condron B.G, Zinn K. Regulated neurite tension as a mechanism for determination of neuronal arbor geometries in vivo. Curr. Biol. 1997;7:813–816. doi: 10.1016/s0960-9822(06)00343-5. doi:10.1016/S0960-9822(06)00343-5 [DOI] [PubMed] [Google Scholar]
- Dabiri G.A, Turnacioglu K.K, Sanger J.M, Sanger J.W. Myofibrillogenesis visualized in living embryonic cardiomyocytes. Proc. Natl Acad. Sci. USA. 1997;94:9493–9498. doi: 10.1073/pnas.94.17.9493. doi:10.1073/pnas.94.17.9493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dike L.E, Chen C.S, Mrksich M, Tien J, Whitesides G.M, Ingber D.E. Geometric control of switching between growth, apoptosis, and differentiation during angiogenesis using micropatterned substrates. In Vitro Cell. Dev. Biol. Anim. 1999;35:441–448. doi: 10.1007/s11626-999-0050-4. doi:10.1007/s11626-999-0050-4 [DOI] [PubMed] [Google Scholar]
- Dlugosz A.A, Antin P.B, Nachmias V.T, Holtzer H. The relationship between stress fiber-like structures and nascent myofibrils in cultured cardiac myocytes. J. Cell Biol. 1984;99:2268–2278. doi: 10.1083/jcb.99.6.2268. doi:10.1083/jcb.99.6.2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehler E, Rothen B.M, Hammerle S.P, Komiyama M, Perriard J.C. Myofibrillogenesis in the developing chicken heart: assembly of Z-disk, M-line and the thick filaments. J. Cell Sci. 1999;112(Pt10):1529–1539. doi: 10.1242/jcs.112.10.1529. [DOI] [PubMed] [Google Scholar]
- Ezzell R.M, Goldmann W.H, Wang N, Parasharama N, Ingber D.E. Vinculin promotes cell spreading by mechanically coupling integrins to the cytoskeleton. Exp. Cell Res. 1997;231:14–26. doi: 10.1006/excr.1996.3451. doi:10.1006/excr.1996.3451 [DOI] [PubMed] [Google Scholar]
- Federspiel W.J, Popel A.S. A theoretical analysis of the effect of the particulate nature of blood on oxygen release in capillaries. Microvasc. Res. 1986;32:164–189. doi: 10.1016/0026-2862(86)90052-x. doi:10.1016/0026-2862(86)90052-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J.J, Hill C.C. From topology to dynamics in biochemical networks. Chaos. 2001;11:809–815. doi: 10.1063/1.1414882. doi:10.1063/1.1414882 [DOI] [PubMed] [Google Scholar]
- Furukawa T, Yamane T, Terai T, Katayama Y, Hiraoka M. Functional linkage of the cardiac ATP-sensitive K+ channel to the actin cytoskeleton. Pflugers Arch. 1996;431:504–512. doi: 10.1007/BF02191896. [DOI] [PubMed] [Google Scholar]
- Galli A, DeFelice L.J. Inactivation of L-type Ca channels in embryonic chick ventricle cells: dependence on the cytoskeletal agents colchicine and taxol. Biophys. J. 1994;67:2296–2304. doi: 10.1016/S0006-3495(94)80715-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudesius G, Miragoli M, Thomas S.P, Rohr S. Coupling of cardiac electrical activity over extended distances by fibroblasts of cardiac origin. Circ. Res. 2003;93:421–428. doi: 10.1161/01.RES.0000089258.40661.0C. doi:10.1161/01.RES.0000089258.40661.0C [DOI] [PubMed] [Google Scholar]
- Glass L, Hill C.C. Ordered and disordered dynamics in random networks. Europhys. Lett. 1998;41:599–604. doi:10.1209/epl/i1998-00199-0 [Google Scholar]
- Gregorio C.C, Antin P.B. To the heart of myofibril assembly. Trends Cell Biol. 2000;10:355–362. doi: 10.1016/s0962-8924(00)01793-1. doi:10.1016/S0962-8924(00)01793-1 [DOI] [PubMed] [Google Scholar]
- Honig C.R, Frierson J.L, Gayeski T.E. Anatomical determinants of O2 flux density at coronary capillaries. Am. J. Physiol. 1989;256:H375–H382. doi: 10.1152/ajpheart.1989.256.2.H375. [DOI] [PubMed] [Google Scholar]
- Hoofd L, Bos C, Turek Z. Modelling erythrocytes as point-like O2 sources in a Kroghian cylinder model. Adv. Exp. Med. Biol. 1994;345:893–900. doi: 10.1007/978-1-4615-2468-7_117. [DOI] [PubMed] [Google Scholar]
- Hu H, Sachs F. Stretch-activated ion channels in the heart. J. Mol. Cell. Cardiol. 1997;29:1511–1523. doi: 10.1006/jmcc.1997.0392. doi:10.1006/jmcc.1997.0392 [DOI] [PubMed] [Google Scholar]
- Hu S, Chen J, Fabry B, Numaguchi Y, Gouldstone A, Ingber D.E, Fredberg J.J, Butler J.P, Wang N. Intracellular stress tomography reveals stress focusing and structural anisotropy in cytoskeleton of living cells. Am. J. Physiol. Cell Physiol. 2003;285:C1082–C1090. doi: 10.1152/ajpcell.00159.2003. [DOI] [PubMed] [Google Scholar]
- Hu S, Chen J, Wang N. Cell spreading controls balance of prestress by microtubules and extracellular matrix. Front. Biosci. 2004;9:2177–2182. doi: 10.2741/1352. doi:10.2741/1352 [DOI] [PubMed] [Google Scholar]
- Huang S, Ingber D.E. Shape-dependent control of cell growth, differentiation, and apoptosis: switching between attractors in cell regulatory networks. Exp. Cell Res. 2000;261:91–103. doi: 10.1006/excr.2000.5044. doi:10.1006/excr.2000.5044 [DOI] [PubMed] [Google Scholar]
- Huang S, Eichler G, Bar-Yaam Y, Ingber D. Cell fate as high-dimensional attractor of a complex gene regulatory network. Phys. Rev. Let. 2005;94:128 701. doi: 10.1103/PhysRevLett.94.128701. doi:10.1103/PhysRevLett.94.128701 [DOI] [PubMed] [Google Scholar]
- Hudlicka O, Brown M, Egginton S. Angiogenesis in skeletal and cardiac muscle. Physiol. Rev. 1992;72:369–417. doi: 10.1152/physrev.1992.72.2.369. [DOI] [PubMed] [Google Scholar]
- Ingber D.E. Tensegrity II. How structural networks influence cellular information processing networks. J. Cell Sci. 2003;116:1397–1408. doi: 10.1242/jcs.00360. doi:10.1242/jcs.00360 [DOI] [PubMed] [Google Scholar]
- Itano N, Okamoto S, Zhang D, Lipton S.A, Ruoslahti E. Cell spreading controls endoplasmic and nuclear calcium: a physical gene regulation pathway from the cell surface to the nucleus. Proc. Natl Acad. Sci. USA. 2003;100:5181–5186. doi: 10.1073/pnas.0531397100. doi:10.1073/pnas.0531397100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong H, Mason S.P, Barabasi A.L, Oltvai Z.N. Lethality and centrality in protein networks. Nature. 2001;411:41–42. doi: 10.1038/35075138. doi:10.1038/35075138 [DOI] [PubMed] [Google Scholar]
- Jiang X, Bruzewicz D.A, Wong A.P, Piel M, Whitesides G.M. Directing cell migration with asymmetric micro-patterns. Proc. Natl Acad. Sci. USA. 2005;102:975–978. doi: 10.1073/pnas.0408954102. doi:10.1073/pnas.0408954102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B.D, Byerly L. Ca2+ channel Ca(2+)-dependent inactivation in a mammalian central neuron involves the cytoskeleton. Pflugers Arch. 1994;429:14–21. doi: 10.1007/BF02584025. doi:10.1007/BF02584025 [DOI] [PubMed] [Google Scholar]
- Joshi H.C, Chu D, Buxbaum R.E, Heidemann S.R. Tension and compression in the cytoskeleton of PC 12 neurites. J. Cell Biol. 1985;101:697–705. doi: 10.1083/jcb.101.3.697. doi:10.1083/jcb.101.3.697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman S.A. Metabolic stability and epigenesis in randomly constructed genetic nets. J. Theor. Biol. 1969;22:437–467. doi: 10.1016/0022-5193(69)90015-0. doi:10.1016/0022-5193(69)90015-0 [DOI] [PubMed] [Google Scholar]
- Kauffman S.A. Oxford University Press; New York, NY: 1993. The origins of order. [Google Scholar]
- Klein C.L, Scholl M, Maelicke A. Neuronal networks in vitro: formation and organization on biofunctionalized surfaces. J. Mater. Sci. Mater. Med. 1999;10:721–727. doi: 10.1023/a:1008975105243. doi:10.1023/A:1008975105243 [DOI] [PubMed] [Google Scholar]
- Kogler H, Hartmann O, Leineweber K, Nguyen van P, Schott P, Brodde O.E, Hasenfuss G. Mechanical load-dependent regulation of gene expression in monocrotaline-induced right ventricular hypertrophy in the rat. Circ. Res. 2003;93:230–237. doi: 10.1161/01.RES.0000085042.89656.C7. doi:10.1161/01.RES.0000085042.89656.C7 [DOI] [PubMed] [Google Scholar]
- Kohl P. Heterogeneous cell coupling in the heart: an electrophysiological role for fibroblasts. Circ. Res. 2003;93:381–383. doi: 10.1161/01.RES.0000091364.90121.0C. doi:10.1161/01.RES.0000091364.90121.0C [DOI] [PubMed] [Google Scholar]
- Korff T, Augustin H.G. Tensional forces in fibrillar extracellular matrices control directional capillary sprouting. J. Cell Sci. 1999;112(19):3249–3258. doi: 10.1242/jcs.112.19.3249. [DOI] [PubMed] [Google Scholar]
- Lader A.S, Kwiatkowski D.J, Cantiello H.F. Role of gelsolin in the actin filament regulation of cardiac L-type calcium channels. Am. J. Physiol. 1999;277:C1277–C1283. doi: 10.1152/ajpcell.1999.277.6.C1277. [DOI] [PubMed] [Google Scholar]
- Lamoureux P, Ruthel G, Buxbaum R.E, Heidemann S.R. Mechanical tension can specify axonal fate in hippocampal neurons. J. Cell Biol. 2002;159:499–508. doi: 10.1083/jcb.200207174. doi:10.1083/jcb.200207174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe P.D, Nguyen B.P, Gil S, Usui M, Olerud J, Takada Y, Carter W.G. Cellular interaction of integrin α3β1 with laminin 5 promotes gap junctional communication. J. Cell Biol. 1998;143:1735–1747. doi: 10.1083/jcb.143.6.1735. doi:10.1083/jcb.143.6.1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen W.J, Tung H.N, Murray S.A, Swenson C.A. Evidence for the participation of actin microfilaments and bristle coats in the internalization of gap junction membrane. J. Cell Biol. 1979;83:576–587. doi: 10.1083/jcb.83.3.576. doi:10.1083/jcb.83.3.576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng T, Wu P, Mehenti N.Z, Bent S.F, Marmor M.F, Blumenkranz M.S, Fishman H.A. Directed retinal nerve cell growth for use in a retinal prosthesis interface. Invest. Ophthalmol. Vis. Sci. 2004;45:4132–4137. doi: 10.1167/iovs.03-1335. doi:10.1167/iovs.03-1335 [DOI] [PubMed] [Google Scholar]
- Manabe I, Shindo T, Nagai R. Gene expression in fibroblasts and fibrosis: involvement in cardiac hypertrophy. Circ. Res. 2002;91:1103–1113. doi: 10.1161/01.res.0000046452.67724.b8. doi:10.1161/01.RES.0000046452.67724.B8 [DOI] [PubMed] [Google Scholar]
- Maniotis A.J, Chen C.S, Ingber D.E. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc. Natl Acad. Sci. USA. 1997;94:849–854. doi: 10.1073/pnas.94.3.849. doi:10.1073/pnas.94.3.849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotte E.M. The path not taken. Nat. Biotechnol. 2001;19:626–627. doi: 10.1038/90222. doi:10.1038/90222 [DOI] [PubMed] [Google Scholar]
- Matsushita T, Oyamada M, Fujimoto K, Yasuda Y, Masuda S, Wada Y, Oka T, Takamatsu T. Remodeling of cell–cell and cell–extracellular matrix interactions at the border zone of rat myocardial infarcts. Circ. Res. 1999;85:1046–1055. doi: 10.1161/01.res.85.11.1046. [DOI] [PubMed] [Google Scholar]
- McBeath R, Pirone D.M, Nelson C.M, Bhadriraju K, Chen C.S. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. doi:10.1016/S1534-5807(04)00075-9 [DOI] [PubMed] [Google Scholar]
- Meyer C.J, Alenghat F.J, Rim P, Fong J.H, Fabry B, Ingber D.E. Mechanical control of cyclic AMP signalling and gene transcription through integrins. Nat. Cell Biol. 2000;2:666–668. doi: 10.1038/35023621. doi:10.1038/35023621 [DOI] [PubMed] [Google Scholar]
- Moore S.W, Keller R.E, Koehl M.A. The dorsal involuting marginal zone stiffens anisotropically during its convergent extension in the gastrula of Xenopus laevis. Development. 1995;121:3131–3140. doi: 10.1242/dev.121.10.3131. [DOI] [PubMed] [Google Scholar]
- Morris C.E. Mechanosensitive ion channels. J. Membr. Biol. 1990;113:93–107. doi: 10.1007/BF01872883. doi:10.1007/BF01872883 [DOI] [PubMed] [Google Scholar]
- Murray S.A, Williams S.Y, Dillard C.Y, Narayanan S.K, McCauley J. Relationship of cytoskeletal filaments to annular gap junction expression in human adrenal cortical tumor cells in culture. Exp. Cell Res. 1997;234:398–404. doi: 10.1006/excr.1997.3628. doi:10.1006/excr.1997.3628 [DOI] [PubMed] [Google Scholar]
- Ojakian G.K, Ratcliffe D.R, Schwimmer R. Integrin regulation of cell–cell adhesion during epithelial tubule formation. J. Cell Sci. 2001;114:941–952. doi: 10.1242/jcs.114.5.941. [DOI] [PubMed] [Google Scholar]
- Parker K.K, Taylor L.K, Atkinson J.B, Hansen D.E, Wikswo J.P. The effects of tubulin-binding agents on stretch-induced ventricular arrhythmias. Eur. J. Pharmacol. 2001;417:131–140. doi: 10.1016/s0014-2999(01)00856-1. doi:10.1016/S0014-2999(01)00856-1 [DOI] [PubMed] [Google Scholar]
- Parker K.K, et al. Directional control of lamellipodia extension by constraining cell shape and orienting cell tractional forces. FASEB J. 2002;16:1195–1204. doi: 10.1096/fj.02-0038com. doi:10.1096/fj.02-0038com [DOI] [PubMed] [Google Scholar]
- Pelouch V. Molecular aspects of regulation of cardiac contraction. Physiol. Res. 1995;44:53–60. [PubMed] [Google Scholar]
- Polte T.R, Eichler G.S, Wang N, Ingber D.E. Extracellular matrix controls myosin light chain phosphorylation and cell contractility through modulation of cell shape and cytoskeletal prestress. Am. J. Physiol. Cell Physiol. 2004;286:C518–C528. doi: 10.1152/ajpcell.00280.2003. doi:10.1152/ajpcell.00280.2003 [DOI] [PubMed] [Google Scholar]
- Rakusan K, Cicutti N, Kolar F. Cardiac function, microvascular structure, and capillary hematocrit in hearts of polycythemic rats. Am. J. Physiol. Heart Circ. Physiol. 2001;281:H2425–H2431. doi: 10.1152/ajpheart.2001.281.6.H2425. [DOI] [PubMed] [Google Scholar]
- Rhee D, Sanger J.M, Sanger J.W. The premyofibril: evidence for its role in myofibrillogenesis. Cell Motil. Cytoskeleton. 1994;28:1–24. doi: 10.1002/cm.970280102. doi:10.1002/cm.970280102 [DOI] [PubMed] [Google Scholar]
- Ross R.S, Borg T.K. Integrins and the myocardium. Circ. Res. 2001;88:1112–1119. doi: 10.1161/hh1101.091862. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Integrins. J. Clin. Invest. 1991;87:1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell B, Motlagh D, Ashley W.W. Form follows function: how muscle shape is regulated by work. J. Appl. Physiol. 2000;88:1127–1132. doi: 10.1152/jappl.2000.88.3.1127. [DOI] [PubMed] [Google Scholar]
- Sadeghi A, Doyle A.D, Johnson B.D. Regulation of the cardiac L-type Ca2+ channel by the actin-binding proteins α-actinin and dystrophin. Am. J. Physiol. Cell Physiol. 2002;282:C1502–C1511. doi: 10.1152/ajpcell.00435.2001. [DOI] [PubMed] [Google Scholar]
- Sadoshima J, Izumo S. Mechanical stretch rapidly activates multiple signal transduction pathways in cardiac myocytes: potential involvement of an autocrine/paracrine mechanism. EMBO J. 1993;12:1681–1692. doi: 10.1002/j.1460-2075.1993.tb05813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoshima J, Jahn L, Takahashi T, Kulik T.J, Izumo S. Molecular characterization of the stretch-induced adaptation of cultured cardiac cells. An in vitro model of load-induced cardiac hypertrophy. J. Biol. Chem. 1992;267:10 551–10 560. [PubMed] [Google Scholar]
- Sharp W.W, Terracio L, Borg T.K, Samarel A.M. Contractile activity modulates actin synthesis and turnover in cultured neonatal rat heart cells. Circ. Res. 1993;73:172–183. doi: 10.1161/01.res.73.1.172. [DOI] [PubMed] [Google Scholar]
- Shiraishi I, Simpson D.G, Carver W, Price R, Hirozane T, Terracio L, Borg T.K. Vinculin is an essential component for normal myofibrillar arrangement in fetal mouse cardiac myocytes. J. Mol. Cell. Cardiol. 1997;29:2041–2052. doi: 10.1006/jmcc.1997.0438. doi:10.1006/jmcc.1997.0438 [DOI] [PubMed] [Google Scholar]
- Singhvi R, Kumar A, Lopez G.P, Stephanopoulos G.N, Wang D.I, Whitesides G.M, Ingber D.E. Engineering cell shape and function. Science. 1994;264:696–698. doi: 10.1126/science.8171320. doi:10.1126/science.8171320 [DOI] [PubMed] [Google Scholar]
- Sodian R, et al. Early in vivo experience with tissue-engineered trileaflet heart valves. Circulation. 2000;102:III22–III29. doi: 10.1161/01.cir.102.suppl_3.iii-22. [DOI] [PubMed] [Google Scholar]
- Tagawa H, Wang N, Narishige T, Ingber D.E, Zile M.R, Cooper G.T. Cytoskeletal mechanics in pressure-overload cardiac hypertrophy. Circ. Res. 1997;80:281–289. doi: 10.1161/01.res.80.2.281. [DOI] [PubMed] [Google Scholar]
- Terzic A, Kurachi Y. Actin microfilament disrupters enhance K(ATP) channel opening in patches from guinea-pig cardiomyocytes. J. Physiol. 1996;492(Pt2):395–404. doi: 10.1113/jphysiol.1996.sp021316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui H, Ishihara K, Cooper G.T. Cytoskeletal role in the contractile dysfunction of hypertrophied myocardium. Science. 1993;260:682–687. doi: 10.1126/science.8097594. doi:10.1126/science.8097594 [DOI] [PubMed] [Google Scholar]
- Undrovinas A.I, Shander G.S, Makielski J.C. Cytoskeleton modulates gating of voltage-dependent sodium channel in heart. Am. J. Physiol. 1995;269:H203–H214. doi: 10.1152/ajpheart.1995.269.1.H203. [DOI] [PubMed] [Google Scholar]
- Wang N, Butler J.P, Ingber D.E. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260:1124–1127. doi: 10.1126/science.7684161. doi:10.1126/science.7684161 [DOI] [PubMed] [Google Scholar]
- Wang Y.G, Samarel A.M, Lipsius S.L. Laminin acts via β1 integrin signalling to alter cholinergic regulation of L-type Ca2+ current in cat atrial myocytes. J. Physiol. 2000;526(Pt1):57–68. doi: 10.1111/j.1469-7793.2000.t01-1-00057.x. doi:10.1111/j.1469-7793.2000.t01-1-00057.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Naruse K, Stamenovic D, Fredberg J.J, Mijailovich S.M, Tolic-Norrelykke I.M, Polte T, Mannix R, Ingber D.E. Mechanical behavior in living cells consistent with the tensegrity model. Proc. Natl Acad. Sci. USA. 2001;98:7765–7770. doi: 10.1073/pnas.141199598. doi:10.1073/pnas.141199598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Ostuni E, Whitesides G.M, Ingber D.E. Micropatterning tractional forces in living cells. Cell Motil. Cytoskeleton. 2002;52:97–106. doi: 10.1002/cm.10037. doi:10.1002/cm.10037 [DOI] [PubMed] [Google Scholar]
- Yan L, Moses M.A, Huang S, Ingber D.E. Adhesion-dependent control of matrix metalloproteinase-2 activation in human capillary endothelial cells. J. Cell Sci. 2000;113(Pt22):3979–3987. doi: 10.1242/jcs.113.22.3979. [DOI] [PubMed] [Google Scholar]
- Zamir E.A, Srinivasan V, Perucchio R, Taber L.A. Mechanical asymmetry in the embryonic chick heart during looping. Ann. Biomed. Eng. 2003;31:1327–1336. doi: 10.1114/1.1623487. doi:10.1114/1.1623487 [DOI] [PubMed] [Google Scholar]
- Zhuang J, Yamada K.A, Saffitz J.E, Kleber A.G. Pulsatile stretch remodels cell-to-cell communication in cultured myocytes. Circ. Res. 2000;87:316–322. doi: 10.1161/01.res.87.4.316. [DOI] [PubMed] [Google Scholar]