Abstract
A crucial step towards the goal of tissue engineering a heart valve will be the choice of scaffold onto which an appropriate cell phenotype can be seeded. Successful scaffold materials should be amenable to modification, have a controlled degradation, be compatible with the cells, lack cytotoxicity and not elicit an immune or inflammatory response. In addition, the scaffold should induce appropriate responses from the cells seeded onto it, such as cell attachment, proliferation and remodelling capacity, all of which should promote the formation of a tissue construct that can mimic the structure and function of the native valve. This paper discusses the various biological scaffolds that have been considered and are being studied for use in tissue engineering a heart valve. Also, strategies to enhance the biological communication between the scaffold and the cells seeded onto it as well as the use of bionanotechnology in the manufacture of scaffolds possessing the desired properties will be discussed.
Keywords: heart valves, tissue engineering, extracellular matrix, bionanotechnology
1. Introduction
The sophisticated functions, regulation and durability of heart valves are closely linked to their inherent biological properties (Yacoub et al. 1999; Yacoub & Cohn 2004). It is becoming increasingly evident that the functions of the native valve cannot be truly emulated by currently available valve substitutes, limiting the clinical benefit, quality of life and survival (Yacoub & Takkenberg 2005). In addition, the burden of heart valve disease is predicted to rise significantly over the next 50 years to over 850 000 cases per year. Thus, there is increasing interest and effort towards reproducing the function of the native valve by developing a living tissue engineered valve. The successful bioengineering of a tissue valve will rely on the completion of a number of integral steps. These include establishing an appropriate choice of cells that will mimic the function of the native cells of the valve, identification of a suitable scaffold material on which to seed the cells and determination of the optimal mechanical conditioning protocols to promote a functioning tissue engineered construct. These steps should culminate in the production and development of a structure that resembles the unique cellular and load-bearing characteristics and exhibits dynamic properties, structure and function similar to the native valve (Hentz & Chang 2001; Yacoub & Cohn 2004; Yacoub & Takkenberg 2005).
This paper discusses different strategies that have been adopted and are being developed to tissue engineer a heart valve derived from a biological scaffold, focusing primarily on the aortic valve. Part of this paper is based on a recent publication by Taylor et al. (2006a).
2. Valve structure and relation to function
Aortic valve leaflets contain about 50% collagen and 13% elastin by dry weight (Bashey et al. 1967), with the principal components of the native valve being collagen (type I (74%), type III (24%) and type V (2%)), elastin and proteoglycans. Each component confers unique physical and mechanical properties crucial for valve function. Collagen fibrils provide mechanical and tensile strength, but are stiff, with limited extensibility. Elastin is highly extensible but has very low stiffness, existing as a sponge-like matrix of fibres and sheets with tubular openings surrounding and interconnecting the collagen fibre bundles (Scott & Vesely 1996). The aortic valve can withstand strains of up to 40%, returning to its original form when unloaded (Vesely & Lozon 1993). It has been proposed that this is owing to the wave-like configuration of collagen in valve leaflets and that elastin maintains the specific collagen fibre orientation, returning it to its original state after loading (Scott & Vesely 1995; Vesely 1998). The highly negatively charged glycosaminoglycan side chains of the proteoglycans produce a hydrated gel-like ground substance which allows interaction of other matrix molecules and deposition of components such as growth factors. In addition, the particular chemical composition of glycosaminoglycans can influence the function and survival of endothelial and, possibly, other cells (Johnson et al. 2004).
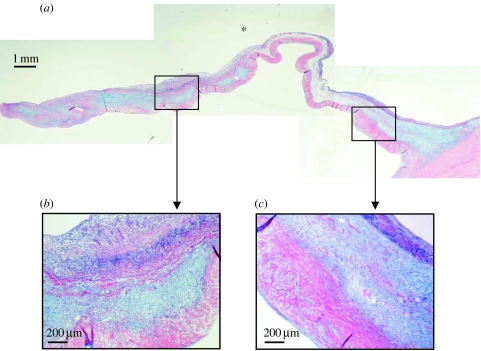
The extracellular matrix of the aortic valve is spatially arranged to give the leaflet three distinct regions (figure 1). These regions are termed the fibrosa, which is found on the aortic side of the cusp, the ventricularis on the ventricular side and the spongiosa in between (Bairati & DeBiasi 1981). The fibrosa is considered to be the main load-carrying structure and is primarily composed of circumferentially arranged densely packed bundles of collagen fibres and a microfibrillar network of elastin. The spongiosa is composed of glycosaminoglycan-containing proteoglycans which absorb water, providing resistance against compressive forces and allowing shearing between the layers. It also contains loosely arranged collagen and a thin mesh of elastic fibres. The thinnest layer, the ventricularis, contains radially arranged elastin, mostly in the form of sheets, with less well-organized collagen. Elegant studies using multiphoton microscopy have provided high-resolution three-dimensional images of elastin and collagen within the extracellular matrix of aortic and pulmonary valves, illustrating their spatial configuration (Konig et al. 2005; Schenke-Layland et al. 2005).
Figure 1.
Human aortic valve stained with Miller's elastin, alcian blue and sirius red, showing leaflet hinge to coapting edge at (a) low power and (b,c) areas identified by the boxes at higher power. The ventricularis, spongiosa and fibrosa can be discerned. Elastin is stained purple, glycosaminoglycans (proteoglycans) pale blue and collagen pink. Asterisk denotes ventricular side of the leaflet.
We previously described the distribution and arrangement of extracellular matrix components in human heart valves and reported that many of these were expressed by cultured valve interstitial cells (ICs; Latif et al. 2005a). A major and vital function of the ICs that populate the valve matrix is the production of extracellular matrix components, allowing continual repair and remodelling of the matrix throughout life. We have also shown that valve structures respond to several humoral mediators (Chester et al. 2000, 2001; Kershaw et al. 2004) and neural impulses (A. H. Chester & J. D. Kershaw 2004, unpublished obervations), and proposed that these responses may play a role in changing the size and shape of different components of the valve during the cardiac cycle, with possible implications for coronary flow, left ventricular function and valve durability (Yacoub et al. 1999; Yacoub & Cohn 2004).
The extracellular matrix plays a crucial role in valve function, dictating the physical and mechanical properties of the valve, maintaining the spatial arrangement of the cells that reside within it and mediating the complex crosstalk that exists between the cells, the matrix and the external forces. Cardiac valves exist in a highly dynamic environment and are subjected to patterns of flow and pressure that are unique in the cardiovascular system. The complex haemodynamic forces generated in the aortic valve in vivo impose different forces on the inflow and outflow surfaces of the valve (Thubrikar et al. 1986; Deck et al. 1988). The ventricular or inflow surface of the aortic valve is exposed to pulsatile laminar flow, whereas the aortic or outflow surface is exposed to turbulent low-shear flow. In addition, the whole of the valve is exposed to diastolic pressure. The deposition and subsequent remodelling of extracellular matrix components within a tissue engineered construct will play a crucial role in its ability to withstand haemodynamic forces and thus determine its ultimate success.
3. The scaffold
A suitable scaffold for tissue engineering must be compatible with the cells that are to be seeded and grown onto it to form a ‘tissue construct’. Successful scaffold materials need to be amenable to modification, have a controlled degradation rate and possess properties that will promote and direct cellular attachment, migration, proliferation and differentiation. The scaffold and its degradation products should neither be cytotoxic nor elicit an immune, inflammatory or thrombogenic response. The scaffold will serve as an initial support for the cells, which would then be replaced by newly synthesized extracellular matrix. The point at which seeded constructs should or could be implanted will depend upon their mechanical and functional properties. This will largely be determined by the capacity of candidate cells to populate and remodel the scaffold and will rely on the conditioning protocols used. However, since tissue engineered valves must have the strength and integrity to function as the native valve when implanted, it is probable that they need to be developed under dynamic conditioning protocols in vitro until mature extracellular matrix characteristic of the native valve has formed. This strategy would have the added advantage of minimizing any deleterious effects of the original scaffold and its degradation products. Clearly, accurate determination of scaffold degradation rate and remodelling will be vital to the success of a tissue engineered living valve that has the ability to emulate the structure and function of the native valve.
Although promising results have been obtained from in vivo animal studies using valves made from synthetic biodegradable polymers repopulated with autologous cells, such constructs have neither been able to withstand aortic pressures nor reproduce many of the sophisticated functions of the native aortic valve (Hoerstrup et al. 2000; Sodian et al. 2000; Stock et al. 2000; Sutherland et al. 2005). A great advantage of synthetic polymers is that they can be easily modified to have a wide range of mechanical and chemical properties, including rate of degradation. Conversely, of major concern is their lack of biocompatibility and biological cues. The latter is substantiated by the demonstration that pre-coating polyglycolic acid scaffolds with human extracellular matrix proteins improves attachment of human aortic myofibroblasts (Ye et al. 2000c). Thus, biological scaffolds have several advantages over synthetic scaffolds as they are a rich source of signalling and cell attachment molecules, which promote and direct cell proliferation, differentiation and function. However, controlling the mechanical properties and degradation rates of biological scaffolds will be challenging, and unfixed naturally derived materials may provoke an undesirable immune response. It is probable that human recombinant proteins rather than animal-derived proteins will be used in order to prevent any risk of transmission of infectious agents such as porcine endogenous retroviruses or prions. Overcoming the challenges involved in using biological materials as scaffolds would appear to be a worthwhile strategy, since it would result in the provision of an environment reminiscent of the native tissue to be replicated.
Communication between the extracellular matrix and the cells within the matrix is important for maintaining cellular function and the spatial organization of the tissue. It is recognized that cell–matrix and cell–cell interactions have the capacity to initiate signalling mechanisms that result in changes in cell function. Interactions between integrins on the cell membrane and receptors on the extracellular matrix or counter receptor on the cell surface are largely responsible for these effects (ffrench-Constant & Colognato 2004; Humphries et al. 2004). We recently described the expression profile of integrins and other adhesion molecules that interact with the extracellular matrix by human valve ICs both in situ and in vitro (Latif et al. 2005b). Integrins are dimeric proteins consisting of α and β chains, with at least 18α subunits and 8β subunits that assemble into 24αβ heterodimeric receptors. Most integrins are not specific for a single ligand and generally bind several ligands, which in turn are recognized by multiple integrins (Hynes 2002). Integrin activation mediates cellular attachment to the extracellular matrix and plays an important role in the translation of mechanical stress from the extracellular matrix to the cell cytoskeleton, providing a link between the outside and inside of the cell and activating signal transduction pathways. These interactions are important during development and throughout life, maintaining specialized cellular and tissue structure and function. Integrins also participate in remodelling the extracellular matrix via integrin-mediated release of matrix metalloproteinases, which in addition to remodelling the extracellular matrix also release extracellular matrix-bound growth factors such as fibroblast growth factor and transforming growth factor-β (McCawley & Matrisian 2001). More recently, it has been demonstrated that an important collaboration or synergy exists between integrins and growth factor receptors, which regulates subsequent cell signal transduction pathways (Comoglio et al. 2003; ffrench-Constant & Colognato 2004). Therefore, tissue engineering strategies using naturally occurring biological scaffolds rich in receptors for integrins may be more successful than those using synthetic scaffolds.
4. Scaffold modifications
As previously discussed, synthetic scaffolds lack the vital temporal and spatial complexity that would be provided by naturally occurring extracellular matrices. In order to direct organization, proliferation and differentiation of cells in tissue engineered constructs, the scaffold must provide chemical and biological cues, allowing crosstalk between the cells and the scaffold, as well as the necessary physical support (Langer & Tirrell 2004). Synthetic scaffolds can be engineered to present a specific profile of signalling molecules and growth factors within the fabric of the scaffold. Such biomaterials, containing bioactive molecules, have been termed ‘biomimetics’. Ideally, these biomaterials would be designed to have the appropriate physical strength, degradation kinetics and biological signals to promote the development of functional tissue engineered constructs. Biomimetics can be synthesized de novo or chemically modified, resulting in the formation of a covalent bond between the biomaterial and the bioactive peptide. The favoured strategy is to use cell-binding peptides rather than whole extracellular matrix proteins, since these are more stable and can be easily synthesized. Using protein engineering and recombinant DNA technology, growth factors and signalling peptides can be modified to express sequences designed to bind specifically to the scaffold. This powerful technology can be used to modify either synthetic or biological scaffolds.
5. Biological matrices
(a) Collagen
There are several biological scaffolds that may be suitable for tissue engineering heart valves. Of these, type I collagen is probably one of the most appropriate, since it is the major extracellular matrix protein of the native valve and provides most of its mechanical and tensile strength. In addition, collagens have a low antigenicity, being only weakly immunogenic largely due to their homology across species and are biodegradable owing to their proteinaceous nature (Chevallay & Herbage 2000). The biodegradability can be reduced, if required, through the introduction of cross links between the polypeptide chains.
Our studies have demonstrated that valve ICs proliferated within type I collagen scaffolds under static conditions and that collagen enhanced the capacity of the cells to express their native phenotype (Taylor et al. 2002). Others have reported the migration of smooth muscle cells into collagen scaffolds, the development of a tissue-like morphology with highly organized, newly synthesized extracellular matrix proteins and the production of collagen and proteoglycans in collagen scaffolds seeded with valve myofibroblasts (Rothenburger et al. 2001, 2002b,a). These studies were carried out under static conditions, but clearly demonstrate the ability of collagen to promote cell responses.
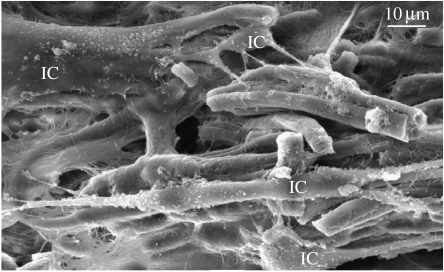
Collagen scaffolds can be made by freezing aqueous-based dispersions of insoluble collagen at −30°C, which results in the formation of pores created by ice crystal growth (Sachlos et al. 2003). The size of the pores is dependent on the freezing temperature, the concentration of collagen and the pH of the solution. Immersion in ethanol dissolves the ice crystals, and critical point drying with liquid carbon dioxide results in a dry collagen scaffold, which can then be lightly cross-linked using a non-toxic, dehydrothermal treatment, resulting in the formation of interchain cross links. This technique does not denature the tertiary structure of collagen (Sachlos et al. 2003). Scanning electron microscopy demonstrated that valve ICs interacted with and spread across collagen scaffolds made using this technique (figure 2). In addition, we found that cells consistently proliferated to a greater extent on 1% type I collagen scaffolds rather than on either 2 or 5% collagen scaffolds (Taylor et al. 2006b). The size of the pores in the 1% scaffolds is larger than those made from 2 and 5% collagen and may be preferred by the cells, since it should allow better diffusion of nutrients and removal of waste products. The advantage of using this technique to produce scaffolds is that other types of collagen and also elastin can be added to the dispersion. Through careful manipulation, scaffolds that contain only elastin in the lower layer can be produced to emulate the native aortic valve, where elastin is found predominantly in the ventricularis.
Figure 2.
Scanning electron micrographs of 1% bovine type I collagen scaffolds seeded with aortic valve interstitial cells (IC) and cultured for 28 days.
The use of animal-derived collagen raises concerns over the possible transmission of infectious agents such as prions. Human recombinant collagen may provide a solution to this problem, but non-mammalian hosts require exogenous expression of prolyl 4-hydroxylase to stabilize the triple helix and native triple-helical collagen is notoriously difficult to work with owing to its insolubility and gelating properties, making it a less attractive option. Thus, other alternatives are being sought. The innovative creation of peptide-based supramolecules designed to mimic the structure and function of collagen presents an attractive alternative approach (Koide 2005).
(b) Xenogenic extracellular matrices
In recent years, decellularized, sterilized, xenogenic, extracellular matrices derived from a variety of tissues have been used as scaffolds for regenerative medicine (Badylak 2004). The unique spatial organization and composition of the extracellular matrix, which retains biologically functional molecules or ‘morphogens’, provide an extremely favourable environment for tissue regeneration by cells. Indeed, the use of decellularized valve matrices is one of the main approaches currently being investigated for tissue engineered heart valves and a comprehensive review is provided by Schmidt et al. (2007) in this issue.
The small intestine submucosa has been reported to encourage migration and spatial organization of cells within its matrix (Badylak et al. 1998). This resorbable, acellular bioscaffold is composed mainly of collagen type I, with small amounts of collagen types III and IV, as well as other extracellular matrix components such as fibronectin, glycosaminoglycans and growth factors. It has been extensively tested in animal studies and has been reported to induce native tissue regeneration in various organs (bladder, urethra, blood vessels, ligaments, tendons and valves).
Promising results have been obtained using porcine small intestine submucosa to replace mature pulmonary leaflets in pigs (Matheny et al. 2000). The implanted matrix was reabsorbed and progressively replaced with a functionally competent leaflet possessing histologically identifiable features similar to native tissue. Although the matrix was not seeded prior to implantation, it became covered with a layer of endothelial cells. Recently, it was reported that prosthetic valves constructed of small intestine submucosa, inserted percutaneously in pigs, showed the potential for longevity, without the need for anticoagulation or immunosuppression (Ruiz et al. 2005). Although the authors demonstrated histological remodelling of the extracellular matrix as well as endothelialization and population of the matrix by fibroblasts and smooth muscle cells, they expressed concern over the progressive thickening of the valves. In addition, the exact phenotype of these cells and whether such autologous repopulation would occur in humans remain to be proven.
(c) Fibrin
Fibrin gels have controllable biodegradable and polymerization characteristics. They have an additional advantage as they can easily be prepared from the patient's blood, resulting in an autologous scaffold that should produce neither toxic degradation products nor inflammatory or immune reactions. In addition, fibrin interacts biologically with cells and can promote proliferation or migration. Using aprotonin to inhibit fibrinolysis, Ye et al. (2000a) demonstrated cell proliferation and collagen production in fibrin gels seeded with aortic-derived myofibroblasts. Further studies revealed that complex structures such as a valve conduit could be formed from fibrin/cell gels using a moulding technique (Jockenhoevel et al. 2001a). However, these composites displayed poor mechanical properties and there were added problems owing to gel shrinkage. Fixation of fibrin gels using poly-l-lysine has been reported to prevent shrinkage and enhance collagen production through creation of inner tension, resulting in improved mechanical properties (Jockenhoevel et al. 2001b). It has also been demonstrated that the biological characteristics of fibrin can be enhanced by immobilization of growth and other active factors, a highly desirable attribute for tissue engineering applications (Schense & Hubbell 1999).
(d) Autologous extracellular matrix
An alternative and novel approach is the production of autologous tissue constructs without the use of a supporting scaffold. Human aortic myofibroblasts are capable of forming sheets in culture, which can be layered and cultured on frames forming solid flexible and elastic tissues (Ye et al. 2000b). Ascorbic acid was added to promote extracellular matrix production and the constructs resembled native tissue possessing bundles of mature collagen fibres. Using the same technique, it was reported that folding and framing the sheets of cells enhanced collagen production through the creation of tension and that cells of aortic origin produced more collagen than those of venous origin (Hoerstrup et al. 2002). Again, although these results are promising and encouraging, the mechanical properties of such constructs have not yet been shown to be sufficient for implantation. However, others have shown that vessels constructed from sheet-based tissue engineering of skin fibroblasts, intended for arterial bypass grafts, demonstrated mechanical properties similar to those of human blood vessels and were mechanically stable in an animal model for up to eight months (L'heureux et al. 2006).
(e) Hydrogels
Hyaluronic acid is a unique type of glycosaminoglycan in that it is a non-sulphated polysaccharide and exists in the free form with no covalently bound protein. Hyaluronan plays an important role in biological processes such as matrix structure, lubrication, cell movement and differentiation (Turley et al. 2002). It is an essential component in cardiac morphogenesis and is the predominant glycosaminoglycan of the native valve leaflet. These characteristics make it an attractive candidate scaffold material for tissue engineering heart valves. Hydrogels can be made by cross-linking hyaluronan using a variety of methods to form non-immunogenic, non-thrombogenic scaffolds. It has been demonstrated that hyaluronan hydrogels significantly increase extracellular matrix production by valve ICs (Masters et al. 2004) and further studies revealed that hydrogel degradation products increased valve IC proliferation and extracellular matrix production (Masters et al. 2005). It has also been reported that seeding cells on such hydrogels induced synthesis of elastin, resulting in elastin matrices resembling those of the native aortic valve (Ramamurthi & Vesely 2005). These results demonstrate the attractiveness of pursuing the use of hyaluronic acid-based scaffolds for tissue engineering heart valves.
Composite type I collagen–chondroitin sulphate hydrogels have also been investigated (Flanagan et al. 2006). When seeded with valve interstitial and valve endothelial cells, the authors reported that chondroitin sulphate increased matrix porosity and positively influenced cell bioactivity and matrix remodelling. They demonstrated an enhanced coverage of gels with endothelial cells and increased extracellular matrix production including fibronectin, laminin collagen and elastin.
6. Bionanotechnology
The recently emerging and expanding field of bionanotechnology promises great potential for the development of scaffolds with specific biological properties. Molecular self-assembly occurs ubiquitously and reproducibly in nature and is exemplified in minerals, biological composites such as pearls, silk, teeth, muscle and extracellular matrix, and in macromolecular assemblies such as haemoglobin, enzymes, membrane channels and ribosomes. These sophisticated fabrication processes pose a daunting challenge for scientists and engineers. Bionanotechnology has been defined as nanotechnology (engineering at the nanometre scale) that uses biological precedents for guidance, deriving inspiration from nature. Using this technology, scaffolds for tissue engineering could be designed and modified at the molecular level incorporating recognition sequences to promote the desired cellular function. This is an attractive and powerful strategy for the production of scaffolds that would result in tissue engineered constructs with the properties and functions of native tissues.
Since nanofibres are around 1000 times smaller than synthetic polymer fibres, they are able to surround cells in a manner similar to the native extracellular matrix. This allows biomolecules to diffuse slowly, creating local molecular gradients. Molecular gradients are known to play a vital and fundamental role in cell differentiation, signal transduction, organ development and many other biological processes (Tabata & Takei 2004). The size of the pores within the nanofibre scaffold is much smaller than the diameter of the cells precluding, or at the very least impeding, the addition of cells after its formation. This has dictated construction of the nanofibre network in situ around the cells, without deleterious effects to the cells, negating the use of techniques such as electrospinning. This has been achieved using amphiphilic peptides that self-assemble in physiological medium (Zhang 2003). Amphiphilic peptides possess hydrophilic and hydrophobic regions and are capable of self-assembly into well-ordered nanofibres and scaffolds of defined diameter and pore size (Zhang et al. 2002, 2005; Zhang 2003). Self-assembly occurs through the formation of non-covalent, reversible, relatively weak interactions such as electrostatic, van der Waals, hydrophobic and hydrogen bonds with stable assemblies resulting from the formation of multiple interactions. The peptides can be formed from either naturally occurring or chemically synthesized amino acids.
A variety of human and animal-derived cells, encapsulated and cultured within peptide scaffolds, have been reported to exhibit proliferation, differentiation, migration and production of their own extracellular matrix (Zhang 2003). Importantly, specific signalling molecules such as extracellular matrix ligands can be engineered into the fabric of the nanofibre scaffold to specifically direct cell differentiation, migration or proliferation. Encapsulating neural progenitor cells in nanofibres containing a neurite-promoting laminin-derived peptide selectively induced differentiation of cells into neurons, rather than astrocytes (Silva et al. 2004). Cells derive additional cues from nanotopography which promote adhesion, spreading and cytoskeletal rearrangement, illustrating another advantage of using nanofibres (Berry et al. 2005).
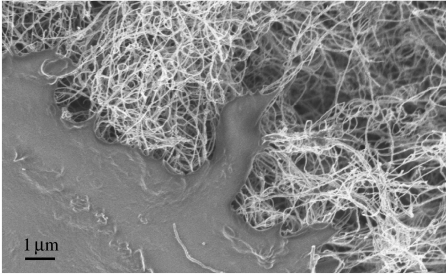
Composite materials of synthetic and naturally occurring polymers are also being developed for use as tissue engineering scaffolds owing to their potential to improve structural properties. One such composite is collagen–carbon nanotubes, which can be made by mixing soluble collagen type I with a solution of single-walled carboxylated carbon nanotubes (MacDonald et al. 2005). Cells incorporated into these matrices during collagen gelation were reported to be viable after 7 days and capable of proliferation. Others have demonstrated that cells were able to attach and proliferate on a network of 35 nm multi-walled carbon nanotubes (figure 3; George et al. 2006). Carbon nanotubes have diameters ranging from 0.7 to 2 nm, but can be up to 1000 times greater in length, having a very high aspect ratio. In addition, they are flexible and among the strongest, stiffest materials known. Whether they will result in the development and production of tissue engineered constructs with structure and function of the native valve remains to be seen.
Figure 3.
Scanning electron micrograph showing interaction of a human osteosarcoma cell (bottom left) with multi-walled carbon nanotubes. Cells attached to and spread across the carbon nanotubes.
Bionanotechnology is in its infancy and shows great promise for a broad range of applications in engineering, electronics and medicine. However, there are concerns over the consequences of nanoparticles on human health, owing to their high surface reactivity and ability to cross cell membranes, which are the very reasons for their exploitation. In the context of tissue engineering, the risks and consequences of nanoparticles and nanotubes that are not fixed or embedded in the support scaffold, as might occur during degradation and remodelling in vivo, need to be investigated and assessed.
7. Future directions
Clearly, there are many issues to be resolved before the realization of a tissue engineered heart valve with the sophisticated structure and function of the native valve. It would appear that strategies employing scaffolds which display biological signalling and adhesion molecules show the most promise.
Acknowledgments
The author would like to thank Dr Padmini Sarathchandra, Imperial College London, for supplying the images in figures 1 and 2, and Mr Julian George, Dr Milo Shaffer and Dr Molly Stevens, Imperial College London, for supplying the image in figure 3.
Footnotes
One contribution of 21 to a Theme Issue ‘Bioengineering the heart’.
References
- Badylak S.F. Xenogeneic extracellular matrix as a scaffold for tissue reconstruction. Transpl. Immunol. 2004;12:367–377. doi: 10.1016/j.trim.2003.12.016. doi:10.1016/j.trim.2003.12.016 [DOI] [PubMed] [Google Scholar]
- Badylak S.F, Record R, Lindberg K, Hodde J, Park K. Small intestinal submucosa: a substrate for in vitro cell growth. J. Biomater. Sci. Polym. Edn. 1998;9:863–878. doi: 10.1163/156856298x00208. [DOI] [PubMed] [Google Scholar]
- Bairati A, DeBiasi S. Presence of a smooth muscle system in aortic valve leaflets. Anat. Embryol.(Berl) 1981;161:329–340. doi: 10.1007/BF00301830. doi:10.1007/BF00301830 [DOI] [PubMed] [Google Scholar]
- Bashey R.I, Torii S, Angrist A. Age-related collagen and elastin content of human heart valves. J. Gerontol. 1967;22:203–208. doi: 10.1093/geronj/22.2.203. [DOI] [PubMed] [Google Scholar]
- Berry C.C, Dalby M.J, McCloy D, Affrossman S. The fibroblast response to tubes exhibiting internal nanotopography. Biomaterials. 2005;26:4985–4992. doi: 10.1016/j.biomaterials.2005.01.046. doi:10.1016/j.biomaterials.2005.01.046 [DOI] [PubMed] [Google Scholar]
- Chester A.H, Misfeld M, Yacoub M.H. Receptor-mediated contraction of aortic valve leaflets. J. Heart Valve Dis. 2000;9:250–254. [PubMed] [Google Scholar]
- Chester A.H, Misfeld M, Sievers H.H, Yacoub M.H. Influence of 5-hydroxytryptamine on aortic valve competence in vitro. J. Heart Valve Dis. 2001;10:822–825. [PubMed] [Google Scholar]
- Chevallay B, Herbage D. Collagen-based biomaterials as 3D scaffold for cell cultures: applications for tissue engineering and gene therapy. Med. Biol. Eng. Comput. 2000;38:211–218. doi: 10.1007/BF02344779. doi:10.1007/BF02344779 [DOI] [PubMed] [Google Scholar]
- Comoglio P.M, Boccaccio C, Trusolino L. Interactions between growth factor receptors and adhesion molecules: breaking the rules. Curr. Opin. Cell Biol. 2003;15:565–571. doi: 10.1016/s0955-0674(03)00096-6. doi:10.1016/S0955-0674(03)00096-6 [DOI] [PubMed] [Google Scholar]
- Deck J.D, Thubrikar M.J, Schneider P.J, Nolan S.P. Structure, stress, and tissue repair in aortic valve leaflets. Cardiovasc. Res. 1988;22:7–16. doi: 10.1093/cvr/22.1.7. [DOI] [PubMed] [Google Scholar]
- ffrench-Constant C, Colognato H. Integrins: versatile integrators of extracellular signals. Trends Cell Biol. 2004;14:678–686. doi: 10.1016/j.tcb.2004.10.005. doi:10.1016/j.tcb.2004.10.005 [DOI] [PubMed] [Google Scholar]
- Flanagan T.C, Wilkins B, Black A, Jockenhoevel S, Smith T.J, Pandit A.S. A collagen-glycosaminoglycan co-culture model for heart valve tissue engineering applications. Biomaterials. 2006;27:2233–2246. doi: 10.1016/j.biomaterials.2005.10.031. doi:10.1016/j.biomaterials.2005.10.031 [DOI] [PubMed] [Google Scholar]
- George J.H, Shaffer M.S, Stevens M.M. Investigating the cellular response to nanofibrous materials by the use of a multi-walled carbon nanotube model. J. Exp. Nanosci. 2006;1:1–12. doi:10.1080/17458080500463149 [Google Scholar]
- Hentz R, Chang J. Tissue engineering for reconstruction of the thumb. N. Engl. J. Med. 2001;344:1547–1548. doi: 10.1056/NEJM200105173442011. doi:10.1056/NEJM200105173442011 [DOI] [PubMed] [Google Scholar]
- Hoerstrup S.P, et al. Functional living trileaflet heart valves grown in vitro. Circulation. 2000;102:III44–III49. doi: 10.1161/01.cir.102.suppl_3.iii-44. [DOI] [PubMed] [Google Scholar]
- Hoerstrup S.P, Zund G, Cheng S, Melnitchouk S, Kadner A, Sodian R, Kolb S.A, Turina M. A new approach to completely autologous cardiovascular tissue in humans. ASAIO J. 2002;48:234–238. doi: 10.1097/00002480-200205000-00006. doi:10.1097/00002480-200205000-00006 [DOI] [PubMed] [Google Scholar]
- Humphries M.J, Travis M.A, Clark K, Mould A.P. Mechanisms of integration of cells and extracellular matrices by integrins. Biochem. Soc. Trans. 2004;32:822–825. doi: 10.1042/BST0320822. doi:10.1042/BST0320407 [DOI] [PubMed] [Google Scholar]
- Hynes R.O. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. doi:10.1016/S0092-8674(02)00971-6 [DOI] [PubMed] [Google Scholar]
- Jockenhoevel S, Chalabi K, Sachweh J.S, Groesdonk H.V, Demircan L, Grossmann M, Zund G, Messmer B.J. Tissue engineering: complete autologous valve conduit—a new moulding technique. Thorac. Cardiovasc. Surg. 2001a;49:287–290. doi: 10.1055/s-2001-17807. doi:10.1055/s-2001-17807 [DOI] [PubMed] [Google Scholar]
- Jockenhoevel S, Zund G, Hoerstrup S.P, Chalabi K, Sachweh J.S, Demircan L, Messmer B.J, Turina M. Fibrin gel—advantages of a new scaffold in cardiovascular tissue engineering. Eur. J. Cardiothorac. Surg. 2001b;19:424–430. doi: 10.1016/s1010-7940(01)00624-8. doi:10.1016/S1010-7940(01)00624-8 [DOI] [PubMed] [Google Scholar]
- Johnson N.A, et al. Endothelial cells preparing to die by apoptosis initiate a program of transcriptome and glycome regulation. FASEB J. 2004;18:188–190. doi: 10.1096/fj.03-0097fje. [DOI] [PubMed] [Google Scholar]
- Kershaw J.D, Misfeld M, Sievers H.H, Yacoub M.H, Chester A.H. Specific regional and directional contractile responses of aortic cusp tissue. J. Heart Valve Dis. 2004;13:798–803. [PubMed] [Google Scholar]
- Koide T. Triple helical collagen-like peptides: engineering and applications in matrix biology. Connect. Tissue Res. 2005;46:131–141. doi: 10.1080/03008200591008518. doi:10.1080/03008200591008518 [DOI] [PubMed] [Google Scholar]
- Konig K, Schenke-Layland K, Riemann I, Stock U.A. Multiphoton autofluorescence imaging of intratissue elastic fibers. Biomaterials. 2005;26:495–500. doi: 10.1016/j.biomaterials.2004.02.059. doi:10.1016/j.biomaterials.2004.02.059 [DOI] [PubMed] [Google Scholar]
- L'heureux N, et al. Human tissue-engineered blood vessels for adult arterial revascularization. Nat. Med. 2006;12:361–365. doi: 10.1038/nm1364. doi:10.1038/nm1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer R, Tirrell D.A. Designing materials for biology and medicine. Nature. 2004;428:487–492. doi: 10.1038/nature02388. doi:10.1038/nature02388 [DOI] [PubMed] [Google Scholar]
- Latif N, Sarathchandra P, Taylor P.M, Antoniw J, Yacoub M.H. Localization and pattern of expression of extracellular matrix components in human heart valves. J. Heart Valve Dis. 2005a;14:218–227. [PubMed] [Google Scholar]
- Latif N, Sarathchandra P, Taylor P.M, Antoniw J, Yacoub M.H. Molecules mediating cell–ECM and cell–cell communication in human heart valves. Cell Biochem. Biophys. 2005b;43:275–288. doi: 10.1385/CBB:43:2:275. doi:10.1385/CBB:43:2:275 [DOI] [PubMed] [Google Scholar]
- MacDonald R.A, Laurenzi B.F, Viswanathan G, Ajayan P.M, Stegemann J.P. Collagen–carbon nanotube composite materials as scaffolds in tissue engineering. J. Biomed. Mater. Res. A. 2005;74:489–496. doi: 10.1002/jbm.a.30386. [DOI] [PubMed] [Google Scholar]
- Masters K.S, Shah D.N, Walker G, Leinwand L.A, Anseth K.S. Designing scaffolds for valvular interstitial cells: cell adhesion and function on naturally derived materials. J. Biomed. Mater. Res. A. 2004;71:172–180. doi: 10.1002/jbm.a.30149. doi:10.1002/jbm.a.30149 [DOI] [PubMed] [Google Scholar]
- Masters K.S, Shah D.N, Leinwand L.A, Anseth K.S. Crosslinked hyaluronan scaffolds as a biologically active carrier for valvular interstitial cells. Biomaterials. 2005;26:2517–2525. doi: 10.1016/j.biomaterials.2004.07.018. doi:10.1016/j.biomaterials.2004.07.018 [DOI] [PubMed] [Google Scholar]
- Matheny R.G, Hutchison M.L, Dryden P.E, Hiles M.D, Shaar C.J. Porcine small intestine submucosa as a pulmonary valve leaflet substitute. J. Heart Valve Dis. 2000;9:769–774. [PubMed] [Google Scholar]
- McCawley L.J, Matrisian L.M. Matrix metalloproteinases: they're not just for matrix anymore! Curr. Opin. Cell Biol. 2001;13:534–540. doi: 10.1016/s0955-0674(00)00248-9. doi:10.1016/S0955-0674(00)00248-9 [DOI] [PubMed] [Google Scholar]
- Ramamurthi A, Vesely I. Evaluation of the matrix-synthesis potential of crosslinked hyaluronan gels for tissue engineering of aortic heart valves. Biomaterials. 2005;26:999–1010. doi: 10.1016/j.biomaterials.2004.04.016. doi:10.1016/j.biomaterials.2004.04.016 [DOI] [PubMed] [Google Scholar]
- Rothenburger M, Vischer P, Volker W, Glasmacher B, Berendes E, Scheld H.H, Deiwick M. In vitro modelling of tissue using isolated vascular cells on a synthetic collagen matrix as a substitute for heart valves. Thorac. Cardiovasc. Surg. 2001;49:204–209. doi: 10.1055/s-2001-16108. doi:10.1055/s-2001-16108 [DOI] [PubMed] [Google Scholar]
- Rothenburger M, Volker W, Vischer J.P, Berendes E, Glasmacher B, Scheld H.H, Deiwick M. Tissue engineering of heart valves: formation of a three-dimensional tissue using porcine heart valve cells. ASAIO J. 2002a;48:586–591. doi: 10.1097/00002480-200211000-00003. doi:10.1097/00002480-200211000-00003 [DOI] [PubMed] [Google Scholar]
- Rothenburger M, Volker W, Vischer P, Glasmacher B, Scheld H.H, Deiwick M. Ultrastructure of proteoglycans in tissue-engineered cardiovascular structures. Tissue Eng. 2002b;8:1049–1056. doi: 10.1089/107632702320934146. doi:10.1089/107632702320934146 [DOI] [PubMed] [Google Scholar]
- Ruiz C.E, et al. Transcatheter placement of a low-profile biodegradable pulmonary valve made of small intestinal submucosa: a long-term study in a swine model. J. Thorac. Cardiovasc. Surg. 2005;130:477–484. doi: 10.1016/j.jtcvs.2005.04.008. doi:10.1016/j.jtcvs.2005.04.008 [DOI] [PubMed] [Google Scholar]
- Sachlos E, Reis N, Ainsley C, Derby B, Czernuszka J.T. Novel collagen scaffolds with predefined internal morphology made by solid freeform fabrication. Biomaterials. 2003;24:1487–1497. doi: 10.1016/s0142-9612(02)00528-8. doi:10.1016/S0142-9612(02)00528-8 [DOI] [PubMed] [Google Scholar]
- Schenke-Layland K, Riemann I, Stock U.A, Konig K. Imaging of cardiovascular structures using near-infrared femtosecond multiphoton laser scanning microscopy. J. Biomed. Opt. 2005;10:024017. doi: 10.1117/1.1896966. doi:10.1117/1.1896966 [DOI] [PubMed] [Google Scholar]
- Schense J.C, Hubbell J.A. Cross-linking exogenous bifunctional peptides into fibrin gels with factor XIIIa. Bioconjug. Chem. 1999;10:75–81. doi: 10.1021/bc9800769. doi:10.1021/bc9800769 [DOI] [PubMed] [Google Scholar]
- Schmidt D, Stock U.A, Hoerstrup S.P. Tissue engineering of heart valves using decellularized xenogenic or polymeric starter matrices. Phil. Trans. R. Soc. B. 2007;362:1505–1512. doi: 10.1098/rstb.2007.2131. doi:10.1098/rstb.2007.2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M, Vesely I. Aortic valve cusp microstructure: the role of elastin. Ann. Thorac. Surg. 1995;60:S391–S394. doi: 10.1016/0003-4975(95)00263-k. doi:10.1016/0003-4975(95)00263-K [DOI] [PubMed] [Google Scholar]
- Scott M.J, Vesely I. Morphology of porcine aortic valve cusp elastin. J. Heart Valve Dis. 1996;5:464–471. [PubMed] [Google Scholar]
- Silva G.A, Czeisler C, Niece K.L, Beniash E, Harrington D.A, Kessler J.A, Stupp S.I. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science. 2004;303:1352–1355. doi: 10.1126/science.1093783. doi:10.1126/science.1093783 [DOI] [PubMed] [Google Scholar]
- Sodian R, et al. Early in vivo experience with tissue-engineered trileaflet heart valves. Circulation. 2000;102:III22–III29. doi: 10.1161/01.cir.102.suppl_3.iii-22. [DOI] [PubMed] [Google Scholar]
- Stock U.A, et al. Tissue-engineered valved conduits in the pulmonary circulation. J. Thorac. Cardiovasc. Surg. 2000;119:732–740. doi: 10.1016/s0022-5223(00)70008-0. doi:10.1016/S0022-5223(00)70008-0 [DOI] [PubMed] [Google Scholar]
- Sutherland F.W, et al. From stem cells to viable autologous semilunar heart valve. Circulation. 2005;111:2783–2791. doi: 10.1161/CIRCULATIONAHA.104.498378. doi:10.1161/CIRCULATIONAHA.104.498378 [DOI] [PubMed] [Google Scholar]
- Tabata T, Takei Y. Morphogens, their identification and regulation. Development. 2004;131:703–712. doi: 10.1242/dev.01043. doi:10.1242/dev.01043 [DOI] [PubMed] [Google Scholar]
- Taylor P.M, Allen S.P, Dreger S.A, Yacoub M.H. Human cardiac valve interstitial cells in collagen sponge: a biological three-dimensional matrix for tissue engineering. J. Heart Valve Dis. 2002;11:298–306. [PubMed] [Google Scholar]
- Taylor P.M, Cass A.E.G, Yacoub M.H. Extracellular matrix scaffolds for tissue engineering heart valves. Prog. Paediatr. Cardiol. 2006a;21:219–225. doi:10.1016/j.ppedcard.2005.11.010 [Google Scholar]
- Taylor P.M, Sachlos E, Dreger S.A, Chester A.H, Czernuszka J.T, Yacoub M.H. Interaction of human valve interstitial cells with collagen matrices manufactured using rapid prototyping. Biomaterials. 2006b;27:2733–2737. doi: 10.1016/j.biomaterials.2005.12.003. doi:10.1016/j.biomaterials.2005.12.003 [DOI] [PubMed] [Google Scholar]
- Thubrikar M.J, Aouad J, Nolan S.P. Comparison of the in vivo and in vitro mechanical properties of aortic valve leaflets. J. Thorac. Cardiovasc. Surg. 1986;92:29–36. [PubMed] [Google Scholar]
- Turley E.A, Noble P.W, Bourguignon L.Y. Signaling properties of hyaluronan receptors. J. Biol. Chem. 2002;277:4589–4592. doi: 10.1074/jbc.R100038200. doi:10.1074/jbc.R100038200 [DOI] [PubMed] [Google Scholar]
- Vesely I. The role of elastin in aortic valve mechanics. J. Biomech. 1998;31:115–123. doi: 10.1016/s0021-9290(97)00122-x. doi:10.1016/S0021-9290(97)00122-X [DOI] [PubMed] [Google Scholar]
- Vesely I, Lozon A. Natural preload of aortic valve leaflet components during glutaraldehyde fixation: effects on tissue mechanics. J. Biomech. 1993;26:121–131. doi: 10.1016/0021-9290(93)90043-e. doi:10.1016/0021-9290(93)90043-E [DOI] [PubMed] [Google Scholar]
- Yacoub M.H, Cohn L.H. Novel approaches to cardiac valve repair: from structure to function: part I. Circulation. 2004;109:942–950. doi: 10.1161/01.CIR.0000115633.19829.5E. doi:10.1161/01.CIR.0000115633.19829.5E [DOI] [PubMed] [Google Scholar]
- Yacoub M.H, Takkenberg J.J.M. Will heart valve tissue engineering change the world? Nat. Clin. Prac. Cardiovasc. Med. 2005;2:60–61. doi: 10.1038/ncpcardio0112. doi:10.1038/ncpcardio0112 [DOI] [PubMed] [Google Scholar]
- Yacoub M.H, Kilner P.J, Birks E.J, Misfeld M. The aortic outflow and root: a tale of dynamism and crosstalk. Ann. Thorac. Surg. 1999;68:S37–S43. doi: 10.1016/s0003-4975(99)00745-6. doi:10.1016/S0003-4975(99)00745-6 [DOI] [PubMed] [Google Scholar]
- Ye Q, Zund G, Benedikt P, Jockenhoevel S, Hoerstrup S.P, Sakyama S, Hubbell J.A, Turina M. Fibrin gel as a three dimensional matrix in cardiovascular tissue engineering. Eur. J. Cardiothorac. Surg. 2000a;17:587–591. doi: 10.1016/s1010-7940(00)00373-0. doi:10.1016/S1010-7940(00)00373-0 [DOI] [PubMed] [Google Scholar]
- Ye Q, Zund G, Jockenhoevel S, Hoerstrup S.P, Schoeberlein A, Grunenfelder J, Turina M. Tissue engineering in cardiovascular surgery: new approach to develop completely human autologous tissue. Eur. J. Cardiothorac. Surg. 2000b;17:449–454. doi: 10.1016/s1010-7940(00)00371-7. doi:10.1016/S1010-7940(00)00371-7 [DOI] [PubMed] [Google Scholar]
- Ye Q, Zund G, Jockenhoevel S, Schoeberlein A, Hoerstrup S.P, Grunenfelder J, Benedikt P, Turina M. Scaffold precoating with human autologous extracellular matrix for improved cell attachment in cardiovascular tissue engineering. ASAIO J. 2000c;46:730–733. doi: 10.1097/00002480-200011000-00014. doi:10.1097/00002480-200011000-00014 [DOI] [PubMed] [Google Scholar]
- Zhang S. Fabrication of novel biomaterials through molecular self-assembly. Nat. Biotechnol. 2003;21:1171–1178. doi: 10.1038/nbt874. doi:10.1038/nbt874 [DOI] [PubMed] [Google Scholar]
- Zhang S, Marini D.M, Hwang W, Santoso S. Design of nanostructured biological materials through self-assembly of peptides and proteins. Curr. Opin. Chem. Biol. 2002;6:865–871. doi: 10.1016/s1367-5931(02)00391-5. doi:10.1016/S1367-5931(02)00391-5 [DOI] [PubMed] [Google Scholar]
- Zhang S, Gelain F, Zhao X. Designer self-assembling peptide nanofiber scaffolds for 3D tissue cell cultures. Semin. Cancer Biol. 2005;15:413–420. doi: 10.1016/j.semcancer.2005.05.007. doi:10.1016/j.semcancer.2005.05.007 [DOI] [PubMed] [Google Scholar]