Abstract
Although recent progress in cardiovascular tissue engineering has generated great expectations for the exploitation of stem cells to restore cardiac form and function, the prospects of a common mass-produced cell resource for clinically viable engineered tissues and organs remain problematic. The refinement of stem cell culture protocols to increase induction of the cardiomyocyte phenotype and the assembly of transplantable vascularized tissue are areas of intense current research, but the problem of immune rejection of heterologous cell type poses perhaps the most significant hurdle to overcome. This article focuses on the potential advantages and problems encountered with various stem cell sources for reconstruction of the damaged or failing myocardium or heart valves and also discusses the need for integrating advances in developmental and stem cell biology, immunology and tissue engineering to achieve the full potential of cardiac tissue engineering. The ultimate goal is to produce ‘off-the-shelf’ cells and tissues capable of inducing specific immune tolerance.
Keywords: stem cells, tolerance, immune response, regenerative medicine, tissue engineering
1. Introduction
Tissue engineering, one of the major components of regenerative medicine, encompasses various disciplines such as biology, material science and engineering, with the single aim of developing living substitutes that can restore and maintain normal function. This involves several strategies including the ex vivo use of three-dimensional biological scaffolds seeded with appropriate cells and cultivated in bioreactors which allow formation of the desired tissue or organ (Stock & Vacanti 2001; Vaananen 2005). A critical factor in these strategies is the choice and source of cells to be used. The use of autologous cells from the host is actively being explored to avoid rejection. However, for many patients with end-stage organ failure this approach is not feasible, since in most cases a tissue biopsy does not yield enough normal cells for expansion, and transplantation and the procedure could be severely hampered by time constraints involved in generating a tissue engineered organ such as heart valve leaflets or functional myocardium. Regenerative therapy, in particular cardiac regeneration, may offer a viable solution (Sanchez et al. 2006; Yacoub et al. 2006).
The use of allogeneic or xenogeneic stem cells to create an ‘off-the-shelf’ organ, suitable for all patients is extremely attractive. However, this poses the significant problem of anti-graft immune responses. During the last few years, a significant amount of knowledge regarding the specific immune responses of stem cells, and to some extent strategies to deal with them, has accumulated. In this article, we will discuss the different sources and types of stem cells that are currently used in both clinical applications and experimental models of cardiac repair in vivo and in vitro, with particular reference to their immunogenicity. This is followed by reviewing current strategies used to enhance engraftment and to overcome rejection.
2. Sources of stem cells for tissue engineering
(a) Embryonic stem cells
Studies carried out on human embryonic stem (ES) cells have demonstrated that they have an enormous potential for generating tissues of therapeutic value, which offers prospects for cell transplantation that may be applicable to a wide range of human diseases. Three main properties make ES cells very attractive for replacement therapies: (i) their ability to grow indefinitely in culture provides a renewable cell source for transplantation and tissue engineering; (ii) ES cells can be genetically manipulated and can theoretically be targeted with transgenes to replace defective genes or to supplement tissues with growth and survival factors; and (iii) the pluripotent capacity of ES cells to generate almost any cell type has been demonstrated through many reported differentiation protocols using established culture conditions (McCloskey et al. 2005; Wobus & Boheler 2005). Although the hope is that human ES cells could be used to treat a wide range of human ailments, the proof of principle has mainly come from studies using animal ES cells where many models are being used to validate their potential use.
(i) ES cells in cardiac repair
The use of ES cells in cardiac repair in animal models has yielded some encouraging results. For instance, the utilization of cardiac-restricted promoters using selectable markers in the selection of cardiomyocytes from differentiating ES cells has been well documented (Klug et al. 1996; Meyer et al. 2000; Muller et al. 2000; Fijnvandraat et al. 2003). Others have demonstrated the therapeutic capacity of mouse ES cell-derived cardiomyocytes by injecting these cells into the ventricular myocardium of adult dystrophic (mdx) mice. ES cell incorporation into the hearts was verified by their presence within the grafts seven weeks after implantation, in the absence of tumour formation (Klug et al. 1996). This study was further corroborated by reports demonstrating ameliorated left ventricular function in post-infarcted rats following implantation of ES-derived ‘beating cells’ (Min et al. 2002). The engrafted cells not only displayed typical striations and markers of mature cardiomyocytes such as the myofilament proteins sarcomeric α-actin, α-myosin heavy chain and troponin I, but were also able to establish themselves among the endogenous cardiomyocytes. Whether the transplanted cardiomyocytes coupled normally with endogenous cells or produced long-term beneficial effects in this study was unclear. However, in a large animal model of xenotransplantation, recent data from Kehat et al. (2004) observed that when they transplanted human ES-derived cardiomyocytes into the left ventricular wall of a pig heart with complete heart block, the grafted cells could electromechanically integrate with the resident cardiomyocytes and functioned to rescue cardiac rhythm, thus acting as pacemaker cells. Recently, progress in developmental biology has identified the involvement of critical factors in cardiomyocyte differentiation, including bone morphogenic protein (Bin et al. 2006) and Wnt signalling proteins (Eisenberg & Eisenberg 1999; Pandur et al. 2002; Terami et al. 2004), and such factors have the potential to improve the efficiency of stem cell induction. Therefore, it remains to be seen whether the full potential of ES-derived cardiomyocytes in the generation of stable cardiac grafts for cardiac therapy will be fulfilled.
Currently, there are no ES cell-based therapies ongoing in humans. Their use in a clinical setting is beset by ethical constraints due to their isolation from in vitro fertilized human embryos, and there are additional concerns such as inefficient differentiation (McLaren 2001) and tumorogenicity (Wobus & Boheler 2005) of human ES cells. In a recent report by Mummery et al. (2003), the problem of inefficient differentiation of human ES cells into functional cardiomyocytes was addressed using a novel strategy. They show that by co-culturing human ES cells, which have not undergone spontaneous cardiogenesis, with visceral-endoderm (VE)-like cells from the mouse, differentiation to beating cardiomyocytes could be initiated within 10 days. This suggested that paracrine factors derived from the VE cells act as a cardiac differentiation factor. However, the nature of the specific factors involved was not elucidated. Nevertheless, the differentiated ES cell-derived cardiomyocytes were fully characterized and were found to exhibit sarcomeric proteins, atrial naturetic peptide, cardiac ion channel genes and the generation of gap junctions, demonstrated by connexion 43 expression and dye-coupling. With regards to tumorogenicity, the neoplastic tendency of embryoid bodies has been proposed to decrease with increased cell differentiation in vitro (Zhang et al. 2001; Chinzei et al. 2002), but the possibility that differentiated ES cell-derived tissues may still harbour undifferentiated ES cells remains a concern. To circumvent this risk, one possibility is to pre-differentiate ES cells in vitro (ideally to 100%) to the desired lineage and/or eliminate undifferentiated cells by positive selection using cell surface markers and fluorescent-activated cell sorting strategies. Such a strategy has been successfully tested in an experimental study (Menard et al. 2005).
Perhaps most importantly, the immunogenicity and the high potential for rejection of differentiated cells derived from ES cells in allogeneic recipients have been major impediments to the approval of clinical trials (Bradley et al. 2002; Drukker & Benvenisty 2004; Swijnenburg et al. 2005). In addition to carrying the risk of infection by non-human pathogens, the exposure of human ES cells to non-human proteins and xenogeneic feeder cells during derivation and propagation may also contribute to their immunogenicity. Non-human sialic acid, Neu5Gc, has been identified as a possible cause for human ES cell immunogenicity (Martin et al. 2005). This protein is abundant in animal-derived serum substitutes and feeder cells to which a vast majority of humans have circulating antibodies. Since both embryoid bodies and human ES cells can metabolically take in considerable amounts of Neu5Gc, their exposure to human sera containing antibodies to Neu5Gc will inevitably result in complement induced cell death in vivo. In an attempt to avoid this possible scenario, human ES cell lines have now been expanded on both human feeder cells (Richards et al. 2002; Amit et al. 2004) and in the absence of feeder cells under serum-free conditions (Lee et al. 2005; Ellerstrom et al. 2006). These newly derived human ES cell lines, free from potential retroviral infections, may then be suitable for eventual therapeutic applications.
(ii) Immunogenicity of ES cells and routes to escape rejection
Human ES cells have the potential to be rejected following post-transplantion due to the fact that they express low levels of major histocompatibility complex (MHC) class I antigen which can increase after differentiation both in vitro and in vivo (Drukker et al. 2002). In addition, interferon gamma treatment has been shown to markedly increase MHC class I expression (Draper et al. 2002; Drukker et al. 2002). Recently, it has also been shown that expression of MHC class I on human ES cells is sufficient for rejection by cytotoxic T cells (Drukker et al. 2006). On the other hand, the absence of MHC class II molecules and low-level expression of MHC class I may also lead to natural killer cell rejection of the transplanted cells (Drukker et al. 2002); however, several studies have reported that this is not always the case (Hori et al. 2003; Mammolenti et al. 2004; Wei et al. 2004). In addition, mismatched minor histocompatibility antigens as well as ABO blood groups should also be considered as potential triggers for rejection. For further reading, the subject of ES cell immunogenicity has been extensively covered in several reviews (Bradley et al. 2002; Drukker & Benvenisty 2004; Fairchild et al. 2004; Boyd et al. 2005).
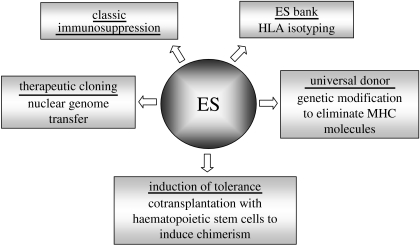
A number of approaches have been proposed to tackle or eliminate ES-derived graft rejection (figure 1). Classic immunosuppression, routinely used for organ transplantation, could reduce the host reactivity to allogeneic ES-derived transplants (Gage 1998), but associated complications such as drug-related toxicities, opportunistic infections (Simon & Levin 2001) and a 100-fold increase in the incidence of malignant tumours (Penn 2000; Buell et al. 2006) may reduce the quality of life for the patients. Another possible route is through the induction of tolerance by cotransplanting both ES-derived graft and haematopoietic stem cells established from the same parental ES cell line. This could lead to specific inhibition of immune rejection (Odorico et al. 2001). Alternatively, genetically modifying ES cells to eliminate MHC class I molecules to generate a ‘universal’ undifferentiated ES cell line has also been suggested (Boheler & Fiszman 1999; Drukker et al. 2002). In addition to the potential consequences of such drastic gene modifications to the graft (Grusby & Glimcher 1995), it is also worth considering that in addition to direct allo-recognition, the absence of MHC molecules may lead to indirect allo-recognition-mediated rejection and/or natural killer cell-mediated cell destruction (McNerney et al. 2006). However, it has been reported that the expression of non-polymorphic class I molecule human leucocyte antigen (HLA)-E on the surface of cells confers protection from death by natural killer cells (Moretta et al. 2002, 2003). Another approach is the generation of an ES stem cell bank containing HLA-isotyped and/or genetically modified human ES cell lines where known HLA backgrounds could be established. But this would involve a massive amount of work to attain pure populations of HLA-defined ES cells, which ultimately may be unachievable.
Figure 1.
Routes for evading immune rejection of human ES cells.
Finally, the process of ‘therapeutic cloning’ has been proposed for generating pluripotent stem cells that carry the nuclear genome of the patient. In this approach, also known as nuclear transfer, the nucleus of a human oocyte is removed and replaced by a nucleus from a somatic cell obtained from the patient. The manipulated oocyte is allowed to proliferate in culture, creating an ES cell population. Inducing these ES cells to differentiate into replacement cells may eliminate the critical problem of immune incompatibility since they contain (with the exception of the mitochondrial genome) the same genetic information as the patient (Lanza et al. 1999). This strategy was successfully extended to an application known as reproductive cloning, used to create Dolly the sheep (Wilmut et al. 1997), although reproductive cloning through nuclear transfer of human cells would not be pursued for ethical reasons. Therapeutic cloning on the other hand is legal in many countries including the United Kingdom (Pincock 2004), other European countries including Sweden and Belgium, and also in certain parts of the United States such as California and New Jersey. Irrespective of what the optimal application proves to be, human ES cells offer an enormous potential for generating tissues of therapeutic value, although further research is required to overcome the problems highlighted earlier, especially those regarding their immunological status following differentiation.
(b) Adult stem/progenitor cells
(i) Sources of adult stem/progenitor cells
Adult stem cells or tissue-derived progenitor cells are a population of cells found within mature tissue that have the capacity to differentiate and expand into mature cells with specific function. However, unlike ES cells which are defined by their origin (the inner cell mass of the blastocyst), adult stem cells cannot be characterized in the same manner, especially since their true origin is still unclear. Adult human stem cells can be isolated from tissues such as periosteum, trabecular bone, adipose tissue, synovium, skeletal muscle, deciduous teeth and, more recently, heart valves (Veinot et al. 2006; Latif et al. submitted) and cardiac tissue (Anversa et al. 2006), in particular the islet-1 positive cells which have been termed as the definitive cardiac progenitor cell (CPC; Laugwitz et al. 2005). The most extensively studied source is the bone marrow which contains the multipotential mesenchymal stem cells (MSCs) or stromal cells, resident at low density within the bone marrow stroma (Le Blanc & Pittenger 2005; Vaananen 2005) as well as haematopoietic stem cells (Bryder et al. 2006) and endothelial progenitor cells (EPCs; Barber & Iruela-Arispe 2006). The plasticity or transdifferentiation ability of these bone marrow stem cells to differentiate into specific cell types of another tissue has been under intense investigation, in particular the use of bone marrow-derived stem cells in cardiac repair.
(ii) Mesenchymal stem cells
The MSC is the most extensively characterized and most potent adult stem cell in terms of differentiation into multiple cell phenotypes including bone, fat, cartilage, muscle, epithelium, neural and cardiomyocytes (Pittenger et al. 2000; Pittenger & Martin 2004; Smits et al. 2005). At present, there is no definitive marker or markers which exclusively identify MSCs, which suggests that the cell populations derived by the current methods are heterogeneous. Morphologically they mostly resemble fibroblast-like cells and are characterized by their positive surface staining for CD105 (SH2 or transforming growth factor-beta (TGFβ) receptor endoglin), CD166 (activated leucocyte-cell adhesion molecule, ALCAM), CD73 (SH3 and SH4 or membrane-bound ecto-5′-nucleotidase) and CD44 (hyaluronan receptor), and negative for haematopoietic surface markers CD34 (haematopoitic stem cells), CD45 (leucocyte common antigen), CD14 (monocytes, macrophages), CD31 (endothelial, T lymphocytes, macrophages) and CD133 (endothelial/stem progenitor cells; Majumdar et al. 2003; Barry & Murphy 2004). Although these cells are considered to be involved mainly in the support of haemapoiesis, their ease of isolation, culture and capacity to be greatly expanded without losing the ability to differentiate into multiple lineages, has highlighted their clinical potential for repair or replacement of damaged tissues (Le Blanc & Pittenger 2005).
Another plentiful source for MSCs is from adipose tissue, which not only provides a rich source of MSCs which can be easily harvested by a simple minimally invasive method, but also of other stem/progenitor cells such as EPCs, providing a plentiful reservoir of MSCs and EPCs which would otherwise be normally discarded following cosmetic surgery. As with bone marrow-derived MSCs, adipose tissue-derived MSCs can also be similarly characterized using the same panel of antibodies. For EPCs, these cells are typically defined on the basis of expression of cell surface markers such as CD34, Flk-1 (vascular endothelial growth factor receptor (VEGFR) 2) and CD133. Of particular interest regarding the use of MSCs and EPCs derived from adipose tissue is the fact that these cells can be isolated in greater numbers (0.001–0.01% versus 1–2%; M. Hedrick 2003, personal communication) and expanded more rapidly than from bone marrow. This may obviate the need for long-term in vitro propagation since clinically relevant stem cell numbers can be extracted from isolates of adipose tissue (De Ugarte et al. 2003; Planat-Benard et al. 2004b; Martinez-Estrada et al. 2005). Recent reports have demonstrated that adipose-derived MSCs can also be induced to differentiate into cardiomyocytes (Planat-Benard et al. 2004a). However, the subject of whether adult bone marrow cells have true in vivo differentiation capacity into cardiac myocytes remains controversial. It will be important to clarify whether the surviving cells within the heart arose from differentiation or fusion and to assess their functional capacity in the heart.
(iii) Cardiac-derived progenitor cells
The most commonly held view about the regenerative capacity of the heart is that it cannot regenerate as it is a post-mitotic organ consisting of terminally differentiated cardiomyocytes. This view is now being challenged by reports from several groups who suggest that the heart, similar to other organs such as skeletal muscle and brain, may contain a resident population of progenitor cells with cardiomyogenic and even myocardiogenic potential (ability to differentiate into myocytes, vascular smooth muscle cells and endothelial cells). CPCs have been identified and characterized in the hearts of mice (Hierlihy et al. 2002; Oh et al. 2003; Matsuura et al. 2004), rats (Beltrami et al. 2003), dogs (Linke et al. 2005) and humans (Evans 1993; Messina et al. 2004; Laugwitz et al. 2005). Some of the first evidence for the presence of these cells came from Hierlihy et al. (2002), who demonstrated that the ‘side population’ cells determined by their ability to take up the stain Hoechst, represented CPC (approx. 1%) in the hearts of post-natal mice. Studies examining phenotypic and functional characteristics indicated that the CPCs were positive for the stem cell marker c-kit (7–10% of the cells expressed early myocyte-related transcription factors Nkx2.5, GATA-4 and MEF2C) and displayed stem cell qualities of self renewal, clonogenicity and multipotency (Beltrami et al. 2003). Furthermore, several studies demonstrated that intramyocardial injection of c-kit positive CPCs, or growth factor activation of local CPCs significantly improved cardiac function (Beltrami et al. 2003; Dawn et al. 2005; Linke et al. 2005). Oh et al. (2003), using a different approach, isolated stem cell antigen-1 positive (Sca-1+, but c-kit negative) cells from mouse hearts and found that after injecting them intravenously, the Sca-1+ cells homed to the site of ischaemia/reperfusion injury where they differentiated and fused with the host cells. Matsuura et al. (2004) confirmed these findings and went on to show that Sca-1+ cells expressed cardiac transcription factors, contractile proteins, sarcomeric structures and spontaneous beating following treatment with oxytocin.
CPCs isolated from human heart (atrial and ventricular biopsies; Messina et al. 2004) express the stem cell markers c-kit and Sca-1, and the endothelial marker CD34, when grown as cardiospheres or clonal spherical clusters, but can also express cardiac differentiation markers when grown on collagen-coated dishes. More recently, the expression of islet-1 (Isl-1), a marker of progenitor cells implicated in the morphogenesis of the embryonic heart (Evans 1993), has been used to identify and isolate another population of CPCs from human, mouse and rat post-natal hearts (Laugwitz et al. 2005). These cells are negative for Sca-1 and c-kit but express early cardiac differentiation markers Nkx2.5 and GATA-4. However, there is a dispute whether these newly identified cells represent a new definitive CPC type important for reconstituting the damaged heart (Anversa et al. 2006). It has been suggested that Isl-1-positive cells have not been detected in the failing heart and furthermore, during development, Isl-1-positive cells are not implicated in left ventricle formation (Anversa et al. 2006). Nevertheless, there appears to be overwhelming evidence that the heart harbours progenitor cells with the capacity to generate cells that exhibit cardiomyocyte characteristics, as well as other non-cardiac cell types, but further studies are required to ascertain their true identities and the relationship between them.
(iv) Clinical studies using autologous adult stem cells
MSCs have become an important tool of tissue regeneration studies and are considered as potential candidates for several clinical applications (Prockop et al. 2003). Several phase I clinical studies have reported to have used autologous whole bone marrow, consisting of both MSCs and endothelial stem cells, in cardiac therapy. Although these studies do not define the percentage of each subset of cell type used, their overall outcome is an improvement in a range of cardiac clinical measurements. For instance, one clinical study followed 10 patients with acute myocardial infarction after administering autologous bone marrow cells into the infarct region for a period of several minutes under increased pressure using a double-balloon catheter. Three-month post-procedure evaluation revealed the infarct region to have significantly reduced as well as an improved wall motion score (Strauer et al. 2002). Another clinical study utilizing autologous mononuclear bone marrow cells, carried out electromechanical mapping to visualize viable myocardium regions (15 sites in total) for optimal placement of the cells (15 million cells) in patients with severe heart failure. Two months after intramyocardial injections of bone marrow-derived stem cells, an enhancement in myocardial blood flow with associated improvement of regional and global left ventricular function was noted (Perin et al. 2003). Also of interest is the use of autologous bone marrow-derived EPCs by Stamm et al. (2004) who isolated EPCs using magnetic beads coated with the AC133 antibody (CD133) and injected them into areas of infarct in patients undergoing coronary artery bypass. An improvement in left ventricular function, revascularization and contractility of the infarct areas was seen; however, the exact mechanism of improvement in left ventricular function is not known. Recent evidence indicates that transdifferentiation to either cardiomyocytes or endothelial cells is unlikely and that paracrine factors play a major role. Taken together, these studies suggest that autologous grafts of MSCs may be possible, but engrafting allogeneic MSCs would be more therapeutically attractive. However, there are concerns regarding the very small improvement in ejection fraction in reports from phase I clinical trials using bone marrow-derived putative stem cells and the apparent inconsistency, particularly in longer term results (Nadal-Ginard & Fuster 2007).
(v) Use of allogeneic or xenogeneic mesenchymal stem cells in cardiac repair and regeneration
Recent studies have shown that the use of allogeneic adult stem cells may be more feasible for clinical applications, as several preclinical and clinical studies have been performed in the field of bone marrow transplantation and immune diseases (Devine 2002; Serakinci & Keith 2006). Long-term engraftment of allogeneic MSC has been demonstrated in several animal studies using a range of tissues in the absence of immunosuppression (Liechty et al. 2000; Bartholomew et al. 2001). Pittenger & Martin (2004) reported the ability of allogeneic MSCs to engraft into infarcted myocardium. Using both rat and porcine animal models, they demonstrated both engraftment of allogeneic MSCs in necrotic myocardium and also improvement in ventricular function after infarction. Furthermore, in the absence of immunosuppressive therapy, they found no evidence of immunological rejection or lymphocytic infiltration. The precise mechanism involved in these animal studies requires further research, but fusion with resident cardiomyocytes has been proposed (Alvarez-Dolado et al. 2003). However, subsequent studies using male bone marrow cell donors in female recipients have demonstrated that bone marrow cells can differentiate directly into cardiac cell lineages (Kajstura et al. 2005). Further evidence of MSC engraftment was observed when xenogeneic human MSCs were injected into experimentally induced ischaemic rat myocardium (Grinnemo et al. 2004). Other studies have documented the presence of resident cardiac stem cells (Beltrami et al. 2003) and some recent new data from several groups have reported that these cells can be expanded and differentiated in vitro and transplanted to improve function (Ott et al. 2005; Tateishi et al. 2005). Although further studies are necessary to determine whether the immune privileged status of MSCs may allow for enhanced clinical applicability, allogeneic MSCs can be readily available and administered with immunological acceptance by the recipient.
(vi) Current non-cardiac therapeutic applications of mesenchymal stem cells
Clinical trials with encouraging results have already been initiated in a variety of other conditions using MSCs. These include the treatment of brittle bone disease or osteogenesis imperfecta (Horwitz et al. 2002; Le Blanc et al. 2005) through systemic transplantation of allogeneic MSC which resulted in the MSCs homing to the bone and an increase in new bone formation. Also, similar clinical improvement has been observed in patients with metachromatic leukodystrophy or Hurler's syndrome (Koc et al. 2002). Furthermore, cotransplantation of MSCs with heterologous bone marrow transplants have been used to enhance engraftment (Koc et al. 2000) as well as to successfully treat severe acute graft-versus-host disease (GVHD; Collins et al. 2000a; Bartholomew et al. 2002; Potian et al. 2003; Le Blanc et al. 2004). These clinical data suggest that MSCs possess an immune privileged position that allows their persistence in an allogeneic environment. These studies strongly suggest that MSC grafting may not only treat and prevent GVHD, but may be incorporated in and extended to future tolerance generating regimes in both transplantation and tissue engineering.
(vii) Immunological obstacles in tissue engineering using adult progenitor cells
In addition to their applications in tissue regeneration, the use of adult stem cells in the field of tissue engineering is also being vigorously pursued. Target organs include liver (Kaihara et al. 2000), skin (Andreadis 2004), bone (Schek et al. 2005), cartilage (Pang et al. 2005), teeth (Modino & Sharpe 2005), small intestine (Choi & Vacanti 1997) and cardiovascular organs such as heart valves (Hoerstrup et al. 2002; Neuenschwander & Hoerstrup 2004). However, very little is known regarding their behaviour and fate following either systemic infusion or local implantation. In our laboratory, we are actively pursuing the tissue engineering of human heart valves. Through our initial studies comparing interstitial cells derived from the native valve leaflet with bone marrow-derived MSCs, we found that these two cell types share many properties. These include similar functional capacity to synthesize collagen when mechanically stretched (Ku et al. 2006), which will be important if MSCs are to be used in tissue engineered heart valves. MSCs were also found to highly resemble valve interstitial cells with respect to phenotype (cell surface molecules including cytoplasmic proteins, ECM components and cell communication molecules; N. Latif 2006, personal communication) and perhaps more importantly, our in vitro studies have shown that, like valve interstitial cells, MSCs also downmodulate T cell responses, suggesting that MSCs may prove to be a very good cell source for tissue engineering heart valve allografts (Batten et al. 2001, 2006).
Unlike ES cells, there is emerging evidence that donor MSCs have profound immunomodulatory function, both in vivo and in vitro, suggesting that the clinical application of MSCs for tissue regeneration could be achieved (Devine 2002; Ryan et al. 2005). As evidenced by both experimental and clinical data, MSCs have unique immunological characteristics that allow their persistence in an allogeneic environment (Liechty et al. 2000; Hoerstrup et al. 2002; Tse et al. 2003). In vivo non-human primate studies have elegantly demonstrated that neither allogeneic nor autologous MSCs are rejected but are engrafted into multiple tissues when transplanted in baboons (Devine et al. 2003). Clinical studies (Le Blanc et al. 2004) have demonstrated that MSCs expanded ex vivo can either engraft into tissues or be infused into patients without being rejected, the best examples of which are bone marrow transplantations for the treatment of leukaemia after myeloablative therapies. In vitro studies have indicated that MSCs can directly suppress T cell immune responses (Tse et al. 2003; Aggarwal & Pittenger 2004), as well as modulate the immune response indirectly through effects on professional antigen presenting cells such as dendritic cells (Beyth et al. 2005) and B cells (Corcione et al. 2006).
The immunogenicity of undifferentiated MSCs conforms to the commonly agreed consensus that they are hypoimmunogenic; it is generally accepted that MSCs are MHC class I positive and MHC class II negative or be it at very low levels. It is important to note that MSCs express MHC class I because without this expression NK cells may exert mechanisms of deletion seen against tumour cells that have downregulated MHC class I molecules (Ruggeri et al. 2001). The absence of MHC class II expression by MSCs may also help protect them from recognition by alloreactive CD4+T cells (Le Blanc et al. 2003; Majumdar et al. 2003). In addition, MSCs do not appear to express the costimulatory molecules CD40, CD40L, CD80 or CD86 required for effector T cell activation (Fijnvandraat et al. 2003; Majumdar et al. 2003; Tse et al. 2003), suggesting that any cognate interaction with the T cell receptor on T helper cells in the absence of costimulation would result in anergy.
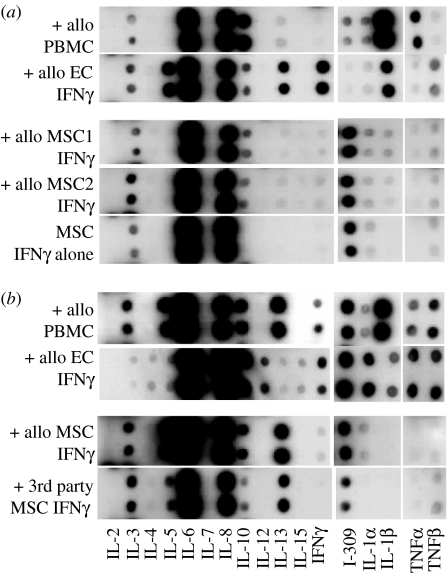
At the cellular level, we have shown that the immunoregulatory mechanisms involved in MSC ability to downregulate T cell responses require both cell-to-cell contact (through T cell receptor/MHC class II interaction) and the release of soluble factors (Batten et al. 2006). We have described the induction of a distinct cytokine profile as a consequence of T cell co-culture with both allogeneic and third party MSCs which included upregulation of anti-inflammatory T helper (Th) 2 cytokines interleukin (IL)-3, IL-5, IL-10, IL-13 and the Th2 chemokine, I-309, a chemoattractant for regulatory T cells. Simultaneously, there was a downregulation of pro-inflammatory Th1 cytokines IL-1α and IL-1β, interferon (IFN)γ and tumour necrosis factor (TNF)α (figure 2). The generation of CD4+CD25lowCD69lowFoxP3+ regulatory T cells following co-culture with MSCs may also contribute to their ability to downmodulate the T cell response (Batten et al. 2006).
Figure 2.
MSCs induce the release of anti-inflammatory Th2 cytokines and the Th2 chemokine, I-309, a major chemoattractant for regulatory T(reg) cells, but downregulate Th1 pro-inflammatory cytokines. Supernatants were collected following 48 h co-culture of either (a) primary T cells with allogeneic peripheral blood mononuclear cells (PBMCs), two isolates of IFNγ-treated MSC (1 and 2) or IFNγ-treated allogeneic endothelial cells (ECs) or (b) primed DR11-specific T cells with allo-specific DR11 expressing PBMCs, IFNγ-treated DR11-expressing ECs, IFNγ-treated DR11-expressing MSCs or IFNγ-treated third party MHC mismatched MSCs. Cytokines detected from 48 h supernatants from T cells alone (primary and primed) and untreated or IFNγ-treated MSCs alone are also shown. Secretion of cytokines was measured using the RayBio Human Cytokine Array III system. Sections of the membrane detecting a range of Th1 and Th2 cytokines are as shown and the results are shown as signal spots.
Although we and others have started to unravel the interactions and factors involved in MSC-mediated T cell suppression, there is still dispute concerning the actual mechanisms involved. For instance, several reports have implicated IL-10, hepatocyte growth factor, TGFβ (Di Nicola et al. 2002; Potian et al. 2003) and more recently prostaglandin E2 (PGE2; Aggarwal & Pittenger 2004) as the soluble factors responsible for the suppressive effects exerted by MSCs, while others did not find the presence of IL-10 or inhibition with anti-TGFβ antibodies (Djouad et al. 2003; Krampera et al. 2003) or reversal of suppression with indomethacin which inhibits PGE2 biosynthesis (Tse et al. 2003). Other disputed mechanisms which have also been suggested to play a role in MSC-mediated immunosuppression include the role of tryptophan catabolism by the enzyme indoleamine 2,3-dioxygenase (IDO; Meisel et al. 2004). In this mechanism, MSCs are thought to deplete tryptophan, essential for cell proliferation from the tissue culture medium via IDO, and thus negate proliferation. However, the suppressive activity of MSCs could not be reversed by the addition of an IDO-specific inhibitor or by the addition of supplementary tryptophan (Tse et al. 2003). These inconsistencies between reports suggest that other as yet undefined mechanisms may also be involved.
Although studies to date have uncovered a range of soluble factors that may induce MSC-mediated downregulation of T cell responses, there are others which also require consideration, such as the chemokines, IL-1 receptor antagonist (IL-1ra) and IL-4ra. For instance, IL-1ra was reported to inhibit the effects of IL-1α and IL-1β by competing for type I and type II IL-1 receptors, resulting in significantly attenuated inflammation (Granowitz et al. 1991) as well as downregulating the inflammatory response following ischaemia-reperfusion injury in the heart (Suzuki et al. 2001). More recently, Murtuza et al. (2004) demonstrated that skeletal myoblasts overexpressing secretory IL-1ra enhanced the beneficial effects of skeletal muscle transplantation resulting in improved cardiac function.
Although in vitro studies investigating the immunogenicity of MSCs demonstrate that they possess immunomodulatory functions, there is some growing evidence from several studies documenting immune-mediated damage to these cells following implantation and differentiation in vivo. In one report, it was demonstrated that implantation of allogeneic murine gene-modified erythropoietin (Epo)-secreting MSCs into non-immunosuppressed classes I and II MHC-mismatched immunocompetent recipients, led to a vigorous and specific cellular immune response (Eliopoulos et al. 2005). This suggests that MSCs become immunogenic following in vivo differentiation. Other studies have demonstrated that although differentiated or undifferentiated MSCs remain non-immunogenic in vitro, they cannot impart their immunomodulatory activities when cotransplanted to protect allogeneic allografts. One such example was demonstrated by Liu et al. (2006) where they showed that osteogenic differentiated MSCs displayed normal immunosuppressive activities in vitro and in vivo, but their immunomodulatory activity in protecting allogeneic skin allografts was lost following transplantation. More recently, the use of MSCs to prolong a rat model of allogeneic heart transplant was shown to be ineffective (Inoue et al. 2006). These studies therefore suggest that upon differentiation in vivo, local growth factors, cytokines and other environmental cues such as mechanotransduction and spatial cues may render MSCs immunogenic and therefore markedly limit their use as a universal donor cell type for tissue engineering. If this is the case, the actual mechanisms involved in ‘switching off’ the non-immunogenic status of MSCs in vivo need to be determined.
3. Strategies to induce tolerance to overcome rejection of allogeneic tissue engineered organs
To achieve this objective, several strategies are currently being pursued. One potential avenue involves genetic manipulation of MSCs. MSCs can be readily transduced by a variety of vectors and maintain transgene expression after in vivo differentiation (Mosca et al. 2000; Toma et al. 2002). This may be used to augment cell engraftment or the extent of differentiation. Gene transfer of tolerogenic molecules such as Fas ligand (FasL) and haemoxygenase-1 (HO-1) may offer protection against rejection. These cytoprotective molecules have recently been implicated in MSC-mediated downregulation of T cell responses (Liu et al. 2004), as well as in enhanced MSC survival following their transplantation in infarcted rat hearts resulting in improved cardiac function (Zhang et al. 2005b). FasL is a type II cell surface protein which has multiple roles (Askenasy et al. 2005). It can be induced on T cells and is constitutively expressed in cells from immune privileged organs such as the Sertoli cells in the testis, epithelial cells in the eye, and in the thymus where it contributes to peripheral tolerance. FasL provides and maintains immunological privilege of these sites by destroying all activated lymphocytes that enter (Singer & Abbas 1994; Bellgrau et al. 1995; Griffith et al. 1995). Studies from our laboratory have demonstrated that tolerance can be induced by FasL through overexpression in human endothelial cells (Li et al. 2000). However, normal human MSCs neither express FasL nor can they be upregulated following cytokine treatment (P. Batten 2005, unpublished data). Perhaps genetic modification of MSCs to express FasL may induce peripheral allograft tolerance by selectively deleting alloreactive T cells. Recent data from Liu et al. (2004) have indicated a role for FasL-mediated immunosuppression of peripheral blood lymphocyte proliferation by MSCs. The consequence of FasL expression on MSCs will therefore be an important immunomodulatory pathway to consider for producing an ‘off-the-shelf’ tissue engineered organ.
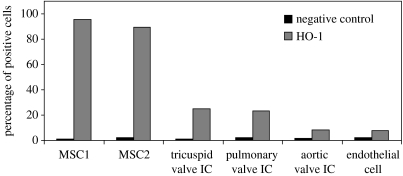
Another likely candidate is the HO-1 system, which may confer cytoprotection. HO-1 has been strongly associated with cytoprotection and immune regulatory functions under conditions of cellular stress such as inflammation, ischaemia, hypoxia, hyperoxia, hyperthermia and radiation (Choi & Alam 1996) as well as in conferring protection from graft rejection in various models of organ transplantation (Katori et al. 2002; Braudeau et al. 2004; Camara & Soares 2005). This cytoprotective molecule has recently been implicated in enhanced MSC survival following transplantation of HO-1-transduced MSC in infarcted rat hearts resulting in improved cardiac function (Zhang et al. 2005b). Although there are three isoforms of HO (HO-1, -2 and -3), only the upregulation of inducible HO-1 or heat shock protein 32 produces the most critical cytoprotective mechanisms activated during times of cellular stress. It is through the elimination of haem and the function of its by-products biliverdin, carbon monoxide (CO) and free iron (Fe2+) that cytoprotection is conferred (Katori et al. 2002). Of particular interest is the ability of HO-1 to modulate T cell activation and proliferation. This was first suggested by the observation that HO-1-deficient mice have higher numbers of circulating activated CD4+T cells when compared with wild-type mice (Poss & Tonegawa 1997a,b). More recently, both upregulation (Pae et al. 2004) and overexpression (Braudeau et al. 2004; Pae et al. 2004) of HO-1 were shown to inhibit CD4+T cell proliferation, which could also be mimicked by adding exogenous CO or biliverdin (Braudeau et al. 2004; Pae et al. 2004). In addition, recent data from Sawle et al. (2005, 2006) have demonstrated that anti-inflammatory responses can be suppressed by novel metal carbonyl-based compounds or CO-releasing molecules (CO-RMs). CO released by CO-RMs may therefore have therapeutic potential since several reports have suggested that they may play a role as vasorelaxants in rat aortic and cardiac tissue (Motterlini et al. 2002; Johnson et al. 2003; Foresti et al. 2004) as well as in decreasing myocardial ischaemia/reperfusion damage (Clark et al. 2003; Guo et al. 2004). Furthermore, in addition to downregulating T cell immune responses directly, expression of HO-1 in other cell types such as immature dendritic cells has been reported to result in indirect inhibition of pro-inflammatory cytokines and allogeneic immune responses (Chauveau et al. 2005). We have preliminary evidence showing MSCs to exhibit high constitutive expression of HO-1 when compared with other cell types such as endothelial cells and valve interstitial cells (figure 3). This novel finding therefore suggests that HO-1 may have a role in MSC immunoregulatory activities.
Figure 3.
MSCs constitutively express high levels of HO-1 when compared with endothelial and valve interstitial cells (IC).
Another molecule which has recently been of interest is Akt, a serine–threonine kinase involved in diverse cellular responses such as differentiation and survival. Activation of Akt in cardiomyocytes has been shown to protect against apoptosis after ischaemia/reperfusion injury (Matsui et al. 2001). Recent data from several groups (Mangi et al. 2003; Lim et al. 2006) have used this molecule to overcome the poor survival rate of implanted cells, critical for improving the efficiency of stem cell therapy. Mangi et al. (2003) demonstrated that transplantation of Akt-transduced MSCs made resistant to apoptosis, enhanced cardiac repair after transplantation. This group used a retroviral vector to overexpress the pro-survival gene akt in MSCs before implantation in infarcted rat myocardium. Akt protein overexpression was reported to not only greatly enhance MSC survival but also prevent pathological remodelling after infarction, with striking improvement in cardiac output. Lim et al. (2006) also demonstrated similar effects in a porcine model.
Finally, another molecule which could be of interest is relaxin. Functionally, relaxin is classically described to improve blood supply to multiple organs including the uterus, mammary gland, lung and heart (Bani 1997). It is also known to increase matrix metalloproteinase expression, resulting in collagen turnover of reproductive tissues and in models of fibrosis (Bani 1997; Ivell & Einspanier 2002; Bathgate et al. 2003). However, it is relaxin's contribution to the regulation of immune homeostasis during pregnancy which suggests it could have a role in future tolerance induction regimes. Evidence comes from relaxin's ability to promote the development of IFNγ-producing Th1 cells to protect the mother against intracellular pathogens as well as inducing the expression of IDO by macrophages at the maternofoetal interface, thereby suppressing T cell activity and preventing the immunological rejection of the foetal allograft (Munn et al. 1998; Piccinni et al. 2000). In addition, it has also been shown to have a role in protecting the heart from ischaemia/reperfusion-induced myocardial injury, whereby free radical-induced myocardial injury, infiltration of inflammatory cells and myocardial fibrosis were prevented, thus improving cardiac function (Zhang et al. 2005a).
Taken together, it seems highly likely that genetic manipulaton of MSCs may provide an attractive strategy to overcome rejection of tissue engineered organs; however, the ethics and clinical outcome of using virally transduced cells in humans remains controversial. Perhaps the use of non-viral transfection techniques (Collins et al. 2000a,b; Patel et al. 2001) which has been successfully used to transfect primary cultures of vascular smooth muscle cells (Li et al. 2001) may provide a solution, but alternative strategies involving the use of novel compounds such as CO-RMs, as mentioned earlier, require further investigation. Given that there are many factors that govern MSC immunomodulatory activities both in vitro and in vivo, it is more than likely that there will not be a single ‘magic bullet’ solution for MSCs to be completely accepted in an allogeneic recipient, suggesting that a multifaceted approach will probably be required, with the need for greater understanding of the mechanisms involved.
Footnotes
One contribution of 21 to a Theme Issue ‘Bioengineering the heart’.
References
- Aggarwal S, Pittenger M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2004;105:1815–1822. doi: 10.1182/blood-2004-04-1559. doi:10.1182/blood-2004-04-1559 [DOI] [PubMed] [Google Scholar]
- Alvarez-Dolado M, Pardal R, Garcia-Verdugo J.M, Fike J.R, Lee H.O, Pfeffer K, Lois C, Morrison S.J, Alvarez-Buylla A. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–973. doi: 10.1038/nature02069. doi:10.1038/nature02069 [DOI] [PubMed] [Google Scholar]
- Amit M, Shariki C, Margulets V, Itskovitz-Eldor J. Feeder layer- and serum-free culture of human embryonic stem cells. Biol. Reprod. 2004;70:837–845. doi: 10.1095/biolreprod.103.021147. doi:10.1095/biolreprod.103.021147 [DOI] [PubMed] [Google Scholar]
- Andreadis S.T. Gene transfer to epidermal stem cells: implications for tissue engineering. Expert Opin. Biol. Ther. 2004;4:783–800. doi: 10.1517/14712598.4.6.783. doi:10.1517/14712598.4.6.783 [DOI] [PubMed] [Google Scholar]
- Anversa P, Leri A, Kajstura J. Cardiac regeneration. J. Am. College Cardiol. 2006;47:1769–1776. doi: 10.1016/j.jacc.2006.02.003. doi:10.1016/j.jacc.2006.02.003 [DOI] [PubMed] [Google Scholar]
- Askenasy N, Yolcu E.S, Yaniv I, Shirwan H. Induction of tolerance using Fas ligand: a double-edged immunomodulator. Blood. 2005;105:1396–1404. doi: 10.1182/blood-2004-06-2364. doi:10.1182/blood-2004-06-2364 [DOI] [PubMed] [Google Scholar]
- Bani D. Relaxin: a pleiotropic hormone. Gen. Pharmacol.: The Vasc. Syst. 1997;28:13–22. doi: 10.1016/s0306-3623(96)00171-1. doi:10.1016/S0306-3623(96)00171-1 [DOI] [PubMed] [Google Scholar]
- Barber C.L, Iruela-Arispe M.L. The ever-elusive endothelial progenitor cell: identities, functions and clinical implications. Pediatr. Res. 2006;59:26R–32R. doi: 10.1203/01.pdr.0000203553.46471.18. doi:10.1203/01.pdr.0000203553.46471.18 [DOI] [PubMed] [Google Scholar]
- Barry F.P, Murphy J.M. Mesenchymal stem cells: clinical applications and biological characterization. Int. J. Biochem. Cell Biol. 2004;36:568–584. doi: 10.1016/j.biocel.2003.11.001. doi:10.1016/j.biocel.2003.11.001 [DOI] [PubMed] [Google Scholar]
- Bartholomew A, et al. Baboon mesenchymal stem cells can be genetically modified to secrete human erythropoietin in vivo. Hum. Gene Ther. 2001;12:1527–1541. doi: 10.1089/10430340152480258. doi:10.1089/10430340152480258 [DOI] [PubMed] [Google Scholar]
- Bartholomew A, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp. Hematol. 2002;30:42–48. doi: 10.1016/s0301-472x(01)00769-x. doi:10.1016/S0301-472X(01)00769-X [DOI] [PubMed] [Google Scholar]
- Bathgate R.A, Samuel C.S, Burazin T.C, Gundlach A.L, Tregear G.W. Relaxin: new peptides, receptors and novel actions. Trends Endocrinol. Metab. 2003;14:207–213. doi: 10.1016/s1043-2760(03)00081-x. doi:10.1016/S1043-2760(03)00081-X [DOI] [PubMed] [Google Scholar]
- Batten P, McCormack A.M, Rose M.L, Yacoub M.H. Valve interstitial cells induce donor-specific T-cell anergy. J. Thorac. Cardiovasc. Surg. 2001;122:129–135. doi: 10.1067/mtc.2001.114940. doi:10.1067/mtc.2001.114940 [DOI] [PubMed] [Google Scholar]
- Batten P, Sarathchandra P, Antoniw J.W, Tay S.S, Lowdell M.W, Taylor P.M, Yacoub M.H. Human mesenchymal stem cells induce T cell anergy and downregulate T cell allo-responses via the th2 pathway: relevance to tissue engineering human heart valves. Tissue Eng. 2006;12:2263–2273. doi: 10.1089/ten.2006.12.2263. doi:10.1089/ten.2006.12.2263 [DOI] [PubMed] [Google Scholar]
- Bellgrau D, Gold D, Selawry H, Moore J, Franzusoff A, Duke R.C. A role for CD95 ligand in preventing graft rejection. Nature. 1995;377:630–632. doi: 10.1038/377630a0. doi:10.1038/377630a0 [DOI] [PubMed] [Google Scholar]
- Beltrami A.P, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. doi:10.1016/S0092-8674(03)00687-1 [DOI] [PubMed] [Google Scholar]
- Beyth S, Borovsky Z, Mevorach D, Liebergall M, Gazit Z, Aslan H, Galun E, Rachmilewitz J. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105:2214–2219. doi: 10.1182/blood-2004-07-2921. doi:10.1182/blood-2004-07-2921 [DOI] [PubMed] [Google Scholar]
- Bin Z, Sheng L.G, Gang Z.C, Hong J, Jun C, Bo Y, Hui S. Efficient cardiomyocyte differentiation of embryonic stem cells by bone morphogenetic protein-2 combined with visceral endoderm-like cells. Cell Biol. Int. 2006;30:769–776. doi: 10.1016/j.cellbi.2006.05.011. doi:10.1016/j.cellbi.2006.05.011 [DOI] [PubMed] [Google Scholar]
- Boheler K.R, Fiszman M.Y. Can exogenous stem cells be used in transplantation? Cells Tissues Organs. 1999;165:237–245. doi: 10.1159/000016684. doi:10.1159/000016684 [DOI] [PubMed] [Google Scholar]
- Boyd A.S, Higashi Y, Wood K.J. Transplanting stem cells: potential targets for immune attack. Modulating the immune response against embryonic stem cell transplantation. Adv. Drug Deliv. Rev. 2005;57:1944–1969. doi: 10.1016/j.addr.2005.08.004. doi:10.1016/j.addr.2005.08.004 [DOI] [PubMed] [Google Scholar]
- Bradley J.A, Bolton E.M, Pedersen R.A. Stem cell medicine encounters the immune system. Nat. Rev. Immunol. 2002;2:859–871. doi: 10.1038/nri934. doi:10.1038/nri934 [DOI] [PubMed] [Google Scholar]
- Braudeau C, Bouchet D, Tesson L, Iyer S, Remy S, Buelow R, Anegon I, Chauveau C. Induction of long-term cardiac allograft survival by heme oxygenase-1 gene transfer. Gene Ther. 2004;11:701–710. doi: 10.1038/sj.gt.3302208. doi:10.1038/sj.gt.3302208 [DOI] [PubMed] [Google Scholar]
- Bryder D, Rossi D.J, Weissman I.L. Hematopoietic stem cells: the paradigmatic tissue-specific stem cell. Am. J. Pathol. 2006;169:338–346. doi: 10.2353/ajpath.2006.060312. doi:10.2353/ajpath.2006.060312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buell J.F, Gross T.G, Thomas M.J, Neff G, Muthiah C, Alloway R, Ryckman F.C, Tiao G.M, Woodle E.S. Malignancy in pediatric transplant recipients. Semin. Pediatr. Surg. 2006;15:179–187. doi: 10.1053/j.sempedsurg.2006.03.005. doi:10.1053/j.sempedsurg.2006.03.005 [DOI] [PubMed] [Google Scholar]
- Camara N.O, Soares M.P. Heme oxygenase-1 (HO-1), a protective gene that prevents chronic graft dysfunction. Free Radic. Biol. Med. 2005;38:426–435. doi: 10.1016/j.freeradbiomed.2004.11.019. doi:10.1016/j.freeradbiomed.2004.11.019 [DOI] [PubMed] [Google Scholar]
- Chauveau C, et al. Heme oxygenase-1 expression inhibits dendritic cell maturation and pro-inflammatory function but conserves IL-10 expression. Blood. 2005;106:1694–1702. doi: 10.1182/blood-2005-02-0494. doi:10.1182/blood-2005-02-0494 [DOI] [PubMed] [Google Scholar]
- Chinzei R, et al. Embryoid-body cells derived from a mouse embryonic stem cell line show differentiation into functional hepatocytes. Hepatology. 2002;36:22–29. doi: 10.1053/jhep.2002.34136. doi:10.1053/jhep.2002.34136 [DOI] [PubMed] [Google Scholar]
- Choi A.M, Alam J. Heme oxygenase-1: function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am. J. Respir. Cell Mol. Biol. 1996;15:9–19. doi: 10.1165/ajrcmb.15.1.8679227. [DOI] [PubMed] [Google Scholar]
- Choi R.S, Vacanti J.P. Preliminary studies of tissue-engineered intestine using isolated epithelial organoid units on tubular synthetic biodegradable scaffolds. Transpl. Proc. 1997;29:848–851. doi: 10.1016/s0041-1345(96)00164-9. doi:10.1016/S0041-1345(96)00164-9 [DOI] [PubMed] [Google Scholar]
- Clark J.E, Naughton P, Shurey S, Green C.J, Johnson T.R, Mann B.E, Foresti R, Motterlini R. Cardioprotective actions by a water-soluble carbon monoxide-releasing molecule. Circ. Res. 2003;93:e2–e8. doi: 10.1161/01.RES.0000084381.86567.08. doi:10.1161/01.RES.0000084381.86567.08 [DOI] [PubMed] [Google Scholar]
- Collins L, Gustafsson K, Fabre J.W. Tissue-binding properties of a synthetic peptide DNA vector targeted to cell membrane integrins: a possible universal nonviral vector for organ and tissue transplantation. Transplantation. 2000a;69:1041–1050. doi: 10.1097/00007890-200003270-00006. doi:10.1097/00007890-200003270-00006 [DOI] [PubMed] [Google Scholar]
- Collins L, Sawyer G.J, Zhang X.H, Gustafsson K, Fabre J.W. In vitro investigation of factors important for the delivery of an integrin-targeted nonviral DNA vector in organ transplantation. Transplantation. 2000b;69:1168–1176. doi: 10.1097/00007890-200003270-00023. doi:10.1097/00007890-200003270-00023 [DOI] [PubMed] [Google Scholar]
- Corcione A, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–372. doi: 10.1182/blood-2005-07-2657. doi:10.1182/blood-2005-07-2657 [DOI] [PubMed] [Google Scholar]
- Dawn B, et al. Cardiac stem cells delivered intravascularly traverse the vessel barrier, regenerate infarcted myocardium, and improve cardiac function. Proc. Natl Acad. Sci. USA. 2005;102:3766–3771. doi: 10.1073/pnas.0405957102. doi:10.1073/pnas.0405957102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ugarte D.A, et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174:101–109. doi: 10.1159/000071150. doi:10.1159/000071150 [DOI] [PubMed] [Google Scholar]
- Devine S.M. Mesenchymal stem cells: will they have a role in the clinic? J. Cell Biochem. Suppl. 2002;38:73–79. doi: 10.1002/jcb.10046. doi:10.1002/jcb.10046 [DOI] [PubMed] [Google Scholar]
- Devine S.M, Cobbs C, Jennings M, Bartholomew A, Hoffman R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. 2003;101:2999–3001. doi: 10.1182/blood-2002-06-1830. doi:10.1182/blood-2002-06-1830 [DOI] [PubMed] [Google Scholar]
- Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni P.D, Matteucci P, Grisanti S, Gianni A.M. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.v99.10.3838. doi:10.1182/blood.V99.10.3838 [DOI] [PubMed] [Google Scholar]
- Djouad F, Plence P, Bony C, Tropel P, Apparailly F, Sany J, Noel D, Jorgensen C. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003;102:3837–3844. doi: 10.1182/blood-2003-04-1193. doi:10.1182/blood-2003-04-1193 [DOI] [PubMed] [Google Scholar]
- Draper J.S, Pigott C, Thomson J.A, Andrews P.W. Surface antigens of human embryonic stem cells: changes upon differentiation in culture. J. Anat. 2002;200:249–258. doi: 10.1046/j.1469-7580.2002.00030.x. doi:10.1046/j.1469-7580.2002.00030.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drukker M, Benvenisty N. The immunogenicity of human embryonic stem-derived cells. Trends Biotechnol. 2004;22:136–141. doi: 10.1016/j.tibtech.2004.01.003. doi:10.1016/j.tibtech.2004.01.003 [DOI] [PubMed] [Google Scholar]
- Drukker M, Katz G, Urbach A, Schuldiner M, Markel G, Itskovitz-Eldor J, Reubinoff B, Mandelboim O, Benvenisty N. Characterization of the expression of MHC proteins in human embryonic stem cells. Proc. Natl Acad. Sci. USA. 2002;99:9864–9869. doi: 10.1073/pnas.142298299. doi:10.1073/pnas.142298299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drukker M, Katchman H, Katz G, Even-Tov Friedman S, Shezen E, Hornstein E, Mandelboim O, Reisner Y, Benvenisty N. Human embryonic stem cells and their differentiated derivatives are less susceptible to immune rejection than adult cells. Stem Cells. 2006;24:221–229. doi: 10.1634/stemcells.2005-0188. doi:10.1634/stemcells.2005-0188 [DOI] [PubMed] [Google Scholar]
- Eisenberg C.A, Eisenberg L.M. WNT11 promotes cardiac tissue formation of early mesoderm. Dev. Dyn. 1999;216:45–58. doi: 10.1002/(SICI)1097-0177(199909)216:1<45::AID-DVDY7>3.0.CO;2-L. doi:10.1002/(SICI)1097-0177(199909)216:1<45::AID-DVDY7>3.0.CO;2-L [DOI] [PubMed] [Google Scholar]
- Eliopoulos N, Stagg J, Lejeune L, Pommey S, Galipeau J. Allogeneic marrow stromal cells are immune rejected by MHC class I- and class II-mismatched recipient mice. Blood. 2005;106:4057–4065. doi: 10.1182/blood-2005-03-1004. doi:10.1182/blood-2005-03-1004 [DOI] [PubMed] [Google Scholar]
- Ellerstrom C, Strehl R, Moya K, Andersson K, Bergh C, Lundin K, Hyllner J, Semb H. Derivation of a xeno-free human ES cell line. Stem Cells. 2006;24:2170–2176. doi: 10.1634/stemcells.2006-0130. doi:10.1634/stemcells.2006-0130 [DOI] [PubMed] [Google Scholar]
- Evans G.A. “MegaYAC” library. Science. 1993;260:877. doi: 10.1126/science.8493512. doi:10.1126/science.8493512 [DOI] [PubMed] [Google Scholar]
- Fairchild P.J, Cartland S, Nolan K.F, Waldmann H. Embryonic stem cells and the challenge of transplantation tolerance. Trends Immunol. 2004;25:465–470. doi: 10.1016/j.it.2004.07.005. doi:10.1016/j.it.2004.07.005 [DOI] [PubMed] [Google Scholar]
- Fijnvandraat A.C, van Ginneken A.C, Schumacher C.A, Boheler K.R, Lekanne Deprez R.H, Christoffels V.M, Moorman A.F. Cardiomyocytes purified from differentiated embryonic stem cells exhibit characteristics of early chamber myocardium. J. Mol. Cell Cardiol. 2003;35:1461–1472. doi: 10.1016/j.yjmcc.2003.09.011. doi:10.1016/j.yjmcc.2003.09.011 [DOI] [PubMed] [Google Scholar]
- Foresti R, Hammad J, Clark J.E, Johnson T.R, Mann B.E, Friebe A, Green C.J, Motterlini R. Vasoactive properties of CORM-3, a novel water-soluble carbon monoxide-releasing molecule. Br. J. Pharmacol. 2004;142:453–460. doi: 10.1038/sj.bjp.0705825. doi:10.1038/sj.bjp.0705825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage F.H. Cell therapy. Nature. 1998;392:18–24. [PubMed] [Google Scholar]
- Granowitz E.V, Clark B.D, Mancilla J, Dinarello C.A. Interleukin-1 receptor antagonist competitively inhibits the binding of interleukin-1 to the type II interleukin-1 receptor. J. Biol. Chem. 1991;266:14 147–14 150. [PubMed] [Google Scholar]
- Griffith T.S, Brunner T, Fletcher S.M, Green D.R, Ferguson T.A. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189–1192. doi: 10.1126/science.270.5239.1189. doi:10.1126/science.270.5239.1189 [DOI] [PubMed] [Google Scholar]
- Grinnemo K.H, et al. Xenoreactivity and engraftment of human mesenchymal stem cells transplanted into infarcted rat myocardium. J. Thorac. Cardiovasc. Surg. 2004;127:1293–1300. doi: 10.1016/j.jtcvs.2003.07.037. doi:10.1016/j.jtcvs.2003.07.037 [DOI] [PubMed] [Google Scholar]
- Grusby M.J, Glimcher L.H. Immune responses in MHC class II-deficient mice. Annu. Rev. Immunol. 1995;13:417–435. doi: 10.1146/annurev.iy.13.040195.002221. doi:10.1146/annurev.iy.13.040195.002221 [DOI] [PubMed] [Google Scholar]
- Guo Y, Stein A.B, Wu W.J, Tan W, Zhu X, Li Q.H, Dawn B, Motterlini R, Bolli R. Administration of a CO-releasing molecule at the time of reperfusion reduces infarct size in vivo. Am. J. Physiol. Heart Circ. Physiol. 2004;286:H1649–H1653. doi: 10.1152/ajpheart.00971.2003. doi:10.1152/ajpheart.00971.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hierlihy A.M, Seale P, Lobe C.G, Rudnicki M.A, Megeney L.A. The post-natal heart contains a myocardial stem cell population. FEBS Lett. 2002;530:239–243. doi: 10.1016/s0014-5793(02)03477-4. doi:10.1016/S0014-5793(02)03477-4 [DOI] [PubMed] [Google Scholar]
- Hoerstrup S.P, et al. Tissue engineering of functional trileaflet heart valves from human marrow stromal cells. Circulation. 2002;106:I143–I150. [PubMed] [Google Scholar]
- Hori J, Ng T.F, Shatos M, Klassen H, Streilein J.W, Young M.J. Neural progenitor cells lack immunogenicity and resist destruction as allografts. Stem Cells. 2003;21:405–416. doi: 10.1634/stemcells.21-4-405. doi:10.1634/stemcells.21-4-405 [DOI] [PubMed] [Google Scholar]
- Horwitz E.M, Gordon P.L, Koo W.K, Marx J.C, Neel M.D, McNall R.Y, Muul L, Hofmann T. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proc. Natl Acad. Sci. USA. 2002;99:8932–8937. doi: 10.1073/pnas.132252399. doi:10.1073/pnas.132252399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S, Popp F.C, Koehl G.E, Piso P, Schlitt H.J, Geissler E.K, Dahlke M.H. Immunomodulatory effects of mesenchymal stem cells in a rat organ transplant model. Transplantation. 2006;81:1589–1595. doi: 10.1097/01.tp.0000209919.90630.7b. doi:10.1097/01.tp.0000209919.90630.7b [DOI] [PubMed] [Google Scholar]
- Ivell R, Einspanier A. Relaxin peptides are new global players. Trends Endocrinol. Metab. 2002;13:343–348. doi: 10.1016/s1043-2760(02)00664-1. doi:10.1016/S1043-2760(02)00664-1 [DOI] [PubMed] [Google Scholar]
- Johnson T.R, Mann B.E, Clark J.E, Foresti R, Green C.J, Motterlini R. Metal carbonyls: a new class of pharmaceuticals? Angew. Chem. Int. Edn. Engl. 2003;42:3722–3729. doi: 10.1002/anie.200301634. doi:10.1002/anie.200301634 [DOI] [PubMed] [Google Scholar]
- Kaihara S, Borenstein J, Koka R, Lalan S, Ochoa E.R, Ravens M, Pien H, Cunningham B, Vacanti J.P. Silicon micromachining to tissue engineer branched vascular channels for liver fabrication. Tissue Eng. 2000;6:105–117. doi: 10.1089/107632700320739. doi:10.1089/107632700320739 [DOI] [PubMed] [Google Scholar]
- Kajstura J, et al. Bone marrow cells differentiate in cardiac cell lineages after infarction independently of cell fusion. Circ. Res. 2005;96:127–137. doi: 10.1161/01.RES.0000151843.79801.60. doi:10.1161/01.RES.0000151843.79801.60 [DOI] [PubMed] [Google Scholar]
- Katori M, Busuttil R.W, Kupiec-Weglinski J.W. Heme oxygenase-1 system in organ transplantation. Transplantation. 2002;74:905–912. doi: 10.1097/00007890-200210150-00001. doi:10.1097/00007890-200210150-00001 [DOI] [PubMed] [Google Scholar]
- Kehat I, et al. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat. Biotechnol. 2004;22:1282–1289. doi: 10.1038/nbt1014. doi:10.1038/nbt1014 [DOI] [PubMed] [Google Scholar]
- Klug M.G, Soonpaa M.H, Koh G.Y, Field L.J. Genetically selected cardiomyocytes from differentiating embronic stem cells form stable intracardiac grafts. J. Clin. Invest. 1996;98:216–224. doi: 10.1172/JCI118769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koc O.N, Gerson S.L, Cooper B.W, Dyhouse S.M, Haynesworth S.E, Caplan A.I, Lazarus H.M. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J. Clin. Oncol. 2000;18:307–316. doi: 10.1200/JCO.2000.18.2.307. [DOI] [PubMed] [Google Scholar]
- Koc O.N, Day J, Nieder M, Gerson S.L, Lazarus H.M, Krivit W. Allogeneic mesenchymal stem cell infusion for treatment of metachromatic leukodystrophy (MLD) and Hurler syndrome (MPS-IH) Bone Marrow Transplant. 2002;30:215–222. doi: 10.1038/sj.bmt.1703650. doi:10.1038/sj.bmt.1703650 [DOI] [PubMed] [Google Scholar]
- Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, Dazzi F. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–3729. doi: 10.1182/blood-2002-07-2104. doi:10.1182/blood-2002-07-2104 [DOI] [PubMed] [Google Scholar]
- Ku C.H, Johnson P.H, Batten P, Sarathchandra P, Chambers R.C, Taylor P.M, Yacoub M.H, Chester A.H. Collagen synthesis by mesenchymal stem cells and aortic valve interstitial cells in response to mechanical stretch. Cardiovasc. Res. 2006;71:548–556. doi: 10.1016/j.cardiores.2006.03.022. doi:10.1016/j.cardiores.2006.03.022 [DOI] [PubMed] [Google Scholar]
- Lanza R.P, Cibelli J.B, West M.D. Prospects for the use of nuclear transfer in human transplantation. Nat. Biotechnol. 1999;17:1171–1174. doi: 10.1038/70709. doi:10.1038/70709 [DOI] [PubMed] [Google Scholar]
- Latif, N. et al Submitted. Human valve interstitial cells demonstrate transdifferentiation potential.
- Laugwitz K.L, et al. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–653. doi: 10.1038/nature03215. doi:10.1038/nature03215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Blanc K, Pittenger M. Mesenchymal stem cells: progress toward promise. Cytotherapy. 2005;7:36–45. doi: 10.1080/14653240510018118. doi:10.1080/14653240510018118 [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringden O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp. Hematol. 2003;31:890–896. doi: 10.1016/s0301-472x(03)00110-3. doi:10.1016/S0301-472X(03)00110-3 [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, Ringden O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. doi:10.1016/S0140-6736(04)16104-7 [DOI] [PubMed] [Google Scholar]
- Le Blanc K, et al. Fetal mesenchymal stem-cell engraftment in bone after in utero transplantation in a patient with severe osteogenesis imperfecta. Transplantation. 2005;79:1607–1614. doi: 10.1097/01.tp.0000159029.48678.93. doi:10.1097/01.TP.0000159029.48678.93 [DOI] [PubMed] [Google Scholar]
- Lee J.B, Lee J.E, Park J.H, Kim S.J, Kim M.K, Roh S.I, Yoon H.S. Establishment and maintenance of human embryonic stem cell lines on human feeder cells derived from uterine endometrium under serum-free condition. Biol. Reprod. 2005;72:42–49. doi: 10.1095/biolreprod.104.033480. doi:10.1095/biolreprod.104.033480 [DOI] [PubMed] [Google Scholar]
- Li Y, McCormack A.M, Brand N.J, Yacoub M.H. A strategy for inducing immune tolerance to valve endothelial cells through gene transfer. J. Heart Valve Dis. 2000;9:439–444. [PubMed] [Google Scholar]
- Li J, Collins L, Zhang X, Gustafsson K, Fabre J.W. Efficient gene delivery to vascular smooth muscle cells using a nontoxic, synthetic peptide vector system targeted to membrane integrins: a first step toward the gene therapy of chronic rejection. Transplant Proc. 2001;33:589–589. doi: 10.1016/s0041-1345(00)02155-2. doi:10.1016/S0041-1345(00)02155-2 [DOI] [PubMed] [Google Scholar]
- Liechty K.W, MacKenzie T.C, Shaaban A.F, Radu A, Moseley A.M, Deans R, Marshak D.R, Flake A.W. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat. Med. 2000;6:1282–1286. doi: 10.1038/81395. doi:10.1038/81395 [DOI] [PubMed] [Google Scholar]
- Lim S.Y, et al. The effects of mesenchymal stem cells transduced with Akt in a porcine myocardial infarction model. Cardiovasc. Res. 2006;70:530–542. doi: 10.1016/j.cardiores.2006.02.016. doi:10.1016/j.cardiores.2006.02.016 [DOI] [PubMed] [Google Scholar]
- Linke A, et al. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc. Natl Acad. Sci. USA. 2005;102:8966–8971. doi: 10.1073/pnas.0502678102. doi:10.1073/pnas.0502678102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, et al. Suppression of human peripheral blood lymphocyte proliferation by immortalized mesenchymal stem cells derived from bone marrow of Banna Minipig inbred-line. Transplant Proc. 2004;36:3272–3275. doi: 10.1016/j.transproceed.2004.11.090. doi:10.1016/j.transproceed.2004.11.090 [DOI] [PubMed] [Google Scholar]
- Liu H, Kemeny D.M, Heng B.C, Ouyang H.W, Melendez A.J, Cao T. The immunogenicity and immunomodulatory function of osteogenic cells differentiated from mesenchymal stem cells. J. Immunol. 2006;176:2864–2871. doi: 10.4049/jimmunol.176.5.2864. [DOI] [PubMed] [Google Scholar]
- Majumdar M.K, Keane-Moore M, Buyaner D, Hardy W.B, Moorman M.A, McIntosh K.R, Mosca J.D. Characterization and functionality of cell surface molecules on human mesenchymal stem cells. J. Biomed. Sci. 2003;10:228–241. doi: 10.1007/BF02256058. doi:10.1007/BF02256058 [DOI] [PubMed] [Google Scholar]
- Mammolenti M, Gajavelli S, Tsoulfas P, Levy R. Absence of major histocompatibility complex class I on neural stem cells does not permit natural killer cell killing and prevents recognition by alloreactive cytotoxic T lymphocytes in vitro. Stem Cells. 2004;22:1101–1110. doi: 10.1634/stemcells.22-6-1101. doi:10.1634/stemcells.22-6-1101 [DOI] [PubMed] [Google Scholar]
- Mangi A.A, Noiseux N, Kong D, He H, Rezvani M, Ingwall J.S, Dzau V.J. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat. Med. 2003;9:1195–1201. doi: 10.1038/nm912. doi:10.1038/nm912 [DOI] [PubMed] [Google Scholar]
- Martin M.J, Muotri A, Gage F, Varki A. Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat. Med. 2005;11:228–232. doi: 10.1038/nm1181. doi:10.1038/nm1181 [DOI] [PubMed] [Google Scholar]
- Martinez-Estrada O.M, Munoz-Santos Y, Julve J, Reina M, Vilaro S. Human adipose tissue as a source of Flk-1+ cells: new method of differentiation and expansion. Cardiovasc. Res. 2005;65:328–333. doi: 10.1016/j.cardiores.2004.11.015. doi:10.1016/j.cardiores.2004.11.015 [DOI] [PubMed] [Google Scholar]
- Matsui T, et al. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation. 2001;104:330–335. doi: 10.1161/01.cir.104.3.330. [DOI] [PubMed] [Google Scholar]
- Matsuura K, et al. Adult cardiac Sca-1-positive cells differentiate into beating cardiomyocytes. J. Biol. Chem. 2004;279:11 384–11 391. doi: 10.1074/jbc.M310822200. doi:10.1074/jbc.M310822200 [DOI] [PubMed] [Google Scholar]
- McCloskey K.E, Gilroy M.E, Nerem R.M. Use of embryonic stem cell-derived endothelial cells as a cell source to generate vessel structures in vitro. Tissue Eng. 2005;11:497–505. doi: 10.1089/ten.2005.11.497. doi:10.1089/ten.2005.11.497 [DOI] [PubMed] [Google Scholar]
- McLaren A. Ethical and social considerations of stem cell research. Nature. 2001;414:129–131. doi: 10.1038/35102194. doi:10.1038/35102194 [DOI] [PubMed] [Google Scholar]
- McNerney M.E, et al. Role of natural killer cell subsets in cardiac allograft rejection. Am. J. Transplant. 2006;6:505–513. doi: 10.1111/j.1600-6143.2005.01226.x. doi:10.1111/j.1600-6143.2005.01226.x [DOI] [PubMed] [Google Scholar]
- Meisel R, Zibert A, Laryea M, Gobel U, Daubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619–4621. doi: 10.1182/blood-2003-11-3909. doi:10.1182/blood-2003-11-3909 [DOI] [PubMed] [Google Scholar]
- Menard C, et al. Transplantation of cardiac-committed mouse embryonic stem cells to infarcted sheep myocardium: a preclinical study. Lancet. 2005;366:1005–1012. doi: 10.1016/S0140-6736(05)67380-1. doi:10.1016/S0140-6736(05)67380-1 [DOI] [PubMed] [Google Scholar]
- Messina E, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ. Res. 2004;95:911–921. doi: 10.1161/01.RES.0000147315.71699.51. doi:10.1161/01.RES.0000147315.71699.51 [DOI] [PubMed] [Google Scholar]
- Meyer N, Jaconi M, Landopoulou A, Fort P, Puceat M. A fluorescent reporter gene as a marker for ventricular specification in ES-derived cardiac cells. FEBS Lett. 2000;478:151–158. doi: 10.1016/s0014-5793(00)01839-1. doi:10.1016/S0014-5793(00)01839-1 [DOI] [PubMed] [Google Scholar]
- Min J.Y, Yang Y, Converso K.L, Liu L, Huang Q, Morgan J.P, Xiao Y.F. Transplantation of embryonic stem cells improves cardiac function in postinfarcted rats. J. Appl. Physiol. 2002;92:288–296. doi: 10.1152/jappl.2002.92.1.288. doi:10.1063/1.1481962 [DOI] [PubMed] [Google Scholar]
- Modino S.A.C, Sharpe P.T. Tissue engineering of teeth using adult stem cells. Arch. Oral Biol. 2005;50:255–258. doi: 10.1016/j.archoralbio.2005.01.002. doi:10.1016/j.archoralbio.2005.01.002 [DOI] [PubMed] [Google Scholar]
- Moretta L, Bottino C, Pende D, Mingari M.C, Biassoni R, Moretta A. Human natural killer cells: their origin, receptors and function. Eur. J. Immunol. 2002;32:1205–1211. doi: 10.1002/1521-4141(200205)32:5<1205::AID-IMMU1205>3.0.CO;2-Y. doi:10.1002/1521-4141(200205)32:5<1205::AID-IMMU1205>3.0.CO;2-Y [DOI] [PubMed] [Google Scholar]
- Moretta L, Romagnani C, Pietra G, Moretta A, Mingari M.C. NK-CTLs, a novel HLA-E-restricted T-cell subset. Trends Immunol. 2003;24:136–143. doi: 10.1016/s1471-4906(03)00031-0. doi:10.1016/S1471-4906(03)00031-0 [DOI] [PubMed] [Google Scholar]
- Mosca J.D, et al. Mesenchymal stem cells as vehicles for gene delivery. Clin. Orthop. Relat. Res. 2000;379:S71–S90. doi: 10.1097/00003086-200010001-00011. doi:10.1097/00003086-200010001-00011 [DOI] [PubMed] [Google Scholar]
- Motterlini R, Clark J.E, Foresti R, Sarathchandra P, Mann B.E, Green C.J. Carbon monoxide-releasing molecules: characterization of biochemical and vascular activities. Circ. Res. 2002;90:E17–E24. doi: 10.1161/hh0202.104530. doi:10.1161/hh0202.104530 [DOI] [PubMed] [Google Scholar]
- Muller M, et al. Selection of ventricular-like cardiomyocytes from ES cells in vitro. FASEB J. 2000;14:2540–2548. doi: 10.1096/fj.00-0002com. doi:10.1096/fj.00-0002com [DOI] [PubMed] [Google Scholar]
- Mummery C, et al. Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm-like cells. Circulation. 2003;107:2733–2740. doi: 10.1161/01.CIR.0000068356.38592.68. doi:10.1161/01.CIR.0000068356.38592.68 [DOI] [PubMed] [Google Scholar]
- Munn D.H, Zhou M, Attwood J.T, Bondarev I, Conway S.J, Marshall B, Brown C, Mellor A.L. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. doi:10.1126/science.281.5380.1191 [DOI] [PubMed] [Google Scholar]
- Murtuza B, Suzuki K, Bou-Gharios G, Beauchamp J.R, Smolenski R.T, Partridge T.A, Yacoub M.H. Transplantation of skeletal myoblasts secreting an IL-1 inhibitor modulates adverse remodeling in infarcted murine myocardium. Proc. Natl Acad. Sci. USA. 2004;101:4216–4221. doi: 10.1073/pnas.0306205101. doi:10.1073/pnas.0306205101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal-Ginard B, Fuster V. Myocardial cell therapy at the crossroads. Nat. Clin. Pract. Cardiovasc. Med. 2007;4:1. doi: 10.1038/ncpcardio0743. doi:10.1038/ncpcardio0743 [DOI] [PubMed] [Google Scholar]
- Neuenschwander S, Hoerstrup S.P. Heart valve tissue engineering. Transpl. Immunol. 2004;12:359–365. doi: 10.1016/j.trim.2003.12.010. doi:10.1016/j.trim.2003.12.010 [DOI] [PubMed] [Google Scholar]
- Odorico J.S, Kaufman D.S, Thomson J.A. Multilineage differentiation from human embryonic stem cell lines. Stem Cells. 2001;19:193–204. doi: 10.1634/stemcells.19-3-193. doi:10.1634/stemcells.19-3-193 [DOI] [PubMed] [Google Scholar]
- Oh H, et al. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc. Natl Acad. Sci. USA. 2003;100:12 313–12 318. doi: 10.1073/pnas.2132126100. doi:10.1073/pnas.2132126100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott C.H, Matthiesen T.S, Brechtken J, Xin X, Barnes S.A, Nelson W, Goh S.-K, Taylor D.A. A novel population of adult derived cardiac progenitor cells is capable of functional myocardial repair. Circulation. 2005;112:II-51. [Google Scholar]
- Pae H.O, Oh G.S, Choi B.M, Chae S.C, Kim Y.M, Chung K.R, Chung H.T. Carbon monoxide produced by heme oxygenase-1 suppresses T cell proliferation via inhibition of IL-2 production. J. Immunol. 2004;172:4744–4751. doi: 10.4049/jimmunol.172.8.4744. [DOI] [PubMed] [Google Scholar]
- Pandur P, Lasche M, Eisenberg L.M, Kuhl M. Wnt-11 activation of a non-canonical Wnt signalling pathway is required for cardiogenesis. Nature. 2002;418:636–641. doi: 10.1038/nature00921. doi:10.1038/nature00921 [DOI] [PubMed] [Google Scholar]
- Pang Y, Cui P, Chen W, Gao P, Zhang H. Quantitative study of tissue-engineered cartilage with human bone marrow mesenchymal stem cells. Arch. Facial Plast. Surg. 2005;7:7–11. doi: 10.1001/archfaci.7.1.7. doi:10.1001/archfaci.7.1.7 [DOI] [PubMed] [Google Scholar]
- Patel S, Zhang X, Collins L, Fabre J.W. A small, synthetic peptide for gene delivery via the serpin–enzyme complex receptor. J. Gene Med. 2001;3:271–279. doi: 10.1002/jgm.180. doi:10.1002/jgm.180 [DOI] [PubMed] [Google Scholar]
- Penn I. Post-transplant malignancy: the role of immunosuppression. Drug Saf. 2000;23:101–113. doi: 10.2165/00002018-200023020-00002. doi:10.2165/00002018-200023020-00002 [DOI] [PubMed] [Google Scholar]
- Perin E.C, et al. Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation. 2003;107:2294–2302. doi: 10.1161/01.CIR.0000070596.30552.8B. doi:10.1161/01.CIR.0000070596.30552.8B [DOI] [PubMed] [Google Scholar]
- Piccinni M.P, Scaletti C, Maggi E, Romagnani S. Role of hormone-controlled Th1- and Th2-type cytokines in successful pregnancy. J. Neuroimmunol. 2000;109:30–33. doi: 10.1016/s0165-5728(00)00299-x. doi:10.1016/S0165-5728(00)00299-X [DOI] [PubMed] [Google Scholar]
- Pincock S. Newcastle centre gains licence for therapeutic cloning. Br. Med. J. 2004;329:417–417. doi: 10.1136/bmj.329.7463.417. doi:10.1136/bmj.329.7463.417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger M.F, Martin B.J. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ. Res. 2004;95:9–20. doi: 10.1161/01.RES.0000135902.99383.6f. doi:10.1161/01.RES.0000135902.99383.6f [DOI] [PubMed] [Google Scholar]
- Pittenger M.F, Mosca J.D, McIntosh K.R. Human mesenchymal stem cells: progenitor cells for cartilage, bone, fat and stroma. Curr. Top. Microbiol. Immunol. 2000;251:3–11. doi: 10.1007/978-3-642-57276-0_1. [DOI] [PubMed] [Google Scholar]
- Planat-Benard V, Menard C, Andre M, Puceat M, Perez A, Garcia-Verdugo J.M, Penicaud L, Casteilla L. Spontaneous cardiomyocyte differentiation from adipose tissue stroma cells. Circ. Res. 2004a;94:223–229. doi: 10.1161/01.RES.0000109792.43271.47. doi:10.1161/01.RES.0000109792.43271.47 [DOI] [PubMed] [Google Scholar]
- Planat-Benard V, et al. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004b;109:656–663. doi: 10.1161/01.CIR.0000114522.38265.61. doi:10.1161/01.CIR.0000114522.38265.61 [DOI] [PubMed] [Google Scholar]
- Poss K.D, Tonegawa S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc. Natl Acad. Sci. USA. 1997a;94:10 919–10 924. doi: 10.1073/pnas.94.20.10919. doi:10.1073/pnas.94.20.10919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poss K.D, Tonegawa S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc. Natl Acad. Sci. USA. 1997b;94:10 925–10 930. doi: 10.1073/pnas.94.20.10925. doi:10.1073/pnas.94.20.10925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potian J.A, Aviv H, Ponzio N.M, Harrison J.S, Rameshwar P. Veto-like activity of mesenchymal stem cells: functional discrimination between cellular responses to alloantigens and recall antigens. J. Immunol. 2003;171:3426–3434. doi: 10.4049/jimmunol.171.7.3426. [DOI] [PubMed] [Google Scholar]
- Prockop D.J, Gregory C.A, Spees J.L. One strategy for cell and gene therapy: harnessing the power of adult stem cells to repair tissues. Proc. Natl Acad. Sci. USA. 2003;100(Suppl. 1):11 917–11 923. doi: 10.1073/pnas.1834138100. doi:10.1073/pnas.1834138100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards M, Fong C.Y, Chan W.K, Wong P.C, Bongso A. Human feeders support prolonged undifferentiated growth of human inner cell masses and embryonic stem cells. Nat. Biotechnol. 2002;20:933–936. doi: 10.1038/nbt726. doi:10.1038/nbt726 [DOI] [PubMed] [Google Scholar]
- Ruggeri L, Capanni M, Martelli M.F, Velardi A. Cellular therapy: exploiting NK cell alloreactivity in transplantation. Curr. Opin. Hematol. 2001;8:355–359. doi: 10.1097/00062752-200111000-00007. doi:10.1097/00062752-200111000-00007 [DOI] [PubMed] [Google Scholar]
- Ryan J, Barry F, Murphy J.M, Mahon B. Mesenchymal stem cells avoid allogeneic rejection. J. Inflamm. 2005;2:8. doi: 10.1186/1476-9255-2-8. doi:10.1186/1476-9255-2-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez P.L, San Roman J.A, Villa A, Fernandez M.E, Fernandez-Aviles F. Contemplating the bright future of stem cell therapy for cardiovascular disease. Nat. Clin. Pract. Cardiovasc. Med. 2006;3(Suppl. 1):S138–S151. doi: 10.1038/ncpcardio0456. doi:10.1038/ncpcardio0456 [DOI] [PubMed] [Google Scholar]
- Sawle P, Foresti R, Mann B.E, Johnson T.R, Green C.J, Motterlini R. Carbon monoxide-releasing molecules (CO-RMs) attenuate the inflammatory response elicited by lipopolysaccharide in RAW264.7 murine macrophages. Br. J. Pharmacol. 2005;145:800–810. doi: 10.1038/sj.bjp.0706241. doi:10.1038/sj.bjp.0706241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawle P, Hammad J, Fairlamb I.J, Moulton B, O'Brien C.T, Lynam J.M, Duhme-Klair A.K, Foresti R, Motterlini R. Bioactive properties of iron-containing carbon monoxide-releasing molecules (CO-RMs) J. Pharmacol. Exp. Ther. 2006;318:403–410. doi: 10.1124/jpet.106.101758. doi:10.1124/jpet.106.101758 [DOI] [PubMed] [Google Scholar]
- Schek R.M, Taboas J.M, Hollister S.J, Krebsbach P.H. Tissue engineering osteochondral implants for temporomandibular joint repair. Orthodont. Craniofac. Res. 2005;8:313–319. doi: 10.1111/j.1601-6343.2005.00354.x. doi:10.1111/j.1601-6343.2005.00354.x [DOI] [PubMed] [Google Scholar]
- Serakinci N, Keith W.N. Therapeutic potential of adult stem cells. Eur. J. Cancer. 2006;42:1243–1246. doi: 10.1016/j.ejca.2006.01.036. doi:10.1016/j.ejca.2006.01.036 [DOI] [PubMed] [Google Scholar]
- Simon D.M, Levin S. Infectious complications of solid organ transplantations. Infect. Dis. Clin. North Am. 2001;15:521–549. doi: 10.1016/s0891-5520(05)70158-6. doi:10.1016/S0891-5520(05)70158-6 [DOI] [PubMed] [Google Scholar]
- Singer G.G, Abbas A.K. The fas antigen is involved in peripheral but not thymic deletion of T lymphocytes in T cell receptor transgenic mice. Immunity. 1994;1:365–371. doi: 10.1016/1074-7613(94)90067-1. doi:10.1016/1074-7613(94)90067-1 [DOI] [PubMed] [Google Scholar]
- Smits A.M, van Vliet P, Hassink R.J, Goumans M.J, Doevendans P.A. The role of stem cells in cardiac regeneration. J. Cell Mol. Med. 2005;9:25–36. doi: 10.1111/j.1582-4934.2005.tb00334.x. doi:10.1111/j.1582-4934.2005.tb00334.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamm C, Kleine H.D, Westphal B, Petzsch M, Kittner C, Nienaber C.A, Freund M, Steinhoff G. CABG and bone marrow stem cell transplantation after myocardial infarction. Thorac. Cardiovasc. Surg. 2004;52:152–158. doi: 10.1055/s-2004-817981. doi:10.1055/s-2004-817981 [DOI] [PubMed] [Google Scholar]
- Stock U.A, Vacanti J.P. Tissue engineering: current state and prospects. Annu. Rev. Med. 2001;52:443–451. doi: 10.1146/annurev.med.52.1.443. doi:10.1146/annurev.med.52.1.443 [DOI] [PubMed] [Google Scholar]
- Strauer B.E, Brehm M, Zeus T, Kostering M, Hernandez A, Sorg R.V, Kogler G, Wernet P. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002;106:1913–1918. doi: 10.1161/01.cir.0000034046.87607.1c. doi:10.1161/01.CIR.0000034046.87607.1C [DOI] [PubMed] [Google Scholar]
- Suzuki K, Murtuza B, Smolenski R.T, Sammut I.A, Suzuki N, Kaneda Y, Yacoub M.H. Overexpression of interleukin-1 receptor antagonist provides cardioprotection against ischemia-reperfusion injury associated with reduction in apoptosis. Circulation. 2001;104:I308–I3I3. doi: 10.1161/hc37t1.094871. [DOI] [PubMed] [Google Scholar]
- Swijnenburg R.J, et al. Embryonic stem cell immunogenicity increases upon differentiation after transplantation into ischemic myocardium. Circulation. 2005;112:I166–I172. doi: 10.1161/CIRCULATIONAHA.104.525824. [DOI] [PubMed] [Google Scholar]
- Tateishi K, Takahashi T, Nomura T, Yamagishi M, Yaku H, Matsubara H, Oh H. Single cardiac stem cells from adult mammalian heart are multipotent and can regenerate ischemic myocardium. Circulation. 2005;112:II-51. [Google Scholar]
- Terami H, Hidaka K, Katsumata T, Iio A, Morisaki T. Wnt11 facilitates embryonic stem cell differentiation to Nkx2.5-positive cardiomyocytes. Biochem. Biophys. Res. Commun. 2004;325:968–975. doi: 10.1016/j.bbrc.2004.10.103. doi:10.1016/j.bbrc.2004.10.103 [DOI] [PubMed] [Google Scholar]
- Toma C, Pittenger M.F, Cahill K.S, Byrne B.J, Kessler P.D. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. doi:10.1161/hc0102.101442 [DOI] [PubMed] [Google Scholar]
- Tse W.T, Pendleton J.D, Beyer W.M, Egalka M.C, Guinan E.C. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75:389–397. doi: 10.1097/01.TP.0000045055.63901.A9. doi:10.1097/01.TP.0000045055.63901.A9 [DOI] [PubMed] [Google Scholar]
- Vaananen H.K. Mesenchymal stem cells. Ann. Med. 2005;37:469–479. doi: 10.1080/07853890500371957. doi:10.1080/07853890500371957 [DOI] [PubMed] [Google Scholar]
- Veinot J.P, Prichett-Pejic W, Song J, Waghray G, Parks W, Mesana T.G, Ruel M. CD117-positive cells and mast cells in adult human cardiac valves—observations and implications for the creation of bioengineered grafts. Cardiovasc. Pathol. 2006;15:36–40. doi: 10.1016/j.carpath.2005.08.005. doi:10.1016/j.carpath.2005.08.005 [DOI] [PubMed] [Google Scholar]
- Wei J, et al. Embryonic endothelial progenitor cells armed with a suicide gene target hypoxic lung metastases after intravenous delivery. Cancer Cell. 2004;5:477–488. doi: 10.1016/s1535-6108(04)00116-3. doi:10.1016/S1535-6108(04)00116-3 [DOI] [PubMed] [Google Scholar]
- Wilmut I, Schnieke A.E, McWhir J, Kind A.J, Campbell K.H. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385:810–813. doi: 10.1038/385810a0. doi:10.1038/385810a0 [DOI] [PubMed] [Google Scholar]
- Wobus A.M, Boheler K.R. Embryonic stem cells: prospects for developmental biology and cell therapy. Physiol. Rev. 2005;85:635–678. doi: 10.1152/physrev.00054.2003. doi:10.1152/physrev.00054.2003 [DOI] [PubMed] [Google Scholar]
- Yacoub M, Suzuki K, Rosenthal N. The future of regenerative therapy in patients with chronic heart failure. Nat. Clin. Pract. Cardiovasc. Med. 2006;3(Suppl. 1):S133–S135. doi: 10.1038/ncpcardio0401. doi:10.1038/ncpcardio0401 [DOI] [PubMed] [Google Scholar]
- Zhang S.C, Wernig M, Duncan I.D, Brustle O, Thomson J.A. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat. Biotechnol. 2001;19:1129–1133. doi: 10.1038/nbt1201-1129. doi:10.1038/nbt1201-1129 [DOI] [PubMed] [Google Scholar]
- Zhang J, Qi Y.F, Geng B, Pan C.S, Zhao J, Chen L, Yang J, Chang J.K, Tang C.S. Effect of relaxin on myocardial ischemia injury induced by isoproterenol. Peptides. 2005a;26:1632–1639. doi: 10.1016/j.peptides.2005.02.008. doi:10.1016/j.peptides.2005.02.008 [DOI] [PubMed] [Google Scholar]
- Zhang S, et al. Increased heme oxygenase-1 expression in infarcted rat hearts following human bone marrow mesenchymal cell transplantation. Microvasc. Res. 2005b;69:64–70. doi: 10.1016/j.mvr.2005.01.004. doi:10.1016/j.mvr.2005.01.004 [DOI] [PubMed] [Google Scholar]