Abstract
Here, we review an approach to tissue engineering of functional myocardium that is biomimetic in nature, as it involves the use of culture systems designed to recapitulate some aspects of the actual in vivo environment. To mimic the capillary network, subpopulations of neonatal rat heart cells were cultured on a highly porous elastomer scaffold with a parallel array of channels perfused with culture medium. To mimic oxygen supply by haemoglobin, the culture medium was supplemented with a perfluorocarbon (PFC) emulsion. Constructs cultivated in the presence of PFC contained higher amounts of DNA and cardiac markers and had significantly better contractile properties than control constructs cultured without PFC. To induce synchronous contractions of cultured constructs, electrical signals mimicking those in native heart were applied. Over only 8 days of cultivation, electrical stimulation induced cell alignment and coupling, markedly increased the amplitude of synchronous construct contractions and resulted in a remarkable level of ultrastructural organization. The biomimetic approach is discussed in the overall context of cardiac tissue engineering, and the possibility to engineer functional human cardiac grafts based on human stem cells.
Keywords: tissue engineering, cardiac, bioreactor, oxygen, vascularization, development
1. Introduction
Cardiovascular disease is responsible for a preponderance of health problems in developed countries. In developed countries, almost one-fourth of the population lives with coronary heart disease, congenital cardiovascular defects and congestive heart failure, and this already high incidence is likely to further increase as the population ages. Heart disease and stroke, the principal components of cardiovascular disease, are the first and the third leading cause of death in the US, accounting for nearly 40% of all deaths. Congenital heart defects, which occur in nearly 14 of every 1000 newborn children (Gillum 1994), are the most common congenital defects and the leading cause of death in the first year of life (Hoffman 1995a,b). Cardiovascular diseases result in substantial disability, and largely contribute to the escalating costs of health care.
Myocardium does not have the ability to regenerate after injury. Cell-based therapies have been considered as a novel and potentially curative treatment option, either by usage of cells alone (Koh et al. 1993; Soonpaa et al. 1994; Li et al. 1996; Taylor et al. 1998; Reinecke et al. 1999; Sakai et al. 1999; Condorelli et al. 2001; Etzion et al. 2001; Orlic et al. 2001; Muller-Ehmsen et al. 2002a,b; Roell et al. 2002; Menasche et al. 2003) or by scaffold-aided tissue engineering cardiac constructs that can be surgically attached to the myocardium (Eschenhagen et al. 1997; Akins et al. 1999; Bursac et al. 1999; Carrier et al. 1999; Li et al. 1999, 2000; Fink et al. 2000; Leor et al. 2000; Papadaki et al. 2001; Kofidis et al. 2002; Shimizu et al. 2002; Zimmermann et al. 2002a,b, 2006; Radisic et al. 2003, 2004a,b, 2006). Transplanted cells included embryonic, foetal and neonatal cardiomyocytes (Leor et al. 1996; Scorsin et al. 1996), skeletal myoblasts (Taylor et al. 1998; Menasche et al. 2003), mesenchymal stem cells from bone marrow (Orlic et al. 2001) and cardiomyocytes derived from human embryonic stem cells (Kehat et al. 2004).
Transplantation of exogenous cells into damaged myocardium was reported to improve myocardial function and vascular supply (Orlic et al. 2001; Menasche et al. 2003; Kehat et al. 2004). However, the extent and mechanisms of myocardial repair are not well established, and several reports raise a cautionary note as they found no evidence of either transdifferentiation of stem cells into cardiac phenotype (Murry et al. 2004), or myocardial regeneration (Balsam et al. 2004). While the application of cells alone is being pursued for treating small-scale injuries, tissue engineering may provide functional cell-based substitutes of the native tissue to treat large defects. As compared with the transplantation of cells alone, engineered cardiac constructs have the potential advantage of immediate functionality. In addition, engineered tissues can serve as high-fidelity in vitro models for the study of normal and pathological cardiac functions, drug screening and effects of hypoxia (Carrier et al. 2002a; Bursac et al. 2003; Radisic et al. 2004a, 2005c).
Three-dimensional cardiac tissue constructs that express structural and physiological features characteristic of native cardiac muscle have been engineered using foetal or neonatal rat cardiac myocytes (CMs) cultured in collagen gels with mechanical stimulation (Eschenhagen et al. 1997; Fink et al. 2000; Zimmermann et al. 2000, 2002b), on collagen fibres (Akins et al. 1999), fibrous polyglycolic acid scaffolds (Bursac et al. 1999; Carrier et al. 1999, a,b; Papadaki et al. 2001) and porous collagen scaffolds (Li et al. 1999, 2000; Radisic et al. 2003). In early studies, cells were seeded onto scaffolds and cultivated in dishes (Carrier et al. 1999; Li et al. 1999; Leor et al. 2000; Papadaki et al. 2001), spinner flasks (Bursac et al. 1999; Carrier et al. 1999; Papadaki et al. 2001) or in rotating vessels (Akins et al. 1999; Carrier et al. 1999; Papadaki et al. 2001). Oxygen dissolved in medium was transported to the cells by molecular diffusion, which could support the viability of only a 100 μm thick outer layer of functional tissue but not the construct interior, which remained largely acellular (Bursac et al. 1999; Carrier et al. 1999; Zimmermann et al. 2000; Papadaki et al. 2001).
In standard culture systems (dishes, flasks and rotating vessels), cardiomyocytes did not align in parallel as in the native heart and they remained poorly differentiated (Bursac et al. 1999; Carrier et al. 1999). Application of contraction alone via cyclic mechanical stretch significantly improved the level of cell differentiation and force of contraction, but some hallmarks of cardiac differentiation were still missing (including M bands and intercalated (IC) discs; Zimmermann et al. 2002b). In addition, the distribution and frequency of gap junctions, critical for electrical signal propagation in the tissue, remained unclear. Notably, excitation–contraction coupling, which is crucial for the development and function of native myocardium (Severs 2000), has not been fully established. More recently, clinically sized (6×8×2 mm thick), compact cardiac constructs with physiological cell densities were engineered in vitro by mimicking various aspects of the in vivo environment in native myocardium, including oxygen supply, by perfusion of culture medium supplemented with oxygen carriers (Radisic et al. 2005a) and the induction of construct contractions by electrical pacing signals (Radisic et al. 2004a).
We discuss here the key parameters of this new ‘biomimetic’ approach to cardiac tissue engineering, involving the cultivation of cells on scaffolds under conditions designed to provide developmental cues found in vivo. The focus is on the implementation of in vivo-like oxygen transport to support physiological concentrations of viable cells in clinically sized constructs, and electrical stimulation of cultured constructs to establish orderly excitation–contraction coupling.
2. Requirements derived from native myocardium
(a) Key parameters
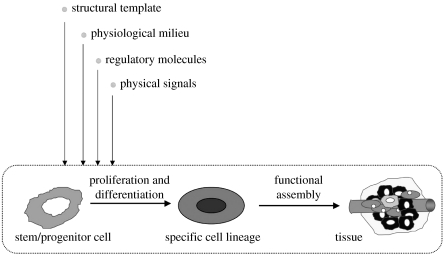
In vivo, the processes of cell differentiation during embryogenesis and adult tissue remodelling are directed by multiple factors acting in concert and according to specific spatial and temporal sequences. Undifferentiated (stem) cells first differentiate into specific cell lineages (bone, muscle, nerves and blood vessels) and then undergo functional assembly into tissue structures. Both phases depend on at least four groups of factors: (i) a structural template for cell attachment and tissue formation, (ii) a physiological milieu, (iii) levels of nutrients, oxygen and metabolites, and (iv) physical regulatory signals (figure 1). It is thought that the cell function in vitro can be modulated by the same factors known to play a role during embryogenesis, by an integrated use of biomaterial scaffolds and bioreactors (figure 2).
Figure 1.
Developmental paradigm. Tissue development and remodelling, in vivo and in vitro, involve the proliferation and differentiation of stem/progenitor cells and their subsequent assembly into a tissue structure. Cell function and the progression of tissue assembly depend on (i) the availability of a scaffold for cell attachment and tissue formation, (ii) the maintenance of physiological conditions in cell/tissue environment, (iii) the supply of nutrients, oxygen, metabolites and growth factors, and (iv) the presence of physical regulatory factors (adapted from Radisic et al. 2005b).
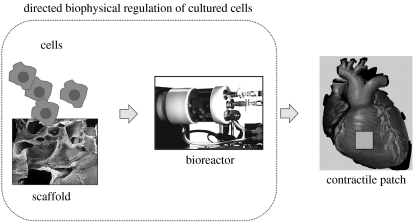
Figure 2.
Tissue engineering paradigm. The regulatory factors of cell differentiation and tissue assembly shown in figure 1 can be used in vitro to engineer functional tissues by an integrated use of isolated cells, biomaterial scaffolds and bioreactors. The cells themselves (either differentiated or progenitor/stem cells seeded onto a scaffold and cultured in a bioreactor) carry out the process of tissue formation in response to regulatory signals. The scaffold provides a structural, mechanical and logistic template for cell attachment and tissue formation. The bioreactor provides the environmental conditions and regulatory signals (biochemical and physical) that induce, enhance or at least support the development of functional tissue constructs (adapted from Radisic et al. 2005b).
Based on these general principles, a biomimetic approach to cardiac tissue engineering has been established, which involves the in vitro creation of immature but functional tissues by an integrated use of (i) populations of cardiac cells, (ii) biomaterial scaffolds that serve as a structural and logistic template for tissue development and biodegrade at a controlled rate, and (iii) bioreactors that provide environmental control and regulatory factors necessary for the cells to regenerate a functional tissue. A bioreactor is designed to perform one or more of the following functions: (i) establish spatially uniform, physiologically high cell concentration within the scaffold, (ii) maintain micro-environmental conditions via efficient mass transfer between the cells and culture medium (e.g. temperature, pH, osmolality, levels of oxygen, nutrients, metabolites and regulatory molecules), and (iii) apply physical signals relevant for cardiac development and function (e.g. interstitial flow, hydrodynamic shear and electrical stimuli). The environmental factors inherent for native myocardium in vivo and their in vitro counterparts are listed in table 1.
Table 1.
Factors governing cardiac tissue development in vivo and in vitro.
| in vivo | in vitro | |
|---|---|---|
| cells | high-density 5×108 cells cm−3 (Mandarim-de-Lacerda & Pereira, 2000) | high-density (0.5–1)×108 cells cm−3 |
| multiple cell types (myocytes, endothelial cells and fibroblasts) | multiple cell types | |
| oxygen supply | blood flow | interstitial flow of culture medium |
| geometry | dense capillary network within the tissue (diameter 10 μm, spacing 20 μm) | scaffold with a parallel array of channels |
| oxygen carrier | haemoglobin | synthetic oxygen carrier |
| perfluorocarbon (PFC) emulsion (Oxygent) | ||
| excitation–contraction coupling | electrical signal propagation | electrical stimulation |
| ventricle contraction | construct contraction |
(b) Cell populations
Native myocardium (cardiac muscle) is a highly differentiated tissue composed of CMs and fibroblasts with a dense supporting vasculature and collagen-based extracellular matrix (ECM), and an average cell density of 1–10×108 cells cm−3. The myocytes form a three-dimensional syncytium that enables propagation of electrical signals across specialized intracellular junctions to produce coordinated mechanical contractions that pump blood forward. Only 20–40% of the cells in the heart are CMs, but they occupy 80–90% of the heart volume (Nag 1980). Cardiac fibroblasts contribute to most of the non-myocytes in the myocardium. The main roles of cardiac fibroblasts are to secrete the components of the ECM and transmit mechanical force by the receptor-mediated connections to the ECM (Sussman et al. 2002). The myocardial ECM consists of a fibrillar collagen network, with predominant collagen types I and III, a basement membrane, proteoglycans, glycosaminoglycans and a variety of other bioactive molecules (Burlew & Weber 2002). The exact composition of the ECM is regulated by a crosstalk between myocytes and fibroblasts (Sussman et al. 2002). Recent studies demonstrated that cardiac fibroblasts propagate electrical stimuli over the distances of the order of 100 μm via gap junction communications (Gaudesius et al. 2003).
CMs and endothelial cells interact during embryonic development and in adult life. Myocardium secretes angiogenic factors that interact with endothelial cells. For example, basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF) that are released by ischemic myocardium act on endothelial cells to promote local angiogenesis. Cardiac myocytes stimulate endothelial cells' production of platelet derived growth factor-beta (PDGF-β), which combines with PDGF-α to induce endothelial cells' expression of von Willebrand factor (Edelberg et al. 1998). Cardiac-induced PDGF stimulates endothelial cells' secretion of VEGF and the VEGF receptor Flk-1, which are critical components of angiogenesis. Conversely, endothelium secretes multiple factors that affect cardiac myocytes. Nitric oxide secreted by endothelial cells causes vasodilatation of coronary vessels, exerts direct effects on myocardium, and decreases isotonic twitch shortening isolated myocytes and enhances myocardial relaxation (Shah et al. 1996; MacCarthy et al. 2001). These effects combine to decrease myocardial oxygen requirements, principally via increases in cGMP in myocytes and inhibition of Ca2+ entry through L-type calcium channels (Parratt et al. 1997). Hypoxic endothelial cells secrete substances that reduce actin-activated cardiac myosin ATPase activity and inhibit myocardial cross-bridge cycling, thereby reducing oxygen usage (Shah et al. 1997).
(c) Oxygen supply
Owing to the high density and high metabolic activity of the cells, myocardium consumes large amounts of oxygen, and cannot tolerate hypoxia for prolonged periods of time (Vander et al. 1985; Schoen 1999). Therefore, nutrients and in particular oxygen are quickly depleted. In native myocardium, oxygen is supplied to the cells through a rich vasculature, with average capillary-to-capillary distances in rat heart of only 17–19 μm, a width of an individual CM (Steinhausen et al. 1978; Korecky et al. 1982; Rakusan & Korecky 1982; Zadeh et al. 1986). The solubility of oxygen in plasma is very low. However, haemoglobin increases the total oxygen content of blood by two orders of magnitude, and thereby increases the mass of tissue that can be supported in a single pass through capillary network. The average oxygen concentration in arterial blood is 130 μM and in the venous blood it is 54 μM (Fournier 1998).
(d) Excitation–contraction coupling
The CM is the most physically energetic cell in the body, contracting more than three billion times in an average human lifespan, and pumping over 7000 l of blood per day along 160 000 km of blood vessels. Morphologically, intact CMs have an elongated rod-shaped appearance. Their contractile apparatus consists of sarcomeres, high metabolic activity is supported by the high density of mitochondria, and electrical signal propagation is provided by specialized intercellular connections, gap junctions (MacKenna et al. 1994; Brilla et al. 1995). The control of heart contractions is almost entirely self-contained, and can be attributed to the groups of specialized CMs (pacemakers), the fastest of which are located in the sinoatrial node. Contraction of the cardiac muscle is driven by the waves of electrical excitation generated by pacing cells that spread rapidly along the membranes of adjoining CMs and trigger release of calcium, which in turn stimulates contraction of the myofibrils. Electromechanical coupling of the myocytes is crucial for their synchronous response to electrical pacing signals (Severs 2000).
3. Tissue engineering
(a) Model system
In a tissue engineering model system we previously established, differentiated heart cells are used in conjunction with a biodegradable scaffold (designed to serve as a structural and logistic template for tissue development) and a bioreactor (designed to enable environmental control and support cell differentiation and functional assembly into an engineered tissue; figure 2). Cells were isolated from 1- to 2-day-old neonatal rat heart ventricles, separated into non-myocyte and myocyte fractions, and inoculated into scaffolds using a gel (Matrigel) as a cell delivery vehicle (Radisic et al. 2003). Scaffolds need to be made of biocompatible materials (preferentially those already approved for clinical use) and degrade with time (such that the final tissue graft does not contain any foreign material). We used scaffolds made either of natural polymers (e.g. collagen in form of a sponge; Radisic et al. 2004b) or synthetic polymers (e.g. polyglycolic acid in form of a fibrous mesh; Carrier et al. 1999) or polyglycerol sebacate in form of a sponge (Radisic et al. 2004b). In all cases, scaffolds had high void volumes (greater than 90%) and contained large interconnected pores.
Scaffold structure determines the transport of nutrients, metabolites and regulatory molecules to and from the cells, whereas the scaffold chemistry has an important role in cell attachment and differentiation. Mechanical properties of the scaffold should ideally match those of the native tissue, to provide mechanical integrity of the forming tissue and support an in vivo-like mechanotransduction between the cells and their environment.
(b) Diffusional limitations of conventional culture systems
The culture vessels most frequently used for cardiac tissue engineering include: Petri dishes (static or mixed), flasks (static or magnetically stirred), rotating vessels and perfused cartridges. Flasks contain constructs that are fixed in place and cultured either statically or with magnetic stirring, with gas exchange through loosened side-arm caps. Rotating vessels contain constructs that are freely suspended in culture medium between two concentric cylinders the inner of which serves as a gas exchange membrane, by adjusting the rate of vessel rotation (Carrier et al. 1999). Perfused cartridges are designed to provide interstitial fluid flow through the construct, with gas exchange in an external fluid loop (Carrier et al. 2002b).
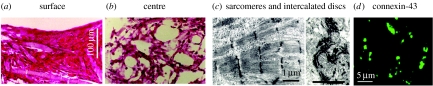
Constructs cultured in bioreactors providing convective flow of culture medium at construct surfaces (Bursac et al. 1999; Carrier et al. 1999) contained only a 100 μm thick peripheral region (figure 3a) around a largely acellular interior (figure 3b), a result of diffusional constraints of oxygen supply (Carrier et al. 1999, 2002b). In the outer tissue layer, cells were electrically connected via the gap junctional protein connexin-43 (Cx-43; figure 3d), exhibited cardiac-specific ultrastructural features (figure 3c), and propagated electrical signals over millimetre distances. Engineered constructs exhibited synchronous contractility, and could be captured and paced at a range of frequencies (20–300 b.p.m.; Bursac et al. 1999; Papadaki et al. 2001).
Figure 3.
Cardiac constructs engineered in a conventional culture system. Constructs seeded and cultured with medium flow at construct surfaces formed (a) approximately 100 μm thick peripheral region (b) around an acellular interior. Cells in the peripheral region were electrically connected via gap junctions (d), immunostain for connexin-43 (c) exhibited cardiac-specific ultrastructural features, and propagated electrical signals over a distance of 5 mm (as recorded by a linear array of electrodes; Papadaki et al. 2001) (adapted from Carrier et al. 1999).
In an attempt to enhance mass transport between culture medium and cells within cultured constructs, we later developed a bioreactor with interstitial medium flow (Carrier et al. 2002b). Cells were seeded onto scaffolds in tissue culture dishes and subsequently cultured with medium perfusion (interstitial flow through the construct). Hence, the transport of oxygen from the medium to the cells occurred via diffusion during cell seeding, and by a combination of diffusion and convection during cultivation. During cultivation, the flow of medium redistributed the cells evenly across the entire volume of the construct, but the cell density remained low due to the limitations in oxygen transport during cell seeding.
(c) Perfusion during seeding enabled physiological cell density
To provide oxygen supply to the cells at levels necessary to maintain their viability, we developed a technique of seeding that involves (i) rapid cell inoculation into collagen sponges using Matrigel as a cell delivery vehicle and (ii) transfer of inoculated scaffolds into perfused cartridges with immediate establishment of the interstitial flow of culture medium. Forward–reverse flow was used for the initial period of 1.5–4.5 h in order to further increase the rate and spatial uniformity of cell seeding (Radisic et al. 2003). Unidirectional flow of culture medium was maintained for the duration of cultivation. In this system, cells were ‘locked’ into the scaffold during a short (10 min) gelation period, and supplied with oxygen at all times during culture.
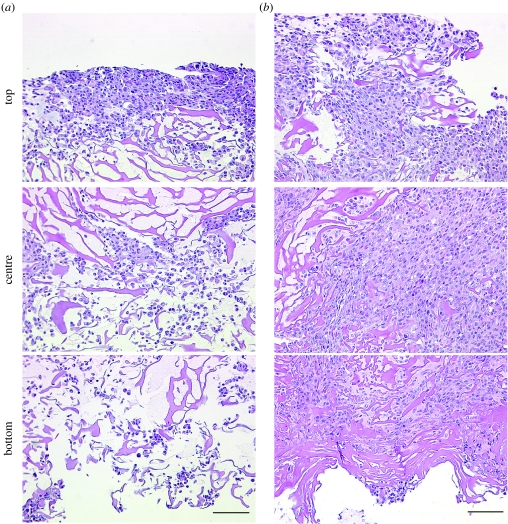
Cell distributions in the top, centre and bottom areas of a 650 μm wide strip extending from one construct surface to the other are shown in figure 4. Constructs seeded in dishes had most cells located within a 100 μm thick layer at the top surface, and only a small number of cells penetrated the entire construct depth. Constructs seeded in perfusion had high and spatially uniform cell density throughout the perfused volume of the construct. Clearly, medium perfusion during seeding was a key for engineering thick constructs with high densities of viable cells, due to enhanced transport of oxygen within the construct.
Figure 4.
Effects of perfusion during seeding and cultivation on cardiac cell distribution. Cross-sections of constructs inoculated with 12 million cells and then transferred for a period of 4.5 h either into (a) dishes (25 r.p.m.) or into (b) perfused cartridges (1.5 ml min−1). The top, centre and bottom areas of a 650 μm wide strip extending from one construct surface to the other are shown. Scale bar, 100 μm (adapted from Radisic et al. 2003).
(d) Perfusion during cultivation enabled cell viability and aerobic metabolism
Throughout the cultivation, the number of live cells in perfused constructs was significantly higher than in dish-grown constructs. Importantly, the final cell viability in perfused constructs cultured for 8 days (81.6±3.7%) was indistinguishable from the viability of the freshly isolated cells (83.8±2.0%) and it was markedly higher than the cell viability in dish-grown constructs (47.4±7.8%; Radisic et al. 2004b). Consistently, the molar ratio of lactate produced to glucose consumed (L/G) was approximately 1 for perfused constructs, indicating aerobic cell metabolism. In contrast, in orbitally mixed dishes, L/G increased progressively from 1 to approximately 2, indicating a transition to anaerobic cell metabolism. Cell damage, assessed by monitoring the activity of lactate dehydrogenase in culture medium, was at all time points significantly lower in perfusion than in dish cultures.
Perfused constructs and native ventricles had more cells in the S-phase than in the G2/M-phases, whereas the cells from dish-grown constructs appeared unable to complete the cell cycle and accumulated in the G2/M-phases. Cells expressing cardiac-specific differentiation markers (sarcomeric α-actin, sarcomeric tropomyosin and cardiac troponin I) were present throughout the perfused constructs, and only within an approximately 100 μm thick surface layer in dish-grown constructs.
Spontaneous contractions were observed in some constructs early in culture and ceased after approximately 5 days of cultivation, indicating the maturation of engineered tissue. In response to electrical stimulation, perfused constructs contracted synchronously, with lower excitation thresholds (ETs) and recovered their baseline function levels following treatment with a gap junction blocker; dish-grown constructs exhibited arrhythmic contractile patterns and failed to recover their baseline levels (Radisic et al. 2004b). However, most cells were round and mononucleated, a situation that was probably due to the exposure of CMs to hydrodynamic shear, in contrast to the native heart muscle where blood is confined within the capillary bed and therefore not in direct contact with CMs. This motivated the design of scaffolds with arrays of channels that provide a separate compartment for medium flow.
(e) In vivo-like oxygen supply: medium perfusion, channelled scaffolds and oxygen carriers
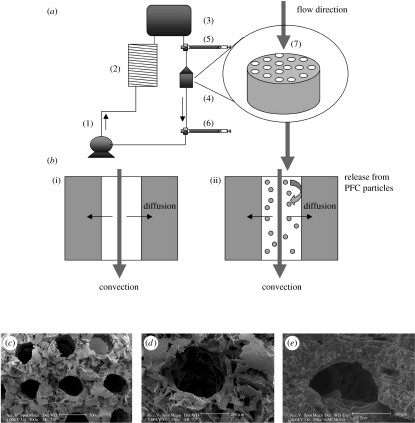
To mimic the capillary network, neonatal rat heart cells were cultured on an elastomer scaffold with an array of parallel channels and perfused with culture medium (figure 5a). Constructs were subjected to unidirectional medium flow at a flow rate of 0.1 ml min−1. To mimic oxygen supply by haemoglobin, culture medium was supplemented with 5.4% (v/v) PFC emulsion (Oxygent, Alliance Pharmaceuticals, San Diego, CA); constructs perfused with unsupplemented culture medium served as controls (figure 5b).
Figure 5.
Oxygen supply: medium perfusion, channelled scaffolds and oxygen carriers. (a) Perfusion loop. Channelled biorubber scaffolds (7) preconditioned with cardiac fibroblasts were seeded with CMs and placed into perfusion cartridges (4) between two debubbling syringes (5,6). Medium flow (0.1 ml min−1) was provided by a multi-channel peristaltic pump (1) and gas exchange was provided by a coil of thin silicone tubing (2). Loops were placed in the 37oC/5% CO2 incubator vertically. (b) Modes of oxygen transport in the channelled construct perfused with culture medium include convection through the channel lumen and diffusion into the tissue space surrounding each channel. In regular culture medium ((i) control group), oxygen dissolved in the aqueous phase during gas exchange in the external loop is transported into the tissue phase and consumed by the cells. In culture medium supplemented with 10% PFC emulsion ((ii) PFC group), oxygen is replenished within the tissue construct by the release of oxygen from the PFC particles into the culture medium phase. Scanning electron micrographs of biorubber scaffold with (c) a parallel channel array and (d) of a single channel are shown at the beginning and (e) after 3 days of cultivation (adapted from Radisic et al. (2005a)).
The elastomer, poly(glycerol sebacate) (PGS) was obtained by condensation of glycerol and sebacic acid, and formed by salt leaching into a three-dimensional network of large interconnected approximately 100 μm pores, high void volume (greater than 95%) and thicknesses of 1–5 mm (Wang et al. 2002). The cross-links and hydrogen bonds contribute to its unique elastic properties resembling vulcanized rubber and being capable of up to 400% elongation before it yields. PGS degrades by hydrolysis of its ester bond into glycerol (probably adsorbed in the body) and sebacic acid (secreted by urine either directly or metabolized into carboxylic acids). In vivo (subcutaneous implantation), PGS scaffold was biocompatible and lost its mass linearly (to approx. 20% of initial over 5 weeks), such that its shape and structural integrity were well maintained.
An array of parallel channels with diameter of approximately 350 μm and wall-to-wall spacing of approximately 220 μm was made using a laser beam (figure 5c). The scaffold was first pretreated with neonatal rat cardiofibroblasts for 3 days and then with rat cardiomyocytes, and cultured with perfusion of culture medium (Radisic et al. 2005a). After only 3 days in vitro, the subpopulations of cells on scaffolds formed constructs that contracted synchronously in response to electrical stimulation. The scaffold pores remained open (figure 5d,e) and the pressure drop measured across the construct was as low as 0.1 kPa mm−1 construct thickness.
As the medium flowed through the channel array, oxygen was depleted from the aqueous phase of the culture medium by diffusion into the construct space where it was used for cell respiration (figure 5b). Depletion of oxygen in the aqueous phase acted as a driving force for the diffusion of dissolved oxygen from the PFC particles, thereby contributing to the maintenance of higher oxygen concentrations in the medium. Owing to the small size of PFC particles, the passive diffusion of dissolved oxygen from the PFC phase into the aqueous phase was very fast, and estimated not to be a rate-limiting step in this system. For comparison, in non-supplemented culture medium, oxygen was depleted faster since there was no oxygen carrier phase that acted as a reservoir (Radisic et al. 2004a,b). In PFC-supplemented medium, the decrease in the partial pressure of oxygen in the aqueous phase was only 50% of that in control medium (28 versus 45 mmHg between the construct inlet and outlet at a flow rate of 0.1 ml min−1). Consistently, constructs cultivated in the presence of PFC had higher amounts of DNA, troponin I and Cx-43, and significantly better contractile properties as compared with control constructs. In both groups, cells were present at the channel surfaces as well as within constructs. Improved construct properties were correlated with the enhanced supply of oxygen to the cells within constructs.
(f) Mathematical model of oxygen transport
In order to rationalize experimental data for oxygen transport and consumption in engineered cardiac constructs with an array of channels, we developed a mathematical model of oxygen distribution in cardiac constructs similar to the Krogh cylinder model. Concentration profiles of oxygen and cells within the constructs were obtained by numerical simulation of the diffusive–convective oxygen transport and its usage by the cells (Radisic et al. 2004a,b). The model was used to define scaffold geometry and flow conditions necessary to cultivate cardiac constructs with clinically relevant thicknesses (5 mm).
Oxygen profiles were modelled in a channel array consisting of channels 100 μm in diameter and 100 μm wall-to-wall spacing at a physiologically high cell density of 1×108 cells cm−3. At a flow rate of 0.05 cm s−1, oxygen concentration increased significantly in both tissue space and channel lumen with the increase in circulating PFC emulsion from 0 to 6%. Although the oxygen concentration in the tissue space increased considerably with the increase of circulating PFC concentration, we had to increase the flow rate, keeping the shear stress in the physiological range approximately 1 dyn cm−2, in order to provide enough oxygen for the entire 0.5 cm thick construct. At the best conditions (0.135 cm s−1 and 6% PFC), oxygen is not depleted at any point in the scaffold and the minimum concentration of 33 μM is approximately five times above the Km.
(g) Excitation–contraction coupling: electrical stimulation
In native heart, mechanical stretch is induced by electrical signals, and the orderly coupling between electrical pacing signals and macroscopic contractions is crucial for the development and function of native myocardium (Severs 2000). We hypothesized that applying electrical signals designed to induce synchronous construct contractions would enhance cell differentiation and functional assembly of engineered tissue via physiologically relevant mechanisms. To test this hypothesis, cardiac constructs prepared by seeding collagen sponges (6×8×1.5 mm) with neonatal rat ventricular cells (5×106) were stimulated using supra-threshold square biphasic pulses (2 ms duration, 1 Hz, 5 V/cm). The stimulation was initiated after 1–5 days of scaffold seeding (3-day period was optimal) and applied for up to 8 days. Over only 8 days in vitro, electrical field stimulation induced cell alignment and coupling, increased the amplitude of synchronous construct contractions by a factor of 7 and resulted in an in vivo-like ultrastructural organization. Development of conductive and contractile properties of cardiac constructs was concurrent, with strong dependence on the initiation and duration of electrical stimulation.
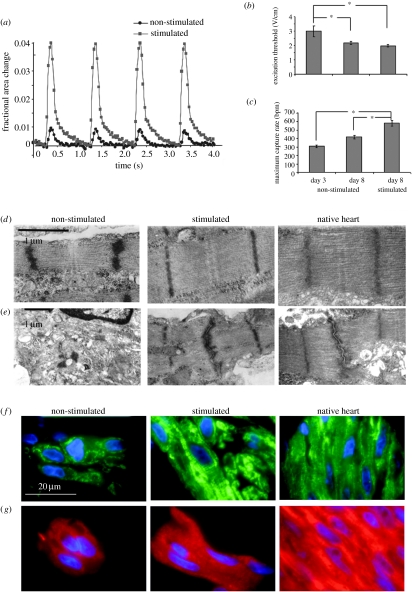
The application of electrical stimulation during construct cultivation markedly enhanced the contractile behaviour. After 8 days of culture, the amplitude of contractions was sevenfold higher in stimulated than in non-stimulated constructs (figure 6a), a result of the progressive increase with the duration of culture. The excitation threshold (ET; the minimum voltage at which the entire construct was observed to beat) decreased (figure 6b) and the maximum capture rate (MCR; the maximum pacing frequency for synchronous construct contractions) increased (figure 6c) both with time and due to electrical stimulation, suggesting functional coupling of the cells. The shape, amplitude (approx. 100 mV) and duration (approx. 200 ms) of the electrical activity recorded for cells in constructs stimulated during culture were similar to action potentials reported for constructs that were mechanically stimulated during culture (Zimmermann et al. 2002a,b). Notably, improved contractile properties of electrically stimulated constructs were not reflected in differences in cell density, cell damage or cell metabolism, but correlated instead with cell differentiation.
Figure 6.
Effects of electrical stimulation on functional assembly of engineered cardiac constructs. (a) Contraction amplitude of constructs cultured for a total of 8 days, shown as a fractional change in the construct size. Electrical stimulation increased the amplitude of contractions by a factor of 7. (b) ET decreased and (c) MCR increased significantly both with time in culture and due to electrical stimulation. (*) denotes statistically significant differences (p<0.05; Tukey's post hoc test with one-way ANOVA, n=5–10 samples per group and time point). (d) The structure of sarcomeres and (e) gap junctions observed in micrographs of stimulated constructs after 8 days of cultivation were remarkably similar to neonatal rat ventricles and markedly better developed than in control (non-stimulated) constructs. Representative sections of constructs stained for (f) Cx-43 (green) and (g) β-MHC (red), cell nuclei are shown in blue (adapted from Radisic et al. (2004a)).
After 8 days, stimulated constructs demonstrated a remarkable level of ultrastructural differentiation, comparable in several respects with that of native myocardium. Myofibres aligned in the direction of the electrical field lines, possibly in an attempt to decrease the apparent ET in response to pacing (Tung et al. 1991). In contrast, cells in non-stimulated constructs stayed round and expressed relatively low levels of cardiac markers. After 8 days, stimulated constructs exhibited a markedly higher density of Cx-43 than either early (3 days) or non-stimulated constructs. Stimulated constructs and neonatal ventricles contained abundant mitochondria positioned between myofibrils, in contrast to non-stimulated constructs containing mitochondria scattered around the cytoplasm, and substantially larger amounts of glycogen.
Electrical stimulation induced the development of long well-aligned registers of sarcomeres that closely resembled those in native myocardium (figure 6d,e, representing a hallmark of maturing cardiomyocytes; Severs 2000). The volume fraction of sarcomeres in stimulated 8-day constructs was indistinguishable from that measured for neonatal ventricles (Olivetti et al. 1980); in contrast, non-stimulated constructs contained only scattered and poorly organized sarcomeres. In stimulated constructs, IC discs were positioned between aligned Z lines and were as frequent as in neonatal ventricles; gap junctions were also better developed and more frequent.
On a molecular level, electrical stimulation elevated the levels of all measured cardiac proteins and enhanced the expression of the corresponding genes, without causing pathological cell hypertrophy (Di Nardo et al. 1993). With time in culture, the ratio of mature and immature forms of myosin heavy chain (α-MHC and β-MHC, respectively) decreased in non-stimulated and increased in stimulated constructs, suggesting that the maturation of cardiomyocytes depended both on culture duration and electrical stimulation.
These studies suggest that electrical stimulation of construct contractions during cultivation progressively enhanced the excitation–contraction coupling and improved the molecular, cellular and functional properties of engineered myocardium.
(h) Summary and ongoing developments
The overall goal of cardiac tissue engineering is to direct the cells to establish the physiological structure and function of the tissue being replaced across different hierarchical scales. To engineer myocardium, biophysical regulation of the cells needs to recapitulate multiple signals present in the native heart. The biomimetic approach to tissue engineering reviewed here focused on two specific aspects that are critical for the development and function of native myocardium: convective–diffusive oxygen transport and excitation–contraction coupling. To mimic the capillary network, culture medium was perfused through a channelled elastomer scaffold containing subpopulations of neonatal rat heart cells seeded at a physiological density. In addition, to mimic oxygen supply by haemoglobin, the culture medium was supplemented with a synthetic oxygen carrier (PFC emulsion). To promote functional assembly and electromechanical cell coupling, synchronous contractions of cultured constructs were induced by applying electrical signals designed to mimic those in native heart. Each of these two aspects substantially enhanced the structural and functional properties of engineered myocardium based on neonatal rat cells, over only 8 days of in vitro cultivation.
One major challenge is to extend the application of the same methods and principles to the cultivation of functional cardiac grafts based on human cells. Various cell sources are currently under consideration, including primitive cells from the adult heart that are capable of dividing and developing into mature heart and vascular cells (Beltrami et al. 2003; Oh et al. 2003; Messina et al. 2004). A recent study revealed relatively unspecialized cells that can both divide while maintaining their own population and give rise to functional heart cells (Laugwitz et al. 2005). These cells were demonstrated to populate the embryonic and postnatal heart and were successfully cultured in vitro, thus representing a potential source of cells for cardiac tissue engineering. It remains to be determined if the progenitors, regardless of their marker, can be isolated from adult human biopsies and if sufficient numbers of cardiomyocytes can be generated in vitro. Human embryonic stem cells represent another potential source of cardiac cells (Thomson et al. 1998). Notably, it was demonstrated that this differentiating system is not limited to the generation of isolated cardiac cells, but can result in a functional cardiac syncytium with a stable pacemaker activity and electrical propagation (Kehat et al. 2004).
Cardiac myocytes can be obtained at potentially unlimited quantities and high purity from embryonic stem cells (Klug et al. 1996). However, nuclear transfer is required to make them autologous and the presence of undifferentiated cells may lead to teratomas upon implantation (Laflamme & Murry 2005). Recent studies report cultivation and implantation of cardiac grafts based on mouse embryonic stem cells (Guo et al. 2006; Caspi et al. 2007).
Another challenge is to further advance the capabilities of our in vitro culture systems. Cultivation with direct perfusion of culture medium containing PFC enabled the maintenance of physiological density of viable differentiated cells in millimetres thick constructs. Electrical field stimulation induced cell alignment and coupling, increased the amplitude of synchronous contractions and enhanced the ultrastructural organization of engineered myocardium. However, our most advanced existing bioreactors can provide either local micro-environmental control of oxygen and pH (via medium perfusion), or the application of physical stimuli (via electrical stimulation). A system providing both factors simultaneously, would have a major impact on advancing our methods of cardiac tissue engineering, by subjecting the cultured cells to multiple signals present in the native heart. The combined application of the in vivo-like oxygen supply and electrical stimulation is expected to further enhance and/or accelerate cell differentiation and functional assembly. An additional advantage of this system is that it could serve as a high-fidelity model for rigorous studies of cardiac development and function. One of the key requirements for the success of the implantation strategies is electrical coupling between the graft and host myocardium (Eschenhagen et al. 2006). Recent studies with engineered heart tissue (Zimmermann et al. 2006) and cell sheets (Furuta et al. 2006) demonstrated such coupling, thus indicating that functional integration between the graft and host tissue, without arrhythmias, is possible. Although these studies demonstrated feasibility of implantation of contractile cardiac patches, more studies are necessary to correlate main in vitro parameters to the in vivo outcome. Questions yet to be answered through in vivo studies include: how long should the constructs be cultivated before implantation, how differentiated should the cardiomyocytes be, and what is the optimal composition of the biomaterial scaffold?
Acknowledgments
The work presented in this paper has been supported by the National Aeronautics and Space Administration (G.V.-N., M.R. and H.P.), the National Institutes of Health (G.V.-N. and C.C.), Poitras Fellowship (M.R.) and Juvenile Diabetes Research Foundation (S.G. and G.V.-N.). The authors would like to thank Sue Kangiser for her help with the manuscript preparation.
Footnotes
One contribution of 21 to a Theme Issue ‘Bioengineering the heart’.
References
- Akins R.E, Boyce R.A, Madonna M.L, Schroedl N.A, Gonda S.R, McLaughlin T.A, Hartzell C.R. Cardiac organogenesis in vitro: reestablishment of three-dimensional tissue architecture by dissociated neonatal rat ventricular cells. Tissue Eng. 1999;5:103–118. doi: 10.1089/ten.1999.5.103. doi:10.1089/ten.1999.5.103 [DOI] [PubMed] [Google Scholar]
- Balsam L.B, Wagers A.J, Christensen J.L, Kofidis T, Weissman I.L, Robbins R.C. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. doi: 10.1038/nature02460. doi:10.1038/nature02460 [DOI] [PubMed] [Google Scholar]
- Beltrami A.P, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. doi:10.1016/S0092-8674(03)00687-1 [DOI] [PubMed] [Google Scholar]
- Brilla C.G, Maisch B, Rupp H, Sunck R, Zhou G, Weber K.T. Pharmacological modulation of cardiac fibroblast function. Herz. 1995;20:127–135. [PubMed] [Google Scholar]
- Burlew B.S, Weber K.T. Cardiac fibrosis as a cause of diastolic dysfunction. Herz. 2002;27:92–98. doi: 10.1007/s00059-002-2354-y. doi:10.1007/s00059-002-2354-y [DOI] [PubMed] [Google Scholar]
- Bursac N, Papadaki M, Cohen R.J, Schoen F.J, Eisenberg S.R, Carrier R, Vunjak-Novakovic G, Freed L.E. Cardiac muscle tissue engineering: toward an in vitro model for electrophysiological studies. Am. J. Physiol: Heart Circ. Physiol. 1999;277:H433–H444. doi: 10.1152/ajpheart.1999.277.2.H433. [DOI] [PubMed] [Google Scholar]
- Bursac N, Papadaki M, White J.A, Eisenberg S.R, Vunjak-Novakovic G, Freed L.E. Cultivation in rotating bioreactors promotes maintenance of cardiac myocyte electrophysiology and molecular properties. Tissue Eng. 2003;9:1243–1253. doi: 10.1089/10763270360728152. doi:10.1089/10763270360728152 [DOI] [PubMed] [Google Scholar]
- Carrier R.L, Papadaki M, Rupnick M, Schoen F.J, Bursac N, Langer R, Freed L.E, Vunjak-Novakovic G. Cardiac tissue engineering: cell seeding, cultivation parameters and tissue construct characterization. Biotechnol. Bioeng. 1999;64:580–589. doi: 10.1002/(sici)1097-0290(19990905)64:5<580::aid-bit8>3.0.co;2-x. doi:10.1002/(SICI)1097-0290(19990905)64:5<580::AID-BIT8>3.0.CO;2-X [DOI] [PubMed] [Google Scholar]
- Carrier R.L, Rupnick M, Langer R, Schoen F.J, Freed L.E, Vunjak-Novakovic G. Effects of oxygen on engineered cardiac muscle. Biotechnol. Bioeng. 2002a;78:617–625. doi: 10.1002/bit.10245. doi:10.1002/bit.10245 [DOI] [PubMed] [Google Scholar]
- Carrier R.L, Rupnick M, Langer R, Schoen F.J, Freed L.E, Vunjak-Novakovic G. Perfusion improves tissue architecture of engineered cardiac muscle. Tissue Eng. 2002b;8:175–188. doi: 10.1089/107632702753724950. doi:10.1089/107632702753724950 [DOI] [PubMed] [Google Scholar]
- Caspi O, Lesman A, Basevitch Y, Gepstein A, Arbel G, Habib I.H.M, Gepstein L, Levenberg S. Tissue engineering of vascularized cardiac muscle from human embryonic stem cells. Circ. Res. 2007;100:263–272. doi: 10.1161/01.RES.0000257776.05673.ff. doi:10.1161/01.RES.0000257776.05673.ff [DOI] [PubMed] [Google Scholar]
- Condorelli G, et al. Cardiomyocytes induce endothelial cells to trans-differentiate into cardiac muscle: implications for myocardium regeneration. Proc. Natl Acad. Sci. USA. 2001;98:10 733–10 738. doi: 10.1073/pnas.191217898. doi:10.1073/pnas.191217898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Nardo P, Minieri M, Carbone A, Maggiano N, Micheletti R, Peruzzi G, Tallarida G. Myocardial expression of atrial natriuretic factor gene in early stages of hamster cardiomyopathy. Mol. Cell. Biochem. 1993;125:179–192. doi: 10.1007/BF00936447. doi:10.1007/BF00936447 [DOI] [PubMed] [Google Scholar]
- Edelberg J.M, Aird W.C, Wu W, Rayburn H, Mamuya W.S, Mercola M, Rosenberg R.D. PDGF mediates cardiac microvascular communication. J. Clin. Invest. 1998;102:837–843. doi: 10.1172/JCI3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschenhagen T, et al. Three-dimensional reconstitution of embryonic cardiomyocytes in a collagen matrix: a new heart model system. FASEB J. 1997;11:683–694. doi: 10.1096/fasebj.11.8.9240969. [DOI] [PubMed] [Google Scholar]
- Eschenhagen T, Zimmermann W.H, Kleber A.G. Electrical coupling of cardiac myocyte cell sheets to the heart. Circ. Res. 2006;98:573–575. doi: 10.1161/01.RES.0000215627.13049.5d. [DOI] [PubMed] [Google Scholar]
- Etzion S, Battler A, Barbash I.M, Cagnano E, Zarin P, Granot Y, Kedes L.H, Kloner R.A, Leor J. Influence of embryonic cardiomyocyte transplantation on the progression of heart failure in a rat model of extensive myocardial infarction. J. Mol. Cell. Cardiol. 2001;33:1321–1330. doi: 10.1006/jmcc.2000.1391. doi:10.1006/jmcc.2000.1391 [DOI] [PubMed] [Google Scholar]
- Fink C, Ergun S, Kralisch D, Remmers U, Weil J, Eschenhagen T. Chronic stretch of engineered heart tissue induces hypertrophy and functional improvement. FASEB J. 2000;14:669–679. doi: 10.1096/fasebj.14.5.669. [DOI] [PubMed] [Google Scholar]
- Fournier R.L. Taylor & Francis; Philadelphia, PA: 1998. Basic transport phenomena in biomedical engineering. [Google Scholar]
- Furuta A, et al. Pulsatile cardiac tissue grafts using a novel three-dimensional cell sheet manipulation technique functionally integrates with the host heart, in vivo. Circ. Res. 2006;98:705–712. doi: 10.1161/01.RES.0000209515.59115.70. doi:10.1161/01.RES.0000209515.59115.70 [DOI] [PubMed] [Google Scholar]
- Gaudesius G, Miragoli M, Thomas S.P, Rohr S. Coupling of cardiac electrical activity over extended distances by fibroblasts of cardiac origin. Circ. Res. 2003;93:421–428. doi: 10.1161/01.RES.0000089258.40661.0C. doi:10.1161/01.RES.0000089258.40661.0C [DOI] [PubMed] [Google Scholar]
- Gillum R.F. Epidemiology of congenital heart disease in the United States. Am. Heart J. 1994;127:919–927. doi: 10.1016/0002-8703(94)90562-2. doi:10.1016/0002-8703(94)90562-2 [DOI] [PubMed] [Google Scholar]
- Guo X.-M, et al. Creation of engineered cardiac tissue in vitro from mouse embryonic stem cells. Circulation. 2006;113:2229–2237. doi: 10.1161/CIRCULATIONAHA.105.583039. doi:10.1161/CIRCULATIONAHA.105.583039 [DOI] [PubMed] [Google Scholar]
- Hoffman J.I. Incidence of congenital heart disease: I. Postnatal incidence. Pediat. Cardiol. 1995a;16:103–113. doi: 10.1007/BF00801907. doi:10.1007/BF00801907 [DOI] [PubMed] [Google Scholar]
- Hoffman J.I. Incidence of congenital heart disease: II. Prenatal incidence. Pediat. Cardiol. 1995b;16:155–165. doi: 10.1007/BF00794186. [DOI] [PubMed] [Google Scholar]
- Kehat I, et al. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat. Biotechnol. 2004;22:1282–1289. doi: 10.1038/nbt1014. doi:10.1038/nbt1014 [DOI] [PubMed] [Google Scholar]
- Klug M.G, Soonpaa M.H, Koh G.Y, Field L.J. Genetically selected cardiomyocytes from differentiating embronic stem cells form stable intracardiac grafts. J. Clin. Invest. 1996;98:216–224. doi: 10.1172/JCI118769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofidis T, et al. In vitro engineering of heart muscle: artificial myocardial tissue. J. Thorac. Cardiovasc. Surg. 2002;124:63–69. doi: 10.1067/mtc.2002.121971. doi:10.1067/mtc.2002.121971 [DOI] [PubMed] [Google Scholar]
- Koh G.Y, Klug M.G, Soonpaa M.H, Field L.J. Differentiation and long-term survival of C2C12 myoblast grafts in heart. J. Clin. Investig. 1993;92:1115–1116. doi: 10.1172/JCI116734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korecky B, Hai C.M, Rakusan K. Functional capillary density in normal and transplanted rat hearts. Can. J. Physiol. Pharmacol. 1982;60:23–32. doi: 10.1139/y82-003. [DOI] [PubMed] [Google Scholar]
- Laflamme M.A, Murry C.E. Regenerating the heart. Nat. Biotechnol. 2005;23:845–856. doi: 10.1038/nbt1117. doi:10.1038/nbt1117 [DOI] [PubMed] [Google Scholar]
- Laugwitz K.L, et al. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–653. doi: 10.1038/nature03215. doi:10.1038/nature03215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leor J, Patterson M, Quinones M, Kedes L, Kloner R. Transplantation of fetal myocardial tissue into the infarcted myocardium of rat. Circulation. 1996;94:II-322–II-336. [PubMed] [Google Scholar]
- Leor J, Aboulafia-Etzion S, Dar A, Shapiro L, Barbash I.M, Battler A, Granot Y, Cohen S. Bioengineerred cardiac grafts: a new approach to repair the infarcted myocardium? Circulation. 2000;102:III56–III61. doi: 10.1161/01.cir.102.suppl_3.iii-56. [DOI] [PubMed] [Google Scholar]
- Li R.K, Jia Z.Q, Weisel M.H, Mickel D.A, Zhang J, Mohabeer M.K, Rao V, Ivanov J. Cardiomyocyte transplantation improved heart function. Ann. Thorac. Surg. 1996;62:654–660. doi: 10.1016/s0003-4975(96)00389-x. doi:10.1016/S0003-4975(96)00389-X [DOI] [PubMed] [Google Scholar]
- Li R.-K, Jia Z.Q, Weisel R.D, Mickle D.A.G, Choi A, Yau T.M. Survival and function of bioengineered cardiac grafts. Circulation. 1999;100:II63–II69. doi: 10.1161/01.cir.100.suppl_2.ii-63. [DOI] [PubMed] [Google Scholar]
- Li R.-K, Yau T.M, Weisel R.D, Mickle D.A.G, Sakai T, Choi A, Jia Z.-Q. Construction of a bioengineered cardiac graft. J. Thorac. Cardiovasc. Surg. 2000;119:368–375. doi: 10.1016/S0022-5223(00)70193-0. doi:10.1016/S0022-5223(00)70193-0 [DOI] [PubMed] [Google Scholar]
- MacCarthy P.A, Grieve D.J, Li J.-M, Dunster C, Kelly F.J, Shah A.M. Impaired endothelial regulation of ventricular relaxation in cardiac hypertrophy. Circulation. 2001;104:2967–2974. doi: 10.1161/hc4901.100382. [DOI] [PubMed] [Google Scholar]
- MacKenna D.A, Omens J.H, McCulloch A.D, Covell J.W. Contribution of collagen matrix to passive left ventricular mechanics in isolated rat heart. Am. J. Physiol. 1994;266:H1007–H1018. doi: 10.1152/ajpheart.1994.266.3.H1007. [DOI] [PubMed] [Google Scholar]
- Mandarim-de-Lacerda C.A, Pereira L.M.M. Numerical density of cardiomyocytes in chronic nitric oxide synthesis inhibition. Pathobiology. 2000;68:36–42. doi: 10.1159/000028113. doi:10.1159/000028113 [DOI] [PubMed] [Google Scholar]
- Menasche P, et al. Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. J. Am. Coll. Cardiol. 2003;41:1078–1083. doi: 10.1016/s0735-1097(03)00092-5. doi:10.1016/S0735-1097(03)00092-5 [DOI] [PubMed] [Google Scholar]
- Messina E, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ. Res. 2004;95:911–921. doi: 10.1161/01.RES.0000147315.71699.51. doi:10.1161/01.RES.0000147315.71699.51 [DOI] [PubMed] [Google Scholar]
- Muller-Ehmsen J, Peterson K.L, Kedes L, Whittaker P, Dow J.S, Long T.I, Laird P.W, Kloner R.A. Rebuilding a damaged heart: long-term survival of transplanted neonatal rat cardiomyocytes after myocardial infarction and effect on cardiac function. Circulation. 2002a;105:1720–1726. doi: 10.1161/01.cir.0000013782.76324.92. doi:10.1161/01.CIR.0000013782.76324.92 [DOI] [PubMed] [Google Scholar]
- Muller-Ehmsen J, Whittaker P, Kloner R.A, Dow J.S, Sakoda T, Long T.I, Laird P.W, Kedes L. Survival and development of neonatal rat cardiomyocytes transplanted into adult myocardium. J. Mol. Cell. Cardiol. 2002b;34:107–116. doi: 10.1006/jmcc.2001.1491. doi:10.1006/jmcc.2001.1491 [DOI] [PubMed] [Google Scholar]
- Murry C.E, et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446. doi:10.1038/nature02446 [DOI] [PubMed] [Google Scholar]
- Nag A.C. Study of non-muscle cells of the adult mammalian heart: a fine structural analysis and distribution. Cytobios. 1980;28:41–61. [PubMed] [Google Scholar]
- Oh H, et al. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc. Natl Acad. Sci. USA. 2003;100:12 313–12 318. doi: 10.1073/pnas.2132126100. doi:10.1073/pnas.2132126100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivetti G, Anversa P, Loud A.V. Morphometric study of early postnatal development in the left and right ventricular myocardium of the rat. II. Tissue composition, capillary growth, and sarcoplasmic alterations. Circ. Res. 1980;46:503–512. doi: 10.1161/01.res.46.4.503. [DOI] [PubMed] [Google Scholar]
- Orlic D, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. doi:10.1038/35070587 [DOI] [PubMed] [Google Scholar]
- Papadaki M, Bursac N, Langer R, Merok J, Vunjak-Novakovic G, Freed L.E. Tissue engineering of functional cardiac muscle: molecular, structural and electrophysiological studies. Am. J. Physiol: Heart Circ. Physiol. 2001;280:H168–H178. doi: 10.1152/ajpheart.2001.280.1.H168. [DOI] [PubMed] [Google Scholar]
- Parratt J.R, Vegh A, Zeitlin I.J, Ahmad M, Oldroyd K, Kaszala K, Papp J.G. Bradykinin and endothelial–cardiac myocyte interactions in ischemic preconditioning. Am. J. Cardiol. 1997;80:124A–131A. doi: 10.1016/s0002-9149(97)00467-0. doi:10.1016/S0002-9149(97)00467-0 [DOI] [PubMed] [Google Scholar]
- Radisic M, Euloth M, Yang L, Langer R, Freed L.E, Vunjak-Novakovic G. High density seeding of myocyte cells for tissue engineering. Biotechnol. Bioeng. 2003;82:403–414. doi: 10.1002/bit.10594. doi:10.1002/bit.10594 [DOI] [PubMed] [Google Scholar]
- Radisic M, Park H, Shing H, Consi T, Schoen F.J, Langer R, Freed L.E, Vunjak-Novakovic G. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc. Natl Acad. Sci. USA. 2004a;101:18 129–18 134. doi: 10.1073/pnas.0407817101. doi:10.1073/pnas.0407817101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radisic M, Yang L, Boublik J, Cohen R.J, Langer R, Freed L.E, Vunjak-Novakovic G. Medium perfusion enables engineering of compact and contractile cardiac tissue. Am. J. Physiol: Heart Circ. Physiol. 2004b;286:H507–H516. doi: 10.1152/ajpheart.00171.2003. doi:10.1152/ajpheart.00171.2003 [DOI] [PubMed] [Google Scholar]
- Radisic M, Deen W, Langer R, Vunjak-Novakovic G. Mathematical model of oxygen distribution in engineered cardiac tissue with parallel channel array perfused with culture medium containing oxygen carriers. Am. J. Physiol: Heart Circ. Physiol. 2005a;288:H1278–H1289. doi: 10.1152/ajpheart.00787.2004. doi:10.1152/ajpheart.00787.2004 [DOI] [PubMed] [Google Scholar]
- Radisic M, Obradovic B, Vunjak-Novakovic G. Functional tissue engineering of cartilage and myocardium: bioreactor aspects. In: Ma P.X, Elisseeff J, editors. Scaffolding in tissue engineering. Marcel Dekker; New York, NY: 2005b. pp. 491–520. ch. 33. [Google Scholar]
- Radisic M, Malda J, Epping E, Geng W, Langer R, Vunjak-Novakovic G. Oxygen gradients correlate with cell density and cell viability in engineered cardiac tissue. Biotechnol. Bioeng. 2005c;93:332–343. doi: 10.1002/bit.20722. doi:10.1002/bit.20722 [DOI] [PubMed] [Google Scholar]
- Radisic M, Park H, Chen F, Wang Y, Dennis R, Langer R, Freed L.E, Vunjak-Novakovic G. Biomimetic approach to cardiac tissue engineering: oxygen carriers and channeled scaffolds. Tissue Eng. 2006;12:2077–2091. doi: 10.1089/ten.2006.12.2077. doi:10.1089/ten.2006.12.2077 [DOI] [PubMed] [Google Scholar]
- Rakusan K, Korecky B. The effect of growth and aging on functional capillary supply of the rat heart. Growth. 1982;46:275–281. [PubMed] [Google Scholar]
- Reinecke H, Zhang M, Bartosek T, Murry C.E. Survival, integration, and differentiation of cardiomyocyte grafts: a study in normal and injured rat hearts. Circulation. 1999;100:193–202. doi: 10.1161/01.cir.100.2.193. [DOI] [PubMed] [Google Scholar]
- Roell W, et al. Cellular cardiomyoplasty improves survival after myocardial injury. Circulation. 2002;105:2435–2441. doi: 10.1161/01.cir.0000016063.66513.bb. doi:10.1161/01.CIR.0000016063.66513.BB [DOI] [PubMed] [Google Scholar]
- Sakai T, Li R.K, Weisel R.D, Mickle D.A, Jia Z.Q, Tomita S, Kim E.J, Yau T.M. Fetal cell transplantation: a comparison of three cell types. J. Thorac. Cardiovasc. Surg. 1999;118:715–724. doi: 10.1016/S0022-5223(99)70018-8. doi:10.1016/S0022-5223(99)70018-8 [DOI] [PubMed] [Google Scholar]
- Schoen F.J. The heart. In: Cotran R.S, Kumar V, Collins T, Robbins S.L, editors. Robbins pathologic basis of disease. W. B. Saunders; Philadelphia, PA: 1999. pp. 543–599. [Google Scholar]
- Scorsin M, Marotte F, Sabri A, Le Dref O, Demirag M, Samuel J.-L, Rappaport L, Measche P. Can grafted cardiomyocytes colonize peri-infarct myocardial areas? Circulation. 1996;94:II337–II340. [PubMed] [Google Scholar]
- Severs N.J. The cardiac muscle cell. Bioessays. 2000;22:188–199. doi: 10.1002/(SICI)1521-1878(200002)22:2<188::AID-BIES10>3.0.CO;2-T. doi:10.1002/(SICI)1521-1878(200002)22:2<188::AID-BIES10>3.0.CO;2-T [DOI] [PubMed] [Google Scholar]
- Shah A.M, Grocott-Mason R.M, Pepper C.B, Mebaza A, Henderson A.H, Lewis M.J, Paulus W.J. The cardiac endothelium: cardioactive mediators. Prog. Cardiovasc. Dis. 1996;39:263–284. doi: 10.1016/s0033-0620(96)80005-3. doi:10.1016/S0033-0620(96)80005-3 [DOI] [PubMed] [Google Scholar]
- Shah A.M, et al. Inhibition of myocardial crossbridge cycling by hypoxic endothelial cells: a potential mechanism for matching oxygen supply and demand? Circ. Res. 1997;80:688–698. doi: 10.1161/01.res.80.5.688. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Yamato M, Isoi Y, Akutsu T, Setomaru T, Abe K, Kikuchi A, Umezu M, Okano T. Fabrication of pulsatile cardiac tissue grafts using a novel 3-dimensional cell sheet manipulation technique and temperature-responsive cell culture surfaces. Circ. Res. 2002;90:e40–e48. doi: 10.1161/hh0302.105722. doi:10.1161/hh0302.105722 [DOI] [PubMed] [Google Scholar]
- Soonpaa M.H, Koh G.Y, Klug M.G, Field L.J. Formation of nascent intercalated disks between grafted fetal cardiomyocytes and host myocardium. Science. 1994;264:98–101. doi: 10.1126/science.8140423. doi:10.1126/science.8140423 [DOI] [PubMed] [Google Scholar]
- Steinhausen M, Tillmanns H, Thederan H. Microcirculation of the epimyocardial layer of the heart. I. A method for in vivo observation of the microcirculation of superficial ventricular myocardium of the heart and capillary flow pattern under normal and hypoxic conditions. Pflugers Arch: Eur. J. Physiol. 1978;374:57–66. doi: 10.1007/BF00581952. doi:10.1007/BF00585697 [DOI] [PubMed] [Google Scholar]
- Sussman M.A, McCulloch A, Borg T.K. Dance band on the Titanic: biomechanical signaling in cardiac hypertrophy. Circ. Res. 2002;91:888–898. doi: 10.1161/01.res.0000041680.43270.f8. doi:10.1161/01.RES.0000041680.43270.F8 [DOI] [PubMed] [Google Scholar]
- Taylor D.A, Atkins B.Z, Hungspreugs P, Jones T.R, Reedy M.C, Hutcheson K.A, Glower D.D, Kraus W.E. Regenerating functional myocardium: improved performance after skeletal myoblast transplantation. Nat. Med. 1998;4:929–933. doi: 10.1038/nm0898-929. doi:10.1038/nm0898-929 [DOI] [PubMed] [Google Scholar]
- Thomson J.A, Itskovitz-Eldor J, Shapiro S.S, Waknitz M.A, Swiergiel J.J, Marshall V.S, Jones J.M. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. doi:10.1126/science.282.5391.1145 [DOI] [PubMed] [Google Scholar]
- Tung L, Sliz N, Mulligan M.R. Influence of electrical axis of stimulation on excitation of cardiac muscle cells. Circ. Res. 1991;69:722–730. doi: 10.1161/01.res.69.3.722. [DOI] [PubMed] [Google Scholar]
- Vander A.J, Sherman J.H, Luciano D.S. McGraw-Hill; New York, NY: 1985. Human physiology. [Google Scholar]
- Wang Y, Ameer G.A, Sheppard B.J, Langer R. A tough biodegradable elastomer. Nat. Biotechnol. 2002;20:602–606. doi: 10.1038/nbt0602-602. doi:10.1038/nbt0602-602 [DOI] [PubMed] [Google Scholar]
- Zadeh B.J, Gonzalez-Sanchez A, Fischman D.A, Bader D.M. Myosin heavy chain expression in embryonic cardiac cell culture. Dev. Biol. 1986;115:204–214. doi: 10.1016/0012-1606(86)90241-1. doi:10.1016/0012-1606(86)90241-1 [DOI] [PubMed] [Google Scholar]
- Zimmermann W.H, Fink C, Kralish D, Remmers U, Weil J, Eschenhagen T. Three-dimensional engineered heart tissue from neonatal rat cardiac myocytes. Biotechnol. Bioeng. 2000;68:106–114. doi:10.1002/(SICI)1097-0290(20000405)68:1<106::AID-BIT13>3.0.CO;2-3 [PubMed] [Google Scholar]
- Zimmermann W.H, et al. Cardiac grafting of engineered heart tissue in syngenic rats. Circulation. 2002a;106:I151–I157. [PubMed] [Google Scholar]
- Zimmermann W.H, Schneiderbanger K, Schubert P, Didie M, Munzel F, Heubach J.F, Kostin S, Nehuber W.L, Eschenhagen T. Tissue engineering of a differentiated cardiac muscle construct. Circ. Res. 2002b;90:223–230. doi: 10.1161/hh0202.103644. doi:10.1161/hh0202.103644 [DOI] [PubMed] [Google Scholar]
- Zimmermann W.H, et al. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat. Med. 2006;12:452–458. doi: 10.1038/nm1394. doi:10.1038/nm1394 [DOI] [PubMed] [Google Scholar]