Abstract
The cells that reside within valve cusps play an integral role in the durability and function of heart valves. There are principally two types of cells found in cusp tissue: the endothelial cells that cover the surface of the cusps and the interstitial cells (ICs) that form a network within the extracellular matrix (ECM) within the body of the cusp. Both cell types exhibit unique functions that are unlike those of other endothelial and ICs found throughout the body. The valve ICs express a complex pattern of cell-surface, cytoskeletal and muscle proteins. They are able to bind to, and communicate with, each other and the ECM. The endothelial cells on the outflow and inflow surfaces of the valve differ from one another. Their individual characteristics and functions reflect the fact that they are exposed to separate patterns of flow and pressure. In addition to providing a structural role in the valve, it is now known that the biological function of valve cells is important in maintaining the integrity of the cusps and the optimum function of the valve. In response to inappropriate stimuli, valve interstitial and endothelial cells may also participate in processes that lead to valve degeneration and calcification. Understanding the complex biology of valve interstitial and endothelial cells is an important requirement in elucidating the mechanisms that regulate valve function in health and disease, as well as setting a benchmark for the function of cells that may be used to tissue engineer a heart valve.
Keywords: heart valves, valve interstitial cells, cell phenotype, cell communication
1. Background
Recent observations have illustrated the complexity of the biological processes that are performed by valve interstitial and endothelial cells. These mechanisms are now being recognized to an important role in the sophisticated function of heart valves. The ability of these cells to communicate with each other, with the extracellular matrix (ECM), and to respond to their environment, underpins the mechanisms that allow heart valves to function in an optimal and efficient manner. Cell types within the valve can be broadly divided into two separate phenotypes: interstitial and endothelial cells. The ICs populate the body of the valve cusp and may in fact represent a heterogeneous population of cells. The endothelial cells cover both sides of the valve cusps and act as an interface between the cusp and the blood.
2. Valve-cusp interstitial cells
Valve ICs are a heterogeneous and dynamic population of specific cell types that are phenotypically different from dermal fibroblasts, and have many unique characteristics (Lester et al. 1988; Messier et al. 1994; Mulholland & Gotlieb 1996; Della et al. 2000; Taylor et al. 2000). It is probable that a family of fibroblast-like cells exists that varies its phenotype as an adaptive response to its microenvironment, as dictated by the ECM, mechanical force and soluble factors (Komuro 1990). These cells synthesize matrix components, such as collagen, elastin, proteoglycans and glycoproteins; growth factors, cytokines and chemokines; as well as matrix remodelling enzymes, the matrix metalloproteinases (MMPs) and their tissue inhibitors (TIMPs; Sappino et al. 1990; Smith et al. 1997).
(a) Phenotypic characteristics
Two cellular phenotypes have been demonstrated by electron microscopy and immunocytochemistry, both in situ and in cells cultured from valves. One type, ‘the myofibroblast’, is characterized by prominent stress fibres associated with smooth muscle α-actin expression (Lester et al. 1988; Messier et al. 1994; Mulholland & Gotlieb 1996; Della et al. 2000; Taylor et al. 2000). A layer of smooth muscle α-actin-positive cells has been identified in the ventricularis of both human and porcine aortic valves (Bertipaglia et al. 2003; Taylor et al. 2003). This contractile phenotype is thought to be proliferative, migratory and capable of remodelling the ECM (Arora & McCulloch 1994). The second phenotype is characterized by prominent synthetic and secretory organelles and is involved in matrix regulation and production. This phenotype is associated with the expression of prolyl 4-hydroxylase, an enzyme essential for stabilization of the collagen triple helix, which indicates that the cells are actively synthesizing collagen (Konttinen et al. 1989; Janin et al. 1990).
Two cell morphologies are observed when valve ICs are cultured: small islands of cuboidal cells and spindle-shaped elongated cells. Once confluent, the cells exhibit a swirling pattern, characteristic of fibroblasts, and start to pile up on each other in layers (Johnson et al. 1987). In our experience, these cells have a slow rate of proliferation even when a variety of growth factors are added, which may reflect an inherently low turnover rate. Whether the same range of phenotypes is present in all of the valve leaflets still remains to be determined. However, our investigations have suggested that cultured valve ICs have a similar phenotypic profile, whether they are from the outflow valves (aortic and pulmonary) or the atrioventricular valves (mitral and tricuspid; Taylor et al. 2000). Our laboratory has recently been able to demonstrate that valve ICs in culture are capable of differentiating into other cell phenotypes including osteoblast, adipocytes and chondrocytes (Osman et al. 2006; N. Latif 2006, personal communication).
It is well documented that ECM and cytokines are important in fibroblast differentiation and function (Sappino et al. 1990; Juliano & Haskill 1993; Arora & McCulloch 1994; Desmouliere & Gabbiani 1994). Fibroblasts synthesize collagen, elastin, proteoglycans, glycoproteins, growth factors, cytokines and chemokines, as well as MMPs and TIMPs (Sappino et al. 1990; Smith et al. 1997). The ability of valve ICs to secrete growth factors, or stimulate their release from the ECM via the action of MMPs (Segura et al. 1998), may play a vital role in regulation of cell phenotype within the cusp. It has been shown that in several valve pathologies, there is increased expression/activity of matrix remodelling enzymes and secretion of cytokines (Rabkin et al. 2001; Kaden et al. 2003, 2004b; Guilherme et al. 2004; Fondard et al. 2005).
Valve ICs have been shown to express a range of skeletal and non-muscle cell markers. Molecular expression of β-myosin heavy chain (βMHC) has been reported, which is associated with the slower contractile isoform of myocytes due to its lower ATPase activity compared with αMHC (Brand et al. 2006). In addition to cardiac muscle markers, valve ICs express skeletal muscle components of the troponin complex. The expression of these skeletal muscle proteins may be regulated by the transcription factor myogenin, which is also expressed by valve ICs (Brand et al. 2006). The expression of these muscle cell markers suggests that valve cusps express the required machinery for contractile responses. These cells also express genes that encode components of the wnt/β-catenin signalling pathway. Members of the Frizzled family of wnt receptors have also been detected in cells from all four heart valves (Brand et al. 2006). Believed to play a role in the embryonic development of the valves (Butcher & Markwald (2007) review developmental signalling in this issue), the components of wnt growth factor signalling have been implicated in pathological mechanisms in mature valves (Hurlstone et al. 2003; Rajamannan et al. 2005).
(b) Cell communication
Communication between valve ICs themselves, and between the ECM and ICs, is a feature that is important to the regulation of valve function. Recently, we identified a range of the molecules involved in communication between valve ICs (Latif et al. 2005b). These markers include components of cell-to-cell junctions, such as cadherens (N-cadherin), desmosomal junctions (desmoglein) and some expression of gap-junction proteins (Connexin-26 and 45), but an absence of proteins associated with tight junctions. The cells that populate valve cusps reside within, and make connections to, the ECM as well as connections to each other (figure 1). This enables them to sense and respond to the mechanical forces experienced by the valve. It has been shown that cells in intact valves and those maintained in culture express a range of integrin molecules, including α1, α2, α3, α4, α5 and, to a lesser extent, α6 and αV. Expression of β1, but not β3 or β4, integrins has also been reported (Latif et al. 2005a). A number of studies have demonstrated the importance of integrins in the regulation of cell behaviour due to their ability to act as sensors and transducers of signals in a bidirectional manner.
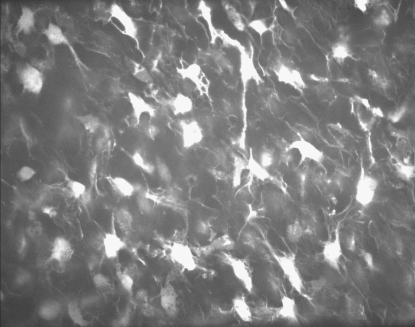
Figure 1.
Photomicrographs of a porcine aortic valve leaflet stained with calcein AM. The panel shows a confocal slice at z=200 μm from ventricular surface showing ICs.
The ability of valve ICs to form a network in vitro is demonstrated by their ability to contract collagen gels in an efficient and unique manner (Butcher et al. 2004). They have been shown to have contractile properties and synthesize matrix components. Unlike some fibroblast-type cells and vascular smooth-muscle cells, valve ICs are able to induce contraction of collagen gels, the magnitude of which was inversely proportional to the number of cells seeded into the gel (Smith et al. in press). Morphologically, the valve ICs within the collagen gels generated force initially by spreading, elongation and finally sending out extension processes. The maintenance and gradual increase in force appeared to be due to continued and extensive formation of cellular processes that created a network of cells within the collagen gel. Given that these cells normally reside in a highly dynamic mechanical environment, the intensive direct cell-to-cell and cell-to-matrix communication may reflect a mechanism that contributes to the maintenance of tension within the valve cusp during systole. Examining the response of cells in collagen gels may be a useful gauge of the similarity of different cells to valve ICs, and therefore aids in the choice of cells with which to tissue engineer a heart valve.
(c) Contractile capacity
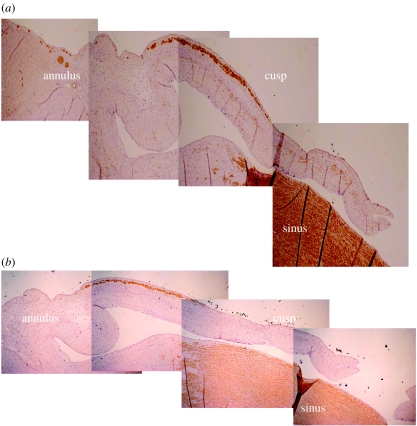
The presence of contractile cell phenotypes within porcine and human aortic valves has been demonstrated via molecular and histological studies (Bairati & DeBiasi 1981; Taylor et al. 2000). In addition, aortic cusp tissue has been shown to exhibit contractile responses to a range of different vasoactive agents, a number of which are also capable of increasing levels of intracellular calcium (Chester et al. 2000; Taylor et al. 2000). Specific receptor systems have been demonstrated to mediate these contractile responses (Wassenaar et al. 1997; Chester et al. 2000, 2001; Misfeld et al. 2002). These include responses to commonly encountered vasoconstrictors such as catecholamines, histamine, 5-hydroxytryptamine and endothelin. In addition to the endothelium-derived vasoconstrictor endothelin, it has also been demonstrated that cusp tissue can relax in response to the release of nitric oxide from the endothelium (Pompilio et al. 1998). The responses to vasoactive mediators of cusp tissue are most probably mediated by contraction of a band of smooth-muscle α-actin-positive cells that can be localized along the ventricular surface of aortic valve cusps (figure 2). The role of these cells, and whether they represent an ‘activated’ cell phenotype, is still a matter of debate. The percentage of cells that are smooth-muscle α-actin-positive increases in cells which are involved with development of the valve or under diseased conditions, compared to normal undiseased adult cells (Rabkin-Aikawa et al. 2004). The pattern of expression of smooth-muscle α-actin in foetal tissue has been shown to represent activated/immature cells (with high expression) that decrease with age as the cells become quiescent, suggesting a remodelling role for these cells as the valve responds to the post-natal haemodynamic environment (Aikawa et al. 2006). It has been demonstrated that valve ICs respond to local tissue stress by increasing their stiffness via changes in their smooth-muscle α-actin content (Merryman et al. 2006), indicating that the contractile machinery in valve cells may assist in tissue homeostasis. The role and relevance of contractile mechanisms in valve tissue remains unclear. Investigation is required to elucidate if certain subpopulations of valve cells mediate specific responses of the valve, such as those to mechanical force, autocrine or paracrine mediators, or neuronal responses (Hafizi et al. 2000; Xing et al. 2004a,b; Ku et al. 2006). Indeed, if certain cells do become activated as part of a pathological response, this suggests that such cells go on to alter their phenotype further and participate in the disease process.
Figure 2.
Composite photomicrograph of sections of porcine aortic roots shown staining (brown colour) for antibodies against (a) smooth muscle cell α-actin and (b) smooth muscle cell myosin.
(d) Secretory properties
Valve ICs have the capacity to synthesize and release mediators that are involved in ECM synthesis, remodelling of the matrix and mediation of inflammatory responses. We have previously shown the expression of MMPs and TIMPs in valve cusps (Dreger et al. 2002). The valve ICs constitutively express these enzymes, indicating their key role in the regulation of the ECM. In addition, valve ICs in culture can secrete collagen in response to stimulation by vasoactive mediators, such as 5-hydroxytryptamine and angiotensin II, and in response to mechanical force (Hafizi et al. 2000; Xing et al. 2004b). The ability of valve ICs to regulate and remodel the ECM is important to maintain the strength and durability of valve cusps. It will be important for any cell type that is used to tissue engineer a heart valve to possess similar properties.
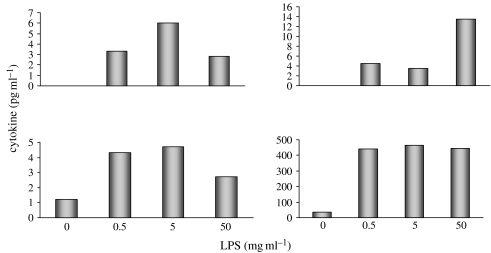
The role of the valve ICs in orchestrating inflammatory responses in valve cusps has not been addressed. There are a number of non-immunological cell types that are thought to play a role in mediating inflammatory reactions due to their capacity to release cytokines and/or chemokines; these include vascular and airway smooth-muscle cells, vascular endothelial cells and lung epithelial cells (Bouma et al. 1996; Saunders et al. 1997; Stanford et al. 2002). It would appear that valve ICs also have the capacity to secrete a range of pro- and anti-inflammatory cytokines under basal conditions. We have obtained evidence that human valve ICs can release significant levels of IL-6 and IL-8 under resting conditions. IL-12, IL-10 and TNF-α were also detectable; in contrast, there was no release of IL-1. The levels of these cytokines increased when the cells were stimulated with lipopolysaccaride (figure 3). Localized disruptions in the balance between the release of anti- and pro-inflammatory cytokines and chemokines may be induced by stimuli associated with valve-disease risk factors.
Figure 3.
Graphs showing the release of IL12, IL10, TNF-α and IL-6 from cultured valve ICs in response to increasing concentration of lipopolysaccride over a 24 h period.
It has been proposed that valve calcification is an inflammatory disease (Durbin & Gotlieb 2002). The ability of valve ICs to participate in the initiation or progression of inflammatory reactions may represent important mechanisms through which these cells are involved in the disease process, and identifies them as potential targets for pharmacological intervention.
(e) Signalling pathways involved in valve calcification
The presence of osteoblast-like phenotypes has been described in calcified aortic valves. This observation suggests that valve ICs are capable of undergoing cell differentiation and expressing osteoblast markers such as osteopontin, bone sialoprotein, osteocalcin and osteoblast-specific transcription factors (O'Brien et al. 1995; Rajamannan et al. 2003). Bone formation in cardiac valves has been associated with expression of bone morphogenetic proteins 2 and 4, as well as infiltration of B- and T-lymphocytes, in areas of ossification (Mohler et al. 2001). The expression of bone markers in degenerating aortic valves and the formation of cartilage in diseased mitral valves have been shown to involve activation of the lipoprotein-receptor-related protein 5 (Lrp5; Caira et al. 2006). Lrp5 is a co-receptor that binds to the secreted glycoprotein wnt and activates β-catenin, a pathway involved in bone formation.
Calcified valves have differential expression of RANKL (ligand for the receptor activator of NF-κB, RANK) and osteoprotegerin (OPG), both of which are members of the cytokine system that regulate bone turnover (Kaden et al. 2004a). Via the stimulation of RANK, RANKL induces osteoclastogenesis and has been shown to increase the DNA binding of osteoblast transcription factor cbfa-1, which is essential for osteoblast differentiation (Kaden et al. 2004a). Importantly, in human valve cells grown in osteogenic media, RANK/RANKL was shown to induce expression of cbfa-1. Interestingly, RANK, RANKL knockout mice and mice deficient in the p50 and p52 subunits of NF-κB all develop severe osteopetrosis (Franzoso et al. 1997; Dougall et al. 1999; Kong et al. 1999). The RANK/RANKL pathway is inhibited by expression of OPG, which serves as a soluble decoy receptor for RANKL to limit activation of RANK. Deletion of the OPG gene results in severe calcification of the vasculature and expression of RANK/RANKL in calcified areas. It has been shown that TNF-α is capable of increasing the responsiveness of RANKL, via induction of RANK (Nanes 2003). In addition, IL-1, IL-4, IL-6, TGF-β1, GM-CSF and IFN-γ have all been shown to affect the regulation of bone synthesis and reabsorption (Riancho et al. 1995; Deyama et al. 2001; Yeh et al. 2002; Postiglione et al. 2003).
There appear to be a number of different potential mediators that are involved in valve function and/or dysfunction. The reason why those mediators that are involved in valve development (BMPs, endothelin) are then implicated in valve disease in a later stage of life, or how multiple mediators can initiate the calcification process, is undefined. It is probable that there are a number of independent signalling pathways which either act in a cooperative manner or merely converge to induce phenotypic changes in valve cells. The precise molecular and cellular mechanisms, that either regulate valve development and function or valve dysfunction, are the subject of intense investigation by a number of groups.
3. Summary
The cells that reside in or on heart valve exhibit a host of unique characteristics and functions. These properties allow them to regulate the complex function of the heart valve by serving as a source of structural proteins or biological mediators. Valve cells also act as a target for biological mediators and enzymes, via the activation of complex signalling pathways. These signalling pathways can mediate both physiological and pathophysiological mechanisms. As our understanding of the properties and function of valve ICs and endothelial cell increases, we are becoming increasingly aware of the challenge that is presented by the need to tissue engineer a heart valve. Replication of valve function will be a fundamental requirement for the successful production of a tissue-engineered heart valve.
Acknowledgments
The author would like to thank Dr Hazel Screen of Department of Engineering Queen Mary, University of London for kindly supplying the photomicrograph used in figure 1.
Footnotes
One contribution of 21 to a Theme Issue ‘Bioengineering the heart’.
References
- Aikawa E, Whittaker P, Farber M, Mendelson K, Padera R.F, Aikawa M, Schoen F.J. Human semilunar cardiac valve remodeling by activated cells from fetus to adult: implications for postnatal adaptation, pathology and tissue engineering. Circulation. 2006;113:1344–1352. doi: 10.1161/CIRCULATIONAHA.105.591768. doi:10.1161/CIRCULATIONAHA.105.591768 [DOI] [PubMed] [Google Scholar]
- Arora P.D, McCulloch C.A. Dependence of collagen remodelling on alpha-smooth muscle actin expression by fibroblasts. J. Cell Physiol. 1994;159:161–175. doi: 10.1002/jcp.1041590120. doi:10.1002/jcp.1041590120 [DOI] [PubMed] [Google Scholar]
- Bairati A, DeBiasi S. Presence of a smooth muscle system in aortic valve leaflets. Anat. Embryol.(Berl.) 1981;161:329–340. doi: 10.1007/BF00301830. doi:10.1007/BF00301830 [DOI] [PubMed] [Google Scholar]
- Bertipaglia B, Ortolani F, Petrelli L, Gerosa G, Spina M, Pauletto P, Casarotto D, Marchini M, Sartore S. Cell characterization of porcine aortic valve and decellularized leaflets repopulated with aortic valve interstitial cells: the VESALIO Project (Vitalitate Exornatum Succedaneum Aorticum Labore Ingenioso Obtenibitur) Ann. Thorac. Surg. 2003;75:1274–1282. doi: 10.1016/s0003-4975(02)04706-9. doi:10.1016/S0003-4975(02)04706-9 [DOI] [PubMed] [Google Scholar]
- Bouma M.G, van den Wildenberg F.A, Buurman W.A. Adenosine inhibits cytokine release and expression of adhesion molecules by activated human endothelial cells. Am. J. Physiol. 1996;270:C522–C529. doi: 10.1152/ajpcell.1996.270.2.C522. [DOI] [PubMed] [Google Scholar]
- Brand N.J, Roy A, Hoare G, Chester A, Yacoub M.H. Cultured interstitial cells from human heart valves express both specific skeletal muscle and non-muscle markers. Int. J. Biochem. Cell Biol. 2006;38:30–42. doi: 10.1016/j.biocel.2005.06.018. doi:10.1016/j.biocel.2005.06.018 [DOI] [PubMed] [Google Scholar]
- Butcher J.T, Markwald R.R. Valvulogenesis—moving target. Phil. Trans. R. Soc. B. 2007;362:1489–1503. doi: 10.1098/rstb.2007.2130. doi:10.1098/rstb.2007.2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher J.T, Penrod A.M, Garcia A.J, Nerem R.M. Unique morphology and focal adhesion development of valvular endothelial cells in static and fluid flow environments. Arterioscler. Thromb. Vasc. Biol. 2004;24:1429–1434. doi: 10.1161/01.ATV.0000130462.50769.5a. doi:10.1161/01.ATV.0000130462.50769.5a [DOI] [PubMed] [Google Scholar]
- Caira F.C, et al. Human degenerative valve disease is associated with up-regulation of low-density lipoprotein receptor-related protein 5 receptor-mediated bone formation. J. Am. Coll. Cardiol. 2006;47:1707–1712. doi: 10.1016/j.jacc.2006.02.040. doi:10.1016/j.jacc.2006.02.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester A.H, Misfeld M, Yacoub M.H. Receptor-mediated contraction of aortic valve leaflets. J. Heart Valve Dis. 2000;9:250–254. [PubMed] [Google Scholar]
- Chester A.H, Misfeld M, Sievers H.H, Yacoub M.H. Influence of 5-hydroxytryptamine on aortic valve competence in vitro. J. Heart Valve Dis. 2001;10:822–825. [PubMed] [Google Scholar]
- Della R.F, Sartore S, Guidolin D, Bertiplaglia B, Gerosa G, Casarotto D, Pauletto P. Cell composition of the human pulmonary valve: a comparative study with the aortic valve—the VESALIO Project. Vitalitate Exornatum Succedaneum Aorticum labore Ingegnoso Obtinebitur. Ann. Thorac. Surg. 2000;70:1594–1600. doi: 10.1016/s0003-4975(00)01979-2. doi:10.1016/S0003-4975(00)01979-2 [DOI] [PubMed] [Google Scholar]
- Desmouliere A, Gabbiani G. Modulation of fibroblastic cytoskeletal features during pathological situations: the role of extracellular matrix and cytokines. Cell Motil. Cytoskeleton. 1994;29:195–203. doi: 10.1002/cm.970290302. doi:10.1002/cm.970290302 [DOI] [PubMed] [Google Scholar]
- Deyama Y, Takeyama S, Suzuki K, Yoshimura Y, Nishikata M, Matsumoto A. Inactivation of NF-kappaB involved in osteoblast development through interleukin-6. Biochem. Biophys. Res. Commun. 2001;282:1080–1084. doi: 10.1006/bbrc.2001.4693. doi:10.1006/bbrc.2001.4693 [DOI] [PubMed] [Google Scholar]
- Dougall W.C, et al. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999;13:2412–2424. doi: 10.1101/gad.13.18.2412. doi:10.1101/gad.13.18.2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreger S.A, Taylor P.M, Allen S.P, Yacoub M.H. Profile and localization of matrix metalloproteinases (MMPs) and their tissue inhibitors (TIMPs) in human heart valves. J. Heart Valve Dis. 2002;11:875–880. [PubMed] [Google Scholar]
- Durbin A.D, Gotlieb A.I. Advances towards understanding heart valve response to injury. Cardiovasc. Pathol. 2002;11:69–77. doi: 10.1016/s1054-8807(01)00109-0. doi:10.1016/S1054-8807(01)00109-0 [DOI] [PubMed] [Google Scholar]
- Fondard O, et al. Extracellular matrix remodelling in human aortic valve disease: the role of matrix metalloproteinases and their tissue inhibitors. Eur. Heart J. 2005;26:1333–1341. doi: 10.1093/eurheartj/ehi248. doi:10.1093/eurheartj/ehi248 [DOI] [PubMed] [Google Scholar]
- Franzoso G, et al. Requirement for NF-kappaB in osteoclast and B-cell development. Genes Dev. 1997;11:3482–3496. doi: 10.1101/gad.11.24.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilherme L, et al. Rheumatic heart disease: proinflammatory cytokines play a role in the progression and maintenance of valvular lesions. Am. J. Pathol. 2004;165:1583–1591. doi: 10.1016/S0002-9440(10)63415-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafizi S, Taylor P.M, Chester A.H, Allen S.P, Yacoub M.H. Mitogenic and secretory responses of human valve interstitial cells to vasoactive agents. J. Heart Valve Dis. 2000;9:454–458. [PubMed] [Google Scholar]
- Hurlstone A.F, et al. The Wnt/beta-catenin pathway regulates cardiac valve formation. Nature. 2003;425:633–637. doi: 10.1038/nature02028. doi:10.1038/nature02028 [DOI] [PubMed] [Google Scholar]
- Janin A, Konttinen Y.T, Gronblad M, Karhunen P, Gosset D, Malmstrom M. Fibroblast markers in labial salivary gland biopsies in progressive systemic sclerosis. Clin. Exp. Rheumatol. 1990;8:237–242. [PubMed] [Google Scholar]
- Johnson C.M, Hanson M.N, Helgeson S.C. Porcine cardiac valvular subendothelial cells in culture: cell isolation and growth characteristics. J. Mol. Cell Cardiol. 1987;19:1185–1193. doi: 10.1016/s0022-2828(87)80529-1. doi:10.1016/S0022-2828(87)80529-1 [DOI] [PubMed] [Google Scholar]
- Juliano R.L, Haskill S. Signal transduction from the extracellular matrix. J. Cell Biol. 1993;120:577–585. doi: 10.1083/jcb.120.3.577. doi:10.1083/jcb.120.3.577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaden J.J, et al. Interleukin-1 beta promotes matrix metalloproteinase expression and cell proliferation in calcific aortic valve stenosis. Atherosclerosis. 2003;170:205–211. doi: 10.1016/s0021-9150(03)00284-3. doi:10.1016/S0021-9150(03)00284-3 [DOI] [PubMed] [Google Scholar]
- Kaden J.J, et al. Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulate aortic valve calcification. J. Mol. Cell Cardiol. 2004a;36:57–66. doi: 10.1016/j.yjmcc.2003.09.015. doi:10.1016/j.yjmcc.2003.09.015 [DOI] [PubMed] [Google Scholar]
- Kaden J.J, et al. Expression and activity of matrix metalloproteinase-2 in calcific aortic stenosis. Z. Kardiol. 2004b;93:124–130. doi: 10.1007/s00392-004-1021-0. doi:10.1007/s00392-004-1021-0 [DOI] [PubMed] [Google Scholar]
- Komuro T. Re-evaluation of fibroblasts and fibroblast-like cells. Anat. Embryol.(Berl.) 1990;182:103–112. doi: 10.1007/BF00174011. doi:10.1007/BF00174011 [DOI] [PubMed] [Google Scholar]
- Kong Y.Y, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–323. doi: 10.1038/16852. doi:10.1038/16852 [DOI] [PubMed] [Google Scholar]
- Konttinen Y.T, Nykanen P, Nordstrom D, Saari H, Sandelin J, Santavirta S, Kouri T. DNA synthesis in prolyl 4-hydroxylase positive fibroblasts in situ in synovial tissue. An autoradiography-immunoperoxidase double labeling study. J. Rheumatol. 1989;16:339–345. [PubMed] [Google Scholar]
- Ku C.H, Johnson P.H, Batten P, Sarathchandra P, Chambers R.C, Taylor P.M, Yacoub M.H, Chester A.H. Collagen synthesis by mesenchymal stem cells and aortic valve interstitial cells in response to mechanical stretch. Cardiovasc. Res. 2006;71:548–556. doi: 10.1016/j.cardiores.2006.03.022. doi:10.1016/j.cardiores.2006.03.022 [DOI] [PubMed] [Google Scholar]
- Latif N, Sarathchandra P, Taylor P.M, Antoniw J, Yacoub M.H. Localization and pattern of expression of extracellular matrix components in human heart valves. J. Heart Valve Dis. 2005a;14:218–227. [PubMed] [Google Scholar]
- Latif N, Sarathchandra P, Taylor P.M, Antoniw J, Yacoub M.H. Molecules mediating cell-ECM and cell-cell communication in human heart valves. Cell Biochem. Biophys. 2005b;43:275–288. doi: 10.1385/CBB:43:2:275. doi:10.1385/CBB:43:2:275 [DOI] [PubMed] [Google Scholar]
- Lester W, Rosenthal A, Granton B, Gotlieb A.I. Porcine mitral valve interstitial cells in culture. Lab Invest. 1988;59:710–719. [PubMed] [Google Scholar]
- Merryman W.D, Youn I, Lukoff H.D, Krueger P.M, Guilak F, Hopkins R.A, Sacks M.S. Correlation between heart valve interstitial cell stiffness and transvalvular pressure: implications for collagen biosynthesis. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H224–H231. doi: 10.1152/ajpheart.00521.2005. doi:10.1152/ajpheart.00521.2005 [DOI] [PubMed] [Google Scholar]
- Messier R.H, Jr, Bass B.L, Aly H.M, Jones J.L, Domkowski P.W, Wallace R.B, Hopkins R.A. Dual structural and functional phenotypes of the porcine aortic valve interstitial population: characteristics of the leaflet myofibroblast. J. Surg. Res. 1994;57:1–21. doi: 10.1006/jsre.1994.1102. doi:10.1006/jsre.1994.1102 [DOI] [PubMed] [Google Scholar]
- Misfeld M, Morrison K, Sievers H, Yacoub M.H, Chester A.H. Localization of immunoreactive endothelin and characterization of its receptors in aortic cusps. J. Heart Valve Dis. 2002;11:472–476. [PubMed] [Google Scholar]
- Mohler E.R, III, Gannon F, Reynolds C, Zimmerman R, Keane M.G, Kaplan F.S. Bone formation and inflammation in cardiac valves. Circulation. 2001;103:1522–1528. doi: 10.1161/01.cir.103.11.1522. [DOI] [PubMed] [Google Scholar]
- Mulholland D.L, Gotlieb A.I. Cell biology of valvular interstitial cells. Can. J. Cardiol. 1996;12:231–236. [PubMed] [Google Scholar]
- Nanes M.S. Tumor necrosis factor-alpha: molecular and cellular mechanisms in skeletal pathology. Gene. 2003;321:1–15. doi: 10.1016/s0378-1119(03)00841-2. doi:10.1016/S0378-1119(03)00841-2 [DOI] [PubMed] [Google Scholar]
- O'Brien K.D, Kuusisto J, Reichenbach D.D, Ferguson M, Giachelli C, Alpers C.E, Otto C.M. Osteopontin is expressed in human aortic valvular lesions. Circulation. 1995;92:2163–2168. doi: 10.1161/01.cir.92.8.2163. [DOI] [PubMed] [Google Scholar]
- Osman L, Yacoub M.H, Latif N, Amrani M, Chester A.H. Role of human valve interstitial cells in valve calcification and their response to atorvastatin. Circulation. 2006;114:I-547–I-552. doi: 10.1161/CIRCULATIONAHA.105.001115. doi:10.1161/CIRCULATIONAHA.105.001115 [DOI] [PubMed] [Google Scholar]
- Pompilio G, Rossoni G, Sala A, Polvani G.L, Berti F, Dainese L, Porqueddu M, Biglioli P. Endothelial-dependent dynamic and antithrombotic properties of porcine aortic and pulmonary valves. Ann. Thorac. Surg. 1998;65:986–992. doi: 10.1016/s0003-4975(98)00075-7. doi:10.1016/S0003-4975(98)00075-7 [DOI] [PubMed] [Google Scholar]
- Postiglione L, Domenico G.D, Montagnani S, Spigna G.D, Salzano S, Castaldo C, Ramaglia L, Sbordone L, Rossi G. Granulocyte-macrophage colony-stimulating factor (GM-CSF) induces the osteoblastic differentiation of the human osteosarcoma cell line SaOS-2. Calcif. Tissue Int. 2003;72:85–97. doi: 10.1007/s00223-001-2088-5. doi:10.1007/s00223-001-2088-5 [DOI] [PubMed] [Google Scholar]
- Rabkin E, Aikawa M, Stone J.R, Fukumoto Y, Libby P, Schoen F.J. Activated interstitial myofibroblasts express catabolic enzymes and mediate matrix remodeling in myxomatous heart valves. Circulation. 2001;104:2525–2532. doi: 10.1161/hc4601.099489. [DOI] [PubMed] [Google Scholar]
- Rabkin-Aikawa E, Farber M, Aikawa M, Schoen F.J. Dynamic and reversible changes of interstitial cell phenotype during remodeling of cardiac valves. J. Heart Valve Dis. 2004;13:841–847. [PubMed] [Google Scholar]
- Rajamannan N.M, et al. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation. 2003;107:2181–2184. doi: 10.1161/01.CIR.0000070591.21548.69. doi:10.1161/01.CIR.0000070591.21548.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajamannan N.M, Subramaniam M, Caira F, Stock S.R, Spelsberg T.C. Atorvastatin inhibits hypercholesterolemia-induced calcification in the aortic valves via the Lrp5 receptor pathway. Circulation. 2005;112:I229–I234. doi: 10.1161/01.CIRCULATIONAHA.104.524306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riancho J.A, Gonzalez-Marcias J, Amado J.A, Olmos J.M, Fernandez-Luna J.L. Interleukin-4 as a bone regulatory factor: effects on murine osteoblast-like cells. J. Endocrinol. Invest. 1995;18:174–179. doi: 10.1007/BF03347799. [DOI] [PubMed] [Google Scholar]
- Sappino A.P, Schurch W, Gabbiani G. Differentiation repertoire of fibroblastic cells: expression of cytoskeletal proteins as marker of phenotypic modulations. Lab Invest. 1990;63:144–161. [PubMed] [Google Scholar]
- Saunders M.A, Mitchell J.A, Seldon P.M, Yacoub M.H, Barnes P.J, Giembycz M.A, Belvisi M.G. Release of granulocyte-macrophage colony stimulating factor by human cultured airway smooth muscle cells: suppression by dexamethasone. Br. J. Pharmacol. 1997;120:545–546. doi: 10.1038/sj.bjp.0700998. doi:10.1038/sj.bjp.0700998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura A.M, Luna R.E, Horiba K, Stetler-Stevenson W.G, McAllister H.A, Jr, Willerson J.T, Ferrans V.J. Immunohistochemistry of matrix metalloproteinases and their inhibitors in thoracic aortic aneurysms and aortic valves of patients with Marfan's syndrome. Circulation. 1998;98:II331–II337. [PubMed] [Google Scholar]
- Smith R.S, Smith T.J, Blieden T.M, Phipps R.P. Fibroblasts as sentinel cells. Synthesis of chemokines and regulation of inflammation. Am. J. Pathol. 1997;151:317–322. [PMC free article] [PubMed] [Google Scholar]
- Smith S. et al. In press. Force generation of different human cardiac valve interstitial cells: relevance to individual valve function and tissue engineering. J. Heart Valve Dis. [PubMed]
- Stanford S.J, Pepper J.R, Mitchell J.A. Cytokine modulation of granulocyte macrophage-CSF and granulocyte-CSF release from stimulated vascular smooth muscle cells. Eur. J. Pharmacol. 2002;436:241–244. doi: 10.1016/s0014-2999(01)01621-1. doi:10.1016/S0014-2999(01)01621-1 [DOI] [PubMed] [Google Scholar]
- Taylor P.M, Allen S.P, Yacoub M.H. Phenotypic and functional characterization of interstitial cells from human heart valves, pericardium and skin. J. Heart Valve Dis. 2000;9:150–158. [PubMed] [Google Scholar]
- Taylor P.M, Batten P, Brand N.J, Thomas P.S, Yacoub M.H. The cardiac valve interstitial cell. Int. J. Biochem. Cell Biol. 2003;35:113–118. doi: 10.1016/s1357-2725(02)00100-0. doi:10.1016/S1357-2725(02)00100-0 [DOI] [PubMed] [Google Scholar]
- Wassenaar C, Bax W.A, van Suylen R.-J, Vuzevski V.D, Bos E. Effects of cryopreservation on contractile properties of porcine isolated aortic valve leaflets and aortic wall. J. Thorac. Cardiovasc. Surg. 1997;113:165–172. doi: 10.1016/S0022-5223(97)70412-4. doi:10.1016/S0022-5223(97)70412-4 [DOI] [PubMed] [Google Scholar]
- Xing Y, He Z, Warnock J.N, Hilbert S.L, Yoganathan A.P. Effects of constant static pressure on the biological properties of porcine aortic valve leaflets. Ann. Biomed. Eng. 2004a;32:555–562. doi: 10.1023/b:abme.0000019175.12013.8f. doi:10.1023/B:ABME.0000019175.12013.8f [DOI] [PubMed] [Google Scholar]
- Xing Y, Warnock J.N, He Z, Hilbert S.L, Yoganathan A.P. Cyclic pressure affects the biological properties of porcine aortic valve leaflets in a magnitude and frequency dependent manner. Ann. Biomed. Eng. 2004b;32:1461–1470. doi: 10.1114/b:abme.0000049031.07512.11. doi:10.1114/B:ABME.0000049031.07512.11 [DOI] [PubMed] [Google Scholar]
- Yeh L.C, Zavala M.C, Lee J.C. Osteogenic protein-1 and interleukin-6 with its soluble receptor synergistically stimulate rat osteoblastic cell differentiation. J. Cell Physiol. 2002;190:322–331. doi: 10.1002/jcp.10064. doi:10.1002/jcp.10064 [DOI] [PubMed] [Google Scholar]