Abstract
Endothelial cells are critical mediators of haemodynamic forces and as such are important foci for initiation of vascular pathology. Valvular leaflets are also lined with endothelial cells, though a similar role in mechanosensing has not been demonstrated. Recent evidence has shown that valvular endothelial cells respond morphologically to shear stress, and several studies have implicated valvular endothelial dysfunction in the pathogenesis of disease. This review seeks to combine what is known about vascular and valvular haemodynamics, endothelial response to mechanical stimuli and the pathogenesis of valvular diseases to form a hypothesis as to how mechanical stimuli can initiate valvular endothelial dysfunction and disease progression. From this analysis, it appears that inflow surface-related bacterial/thrombotic vegetative endocarditis is a high shear-driven endothelial denudation phenomenon, while the outflow surface with its related calcific/atherosclerotic degeneration is a low/oscillatory shear-driven endothelial activation phenomenon. Further understanding of these mechanisms may help lead to earlier diagnostic tools and therapeutic strategies.
Keywords: shear stress, strain, gene, calcification, vegetations
1. Introduction
Heart valve disease is a significant contributor to cardiovascular deficiencies and a strong predictor of mortality (Trenouth et al. 1976; Schwarz et al. 1979; Lund et al. 1997; Mehta et al. 2001). Valvular disease in Western nations is predominantly of a degenerative (senile, calcific) nature (Passik et al. 1987; Dare et al. 1993), while in developing nations, rheumatic/bacterial heart disease dominates (Akinkugbe et al. 1991; da Silva & Pereira 1997). In addition, congenital heart defects, which affect 1% of all live births, can result in predispositions to these pathologies. Unfortunately, most valvular pathologies are diagnosed by echocardiography, which identifies structural deficiencies at an advanced state of disease progression (Baxley 1994; Heper & Yorukoglu 2002), often requiring surgical valve replacement with a non-living substitute valve. These procedures have greatly enhanced the length and quality of life in these patients, but patients eventually suffer from similar degenerative (calcific) and/or endocarditis (bacterial/thrombotic) events (Borkon et al. 1987; Oxenham et al. 2003).
Advances in vascular biology have contributed greatly to the discovery of vascular disease pathogenesis, but similar exploration of valvular pathology has lagged far behind. Research over the last 30 years has demonstrated that the most prevalent vascular disease, atherosclerosis, is initiated at specific regions of the vasculature identified by certain haemodynamic patterns (Baxley 1994; Heper & Yorukoglu 2002). Heart valves, particularly the aortic valve, experience the greatest and most complex stresses within the cardiovascular system, and we are now beginning only to appreciate the complex interactions between the native valvular cells that enable the leaflets to open and close 100 000 times a day for a lifetime (Schneider & Deck 1981; Deck et al. 1988).
Recent evidence is beginning to suggest that there may be very important roles for mechanics in the stimulation of valvular diseases. Certain valvular lesions develop preferentially on one side of the leaflet or the other, and these regions are exposed to vastly different mechanical forces. Endothelial cells line all blood contacting surfaces, including heart valve leaflets and large arteries. Endothelial cells have the ability to sense and respond to different mechanical and haemodynamic environments, and the ability to rapidly alter their behaviour with changes in these stimuli. Recent evidence has implicated systemic endothelial dysfunction in the initiation of pathology (Poggianti et al. 2003), but to date there has been little mechanistic connection to the haemodynamic environment. Some of our recent in vitro data suggest that valvular endothelial cells are in fact distinct from vascular endothelial cells in their response to haemodynamics, which suggests that these cells are uniquely designed to function on valvular leaflets (Butcher et al. 2004). It is therefore probable that local haemodynamics plays an important role in valvular endothelial biology and dysfunction.
The purpose of this review therefore is to combine what is known about valvular and vascular mechanics with the knowledge of valvular disease pathogenesis, paying particular attention to the role of valvular endothelium, and formulate a hypothesis as to how mechanics and haemodynamics could be acting on the valvular endothelium to both maintain normal function and initiate dysfunction. The review will conclude with an assessment of how this insight can be applied to future diagnostic and therapeutic agents.
2. Differences in haemodynamic environment of heart valves and large arteries
The blood flow patterns in the vasculature are determined by the complex interrelationship between cardiac function, the viscous nature of blood, the mechanical properties of the vessel walls and local geometry. Flow in the ascending aorta is just barely laminar (mean Reynolds number∼2000, peak∼4500) and highly pulsatile (Wormersley number∼15), but both these numbers gradually decrease further down the aorta (Caro et al. 1974).
Wall shear stress, considered the most important haemodynamic parameter for endothelial cells, defined as the gradient of flow velocity multiplied by viscosity, can be determined analytically for a Newtonian fluid and straight tubular arteries from the following equation:
Here, Q is the volumetric flow rate, μ the viscosity, and R the vessel radius (Poiseuille flow). The mean wall shear stress in a large artery is believed to be within a remarkably small range (10–20 dynes cm−2). Actual wall shear stresses are much more difficult to measure, especially at the level of the valve. Local changes in geometry can dramatically increase or reduce shear stresses, notably apparent at stenoses (increase) and bifurcations (reduce). Shear stresses on the surface of valve leaflets are much harder to measure. Reports of peak wall shear stress vary from 20 dynes cm−2 to over 1000 dynes cm−2 (Walburn & Stein 1984; Woo & Yoganathan 1985; Nandy & Tarbell 1988; Einav et al. 1990; Weston et al. 1999). Part of the reason for the differences in these measurements can be explained by the differences in how far downstream of the valve the measurements were made, what type of flow in which the valve was functioning and whether the valve was natural or bioprosthetic. It is known that haemolysis occurs at shear stresses in excess of approximately 400 dynes cm−2 (Paul et al. 2003), and conservative analytical solutions in steady flow suggest a lower bound of 20 dynes cm−2 for peak shear stress. Therefore, it is probable that the peak shear stress on the inflow surface of valve leaflets are in the range of 100–200 dynes cm−2. The inflow surface experiences these extremely high shear stresses only for a very brief period of the cardiac cycle, and it is unclear how significant this peak in shear stress is. If the blood flow velocity through the valve is averaged across the cardiac cycle (aka steady flow), then the resulting shear stress is much less, approximately 20 dynes cm−2 (Weston et al. 1999). It is important to note that the velocity gradients are very different between the two sides of a leaflet. While the inflow surface experiences a strong pulsatile unidirectional shear stress, the outflow surface experiences a much lower recirculating shear stress. The exact measurement of these shear stress profiles will require more advanced measurement techniques and rigorous fluid–structure coupled computational models, the foundation of which has already been laid (Aluri & Chandran 2001; Leuprecht et al. 2002).
In addition to the wall shear stress, there are other components to the mechanical environment of the valve leaflet. These include normal pressure stress and mostly planar tissue strains. Ventricular and aortic pressure waves are different in that the aorta remains pressurized at all times, while ventricular pressure reduces to near zero in diastole. This creates different pressure profiles for valvular leaflets and large arteries. While transaortic pressures range from 80 to 120 mm Hg, transvalvular pressure ranges from approximately 0 to 80 mm Hg. Maximum pressure stress in the aorta occurs in systole, whereas in leaflets it is during diastole, making tissue strains out of phase between these two locations. Strain in both tissues is a function of both this pressure pulse and the motions of adjacent structures. Measurements of the motions of aortic segments in vivo have determined that vascular tissues experience pulsatile circumferential strains of the order of 10%, with minimal axial and radial strains (Holzapfel & Weizsacker 1998; Wedding et al. 2002; Draney et al. 2004; Stalhand et al. 2004). Valvular leaflets, in contrast, are strained in a more complicated biaxial pattern. Peak circumferential strains are approximately 10%, while radial strains are as high as 40% (Thubrikar et al. 1980, 1986a). These strains are also imposed at very high rates (200% s−1) in valvular leaflets (Brewer et al. 1977). In addition to planar strains, leaflets are also bent to very high radii of curvature during opening, which results in an additional compressive strain in the fibrosa and tensile strain in the ventricularis (Smith et al. 2000). Thus, the endothelium on the two sides of the leaflet experiences a very different general strain pattern. It is also known that both valvular and vascular tissues have residual strains in unloaded conditions, both of the order of 10% (Vaishnav & Vossoughi 1987; Vesely et al. 1993). It is not yet known whether these residual tissue strains correspond to states of strain in the cells. The surface strains on both arteries and valve leaflets are not homogeneous, but vary between leaflet and around the aortic circumference (Dagum et al. 1999). Indeed, local changes in geometry will again have an important effect on the magnitude of strains felt by the endothelial cells. Thubrikar and colleagues also demonstrated that the aortic sinus plays an important role in shielding stress accumulation in valve leaflets and will also have different effects on the ventricularis and fibrosa (Thubrikar et al. 1986b).
3. Response of endothelial cells to mechanical environment
(a) Morphology
Endothelial cell shape varies spatially throughout the vascular tree, even between adjacent cells, and much work over the last 30 years has demonstrated a strong correlation between endothelial cell shape and the mechanical environment. In vivo, vascular endothelial cells resemble elongated ellipsoids, with the long axis of the cells aligned parallel to the direction of flow (Wong et al. 1983). At sites of bifurcations and high curvatures, vascular endothelial cells appear to be larger and more rounded (Langille & Adamson 1981; Kim et al. 1989). In vitro experimentation has shown a remarkable consistency of endothelial shape change to different patterns of shear stress for a variety of arterial and venous locations, while both pulsatile and steady laminar shear stresses result in elongated highly aligned cells. For a purely oscillatory or turbulent shear stress, the monolayer is composed of rounded cell bodies with no discernable alignment (Helmlinger et al. 1991). In relation to underlying cells and matrix, vascular endothelial alignment is parallel to underlying matrix orientation, but switches to parallel to the flow direction once shear stress is applied regardless of matrix orientation (Imberti et al. 2002).
Scanning electron microscopy of valvular endothelial cell alignment shows, however, a remarkably different pattern. Valvular endothelial cells appear to be aligned with their long axis parallel to the circumferentially aligned underlying tissue, and thus perpendicular to the direction of flow (Deck 1986). Both the ventricular and aortic surfaces are aligned in this manner, but in some cases, the middle portion of the aortic surface contains rounded cells without discernable alignment (Inai et al. 2004). Recent in vitro evidence indicates that valvular endothelial cells align perpendicular to flow even without the presence of an aligned substrate (Butcher & Nerem 2006). Similar to vascular endothelial cells, this response was dependent on changes in cytoskeletal actin filaments and resulted in prominent stress fibres along the long axis of the cell (Butcher et al. 2004).
Endothelial cells also respond morphologically to substrate strain patterns. In vitro experiments have shown that vascular endothelial cells align with their major axis perpendicular to the principal axis of stretch (Terracio et al. 1988; Sipkema et al. 2003; Moretti et al. 2004). Equibiaxial strain of vascular endothelial cells results in a rounded endothelial cell shape with a tent-like cytoskeletal actin structure (Gorfien et al. 1990). To date, no in vitro data exist on the morphological response of valvular endothelial cells to substrate strain.
Interestingly, the combination of tissue strain and fluid shear stress may result in the endothelial cell morphologies observed in vivo. Since blood vessels experience mainly a circumferential tissue strain, vascular endothelial cells would align perpendicular to this stimulus, which is also parallel to the direction of blood flow. In valvular endothelial cells, however, the principal strain direction is radial, and cells would align perpendicular to this, or circumferentially. This alignment would be perpendicular to the direction of flow. The in vivo situation is much more complex than this, with matrix strains and fluid shear stresses often out of phase with each other. While almost all in vitro endothelial cell morphology studies involved only one mechanical stimulus, the interplay between strain and shear stress in endothelial biology may be as important as their effects individually (Wang et al. 2001), and the fact that this interplay may unify the seemingly divergent morphological responses seen by these two different types of endothelial cells warrants further study.
(b) Gene and protein expression
In addition to morphological changes, endothelial cells respond to both mechanical strain and fluid shear stress by the modulation of gene and protein expression. The regulation of gene and protein expression in endothelial cells is dependent on the type, magnitude and duration of mechanical stimulus and, to a lesser extent, the vascular location of the cells of interest. The now widespread use of microarray technology has caused a dramatic increase in information in this field. While the expression of thousands of genes can be assayed at once, the differences in microarray platform, cell types, mechanical stimuli, duration and statistical analysis make integration of this information truly difficult. Endothelial cells modulate many genes and proteins in reacting to a change from residing in a static culture environment to one with flow, and these transient expression changes may be of little relevance to in vivo endothelial biology. Therefore, only phenotype modulation to a more ‘chronic’ duration of shear stress will be discussed in this review. Using morphology as an indicator, vascular endothelial alignment is largely completed by 24 h, while valvular endothelial alignment is completed by 48 h. An analysis of several microarray studies using vascular endothelial cells isolated from different regions reveals many genes modified by similar mechanical stimuli. Those genes for which confirmational data (Western, RT-PCR) exist are summarized in table 1. It appears that the effects of pulsatile and steady laminar shear are similar in terms of genes changed, while oscillatory and disturbed (turbulent) flows modulate gene expression similarly. Cyclic stretch, however, modulates some genes in a manner similar to unidirectional flow and others like disturbed flow. Unidirectional shear stress downregulates adhesion proteins (VCAM-1 and PECAM-1) and chemokines (IL-1β and IL-8) and upregulates redox proteins (CYP1A1, CYP1B1 and Cu/ZN-SOD) and growth factors (TGFβ and VEGF). Oscillatory or turbulent shear stress has the opposite effect, upregulating adhesion proteins and chemokines. Steady and pulsatile shear stresses therefore seem to promote an atheroprotective phenotype, while oscillatory or turbulent shear stress promotes an atherogenic phenotype. However, the effects of mechanical strain are much more complicated, indicating both atheroprotective- and atherogenic-like responses.
Table 1.
Endothelial protein expression in response to chronic shear stress profiles. (see table 3 in the electronic supplementary material for references and full gene names)
| category | steady | pulsatile | oscillatory | turbulent | strain |
|---|---|---|---|---|---|
| adhesion | |||||
| ICAM-1 | down | down | up | up | up |
| VCAM-1 | down | down | up | up | up |
| E-selectin | down | ? | up | up | up |
| MCP-1 | down | down | up | ? | up |
| PECAM (CD31) | up | up | ? | ? | ? |
| coagulation | |||||
| tissue factor | down | down | up | ? | up |
| t-PA | up | up | ? | n.s. | up |
| thrombomodulin | down | down | n.s. | down | |
| PAI-1 | down | down | ? | n.s. | up |
| TF pathway inhibitor | up | up | down | ? | ? |
| TSP-1 | down | down | up | up | up |
| redox | |||||
| CYP1A1 | up | up | ? | ? | up |
| CYP1B1 | up | up | ? | ? | up |
| Cu/Zn-SOD | up | up | n.s. | n.s. | ? |
| NADPH | n.s. | up | up | ? | up |
| growth | |||||
| bFGF | n.s. | n.s. | n.s. | n.s. | n.s. |
| TGFβ1 | up | up | up | up | ? |
| VEGF | up | up | down | ? | up |
| vasoactivity | |||||
| eNOS | up | up | n.s. | n.s. | up |
| ET-1 | down | down | up | down | up |
| prostacyclin | n.s. | n.s. | ? | ? | up |
| extracellular matrix | |||||
| BMP-4 | down | down | up | ? | ? |
| MMP-9 | n.s. | up | up | ? | up |
| collagen IV | up | up | up | ||
| MMP-2 | n.s. | up | ? | ? | up |
| chemokine | |||||
| IL-8 | down | down | up | up | up |
| IL-13 | down | down | up | up | up |
| other | |||||
| Kruppel-like lung factor | up | up | ? | ? | ? |
| Cxn 43 | up | up | down | down | up |
| CxN 37 | up | up | down | down | n.s. |
To begin to ascertain similarities and differences between vascular and valvular endothelial cell response to shear stress, we performed a cDNA microarray analysis of cultured porcine aortic and aortic valve endothelial cells exposed to 20 dynes cm−2 steady laminar shear stress for 48 h, with static cultures serving as controls (figure 1). This shear stress level was determined based on the mean shear stress across the aortic valve averaged across the cardiac cycle as determined by Weston et al. (1999).
Figure 1.
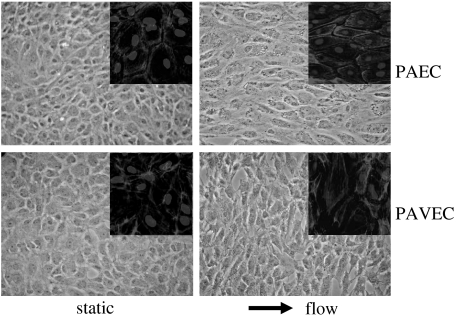
Valvular and vascular endothelial cell morphology in static and fluid flow environments. Insets depict F-actin filament organization (light grey) counterstained for cell nuclei (dark grey) at 40×. Phase contrast images at 20× magnification. PAEC, porcine aortic endothelial cells; PAVEC, porcine aortic valve endothelial cells.
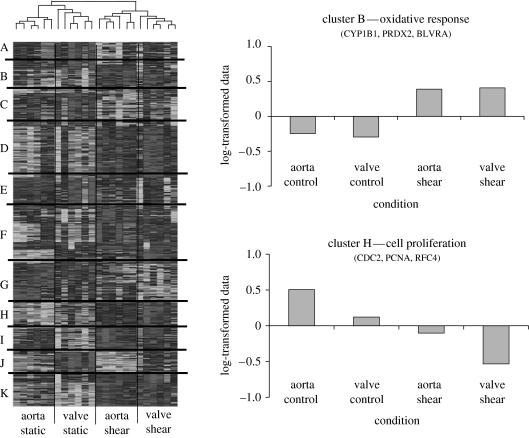
Based on our morphological studies, valvular and vascular endothelial cells complete their morphological alignment to flow by 48 h, and thus the gene profile is more likely to represent a more ‘chronic’ shear response (Butcher et al. 2004). Although not perfectly mimicking the native wall shear stress flow condition, this profile can serve as a reasonable approximation. The microarray results were analysed statistically (Butcher et al. 2004) and clustered according to similarity in expression patterns. Some results of this analysis are shown in figure 2. Eleven different groups of genes were found to have similar expression levels across the shear stress and cell type conditions. Surprisingly, when the individual arrays were clustered according to similar gene expression profiles, all of the arrays representing a particular condition were grouped together, suggesting a repeatable and unique gene expression profile. It appears that shear stress condition was more of a distinguishing factor than cell type, but several expression groups show potentially important differences in regulation. The expressions of several genes regulated by shear stress for both vascular and valvular endothelial cells are given in table 2.
Figure 2.
Heatmap rendering of the clustered microarray data of valvular and vascular endothelial cells in static and fluid flow environments. Eleven different groups with unique expression profiles are identified A–K. Normalized log-transformed mean values for each cluster are plotted across conditions.
Table 2.
Gene expression in valvular and vascular endothelial cells in response to steady laminar shear stress. (U, upregulation; D, downregulation; s.d., standard deviation)
| gene | aortic valve EC | aortic EC | ||||
|---|---|---|---|---|---|---|
| fold | s.d. | p value | fold | s.d. | p value | |
| VCAM-1 | D 1.25 | 0.18 | 0.003 | D 1.06 | 0.15 | 0.732 |
| Cu/Zn SOD | U 1.70 | 0.17 | 0.007 | U 1.44 | 0.14 | <0.001 |
| CYP1A1 | U 8.38 | 1.27 | <0.001 | U 9.33 | 2.87 | <0.001 |
| eNOS | U 1.18 | 0.22 | 0.06 | U 1.14 | 0.18 | 0.162 |
| Cxn 37 | D 1.25 | 0.09 | 0.07 | D 1.29 | 0.12 | 0.17 |
| BMP-4 | D 2.05 | 0.02 | <0.001 | D 2.07 | 0.91 | <0.001 |
(c) Mechanotransduction
The responses of endothelial cells to different mechanical stimuli (shear stress, strain and combinations) are the results of complex signal cascades that initiate within the first seconds of stimulations. While mechanotransduction pathways are numerous and probably redundant, specific endothelial responses to shear stress can be traced to specific pathways. Endothelial cells can sense shear stress through several ways: phosphorylation of receptors that are recruited and/or clustered on the flow surface (Takahashi et al. 1997); changes in focal adhesion organization on the basal surface (Shyy & Chien 2002); and by changes in local strain patterns within individual cells (Helmke et al. 2001). While numerous reviews exist detailing vascular endothelial mechanotransduction to shear stress, it is unclear whether these pathways are similarly followed in valvular endothelial cells. We used focal adhesion arrangement and morphological alignment as an indicator of response, and compared the response of valvular and vascular endothelial cells with shear stress in the presence of inhibitors to prominent signal pathways (Butcher et al. 2004). Both valvular and vascular endothelial morphological alignment is preceded by reorganization of focal adhesion proteins, including β1 integrin, vinculin and focal adhesion kinase (figure 3, arrows), yet the pattern of reorganization is somewhat different between the two types of cells. Plaques in both cells are localized prominently at the ends of the long axis of the cells, but this orientation is parallel to flow in vascular endothelium and perpendicular in valvular endothelial cells.
Figure 3.
Mechanotransduction differences between valvular and vascular endothelial cells. Valvular endothelial cells align perpendicular to flow independent of PI-3 kinase, which is required for vascular endothelial cell alignment. Alignment of both endothelial cells is dependent on focal adhesion rearrangement. Modified from Butcher et al. (2004).
We found that morphological alignment is dependent on the action of Rho-kinase, phosphoinositide-3 kinase (PI-3 kinase) and calpain in vascular endothelial cells. Valvular endothelial cells require Rho-kinase and calpain, but not PI-3 kinase to align perpendicular to the direction of flow (figure 3). Disruption of the aforementioned pathways also inhibited focal adhesion reorganization. These results suggest that valvular endothelial cells share some, but not all mechanotransduction pathways with vascular endothelial cells. Other researchers have shown the importance of Rho-kinase in mediating vascular endothelial cell polarization, but not PI 3-kinase (Wojciak-Stothard & Ridley 2003). PI 3-kinase, on the other hand, is critical to the production of nitric oxide (Zeng & Quon 1996; Wojciak-Stothard & Ridley 2003). Calpain is a cystein protease important for the breakdown and reorganization of focal adhesion proteins, particularly focal adhesion kinase.
4. Physiological roles of valvular endothelial cells
Endothelial cells perform many functions critical for blood and tissue homeostasis. Since all blood vessels and heart valves are lined with a surface layer of endothelial cells, it is probable that several functions are conserved regardless of location along the vascular tree. Physiological roles of endothelial cells can be broadly separated into three categories: regulation of coagulation; regulation of underlying cells/tissue; and transmittance/clearance of agents from the blood. Each of these topics will be discussed in context of valvular endothelial cell function.
(a) Regulation of coagulation
Endothelial cells are the primary means of controlling coagulative responses to ensure free-flowing blood in normal function and clotting only during injury. The coagulation regulation pathway is truly complex, with a myriad of factors, catalysts and antagonists, allowing clotting kinetics to be precisely regulated. Both valvular and vascular endothelial cells express von Willebrand factor (vWF; Manduteanu et al. 1988), secrete tissue factor (TF; Drake & Pang 1989) and seem to modulate expression of coagulative factors in response to shear stress (table 2). Valvular endothelial cells, like vascular EC, are normally non-thrombogenic, but secrete TF when stimulated by inflammatory cytokines or lipopolysaccharide. Valvular interstitial cells, on the other hand, express high levels of TF constitutively (Drake & Pang 1989). It is not clear whether important differences exist in the coagulative states of valvular and vascular cells in vivo, but a study by Welters et al. (1998) showed that some coagulation factors are upregulated during coronary artery bypass graft surgeries compared with during aortic valve repair, suggesting that ECs from these vessels are in a more hypercoagulative state. Platelet adhesion is enhanced at higher shear rates, and vWF has been shown to unfold in the presence of high shear stress, promoting cleavage by ADAMTS13 and subsequent adhesion of platelets and fibrin (Tsai 2003). As discussed earlier, the inflow surfaces of valve leaflets, particularly the aortic valve, experience very high shear stresses, which may enhance coagulation if there is endothelial denudation. Valvular endothelial cells may be conditioned for survival in a higher shear environment in vivo in comparison with vascular endothelial cells, and may therefore resist denudation and the secretion of coagulative factors.
(b) Regulation of underlying cells
Both valvular and vascular endothelial cells have a basal lamina separating them from underlying cells (Manduteanu et al. 1988), but the composition of the underlying cells is very different between the two tissues. Much is known about vascular medial wall cells, which are almost exclusively smooth muscle in nature in arteries, expressing contractile proteins such as α-smooth muscle actin, calponin and myosin heavy chain (Stegemann & Nerem 2003; Stegemann et al. 2004). Valvular interstitial cells, on the other hand, are much more fibroblastic in nature, expressing only a small amount of these markers in a subendothelial section of the ventricularis in vivo (Cimini et al. 2003). The subendothelial matrix composition is also different between vascular and valvular tissues. While vascular tissues contain a lamellar network of circumferentially aligned elastin and smooth muscle cells for much of the medial wall, valvular tissues contain a somewhat lamellar elastin–myofibroblast network, but only on the ventricularis. The fibrosa contains large collagenous bundles that are mostly circumferentially aligned (Vesely 1998). It is well known that the vascular endothelium regulates blood vessel lumen size by secretion of vasoactive agents such as nitric oxide (dilator) and endothelin (constrictor). Studies using fistulas and vein grafts have documented the ability of vascular endothelial cells to sense fluid flow and secrete agents in order to maintain a specific range of wall shear stress (Cambria et al. 1994). A similar role for valvular endothelial cells has yet to be demonstrated in vivo. Valvular endothelial cells and excised leaflets respond to vasoactive agents by alterations in calcium currents and the production of prostaglandin (Laskey et al. 1994) and can contract and dilate in vitro (Pompilio et al. 1998; Bowen et al. 2004). These contractions can be inhibited by blocking specific agonist receptors, suggesting innate capability and vasoactive feedback mechanisms. Interstitial cells have been shown to contract and relax in culture when stimulated with these agents (Messier et al. 1994) in vitro, suggesting that valvular interstitium has the capability to modulate tissue dimensions. Different regions of the aortic valve are more responsive to these agents than others, suggesting the potential for a complex signal regulation. However, a connection to mechanical or fluid dynamic stimuli is still controversial. In vitro experiments similar to those conducted with vascular endothelial cells may help answer this question.
In addition to vasoactivity, vascular endothelial cells have been shown to promote a quiescent smooth muscle cell phenotype by inhibiting cell proliferation and matrix synthesis, and maintenance of smooth muscle markers. In vitro and in vivo evidence has demonstrated that this regulation is optimized when endothelial cells are subjected to unidirectional laminar shear stress. Conversely, valvular interstitial cells are considered quiescent when not expressing smooth muscle markers (Rabkin et al. 2001) and evidence suggests that matrix synthesis and remodelling occurs at an increased constitutive rate in aortic valve leaflets (Schneider & Deck 1981). Valvular endothelial denudation does stimulate interstitial cell proliferation in organ cultures similar to what is seen in vascular endothelial denudation (Lester et al. 1992). We recently conducted a study assessing the effects of steady laminar shear stress in valvular endothelial–interstitial cell interactions in a tissue-engineered valvular leaflet model (Butcher & Nerem 2006). We report that valvular endothelial cells inhibit interstitial cell proliferation, while increasing matrix synthesis, and that these effects are enhanced with shear stress. These data suggest that mechanical factors play a role in the valvular endothelial cell regulation of interstitial cell biology.
(c) Transmittance/clearance of agents from the blood
In addition to coagulative factors, nutrients and molecular stimulants circulate within the blood, and another major role of endothelial cells is the interpretation and response to these agents. Experiments have determined that the diffusion limits through tissues is approximately 250 μm, which is within the range of small blood vessels and most of the valve leaflet. It appears that hypoxic conditions stimulate angiogenesis in both vessels and leaflets, but almost no blood vessels are present in normal valve leaflets (Tompkins et al. 1989). Endothelial cells are also responsible for clearing circulating lipids from the bloodstream, namely low-density lipoproteins (LDL). Experiments by Tompkins et al. demonstrated that valvular endothelium has an increased permeability than vascular endothelium, but their permeability to LDL seems to be much lower, suggesting that LDL concentration in aortic valve leaflets is near saturated (Tompkins et al. 1989). These results suggest that valvular endothelial cells may participate differently than vascular endothelial cells in these processes.
5. Mechanical basis for valvular pathology
The primary concern for researchers studying endothelial biology is to determine mechanisms of and treatment for pathologies affecting these tissues. Atherosclerosis and the associated coronary artery disease is the leading cause of death in the Western world (AHA 2001). Research over the last 30 years has determined that atherosclerosis is an inflammatory disease that is localized to regions of turbulent and/or oscillatory shear stress, namely arterial branches, inner curvatures and sinuses. In vitro research has implicated endothelial dysfunction as a principal initiator. Increased oxidative stress stimulates endothelial activation characterized by the expression of monocyte adhesion receptors (VCAM-1, ICAM-1 and E-selectin), and this results in the recruitment of circulating monocytes and leukocytes. These cells then cross the endothelial monolayer, transdifferentiate into macrophages and foam cells and attempt to ingest accumulating lipids. These cells also secrete cytokines that stimulate smooth muscle cell differentiation, matrix synthesis and proliferation. Advanced lesions are also associated with necrotic cell cores, angiogenesis and unstable fibrous caps that can spontaneously rupture, leading to thromboemboli and local tissue infarction. Vascular calcification is also correlated with atherosclerosis, especially in older patients (Allison & Wright 2005) and patients with hypercalcaemia or hyperphosphataemia (Nishizawa et al. 2004).
Valvular pathology can be primarily separated into two classes: sclerotic degeneration and vegetative endocarditis. Sclerotic degeneration bears a striking similarity to atherosclerosis. Valvular endothelial cells in diseased valves appear activated, with increased expression of VCAM-1, ICAM-1 and E-selectin (Müller et al. 2000). Infiltration of foam cells also appears to be concomitant with endothelial defenestration (Sarphie 1985). Vegetative endocarditis is somewhat unique to valvular leaflets and can be either bacterial or non-bacterial in nature. Generally, endothelial dysfunction and focal defenestration causes upregulation of coagulation factors, which stimulates platelet adhesion and synthesis of fibrin. Circulating bacteria can preferentially adhere to these regions (Allen et al. 2002) and proliferate under the protection of additional clot formation (Sullam et al. 1985).
What has not been investigated mechanistically is the role of mechanical forces in stimulating these events in valvular tissue. Since mechanical factors are strong initiators of the inflammatory cascade in vascular endothelium, it is probable that valvular endothelial cells can also be stimulated by abnormal haemodynamics and tissue mechanics. Based on the differences in haemodynamic environment between the surfaces of the valve leaflet and observations of pathogenesis on these surfaces, the following hypothesis is proposed for the role of mechanical forces in the development of valvular pathology. This hypothesis is discussed below and diagrammed in figure 4 and split into two portions: inflow and outflow surfaces. Although the aortic valve will be considered as the model tissue for simplicity, much of the pathology is observed similarly in the mitral valve.
Figure 4.
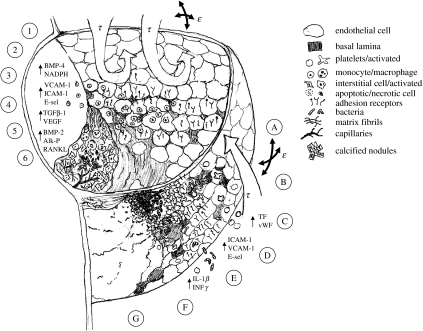
Schematic illustrating the potential roles for mechanical forces in the initiation of valvular pathologies. The inflow surface experiences high-magnitude pulsatile shear stresses and is the predominant location for thrombotic/bacterial endocarditis (A–G). The outflow surface experiences much lower, oscillatory shear stresses and is the predominant location for sclerotic/calcific lesion formation (1–6).
(a) Inflow surface and endocarditis
Investigation of endocarditis on valve leaflets shows a strong preference to the inflow surfaces of valves (Harasaki et al. 1978; Corcoran et al. 2004), where high pulsatile shear stresses dominate. Several stages, labelled A to G in figure 4 and now described, depict the progression of this pathology in relation to the aforementioned mechanical environment and endothelial response. (A) Normal blood flow in this region is strongly unidirectional, pulsatile, and just barely laminar. Any increase in pressure or viscosity, as would be the case in hypertensive or hyperlipidemic conditions, could very easily push the flow into the turbulent regime. (B) This transition would dramatically increase already high shear stresses in the vicinity of the leaflet. These shear stresses could cause either injury/denudation of the valvular endothelial cells and/or platelet rupture. (C) Platelets and platelet particles could then adhere to the valve inflow surface, especially in regions of denudation (Allen et al. 2002; Leask et al. 2003 for references). Studies show that platelet adhesion is actually enhanced at higher shear rates, which would most probably occur at the leaflet-free edge for their proximity to maximum bloodstream velocities. Constitutively secreted and matrix-associated TF and vWF would then be released to the bloodstream, further activating the coagulation cascade. (D) The surrounding endothelium will become activated in the presence of the platelets and coagulation, upregulating receptors for circulating cells (VCAM-1, ICAM-1 and E-selectin). (E) In the case of bacterial endocarditis, circulating bacteria adhere very well to the fibrin–platelet matrix. Bacterial survival is promoted through endothelially derived coagulation factor stimulation of additional clot formation, which would protect the growing bacterial vegetations. (F) The micro-organisms would then begin to breakdown the underlying matrix through secretion of matrix metalloproteinases (Edep et al. 2000; Soini et al. 2001), causing localized tissue damage and cell death. (G) Eventually, the growing vegetations would cover most of the leaflet surface, and the disorganized fibrous healing associated with cell-mediated remodelling (Moreillon et al. 2002) would cause leaflet stiffening and thickening, leading to cell death from diffusion limitations and/or angiogenic responses. The patient would experience increasing stenosis from the constricting annulus and regurgitation from the inability of vegetated leaflets to coapt. Non-bacterial thrombotic endocarditis (NBTE) works in a similar way, except bacterial lesions are not present. In this case, the thrombotic vegetations would increase with the clot, adding mass to the leaflets, altering coaptation and stimulating pathological tissue remodelling, which would result in a similar conclusion.
(b) Outflow surface and degenerative sclerosis
The progression of outflow-related pathology is next described and diagrammed in figure 4 through stages labelled 1 to 6. (1) The outflow surface of valves experiences a much lower magnitude, recirculating flow regime, similar to branching regions in the aorta known to cause vascular sclerosis. Porat et al. mapped the location of the Tie-1 promoter in the cardiovascular system and found that it was colocalized with bifurcations and inner curvatures, and strikingly only on the outflow surfaces of the valves (Porat et al. 2004). (2) Valvular endothelial cells on the outflow surface of valves sometimes appear larger and more rounded (Sarphie 1982, 1985), similar to the endothelial morphology in atherogenic vascular regions. Low shear oscillatory blood flow on the fibrosa surfaces would result from poor drainage of blood into the coronary ostia, as would be the case if there is increased resistance from these vessels due to stenotic occlusion. (3) Valvular endothelial cells become activated in response to this flow pattern, upregulating receptors for monocytes, leukocytes and T-cells (VCAM-1, ICAM-1 and E-selectin; Ghaisas et al. 2000). This activation could be preceded by increases in oxidative stress as mediated by inflammatory agents such as BMP-4, as is the case with vascular endothelial cells in oscillatory shear. (4) Adherent leukocytes also secrete inflammatory cytokines such as IL-1β and INFγ, which induce endothelial cell apoptosis, interstitial cell proliferation and secretion of MMP-1 and MMP-2 (Kaden et al. 2003) and these begin to degrade the collagen and elastin network. In hypercholesteremic rabbits, the non-flow surface contains many endothelial fenestrations and caveolae, promoting cell infiltration (Sarphie 1982). Increase in oxidative factors further promotes cell apoptosis and oxidation of LDL (Prasad & Bhalodkar 2004). Endothelial denudation also stimulates interstitial cell proliferation and matrix synthesis concomitant with a wound response. This thickening in turn reduces the ability of valve leaflets to be fed through diffusion, resulting in hypoxia-induced cell necrosis and/or angiogenesis (Chalajour et al. 2004). (5) The resulting necrotic cell fragments can then be nucleating sites for calcification nodules (Müller et al. 2000). Calcification appears to be localized to regions of endothelial denudation (Müller et al. 2000), and is further propagated by the expression of BMP-2 and TGFβ1 from interstitial cells and macrophages (Mohler et al. 1999). TGFβ1 can also induce endothelial transdifferentiation to an activated mesenchymal phenotype as evidenced by the expression of α-smooth muscle actin. An activated interstitial cell phenotype may contribute to tissue contraction and stiffening. TGFβ1-induced expression of RANKL may also enhance calcification and stimulate pathological angiogenesis. (6) Each of these positive feedback loops contributes to irreversible tissue thickening and stiffening, concomitant with the upregulation of angiogenic factors (VEGF) in regions of hypoxia, causing increasingly pronounced stenosis and/or regurgitation.
In addition to haemodynamic forces, alterations in tissue strains can induce pathological endothelial activation. As shown in table 2, vascular endothelial cells respond to stretch by expressing both atherogenic and atheroprotective proteins. It is currently unknown what types of proteins valvular endothelial cells express in response to stretch. Types of strain, magnitude, frequency and duration have all been shown to be independent stimulators of cell function. Mechanical strain can also interact with fluid shear stress synergistically. Endothelial cells subjected to uniaxial stretch and fluid shear stress respond differently when these stimuli are applied at different phases (Dancu et al. 2004). If they are in phase with each other, cells respond by increasing production of eNOS and COX-2 and decreasing ET-1, thus exhibiting an atheroprotective phenotype. If these stimuli are 180° out of phase, the opposite response occurs. Peak fluid shear stress and tissue strains are commonly out of phase in valvular leaflets, but it is unclear if valvular cells respond similarly to different patterns of stimuli.
6. Roles for tissue engineering
The importance of appropriate valvular endothelial cell phenotype is also of critical importance in many if not all valvular replacement strategies. Clinical results involving pulmonary autograft (Ross procedure) have demonstrated long-term effectiveness in adults as well as children, representing the gold standard for future valve replacements (Affonso da Costa et al. 1998; Oury et al. 1998b). These valves contain native tissue structure and appropriate cell phenotypes, which are undoubtedly responsible for their success. Although the pulmonary circulation is considerably less severe than the aortic circulation after birth, the complex haemodynamics of the aortic root may be proportionally similar to the pulmonary root. Thus, it is probable that pulmonary valve endothelium functions similarly to aortic valve endothelium to interpret the mechanical and haemodynamic signals and stimulate interstitial cell remodelling of pulmonary tissue into aortic tissue (Oury et al. 1998a).
The increased demand for the Ross procedure coupled with the relative lack of experienced surgeons capable of performing these surgeries necessitates the development of other useful options for valvular replacements, particularly for the majority of patients living in the developing world. Current mechanical and bioprosthetic valves can give 15–20 years of adequate function in adults if tailored to specific patient demographics (Hammermeister et al. 2000), but performance is dramatically reduced in growing children, who require several reoperations to accommodate larger valve diameters (Turrentine et al. 2001). Thus, a great need still exists for better replacement options.
Tissue engineering has great potential to extend the functional life of both mechanical and bioprosthetic valves, as well as to fabricate implantable valves from living tissue in vitro. Mechanical valves suffer from bleeding events that are not completely controllable through anticoagulation therapy, not to mention the limitations of activity placed on the patients. Bioprosthetic valves are less thrombogenic, but eventually fail from structural degradation (Hammermeister et al. 2000). In both cases, tissue engineering can be used to modify the surfaces of these valves to accommodate endothelial cell adhesion and function, and some work has been initiated to this end (Fischlein & Fasol 1996; Jansson et al. 2001). Recent work with tissue-engineered living valves has shown proof of concept by 20 weeks of function in the sheep pulmonary circulation with apparent replication of native cell phenotypes and trivial to mild regurgitation (Hoerstrup et al. 2000). Similar valves have begun to be implanted in humans in Europe and longer-term results of these studies are awaited (Dohmen et al. 2002).
With this rapid progress, comes concern that long-term integration may be sacrificed for short-term feasibility. Current living valve replacements use cells isolated from available autologous vascular sources or mesenchymal stem cells. What is becoming clear from this review is that not all endothelial cells are alike, and therefore not all may function similarly in similar mechanical environments. Although stem cells have the potential to be differentiated into native valvular phenotypes, it is still unclear what those valvular phenotypes are or what stimuli are capable of initiating this differentiation. Results have shown that in vitro conditioning is beneficial in preparing living conduits for implantation, suggesting that a complex combination of stimuli are probably responsible (Hoerstrup et al. 2002).
Tissue engineering can also help advance our understanding of these issues in a laboratory setting. Engineered leaflet models containing isolated native valvular cells in defined matrices can be exposed to well-controlled mechanical and/or biochemical stimuli to ascertain their effects. In this way, native valvular cell interactions and biomechanical responses can be ascertained, which will serve as benchmarks for the differentiation of more usable cell sources such as available autologous sources, circulating progenitors cells and stem cells. Results can be interpreted in a positive feedback loop to reduce the need for more expensive animal implantations, potentially reducing the time required to generate successful valvular substitutes. In addition, mechanisms of valvular cell pathology initiation and progression can be studied more effectively in these controlled environments, potentially contributing to earlier diagnosis, prediction and treatment options.
In summary, the mechanical environment imposed by the haemodynamics of the aortic valve is much more complex than that of large arteries and varies dramatically along the surfaces of the valve. These stimuli may lead to different types of pathology on the two different sides of the valve. Further investigation of the role of mechanics in the initiation of these diseases may enable the discovery of key regulating molecules, the expression of which could be selectively targeted through pharmacological intervention before gross structural deterioration occurs.
Footnotes
One contribution of 21 to a Theme Issue ‘Bioengineering the heart’.
Supplementary Material
References and full gene names of endothelial protein expression
References
- Affonso da Costa F.D, et al. The Ross procedure: is it the ideal operation for the young with aortic valve disease? Heart Surg. Forum. 1998;1:116–124. [PubMed] [Google Scholar]
- Akinkugbe O.O, Nicholson G.D, Cruickshank J.K. Heart disease in blacks of Africa and the Caribbean. Cardiovasc. Clin. 1991;21:377–391. [PubMed] [Google Scholar]
- Allen B.L, Katz B, Hook M. Streptococcus anginosus adheres to vascular endothelium basement membrane and purified extracellular matrix proteins. Microb. Pathog. 2002;32:191–204. doi: 10.1006/mpat.2002.0496. doi:10.1006/mpat.2002.0496 [DOI] [PubMed] [Google Scholar]
- Allison M.A, Wright C.M. Age and gender are the strongest clinical correlates of prevalent coronary calcification (R1) Int. J. Cardiol. 2005;98:325–330. doi: 10.1016/j.ijcard.2004.03.015. doi:10.1016/j.ijcard.2004.03.015 [DOI] [PubMed] [Google Scholar]
- Aluri S, Chandran K.B. Numerical simulation of mechanical mitral heart valve closure. Ann. Biomed. Eng. 2001;29:665–676. doi: 10.1114/1.1385810. doi:10.1114/1.1385810 [DOI] [PubMed] [Google Scholar]
- Baxley W.A. Aortic valve disease. Curr. Opin. Cardiol. 1994;9:152–157. doi: 10.1097/00001573-199403000-00003. doi:10.1097/00001573-199403000-00003 [DOI] [PubMed] [Google Scholar]
- Borkon A.M, Soule L.M, Baughman K.L, Aoun H, Baumgartner W.A, Gardner T.J, Watkins L, Jr, Gott V.L, Reitz B.A. Comparative analysis of mechanical and bioprosthetic valves after aortic valve replacement. J. Thorac. Cardiovasc. Surg. 1987;94:20–33. [PubMed] [Google Scholar]
- Bowen I.M, Marr C.M, Chester A.H, Wheeler-Jones C.P, Elliott J. In-vitro contraction of the equine aortic valve. J. Heart Valve Dis. 2004;13:593–599. [PubMed] [Google Scholar]
- Brewer R, Mentzer R, Jr, Deck J.D, Ritter R.C, Trefil J.S, Nolan S.P. An in vivo study of the dimensional changes of the aortic valve leaflets during the cardiac cycle. J. Thorac. Cardiovasc. Surg. 1977;74:645–650. [PubMed] [Google Scholar]
- Butcher J.T, Nerem R.M. Valvular endothelial cells regulate the phenotype of interstitial cells in co-culture: effects of steady shear stress. Tissue Eng. 2006;12:905–915. doi: 10.1089/ten.2006.12.905. doi:10.1089/ten.2006.12.905 [DOI] [PubMed] [Google Scholar]
- Butcher J.T, Penrod A.M, Garcia A.J, Nerem R.M. Unique morphology and focal adhesion development of valvular endothelial cells in static and fluid flow environments. Arterioscl. Thromb. Vasc. Biol. 2004;24:1429–1434. doi: 10.1161/01.ATV.0000130462.50769.5a. doi:10.1161/01.ATV.0000130462.50769.5a [DOI] [PubMed] [Google Scholar]
- Cambria R.A, Lowell R.C, Gloviczki P, Miller V.M. Chronic changes in blood flow alter endothelium-dependent responses in autogenous vein grafts in dogs. J. Vasc. Surg. 1994;20:765–773. doi: 10.1016/s0741-5214(94)70164-4. [DOI] [PubMed] [Google Scholar]
- Caro C, Pedley T, Seed W.A. Mechanics of the circulation. In: Guyton A.C, editor. Cardiovascular physiology. ch. 1. Medical and Technical Publishers; London, UK: 1974. [Google Scholar]
- Chalajour F, Treede H, Ebrahimnejad A, Lauke H, Reichenspurner H, Ergun S. Angiogenic activation of valvular endothelial cells in aortic valve stenosis. Exp. Cell Res. 2004;298:455–464. doi: 10.1016/j.yexcr.2004.04.034. doi:10.1016/j.yexcr.2004.04.034 [DOI] [PubMed] [Google Scholar]
- Cimini M, Rogers K.A, Boughner D.R. Smoothelin-positive cells in human and porcine semilunar valves. Histochem. Cell Biol. 2003;120:307–317. doi: 10.1007/s00418-003-0570-z. doi:10.1007/s00418-003-0570-z [DOI] [PubMed] [Google Scholar]
- Corcoran B.M, Black A, Anderson H, McEwan J.D, French A, Smith P, Devine C. Identification of surface morphologic changes in the mitral valve leaflets and chordae tendineae of dogs with myxomatous degeneration. Am. J. Vet. Res. 2004;65:198–206. doi: 10.2460/ajvr.2004.65.198. doi:10.2460/ajvr.2004.65.198 [DOI] [PubMed] [Google Scholar]
- da Silva N.A, Pereira B.A. Acute rheumatic fever. Still a challenge. Rheum. Dis. Clin. North Am. 1997;23:545–568. doi: 10.1016/s0889-857x(05)70347-1. doi:10.1016/S0889-857X(05)70347-1 [DOI] [PubMed] [Google Scholar]
- Dagum P, Green G.R, Nistal F.J, Daughters G.T, Timek T.A, Foppiano L.E, Bolger A.F, Ingels N.B, Jr, Miller D.C. Deformational dynamics of the aortic root: modes and physiologic determinants. Circulation. 1999;100:II54–II62. doi: 10.1161/01.cir.100.suppl_2.ii-54. [DOI] [PubMed] [Google Scholar]
- Dancu M.B, Berardi D.E, Vanden Heuvel J.P, Tarbell J.M. Asynchronous shear stress and circumferential strain reduces endothelial NO synthase and cyclooxygenase-2 but induces endothelin-1 gene expression in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2004;24:2088–2094. doi: 10.1161/01.ATV.0000143855.85343.0e. doi:10.1161/01.ATV.0000143855.85343.0e [DOI] [PubMed] [Google Scholar]
- Dare A.J, Veinot J.P, Edwards W.D, Tazelaar H.D, Schaff H.V. New observations on the etiology of aortic valve disease: a surgical pathologic study of 236 cases from 1990. Hum. Pathol. 1993;24:1330–1338. doi: 10.1016/0046-8177(93)90267-k. doi:10.1016/0046-8177(93)90267-K [DOI] [PubMed] [Google Scholar]
- Deck J.D. Endothelial cell orientation on aortic valve leaflets. Cardiovasc. Res. 1986;20:760–767. doi: 10.1093/cvr/20.10.760. [DOI] [PubMed] [Google Scholar]
- Deck J.D, Thubrikar M.J, Schneider P.J, Nolan S.P. Structure, stress, and tissue repair in aortic valve leaflets. Cardiovasc. Res. 1988;22:7–16. doi: 10.1093/cvr/22.1.7. [DOI] [PubMed] [Google Scholar]
- Dohmen P.M, Lembcke A, Hotz H, Kivelitz D, Konertz W.F. Ross operation with a tissue-engineered heart valve. Ann. Thorac. Surg. 2002;74:1438–1442. doi: 10.1016/s0003-4975(02)03881-x. doi:10.1016/S0003-4975(02)03881-X [DOI] [PubMed] [Google Scholar]
- Drake T.A, Pang M. Effects of interleukin-1, lipopolysaccharide, and streptococci on procoagulant activity of cultured human cardiac valve endothelial and stromal cells. Infect. Immun. 1989;57:507–512. doi: 10.1128/iai.57.2.507-512.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draney M.T, Arko F.R, Alley M.T, Markl M, Herfkens R.J, Pelc N.J, Zarins C.K, Taylor C.A. Quantification of vessel wall motion and cyclic strain using cine phase contrast MRI: in vivo validation in the porcine aorta. Magn. Reson. Med. 2004;52:286–295. doi: 10.1002/mrm.20137. doi:10.1002/mrm.20137 [DOI] [PubMed] [Google Scholar]
- Edep M.E, Shirani J, Wolf P, Brown D.L. Matrix metalloproteinase expression in nonrheumatic aortic stenosis. Cardiovasc. Pathol. 2000;9:281–286. doi: 10.1016/s1054-8807(00)00043-0. doi:10.1016/S1054-8807(00)00043-0 [DOI] [PubMed] [Google Scholar]
- Einav S, Stolero D, Avidor J.M, Elad D, Talbot L. Wall shear stress distribution along the cusp of a tri-leaflet prosthetic valve. J. Biomed. Eng. 1990;12:13–18. doi: 10.1016/0141-5425(90)90108-y. doi:10.1016/0141-5425(90)90108-Y [DOI] [PubMed] [Google Scholar]
- Fischlein T, Fasol R. In vitro endothelialization of bioprosthetic heart valves. J. Heart Valve Dis. 1996;5:58–65. [PubMed] [Google Scholar]
- Ghaisas N.K, Foley J.B, O'Briain D.S, Crean P, Kelleher D, Walsh M. Adhesion molecules in nonrheumatic aortic valve disease: endothelial expression, serum levels and effects of valve replacement. J. Am. Coll. Cardiol. 2000;36:2257–2262. doi: 10.1016/s0735-1097(00)00998-0. doi:10.1016/S0735-1097(00)00998-0 [DOI] [PubMed] [Google Scholar]
- Gorfien S.F, Howard P.S, Myers J.C, Macarak E.J. Cyclic biaxial strain of pulmonary artery endothelial cells causes an increase in cell layer-associated fibronectin. Am. J. Respir. Cell Mol. Biol. 1990;3:421–429. doi: 10.1165/ajrcmb/3.5.421. [DOI] [PubMed] [Google Scholar]
- Hammermeister K, Sethi G.K, Henderson W.G, Grover F.L, Oprian C, Rahimtoola S.H. Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: final report of the Veterans Affairs randomized trial. J. Am. Coll. Cardiol. 2000;36:1152–1158. doi: 10.1016/s0735-1097(00)00834-2. doi:10.1016/S0735-1097(00)00834-2 [DOI] [PubMed] [Google Scholar]
- Harasaki H, Hanano H, Tanaka J, Tokunaga K, Torisu M. Surface structure of the human cardiac valve. A comparative study of normal and diseased valves. J. Cardiovasc. Surg. (Torino) 1978;19:281–290. [PubMed] [Google Scholar]
- Helmke B.P, Thakker D.B, Goldman R.D, Davies P.F. Spatiotemporal analysis of flow-induced intermediate filament displacement in living endothelial cells. Biophys. J. 2001;80:184–194. doi: 10.1016/S0006-3495(01)76006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmlinger G, Geiger R.V, Schreck S, Nerem R.M. Effects of pulsatile flow on cultured vascular endothelial cell morphology. J. Biomech. Eng. 1991;113:123–131. doi: 10.1115/1.2891226. [DOI] [PubMed] [Google Scholar]
- Heper G, Yorukoglu Y. Clinical, bacteriologic and echocardiographic evaluation of infective endocarditis in Ankara, Turkey. Angiology. 2002;53:191–197. doi: 10.1177/000331970205300210. [DOI] [PubMed] [Google Scholar]
- Hoerstrup S.P, et al. Functional living trileaflet heart valves grown in vitro. Circulation. 2000;102(Suppl. 3):III44–III49. doi: 10.1161/01.cir.102.suppl_3.iii-44. [DOI] [PubMed] [Google Scholar]
- Hoerstrup S.P, et al. Tissue engineering of functional trileaflet heart valves from human marrow stromal cells. Circulation. 2002;106(Suppl. 1):I143–I150. [PubMed] [Google Scholar]
- Holzapfel G.A, Weizsacker H.W. Biomechanical behavior of the arterial wall and its numerical characterization. Comput. Biol. Med. 1998;28:377–392. doi: 10.1016/s0010-4825(98)00022-5. doi:10.1016/S0010-4825(98)00022-5 [DOI] [PubMed] [Google Scholar]
- Imberti B, Seliktar D, Nerem R.M, Remuzzi A. The response of endothelial cells to fluid shear stress using a co-culture model of the arterial wall. Endothelium. 2002;9:11–23. doi: 10.1080/10623320210714. doi:10.1080/10623320210714 [DOI] [PubMed] [Google Scholar]
- Inai T, Mancuso M.R, McDonald D.M, Kobayashi J, Nakamura K, Shibata Y. Shear stress-induced upregulation of connexin 43 expression in endothelial cells on upstream surfaces of rat cardiac valves. Histochem. Cell Biol. 2004;122:477–483. doi: 10.1007/s00418-004-0717-6. doi:10.1007/s00418-004-0717-6 [DOI] [PubMed] [Google Scholar]
- Jansson K, Bengtsson L, Swedenborg J, Haegerstrand A. In vitro endothelialization of bioprosthetic heart valves provides a cell monolayer with proliferative capacities and resistance to pulsatile flow. J. Thorac. Cardiovasc. Surg. 2001;121:108–115. doi: 10.1067/mtc.2001.110251. doi:10.1067/mtc.2001.110251 [DOI] [PubMed] [Google Scholar]
- Kaden J.J, Dempfle C.E, Grobholz R, Tran H.T, Kiliç R, Sarikoç A, Brueckmann M, Vahl C, Hagl S, Haase K.K, Borggrefe M. Interleukin-1 beta promotes matrix metalloproteinase expression and cell proliferation in calcific aortic valve stenosis. Atherosclerosis. 2003;170:205–211. doi: 10.1016/s0021-9150(03)00284-3. doi:10.1016/S0021-9150(03)00284-3 [DOI] [PubMed] [Google Scholar]
- Kim D.W, Langille B.L, Wong M.K, Gotlieb A.I. Patterns of endothelial microfilament distribution in the rabbit aorta in situ. Circ. Res. 1989;64:21–31. doi: 10.1161/01.res.64.1.21. [DOI] [PubMed] [Google Scholar]
- Langille B.L, Adamson S.L. Relationship between blood flow direction and endothelial cell orientation at arterial branch sites in rabbits and mice. Circ. Res. 1981;48:481–488. doi: 10.1161/01.res.48.4.481. [DOI] [PubMed] [Google Scholar]
- Laskey R.E, Adams D.J, van Breemen C. Cytosolic [Ca2+] measurements in endothelium of rabbit cardiac valves using imaging fluorescence microscopy. Am. J. Physiol. 1994;266:H2130–H2135. doi: 10.1152/ajpheart.1994.266.5.H2130. [DOI] [PubMed] [Google Scholar]
- Leask R.L, Jain N, Butany J. Endothelium and valvular diseases of the heart. Microsc. Res. Tech. 2003;60:129–137. doi: 10.1002/jemt.10251. doi:10.1002/jemt.10251 [DOI] [PubMed] [Google Scholar]
- Lester W.M, Damji A.A, Tanaka M, Gedeon I. Bovine mitral valve organ culture: role of interstitial cells in repair of valvular injury. J. Mol. Cell Cardiol. 1992;24:43–53. doi: 10.1016/0022-2828(92)91158-2. doi:10.1016/0022-2828(92)91158-2 [DOI] [PubMed] [Google Scholar]
- Leuprecht A, Perktold K, Kozerke S, Boesiger P. Combined CFD and MRI study of blood flow in a human ascending aorta model. Biorheology. 2002;39:425–429. [PubMed] [Google Scholar]
- Lund O, Nielsen T.T, Emmertsen K, Pilegaard H, Knudsen M, Magnussen K. M-mode echocardiography in aortic stenosis. Clinical correlates and prognostic significance after valve replacement. Scand. Cardiovasc. J. 1997;31:17–23. doi: 10.3109/14017439709058064. [DOI] [PubMed] [Google Scholar]
- Manduteanu I, Popov D, Radu A, Simionescu M. Calf cardiac valvular endothelial cells in culture: production of glycosaminoglycans, prostacyclin and fibronectin. J. Mol. Cell Cardiol. 1988;20:103–118. doi: 10.1016/s0022-2828(88)80024-5. doi:10.1016/S0022-2828(88)80024-5 [DOI] [PubMed] [Google Scholar]
- Mehta R.H, et al. Implications of increased left ventricular mass index on in-hospital outcomes in patients undergoing aortic valve surgery. J. Thorac. Cardiovasc. Surg. 2001;122:919–928. doi: 10.1067/mtc.2001.116558. doi:10.1067/mtc.2001.116558 [DOI] [PubMed] [Google Scholar]
- Messier R.H, Jr, Bass B.L, Aly H.M, Jones J.L, Domkowski P.W, Wallace R.B, Hopkins R.A. Dual structural and functional phenotypes of the porcine aortic valve interstitial population: characteristics of the leaflet myofibroblast. J. Surg. Res. 1994;57:1–21. doi: 10.1006/jsre.1994.1102. doi:10.1006/jsre.1994.1102 [DOI] [PubMed] [Google Scholar]
- Mohler E.R, III, Chawla M.K, Chang A.W, Vyavahare N, Levy R.J, Graham L, Gannon F.H. Identification and characterization of calcifying valve cells from human and canine aortic valves. J. Heart Valve Dis. 1999;8:254–260. [PubMed] [Google Scholar]
- Moreillon P, Que Y.A, Bayer A.S. Pathogenesis of streptococcal and staphylococcal endocarditis. Infect. Dis. Clin. North Am. 2002;16:297–318. doi: 10.1016/s0891-5520(01)00009-5. doi:10.1016/S0891-5520(01)00009-5 [DOI] [PubMed] [Google Scholar]
- Moretti M, Prina-Mello A, Reid A.J, Barron V, Prendergast P.J. Endothelial cell alignment on cyclically-stretched silicone surfaces. J. Mater. Sci. Mater. Med. 2004;15:1159–1164. doi: 10.1023/B:JMSM.0000046400.18607.72. doi:10.1023/B:JMSM.0000046400.18607.72 [DOI] [PubMed] [Google Scholar]
- Müller A.M, Cronen C, Kupferwasser L.I, Oelert H, Müller K.M, Kirkpatrick C.J. Expression of endothelial cell adhesion molecules on heart valves: up-regulation in degeneration as well as acute endocarditis. J. Pathol. 2000;191:54–60. doi: 10.1002/(SICI)1096-9896(200005)191:1<54::AID-PATH568>3.0.CO;2-Y. doi:10.1002/(SICI)1096-9896(200005)191:1<54::AID-PATH568>3.0.CO;2-Y [DOI] [PubMed] [Google Scholar]
- Nandy S, Tarbell J.M. Measurement of wall shear stress distal to a tri-leaflet valve in a rigid model of the aortic arch with branch flows. J. Biomech. Eng. 1988;110:172–179. doi: 10.1115/1.3108427. [DOI] [PubMed] [Google Scholar]
- Nishizawa Y, Jono S, Ishimura E, Shioi A. Hyperphosphatemia and vascular calcification in end-stage renal disease. J. Ren. Nutr. 2005;15:178–182. doi: 10.1053/j.jrn.2004.09.027. doi:10.1053/j.jrn.2004.09.027 [DOI] [PubMed] [Google Scholar]
- Oury J.H, Doty D.B, Oswalt J.D, Knapp J.F, Mackey S.K, Duran C.M. Cardiopulmonary response to maximal exercise in young athletes following the Ross procedure. Ann. Thorac. Surg. 1998a;66(Suppl. 6):S153–S154. doi: 10.1016/s0003-4975(98)01029-7. doi:10.1016/S0003-4975(98)01029-7 [DOI] [PubMed] [Google Scholar]
- Oury J.H, Hiro S.P, Maxwell J.M, Lamberti J.J, Duran C.M. The Ross procedure: current registry results. Ann. Thorac. Surg. 1998b;66(Suppl. 6):S162–S165. doi: 10.1016/s0003-4975(98)01028-5. doi:10.1016/S0003-4975(98)01028-5 [DOI] [PubMed] [Google Scholar]
- Oxenham H, Bloomfield P, Wheatley D.J, Lee R.J, Cunningham J, Prescott R.J, Miller H.C. Twenty year comparison of a Bjork–Shiley mechanical heart valve with porcine bioprostheses. Heart. 2003;89:715–721. doi: 10.1136/heart.89.7.715. doi:10.1136/heart.89.7.715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passik C.S, Ackermann D.M, Pluth J.R, Edwards W.D. Temporal changes in the causes of aortic stenosis: a surgical pathologic study of 646 cases. Mayo Clin. Proc. 1987;62:119–123. doi: 10.1016/s0025-6196(12)61880-1. [DOI] [PubMed] [Google Scholar]
- Paul R, Apel J, Klaus S, Schugner F, Schwindke P, Reul H. Shear stress related blood damage in laminar couette flow. Artif. Organs. 2003;27:517–529. doi: 10.1046/j.1525-1594.2003.07103.x. doi:10.1046/j.1525-1594.2003.07103.x [DOI] [PubMed] [Google Scholar]
- Poggianti E, Venneri L, Chubuchny V, Jambrik Z, Baroncini L.A, Picano E. Aortic valve sclerosis is associated with systemic endothelial dysfunction. J. Am. Coll. Cardiol. 2003;41:136–141. doi: 10.1016/s0735-1097(02)02622-0. doi:10.1016/S0735-1097(02)02622-0 [DOI] [PubMed] [Google Scholar]
- Pompilio G, Rossoni G, Sala A, Polvani G.L, Berti F, Dainese L, Porqueddu M, Biglioli P. Endothelial-dependent dynamic and antithrombotic properties of porcine aortic and pulmonary valves. Ann. Thorac. Surg. 1998;65:986–992. doi: 10.1016/s0003-4975(98)00075-7. doi:10.1016/S0003-4975(98)00075-7 [DOI] [PubMed] [Google Scholar]
- Porat R.M, Grunewald M, Globerman A, Itin A, Barshtein G, Alhonen L, Alitalo K, Keshet E. Specific induction of tie1 promoter by disturbed flow in atherosclerosis-prone vascular niches and flow-obstructing pathologies. Circ. Res. 2004;94:394–401. doi: 10.1161/01.RES.0000111803.92923.D6. doi:10.1161/01.RES.0000111803.92923.D6 [DOI] [PubMed] [Google Scholar]
- Prasad Y, Bhalodkar N.C. Aortic sclerosis—a marker of coronary atherosclerosis. Clin. Cardiol. 2004;27:671–673. doi: 10.1002/clc.4960271202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabkin E, Aikawa M, Stone J.R, Fukumoto Y, Libby P, Schoen F.J. Activated interstitial myofibroblasts express catabolic enzymes and mediate matrix remodeling in myxomatous heart valves. Circulation. 2001;104:2525–2532. doi: 10.1161/hc4601.099489. [DOI] [PubMed] [Google Scholar]
- Sarphie T.G. Surface topography of mitral valve endothelium from diet-induced hypercholesterolemic rabbits. Atherosclerosis. 1982;45:203–220. doi: 10.1016/0021-9150(82)90139-3. doi:10.1016/0021-9150(82)90139-3 [DOI] [PubMed] [Google Scholar]
- Sarphie T.G. Surface responses of aortic valve endothelia from diet-induced, hypercholesterolemic rabbits. Atherosclerosis. 1985;54:283–299. doi: 10.1016/0021-9150(85)90122-4. doi:10.1016/0021-9150(85)90122-4 [DOI] [PubMed] [Google Scholar]
- Schneider P.J, Deck J.D. Tissue and cell renewal in the natural aortic valve of rats: an autoradiographic study. Cardiovasc. Res. 1981;15:181–189. doi: 10.1093/cvr/15.4.181. [DOI] [PubMed] [Google Scholar]
- Schwarz F, Flameng W, Langebartels F, Sesto M, Walter P, Schlepper M. Impaired left ventricular function in chronic aortic valve disease: survival and function after replacement by Bjork–Shiley prosthesis. Circulation. 1979;60:48–58. doi: 10.1161/01.cir.60.1.48. [DOI] [PubMed] [Google Scholar]
- Shyy J.Y, Chien S. Role of integrins in endothelial mechanosensing of shear stress. Circ. Res. 2002;91:769–775. doi: 10.1161/01.res.0000038487.19924.18. doi:10.1161/01.RES.0000038487.19924.18 [DOI] [PubMed] [Google Scholar]
- Sipkema P, van der Linden P.J, Westerhof N, Yin F.C. Effect of cyclic axial stretch of rat arteries on endothelial cytoskeletal morphology and vascular reactivity. J. Biomech. 2003;36:653–659. doi: 10.1016/s0021-9290(02)00443-8. doi:10.1016/S0021-9290(02)00443-8 [DOI] [PubMed] [Google Scholar]
- Smith D.B, Sacks M.S, Vorp D.A, Thornton M. Surface geometric analysis of anatomic structures using biquintic finite element interpolation. Ann. Biomed. Eng. 2000;28:598–611. doi: 10.1114/1.1306342. doi:10.1114/1.1306342 [DOI] [PubMed] [Google Scholar]
- Soini Y, Satta J, Maatta M, Autio-Harmainen H. Expression of MMP2, MMP9, MT1-MMP, TIMP1, and TIMP2 mRNA in valvular lesions of the heart. J. Pathol. 2001;194:225–231. doi: 10.1002/path.850. doi:10.1002/path.850 [DOI] [PubMed] [Google Scholar]
- Stalhand J, Klarbring A, Karlsson M. Towards in vivo aorta material identification and stress estimation. Biomech. Model Mechanobiol. 2004;2:169–186. doi: 10.1007/s10237-003-0038-z. doi:10.1007/s10237-003-0038-z [DOI] [PubMed] [Google Scholar]
- Stegemann J.P, Nerem R.M. Phenotype modulation in vascular tissue engineering using biochemical and mechanical stimulation. Ann. Biomed. Eng. 2003;31:391–402. doi: 10.1114/1.1558031. doi:10.1114/1.1558031 [DOI] [PubMed] [Google Scholar]
- Stegemann J.P, Dey N.B, Lincoln T.M, Nerem R.M. Genetic modification of smooth muscle cells to control phenotype and function in vascular tissue engineering. Tissue Eng. 2004;10:189–199. doi: 10.1089/107632704322791844. doi:10.1089/107632704322791844 [DOI] [PubMed] [Google Scholar]
- Sullam P.M, Drake T.A, Sande M.A. Pathogenesis of endocarditis. Am. J. Med. 1985;78:110–115. doi: 10.1016/0002-9343(85)90373-0. doi:10.1016/0002-9343(85)90373-0 [DOI] [PubMed] [Google Scholar]
- Takahashi M, Ishida T, Traub O, Corson M.A, Berk B.C. Mechanotransduction in endothelial cells: temporal signaling events in response to shear stress. J. Vasc. Res. 1997;34:212–219. doi: 10.1159/000159225. [DOI] [PubMed] [Google Scholar]
- Terracio L, Miller B, Borg T.K. Effects of cyclic mechanical stimulation of the cellular components of the heart: in vitro. In vitro Cell Dev. Biol. 1988;24:53–58. doi: 10.1007/BF02623815. doi:10.1007/BF02623815 [DOI] [PubMed] [Google Scholar]
- Thubrikar M, Piepgrass W.C, Bosher L.P, Nolan S.P. The elastic modulus of canine aortic valve leaflets in vivo and in vitro. Circ. Res. 1980;47:792–800. doi: 10.1161/01.res.47.5.792. [DOI] [PubMed] [Google Scholar]
- Thubrikar M.J, Aouad J, Nolan S.P. Comparison of the in vivo and in vitro mechanical properties of aortic valve leaflets. J. Thorac. Cardiovasc. Surg. 1986a;92:29–36. [PubMed] [Google Scholar]
- Thubrikar M.J, Nolan S.P, Aouad J, Deck J.D. Stress sharing between the sinus and leaflets of canine aortic valve. Ann. Thorac. Surg. 1986b;42:434–440. doi: 10.1016/s0003-4975(10)60554-1. [DOI] [PubMed] [Google Scholar]
- Tompkins R.G, Schnitzer J.J, Yarmush M.L. Macromolecular transport within heart valves. Circ. Res. 1989;64:1213–1223. doi: 10.1161/01.res.64.6.1213. [DOI] [PubMed] [Google Scholar]
- Trenouth R.S, Phelps N.C, Neill W.A. Determinants of left ventricular hypertrophy and oxygen supply in chronic aortic valve disease. Circulation. 1976;53:644–650. doi: 10.1161/01.cir.53.4.644. [DOI] [PubMed] [Google Scholar]
- Tsai H.M. Shear stress and von Willebrand factor in health and disease. Semin. Thromb. Hemost. 2003;29:479–488. doi: 10.1055/s-2003-44556. doi:10.1055/s-2003-44556 [DOI] [PubMed] [Google Scholar]
- Turrentine M.W, Ruzmetov M, Vijay P, Bills R.G, Brown J.W. Biological versus mechanical aortic valve replacement in children. Ann. Thorac. Surg. 2001;71(Suppl. 5):S356–S360. doi: 10.1016/s0003-4975(01)02507-3. doi:10.1016/S0003-4975(01)02507-3 [DOI] [PubMed] [Google Scholar]
- Vaishnav R.N, Vossoughi J. Residual stress and strain in aortic segments. J. Biomech. 1987;20:235–239. doi: 10.1016/0021-9290(87)90290-9. doi:10.1016/0021-9290(87)90290-9 [DOI] [PubMed] [Google Scholar]
- Vesely I. The role of elastin in aortic valve mechanics. J. Biomech. 1998;31:115–123. doi: 10.1016/s0021-9290(97)00122-x. doi:10.1016/S0021-9290(97)00122-X [DOI] [PubMed] [Google Scholar]
- Vesely I, Lozon A, Talman E. Is zero-pressure fixation of bioprosthetic valves truly stress free? J. Thorac. Cardiovasc. Surg. 1993;106:288–298. [PubMed] [Google Scholar]
- Walburn F.J, Stein P.D. Wall shear stress during pulsatile flow distal to a normal porcine aortic valve. J. Biomech. 1984;17:97–102. doi: 10.1016/0021-9290(84)90127-1. doi:10.1016/0021-9290(84)90127-1 [DOI] [PubMed] [Google Scholar]
- Wang J.H, Goldschmidt-Clermont P, Wille J, Yin F.C. Specificity of endothelial cell reorientation in response to cyclic mechanical stretching. J. Biomech. 2001;34:1563–1572. doi: 10.1016/s0021-9290(01)00150-6. doi:10.1016/S0021-9290(01)00150-6 [DOI] [PubMed] [Google Scholar]
- Wedding K.L, Draney M.T, Herfkens R.J, Zarins C.K, Taylor C.A, Pelc N.J. Measurement of vessel wall strain using cine phase contrast MRI. J. Magn. Reson. Imaging. 2002;15:418–428. doi: 10.1002/jmri.10077. doi:10.1002/jmri.10077 [DOI] [PubMed] [Google Scholar]
- Welters I, Menges T, Ballesteros M, Knothe C, Ruwoldt R, Gorlach G, Hempelmann G. Thrombin generation and activation of the thrombomodulin protein C system in open heart surgery depend on the underlying cardiac disease. Thromb. Res. 1998;92:1–9. doi: 10.1016/s0049-3848(98)00095-4. doi:10.1016/S0049-3848(98)00095-4 [DOI] [PubMed] [Google Scholar]
- Weston M.W, LaBorde D.V, Yoganathan A.P. Estimation of the shear stress on the surface of an aortic valve leaflet. Ann. Biomed. Eng. 1999;27:572–579. doi: 10.1114/1.199. doi:10.1114/1.199 [DOI] [PubMed] [Google Scholar]
- Wojciak-Stothard B, Ridley A.J. Shear stress-induced endothelial cell polarization is mediated by Rho and Rac but not Cdc42 or PI 3-kinases. J. Cell Biol. 2003;161:429–439. doi: 10.1083/jcb.200210135. doi:10.1083/jcb.200210135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong A.J, Pollard T.D, Herman I.M. Actin filament stress fibers in vascular endothelial cells in vivo. Science. 1983;219:867–869. doi: 10.1126/science.6681677. doi:10.1126/science.6681677 [DOI] [PubMed] [Google Scholar]
- Woo Y.R, Yoganathan A.P. In vitro pulsatile flow velocity and turbulent shear stress measurements in the vicinity of mechanical aortic heart valve prostheses. Life Support Syst. 1985;3:283–312. [PubMed] [Google Scholar]
- Zeng G, Quon M.J. Insulin-stimulated production of nitric oxide is inhibited by wortmannin. Direct measurement in vascular endothelial cells. J. Clin. Invest. 1996;98:894–898. doi: 10.1172/JCI118871. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
References and full gene names of endothelial protein expression