Abstract
Valvulogenesis is an extremely complex process by which a fragile gelatinous matrix is populated and remodelled during embryonic development into thin fibrous leaflets capable of maintaining unidirectional flow over a lifetime. This process occurs during exposure to constantly changing haemodynamic forces, with a success rate of approximately 99%. Defective valvulogenesis results in impaired cardiac function and lifelong complications. This review integrates what is known about the roles of genetics and mechanics in the development of valves and how changes in either result in impaired morphogenesis. It is hoped that appropriate developmental cues and phenotypic endpoints could help engineers and clinicians in their efforts to regenerate living valve alternatives.
Keywords: morphogenesis, signal transduction, mechanics, genes, pathways, embryo
1. Introduction
Diseases of the heart valves are often a lifelong struggle as approximately 1% of all Americans are currently living with heart defects acquired during embryonic development. Many of these defects are severely debilitating, necessitating invasive surgery and rigorous pharmacological treatment, while others lie largely undetected until later in life, when earlier than expected tissue failure demands immediate medical attention. The mechanisms by which these tissues fail are largely unknown, but the complex mechanical environment in which they thrive suggests that some deficiency exists in the resident cells' ability to repair damage and/or adapt to changes in haemodynamics (Deck et al. 1988). By far the most common treatment for damaged valves is replacement with a biological or mechanical prosthetic tailored to the particular conditions of the patient (Hammermeister et al. 2000). These valves are capable of upwards of 20 years of function, which while satisfactory in elderly patients, is far from ideal for paediatric populations. Recent advances in homograft valve preservation and growing availability of autograft valve procedures have improved the long-term prognosis for children (Sako 2004), but still more improvement is needed.
Tissue engineering has emerged as a potential way to solve this crisis. By creating autologous cell-based living tissue replacements, the hope is that these surrogates would remodel seamlessly into the patients system with no traces of previous pathology. Recent success in this area has been limited but encouraging (Hoerstrup et al. 2000; Sutherland et al. 2005). It is clear from these initial results that the complex relationships between mechanical forces and valvular-specific cell biology must be understood before further advancement can be realized. One key scientific interaction that has been lacking in many aspects is collaboration between developmental biologists and engineers. Both of these disciplines share the same goal, that is the eradication of heart and heart valve disease, but have different research foci. While engineers focus more on the identification of candidate scaffolds, cell sources and stimuli, developmental biologists seek to understand how the heart and heart valves develop naturally. The human body is 99% successful in creating normal hearts with functioning valves, but amazingly little of this process and its regulation have been unearthed.
The purpose of this review is, therefore, to summarize what is known about valvulogenesis with particular emphasis on aspects that are relevant to engineer/scientists who seek to reconstruct them. The morphogenesis of these structures is then followed by an analysis of the identified signalling mechanisms, followed by a discussion of what is known about the influence of mechanical forces, and concluded with a discussion of future efforts in valvular developmental biology and connections to tissue engineering.
(a) Valvular morphogenesis
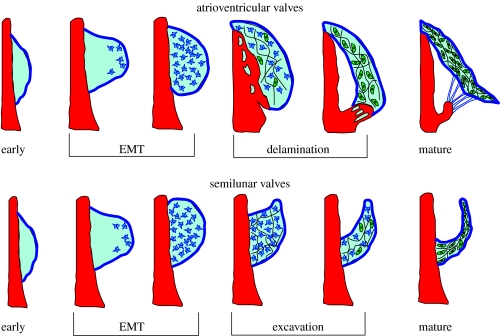
The morphogenesis of the atrioventricular and semilunar valves is a complex process that occurs concomitantly with changing cardiac morphology and haemodynamics. The early embryonic heart is a single tube of endocardial cells surrounded by primary myocardium. During the looping process, the primary myocardium secretes a hyaluronan-rich gelatinous matrix, called cardiac jelly, forming swellings that project into the lumen at the levels of the atrioventricular junction and the outflow tract (OFT; figure 1). A subset of myocardial cells lining these regions then secretes factors that activate the overlaying endocardium. These factors include members of the TGF-β family, including TFG-β1–3 and BMP-2, 4, which is explained in greater detail in a subsequent section. Beginning at Hamburger and Hamilton chick embryo developmental stage 14 (HH14, E9.0 in mouse; Hamburger & Hamilton 1992), activated endocardial cells downregulate cell–cell contacts (PECAM1, NCAM1, DS-CAM) and upregulate cell–matrix adhesions (integrins). We have identified three antigens ES130, coding for a ribosomal receptor protein, JB3/fibrillin and TGF-β3 that are expressed only by activated endocardial cells (Wunsch et al. 1994; Sinning & Hewitt 1996; Ramsdell & Markwald 1997). Activated endocardial cells then change from a polygonal quiescent epithelial phenotype to spindle shaped migratory cells and begin to invade the hyaluronan-rich cardiac jelly matrix transforming into a mesenchymal phenotype characterized by the expression of α-smooth muscle actin (Nakajima et al. 1997). They also digest the hyaluronan and form a denser matrix in its place, which comprises collagens I, II and III, versican and other proteoglycans (Person et al. 2005). Continued expansion of this mesenchymal population creates the swellings that eventually form valvular and septal structures, dubbed ‘cushions’ owing to their appearance on the myocardial wall and apparent softness. The cushions extend into the lumen driven by increased mesenchymal cell proliferation and matrix deposition, and appose to help maintain unidirectional blood flow (Moorman & Christoffels 2003). The originally gelatinous tissue at HH17 becomes significantly more rigid by HH25, but the cell phenotype is still exclusively mesenchymal. The maturation process of the atrioventricular and semilunar valves differs owing to the different end morphology: the atrioventricular valves have tendinous chords while the semilunar valves have free edges. The morphogenesis of each is considered separately.
Figure 1.
General stages of atrioventricular and outflow tract valve development.
(i) Atrioventricular valves
The left and right atrioventricular valves, termed the mitral and tricuspid valves respectively, are formed by a series of cushions that line the atrioventricular canal. Initially two cushions are present, one dorsal/inferior and other ventral/superior. Beginning at approximately HH19 (E9.5), a ring of specialized atrioventricular myocardium, characterized by expression of GATA-6 in the original heart forming fields and GIN2 in later stages, surrounds the atrioventricular canal (Kim et al. 2001a; Adamo et al. 2004). Myocardial protrusions form on the left and right lateral sides of the AV canal, expressing Tbx3 (Moorman et al. 2004). The AV mesenchyme then expands (probably through a combination of proliferation and migration) around the entire circumference of the AV canal, but thickens preferentially at the protrusions, forming left and right lateral cushions. The fusion of the midline cushions at HH25 divides the AV canal into right and left sides, and different portions of these fused cushions contribute to the resulting AV valves. The dorsal/inferior AV cushion forms the basal portion of the anteroseptal leaflet of the left AV (mitral) valve and the septal leaflet in the right AV valve (tricuspid; De la Cruz & Markwald 2000). The ventral/superior AV cushion mainly forms the leaflet associated with the mitral–aortic continuity. The mural/lateral cushions form the mural leaflets. The right mural cushion of the chick becomes muscularized (like the conus region of the OT) forming a large muscular flap, while the right AV septal leaflet is only a ‘micro-leaflet’ (Sedmera et al. 1997). The right AV valves in the mouse, however, are similar in structure and morphogenesis to the tricuspid valve of the human (Wessels & Sedmera 2003).
Formation of the septal and mural atrioventricular leaflets occurs by a process of proliferation, extension, condensation and delamination (figure 1). The AV myocardium forms a fold at its junction with the ventricular myocardium, creating a substrate for the extension of the mural leaflets into the lumen, while the substrate for the septal leaflets is the muscular ventricular septum (the left septal leaflet is not attached to the myocardial wall except at the extreme anterior and posterior edges; Oosthoek et al. 1998). Beginning at HH26 (E12.5 in the mouse) by a process incompletely understood but potentially similar to the extension of the mesenchyme in limb buds, a proliferative zone in immediately subendocardial portion of the AV cushions expands and extends the cushions along their AV myocardial substrates, mediated by FGF-4 secretion by the endocardium (Sugi et al. 2003). That portion of the cushions contacting the myocardium substrate forms a progressive zone that begins to differentiate into a fibroblastic phenotype (De la Cruz & Markwald 2000). This differentiated cushion cell phenotype begins to condense the cushion matrix to form a thinner fibrous tissue. Fenestrations begin to develop in the myocardial layer under the elongating cushions by HH30 (E13.5 in the mouse) through an incompletely understood mechanism potentially due to expansion of the ventricular cavities and/or changes in haemodynamic loading. These fenestrations coalesce, resulting in complete ‘delamination’ of the AV myocardial/cushion hybrid valve tissue from the ventricular myocardial wall, maintaining contacts only at the cranial and caudal edges. Additional fenestrations develop within the residual distal/luminal mesenchymal contacts between cushion and myocardium at the site of the developing papillary muscles. This creates mesenchymal tissue strands that develop into the tendinous chords of the AV valves by HH34 (E15; Icardo & Colvee 1995). By unknown mechanisms, the myocardial tissue of the hybrid AV valves disappears, followed by further condensation into thin fibrous leaflets populated by predominantly fibroblastic cells (de Lange et al. 2004). Labelling of extracellular matrix throughout development of the AV valves shows that the subendocardial surface of the cushions is largely laminin positive, while the ventricular side is predominantly collagen III (at HH25). The cushion matrix also comprises other collagens (I, II, III, V and VI). Between this stage and HH36 (E15), the laminin-filled subendocardial layer increases in thickness, and a lamellar network of elastin and collagen forms (Hurle et al. 1994). Underneath this layer remains a largely undifferentiated mesenchymal matrix, with dense fibrous bundles of collagen and fibroblastic cells on the layer bordering the ventricular side of the leaflet. By HH36, these layers become the mature atrialis/spongiosa, fibrosa and ventricularis of the atrioventricular leaflets. The human mitral leaflet has a large anterior leaflet with a small posterior leaflet, both supported by tendinous chords with multiple orders of branches, supporting leaflet closure like a parachute. The human tricuspid valve is actually a continuous leaflet that encircles the right AV supported by three clusters of chordae (Lamers et al. 1995).
(ii) Semilunar valves
The formation of the semilunar valves progresses along a similar pathway as the AV valves in terms of mesenchymal transformation of the endocardial cells of the distal outflow tract cushions, but instead of delaminating from the muscular walls, the cushions become excavated from the aortic side inward (figure 1). The outflow tract begins as a conically shaped, tubular structure extending from the right ventricle to the opening into aortic sac and is completely enclosed by myocardium. Similar to the AV canal, mesenchymalized cushions form in the lumen of the outflow tract, with two cushions proximal to the right ventricle and three distal cushions. The outflow tract has historically been divided into two regions: the proximal conus and a distal (downstream) truncus, delineated by a characteristic ‘dogleg’ bend between these two regions of the OT (Webb et al. 2003). Recent evidence has demonstrated that the outflow tract is developed from a secondary anterior heart field separate from the original heart forming cell fields (Mjaatvedt et al. 2001; Yelbuz et al. 2003), and this bend may be a demarcation of these lineages. Between HH17 and HH26 (E10–E12), the conal and truncal cushions become invaded and populated by activated endocardially derived cells in a similar manner as in the AV cushions, but the process deviates somewhat once these cushions fuse. Beginning at the level of the fourth and sixth aortic arches (HH30, E13.5), a population of cells derived from the neural crest begins to migrate caudally through the walls of the aortic sac and into the fused truncal cushions, creating a crescent-shaped septal wedge that divides the outflow tract into aortic and pulmonary components (Kirby et al. 1983). The aorticopulmonary (AP) septum spirals through the OFT lumen with anticlockwise rotation of the outflow tract, so that the right portion of the semilunar ring is derived from the original left side of the primitive outflow tract (Thompson et al. 1987; Bajolle et al. 2006). Concomitant with the caudal movement of the septum, the myocardial casing ‘retracts’ through an as yet unknown mechanism. There is evidence of both myocardial apoptosis and myocardial to endocardial differentiation, but there is no clear consensus as of yet (Thompson et al. 1987; Ya et al. 1998; van den Hoff et al. 1999; Rothenberg et al. 2003). The splitting of the parietal and septal distal truncal cushions by the AP septum, combined with the intercalated cushions of the distal outflow tract, creates the six cushions required for the formation of the semilunar valves. The caudal progression of the AP septum also brings down with it the primitive pulmonary bifurcation and the aortic arches from the aortic sac that is surrounded by vascular smooth muscle and not myocardium (Kirby et al. 1983). The process is complete by HH34 (E14.5). Between HH29 and HH33, the endothelium and several subendothelial layers lining the aortic surface of the valves become rounded, apoptotic and flake off the cushion, leaving a small depression (Garcia-Martinez et al. 1991). The ventricular endothelial surface maintains a flat elongated profile. The tissue of the arterial side of the cusp then becomes a more condensed fibrous matrix as the small depression continues to deepen, sculpting the leaflet cusps. By HH39–40, the leaflets begin to appear trilaminar in nature, with an elastin–collagen lamellar structure forming at the ventricular surface. By HH45, fibrous tissue is seen radiating from the attachment of the valve cusps into the aortic wall from the base to the level of the commissures, creating an anchoring ring of fibrocartilaginous tissue around the sinuses (Garcia-Martinez et al. 1991). The only difference between the semilunar valves present at birth is the insertion of the coronary ostia into the left and right sinuses of the aortic valve. After birth, increases in aortic blood pressure and a concomitant drop in pulmonary artery pressure cause the further strengthening of the aorta and aortic valve cusps by increasing the number of collagen–elastin lamellae (Colvee & Hurle 1981).
(b) Cell types involved in valvulogenesis
Multiple cell populations are involved in the development of the valves of the heart in addition to the endocardially derived mesenchyme. They are outlined as follows with their currently known roles.
Cardiac neural crest. Pioneering work by Kirby has identified the neural crest as the origin of the AP septum that impales the fused outflow tract cushions (Kirby et al. 1983). By HH27 (E12.5 in mouse), a significant proportion of the distal outflow tract cushions. Ablation of these cells inhibits the septation of the outflow tract, resulting in a variety of outflow tract defects, including common outlet and transposition of the great arteries (Kirby 1988). There is currently no evidence that neural crest cells derived from the AP septum contribute to the development of the atrioventricular valves. Fate mapping studies using wnt-1 as a definitive marker of neural crest lineage have determined that no residual neural crest cells persist in the outflow tract (de Lange et al. 2004). Recent evidence does suggest in mice that melanocytes, a neural crest related cell type, does populate portions of the post-natal inflow and outlet valves (Mjaatvedt et al. 2005), though a functional mechanism has yet to be determined.
Proepicardium. A series of studies have firmly demonstrated that significant numbers of cells from the proepicardium (termed epicardially derived cells ‘EPDC’) migrate into the atrioventricular cushions and reside at the myocardial/cushion interface and subendocardial regions (Gittenberger-de Groot et al. 2000). However, these cells do not appear to contribute to the outflow cushions. Quail–chick chimeras or proepicardial ablations studies limit embryonic viability due to effects upon coronary vasculature and, thus, have failed to clarify the role of the EPDC in mature AV valves (Lie-Venema et al. 2005).
Dorsal mesocardium. An additional mesenchymal population contributes to AV valves from the venous pole via the remnants of the dorsal mesocardium. This mesenchymal population (spina vestibuli) forms a cap on the anterior surface of the muscular interatrial septum which eventually contacts the fused AV cushions to contribute cells to the developing valves as well as to the closure of the foramen within the muscular atrial septum (Wessels et al. 2000).
2. Molecular regulation of valvulogenesis
Much of the research investigating the molecular regulatory events in valvulogenesis focuses on the initial stage (figure 2a), termed ‘endocardial to mesenchymal transformation’ or EMT. In order for valves to form in the heart, the specific region of endocardial cells lining valve-forming regions undergo a process of activation, migration and invasion into the cardiac jelly. We developed an in vitro assay over 20 years ago where these endocardial regions (and the subadjacent myocardium) can be explanted onto hydrated collagen gels, and the mesenchymal transformation will occur (Bernanke & Markwald 1982). Similarly removed sections of ventricular endocardium/myocardium do not undergo EMT, suggesting that the regulation of this process is a localized molecular event. Manipulation of this model system in mouse and chick coupled with the development of mutant genetic mouse models has dramatically increased our understanding of this critical process. Numerous markers for endocardial cells at various stages of mesenchymal transformation have been identified. Early endothelial activation is represented by expression of ES130, a ribosomal receptor protein, JB3/fibrillin and TGF-β3. Further activation results in endocardial hypertrophy and separation, coupled with downregulation of endothelial markers PECAM1, NCAM1 and VE-cadherin, and upregulation of mesenchymal markers such as smooth muscle α-actin (α-SMA). Migratory mesenchymally transformed endocardial cells secrete metalloproteinases that degrade the endocardial basal lamina (primarily collagen IV), enabling invasion into the cardiac jelly.
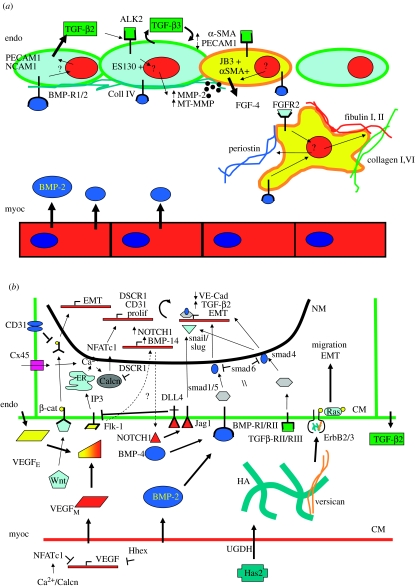
Figure 2.
Molecular regulation of endocardial to mesenchymal transformation (EMT) as discovered in (a) chick and (b) mouse models. The coordinated interaction of VEGF, TGF-β, BMP, NOTCH, hyaluronan and wnt/β-catenin result in proper mesenchymal invasion into the cardiac jelly. CM, cell membrane; NM, nuclear membrane.
(a) Endocardial to mesenchymal transformation
Initiation of mesenchymal transformation involves the coordination of several growth factor-mediated signal pathways originating from both myocardial and endocardial sources. These include VEGF, BMP, TGF-β, EGF and NOTCH. The specific roles of each pathway will be addressed separately (figure. 2b).
(i) VEGF. Vascular endothelial growth factor, initially identified for its role in mediating angiogenesis, is also a critical mediator of EMT. VEGF isoforms are expressed in both the endocardium and myocardium of the AV and OFT valve forming regions in the mouse during the valve forming period (E9–E15; Miquerol et al. 1999). Research from a number of groups has shown that there is a particularly narrow window of VEGF expression required for proper EMT to occur. While 50% reductions in VEGF expression are lethal at E9.5 in the mouse, two- to threefold exogenous overexpression of VEGF also results in severe cardiac abnormalities and death by E14 (Miquerol et al. 2000). The cellular source of VEGF is also a determinant of EMT. VEGF secreted from myocardium is an antagonist of EMT, shown by Chang et al. (2004) to be blocked by the transcriptional regulator NFATc1. Deficient myocardial or endocardial NFATc1 expression results in hypoplastic cushions with limited EMT, and similar results occur by blocking calcineurin signalling. The homeobox gene Hhex is also an antagonist of myocardial VEGF expression, disruption of which also results in dramatically reduced EMT (Hallaq et al. 2004). Experiments in zebrafish, however, show that VEGF receptor signalling is still required for EMT, and is associated with expression of NOTCH1 and BMP-4 (Lee et al. 2006). These results suggest that some basal level of VEGF expression from the endocardium is required for EMT, potentially to maintain endocardial integrity during the transformation process. VEGF acts on endocardium through its receptor Flk-1 to transmit calcium to calcineurin from the endoplasmic reticulum by the IP3 second messenger pathway. Calcineurin then phosphorylates NFATc1-mediated proliferation and PECAM1 (CD31) expression (Johnson et al. 2003). NFATc1 also upregulates DSCR1, which has been shown to antagonize NFATc1 expression in a potential negative feedback loop (Lange et al. 2004). Another critical source of calcium entry into this pathway is mediated through connexin 45 in endocardial cell contacts. The connexin 45 null mutants do not undergo EMT due to inhibited NFATc1 signalling (Kumai et al. 2000) indicating the importance of the latter in regulating EMT.
Wnt/β-catenin. Wnt/β-catenin signalling, originally identified in Drosophila cell fate determination, was recently shown to be critical for valvulogenesis in zebrafish. Inhibition of Wnt signalling by Dickkopf 1 (Dkk1) overexpression resulted in no EMT, while overexpression of constitutively active Wnt causes excessive cushion formation throughout the endocardium (Hurlstone et al. 2003). Wnt/β-catenin signalling was shown to be normally restricted to the endocardium above valve-forming regions in zebrafish and mouse (Gitler et al. 2003a), therefore, suggesting that proper expression of this pathway is also critical for valvulogenesis. Controlled inhibition of this pathway is potentially mediated through PECAM1 expressed on the endocardial surface, which can sequester and inactivate phosphorylated β-catenin (Ilan et al. 1999).
NOTCH. Notch is another cell fate determining signal pathway expressed in embryonic and post-natal tissues, and is also expressed during mesenchymal and oncogenic transformation. NOTCH1 is expressed in the valvulogenic regions of zebrafish and mouse endocardium, and induces EMT by a selective induction of the TGF-β signalling pathway through transcription factor snail repression of endothelial markers (VE-cadherin, TIE, TEK and PECAM1), upregulation of mesenchymal markers (α-SMA, PDGFR and fibronectin), which results in an activated and destabilized endocardium that transforms to mesenchyme (Timmerman et al. 2004). Constitutive activation of NOTCH1 results in hypercellular valves, with disruption of NOTCH signalling causing no EMT to occur. Activation/repression of the NOTCH receptor Jagged-1 (Jag1) gives similar results (Noseda et al. 2004). Another NOTCH ligand, Dll4, is expressed by developing endothelium and has been shown to repress VEGF signalling by downregulating receptor activity (Williams et al. 2006). Dll4 expression may therefore serve as a negative feedback loop to control endocardial VEGF expression during EMT.
BMP. In addition to their osteogenic properties, bone morphogenetic proteins are important mediators of early valvulogenic events. Several BMPs are expressed in the heart through development, two with particular expression patterns and functions in valvulogenic regions: BMP-2 and BMP-4 (Abdelwahid et al. 2001). Both have similar functions, but different regions of action; BMP-2 at the atrioventricular canal and BMP-4 in the outflow tract. Before the onset of EMT, these proteins are expressed in the myocardium. In vitro assays using both chick and mouse AV explants show that myocardial BMP-2 expression (and probably BMP-4 in the OFT) is necessary and sufficient to induce endocardial transformation (Sugi et al. 2004). BMP-2 activates BMP-RI/RII receptor heterodimers, phosphorylates smad1/5, which coupled with smad4 drives transcription of mesenchymal differentiation (increased α-SMA, decreased VE-cadherin; Chen & Massague 1999). Although BMP-4 null mice die before onset of valve formation (Winnier et al. 1995), inhibition of BMP signalling through noggin results in complete inhibition of EMT (Sugi et al. 2004). Defects in BMP receptors are either similarly lethal or result in inability to form outflow tract valves, suggesting that BMP signalling may be more significant for outflow tract valves (Delot et al. 2003). While knockouts of other BMPs are relatively benign, a double knockout of BMP-6 and BMP-7 results in similar deficiencies in outflow tract valve development, suggesting compensation between BMPs (Kim et al. 2001b).
TGF-β. BMPs are part of the transforming growth factor beta superfamily. TGF-β ligands are mediators of many important physiological and pathological cellular differentiation events. TGF ligands are expressed in the heart during development, but there are subtle differences between chick and mouse embryos. TFG-β2 and TGF-β3 are expressed in chick (Boyer et al. 1999), while TGF-β1 and TGF-β2 are expressed in mouse (Millan et al. 1991). Antagonists to TGF-β2 and TGF-β3 show that they play distinct but complementary roles in regulating the initial transformation events in chick (figure 2a). Endocardial TGF-β2 expression is required for cell separation and hypertrophy, while TGF-β3 signalling is required for mesenchymal transformation and migration (Boyer et al. 1999). TGF-β3 induces expression of MMP-2 and MT-MMP, which digest the collagen IV endocardial matrix, permitting invasion (Song et al. 2000). TGF-β2 is secreted from both the myocardium and endocardium in chick, but TGF-β3 is secreted by the endocardium in an autocrine manner upon induction by BMP-2 and TGF-β2 (Boyer et al. 1999; Nakajima et al. 2000). TGF-β binds heterodimers of type II (TBRII) and type III (TBRIII) receptors in both chick and mouse endocardium, and antisera to these receptors blocks EMT in these models (Brown et al. 1996; Brown et al. 1999). TGF-β2 in the mouse has been shown to activate mesenchymal transformation through phosphorylation of smad2/3 (figure 2b), which then activates transcription through snail/slug (Romano & Runyan 2000) or by complexing with smad4 (Greene et al. 2003).
Extracellular matrix. The cardiac jelly underlying the valvulogenic endocardium comprises the glycosaminoglycan hyaluronan (HA) primarily. HA is an important extracellular matrix component in both valvulogenesis and tumorigenesis due to its ability to induce cell signalling (Toole 1997). The majority of the hyaluronan in the cardiac jelly is secreted by the myocardium, a process involving UDP-glucose dehydrogenase (UGDH) and hyaluronan synthase 2 (Walsh & Stainier 2001). Digestion of cultured embryos with hyaluronidase (Baldwin et al. 1994) or disruption of hyaluronan synthesis results in no cushion formation, but could be restored with the addition of exogenous HA (Camenisch et al. 2000). Additionally, disruption of Ras activation causes a similar phenotype, and expression of constitutively active Ras can rescue EMT in Has2 null explants. HA in valvulogenesis activates Ras through the phosphorylation of heterodimeric ErbB receptors, which is also achieved by exogenous treatment of heregulin, which restores EMT in Has2 null explants (Camenisch et al. 2002). The proteoglycan versican may also be involved in this extracellular signalling pathway, as versican null mice do not make valvular cushions (Mjaatvedt et al. 1998). Versican serves as a linking protein between hyaluronan and collagen, and may therefore play a critical structural role in the cardiac jelly. The precise role of versican in valvulogenesis, however, remains to be determined.
3. Post-EMT cushion valvular maturation
The fully mesenchymalized cushions that act as primitive valves during early cardiogenesis are in no way sufficient as functioning valves in the mature heart. As described before, mature valves are thin fibrous leaflets with largely fibroblastic cells entombed in a highly organized matrix. The process by which these cushions differentiate into valves is largely unknown, but recent mutant mouse models have revealed important signal pathways that are involved (figure 3). Mouse embryos survive for only a few hours in culture after E12, limiting their use for more controlled ex vivo manipulation (Kubalak et al. 2002). Chick embryos, on the other hand, are readily available, and the maturation of the left AV valve and semilunar valves closely parallels those of humans. Many of the same factors that initiate valvular formation are also involved in its termination, but cross-talk and feedback loops between these pathways are probably responsible for normal valvular maturation. For clarity, the roles of each individual pathway and their connections will again be addressed separately.
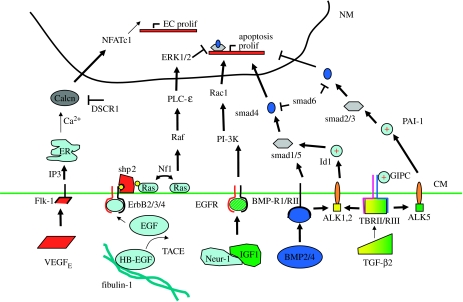
Figure 3.
The regulation of post-cushion valve maturation to thin fibrous leaflets also includes EGF receptor signalling in addition to differential signalling of the pathways in figure 2c. CM, cell membrane; NM, nuclear membrane. (+) denotes positive potentiometer of particular pathway.
FGF. Continued invasion and proliferation of the transformed mesenchyme are controlled at least in part by growth factor signalling from the endocardium. Recent evidence implicates fibroblast growth factor-4 (FGF-4) in mediating this process. FGF-4 is present in mesenchymal cells during avian cushion morphogenesis, as well as FGF receptors 1, 2 and 3 (Sugi et al. 2003). Retroviral overexpression or exogenous addition of FGF-4 resulted in abundant mesenchymal proliferation as indicated by BRDU staining both in vitro and in vivo. FGF-induced mesenchymal proliferation continues to the end of valvular maturation (HH36). FGF-2 has also been shown to inhibit apoptosis of cushion mesenchyme (Zhao & Rivkees 2000). These results suggest that FGF signalling promotes mesenchyme proliferation throughout valvular development and maturation.
VEGF. VEGF expression is markedly increased in the endocardium and cushion mesenchyme after the onset of EMT (E9.5) but is expressed only in the endocardium by E14.5. In addition to its previously described roles, VEGF signalling induces endocardial/endothelial cell proliferation in adult valves mediated by activation of transcription factor NFATc1 (Johnson et al. 2003). By using inhibitors of NFATc1 or calcineurin, Chang et al. (2004) determined that a field of endocardial NFATc1 expression is necessary for proper elongation and condensation of cushion mesenchyme into thin leaflets, and blocking this expression results in persistent rounded hypercellular cushions. Coupled with the fact that high levels of VEGF expression correlate with a lack of mesenchymal transformation (Dor et al. 2001), these results suggest that endocardially expressed VEGF may act through NFATc1 to induce endocardial proliferation necessary to accommodate the dramatically increasing leaflet surface area while inhibiting mesenchymal proliferation.
BMP. BMPs are expressed in valve mesenchyme during the elongation process (BMP-2 in AV region, BMP-4 in the outflow tract). In addition to promoting mesenchyme formation through smad1/5 signalling, BMP2/4 expression correlates with apoptosis in the maturing valves (Abdelwahid et al. 2001), but a mechanism for this has not been determined.
TGF-β. Transforming growth factor beta signalling in the maturing valve is one of the more confusing, because both pro- and anti-proliferative responses have been reported. TGF-β2 deletion results in thickened hypercellular valves among other cardiac defects, and this phenotype is not mimicked by either TGF-β1-/- or TGF-β3-/- mice (Sanford et al. 1997). TGF-β2 ligands bind to TGF-β receptors II and III and modulate mesenchymal cell response depending on how it complexes with additional activin-like kinase (ALK) receptors (Desgrosellier et al. 2005). TGF-β2 stimulates proliferation and migration through complexing with Alk1 or Alk2 (which also interact with BMP receptors) and activating the smad1/5 transduction pathway, potentiated by Id1 (Goumans et al. 2002). The inhibition of proliferation and migration by TGF-β2 were determined through receptor complexing with Alk5 and activation of smad2/3, potentiated by PAI-1 (Lai et al. 2000). The transition between these two pathways may be mediated by GIPC (GAIP-interacting protein, C terminus), which has been shown to enhance TBRIII sensitivity to TGF-β signalling, promoting the PAI-1-mediated inhibition of proliferation (Blobe et al. 2001). Additional evidence supports a dose response mechanism by which low levels of TGF-β2 activate proliferation via smad1/5, while higher levels inhibit proliferation through the smad2/3 pathway (Goumans et al. 2002). Taken together, these results suggest that increasingly greater TGF-β2 expression is required during the later stages of valvulogenesis to ensure appropriate reduction in mesenchymal proliferation.
EGF. Epidermal growth factor signalling, while not a significant regulator of initial EMT, is a significant mediator of valve maturation. EGF is both secreted and released from matrix bound forms, such as HB–EGF (Iwamoto et al. 2003), through proteases like TACE/Adam17 (Jackson et al. 2003). EGF binds to a heterodimeric receptor containing EGF and ErbB receptor partners. Binding-induced phosphorylation also activates the tyrosine phosphatase shp2, which in turn signals ERK-mediated inhibition of proliferation by a Ras-specific pathway (Araki et al. 2004). Disruption of HB–EGF, TACE/Adam17, EGFR, ErbB2 or ErbB3 all result in thickened hyperplastic valves that contain overabundant mesenchyme proliferation but are defective in maintaining unidirectional blood flow (Erickson et al. 1997; Chen et al. 2000; Jackson et al. 2003). A similar phenotype is observed with constitutively active Ras or shp2 (Chen et al. 2000). Deletion of phospholipase C-epsilon, downstream of Ras, also results in hyperplastic valves, but with a concomitant increase in BMP signalling through smad1/5 (Tadano et al. 2005). Indeed, ERK phosphorylation specifically inhibits smad1 accumulation in the nucleus (Kretzschmar et al. 1997), suggesting that the expression of these pathways maintains a well-controlled balance during valvular maturation with ERK-mediated cessation of proliferation and apoptosis required. An additional feedback loop of this pathway is achieved through neurofibromatosis 1 (NF1), which regulates the dephosphorylation of Ras. Deletion of NF1 causes elevated Ras levels, resulting in overabundant mesenchymal cushions due to increased proliferation and a lack of apoptosis (Lakkis & Epstein 1998). AV explants from NF1-/- mice also have greater nuclear localization of NFATc1, suggesting that elevated Ras may also effect VEGF signalling though NFATc1 (Gitler et al. 2003b). Additional growth factor signalling through EGF receptors is achieved by neuregulin and insulin growth factor-1. Combined treatment of Neur-1 and IGF-1 in AV explants induced mesenchymal proliferation via PI 3-kinase and Rac1 (Hertig et al. 1999). Competition for EGF receptors by EGF and Neuregulin may account for yet another feedback loop for valvular maturation.
4. Delamination/maturation of the atrioventricular valves—the Periostin hypothesis
Critical steps in the development of the atrioventricular valves that have yet to be elucidated are how the atrioventricular valves delaminate from the ventricular walls and why cushion cells differentiate only into fibroblastic and myofibroblastic cells. We have previously shown that cushion mesenchyme can differentiate de novo into myocardial cells consistent with the presence of sarcomeric protein expression of some interstitial cells in adult valves (van den Hoff et al. 1999). High-density (pooled 20 or more AV canals) cultures of HH26 AV cushion cells can also differentiate into cartilaginous cells expressing type II collagen, which can be inhibited by conditioned medium from low-density cultured cushions (figure 4a,b). Additionally, valves from smad6 null mice exhibit cartilage, bone and pockets of bone marrow in the leaflets (Galvin et al. 2000). Some secreted protein must be responsible for the inhibition of this tendency to form cells of myocardial or osteo/chondral lineage.
Figure 4.
Periostin is a strong candidate protein involved in the delamination of primitive cushions from the myocardial wall and maintenance of a fibroblastic phenotype. AV cushion mesenchyme cultured in vitro (a) spontaneously differentiates into cartilage as demonstrated by Alcain blue staining in (b), which can be inhibited by a conditioned medium from lower density cultures, suggesting the presence of a secreted inhibitor. Periostin (green) is expressed strongly at (c) the myocardial/mesenchymal boarder in both outflow and (d) atrioventricular valves. (e) Cushion micromass cultures treated with periostin overexpressing virus remain mesenchymal, (f) but the addition of exogenous BMP-2 drastically reduces periostin expression concomitant with osteogenic differentiation.
To identify possible transcripts involved in post-EMT valve remodelling, we conducted a microarray comparison of E10.5 mouse hearts and whole embryos, and found several robustly expressed genes in the heart, one of which was periostin, related to the Drosophila fasciclin gene family. In situ hybridization and immunohistochemistry have shown abundant expression of periostin in the atrioventricular cushion mesenchyme, with strong staining at the subendocardial layer and at the cushion myocardial interface in both the inflow and outflow cushions (Kruzynska-Frejtag et al. 2001; Norris et al. 2004; Kern et al. 2005; figure 4c,d). Expression continues through the delamination period, with staining on both sides of the fenestrating myocardium. By HH 34 (E15 in the mouse), prominent expression is found in the tendinous chords, with fibres anchoring into the papillary myocardium and extending into the leaflets. This expression pattern puts periostin at ‘the scene of the crime’ of cushion delamination. Periostin has been previously identified at sites of mesenchymal stem or ‘blast’ cells that have the potential to form bone but do not so long as they reside in a periostin-rich matrix (Horiuchi et al. 1999; Kruzynska-Frejtag et al. 2004). Periostin is also highly expressed in preosteoblastic cell lines (e.g. MC3T3) cells, but the expression is downregulated once the cells are induced to form bone or cartilage (Litvin et al. 2004). These results suggest, therefore, that periostin may be the secreted protein that may be responsible for these critical processes. Periostin primarily may act to enhance or maintain a fibroblastic cell lineage, and therefore inhibit myocardial and/or osteochondral differentiation. To test this hypothesis, additional high-density cushion cell cultures were infected with a periostin overexpressing virus and remained mesenchymal (figure 4e). These were then treated with abnormally high concentrations of BMP-2, which resulted in almost a complete downregulation of periostin expression (figure 4f). These results show that periostin expression is consistent with a fibroblastic phenotype, and loss of this expression results in the enhancement and/or persistence of myocardial, chondrogenic and osteogenic phenotype. Further experiments will help confirm this hypothesis.
5. Mechanics and valvulogenesis
The embryonic heart is lined continuously with endocardium that may sense and responds to changing haemodynamics, but this remains a vastly understudied area. Scanning electron microscopy of excised hearts shows prominent endocardial cell alignment with the direction of flow at particular areas of the heart such as the atrial floor entering the atrioventricular canal (Icardo & Colvee 1995). Additionally, ligation of a vitelline vein alters the endocardial cell alignment in the atria near the point of venous entry. Experiments with adult valvular endothelial cells show that these cells align perpendicular to flow, potentially complicating the relationship between haemodynamic flow direction and endocardial cell alignment (Butcher et al. 2004). Adult ventricular endocardial cells exposed to controlled shear stresses respond with vasoactive prostaglandin secretion (Hanada et al. 2000). Three genes noted for vascular haemodynamic regulation, endothelial nitric oxide synthase (eNOS), kruppel-like lung factor (KLF2) and endothelin-1 (ET-1) have also been profiled through cardiac development (Groenendijk et al. 2004). eNOS and KLF2 are colocalized in regions of narrowed cardiac lumens, potentially indicative of high wall shear stress. ET-1, on the other hand, was expressed preferentially at sites corresponding to lower wall shear stresses. Complementary expression of these genes along the same cardiac circumference suggests highly localized endocardial cell response to haemodynamics. Changes in the expression patterns of these genes was clearly evident by vitelline vein ligation (Groenendijk et al. 2005), further suggesting that endocardial cells can sense localized changes in haemodynamics and respond with transcriptional changes.
The most explored endocardially mediated phenomenon is the development of the heart valves, which raises the question: Does haemodynamics regulate valvulogenesis? This question has received very little attention in the past, but recent evidence suggests that this question is more complicated than it sounds. Occlusion of the primitive cardiac tube at the inlet or outlet results in disrupted valvulogenesis and cardiac dysfunction, with wall shear stresses approximately 10-fold less than normal (Hove et al. 2003). Unfortunately, the myocardial wall was also severely affected by the occlusion, making it difficult to separate the endocardial and myocardial effects. Another study by Bartman et al. (2004) determined a lack of endocardial cell formation at the atrioventricular canal in zebrafish when myocardial contractile proteins are defective. Pharmacological impairment of myocardial function determined a range of impaired myocardial function that results in no endocardial ring formation. No haemodynamic assessments were conducted on these embryos to determine whether or not flow was present, thus the same difficulty persists. Endocardial cell formation occurs in chick and mice regardless of valvular development, suggesting that this step in valvulogenesis is specific only to zebrafish (Walsh & Stainier 2001). However, mice deficient in erythrocyte tropomodulin (E-Tmod) also have no ventricular contraction (but they do have atrial contractions) and an apparent inability to establish blood flow (Sibilia et al. 2003). These mice die at E10.5, which is after the initiation of valvulogenesis, but it is unclear to what degree mesenchymal transformation is impaired. To further complicate matters, several mutant mouse models with absent valvulogenesis such as NFATc1-/- appear to have normal contraction patterns (Phoon et al. 2004). Furthermore, many of the factors directly responsible for the initiation of mesenchymal transformation (VEGF, BMP, TGF-β, NOTCH) have been demonstrated to be regulated by both shear stress and mechanical strain (Butcher & Nerem 2007) in adult endothelial cells. These results suggest that both cell strain and fluid shear stress have the capability to induce the expression of factors leading to mesenchymal transformation. More controlled in vitro experiments are required to appropriately separate the contributions of these factors in order to understand their unique contributions.
Mechanical forces may also play a role in the remodelling of the primitive valvular cushions into fibrous leaflets. Endothelial changes with the formation of the semilunar valves show striking correlation to changes in local haemodynamics, suggesting that haemodynamics may regulate the excavation of these cushions (Colvee & Hurle 1983). Haemodynamic changes associated with conotruncal banding in chick result in persistant right atrioventricular cushions, suggesting impaired myocardialization of these cushions (Sedmera et al. 1999). Mechanical inhibition of the impalement of the outflow tract into the interventricular groove results in hyperplastic inflow and outflow valve cushions (Colvee & Hurle 1983). It is unclear what effects-specific changes in flow have because neither of these models has been quantitatively characterized. These results demonstrate that mechanical forces play an important, but as yet undetermined role in this process, and further studies will help reveal these functions.
6. Conclusions
Valvulogenesis occurs under a continuous onslaught of fluid shear stress and mechanical strain. The changing shape of the heart and extension of the valves into the flow field suggests that these forces are playing a critical role in this process, but much more work needs to be done. What is becoming more appreciated is that changes (increases) in tissue stress correlates with growth and mechanical strengthening. Primitive valvular cushions begin as poorly organized isotropic swellings of undifferentiated mesenchymal cells, much like the initial makeup of engineered tissue constructs. Mechanical conditioning paradigms have shown results in ‘maturing’ this tissue, but these exercise regimes have not been based on any developmentally relevant scheme. In fact, many of these studies focus on engineering adult valve structure and then strengthen them through mechanical conditioning. An approach that has been paid far less attention is to instead create immature developmental tissue and stimulate maturation by using the monotonically increasing embryonic haemodynamic regime reviewed here. Preliminary evidence suggests that embryonic cushions can remodel in a haemodynamic environment, lengthening into the lumen, even contacting the opposing surface (Goodwin et al. 2005).
One major concern that will need to be overcome for the success of engineered valvular tissue is a cell source. Endocardially derived cushion mesenchyme has the potential to differentiate into cartilage, bone and muscle, but usually becomes largely fibroblastic. While one study suggests the feasibility of achieving useful cell numbers by tricuspid valve biopsy, the potential to damage this valve in the process mitigates the effectiveness of this technique. Immune response limitations suggest that an autologous cell source is needed. Stem and precursor cells have been identified in many tissues, and recently we discovered that approximately 10–20% of the cells that make up adult valves come from haematopoetic origins (Visconti et al. 2006). This suggests that stem cells may be able to repopulate valvular tissues and recapitulate valvular cell phenotype. What is unknown is what point along the continuous spectrum of valvular cell phenotype is to be targeted and how to stimulate appropriate mature phenotype.
In summary, engineers and clinicians could well benefit from the information that developmental biologists bring to the table in their pursuit of cardiac and valvular cell regenerative strategies. The continued understanding of how valves mature embryonically can be fed to engineers as they design new scaffolds and stimulation regimes that mimic this proven system. Results from animal implant models and manipulated embryo models can be compared together to find common pathways. Together, the future goal of functional living valve regeneration can be realized sooner.
Acknowledgments
All studies using mice were performed using protocols approved by institutional review boards and the research facilities are all IACUC approved. Studies using chick embryos were all performed prior to embryonic day 14, and are therefore exempt from institutional review.
Footnotes
One contribution of 21 to a Theme Issue ‘Bioengineering the heart’.
References
- Abdelwahid E, Rice D, Pelliniemi L.J, Jokinen E. Overlapping and differential localization of BMP-2, BMP-4, MSX-2 and apoptosis in the endocardial cushion and adjacent tissues of the developing mouse heart. Cell Tissue Res. 2001;305:67–78. doi: 10.1007/s004410100399. doi:10.1007/s004410100399 [DOI] [PubMed] [Google Scholar]
- Adamo R.F, Guay C.L, Edwards A.V, Wessels A, Burch J.B. GATA-6 gene enhancer contains nested regulatory modules for primary myocardium and the embedded nascent atrioventricular conduction system. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2004;280:1062–1071. doi: 10.1002/ar.a.20105. doi:10.1002/ar.a.20105 [DOI] [PubMed] [Google Scholar]
- Araki T, et al. Mouse model of Noonan syndrome reveals cell type- and gene dosage-dependent effects of Ptpn11 mutation. Nat. Med. 2004;10:849–857. doi: 10.1038/nm1084. doi:10.1038/nm1084 [DOI] [PubMed] [Google Scholar]
- Bajolle F, Zaffran S, Kelly R.G, Hadchouel J, Bonnet D, Brown N.A, Buckingham M. Rotation of the myocardial wall of the outflow tract is implicated in the normal positioning of the great arteries. Circ. Res. 2006;98:421–428. doi: 10.1161/01.RES.0000202800.85341.6e. doi:10.1161/01.RES.0000202800.85341.6e [DOI] [PubMed] [Google Scholar]
- Baldwin H.S, Lloyd T.R, Solursh M. Hyaluronate degradation affects ventricular function of the early postlooped embryonic rat heart in situ. Circ. Res. 1994;74:244–252. doi: 10.1161/01.res.74.2.244. [DOI] [PubMed] [Google Scholar]
- Bartman T, Walsh E.C, Wen K.K, McKane M, Ren J, Alexander J, Rubenstein P.A, Stainier D.Y. Early myocardial function affects endocardial cushion development in zebrafish. PLoS Biol. 2004;2:E129. doi: 10.1371/journal.pbio.0020129. doi:10.1371/journal.pbio.0020129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernanke D.H, Markwald R.R. Migratory behavior of cardiac cushion tissue cells in a collagen-lattice culture system. Dev. Biol. 1982;91:235–245. doi: 10.1016/0012-1606(82)90030-6. doi:10.1016/0012-1606(82)90030-6 [DOI] [PubMed] [Google Scholar]
- Blobe G.C, Liu X, Fang S.J, How T, Lodish H.F. A novel mechanism for regulating transforming growth factor β (TGF-β) signaling. Functional modulation of type III TGF-beta receptor expression through interaction with the PDZ domain protein, GIPC. J. Biol. Chem. 2001;276:39 608–39 617. doi: 10.1074/jbc.M106831200. doi:10.1074/jbc.M106831200 [DOI] [PubMed] [Google Scholar]
- Boyer A.S, Ayerinskas I.I, Vincent E.B, McKinney L.A, Weeks D.L, Runyan R.B. TGFβ2 and TGFβ3 have separate and sequential activities during epithelial–mesenchymal cell transformation in the embryonic heart. Dev. Biol. 1999;208:530–545. doi: 10.1006/dbio.1999.9211. doi:10.1006/dbio.1999.9211 [DOI] [PubMed] [Google Scholar]
- Brown C.B, Boyer A.S, Runyan R.B, Barnett J.V. Antibodies to the Type II TGFβ receptor block cell activation and migration during atrioventricular cushion transformation in the heart. Dev. Biol. 1996;174:248–257. doi: 10.1006/dbio.1996.0070. doi:10.1006/dbio.1996.0070 [DOI] [PubMed] [Google Scholar]
- Brown C.B, Boyer A.S, Runyan R.B, Barnett J.V. Requirement of type III TGF-β receptor for endocardial cell transformation in the heart. Science. 1999;283:2080–2082. doi: 10.1126/science.283.5410.2080. doi:10.1126/science.283.5410.2080 [DOI] [PubMed] [Google Scholar]
- Butcher J.T, Nerem R.M. Valvular endothelial cells and the mechanoregulation of valvular pathology. Phil. Trans. R. Soc. B. 2007;362:1445–1457. doi: 10.1098/rstb.2007.2127. doi:10.1098/rstb.2007.2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher J.T, Penrod A.M, Garcia A.J, Nerem R.M. Unique morphology and focal adhesion development of valvular endothelial cells in static and fluid flow environments. Arterioscler. Thromb. Vasc. Biol. 2004;24:1429–1434. doi: 10.1161/01.ATV.0000130462.50769.5a. doi:10.1161/01.ATV.0000130462.50769.5a [DOI] [PubMed] [Google Scholar]
- Camenisch T.D, Spicer A.P, Brehm-Gibson T, Biesterfeldt J, Augustine M.L, Calabro A, Jr, Kubalak S, Klewer S.E, McDonald J.A. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J. Clin. Invest. 2000;106:349–360. doi: 10.1172/JCI10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camenisch T.D, Schroeder J.A, Bradley J, Klewer S.E, McDonald J.A. Heart-valve mesenchyme formation is dependent on hyaluronan-augmented activation of ErbB2–ErbB3 receptors. Nat. Med. 2002;8:850–855. doi: 10.1038/nm742. [DOI] [PubMed] [Google Scholar]
- Chang C.P, Neilson J.R, Bayle J.H, Gestwicki J.E, Kuo A, Stankunas K, Graef I.A, Crabtree G.R. A field of myocardial-endocardial NFAT signaling underlies heart valve morphogenesis. Cell. 2004;118:649–663. doi: 10.1016/j.cell.2004.08.010. doi:10.1016/j.cell.2004.08.010 [DOI] [PubMed] [Google Scholar]
- Chen Y.G, Massague J. Smad1 recognition and activation by the ALK1 group of transforming growth factor-beta family receptors. J. Biol. Chem. 1999;274:3672–3677. doi: 10.1074/jbc.274.6.3672. doi:10.1074/jbc.274.6.3672 [DOI] [PubMed] [Google Scholar]
- Chen B, et al. Mice mutant for Egfr and Shp2 have defective cardiac semilunar valvulogenesis. Nat. Genet. 2000;24:296–299. doi: 10.1038/73528. doi:10.1038/73528 [DOI] [PubMed] [Google Scholar]
- Colvee E, Hurle J.M. Maturation of the extracellular material of the semilunar heart values in the mouse. A histochemical analysis of collagen and mucopolysaccharides. Anat. Embryol. (Berl.) 1981;162:343–352. doi: 10.1007/BF00299977. [DOI] [PubMed] [Google Scholar]
- Colvee E, Hurle J.M. Malformations of the semilunar valves produced in chick embryos by mechanical interference with cardiogenesis. An experimental approach to the role of hemodynamics in valvular development. Anat. Embryol. (Berl.) 1983;168:59–71. doi: 10.1007/BF00305399. doi:10.1007/BF00305399 [DOI] [PubMed] [Google Scholar]
- De la Cruz, M. V. & Markwald, R. R 2000 Embryological development of the ventricular inlets. Septation and atrioventricular valve apparatus. In Living morphogenesis of the heart (eds M. V. De la Cruz & R. R. Markwald), pp. 131–157. Boston, MA: Birkhauser.
- de Lange F.J. Lineage and morphogenetic analysis of the cardiac valves. Circ. Res. 2004;95:645–654. doi: 10.1161/01.RES.0000141429.13560.cb. doi:10.1161/01.RES.0000141429.13560.cb [DOI] [PubMed] [Google Scholar]
- Deck J.D, Thubrikar M.J, Garcia-Martinez V, Rojo M, Hurlé J.M. Structure, stress, and tissue repair in aortic valve leaflets. Cardiovasc. Res. 1988;22:7–16. doi: 10.1093/cvr/22.1.7. [DOI] [PubMed] [Google Scholar]
- Delot E.C, Bahamonde M.E, Zhao M, Lyons K.M. BMP signaling is required for septation of the outflow tract of the mammalian heart. Development. 2003;130:209–220. doi: 10.1242/dev.00181. doi:10.1242/dev.00181 [DOI] [PubMed] [Google Scholar]
- Desgrosellier J.S, Mundell N.A, McDonnell M.A, Moses H.L, Barnett J.V. Activin receptor-like kinase 2 and Smad6 regulate epithelial–mesenchymal transformation during cardiac valve formation. Dev. Biol. 2005;280:201–210. doi: 10.1016/j.ydbio.2004.12.037. doi:10.1016/j.ydbio.2004.12.037 [DOI] [PubMed] [Google Scholar]
- Dor Y, Camenisch T.D, Itin A, Fishman G.I, McDonald J.A, Carmeliet P, Keshet E. A novel role for VEGF in endocardial cushion formation and its potential contribution to congenital heart defects. Development. 2001;128:1531–1538. doi: 10.1242/dev.128.9.1531. [DOI] [PubMed] [Google Scholar]
- Erickson S.L, O'Shea K.S, Ghaboosi N, Loverro L, Frantz G, Bauer M, Lu L.H, Moore M.W. ErbB3 is required for normal cerebellar and cardiac development: a comparison with ErbB2-and heregulin-deficient mice. Development. 1997;124:4999–5011. doi: 10.1242/dev.124.24.4999. [DOI] [PubMed] [Google Scholar]
- Galvin K.M, et al. A role for smad6 in development and homeostasis of the cardiovascular system. Nat. Genet. 2000;24:171–174. doi: 10.1038/72835. doi:10.1038/72835 [DOI] [PubMed] [Google Scholar]
- Garcia-Martinez V, Sanchez-Quintana D, Hurle J.M. Histochemical and ultrastructural changes in the extracellular matrix of the developing chick semilunar heart valves. Acta. Anat. (Basel) 1991;142:87–96. doi: 10.1159/000147166. [DOI] [PubMed] [Google Scholar]
- Gitler A.D, Lu M.M, Jiang Y.Q, Epstein J.A, Gruber P.J. Molecular markers of cardiac endocardial cushion development. Dev. Dyn. 2003a;228:643–650. doi: 10.1002/dvdy.10418. doi:10.1002/dvdy.10418 [DOI] [PubMed] [Google Scholar]
- Gitler A.D, Zhu Y, Ismat F.A, Lu M.M, Yamauchi Y, Parada L.F, Epstein J.A. Nf1 has an essential role in endothelial cells. Nat. Genet. 2003b;33:75–79. doi: 10.1038/ng1059. doi:10.1038/ng1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittenberger-de Groot A.C, Vrancken Peeters M.P, Bergwerff M, Mentink M.M, Poelmann R.E. Epicardial outgrowth inhibition leads to compensatory mesothelial outflow tract collar and abnormal cardiac septation and coronary formation. Circ. Res. 2000;87:969–971. doi: 10.1161/01.res.87.11.969. [DOI] [PubMed] [Google Scholar]
- Goodwin R.L, Nesbitt T, Price R.L, Wells J.C, Yost M.J, Potts J.D. Three-dimensional model system of valvulogenesis. Dev. Dyn. 2005;233:122–129. doi: 10.1002/dvdy.20326. doi:10.1002/dvdy.20326 [DOI] [PubMed] [Google Scholar]
- Goumans M.J, Valdimarsdottir G, Itoh S, Rosendahl A, Sideras P, ten Dijke P. Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J. 2002;21:1743–1753. doi: 10.1093/emboj/21.7.1743. doi:10.1093/emboj/21.7.1743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene R.M, Nugent P, Mukhopadhyay P, Warner D.R, Pisano M.M. Intracellular dynamics of Smad-mediated TGFbeta signaling. J. Cell Physiol. 2003;197:261–271. doi: 10.1002/jcp.10355. doi:10.1002/jcp.10355 [DOI] [PubMed] [Google Scholar]
- Groenendijk B.C, Hierck B.P, Gittenberger-De Groot A.C, Poelmann R.E. Development-related changes in the expression of shear stress responsive genes KLF-2, ET-1, and NOS-3 in the developing cardiovascular system of chicken embryos. Dev. Dyn. 2004;230:57–68. doi: 10.1002/dvdy.20029. doi:10.1002/dvdy.20029 [DOI] [PubMed] [Google Scholar]
- Groenendijk B.C, Hierck B.P, Vrolijk J, Baiker M, Pourquie M.J.B.M, Gittenberger-de Groot A.C, Poelmann R.E. Changes in shear stress-related gene expression after experimentally altered venous return in the chicken embryo. Circ. Res. 2005;96:1291–1298. doi: 10.1161/01.RES.0000171901.40952.0d. doi:10.1161/01.RES.0000171901.40952.0d [DOI] [PubMed] [Google Scholar]
- Hallaq H, et al. A null mutation of Hhex results in abnormal cardiac development, defective vasculogenesis and elevated VEGFa levels. Development. 2004;131:5197–5209. doi: 10.1242/dev.01393. doi:10.1242/dev.01393 [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton H.L. A series of normal stages in the development of the chick embryo. 1951. Dev. Dyn. 1992;195:231–272. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- Hammermeister K, Sethi G.K, Henderson W.G, Grover F.L, Oprian C, Rahimtoola S.H. Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: final report of the Veterans Affairs randomized trial. J. Am. Coll. Cardiol. 2000;36:1152–1158. doi: 10.1016/s0735-1097(00)00834-2. doi:10.1016/S0735-1097(00)00834-2 [DOI] [PubMed] [Google Scholar]
- Hanada T, Hashimoto M, Nosaka S, Sasaki T, Nakayama K, Masumura S, Yamauchi M, Tamura K. Shear stress enhances prostacyclin release from endocardial endothelial cells. Life Sci. 2000;66:215–220. doi: 10.1016/s0024-3205(99)00583-4. doi:10.1016/S0024-3205(99)00583-4 [DOI] [PubMed] [Google Scholar]
- Hertig C.M, Kubalak S.W, Wang Y, Chien K.R. Synergistic roles of neuregulin-1 and insulin-like growth factor-I in activation of the phosphatidylinositol 3-kinase pathway and cardiac chamber morphogenesis. J. Biol. Chem. 1999;274:37362–37369. doi: 10.1074/jbc.274.52.37362. doi:10.1074/jbc.274.52.37362 [DOI] [PubMed] [Google Scholar]
- Hoerstrup S.P, et al. Functional living trileaflet heart valves grown in vitro. Circulation. 2000;102:III44–III49. doi: 10.1161/01.cir.102.suppl_3.iii-44. [DOI] [PubMed] [Google Scholar]
- Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, Toyama Y, Bonewald L.F, Kudo A. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J. Bone Miner. Res. 1999;14:1239–1249. doi: 10.1359/jbmr.1999.14.7.1239. doi:10.1359/jbmr.1999.14.7.1239 [DOI] [PubMed] [Google Scholar]
- Hove J.R, Koster R.W, Forouhar A.S, Acevedo-Bolton G, Fraser S.E, Gharib M. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature. 2003;421:172–177. doi: 10.1038/nature01282. doi:10.1038/nature01282 [DOI] [PubMed] [Google Scholar]
- Hurle J.M, Kitten G.T, Sakai L.Y, Volpin D, Solursh M. Elastic extracellular matrix of the embryonic chick heart: an immunohistological study using laser confocal microscopy. Dev. Dyn. 1994;200:321–332. doi: 10.1002/aja.1002000407. [DOI] [PubMed] [Google Scholar]
- Hurlstone A.F, et al. The Wnt/beta-catenin pathway regulates cardiac valve formation. Nature. 2003;425:633–637. doi: 10.1038/nature02028. doi:10.1038/nature02028 [DOI] [PubMed] [Google Scholar]
- Icardo J.M, Colvee E. Atrioventricular valves of the mouse: III. Collagenous skeleton and myotendinous junction. Anat. Rec. 1995;243:367–375. doi: 10.1002/ar.1092430311. doi:10.1002/ar.1092430311 [DOI] [PubMed] [Google Scholar]
- Ilan N, Mahooti S, Rimm D.L, Madri J.A. PECAM-1 (CD31) functions as a reservoir for and a modulator of tyrosine-phosphorylated beta-catenin. J. Cell Sci. 1999;18:3005–3014. doi: 10.1242/jcs.112.18.3005. [DOI] [PubMed] [Google Scholar]
- Iwamoto R, et al. Heparin-binding EGF-like growth factor and ErbB signaling is essential for heart function. Proc. Natl Acad. Sci. USA. 2003;100:3221–3226. doi: 10.1073/pnas.0537588100. doi:10.1073/pnas.0537588100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson L.F, Qiu T.H, Sunnarborg S.W, Chang A, Zhang C, Patterson C, Lee D.C. Defective valvulogenesis in HB-EGF and TACE-null mice is associated with aberrant BMP signaling. EMBO J. 2003;22:2704–2716. doi: 10.1093/emboj/cdg264. doi:10.1093/emboj/cdg264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E.N, Lee Y.M, Sander T.L, Rabkin E, Schoen F.J, Kaushal S, Bischoff J. NFATc1 mediates vascular endothelial growth factor-induced proliferation of human pulmonary valve endothelial cells. J. Biol. Chem. 2003;278:1686–1692. doi: 10.1074/jbc.M210250200. doi:10.1074/jbc.M210250200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern C.B, Hoffman S, Moreno R, Damon B.J, Norris R.A, Krug E.L, Markwald R.R, Mjaatvedt C.H. Immunolocalization of chick periostin protein in the developing heart. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2005;284:415–423. doi: 10.1002/ar.a.20193. [DOI] [PubMed] [Google Scholar]
- Kim J.S, Viragh S, Moorman A.F, Anderson R.H, Lamers W.H. Development of the myocardium of the atrioventricular canal and the vestibular spine in the human heart. Circ. Res. 2001a;88:395–402. doi: 10.1161/01.res.88.4.395. [DOI] [PubMed] [Google Scholar]
- Kim R.Y, Robertson J.E, Solloway M.J. BMP6 and BMP7 are required for cushion formation and septation in the developing mouse heart. Dev. Biol. 2001b;235:449–466. doi: 10.1006/dbio.2001.0284. doi:10.1006/dbio.2001.0284 [DOI] [PubMed] [Google Scholar]
- Kirby M.L. Role of extracardiac factors in heart development. Experientia. 1988;44:944–951. doi: 10.1007/BF01939888. doi:10.1007/BF01939888 [DOI] [PubMed] [Google Scholar]
- Kirby M.L, Gale T.F, Stewart D.E. Neural crest cells contribute to normal aorticopulmonary septation. Science. 1983;220:1059–1061. doi: 10.1126/science.6844926. doi:10.1126/science.6844926 [DOI] [PubMed] [Google Scholar]
- Kretzschmar M, Doody J, Massague J. Opposing BMP and EGF signalling pathways converge on the TGF-beta family mediator Smad1. Nature. 1997;389:618–622. doi: 10.1038/39348. doi:10.1038/39348 [DOI] [PubMed] [Google Scholar]
- Kruzynska-Frejtag A, Machnicki M, Rogers R, Markwald R, Conway S.J. Periostin (an osteoblast-specific factor) is expressed within the embryonic mouse heart during valve formation. Mech. Dev. 2001;103:183–188. doi: 10.1016/s0925-4773(01)00356-2. doi:10.1016/S0925-4773(01)00356-2 [DOI] [PubMed] [Google Scholar]
- Kruzynska-Frejtag A, Wang J, Maeda M, Rogers R, Krug E, Hoffman S, Markwald R.R, Conway S.J. Periostin is expressed within the developing teeth at the sites of epithelial–mesenchymal interaction. Dev. Dyn. 2004;229:857–868. doi: 10.1002/dvdy.10453. doi:10.1002/dvdy.10453 [DOI] [PubMed] [Google Scholar]
- Kubalak S.W, Hutson D.R, Scott K.K, Shannon R.A. Elevated transforming growth factor beta2 enhances apoptosis and contributes to abnormal outflow tract and aortic sac development in retinoic X receptor alpha knockout embryos. Development. 2002;129:733–746. doi: 10.1242/dev.129.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumai M, Nishii K, Nakamura K, Takeda N, Suzuki M, Shibata Y. Loss of connexin45 causes a cushion defect in early cardiogenesis. Development. 2000;127:3501–3512. doi: 10.1242/dev.127.16.3501. [DOI] [PubMed] [Google Scholar]
- Lai Y.T, Beason K.B, Brames G.P, Desgrosellier J.S, Cleggett M.C, Shaw M.V, Brown C.B, Barnett J.V. Activin receptor-like kinase 2 can mediate atrioventricular cushion transformation. Dev. Biol. 2000;222:1–11. doi: 10.1006/dbio.2000.9698. doi:10.1006/dbio.2000.9698 [DOI] [PubMed] [Google Scholar]
- Lakkis M.M, Epstein J.A. Neurofibromin modulation of ras activity is required for normal endocardial–mesenchymal transformation in the developing heart. Development. 1998;125:4359–4367. doi: 10.1242/dev.125.22.4359. [DOI] [PubMed] [Google Scholar]
- Lamers W.H, Viragh S, Verbeek F.J, Moorman A.F, Viragh S, Wenink A.C, Gittenberger-de Groot A.C, Anderson R.H. Formation of the tricuspid valve in the human heart. Circulation. 1995;91:111–121. doi: 10.1161/01.cir.91.1.111. [DOI] [PubMed] [Google Scholar]
- Lange A.W, Molkentin J.D, Yutzey K.E. DSCR1 gene expression is dependent on NFATc1 during cardiac valve formation and colocalizes with anomalous organ development in trisomy 16 mice. Dev. Biol. 2004;266:346–360. doi: 10.1016/j.ydbio.2003.10.036. doi:10.1016/j.ydbio.2003.10.036 [DOI] [PubMed] [Google Scholar]
- Lee Y.M, Cope J.J, Ackermann G.E, Goishi K, Armstrong E.J, Paw B.H, Bischoff J. Vascular endothelial growth factor receptor signaling is required for cardiac valve formation in zebrafish. Dev. Dyn. 2006;235:29–37. doi: 10.1002/dvdy.20559. doi:10.1002/dvdy.20559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie-Venema H, Eralp I, Maas S, Gittenberger-De Groot A.C, Poelmann R.E, DeRuiter M.C. Myocardial heterogeneity in permissiveness for epicardium-derived cells and endothelial precursor cells along the developing heart tube at the onset of coronary vascularization. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2005;282:120–129. doi: 10.1002/ar.a.20154. [DOI] [PubMed] [Google Scholar]
- Litvin J, Selim A.H, Montgomery M.O, Lehmann K, Rico M.C, Devlin H, Bednarik D.P, Safadi F.F. Expression and function of periostin-isoforms in bone. J. Cell Biochem. 2004;92:1044–1061. doi: 10.1002/jcb.20115. doi:10.1002/jcb.20115 [DOI] [PubMed] [Google Scholar]
- Millan F.A, Denhez F, Kondaiah P, Akhurst R.J. Embryonic gene expression patterns of TGF beta 1, beta 2 and beta 3 suggest different developmental functions in vivo. Development. 1991;111:131–143. doi: 10.1242/dev.111.1.131. [DOI] [PubMed] [Google Scholar]
- Miquerol L, Gertsenstein M, Harpal K, Rossant J, Nagy A. Multiple developmental roles of VEGF suggested by a LacZ-tagged allele. Dev. Biol. 1999;212:307–322. doi: 10.1006/dbio.1999.9355. doi:10.1006/dbio.1999.9355 [DOI] [PubMed] [Google Scholar]
- Miquerol L, Langille B.L, Nagy A. Embryonic development is disrupted by modest increases in vascular endothelial growth factor gene expression. Development. 2000;127:3941–3946. doi: 10.1242/dev.127.18.3941. [DOI] [PubMed] [Google Scholar]
- Mjaatvedt C.H, Yamamura H, Capehart A.A, Turner D, Markwald R.R. The Cspg2 gene, disrupted in the hdf mutant, is required for right cardiac chamber and endocardial cushion formation. Dev. Biol. 1998;202:56–66. doi: 10.1006/dbio.1998.9001. doi:10.1006/dbio.1998.9001 [DOI] [PubMed] [Google Scholar]
- Mjaatvedt C.H, Nakaoka T, Moreno-Rodriguez R, Norris R.A, Kern M.J, Eisenberg C.A, Turner D, Markwald R.R. The outflow tract of the heart is recruited from a novel heart-forming field. Dev. Biol. 2001;238:97–109. doi: 10.1006/dbio.2001.0409. doi:10.1006/dbio.2001.0409 [DOI] [PubMed] [Google Scholar]
- Mjaatvedt C.H, Kern C.B, Norris R.A, Fairey S, Cave C.L. Normal distribution of melanocytes in the mouse heart. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2005;285:748–757. doi: 10.1002/ar.a.20210. [DOI] [PubMed] [Google Scholar]
- Moorman A.F, Christoffels V.M. Cardiac chamber formation: development, genes, and evolution. Physiol. Rev. 2003;83:1223–1267. doi: 10.1152/physrev.00006.2003. [DOI] [PubMed] [Google Scholar]
- Moorman A.F, Soufan A.T, Hagoort J, de Boer P.A, Christoffels V.M. Development of the building plan of the heart. Ann. NY Acad. Sci. 2004;1015:171–181. doi: 10.1196/annals.1302.014. doi:10.1196/annals.1302.014 [DOI] [PubMed] [Google Scholar]
- Nakajima Y, Mironov V, Yamagishi T, Nakamura H, Markwald R.R. Expression of smooth muscle alpha-actin in mesenchymal cells during formation of avian endocardial cushion tissue: a role for transforming growth factor beta3. Dev. Dyn. 1997;209:296–309. doi: 10.1002/(SICI)1097-0177(199707)209:3<296::AID-AJA5>3.0.CO;2-D. doi:10.1002/(SICI)1097-0177(199707)209:3<296::AID-AJA5>3.0.CO;2-D [DOI] [PubMed] [Google Scholar]
- Nakajima Y, Yamagishi T, Hokari S, Nakamura H. Mechanisms involved in valvuloseptal endocardial cushion formation in early cardiogenesis: roles of transforming growth factor (TGF)-β and bone morphogenetic protein (BMP) Anat. Rec. 2000;258:119–127. doi: 10.1002/(SICI)1097-0185(20000201)258:2<119::AID-AR1>3.0.CO;2-U. doi:10.1002/(SICI)1097-0185(20000201)258:2<119::AID-AR1>3.0.CO;2-U [DOI] [PubMed] [Google Scholar]
- Norris R.A, Kern C.B, Wessels A, Moralez E.I, Markwald R.R, Mjaatvedt C.H. Identification and detection of the periostin gene in cardiac development. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2004;281:1227–1233. doi: 10.1002/ar.a.20135. doi:10.1002/ar.a.20135 [DOI] [PubMed] [Google Scholar]
- Noseda M, et al. Notch activation results in phenotypic and functional changes consistent with endothelial-to-mesenchymal transformation. Circ. Res. 2004;94:910–917. doi: 10.1161/01.RES.0000124300.76171.C9. doi:10.1161/01.RES.0000124300.76171.C9 [DOI] [PubMed] [Google Scholar]
- Oosthoek P.W, Wenink A.C, Vrolijk B.C, Wisse L.J, DeRuiter M.C, Poelmann R.E, Gittenberger-de Groot A.C. Development of the atrioventricular valve tension apparatus in the human heart. Anat. Embryol. (Berl.) 1998;198:317–329. doi: 10.1007/s004290050187. doi:10.1007/s004290050187 [DOI] [PubMed] [Google Scholar]
- Person A.D, Klewer S.E, Runyan R.B. Cell biology of cardiac cushion development. Int. Rev. Cytol. 2005;243:287–335. doi: 10.1016/S0074-7696(05)43005-3. doi:10.1016/S0074-7696(05)43005-3 [DOI] [PubMed] [Google Scholar]
- Phoon C.K, Ji R.P, Aristizabal O, Worrad D.M, Zhou B, Baldwin H.S, Turnbull D.H. Embryonic heart failure in NFATc1-/- mice: novel mechanistic insights from in utero ultrasound biomicroscopy. Circ. Res. 2004;95:92–99. doi: 10.1161/01.RES.0000133681.99617.28. doi:10.1161/01.RES.0000133681.99617.28 [DOI] [PubMed] [Google Scholar]
- Ramsdell A.F, Markwald R.R. Induction of endocardial cushion tissue in the avian heart is regulated, in part, by TGFβ-3-mediated autocrine signaling. Dev. Biol. 1997;188:64–74. doi: 10.1006/dbio.1997.8637. doi:10.1006/dbio.1997.8637 [DOI] [PubMed] [Google Scholar]
- Romano L.A, Runyan R.B. Slug is an essential target of TGFβ2 signaling in the developing chicken heart. Dev. Biol. 2000;223:91–102. doi: 10.1006/dbio.2000.9750. doi:10.1006/dbio.2000.9750 [DOI] [PubMed] [Google Scholar]
- Rothenberg F, Fisher S.A, Watanabe M. Sculpting the cardiac outflow tract. Birth Defects Res. C Embryo Today. 2003;69:38–45. doi: 10.1002/bdrc.10007. doi:10.1002/bdrc.10007 [DOI] [PubMed] [Google Scholar]
- Sako E.Y. Newer concepts in the surgical treatment of valvular heart disease. Curr. Cardiol. Rep. 2004;6:100–105. doi: 10.1007/s11886-004-0006-y. [DOI] [PubMed] [Google Scholar]
- Sanford L.P, Ormsby I, Gittenberger-de Groot A.C, Sariola H, Friedman R, Boivin G.P, Cardell E.L, Doetschman T. TGFβ2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development. 1997;124:2659–2670. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedmera D, Pexieder T, Hu N, Clark E.B. Developmental changes in the myocardial architecture of the chick. Anat. Rec. 1997;248:421–432. doi: 10.1002/(SICI)1097-0185(199707)248:3<421::AID-AR15>3.0.CO;2-R. doi:10.1002/(SICI)1097-0185(199707)248:3<421::AID-AR15>3.0.CO;2-R [DOI] [PubMed] [Google Scholar]
- Sedmera D, Pexieder T, Rychterova V, Hu N, Clark E.B. Remodeling of chick embryonic ventricular myoarchitecture under experimentally changed loading conditions. Anat. Rec. 1999;254:238–252. doi: 10.1002/(SICI)1097-0185(19990201)254:2<238::AID-AR10>3.0.CO;2-V. doi:10.1002/(SICI)1097-0185(19990201)254:2<238::AID-AR10>3.0.CO;2-V [DOI] [PubMed] [Google Scholar]
- Sibilia M, Wagner B, Hoebertz A, Elliott C, Marino S, Jochum W, Wagner E.F. Mice humanised for the EGF receptor display hypomorphic phenotypes in skin, bone and heart. Development. 2003;130:4515–4525. doi: 10.1242/dev.00664. doi:10.1242/dev.00664 [DOI] [PubMed] [Google Scholar]
- Sinning A.R, Hewitt C.C. Identification of a 283-kDa protein component of the particulate matrix associated with cardiac mesenchyme formation. Acta Anat. (Basel) 1996;155:219–230. doi: 10.1159/000147810. [DOI] [PubMed] [Google Scholar]
- Song W, Jackson K, McGuire P.G. Degradation of type IV collagen by matrix metalloproteinases is an important step in the epithelial–mesenchymal transformation of the endocardial cushions. Dev. Biol. 2000;227:606–617. doi: 10.1006/dbio.2000.9919. doi:10.1006/dbio.2000.9919 [DOI] [PubMed] [Google Scholar]
- Sugi Y, Ito N, Szebenyi G, Myers K, Fallon J.F, Mikawa T, Markwald R.R. Fibroblast growth factor (FGF)-4 can induce proliferation of cardiac cushion mesenchymal cells during early valve leaflet formation. Dev. Biol. 2003;258:252–263. doi: 10.1016/s0012-1606(03)00099-x. doi:10.1016/S0012-1606(03)00099-X [DOI] [PubMed] [Google Scholar]
- Sugi Y, Yamamura H, Okagawa H, Markwald R.R. Bone morphogenetic protein-2 can mediate myocardial regulation of atrioventricular cushion mesenchymal cell formation in mice. Dev. Biol. 2004;269:505–518. doi: 10.1016/j.ydbio.2004.01.045. doi:10.1016/j.ydbio.2004.01.045 [DOI] [PubMed] [Google Scholar]
- Sutherland F.W, et al. From stem cells to viable autologous semilunar heart valve. Circulation. 2005;111:2783–2791. doi: 10.1161/CIRCULATIONAHA.104.498378. doi:10.1161/CIRCULATIONAHA.104.498378 [DOI] [PubMed] [Google Scholar]
- Tadano M, et al. Congenital semilunar valvulogenesis defect in mice deficient in phospholipase C epsilon. Mol. Cell Biol. 2005;25:2191–2199. doi: 10.1128/MCB.25.6.2191-2199.2005. doi:10.1128/MCB.25.6.2191-2199.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R.P, Abercrombie V, Wong M. Morphogenesis of the truncus arteriosus of the chick embryo heart: movements of autoradiographic tattoos during septation. Anat. Rec. 1987;218:434–440. doi: 10.1002/ar.1092180411. doi:10.1002/ar.1092180411 pp. 394–395. [DOI] [PubMed] [Google Scholar]
- Timmerman L.A, et al. Notch promotes epithelial–mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev. 2004;18:99–115. doi: 10.1101/gad.276304. doi:10.1101/gad.276304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toole B.P. Hyaluronan in morphogenesis. J. Intern. Med. 1997;242:35–40. doi: 10.1046/j.1365-2796.1997.00171.x. doi:10.1046/j.1365-2796.1997.00171.x [DOI] [PubMed] [Google Scholar]
- van den Hoff M.J, Moorman A.F, Ruijter J.M, Lamers W.H, Bennington R.W, Markwald R.R, Wessels A. Myocardialization of the cardiac outflow tract. Dev. Biol. 1999;212:477–490. doi: 10.1006/dbio.1999.9366. doi:10.1006/dbio.1999.9366 [DOI] [PubMed] [Google Scholar]
- Visconti R.P, et al. An in vivo analysis of hematopoietic stem cell potential. Hematopoietic origin of cardiac valve interstitial cells. Circ. Res. 2006;98:690–696. doi: 10.1161/01.RES.0000207384.81818.d4. doi:10.1161/01.RES.0000207384.81818.d4 [DOI] [PubMed] [Google Scholar]
- Walsh E.C, Stainier D.Y. UDP-glucose dehydrogenase required for cardiac valve formation in zebrafish. Science. 2001;293:1670–1673. doi: 10.1126/science.293.5535.1670. doi:10.1126/science.293.5535.1670 [DOI] [PubMed] [Google Scholar]
- Webb S, Qayyum S.R, Anderson R.H, Lamers W.H, Richardson M.K. Septation and separation within the outflow tract of the developing heart. J. Anat. 2003;202:327–342. doi: 10.1046/j.1469-7580.2003.00168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels A, Sedmera D. Developmental anatomy of the heart: a tale of mice and man. Physiol. Genomics. 2003;15:165–176. doi: 10.1152/physiolgenomics.00033.2003. [DOI] [PubMed] [Google Scholar]
- Wessels A, Anderson R.H, Markwald R.R, Webb S, Brown N.A, Viragh S, Moorman A.F, Lamers W.H. Atrial development in the human heart: an immunohistochemical study with emphasis on the role of mesenchymal tissues. Anat. Rec. 2000;259:288–300. doi: 10.1002/1097-0185(20000701)259:3<288::AID-AR60>3.0.CO;2-D. doi:10.1002/1097-0185(20000701)259:3<288::AID-AR60>3.0.CO;2-D [DOI] [PubMed] [Google Scholar]
- Williams C.K, Li J.L, Murga M, Harris A.L, Tosato G. Up-regulation of the Notch ligand Delta-like 4 inhibits VEGF-induced endothelial cell function. Blood. 2006;107:931–939. doi: 10.1182/blood-2005-03-1000. doi:10.1182/blood-2005-03-1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winnier G, Blessing M, Labosky P.A, Hogan B.L. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- Wunsch A.M, Little C.D, Markwald R.R. Cardiac endothelial heterogeneity defines valvular development as demonstrated by the diverse expression of JB3, an antigen of the endocardial cushion tissue. Dev. Biol. 1994;165:585–601. doi: 10.1006/dbio.1994.1278. doi:10.1006/dbio.1994.1278 [DOI] [PubMed] [Google Scholar]
- Ya J, van den Hoff J.M, de Boer P.A, Tesink-Taekema S, Franco D, Moorman A.F, Lamers W.H. Normal development of the outflow tract in the rat. Circ. Res. 1998;82:464–472. doi: 10.1161/01.res.82.4.464. [DOI] [PubMed] [Google Scholar]
- Yelbuz T.M, Waldo K.L, Zhang X, Zdanowicz M, Parker J, Creazzo T.L, Johnson G.A, Kirby M.L. Myocardial volume and organization are changed by failure of addition of secondary heart field myocardium to the cardiac outflow tract. Dev. Dyn. 2003;228:152–160. doi: 10.1002/dvdy.10364. doi:10.1002/dvdy.10364 [DOI] [PubMed] [Google Scholar]
- Zhao Z, Rivkees S.A. Programmed cell death in the developing heart: regulation by BMP4 and FGF2. Dev. Dyn. 2000;217:388–400. doi: 10.1002/(SICI)1097-0177(200004)217:4<388::AID-DVDY6>3.0.CO;2-N. doi:10.1002/(SICI)1097-0177(200004)217:4<388::AID-DVDY6>3.0.CO;2-N [DOI] [PubMed] [Google Scholar]