Abstract
Heart valve replacement represents the most common surgical therapy for end-stage valvular heart diseases. A major drawback that all contemporary heart valve replacements have in common is the lack of growth, repair and remodelling capability. In order to overcome these limitations, the emerging new field of tissue engineering is focusing on the in vitro generation of functional, living heart valve replacements. The basic approach uses starter matrices either of decellularized xenogeneic or polymeric materials configured in the shape of the heart valve and subsequent cell seeding. This manuscript will give a detailed overview of these two concepts without giving favour to one or the other. The concluding discussion will focus on current limitations and studies as well as future challenges prior to safe clinical application.
Keywords: tissue engineering, heart valves, heart valve prosthesis, decellularized xenogeneic matrix, biomaterials
1. Introduction
The most common treatment for end-stage valvular diseases is surgical replacement by either mechanical or bioprosthetic heart valves. Mechanical valves display good structural durability but are associated with the risk of prosthetic valve endocarditis, and thromboembolic complications caused by their non-physiological surfaces and flow abnormalities. Life-long anticoagulation therapy is necessary for these patients, associated with a substantial risk of spontaneous bleeding and embolism, particularly in patients over 70 years (Senthilnathan et al. 1999).
Bioprosthetic heart valve replacements are either of animal origin (xenografts), such as porcine aortic valves and bovine pericardial valves, or taken from human donors (homografts). Xenografts are chemically cross-linked, which inhibits autolysis, enhances the mechanical stability and creates the possibility of having valves of different sizes stored and available off-the-shelf. Unfortunately, these valves differ in many respects from native valves, for example in their opening and closing behaviour due to the above-mentioned chemical pretreatment (Schoen & Levy 1999). The risk of thromboembolic complications is much lower when compared with mechanical prostheses but their durability is limited. Structural failure is strongly age dependent, making them suitable primarily for the elderly and less for children and young adults (Schoen & Levy 1999). Cryopreserved donor valves are the heart valve replacements closest to the natural valve, being non-thrombogenic and having a low risk of infection. They are not chemically cross-linked and exhibit good mechanical properties (Lee & Mooney 2001). Disadvantages are their limited availability, more difficult implantation techniques (Senthilnathan et al. 1999) and failure associated with a specific immune response, especially in young individuals (Rajani et al. 1998).
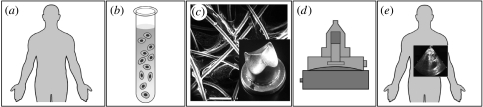
Several attempts have been made to create functional heart valve replacements, with the ability to grow, repair and to remodel, using the concept of tissue engineering (figure 1). In the tissue engineering approach, the patient's own cells isolated, e.g. from a blood vessel, and expanded using standard cell culture techniques, are seeded onto appropriate starter matrices, in the shape of a heart valve. Appropriate starter matrices must be able to support cell growth and cell-to-cell interaction guiding the tissue formation into a functional organ with organotypic extracellular matrix (ECM). The surfaces of these starter vehicles must be biocompatible, allowing cellular ingrowth and the formation of antithrombogenic layers. Currently, two principal types of starter matrices have been applied for the tissue engineering approach: xenogeneic or allogeneic decellularized fixed heart valves and synthetic biodegradable polymers.
Figure 1.
Overview of heart valve tissue engineering process: autologous cells are harvested from the patient (a) and isolated (b). (c) After culturing and expanding in vitro, cells are seeded onto a heart valve starter matrix that could be polymeric or xenogeneic. (d) Seeded construct are positioned into a biomimetic system bioreactor and conditioned. (e) After in vitro maturation, tissue-engineered heart valves are implanted into the patient. (Schmidt & Hoerstrup 2006)
2. Tissue-engineered heart valves based on decellularized xenogeneic starter matrices
One concept in heart-valve tissue engineering is to use decellularized biologic matrices with autologous cell reseeding. These matrices consist of allogeneic or xenogeneic materials that are decellularized either enzymatically or by detergent methods. As the availability of autologous heart valves is limited in the context of worldwide organ scarcity, current research focuses on xenogeneic tissue. The decellularization process is crucial as all cells need to be removed in order to avoid any adverse cellular immune response post-implantation. Conversely, the decellularization process must also preserve the structural components of the ECM relatively unaltered to provide a biomechanically sufficient scaffold and foster efficient reseeding. In a physiologic environment, ECM of heart valves is continuously remodelled by matrix metalloproteinases (MMPs; Stock et al. 2001). Decellularized matrices do not require ECM cross-linking protocols such as glutaraldehyde fixation. This enables degradation or metabolization by MMPs. At least four different methods have been used to produce a decellularized matrix. The most common approach is an enzymatic cell removal (Steinhoff et al. 2000). However, freeze drying (Curtil et al. 1997), osmotic gradients (Wilson et al. 1995) and combinations (Rieder et al. 2004) have been used as well. A recent study demonstrated that complete decellularization of porcine pulmonary heart valves with preservation of the ECM architecture and subsequent ovine reseeding is feasibile (Schenke-Layland et al. 2003a).
(a) Decellularization of xenogeneic heart valves using an enzymatic protocol
Even though the decellularization approach differs, the general concept is rather similar. In general, donor hearts (pig or sheep) are obtained under clean conditions from a local abattoir within 15 min of slaughter and are immediately transferred to the laboratory. It is crucial to reduce the time between slaughter and processing to minimize autolysis. For in vivo trials, size-matched donor animals and sterile harvest procedures are required. As porcine aortic valves have a muscular attachment at the right coronary leaflet, the majority of groups focus on either pulmonary valves (PVs) or assemble a three-leaflet heart valve from three non-coronary cusps. PVs are excised and freed of adherent fat and most of the myocardium, leaving only a thin ridge of subvalvular muscle tissue and the pulmonary artery. In order to reduce potential bacterial contamination, the valves are immediately washed for 30 min at room temperature in antimicrobial solutions such as povidone–iodine solution and sterile PBS followed by an overnight incubation at 4°C in an antibiotic solution (such as 1.2 mg amikacin; 3 mg flucytosin; 1.2 mg vancomycin; 0.3 mg ciprofloxacin; and 1.2 mg metronidazol in 1 ml aqua ad inject). Following this ‘antimicrobic pretreatment’ procedure, the valves are ready for the actual decellularization process. If this is achieved by enzymatic cell lysis, the valves are placed in a solution of 0.05% trypsin and 0.02% EDTA at 37°C and 5% CO2 for 24 h under continuous three-dimensional shaking. Following cell lysis, the cell detritus needs to be removed by a washing step with PBS for another 24 h. The decellularized valve matrices are finally stored in Hanks balanced salt solution at 4°C prior to further processing and seeding (Schenke-Layland et al. 2003b, 2004).
(b) Reseeding and dynamic culture protocol of decellularized heart valves
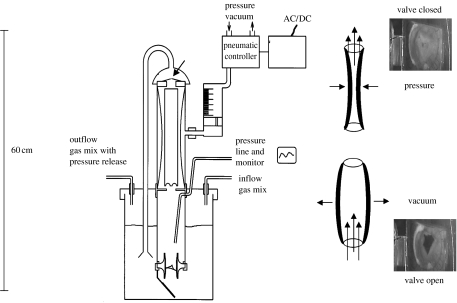
Following expansion, cells equivalent to valve interstitial cells, such as myofibroblasts, are trypsinized, resuspended in culture medium and seeded onto the decellularized PVs. The seeding normally requires a repeated static drip seeding on the inner and outer surface. In order to improve cell migration, proliferation and ECM production, mechanical load by means of shear stress and/or strain has been applied to the seeded valves in various bioreactors (Hoerstrup et al. 2000a; Mol et al. 2003; figure 2). This kind of preimplantation conditioning requires exposure to defined mechanical load over an extended time period (e.g. 16 days). Shorter time periods would result in an incomplete surface and cell ingrowth. Finally, endothelial cells (EC) are seeded under static conditions, allowing the cells to attach on the valve root and the leaflets. The entire culture period is conducted in a standard incubator at 37°C and 5% CO2. A crucial limitation of all long-term in vitro tissue engineering is a constant risk of infections. Accordingly, sterile handling is supported with repeated antibiotic supplements (Schenke-Layland et al. 2003a).
Figure 2.
Schematic of a pulsatile bioreactor. The tissue-engineered heart valve (arrow) is perfused with nutrient media in a pulsatile mode. The pulsatile flow is generated via a pneumatic system. Pressure conditions and gas exchange are monitored.
(c) In vivo evaluation of reseeded heart valves
In vivo evaluation of the decellularized reseeded heart valves has been mainly performed in the pulmonary circulation. As the clinical demand is focused on aortic valves, we describe an animal model in the systemic circulation of juvenile sheep. Carotid artery segments were obtained under sterile conditions. Owing to anatomical limitations, an orthotopic implantation of the tissue-engineered valves is extremely complicated. Accordingly, for an initial feasibility study, the valves were implanted in the descending thoracic aorta. To prevent distal ischaemia during cross-clamping, a modified Gott shunt was established. A roller pump maintained a distal perfusion of approximately 1.5 l flow min−1 and a mean blood pressure of 35 mmHg.
Valve cusps in the descending aorta simply flutter throughout the cardiac cycle due to the missing after-load. A shunt between the distal aortic arch and the left atrium was implanted to increase the aortic pulse pressure by decreasing the diastolic pressure (Hilbert et al. 1999). The placement of this shunt provides the necessary haemodynamic conditions to enable complete leaflet opening and coaptation.
An overall of six valves with autologous cell seeding were implanted. Two valves were seeded explicitly with EC and four with myofibroblasts and EC. All valves were explanted at 12 weeks. Gross morphology showed a smooth internal surface without thrombus formation and no pathologic dilatation. Even though initial leaflet movement was confirmed by Doppler echocardiography and at the time of explantation the shunts were operational, the leaflets did not move. A pathophysiologic explanation for this observation is an adaptation of the sheep circulation.
3. Tissue-engineered heart valves based on polymeric starter matrices
The use of synthetic materials as starter matrix has already been broadly demonstrated for cardiovascular tissue engineering. An ideal synthetic material serves as a temporary scaffold guiding tissue growth and formation. It should be at least 90% porous (Agrawal & Ray 2001) and must possess an interconnected pore network that is essential for cell growth, nutrient supply and removal of metabolic waste products. Currently, several techniques have been used to fabricate such porous structures, for example the salt-leaching technique, weaving of fibre meshes, producing non-woven meshes (Agrawal & Ray 2001; Hutmacher et al. 2001), rapid prototyping (Hutmacher et al. 2001), phase separation (Nam & Park 1999) and electrospinning (Buchko et al. 1999). Besides being biocompatible, biodegradable and reproducible, the scaffold should also exhibit a cell-favourable surface chemistry and match the mechanical properties of the native tissue. In order to guarantee mechanical stability over time, the degradation rate of the scaffold should be controllable and proportional to the rate of tissue formation.
Several synthetic biodegradable polymers, such as polyglactin (Shinoka et al. 1995), polyglycolic acid (PGA; Shinoka et al. 1996), polylactic acid (PLA; Shinoka et al. 1998), polyhydroxyalkanoates (PHA; Sodian et al. 2000a) and a copolymer of PGA and PLA (PGLA; Zund et al. 1997), have been investigated as a starter matrix for heart-valve tissue engineering.
(a) Aliphatic polyesters
Polyglactin, PGA and PLA belong to the family of aliphatic polyesters, which degrade by cleavage of the polymer chains by hydrolysis of their ester bonds. The resulting monomer is either secreted in the urine or enters the tricarboxylic acid cycle (Agrawal & Ray 2001). Initial attempts to create single heart-valve leaflets were based on combinations of aliphatic polyesters as well as, for example, woven polyglactin and non-woven PGA meshes and layers of PLGA and non-woven PGA meshes. The major limitations of aliphatic polyesters are their thickness, initial stiffness and non-pliability, making it difficult to fabricate a trileaflet heart valve.
(b) Polyhydroxyalcanoates
The family of PHAs consists of polyesters built up from hydroxyacids produced as intracellular granules by various bacteria (Kessler & Withold 2001). Both polyhydroxyoctanoate (Sodian et al. 2000b,c) and poly-4-hydroxybutyrate (P4HB) have been used (Sodian et al. 2000a,b) to create trileaflet heart-valve conduits. These materials possess thermoplastic properties and can therefore be easily moulded into any desired shape, as shown by using stereolithography (Sodian et al. 2002). The general drawback of PHAs is their slow degradation. Combinations of aliphatic polyesters and PHAs have been tested as alternative composite polymers (Hoerstrup 2000b; Stock et al. 2000; Rabkin et al. 2002). In particular, the use of PGA coated with P4HB, combining the high porosity of PGA and the thermoplastic properties of P4HB (Hoerstrup 2000b; Rabkin et al. 2002), for fabrication of complete trileaflet heart valves has demonstrated promising results in a rapidly growing sheep model (Hoerstrup 2000b).
(c) Proof-of-principle study
In a proof-of-principle study investigating a completely autologous tissue-engineered heart valve based on PGA/P4HB starter matrices in a long-term animal model, the following experimental steps were performed (Hoerstrup et al. 2000b).
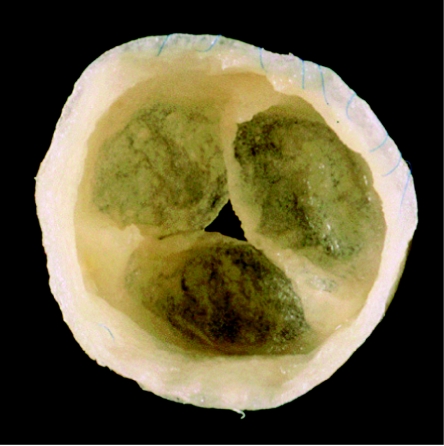
Trileaflet heart-valve starter matrices were fabricated from PGA/P4HB bioabsorbable polymers and sequentially seeded with autologous ovine myofibroblasts and EC. The constructs were grown for 14 days in a pulse duplicator in vitro system under gradually increasing flow and pressure conditions (figure 3). Using cardiopulmonary bypass, the native pulmonary leaflets were resected and the valve constructs were implanted into six lambs (weight=19±2.8 kg). All animals had uneventful post-operative courses and the valves were explanted at 1 day and 4, 6, 8, 16 and 20 weeks. Echocardiography demonstrated mobile, functioning leaflets without stenosis, thrombus or aneurysm up to 20 weeks. Histology showed uniform, layered cuspal tissue with endothelium. Environmental scanning electron microscopy revealed a confluent smooth valve surface. Mechanical properties were comparable to those of native tissue at 20 weeks. Complete degradation of the polymers occurred in eight weeks. The content of ECM (collagen, glycosaminoglycans and elastin) and DNA increased to levels similar to those of native tissue and were even higher at 20 weeks.
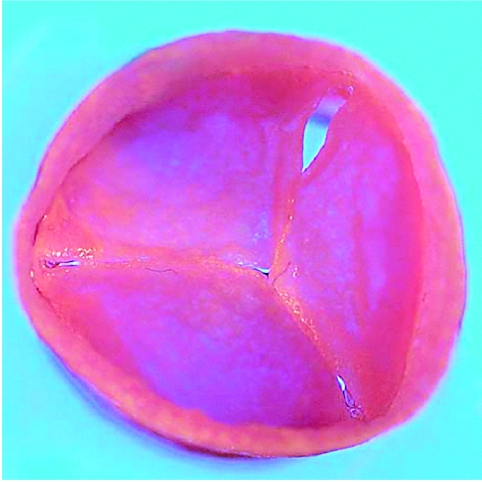
Figure 3.
Autologous tissue-engineered heart valves before implantation in an animal model. (Hoerstrup et al. 2000b)
This study demonstrated in vitro generation of implantable living heart valves based on a seeded synthetic polymeric starter matrix preconditioned in a biomimetic flow culture system. These autologous tissue-engineered valves functioned for up to five months in vivo and resembled normal heart valves as to microstructure, mechanical properties and ECM formation.
(d) From animal to human
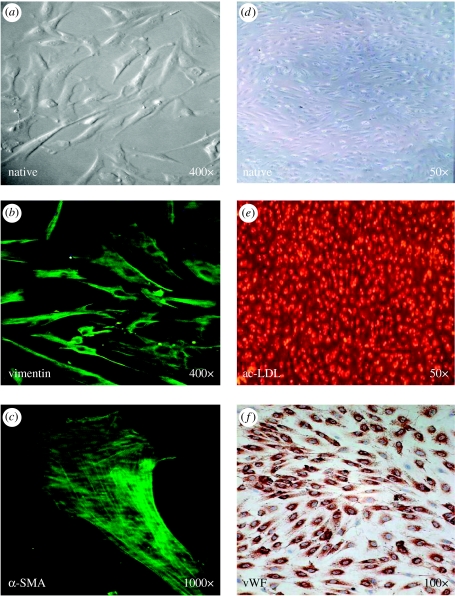
As the principle of using synthetic starter matrices has been carried out successfully in an animal model, the methodology has been transferred to human in vitro systems. In most cardiovascular tissue-engineering approaches, cells are harvested from donor tissues, e.g. from peripheral arteries and mixed vascular cell populations consisting of myofibroblasts and EC. Out of these, pure viable cell lines can be easily isolated by cell sorters (Hoerstrup et al. 1998), and the subsequent seeding onto the biodegradable starter matrices is undertaken in two steps. First, the myofibroblasts are seeded and grown in vitro. Second, the EC are seeded on the surface of the generated neo-tissue leading to the formation of a native-leaflet-analogous histological structure. With regard to clinical applications, several human cell sources have been investigated. Among the most promising are vascular-derived cells, bone-marrow-derived cells (figure 4a–c), blood-derived cells (figure 4d–f) and umbilical-cord-derived cells, particularly for paediatric application. Recently, cells derived from bone marrow or umbilical cords have been successfully used to generate heart valves (figure 5) and conduits in vitro (Hoerstrup et al. 2002a,b). In contrast to vascular cells, these cells can be obtained without surgical interventions representing an easy-to-access cell source in a possible routine clinical scenario. Owing to their good proliferation and progenitor potential, these cells are expected to be an attractive alternative for cardiovascular tissue-engineering applications. In recent studies, the use of umbilical-cord blood-derived endothelial progenitor cells as a promising cell source for paediatric cardiovascular tissue engineering has been demonstrated, when they showed excellent attachment to synthetic matrices PGA/P4HB (Schmidt et al. 2004) as well as formation of living endothelia on tissue-engineered patches and heart valves based on PGA/P4HB starter matrices and umbilical-cord-derived myofibroblasts or chorionic-villi-derived progenitor cells (Schmidt et al. 2005, 2006a,b).
Figure 4.
Human bone-marrow-derived cells 14 days after isolation (a), magnification 400×; expressing vimentin (b), magnification 400×; and α-SMA (c), magnification 1000×; and human umbilical-cord blood-derived endothelial progenitor cells 21 days after isolation (d), magnification 50×; demonstrating ac-LDL uptake (e), magnification 50×; and von Willebrand factor (f), magnification 100×, for fabrication of living autologous heart valves. (Hoerstrup et al. (2002a); Schmidt et al. (2004))
Figure 5.
Tissue-engineered heart valve generated from human marrow stromal cells. (Hoerstrup et al. (2002a))
4. Tissue-engineered heart valves based on biological/polymeric hybrid starter matrices
A further strategy in tissue engineering that has recently been introduced is the use of biological/polymeric composite materials as starter matrices. Such hybrids can be complex structures such as heart valves, e.g. fabricated from decellularized porcine aortic valves and dip coated with a biodegradable polymer (Stamm 2004). Moreover, scaffolds equipped with molecular cues mimicking certain aspects of structure or function of natural extracellular microenvironments have been developed in recent years (Lutolf & Hubbell 2005).
5. Discussion
(a) Inflammation to matrix
Remaining issues include an inflammatory response either as an immunological or foreign body reaction. In 2003, a group of cardiac surgeons in Vienna published their catastrophic results of decellularized porcine heart valves in paediatric patients (Simon et al. 2003). Three of four patients died in the first year after surgical treatment due to valve-related complications. Their histology revealed severe inflammation from outside to inside with subsequent degeneration. They furthermore identified areas of incomplete decellularization. Whether the observed inflammation is to be seen as an immunological or foreign body response to remaining xenogeneic cells or ECM is subject to controversial discussions and ongoing research.
(b) Safety of decellularized xenogeneic tissue
Another critical issue is the potential transmission of microbiological hazards of xenotransplanted tissues, such as porcine endogenous retroviruses and prionic diseases. Recently, epidemiological data have strongly indicated transfer of Creutzfeld-Jakob disease from cattle into humans either via infected meat, surgical material derived from bovine gut or drugs, or vaccines prepared using foetal calf serum (Knight & Collins 2001). In contrast, a more recent study (Kallenbach et al. 2004) revealed that transmission of porcine endogenous retroviruses from pig to human is highly unlikely.
(c) Preconditioning of tissue-engineered heart valves prior to implantation
Mechanical and biological stimuli do interact in a very complex way in the regulation of tissue behaviour. In order to stimulate these interactions several conditioning protocols have been developed. Mechanical conditioning comprises the uses of mechanical stimuli in in vitro bioreactors such as flow, shear stress or strain applied to the developing tissue. For heart-valve tissue engineering, the most commonly used bioreactor is a pulse duplicator system mimicking the biological environment and enabling the opening and closing behaviour of the valve. Subsequent in vitro cultivation providing biological (growth factors) or mechanical stimuli (bioreactors) promotes tissue development (Niklason et al. 1999).
(d) Animal models for pulmonary and aortic circulation
A worldwide established and accepted animal model to test biological heart-valve devices in an in vivo environment is the growing lamb. Lambs have the fastest calcifying valvular system and the growth dynamic of the heart and the great vessels are equivalent to the human. Implantation of valvular substitutes in the pulmonary circulation is relatively easy to accomplish and has a low morbidity (Stock et al. 2000). However, the evaluation of aortic heart valves requires evaluation in the systemic circulation. Owing to anatomical issues orthotopic implantation in the subcoronary position of lamb is extremely difficult and complicated by a high morbidity and mortality rate. Alternative implantation sides focus on the heterotopic descending aorta (Hilbert et al. 1999). Even though initial success by means of competent opening and closing throughout the cardiac cycle was confirmed by ultrasound, mid-term function is diminished due to adaptation of the animals to the altered diastolic/systolic pressure gradient. Accordingly, there might be a need to study different issues of valve function and degeneration in different animal models. One option is the investigation of calicification in an ovine pulmonary circulation and biomechanical wear testing in the mitral position of pigs.
6. Future perspectives
(a) What kind of valve will be required?
The concept of heart-valve tissue engineering was initiated by paediatric surgeons. They were motivated by the lack of adequate substitutes for patients with absent or severely malformed native valve tissue. The majority of these patients require valved conduits. Implantation of these conduits, either isolated or as pulmonary substitutes during pulmonary autograft operations, requires great surgical skills, even in the adult. As the complication rate is higher than in prothestic or stented xenovalves, the worldwide acceptance of these valves shows a recent decrease. A tissue-engineered living autologous valve with the capacity for growth, repair and regeneration would overcome the lack of appropriate replacement material and would avoid reoperations.
The majority of patients requiring valve replacement are older than 70 years. With increasing life expectancy, more and more patients demand valvular substitutes with long-term function exceeding that of currently available glutaraldehyde-fixed biological valves. The development of tissue-engineered heart valves based on a stented design might be an important step in achieving this goal.
Ideally, when tissue-engineering techniques are introduced in future clinical routine, the schedule for an older patient diagnosed with valvular disease may be: (i) cell harvest by, for example, a bone marrow puncture under local anaesthesia; (ii) differentiation and expansion of the cells and tissue engineering of a heart valve prosthesis in vitro; and (iii) following yet to be defined quality criteria (biochemical, histological, bio-safety, etc.), implantation of a living autologous valve replacement into the patient after a time period of six to eight weeks.
Footnotes
One contribution of 21 to a Theme Issue ‘Bioengineering the heart’.
References
- Agrawal C.M, Ray R.B. Biodegradable polymeric scaffolds for musculoskeletal tissue engineering. J. Biomed. Mater. Res. 2001;55:141–150. doi: 10.1002/1097-4636(200105)55:2<141::aid-jbm1000>3.0.co;2-j. doi:10.1002/1097-4636(200105)55:2<141::AID-JBM1000>3.0.CO;2-J [DOI] [PubMed] [Google Scholar]
- Buchko C.J, Chen L.C, Shen Y, Martin D.C. Processing and microstructural characterization of porous biocompatible protein polymer thin films. Polymer. 1999;40:7397–7407. doi:10.1016/S0032-3861(98)00866-0 [Google Scholar]
- Curtil A, Pegg D.E, Wilson A. Freeze drying of cardiac valves in preparation for cellular repopulation. Cryobiology. 1997;34:13–22. doi: 10.1006/cryo.1996.1982. doi:10.1006/cryo.1996.1982 [DOI] [PubMed] [Google Scholar]
- Hilbert S.L, Luna R.E, Zhang J, Wang Y, Hopkins R.A, Yu Z.X, Ferrans V.J. Allograft heart valves: the role of apoptosis-mediated cell loss. J. Thorac. Cardiovasc. Surg. 1999;117:454–462. doi: 10.1016/s0022-5223(99)70324-7. doi:10.1016/S0022-5223(99)70324-7 [DOI] [PubMed] [Google Scholar]
- Hoerstrup S.P, Zund G, Schoeberlein A, Ye Q, Vogt P.R, Turina M.I. Fluorescence activated cell sorting: a reliable method in tissue engineering of a bioprosthetic heart valve. Ann. Thorac. Surg. 1998;66:1653–1657. doi: 10.1016/s0003-4975(98)00796-6. doi:10.1016/S0003-4975(98)00796-6 [DOI] [PubMed] [Google Scholar]
- Hoerstrup S.P, Sodian R, Sperling J.S, Vacanti J.P, Mayer J.E., Jr New pulsatile bioreactor for in vitro formation of tissue engineered heart valves. Tissue Eng. 2000a;6:75–79. doi: 10.1089/107632700320919. doi:10.1089/107632700320919 [DOI] [PubMed] [Google Scholar]
- Hoerstrup S.P, et al. Functional living trileaflet heart valves grown in vitro. Circulation. 2000b;102:III-44–III-49. doi: 10.1161/01.cir.102.suppl_3.iii-44. [DOI] [PubMed] [Google Scholar]
- Hoerstrup S.P, et al. Tissue engineering of functional trileaflet heart valves from human marrow stromal cells. Circulation. 2002a;106:I-143–I-150. [PubMed] [Google Scholar]
- Hoerstrup S.P, Kadner A, Breymann C.I, Maurus C.F, Guenter C.I, Sodian R, Visjager J.F, Zund G, Turina M.I. Living, autologous pulmonary artery conduits tissue engineered from human umbilical cord cells. Ann. Thorac. Surg. 2002b;74:46–52. doi: 10.1016/s0003-4975(02)03649-4. doi:10.1016/S0003-4975(02)03649-4 [DOI] [PubMed] [Google Scholar]
- Hutmacher D.W, Schantz T, Zein I, Ng K.W, Teoh S.H, Tan K.C. Mechanical properties and cell cultural response of polycaprolactone scaffolds designed and fabricated via fused deposition modeling. J. Biomed. Mater. Res. 2001;55:203–216. doi: 10.1002/1097-4636(200105)55:2<203::aid-jbm1007>3.0.co;2-7. doi:10.1002/1097-4636(200105)55:2<203::AID-JBM1007>3.0.CO;2-7 [DOI] [PubMed] [Google Scholar]
- Kallenbach K, Leyh R.G, Lefik E, Walles T, Wilhelmi M, Cebotari S, Schmiedl A, Haverich A, Mertsching H. Guided tissue regeneration: porcine matrix does not transmit PERV. Biomaterials. 2004;25:3613–3620. doi: 10.1016/j.biomaterials.2003.10.040. doi:10.1016/j.biomaterials.2003.10.040 [DOI] [PubMed] [Google Scholar]
- Kessler B, Withold B. Factors involved in the regulatory network of polyhydroxyalkanoate metabolism. J. Biotechnol. 2001;86:97–104. doi: 10.1016/s0168-1656(00)00404-1. doi:10.1016/S0168-1656(00)00404-1 [DOI] [PubMed] [Google Scholar]
- Knight R, Collins S. Human prion diseases: cause, clinical and diagnostic aspects. Contrib. Microbiol. 2001;7:68–92. doi: 10.1159/000060377. [DOI] [PubMed] [Google Scholar]
- Lee K.Y, Mooney D.J. Hydrogels for tissue engineering. Chem. Rev. 2001;124:37–43. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- Lutolf M.P, Hubbell J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. doi:10.1038/nbt1055 [DOI] [PubMed] [Google Scholar]
- Mol A, Bouten C.V, Zund G, Gunter C.I, Visjager J.F, Turina M.I, Baaijens F.P, Hoerstrup S.P. The relevance of large strains in functional tissue engineering of heart valves. J. Thorac. Cardiovasc. Surg. 2003;51:78–83. doi: 10.1055/s-2003-38993. doi:10.1055/s-2003-38993 [DOI] [PubMed] [Google Scholar]
- Nam Y.S, Park T.G. Biodegradable polymeric microcellular foams by modified thermally induced phase separation. Biomaterials. 1999;20:783–790. doi: 10.1016/s0142-9612(99)00073-3. doi:10.1016/S0142-9612(99)00073-3 [DOI] [PubMed] [Google Scholar]
- Niklason L, Gao J, Abbott W.M, Hirschi K.K, Houser S, Marini R, Langer R. Functional arteries grown in vitro. Science. 1999;284:498–493. doi: 10.1126/science.284.5413.489. doi:10.1126/science.284.5413.489 [DOI] [PubMed] [Google Scholar]
- Rabkin E, Hoerstrup S.P, Aikawa M, Mayer J.E, Jr, Schoen F.J. Evolution of cell phenotype and extracellular matrix in tissue-engineered heart valves during in-vitro maturation and remodeling. J. Heart Valve Dis. 2002;11:308–314. [PubMed] [Google Scholar]
- Rajani B, Mee R.B, Ratcliff N.B. Evidence for rejection of homograft cardiac valves in infants. J. Thorac. Cardiovasc. Surg. 1998;115:111–117. doi: 10.1016/s0022-5223(98)70449-0. doi:10.1016/S0022-5223(98)70449-0 [DOI] [PubMed] [Google Scholar]
- Rieder E, Kasimir M.T, Silberhumer G, Seebacher G, Wolner E, Simon P, Weigel G. Decellularization protocols of porcine heart valves differ importantly in efficiency of cell removal and susceptibility of the matrix to recellularization with human vascular cells. J. Thorac. Cardiovasc. Surg. 2004;127:399–405. doi: 10.1016/j.jtcvs.2003.06.017. doi:10.1016/j.jtcvs.2003.06.017 [DOI] [PubMed] [Google Scholar]
- Schenke-Layland K, Opitz F, Gross M, Doring C, Halbhuber K.J, Schirrmeister F, Wahlers T, Stock U.A. Complete repopulation of decellularized heart valves by application of defined physical signals—an in vitro study. Cardiovasc. Res. 2003a;60:497–509. doi: 10.1016/j.cardiores.2003.09.002. doi:10.1016/j.cardiores.2003.09.002 [DOI] [PubMed] [Google Scholar]
- Schenke-Layland K, Vasilevski O, Opitz F, Konig K, Riemann I, Halbhuber K.J, Wahlers T, Stock U.A. Impact of decellularization of xenogeneic tissue on extracellular matrix integrity for tissue engineering of heart valves. J. Struct. Biol. 2003b;143:201–208. doi: 10.1016/j.jsb.2003.08.002. doi:10.1016/j.jsb.2003.08.002 [DOI] [PubMed] [Google Scholar]
- Schenke-Layland K, Riemann I, Opitz F, Konig K, Halbhuber K.J, Stock U.A. Comparative study of cellular and extracellular matrix composition of native and tissue engineered heart valves. Matrix Biol. 2004;23:113–125. doi: 10.1016/j.matbio.2004.03.005. doi:10.1016/j.matbio.2004.03.005 [DOI] [PubMed] [Google Scholar]
- Schmidt D, Hoerstrup S.P. Heart valve tissue engineering: choosing the right cell source. In: Hijadzi Z.M, Bonhoeffer P, Feldmann T, Ruiz C.E, editors. Transcather valve repair. Taylor & Francis; Abingdon, UK: 2006. pp. 347–356. [Google Scholar]
- Schmidt D, Breymann C, Weber A, Guenter C.I, Neuenschwander S, Zund G, Turina M, Hoerstrup S.P. Umbilical cord blood derived endothelial progenitor cells for tissue engineering of vascular grafts. Ann. Thorac. Surg. 2004;78:2094–2098. doi: 10.1016/j.athoracsur.2004.06.052. doi:10.1016/j.athoracsur.2004.06.052 [DOI] [PubMed] [Google Scholar]
- Schmidt D, Mol A, Neuenschwander S, Breymann C, Gössi M, Zund G, Turina M, Hoerstrup S.P. Living patches engineered from human umbilical cord derived fibroblasts and endothelial progenitor cells. Eur. J. Cardiothorac. Surg. 2005;27:795–800. doi: 10.1016/j.ejcts.2005.01.064. doi:10.1016/j.ejcts.2005.01.064 [DOI] [PubMed] [Google Scholar]
- Schmidt D, Mol A, Odermatt B, Neuenschwander S, Breymann C, Gössi M, Genoni M, Zund G, Hoerstrup S.P. Engineering of biologically active living heart valve leaflets using human umbilical cord-derived progenitor cells. Tissue Eng. 2006a;12:3223–3232. doi: 10.1089/ten.2006.12.3223. doi:10.1089/ten.2006.12.3223 [DOI] [PubMed] [Google Scholar]
- Schmidt D, et al. Living, autologous heart valves engineered from human prenatally harvested progenitors. Circulation. 2006b;114:I-125–I-131. doi: 10.1161/CIRCULATIONAHA.105.001040. doi:10.1161/CIRCULATIONAHA.105.001040 [DOI] [PubMed] [Google Scholar]
- Schoen F.J, Levy R.J. Founder's Award, 25th Annual Meeting of the Society for Biomaterials, Perspectives. Providence, RI, 28 April–2 May. Tissue heart valves: current challenges and future research perspectives. J. Biomed. Mater. Res. 1999;47:439–465. doi: 10.1002/(sici)1097-4636(19991215)47:4<439::aid-jbm1>3.0.co;2-o. doi:10.1002/(SICI)1097-4636(19991215)47:4<439::AID-JBM1>3.0.CO;2-O [DOI] [PubMed] [Google Scholar]
- Senthilnathan V, Treasure T, Grunkemayer G, Starr A. Heart valves: which is the best choice? Cardiovasc. Surg. 1999;7:393–397. doi: 10.1016/s0967-2109(99)00026-5. doi:10.1016/S0967-2109(99)00026-5 [DOI] [PubMed] [Google Scholar]
- Shinoka T, Breuer C.K, Tanel R.E, Zund G, Miura T, Ma P.X, Langer R, Vacanti J.P, Mayer J.E. Tissue engineering heart valves: valve leaflet replacement study in a lamb model. Ann. Thorac. Surg. 1995;60(Suppl. 3):S513–S516. doi: 10.1016/0003-4975(95)00733-4. doi:10.1016/0003-4975(95)00733-4 [DOI] [PubMed] [Google Scholar]
- Shinoka T, Ma P.X, Shum-Tim D, Breuer C.K, Cusick R.A, Zund G, Langer R, Vacanti J.P, Mayer J.E., Jr Tissue engineered heart valves. Autologous valve leaflet replacement study in a lamb model. Circulation. 1996;94(Suppl.):II164–II168. [PubMed] [Google Scholar]
- Shinoka T, Shum-Tim D, Ma P.X, Tanel R.E, Isogai N, Langer R, Vacanti J.P, Mayer J.E., Jr Creation of viable pulmonary artery autografts through tissue engineering. J. Thorac. Cardiovasc. Surg. 1998;115:536–546. doi: 10.1016/S0022-5223(98)70315-0. doi:10.1016/S0022-5223(98)70315-0 [DOI] [PubMed] [Google Scholar]
- Simon P, Kasimir M.T, Seebacher G, Weigel G, Ullrich R, Salzer-Muhar U, Rieder E, Wolner E. Early failure of the tissue engineered porcine heart valve SYNERGRAFT in pediatric patients. Eur. J. Cardiothorac. Surg. 2003;23:1002–1006. doi: 10.1016/s1010-7940(03)00094-0. doi:10.1016/S1010-7940(03)00094-0 [DOI] [PubMed] [Google Scholar]
- Sodian R, et al. Early in vivo experience with tissue-engineered trileaflet heart valves. Circulation. 2000a;102:III22–III29. doi: 10.1161/01.cir.102.suppl_3.iii-22. [DOI] [PubMed] [Google Scholar]
- Sodian R, Hoerstrup S.P, Sperling J.S, Martin D.P, Daebritz S, Mayer J.E, Jr, Vacanti J.P. Evaluation of biodegradable, three-dimensional matrices for tissue engineering of heart valves. ASAIO J. 2000b;46:107–110. doi: 10.1097/00002480-200001000-00025. doi:10.1097/00002480-200001000-00025 [DOI] [PubMed] [Google Scholar]
- Sodian R, Sperling J.S, Martin D.P, Egozy A, Stock U, Mayer J.E, Jr, Vacanti J.P. Fabrication of trileaflet heart valves from polyhydroxyalkanoate biopolyester for use in tissue engineering. Tissue Eng. 2000c;6:183–188. doi: 10.1089/107632700320793. doi:10.1089/107632700320793 [DOI] [PubMed] [Google Scholar]
- Sodian R, Loebe M, Hein A, Martin D.P, Hoerstrup S.P, Potapov E.V, Hausmann H, Lueth T, Hetzer R. Application of stereolithography for scaffold fabrication for tissue engineered heart valves. ASAIO J. 2002;48:12–16. doi: 10.1097/00002480-200201000-00004. doi:10.1097/00002480-200201000-00004 [DOI] [PubMed] [Google Scholar]
- Stamm C, et al. Biomatrix/polymer composite material for heart valve tissue engineering. Ann. Thorac. Surg. 2004;78:2084–2093. doi: 10.1016/j.athoracsur.2004.03.106. doi:10.1016/j.athoracsur.2004.03.106 [DOI] [PubMed] [Google Scholar]
- Steinhoff G, Stock U, Karim N, Mertsching H, Timke A, Meliss R.R, Pethig K, Haverich A, Bader A. Tissue engineering of pulmonary heart valves on allogenic acellular matrix conduits: in vivo restoration of valve tissue. Circulation. 2000;102(Suppl. III):III-50–III-55. doi: 10.1161/01.cir.102.suppl_3.iii-50. [DOI] [PubMed] [Google Scholar]
- Stock U.A, et al. Tissue engineered valved conduits in the pulmonary circulation. J. Thorac. Cardiovasc. Surg. 2000;119:732–740. doi: 10.1016/s0022-5223(00)70008-0. doi:10.1016/S0022-5223(00)70008-0 [DOI] [PubMed] [Google Scholar]
- Stock U.A, Wiederschain D, Kelly S, Shum-Tim D, Khalil P.N, Vacanti J.P, Mayer J.E, Jr, Moses M.A. Dynamics of extra-cellular matrix production and turnover in tissue engineered cardiovascular structures. J. Cell. Biochem. 2001;81:220–228. doi: 10.1002/1097-4644(20010501)81:2<220::aid-jcb1037>3.0.co;2-o. doi:10.1002/1097-4644(20010501)81:2<220::AID-JCB1037>3.0.CO;2-O [DOI] [PubMed] [Google Scholar]
- Wilson G.J, Courtman D.W, Klement P, Lee J.M, Yeger H. Acellular matrix: a biomaterials approach for coronary artery bypass and heart valve replacement. Ann. Thorac. Surg. 1995;60:S353–S358. doi: 10.1016/0003-4975(95)98967-y. doi:10.1016/0003-4975(95)98967-Y [DOI] [PubMed] [Google Scholar]
- Zund G, Breuer C.K, Shinoka T, Ma P.X, Langer R, Mayer J.E, Vacanti J.P. The in vitro construction of a tissue engineered bioprosthetic heart valve. Eur. J. Cardiothorac. Surg. 1997;11:493–497. doi: 10.1016/s1010-7940(96)01005-6. doi:10.1016/S1010-7940(96)01005-6 [DOI] [PubMed] [Google Scholar]