Abstract
For the treatment of vascular disease, the major cause of death in Western society, there is an urgent need for tissue-engineered, biocompatible, small calibre artery substitutes that restore biological function. Vascular tissue engineering of such grafts involves the development of compliant synthetic or biomaterial scaffolds that incorporate vascular cells and extracellular matrix. Elastic fibres are major structural elements of arterial walls that can enhance vascular graft design and patency. In blood vessels, they endow vessels with the critical property of elastic recoil. They also influence vascular cell behaviour through direct interactions and by regulating growth factor activation. This review addresses physiological elastic fibre assembly and contributions to vessel structure and function, and how elastic fibre biology is now being exploited in small diameter vascular graft design.
Keywords: elastic fibres, blood vessels, tissue engineering, elastin, fibrillin, fibulin
1. Introduction
Vascular disease is the major cause of death in Western society. In the USA alone, more than 600 000 arterial bypass operations using autogenous grafts are conducted per annum, while valvular dysfunction results in well over 60 000 valve replacement operations per annum (American Heart Association 2005 update). Coronary artery bypass operations relieve the symptoms of angina and life-threatening vascular disease, but have significant associated morbidity and cost. Many patients do not have suitable autologous vessels (usually internal mammary artery or saphenous vein), there is compliance mismatch between veins and arteries, and thrombosis is a serious problem. At the cellular level, major problems associated with small diameter vessels include endothelial cell (EC) detachment leading to thrombosis, altered smooth muscle cell (SMC) phenotype and limited deposition of functional vascular extracellular matrix (ECM). Valve replacement surgery currently uses mechanical valves that are rigid and do not ‘grow’ with young patients, require chronic anti-coagulant therapy and can fail suddenly; or bioprosthetic valves such as fixed xenografts or cryopreserved homografts that have poor long-term durability due to tissue calcification and mechanical damage and risk of thromboembolism, and generally require re-replacement. Thus, there is an urgent need for stable tissue-engineered patent cardiovascular prostheses that retain long-term biological function and are biocompatible.
Vascular tissue engineering of small calibre artery substitutes that restore and sustain biological function involves the development of compliant synthetic or biomaterial scaffolds, incorporating vascular cells and ECM, with long-term stability and functional properties. Since elastic fibres are major structural elements of arterial walls that also strongly influence vascular cell behaviour through direct interactions and natural growth factor delivery, elastic fibre biology is now being exploited in vascular tissue engineering. This review focuses on how elastic fibre molecules can enhance vascular graft design and patency. First, elastic fibre assembly, structure and function, and contributions to normal vessel walls are outlined, and then applications of elastic fibres in vascular tissue engineering are addressed in the context of small diameter vascular graft design.
2. Elastic fibres
Elastic fibres are major insoluble ECM assemblies that are deposited in blood vessel walls during early postnatal life. They are multimolecular complexes comprising a cross-linked elastin core surrounded by a mantle of fibrillin-rich microfibrils and several other associated molecules such as fibulin-5 (Kielty et al. 2002; Kielty 2006; figure 1).
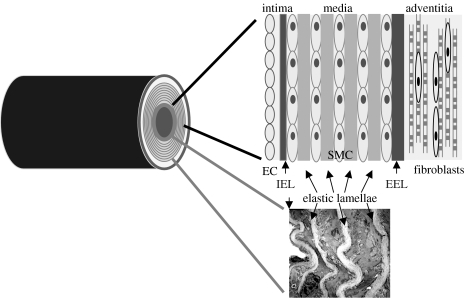
Figure 1.
Cartoon of a cross section through an artery. The tunica intima, tunica media and tunica adventitia, and positions of the internal elastic lamina (IEL), external elastic lamina (EEL) and medial elastic lamellae are shown, together with a transmission electron micrograph of an arterial wall.
(a) Molecular components
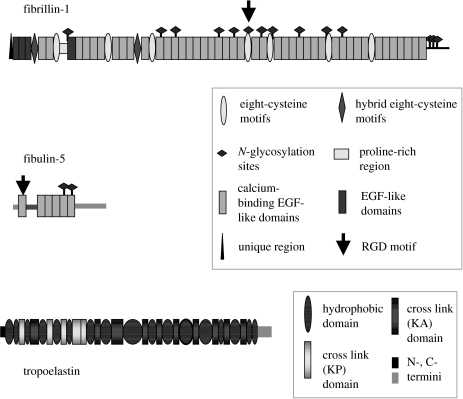
Elastin, the most abundant component of elastic fibres, is secreted as soluble tropoelastin (70 kDa; Mecham & Davis 1994; Mithieux & Weiss 2005; figure 2). It comprises alternating hydrophobic and lysine-rich cross-linking domains that are critical for extracellular assembly and elastic function.
Figure 2.
Domain structures of fibrillin-1, fibulin-5 and tropoelastin.
Fibrillins, the principal structural molecules of microfibrils, are large multidomain glycoproteins (approx. 350 kDa) comprising calcium-binding epidermal growth factor (cbEGF)-like domain arrays interspersed with eight-cysteine (TB) motifs (Pereira et al. 1993; Zhang et al. 1994; Corson et al. 2004; Kielty et al. 2005a), and containing an arg-gly-asp (RGD) cell adhesion motif (figure 2). Furin processing of fibrillin-1 precedes extracellular microfibril deposition. Genetic linkage of fibrillin-1 mutations to Marfan syndrome (Robinson et al. 2002), its abundance in developing and adult tissues (Quondamatteo et al. 2002) and proteomic analysis of isolated microfibrils (Cain et al. 2006) confirm its major contribution to elastic fibres.
There are several other molecules that co-localize or co-purify with microfibrils (microfibril-associated glycoproteins 1 and 2, MAGP-1 and MAGP-2; latent TGFβ-binding proteins, LTBPs), or occur at elastin–microfibril or elastic fibre–cell interfaces (fibulin-5, fibulin-2, emilin-1, decorin) or within the elastin core (biglycan), and stabilize elastic fibres (tissue transglutaminase, TG2; lysyl oxidase, LOX; Kielty et al. 2002; Kielty 2006). Collagen VIII also co-localizes with elastic fibres; this collagen forms specialized hexagonal basement membrane-associated arrays and is strongly expressed by SMC following arterial injury (Sinha et al. 2001).
Fibulin-5, a glycoprotein of approximately 55 kDa containing an RGD motif, is expressed by vascular SMC and EC, mediates vascular cell adhesion through integrin receptors, influences cell growth and motility in development and wound healing, and regulates elastin fibrillogenesis (Nakamura et al. 2002; Yanagisawa et al. 2002; Chu & Tsuda 2004; figure 2). It antagonizes angiogenesis and localizes on elastic lamina surfaces adjacent to the EC and in the aortic media. Its expression is markedly downregulated in adult arteries, but is highly induced in SMC and EC in response to injury in atherosclerotic cells and neointimal cells following balloon angioplasty (Kowal et al. 1999). Fibulin-5 interacts with apolipoprotein(a) and superoxide dismutase in arterial walls (Nguyen et al. 2004). Overexpression of fibulin-5 promotes wound healing, and its expression is upregulated by transforming growth factor beta (TGFβ; Jean et al. 2002).
(b) Deposition
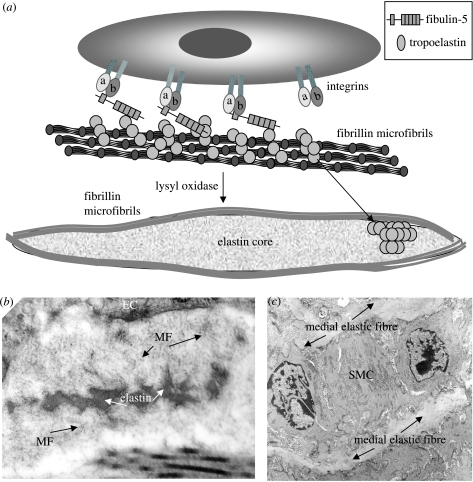
Vascular elastic fibres are deposited developmentally to reinforce the high pressure circulation (Faury 2001). An early step in elastic fibre formation is the pericellular deposition of fibrillin-1 rich microfibrils as a template for soluble tropoelastin (Kielty et al. 2002; figure 3). In foetal development, parallel arrays of fibrillin-rich microfibrils are apparent in the extracellular space juxtaposed to cell membranes. Elastin is then deposited as small aggregates of amorphous material within microfibril arrays, and subsequently coalesces to form mature elastic fibres (Mecham & Davis 1994). Recent studies indicate that an early step in elastic fibre formation is the appearance of small cell surface-associated elastin globules that coalesce hierarchically into larger fibres over time, coupled with cell motion (Czirok et al. 2006; Kozel et al. 2006). In the extracellular space, elastin crosslinking by LOX and lysyl oxidase-like 1 (LOXL1) is required for normal elastic fibre integrity (Maki et al. 2005; Thomassin et al. 2005). The proportion of microfibrils to elastin declines with age, with adult elastic fibres often having only a sparse peripheral mantle of microfibrillar material. Tissue-specific elastic fibre architectures, such as arterial medial lamellae, reflect the organization of the microfibril template, in turn dictated by cells and the strength and direction of forces put upon the tissue. In developing aorta, subendothelial microfibril bundles run parallel to the direction of blood flow and provide elastic anchorage for ECs in flow conditions (Davis 1993a).
Figure 3.
Elastic fibre formation (a) Cartoon depicting the deposition of tropoelastin on fibrillin microfibrils in the pericellular space, in potential association with fibulin-5. (b) Transmission electron micrograph showing elastic fibre formation in the subendothelium (MF, microfibrils; EC, endothelial cell). (c) Transmission electron micrograph showing mature elastic fibre lamellae juxtaposed to medial smooth muscle cell (SMC).
At molecular and cellular levels, the assembly of fibrillin microfibrils is a poorly understood process. Secreted furin-processed fibrillin molecules are thought to accrete both head-to-tail and laterally, to form beaded microfibrils (Reinhardt et al. 1996; Marson et al. 2005) which are then stabilized by transglutaminase cross links (tissue transglutaminase, TG2). We have shown that tropoelastin binds strongly to fibrillin-1 and that transglutaminase can be cross-linked to a specific fibrillin-1 sequence (Rock et al. 2004; Clarke et al. 2005). Elastin may also interact with microfibril-associated molecules such as microfibril-associated (MAGP-1) or biglycan (Trask et al. 2000; Jensen et al. 2001; Rock et al. 2004). These interactions may be regulated by cell surface receptors. Our model of elastic fibre assembly is that tropoelastin first binds periodically along microfibrils at TG2 cross-link sites, and then further tropoelastin molecules accrete and are covalently linked by LOX. Heparan sulphate binds and profoundly influences microfibril and elastic fibre formation (Tiedemann et al. 2001; Cain et al. 2005). The roles of the other elastic fibre-associated molecules remain unclear. Fibrillin-rich microfibrils probably integrate into vascular basement membranes since they can strongly interact with perlecan (Tiedemann et al. 2005).
A 67 kDa elastin-binding protein (EBP) which acts as a recyclable chaperone facilitates the secretion of tropoelastin (Privitera et al. 1998). EBP forms a cell surface-targeted molecular complex with lysosomal sialidase, the activity of which is needed for elastogenesis. It is thought to remove terminal sialic acids from microfibril glycoprotein carbohydrates, thereby unmasking galactosugars to act with the galactolectin domain of EBP, allowing the release of tropoelastin onto microfibrils (Hinek et al. 2006).
The critical role of fibulin-5 in elastic fibre and blood vessel formation is poorly defined. LOXL1 isoform, which is important in elastic fibre renewal in adult tissues, co-localized with fibulin-5 and bound a C-terminal region of fibulin-5 (Liu et al. 2004). This juxtaposition of fibulin-5 to tropoelastin and LOXL1 may allow efficient elastin core cross-linking. Recombinant rat fibulin-5 bound tropoelastin in a calcium-dependent manner, but no significant binding was observed for fibrillin-1, laminin or collagen I (Yanagisawa et al. 2002). In another study, recombinant fibulin-5 associated with pre-formed elastic fibres deposited by cultured cells (Nakamura et al. 2002). Fibulin-5 also interacts with emilin-1 (Zanetti et al. 2004). In our pilot studies using recombinant human fibulin-5, fibulin-5 bound to tropoelastin in a calcium-independent manner and to a conformation-sensitive N-terminal fibrillin-1 fragment (PF1; Freeman et al. 2005). Scanning transmission electron microscopy mass mapping revealed that fibulin-5 binds microfibrils, confirming a physiological role. Our data thus suggest a role for fibulin-5–microfibrillar interactions during elastic fibre formation. Fibulin-5-deficient mice also reveal a role of fibulin-5 in SMC proliferation and migration (Spencer et al. 2005).
3. Elastic fibres and arterial wall organization
Elastic fibres are major structural elements of arterial walls. Their abundance, structure and functions are outlined below, in the context of normal vessel wall organization.
(a) Arterial wall organization
Arteries comprise three major layers: the tunica intima, tunica media, and tunica adventitia (Quaglino & Pasquali-Ronchetti 2002); elastic fibres are components of all three layers (figure 1). The intima comprises an EC monolayer underpinned by subendothelial ECM. It provides an anti-thrombogenic inner lining to blood vessels, a barrier to mononuclear blood cells and a diffusion gradient to systemic cytokines and growth factors. A thick continuous elastic fibre (internal elastic lamina, IEL) forms a physical boundary between intimal EC and medial SMC. We have shown that, in vitro, direct EC contact profoundly disrupts the cytoskeletal organization of precursor SMC (Ball et al. 2004), and a major function of the IEL may be to prevent direct contact between these cells. The media comprises alternating layers of contractile SMC interspersed with concentrically arranged elastic fibres, as well as some collagen fibre bundles and proteoglycans. The elastic fibre and SMC lamellae endow elastic recoil and contractility on vessel walls. The number of elastic lamellae varies depending on the vessel type. The human aortic and elastic (conducting) arteries have between 40 and 70 elastic lamellae; they expand in response to blood flow and then have passive elastic recoil that allows for the maintenance of diastolic pressure. Coronary, peripheral and other muscular arteries have fewer (3–40) elastic lamellar layers. The external elastic lamina is a thick elastic fibre layer delineating the outer boundary of the tunica media. The outer adventitial layer is a thin dense connective tissue containing smooth muscle-like myofibroblasts, abundant collagen fibres, dispersed elastic fibres and vasa vasorum. It exerts homeostatic control on the vessel, limits wall extensibility and integrates the blood vessel into the surrounding tissue.
(b) Vascular cells
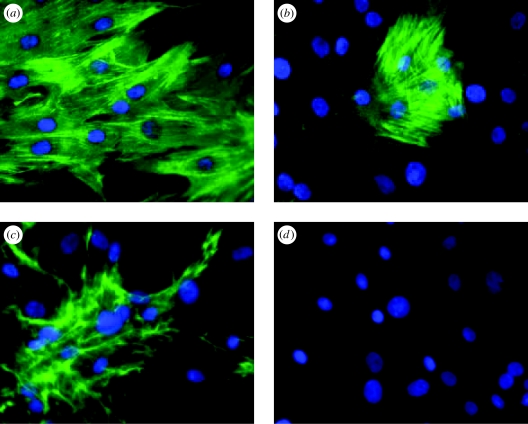
Three major cell types populate the vessel wall, regulate its physiology and deposit its ECM. Intimal ECs exhibit classic cobblestone morphology in culture but, in vessels, are somewhat more elongated with their long axis parallel to blood flow. EC monolayers line the lumen and provide a barrier between the blood and the vessel wall, control vessel tone, and regulate leucocyte adhesion and migration through the vessel wall. They also contribute synthetically to the deposition of the IEL that prevents direct interactions between SMCs and EC. During development, SMCs are responsible for the deposition of most of the vessel wall ECM, especially the medial elastic lamellae and collagen fibrils. In the mature media, SMCs are spindle-shaped cells that adopt a distinctive contractile phenotype characterized by SMC-specific cytoskeletal elements such as SM α-actin, SM myosin, smoothelin and SM22α (Owens 1995; Hungerford & Little 1999) as well as vascular matrix molecules (figure 4). They are surrounded by a specialized basal lamina except where they are connected to one another by gap junctions and to elastic fibres at prominent dense plaques. SMCs often have a ‘feathered’ appearance indicating that they are ‘tethered’ to the medial elastic fibre lamellae that intercalate the SMC layers (Davis 1993b). In culture, SMC phenotype is much more variable, and they usually adopt more synthetic proliferative ‘hills and valleys’ phenotype. Adventitial myofibroblasts express SM α-actin (Shi et al. 1996; Gao et al. 2003), deposit abundant collagen fibres and some elastic fibres and, following vascular injury, orchestrate adventitial remodelling. In vascular graft design, their growth, differentiation and functional characteristics need to be tightly regulated and monitored.
Figure 4.
SMC: immunofluorescence analysis of cytoskeletal and ECM elements. Cultured porcine aortic SMCs immunostained for (a) smooth muscle α-actin, (b) SMC myosin heavy chain, (c) fibronectin and (d) control omitting primary antibody. Images are 400× magnification.
(c) Vascular extracellular matrix
Elastic fibres represent a major component of the vascular ECM. They comprise a cross-linked elastin core surrounded by an outer mantle of fibrillin microfibrils (figure 3). Other key components, many of which interact with elastic fibre molecules, include various collagens and proteoglycans. Collagen fibres (mainly types I and III) represent more than 30% of the dry weight of blood vessels (Quaglino & Pasquali-Ronchetti 2002). Subendothelial and SMC basal laminae contain network-forming collagens IV and VIII. Collagen types XV and XVIII are associated with subendothelial basement membranes; their C-terminal ‘endostatin’ fragments influence angiogenesis (Morbidelli et al. 2003; Ricard-Blum et al. 2003). Collagen VI microfibrils are major cell–matrix linking polymers present throughout the vessel wall (Kielty & Grant 2002). Vascular cell adhesion glycoproteins include laminin in basement membranes, fibronectin and vitronectin. Proteoglycans function as structural elements of the vessel wall, and modulate cell adhesion and vascular growth factor availability. Their biological roles arise from their protein and carbohydrate (glycosaminoglycan) components. They form a hydrated scaffold and are especially abundant in the subendothelium. The heparan sulphate proteoglycan, perlecan, is an intrinsic component of subendothelial and SMC basement membranes. Heparin (a highly sulphated form of heparan sulphate) can inhibit SMC proliferation and migration, and it binds the growth factor FGF, fibrillin-1 (Cain et al. 2005) and elastin (Broekelmann et al. 2005; Gheduzzi et al. 2005), and influences elastic fibre deposition. The small leucine-rich proteoglycans decorin and biglycan are directly implicated in the process of elastic fibre formation (Reinboth et al. 2002) and also regulate collagen packing (Danielson et al. 1997; Wiberg et al. 2002). Some elastic fibre molecules bind TGFβ growth factors and regulate their bioavailability. Transmembrane proteoglycan receptors (syndecans) present on the medial SMC contribute, together with integrins, to elastic fibre-mediated cell–matrix communication and signalling.
(d) Elastic fibres in vascular development
Fibrillin microfibrils and elastic fibres are important players in blood vessel formation. In early vasculogenesis, primitive EC tubes are formed (Hungerford & Little 1999; Jain 2003); then a flexible subendothelial matrix is deposited that supports the functional EC monolayer. Mesenchymal smooth muscle precursor cells are recruited from surrounding tissues, through the actions of vascular growth factors such as platelet-derived growth factor (PDGF)-BB and TGFβ1 (Hellstrom et al. 1999); they deposit abundant vascular wall ECM, including elastic fibres. In mature vessels, medial SMC intercalated between elastic lamellae are contractile and quiescent. Abundant fibrillin microfibrils, or ‘connecting filaments’, are laid down by EC during vasculogenesis, in the direction of blood flow (Davis 1993b). These filaments are closely associated with the EC subluminal surface and may act as a long-range biomechanically flexible anchorage for EC. Microfibril bundles act as a template for tropoelastin deposition throughout the vessel wall. Developmental thickening of the tunica media and tunica adventitia reflects abundant deposition of elastic fibres and also collagen fibres (mainly collagens I, III), and occurs during the first years of life. During this time, microfibrils may also act as a conduit for LTBPs that sequester TGFβ and regulate its availability during vessel formation and repair (Isogai et al. 2003).
4. Biological roles of elastic fibres
Elastic fibres perform at least three critical functions in arteries. An obvious major role is the provision of elastic recoil to blood vessels. Fibrillin microfibrils and elastic fibres also regulate the activity of TGFβ family growth factors in vascular and other elastic tissues. Furthermore, elastic fibre molecules contain specific cell attachment motifs that mediate cell attachment, migration, survival and differentiation, and elastin profoundly influences cells in vascular pathology (Brooke et al. 2003). For these reasons, we and others are now exploiting elastic fibre biology in vascular tissue engineering.
(a) Elastic recoil
Fibrillin-rich microfibrils and elastic fibres profoundly influence vessel wall mechanics. Invertebrate and mammalian microfibril studies have shown that isolated microfibrils and microfibril bundles have elastic properties (McConnell et al. 1997; Thurmond et al. 1997; Megill et al. 2005). Molecular combing revealed that individual microfibrils are relatively stiff reinforcing fibres with Young's modulus of approximately 70 mPa when compared with that of elastin (approx. 1 mPa; Sherratt et al. 2003). Tropoelastin comprises alternating hydrophobic and lysine-rich domains which, in their cross-linked polymerized state, impart the physical properties of extensibility and elastic recoil on arteries (Faury 2001).
(b) Growth factor sequestration and activation
Genetic evidence in humans and mice, and biochemical studies strongly implicate fibrillin microfibrils in the extracellular control of TGFβ and bone morphogenetic protein (BMP) activation and signalling in cardiovascular and other elastic connective tissues (Charbonneau et al. 2004). These growth factors are potent regulators of cell survival and differentiation, tissue morphogenesis and homeostasis, and of cellular responses to injury (Massague & Chen 2000). Fibrillin-1 mutations cause Marfan syndrome and related fibrillinopathies, with symptoms ranging from tall to short stature, hypermobile joints to joint contractures and stiffness, and severe to mild or no cardiovascular defects (Robinson et al. 2002; Dietz et al. 2005). TGFβ family growth factor dysregulation may contribute to this phenotypic variability. Mitral valve defects in a murine model of Marfan syndrome were rescued by TGFβ neutralizing antibodies, confirming a causal relationship between mutant fibrillin-1 and TGFβ dysregulation (Ng et al. 2004; Weyman & Scherrer-Crosbie 2004). Fibrillin-1 deficiencies due to TGFβ dysregulation also caused failure in distal alveolar septation, and apoptosis in the developing lung, leading to emphysema and pneumothorax (Neptune et al. 2003). Mutations in TGFBR1 or TGFBR2 cause a new syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development (Loeys et al. 2005). Null mice models also revealed that fibrillin-2 and BMPs 4 and 7, a member of the TGFβ superfamily, are functionally and genetically linked (Chaudhry et al. 2001). Fibrillin-1 may regulate TGFβ1 availability through tissue and development-specific associations with LTBPs (Charbonneau et al. 2004). We recently showed that fibrillin-1 directly regulates TGFβ1 bioavailability (Chaudhri et al. 2007). Elastin has also been referred to as a matrikine (Duca et al. 2004).
TGFβ activity is regulated by small and large latent complexes (Rifkin 2005). LTBP-1 intracellularly forms a large latent complex with TGFβ1, which is stabilized by a disulphide bond between the latency-associated peptide (LAP) of TGFβ and the third TB module of LTBP-1 (Hyytiäinen et al. 2004; Rifkin 2005). The large latent complex is efficiently secreted, and LTBP-1 has been shown to bind the N-terminal region of fibrillin-1 (Isogai et al. 2003). Active TGFβ1 can then be released by the actions of integrins, protease, heat or thrombospondin-1. The nuclear magnetic resonance (NMR) structure of the TB3 domain in LTBP-1 has revealed the amino acid residues responsible for interactions with LAP; they include two hydrophobic and five acidic residues (Chen et al. 2005). The third TB module of LTBP-3 has TGFβ1-binding residues similar to those of LTBP-1, and it also strongly binds TGFβ (Saharinen & Keski-Oja 2000). However, LTBP-4 binds LAP–TGFβ only weakly, and LTBP-2 not at all. Significantly, although fibrillin microfibrils and LTBP-1 often co-localize, particularly in developing tissues, they are not always juxtaposed. Nevertheless, this interaction is proposed to underlie fibrillin-1-mediated TGFβ1 regulation (Rifkin 2005). Another member of the TGFβ superfamily BMP-7 can bind fibrillin-1 directly (Charbonneau et al. 2004).
(c) Cell adhesion and signalling
Elastic fibres have important vascular cell adhesion functions. Electron microscopy and biochemical studies have highlighted that ECs interact strongly with their subendothelial elastic fibre-containing matrix, and SMCs interact with juxtaposed elastic fibre lamellae at cell surface dense plaques (Davis 1993a,b). These interactions are mediated mainly through integrins, which are heterodimeric transmembrane receptors (Mould & Humphries 2004), and are critical both for a stable endothelium and normal EC vasoactive functions, and for regulating SMC contractile function within the media. Following integrin ligation of vascular ECM molecules, the integrin β-subunit cytoplasmic tail interacts with talin, which in turn binds vinculin that is associated with α-actinin, actin stress fibres and focal adhesions. In this way, the vascular ECM is linked directly, through integrins, to the cellular cytoskeletal framework. Thus, integrin-mediated cell–matrix interactions regulate vascular cell survival, phenotype proliferation, migration and ECM expression and deposition. Integrins expressed by EC and SMC include integrins α1β1, α2β1, α5β1, α6β1 and αvβ3 (Vorp et al. 2005). The major elastic fibre molecules that mediate cell adhesion are outlined below.
(i) Elastin
Cells interact directly with elastin through at least two different mechanisms. The EBP (an alternatively spliced form of β-galactosidase; 67 kDa) has the repeat hexapeptide in tropoelastin, VGVAPG, as its main ligand, and signalling through this receptor profoundly influences SMC proliferation and phenotype (Mochizuki et al. 2002). The C-terminus of elastin also interacts with integrin αvβ3 in a saturable, divalent cation-dependent manner; however, this is not an RGD-mediated mechanism since elastin lacks this motif (Rodgers & Weiss 2004). Certain elastin proteolytic fragments are also highly chemotactic (Bisaccia et al. 1994; Uemura & Okamoto 1997). Using recombinant human tropoelastin (full-length and overlapping fragments), we have demonstrated strong SMC and EC adhesion to elastin-coated substrata, with associated increases in proliferation (Williamson et al. submitted; figure 5).
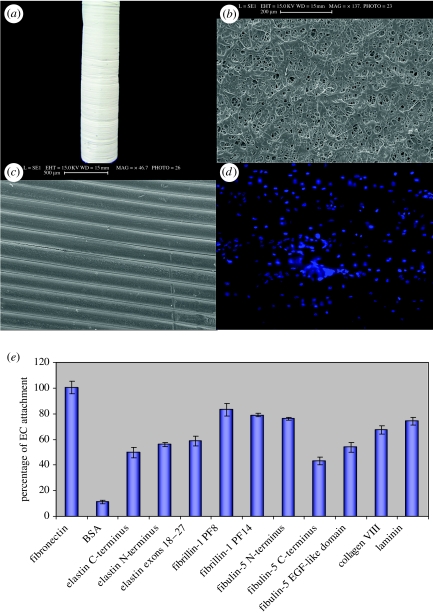
Figure 5.
(a–d) Images of our PU–PCL composite scaffold. (a) Composite scaffold on electrospinning mandrel. (b) Scanning electron microscopy (SEM) image of the anti-lumen surface; (c) SEM image of the lumen surface. (d) Human umbilical vein endothelial cells (HUVECs) cultured on the lumen surface for seven days, stained with DAPI; (e) HUVECs attached well to recombinant vascular matrix molecules coated onto the composite scaffold. Maximal percent cell attachment occurred after 45 min. The fragments used were (i) tropoelastin N-terminal fragment encoded by exons 1–18 and C-terminal fragments encoded by exons 18–36 and 18–27; (ii) fibrillin-1 fragments encoded by exons 30–38 and 33–40; (iii) fibulin-5 N- and C-terminal halves, and the N-terminal calcium-binding epidermal growth factor-like domain that contains the RGD motif. Recombinant collagen VIII (a product of EC and SMC) and laminin (from Sigma Chemical Co., UK) were also tested.
(ii) Fibrillin-1
We were the first to show that human SMCs exhibit RGD and cation-dependent adhesion to microfibrils (Kielty et al. 1992). We and others have since shown that fibrillin-1 is a major cell adhesion molecule, mediating adhesion mainly through a single active RGD motif in its sixth TB module (Pfaff et al. 1996; Sakamoto et al. 1996; Bax et al. 2003; Lee et al. 2004). The corresponding RGD motif in fibrillin-2 is also active. Fibrillin-1 was shown to interact with cells through αvβ3, and we have shown that it also ligates the α5β1 receptor. These interactions profoundly influence cell adhesion and spreading. Cell adhesion to fibrillin-1 specifically modifies gene expression levels—we showed at mRNA and protein levels that there is enhanced fibrillin-1 self-expression when cells are attached to the fibrillin-1 RGD motif but not to fibronectin, which also ligates α5β1 (Bax et al. 2003). Another group recently showed enhanced matrix metalloproteinase (MMP) expression in cells plated on fibrillin-1 RGD-containing peptides (Booms et al. 2005). The molecular bases of these phenotypic effects are unknown, but certainly reflect integrin signalling. We and others have now demonstrated a requirement for domains upstream to the RGD motif for optimal cell adhesion (Lee et al. 2004; Bax et al. 2007); we are conducting domain-swop experiments to establish whether enhanced cell adhesion reflects engagement of a synergy sequence. By defining these cell adhesion mechanisms, we have been able to optimize fibrillin-1 fragments that can now be exploited in vascular tissue engineering.
(iii) Fibulin-5
Fibulin-5 interacts directly with vascular cells, a function that may contribute to its critical role in elastic fibre deposition. Recombinant fibulin-5 expressed in a bacterial system was reported to interact directly with integrins αvβ3 and αvβ5 (RGD-dependent) and α9β1 (not RGD-dependent; integrin subgroup with α4β1) on integrin-overexpressing Chinese hamster ovary cells (Yanagisawa et al. 2002). We have conducted extensive human SMC adhesion studies using recombinant full-length human fibulin-5 (Freeman et al. 2005). We have identified the receptors used by SMCs and ECs on fibulin-5, and associated cellular organization and phenotype associated with low proliferation and migration (Lomas et al. in press).
5. Tissue-engineered vascular grafts
Most current blood vessel tissue engineering approaches are based on the ex vivo generation of living prostheses, colonized with contractile SMC and with vasoactive anti-thrombotic endothelium, which mimic natural vessels and can undergo growth, remodelling and repair (L'Heureux et al. 1998; Niklason et al. 1999; Mitchell & Niklason 2003; Daly et al. 2004; Borschel et al. 2005; Swartz et al. 2005). Most grafts are, in effect, tissue engineered in ‘reverse’ of normal vessel development, since they start with a strong ‘tunica media equivalent’ with appropriate elastic and non-porous properties essential for immediate graft patency. During graft pre-conditioning, SMCs are encouraged to deposit abundant ECM with native architecture and the essential biomechanical properties of elastic recoil (elastic fibres) and tensile strength (collagen fibres), so as to gradually replace biodegradable scaffolds.
A fundamental requirement of tissue-engineered grafts is a compliant polymer scaffold to which attached ECs provide an anti-thrombogenic luminal surface and vasoactive properties, and within which SMC can migrate and deposit functional elastic fibre-rich vascular ECM. Strong cell attachment is essential, and it can be enhanced by coating scaffolds with cell–matrix motifs; however, scaffold surface chemistry profoundly influences the adsorption, conformation and functional properties of biological molecules. Since developmental biomechanical forces direct SMCs to lay down an elastic matrix during vasculogenesis, their phenotype within pre-compliant scaffolds often requires experimental modulation through vascular growth factor signalling. Elastic fibre molecules are central to these strategies. As outlined above, they deliver elastic recoil to arterial walls; profoundly influence cell survival, migration and differentiation through specific cell–matrix interactions; and play a central role in regulating the availability of TGFβ growth factors (Kielty et al. 2002).
(a) Polymer scaffolds
Several small diameter graft models are being developed that use polymer scaffolds to provide mechanical integrity to ensure that they will not rupture when subjected to physiological arterial pressure and flow. Existing synthetic scaffold polymers include inert polyester Dacron and expanded polytetrafluoroethylene (Kannan et al. 2005; Kielty et al. 2005b; Vorp et al. 2005). These materials, however, are not particularly suitable for small diameter grafts owing to a tendency for thrombus induction, embolism and occlusion of the graft lumen, lack of compliance and excessive intimal hyperplasia at anastomoses. They do not allow vessel remodelling or vascular physiological responsiveness, and are prone to infection. Polyurethane (PU) is a promising biocompatible synthetic scaffold material, since it is elastomeric with suitable compliance and controlled degradation characteristics (Zhang et al. 2004; Kannan et al. 2005; Vara et al. 2005). In its unmodified state, PU is uncharged, but can be modified to become cationic, anionic or zwitterionic. PU has a tendency to degrade, causing aneurysm formation. However, carbonate-based PU with no ester linkages has improved stability and endothelialization characteristics (Kannan et al. 2005). Poly(ester-urethane)ureas are more flexible and stronger but have reduced cell adhesion and oxidative biodegradation. We have developed a composite PU–polycaprolactone (PU–PCL) scaffold that shows good EC attachment to the PCL luminal surface (Williamson et al. submitted; figure 5). These synthetic scaffolds are often treated with biological molecules such as collagen, heparin, laminin, synthetic RGD cell adhesive peptides (see below), fibrin–gelatin, growth factors, anti-coagulant peptides and dextran derivatives (Krijgsman et al. 2002; Rashid et al. 2004; Kannan et al. 2005) and with antibiotics (Cagiannos et al. 2005) to regulate cell behaviour through integrin-mediated cell adhesion signalling and to prevent infection and intimal hyperplasia. Biodegradable biomaterials such as polylactic acid, poly(d,l-lactide-co-glycolic acid) (PLGA) and poly(ester-urethane)urea may be used as temporary graft scaffolds but require ingrowth of cells and deposition of crosslinked matrix (Kannan et al. 2005; Kielty et al. 2005a,b; Vorp et al. 2005).
In addition to synthetic polymer scaffolds, decellularized arteries and veins which retain elastic lamellar structure have been tested with some success, although such grafts are susceptible to degradation and aneurysm formation (Walles et al. 2003). Burst strengths of such grafts are often low. However, recent studies of decellularized vein allografts have shown such scaffolds to have burst strengths similar to native vessels (Schaner et al. 2004; Martin et al. 2005). A recent development is decellularized scaffolds based on pure elastin or collagen which were both populated by host cells in vivo and remodelled to contain numerous collagen and elastic fibres (Simionescu et al. 2006). Tissue-engineered vessels based on rolled up sheets of SMC and fibroblasts embedded within their own matrix to form tubes of media coated in adventitia have higher burst strength but take at least three months to prepare (L'Heureux et al. 1998, 2001). Autologous artificial vessels generated by the formation of a matrix-rich granulation tissue capsule surrounding biocompatible tubing placed within the peritoneal cavity also have high burst strengths, can be prepared within two to three weeks and are not rejected (Thomas et al. 2003; Chue et al. 2004).
Our small diameter vascular graft model is based on elastomeric porous scaffolds fabricated from slowly biodegradable PU or PU–PCL composite grafts (figure 5), with SMC seeded within the scaffolds and ECs on the luminal surface. Using a caprine carotid PU interposition graft, we have demonstrated that delivery of TGFβ3 attenuates myointimal hyperplasia and reduces elastin in anastomoses (Ghosh et al. 2006). We are also exploiting elastic fibre molecules to control SMC and EC attachment, migration, proliferation and differentiation within the scaffold. To this end, we have developed a library of recombinant elastic fibre molecules, and characterized SMC and EC integrin-mediated cell adhesion, spreading, proliferation and migration on these molecules (Bax et al. 2003; 2007; Lomas et al. in press; Williamson et al. 2006). We are investigating whether specific elastic fibre motifs can be used for slow release of TGFβ1, and how mechanical forces (cyclic stretch, shear stress) influence SMC proliferation and elastic fibre deposition.
(b) Adsorption of vascular adhesion motifs on scaffolds
Synthetic polymers such as PU do not bear epitopes that directly bind cell adhesion receptors, so many studies have focused on modifying polymers with cell–matrix adhesion motifs to ensure cell and tissue biocompatibility. Since integrin receptors are major vascular cell–matrix receptors (Mould & Humphries 2004), attention has been focused on the attachment of the RGD tripeptide cell adhesion motif to scaffold materials. Adhesion peptides can be stably adsorbed by grafting to the termini of surface-active polymers that adsorb to hydrophobic or anionic surfaces; the surface can then be passivated against non-specific interactions. As well as achieving good surface density of peptides, clustering of peptides is advantageous for cell adhesion. An aqueous-based process has been reported for surface functionalization of RGD moieties onto the luminal surface of a prefabricated cardiovascular graft made of poly(carbonate-urea)urethane (Salacinski et al. 2003; Tiwari et al. 2003). Other immobilized cell adhesion peptides (KQAGDV from fibrinogen, recognized by integrin αIIbβ3; VGVAPG from elastin) have been adsorbed onto scaffolds. In general, cells adhere more strongly to surfaces modified with these adhesive ligands than to control surfaces (Mann & West 1999). Given the exquisite specificity of integrin-RGD recognition and ligation in native molecular context, and the highly specific resultant cellular signalling responses, there is significant potential to exert much greater regulation of cell behaviour by controlling the conformation of selected cell–matrix adhesion motifs on scaffold surfaces.
(c) Surface effects on ECM protein conformation and function
Different scaffold surfaces have distinct chemistries, roughness indices and abilities to adsorb ECM molecules in native conformation and to support cell adhesion. Several recent studies provide critical insights into how adsorption of proteins to different surfaces affects their conformation and biological functions, especially cell adhesion.
Owing to their amphiphilic nature, proteins adsorb to most surfaces over a wide range of solution conditions both in vitro and in vivo (Vandulm & Norde 1983). Such non-specific physiosorption, driven by van der Waals' forces, the electrostatic double layer force and the hydrophobic effect, is influenced by substrate topography and charge, the concentration and valency of the solute and the unique charge distribution of each protein (Haynes & Norde 1995; Muller et al. 1997). Substrate chemistry and topography-dependent conformational changes have been demonstrated for many proteins including the ECM components fibronectin, type I collagen, fibrillin microfibrils (see below) and recombinant elastin peptides (Denis et al. 2002; Yang et al. 2002; Bergkvist et al. 2003; Sherratt et al. 2004). Conformational effects may be limited to alterations in relative domain positions, masking or revealing biologically active sites, or may extend to the secondary structure disrupting α-helical content (Krammer et al. 2002; Bergkvist et al. 2003). The ability of adsorbed fibronectin to bind specific antibodies and to support cell attachment and spreading cell is dependent on the hydrophilicity/hydrophobicity of the substrate (Garcia et al. 1999; Michael et al. 2003).
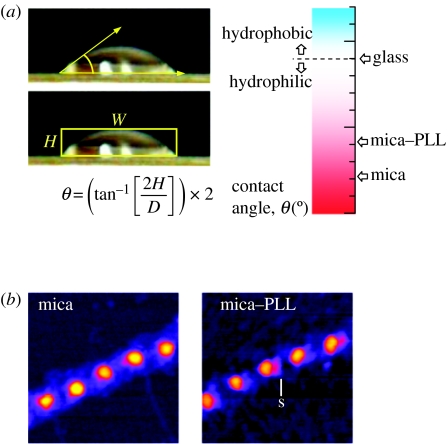
Substrate hydrophobicity/hydrophilicity is commonly quantified by measuring water drop contact angles (Sherratt et al. 2004; figure 6). For a sessile liquid drop on a substrate, the angle formed between the liquid–solid and liquid–vapour interfaces is called the wetting or contact angle (θ). Surfaces are defined as hydrophilic if θ<90° and hydrophobic if θ>90°. Although contact angle measurements may be made on sessile drops, on most solid surfaces the measured contact angle as the drop advances differs from the angle measured as the drop recedes (Bachmann et al. 2000). This contact angle hysteresis may be induced by surface roughness, which is also known to influence both protein adsorption and cellular adhesion (de Gennes 1985; WojciakStothard et al. 1996; Denis et al. 2002). Nanoscale surface roughness is readily quantified using atomic force microscopy (AFM; Bottomley 1998). Substrate roughness and protein conformation can be determined from AFM height maps before and after protein adsorption, respectively.
Figure 6.
Substrate hydrophilicity/hydrophobicity and fibrillin microfibril morphology. (a) Drops of liquid on a surface assume a shape which depends on the relation between the free energies of the three involved surfaces. The contact angle (θ), determined from the height (H) and width (W) of the drop, increases with decreasing substrate hydrophilicity. (b) Foetal bovine aorta fibrillin microfibrils adsorbed on hydrophilic mica and less hydrophilic mica+poly-l-lysine (mica–PLL). Intermittent contact mode atomic force microscopy (AFM) height images, 250×250 nm, Z-scale 10 nm. Fibrillin microfibrils are more diffuse on mica and lack the shoulder region (s) which is present in microfibrils adsorbed on mica–PLL.
Scaffold surface-patterning techniques, such as inkjet printing of ECM molecules in different geometries, offer a powerful means of controlling the cell-adhesive properties of grafts. Self-assembled networks of the basement membrane molecule laminin-1 have recently been obtained on glass substrates by physisorption-assisted microcontact printing (Sgarbi et al. 2004). Recent advances in AFM technology have led to the development of dip-pen nanolithography which employs an AFM tip as a ‘nib’, a solid substrate as the ‘paper’ and molecules with affinity for the substrate as the ‘ink’ (Piner et al. 1999). In this way, substrates can be patterned with linewidths in the range 10–100 nm, generating biologically active multicomponent nanostructures and arrays (Zhang et al. 2002; Lee et al. 2003).
Exploiting elastic fibre molecules in vascular tissue engineering requires detailed molecular knowledge of their assembly and biological interactions. This information throws light into scaffold surface design, supports the development of scaffold coatings of template molecules such as fibrillin-1 to encourage elastin deposition in grafts and support cell adhesion, and provides insights into how vascular cells in vivo can be encouraged to deposit ordered elastic fibres.
6. Elastic fibres in vascular tissue engineering
Several groups are exploiting elastin and microfibrillar molecules to address a major challenge in tissue-engineered graft design, namely, to regulate vascular cell phenotype within the construct. We and others have developed recombinant and purified resources of human elastic fibre molecules that we are using to understand the molecular basis of elastic fibre assembly and function, and to exploit directly in vascular grafts as elastin-based materials and cell-seeding gels (Kielty et al. 2005b).
(a) Understanding elastic fibre formation
In order to better exploit elastic fibre molecules in tissue engineering, there is a need to understand the molecular interactions that normally occur during elastic fibre formation in vivo. For initial microfibril assembly, homotypic interactions of fibrillin-1 appear to be critical. Our recent studies have demonstrated specific high affinity binding between amino- and carboxy-terminal fragments that may form the basis of head-to-tail linear assembly of fibrillin-1 (Marson et al. 2005). MAGP-1 showed calcium-dependent binding to the fibrillin-1 N-terminal region (encoded by exons 1–8), which localizes to the beads. These fibrillin-1 interactions may regulate pericellular microfibril assembly.
The next stage in elastic fibre assembly is the deposition of tropoelastin on microfibrils. The molecular basis of fibrillin-1 interactions with tropoelastin and MAGP-1 has also been defined (Jensen et al. 2001; Rock et al. 2004). Binding assays revealed high affinity calcium-independent binding of two overlapping fibrillin-1 fragments (encoded by central exons 18–25, 24–30) to tropoelastin, which, in microfibrils, map to an exposed ‘arms’ feature adjacent to the beads. A further binding site within an adjacent fragment (encoded by exons 9–17) was within an eight-cysteine motif designated TB2 (encoded by exons 16 and 17). A novel transglutaminase (TG2) cross link between tropoelastin and fibrillin-1 fragment (encoded by exons 9–17) was localized by mass spectrometry to a sequence encoded by exon 17. The high affinity binding and cross-linking of tropoelastin to a central fibrillin-1 sequence imply that this association is fundamental to elastic fibre formation. MAGP-1 may form secondary interactions with adjacent microfibril-bound tropoelastin.
Fibrillin-1 has also been shown to interact with LTBP-1 (Isogai et al. 2003), fibulin-2 (Reinhardt et al. 1996), versican (Isogai et al. 2002), small chondroitin sulphate proteoglycans (Kielty et al. 1996) and with heparin and heparan sulphate. Heparin inhibits microfibril assembly and was shown to interact with fibrillin-1 at three sites (Tiedemann et al. 2001). Using BIAcore 3000 technology to investigate fibrillin-1 interactions with heparin, and with heparin saccharides analogous to S-domains of heparan sulphate, we have now shown that there are four high-affinity heparin-binding sites on fibrillin-1, located in three of these sites and defined their binding kinetics (Cain et al. 2005). Heparin does not inhibit fibrillin-1 N- and C-terminal interactions, but heparin and MAGP-1 compete for binding to the fibrillin-1 N-terminus, and heparin and tropoelastin compete for binding to a central fibrillin-1 sequence. By modulating these interactions, heparin may regulate microfibril and elastic fibre assembly.
(b) Effects of surface chemistry on microfibril organization
Current models of fibrillin-1 alignment within microfibrils are based on the prominent 56 nm periodic beaded morphology of isolated, often apparent by rotary shadowing, microfibrils on mica and by negative staining microfibrils on carbon-coated grids (Baldock et al. 2001). However, surface chemistry, especially hydrophobicity/hydrophilicity, has a profound effect on microfibril beaded morphology and presumably also functional interactions (Sherratt et al. 2004, 2005; figure 6). We showed that microfibrils adsorbed to a hydrophilic mica substrate adopted a diffuse morphology, but adsorbed to mica coated with poly-l-lysine (PLL) or to borosilicate glass substrates had a more compact morphology and a directional asymmetry to the bead, which was not present on mica alone. Intermediate morphologies were observed along a substrate gradient. We also examined the morphology of microfibrils on topographically similar, chemically homogeneous substrates with a larger amphiphilic range, silicon oxide and silicon oxide coated with polymer films of PLGA and self-assembled monolayers of octadecyl trimethylchlorosilane. Extremes of substrate amphiphilicity profoundly altered periodicity and curvature and induced lateral spreading, implying the disruption of domain structure. Microfibrils adsorbed to a substrate with an intermediate amphiphilicity (PLGA) were similar in dimensions and morphology to hydrated microfibrils. Substrate-induced conformational changes are thus highly likely to direct the biological properties of elastic fibre components adsorbed to artificial surfaces for tissue engineering purposes (Yang et al. 2002; Sherratt et al. 2004, 2005).
(c) Elastin coacervates
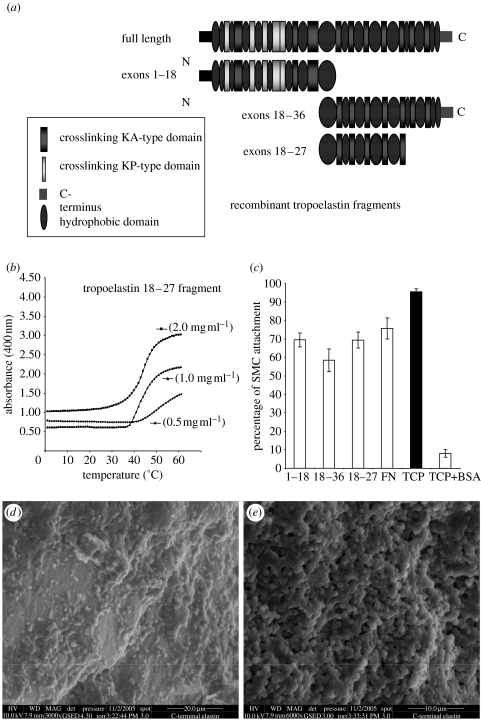
Tropoelastin is a highly non-polar molecule, consisting essentially of 34 alternating hydrophobic and crosslinking domains (Kielty et al. 2002; Mithieux & Weiss 2005). The cross-linking domains contain the lysine residues destined to form the covalent intermolecular cross links through the actions of LOXs that stabilize the assembled polymer. The hydrophobic domains are thought to be sites of interactions that contribute to the juxtaposition of lysine residues in preparation for cross link formation. In vitro, tropoelastin has an intrinsic capacity to form an ordered assembly through a process of hydrophobic self-aggregation or ‘coacervation’, in which the protein comes out of solution as a second phase on an increase in solution temperature. The kinetics of the transition appears to be that of a nucleation process. The temperature at which this transition takes place is dependent on elastin concentration, ionic strength and pH (figure 7). Analysis of the ability of individual elastin domains to coacervate and self-assemble has revealed that most domains can self-assemble but only a few (especially domains encoded by exons 18, 20 and 24) can coacervate (Pepe et al. 2005; Tamburro et al. 2005a). New evidence for contact points between tropoelastin monomers (domains encoded by exons 19–25) has been obtained using cross-linking, protease digestion and mass spectrometry (Mithieux et al. 2005; Wise et al. 2005).
Figure 7.
(a) Schematic of full-length human tropoelastin, and recombinant tropoelastin fragments that we have expressed and purified for vascular tissue engineering. (b) Concentration-dependent coacervation, at increasing temperature, of a recombinant human tropoelastin fragment encoded by exons 18–36, monitored at OD400. (c) Human aortic SMC attached well to tropoelastin fragments (encoded by exons 1–18, 18–36 and 18–27) that were coated onto tissue culture plastic (TCP). Fibronectin (FN) and untreated TCP were positive controls; cells blocked with bovine serum albumin (BSA) were negative controls. (d,e) Environmental scanning electron microscopy images of 50°C coacervate of recombinant human tropoelastin fragment encoded by exons 18–36 (2 mg ml−1). The coacervate had been crosslinked using 3,3′-dithiobis-(sulphosuccinimidylpropionate).
The coacervation behaviour of short recombinant fragments of tropoelastin has been described in detail. As few as three hydrophobic domains flanking two cross-linking domains of human tropoelastin are sufficient to support a self-assembly process that aligns lysines for zero-length cross-linking, resulting in the formation of the cross links of native elastin and with solubility and mechanical properties similar to native elastin (Bellingham et al. 2001; Keeley et al. 2002; Miao et al. 2003). The structures of both cross-linking and hydrophobic domains have significant effects on the assembly of these short elastin polypeptides (Miao et al. 2005). This process provides the basis for fabrication of vascular polymeric elastin-like matrices for incorporating into vascular grafts. Interestingly, in a variable temperature in situ AFM study, these elastin peptides self-assembled in a substrate-dependent manner. On hydrophilic mica surfaces, the peptides were adsorbed as discrete, rounded aggregates. Adsorption to hydrophobic highly ordered pyrolitic graphite (HOPG) induced a fibrillar arrangement (Yang et al. 2002). The order observed on HOPG substrates may be due to hydrophobic peptide–substrate interactions, which form an energetically close-packed arrangement acting as a template for fibril growth.
Another group has recently described the production and properties of massive synthetic elastin assemblies formed by chemically cross-linking recombinant human tropoelastin with bis(sulphosuccinimidyl)- suberate, permitting the construction of elastic sponges, sheets and tubes (Mithieux et al. 2004). These synthetic elastin constructs also had similar extensibility properties to those of native elastin, with Young's modulus ranging from 220 to 280 kPa with linearity of extension to at least 150%. The constructs behaved as hydrogels and displayed stimuli-responsive characteristics towards temperature and salt concentrations. Growth and proliferation of cells were supported in vitro, while in vivo implants were well tolerated. A separate study revealed that the polypeptide sequence encoded by human tropoelastin exon 30 assembles with an ultrastructural organization similar to amyloid networks, with anti-parallel β-sheet conformation predominant in the exon 30 fibres (Tamburro et al. 2005b).
We have produced recombinant human tropoelastin molecules and fragments, and have characterized their coacervation and cross-linking characteristics in the absence or presence of other elastic fibre molecules including fibrillin-1 (figure 7). We are now developing elastin–fibrillin composites for cell-seeding gels and scaffold coatings. In a collaborative study with A. S. Weiss (Sydney, Australia), we have shown that elastin coacervation occurs at a lower temperature in the presence of a fibrillin-1 fragment which contains the transglutaminase cross link site to elastin (Clarke et al. 2005).
(d) Elastin-based vascular graft coatings and cell-seeding gels
Since the ECM is the authentic substrate for connective tissue cells including SMCs and ECs, cells cultured within such a biological matrix experience a richer, more complex physical environment and markedly different geometry than cells on flat surfaces (Cukierman et al. 2001, 2002). Cells sense their surrounding ECM, are directly anchored to it, respond to ECM through cell–matrix adhesion signals that influence cell growth, migration, differentiation, survival, tissue organization and remodel their surrounding matrix. Three-dimensional vascular ECM materials are thus powerful tools for modulating grafts and regulating vascular cell phenotype within them (Gobin & West 2003; Grinnell 2003; Hubbell 2003).
There is currently much interest in the engineering of polypeptides incorporating elastin motifs to generate novel hydrogels and drug-delivery polymers (Bandiera et al. 2005; Haider et al. 2005; Herrero-Vanrell et al. 2005; Junger et al. 2005). We are developing cell-seeding gels based on cross-linked elastin matrices (figure 7) that can be used to deliver cells onto and within our vascular graft scaffolds. Such gels may be used to direct cell proliferation, regulate cell migration and ECM deposition within the scaffold, and control the delivery and release of TGFβ1 growth factors. Elastin composite gels can be formed by incorporating other vascular cell–matrix motifs, including fibrillin-1 and fibulin-5 fragments.
Recombinant vascular matrix molecules generated in our laboratory have been used to coat vascular scaffolds, in order to enhance SMC and EC attachment and to regulate their function. We have shown that SMCs adhere well to tropoelastin (figure 7), fibrillin-1 (Bax et al. 2003) and fibulin-5 (Lomas et al. in press), while ECs adhere to tropoelastin fragments, as well as to fibrillin-1 and fibulin-5 in an RGD-dependent manner (figure 5).
(e) Elastic fibres in the controlled delivery of vascular growth factors
In natural vessels, growth factors influence vascular cells and ECM deposition, and their activation is, in turn, tightly regulated by ECM. Crosstalk between growth factor and integrin receptor signalling pathways profoundly influences growth factor effects. Thus, it is important that strategies are devised which allow the controlled release of vascular growth factors during graft engineering. Bioactive polymers are now being engineered which not only provide physical support and a template for cells but also closely mimic the in vivo release mechanisms of vascular growth factors from the ECM (Zisch 2004).
TGFβ family growth factors are particularly important in the vasculature and in vascular tissue engineering (Ghosh et al. 2005). TGFβ1 stimulates SMC proliferation and ECM deposition, and roles required of SMCs during graft pre-conditioning. TGFβ3 has anti-scarring properties, and we have shown that it reduces scarring at carotid artery anastomotic sites after grafting (Ghosh et al. 2006). Elastic fibre molecules have great potential for regulating TGFβ activity in grafts. The molecular mechanism of LTBP-1 (which interacts with fibrillin microfibrils) binding to the TGFβ1 latency-associated peptide (LAP) has recently been resolved (Chen et al. 2005). We are investigating whether LTBP-3 can be used for controlled delivery of TGFβ isoforms within grafts. Using recombinant fragments, we have shown that that it interacts with a fibrillin-1 sequence, and can, in this way, be stably incorporated into elastic fibre materials. It may also be possible to exploit the heparan sulphate-binding properties of elastin and fibrillin-1 to regulate the delivery of vascular endothelial growth factor and FGF-2 in grafts.
(f) Elastin products in vascular graft fabrication
Purified elastin products can be used directly in vascular graft fabrication. Elastin blends, containing collagen and PLGA, have been electrospun (Stitzel et al. 2006). This technology controls composition, structure and mechanical properties of the resulting biomaterial. These scaffolds possess biocompatibility and mechanical properties similar to native vessels (Stitzel et al. 2006). Controlling the shape and size (diameter or width) of electrospun fibres by varying solute concentration and polymer delivery rate yielded α-elastin and tropoelastin fibres that were several microns in width (Li et al. 2005). These fibres supported attachment and growth of human embryonic mesenchymal cells. Intact purified elastin was incorporated into collagen-based tissue-engineered blood vessels, forming hybrid constructs that mimicked arterial physiology and exhibited improved mechanical properties (Berglund et al. 2004). Porous scaffolds composed of elastin and collagen prepared by freeze drying showed good strain recovery; these products could be chemically cross-linked, which gives increased stiffness while preserving open porous structure during cross-linking (Buttafoco et al. 2006).
(g) Role of elastin as a non-thrombogenic coating
It has been shown that a recombinant human elastin polypeptide can act as an anti-thrombogenic coating on synthetic scaffolds (Woodhouse et al. 2004). Three commercially available synthetic materials coated with adsorbed elastin all demonstrated reduced platelet activation and adhesion in platelet rich plasma in vitro. When compared with non-coated controls, there was a significant decrease in platelet microparticle release and P-selectin expression for the polypeptide-coated surfaces. Scanning electron microscopy showed fewer adhering platelets on coated surfaces when compared with non-coated controls. In vivo evaluations of PU catheters coated with the polypeptide showed a marked increase in catheter patency and a significant decrease in fibrin accretion and embolism when compared with uncoated controls. Thus, this and other tropoelastin polypeptides show a strong potential for use as a non-thrombogenic coating for small diameter vascular grafts.
7. Elastic fibre deposition in vessel regeneration
The deposition of elastic fibres is tightly developmentally regulated, and in adult tissues elastic fibre regeneration is limited. However, several recent strategies suggest new approaches to stimulate the deposition of ordered functional elastic fibres for vascular repair.
(a) Stimulation of adult vascular elastic fibre deposition by versican V3 isoform
Retroviral-mediated overexpression of the versican proteoglycan variant V3, which lacks chondroitin sulphate chains, has been shown to alter arterial SMC phenotype in short-term cell culture. These cells exhibited significantly increased expression of tropoelastin and increased formation of elastic fibres in long-term cell cultures (Merrilees et al. 2002). When V3-overexpressing SMCs were seeded into ballooned rat carotid arteries, by four weeks they had produced a highly structured neointima significantly enriched in ordered elastic fibre lamellae containing elongated SMC arranged in parallel arrays and separated by densely packed elastic fibres and collagen bundles. Reduction of versican using an antisense approach caused flattened SMC morphology, reduced cell proliferation and migration, increased tropoelastin synthesis, increased EBP and increased the deposition of elastic fibres in long-term cultures, but incorporation of chondroitin sulphate reversed these effects. Thus, versican and its constituent chondroitin sulphate chains play a central role in controlling cell phenotype, elastogenesis and intimal structure (Huang et al. 2006). The V3 variant of versican thus offers a powerful new therapeutic approach for the deposition of elastic fibres in vascular conduits.
(b) Using fibrin gels to enhance elastic fibre deposition
It was recently reported that SMCs seeded within fibrin gels remodel their ECM environment over four weeks and deposit abundant elastic fibres (Long & Tranquillo 2003). This was the first observation of ordered, cross-linked elastic fibre deposition in tissue-engineered replacements fabricated in vitro with SMC, and indicates that in vitro elastogenesis can be achieved for three-dimensional elastic structures.
Recently, small diameter vessels based on ovine SMCs and ECs embedded in fibrin gels have been described (Swartz et al. 2005). Vessels implanted into lamb jugular veins demonstrated patency and similar blood flow rates as native vessels. By 15 weeks post implantation, they exhibited remarkable matrix remodelling with the production of abundant elastic and collagen fibres and orientation of SMC perpendicular to the direction of blood flow. Thus, fibrin-based graft models hold significant promise for vascular graft design.
(c) Biomechanical forces to regulate elastic fibre molecular expression
In the healthy artery, radial stretch of the vessel wall is the predominant physical variable that influences SMC phenotype, since shear forces are separated from the tunica media by an intact intima (Baguneid et al. 2004; Hamilton et al. 2004). Although SMCs are not normally exposed directly to the shear stresses of flowing blood, EC desquamation in diseased or injured arteries exposes underlying SMCs to uncharacteristically high shear forces. Interstitial flow across the vessel wall, driven by the transmural pressure differential, also influences SMCs (Wang & Tarbell 1995; Civelek et al. 2002). Although its superficial velocity is typically very low, the interstitial spaces in the tissue are small and the shear stress on SMCs can be significant, up to 1–3 dyn cm−2 in rabbit aortas (Wang & Tarbell 1995; Civelek et al. 2002). Fluid flow of blood through the vascular system has been variously reported to affect SMCs in terms of gene expression, increased proliferation (Kim et al. 1999), decreased proliferation (Ueba et al. 1997; Liu et al. 2003), increased contraction (Civelek et al. 2002), alignment perpendicular to flow (Lee et al. 2002), alignment parallel to flow (Mcintire et al. 1998), increase in apoptosis (Apenberg et al. 2003) and increase in nitrate production. These various flow-induced changes to SMCs may be dependent on differences in tissue origin, species, culture conditions, and the type and intensity of shear stress applied.
Since shear stress influences SMC phenotype and may be exploited to modify SMC in grafts, we have examined the influence of in vitro shear stress on the expression and deposition of elastic fibre components by human coronary artery SMC, and the role of TGFβ1 in regulating these effects (Ghosh et al. 2005). In static culture, elastin and fibrillin-1 mRNAs were in very low abundance, but after exposure to 3 h of shear stress at 15 dyn cm−2 both mRNAs were readily detected. These effects were mediated by TGFβ since neutralizing antibodies blocked the upregulation. We are currently investigating the effects of cyclical stretch on elastic fibre deposition by SMC.
8. Conclusion
Tissue-engineered vascular graft design has advanced greatly in recent years, but several major issues remain to be addressed. These include improving scaffold compliance match, strong luminal attachment of ECs that retain anti-thrombotic and vasoactive properties, population of grafts with contractile SMCs and deposition of an ordered functional elastic fibre-rich vascular matrix that contributes to long-term graft contractile function. This review has outlined how elastic fibre biology can be used to address all of these concerns, since elastic fibres are major elastomeric components of vascular walls, prominent vascular cell adhesion macromolecules and natural delivery systems for vascular growth factors. Moreover, the unique aggregation characteristics of elastin itself are now providing the basis for many novel cell-seeding and drug-delivery hydrogels, and composite gels incorporating specific cell–matrix signals are being developed.
Acknowledgments
C.M.K. is supported by the MRC (UK), and is a Royal Society–Wolfson Research Merit Award holder. Our tissue engineering research referred to in this review is funded by the UK Centre for Tissue Engineering (BBSRC, MRC, EPSRC). Dr Carolyn J. P. Jones carried out the transmission electron microscopy. Dr Claire Crouchley conducted the porcine SMC immunofluorescence imaging.
Footnotes
One contribution of 21 to a Theme Issue ‘Bioengineering the heart’.
References
- American Heart Association 2004 Heart disease and stroke statics—2005 update. Dallas, TX.
- Apenberg S, Freyberg M.A, Friedl P. Shear stress induces apoptosis in vascular smooth muscle cells via an autocrine Fas/FasL pathway. Biochem. Biophys. Res. Commun. 2003;310:355–359. doi: 10.1016/j.bbrc.2003.09.025. doi:10.1016/j.bbrc.2003.09.025 [DOI] [PubMed] [Google Scholar]
- Bachmann J, Ellies A, Hartge K.H. Development and application of a new sessile drop contact angle method to assess soil water repellency. J. Hydrol. 2000;231–232:66–75. doi:10.1016/S0022-1694(00)00184-0 [Google Scholar]
- Baguneid M, Murray D, Salacinski H.J, Fuller B, Hamilton G, Walker M, Seifalian A.M. Shear-stress preconditioning and tissue-engineering-based paradigms for generating arterial substitutes. Biotechnol. Appl. Biochem. 2004;39:151–157. doi: 10.1042/BA20030148. doi:10.1042/BA20030148 [DOI] [PubMed] [Google Scholar]
- Baldock C, Koster A.J, Ziese U, Rock M.J, Sherratt M.J, Kadler K.E, Shuttleworth C.A, Kielty C.M. The supramolecular organization of fibrillin-rich microfibrils. J. Cell Biol. 2001;152:1045–1056. doi: 10.1083/jcb.152.5.1045. doi:10.1083/jcb.152.5.1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball S.G, Shuttleworth C.A, Kielty C.M. Direct cell contact influences bone marrow mesenchymal stem cell fate. Int. J. Biochem. Cell Biol. 2004;36:714–727. doi: 10.1016/j.biocel.2003.10.015. doi:10.1016/j.biocel.2003.10.015 [DOI] [PubMed] [Google Scholar]
- Bandiera A, Taglienti A, Micali F, Pani B, Tamaro M, Crescenzi V, Manzini G. Expression and characterization of human-elastin-repeat-based temperature-responsive protein polymers for biotechnological purposes. Biotechnol. Appl. Biochem. 2005;42:247–256. doi: 10.1042/BA20050114. doi:10.1042/BA20050114 [DOI] [PubMed] [Google Scholar]
- Bax D.V, Bernard S.E, Morgan A, Shuttleworth C.A, Humphries M.J, Kielty C.M. Cell adhesion to fibrillin-1 molecules and microfibrils is mediated by alpha5 beta1 and alphav beta3 integrins. J. Biol. Chem. 2003;278:34 605–34 616. doi: 10.1074/jbc.M303159200. doi:10.1074/jbc.M303159200 [DOI] [PubMed] [Google Scholar]
- Bax D.V, et al. Cell adhesion to fibrillin-1: identification of an Arg-Gly-Asp-dependent synergy region and a heparin-binding site that regulates focal adhesion formation. J. Cell. Sci. 2007;120:1383–1392. doi: 10.1242/jcs.003954. doi:10.1242/jcs.003954 [DOI] [PubMed] [Google Scholar]
- Bellingham C.M, Woodhouse K.A, Robson P, Rothstein S.J, Keeley F.W. Self-aggregation characteristics of recombinantly expressed human elastin polypeptides. Biochim. Biophys. Acta. 2001;1550:6–19. doi: 10.1016/s0167-4838(01)00262-x. [DOI] [PubMed] [Google Scholar]
- Bergkvist M, Carlsson J, Oscarsson S. Surface-dependent conformations of human plasma fibronectin adsorbed to silica, mica, and hydrophobic surfaces, studied with use of atomic force microscopy. J. Biomed. Mater. Res. A. 2003;64:349–356. doi: 10.1002/jbm.a.10423. doi:10.1002/jbm.a.10423 [DOI] [PubMed] [Google Scholar]
- Berglund J.D, Nerem R.M, Sambanis A. Incorporation of intact elastin scaffolds in tissue-engineered collagen-based vascular grafts. Tissue Eng. 2004;10:1526–1535. doi: 10.1089/ten.2004.10.1526. [DOI] [PubMed] [Google Scholar]
- Bisaccia F, Morelli M.A, De Biasi M, Traniello S, Spisani S, Tamburro A.M. Migration of monocytes in the presence of elastolytic fragments of elastin and in synthetic derivates. Structure–activity relationships. Int. J. Pept. Protein Res. 1994;44:332–341. doi: 10.1111/j.1399-3011.1994.tb01017.x. [DOI] [PubMed] [Google Scholar]
- Booms P, Pregla R, Ney A, Barthel F, Reinhardt D.P, Pletschacher A, Mundlos S, Robinson P.N. RGD-containing fibrillin-1 fragments upregulate matrix metalloproteinase expression in cell culture: a potential factor in the pathogenesis of the Marfan syndrome. Hum. Genet. 2005;116:51–61. doi: 10.1007/s00439-004-1194-7. doi:10.1007/s00439-004-1194-7 [DOI] [PubMed] [Google Scholar]
- Borschel G.H, Huang Y.C, Calve S, Arruda E.M, Lynch J.B, Dow D.E, Kuzon W.M, Dennis R.G, Brown D.L. Tissue engineering of recellularized small-diameter vascular grafts. Tissue Eng. 2005;11:778–786. doi: 10.1089/ten.2005.11.778. doi:10.1089/ten.2005.11.778 [DOI] [PubMed] [Google Scholar]
- Bottomley L.A. Scanning probe microscopy. Anal. Chem. 1998;70:425R–475R. doi: 10.1021/a10000108. doi:10.1021/a1980011o [DOI] [PubMed] [Google Scholar]
- Broekelmann T.J, Kozel B.A, Ishibashi H, Werneck C.C, Keeley F.W, Zhang L, Mecham R.P. Tropoelastin interacts with cell-surface glycosaminoglycans via its COOH-terminal domain. J. Biol. Chem. 2005;280:40 939–40 947. doi: 10.1074/jbc.M507309200. doi:10.1074/jbc.M507309200 [DOI] [PubMed] [Google Scholar]
- Brooke B.S, Bayes-Genis A, Li D.Y. New insights into elastin and vascular disease. Trends Cardiovasc. Med. 2003;13:176–181. doi: 10.1016/s1050-1738(03)00065-3. doi:10.1016/S1050-1738(03)00065-3 [DOI] [PubMed] [Google Scholar]
- Buttafoco L, Kolkman N.G, Engbers-Buijtenhuijs P, Poot A.A, Dijkstra P.J, Vermes I, Feijen J. Electrospinning of collagen and elastin for tissue engineering applications. Biomaterials. 2006;27:724–734. doi: 10.1016/j.biomaterials.2005.06.024. doi:10.1016/j.biomaterials.2005.06.024 [DOI] [PubMed] [Google Scholar]
- Cagiannos C, Abul-Khoudoud O.R, DeRijk W, Shell D.H, Jennings L.K, Tolley E.A, Handorf C.R, Fabian T.C. Rapamycin-coated expanded polytetrafluoroethylene bypass grafts exhibit decreased anastomotic neointimal hyperplasia in a porcine model. J. Vasc. Surg. 2005;42:980–988. doi: 10.1016/j.jvs.2005.06.018. doi:10.1016/j.jvs.2005.06.018 [DOI] [PubMed] [Google Scholar]
- Cain S.A, Baldock C, Gallagher J, Morgan A, Bax D.V, Weiss A.S, Shuttleworth C.A, Kielty C.M. Fibrillin-1 interactions with heparin. Implications for microfibril and elastic fiber assembly. J. Biol. Chem. 2005;280:30 526–30 537. doi: 10.1074/jbc.M501390200. doi:10.1074/jbc.M501390200 [DOI] [PubMed] [Google Scholar]
- Cain S.A, Morgan A, Sherratt M.J, Ball S.G, Shuttleworth C.A, Kielty C.M. Proteomic analysis of fibrillin-rich microfibrils. Proteomics. 2006;6:111–122. doi: 10.1002/pmic.200401340. [DOI] [PubMed] [Google Scholar]
- Charbonneau N.L, Ono R.N, Corson G.M, Keene D.R, Sakai L.Y. Fine tuning of growth factor signals depends on fibrillin microfibril networks. Birth Defects Res. C Embryo Today. 2004;72:37–50. doi: 10.1002/bdrc.20000. doi:10.1002/bdrc.20000 [DOI] [PubMed] [Google Scholar]
- Chaudhry S.S, Gazzard J, Baldock C, Dixon J, Rock M.J, Skinner G.C, Steel K.P, Kielty C.M, Dixon M.J. Mutation of the gene encoding fibrillin-2 results in syndactyly in mice. Hum. Mol. Genet. 2001;10:835–843. doi: 10.1093/hmg/10.8.835. doi:10.1093/hmg/10.8.835 [DOI] [PubMed] [Google Scholar]
- Chaudhry S.S, Cain S.A, Morgan A, Dallas S.L, Shuttleworth C.A, Kielty C.M. Fibrillin-1 regulates the bioavailability of TGFβ1. J. Cell Biol. 2007;176:355–367. doi: 10.1083/jcb.200608167. doi:10.1083/jcb.200608167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Ali T, Todorovic V, O'Leary J.M, Downing A.K, Rifkin D.B. Amino acid requirements for formation of the TGF-beta-latent TGF-beta binding protein complexes. J. Mol. Biol. 2005;345:175–186. doi: 10.1016/j.jmb.2004.10.039. doi:10.1016/j.jmb.2004.10.039 [DOI] [PubMed] [Google Scholar]
- Chu M.L, Tsuda T. Fibulins in development and heritable disease. Birth Defects Res. C Embryo Today. 2004;72:25–36. doi: 10.1002/bdrc.20003. doi:10.1002/bdrc.20003 [DOI] [PubMed] [Google Scholar]
- Chue W.L, Campbell G.R, Caplice N, Muhammed A, Berry C.L, Thomas A.C, Bennett M.B, Campbell J.H. Dog peritoneal and pleural cavities as bioreactors to grow autologous vascular grafts. J. Vasc. Surg. 2004;39:859–867. doi: 10.1016/j.jvs.2003.03.003. doi:10.1016/j.jvs.2003.03.003 [DOI] [PubMed] [Google Scholar]
- Civelek M, Ainslie K, Garanich J.S, Tarbell J.M. Smooth muscle cells contract in response to fluid flow via a Ca2+-independent signaling mechanism. J. Appl. Physiol. 2002;93:1907–1917. doi: 10.1152/japplphysiol.00988.2001. [DOI] [PubMed] [Google Scholar]
- Clarke A.W, Wise S.G, Cain S.A, Kielty C.M, Weiss A.S. Coacervation is promoted by molecular interactions between the PF2 segment of fibrillin-1 and the domain 4 region of tropoelastin. Biochemistry. 2005;44:10 271–10 281. doi: 10.1021/bi050530d. doi:10.1021/bi050530d [DOI] [PubMed] [Google Scholar]
- Corson G.M, Charbonneau N.L, Keene D.R, Sakai L.Y. Differential expression of fibrillin-3 adds to microfibril variety in human and avian, but not rodent, connective tissues. Genomics. 2004;83:461–472. doi: 10.1016/j.ygeno.2003.08.023. doi:10.1016/j.ygeno.2003.08.023 [DOI] [PubMed] [Google Scholar]
- Cukierman E, Pankov R, Stevens D.R, Yamada K.M. Taking cell–matrix interactions to the third dimension. Science. 2001;294:1708–1712. doi: 10.1126/science.1064829. doi:10.1126/science.1064829 [DOI] [PubMed] [Google Scholar]
- Cukierman E, Pankov R, Yamada K.M. Cell interactions with three-dimensional matrices. Curr. Opin. Cell Biol. 2002;14:633–639. doi: 10.1016/s0955-0674(02)00364-2. doi:10.1016/S0955-0674(02)00364-2 [DOI] [PubMed] [Google Scholar]
- Czirok A, Zach J, Kozel B.A, Mecham R.P, Davis E.C, Rongish B.J. Elastic fiber macro-assembly is a hierarchical, cell motion-mediated process. J. Cell Physiol. 2006;207:97–106. doi: 10.1002/jcp.20573. doi:10.1002/jcp.20573 [DOI] [PubMed] [Google Scholar]
- Daly C.D, Campbell G.R, Walker P.J, Campbell J.H. In vivo engineering of blood vessels. Front. Biosci. 2004;9:1915–1924. doi: 10.2741/1384. doi:10.2741/1384 [DOI] [PubMed] [Google Scholar]
- Danielson K.G, Baribault H, Holmes D.F, Graham H, Kadler K.E, Iozzo R.V. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J. Cell Biol. 1997;136:729–743. doi: 10.1083/jcb.136.3.729. doi:10.1083/jcb.136.3.729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis E.C. Endothelial cell connecting filaments anchor endothelial cells to the subjacent elastic lamina in the developing aortic intima of the mouse. Cell Tissue Res. 1993a;272:211–219. doi: 10.1007/BF00302726. doi:10.1007/BF00302726 [DOI] [PubMed] [Google Scholar]
- Davis E.C. Smooth muscle cell to elastic lamina connections in developing mouse aorta: role in aortic medial organization. Lab. Invest. 1993b;68:89–99. [PubMed] [Google Scholar]
- de Gennes P.G. Wetting: statics and dynamics. Rev. Mod. Phys. 1985;57:827–863. doi:10.1103/RevModPhys.57.827 [Google Scholar]
- Denis F.A, Hanarp P, Sutherland D.S, Gold J, Mustin C, Rouxhet P.G, Dufrene Y.F. Protein adsorption on model surfaces with controlled nanotopography and chemistry. Langmuir. 2002;18:819–828. doi:10.1021/la011011o [Google Scholar]
- Dietz H.C, Loeys B, Carta L, Ramirez F. Recent progress towards a molecular understanding of Marfan syndrome. Am. J. Med. Genet. C Semin. Med. Genet. 2005;139:4–9. doi: 10.1002/ajmg.c.30068. [DOI] [PubMed] [Google Scholar]
- Duca L, Floquet N, Alix A.J, Haye B, Debelle L. Elastin as a matrikine. Crit. Rev. Oncol. Hematol. 2004;49:235–244. doi: 10.1016/j.critrevonc.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Faury G. Function–structure relationship of elastic arteries in evolution: from microfibrils to elastin and elastic fibres. Pathol. Biol. (Paris) 2001;49:310–325. doi: 10.1016/s0369-8114(01)00147-x. [DOI] [PubMed] [Google Scholar]
- Freeman L.J, Lomas A, Hodson N, Sherratt M.J, Mellody K.T, Weiss A.S, Shuttleworth A, Kielty C.M. Fibulin-5 interacts with fibrillin-1 molecules and microfibrils. Biochem. J. 2005;388:1–5. doi: 10.1042/BJ20050368. doi:10.1042/BJ20050368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P.J, Li Y, Sun A.J, Liu J.J, Ji K.D, Zhang Y.Z, Sun W.L, Marche P, Zhu D.L. Differentiation of vascular myofibroblasts induced by transforming growth factor-beta1 requires the involvement of protein kinase C alpha. J. Mol. Cell. Cardiol. 2003;35:1105–1112. doi: 10.1016/s0022-2828(03)00207-4. doi:10.1016/S0022-2828(03)00207-4 [DOI] [PubMed] [Google Scholar]
- Garcia A.J, Vega M.D, Boettiger D. Modulation of cell proliferation and differentiation through substrate-dependent changes in fibronectin conformation. Mol. Biol. Cell. 1999;10:785–798. doi: 10.1091/mbc.10.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheduzzi D, Guerra D, Bochicchio B, Pepe A, Tamburro A.M, Quaglino D, Mithieux S, Weiss A.S, Pasquali Ronchetti I. Heparan sulphate interacts with tropoelastin, with some tropoelastin peptides and is present in human dermis elastic fibers. Matrix Biol. 2005;24:15–25. doi: 10.1016/j.matbio.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Ghosh J, Murphy M.O, Turner N, Khwaja N, Halka A, Kielty C.M, Walker M.G. The role of transforming growth factor beta1 in the vascular system. Cardiovasc. Pathol. 2005;14:28–36. doi: 10.1016/j.carpath.2004.11.005. doi:10.1016/j.carpath.2004.11.005 [DOI] [PubMed] [Google Scholar]
- Ghosh J, Baguneid M, Murphy M.O, Turner N, Ferguson M.W, Kielty C.M, Walker M.G. Reduction of myointimal hyperplasia following arterial anastomosis by TGF β3. J. Vasc. Surg. 2006;43:142–149. doi: 10.1016/j.jvs.2005.08.041. doi:10.1016/j.jvs.2005.08.041 [DOI] [PubMed] [Google Scholar]
- Gobin A.S, West J.L. Val-ala-pro-gly, an elastin-derived non-integrin ligand: smooth muscle cell adhesion and specificity. J. Biomed. Mater. Res. A. 2003;67:255–259. doi: 10.1002/jbm.a.10110. doi:10.1002/jbm.a.10110 [DOI] [PubMed] [Google Scholar]
- Grinnell F. Fibroblast biology in three-dimensional collagen matrices. Trends Cell Biol. 2003;13:264–269. doi: 10.1016/s0962-8924(03)00057-6. doi:10.1016/S0962-8924(03)00057-6 [DOI] [PubMed] [Google Scholar]
- Haider M, Leung V, Ferrari F, Crissman J, Powell J, Cappello J, Ghandehari H. Molecular engineering of silk-elastinlike polymers for matrix-mediated gene delivery: biosynthesis and characterization. Mol. Pharm. 2005;2:139–150. doi: 10.1021/mp049906s. doi:10.1021/mp049906s [DOI] [PubMed] [Google Scholar]
- Hamilton D.W, Maul T.M, Vorp D.A. Characterization of the response of bone marrow-derived progenitor cells to cyclic strain: implications for vascular tissue-engineering applications. Tissue Eng. 2004;10:361–369. doi: 10.1089/107632704323061726. doi:10.1089/107632704323061726 [DOI] [PubMed] [Google Scholar]
- Haynes C.A, Norde W. Structures and stabilities of adsorbed proteins. J. Colloid Interface Sci. 1995;169:313–328. doi:10.1006/jcis.1995.1039 [Google Scholar]
- Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-β in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047–3055. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- Herrero-Vanrell R, Rincon A.C, Alonso M, Reboto V, Molina-Martinez I.T, Rodriguez-Cabello J.C. Self-assembled particles of an elastin-like polymer as vehicles for controlled drug release. J. Control. Release. 2005;102:113–122. doi: 10.1016/j.jconrel.2004.10.001. doi:10.1016/j.jconrel.2004.10.001 [DOI] [PubMed] [Google Scholar]
- Hinek A, Pshezhetsky A.V, von Itzstein M, Starcher B. Lysosomal sialidase (neuraminidase 1) is targeted to cell surface in the multiprotein complex that facilitates elastic fiber assembly. J. Biol. Chem. 2006;281:3698–3710. doi: 10.1074/jbc.M508736200. doi:10.1074/jbc.M508736200 [DOI] [PubMed] [Google Scholar]
- Huang R, Merrilees M.J, Braun K, Beaumont B, Lemire J, Clowes A.W, Hinek A, Wight T.N. Inhibition of versican synthesis by antisense alters smooth muscle cell phenotype and induces elastic fiber formation in vitro and in neointima after vessel injury. Circ. Res. 2006;98:370–377. doi: 10.1161/01.RES.0000202051.28319.c8. doi:10.1161/01.RES.0000202051.28319.c8 [DOI] [PubMed] [Google Scholar]
- Hubbell J.A. Materials as morphogenetic guides in tissue engineering. Curr. Opin. Biotechnol. 2003;14:551–558. doi: 10.1016/j.copbio.2003.09.004. doi:10.1016/j.copbio.2003.09.004 [DOI] [PubMed] [Google Scholar]
- Hungerford J.E, Little C.D. Developmental biology of the vascular smooth muscle cell: building a multilayered vessel wall. J. Vasc. Res. 1999;36:2–27. doi: 10.1159/000025622. doi:10.1159/000025622 [DOI] [PubMed] [Google Scholar]
- Hyytiäinen M, Penttinen C, Keski-Oja J. Latent TGF-beta binding proteins: extracellular matrix association and roles in TGF-beta activation. Crit. Rev. Clin. Lab. Sci. 2004;41:233–264. doi: 10.1080/10408360490460933. doi:10.1080/10408360490460933 [DOI] [PubMed] [Google Scholar]
- Isogai Z, Aspberg A, Keene D.R, Ono R.N, Reinhardt D.P, Sakai L.Y. Versican interacts with fibrillin-1 and links extracellular microfibrils to other connective tissue networks. J. Biol. Chem. 2002;277:4565–4572. doi: 10.1074/jbc.M110583200. doi:10.1074/jbc.M110583200 [DOI] [PubMed] [Google Scholar]
- Isogai Z, et al. Latent transforming growth factor beta-binding protein 1 interacts with fibrillin and is a microfibril-associated protein. J. Biol. Chem. 2003;278:2750–2757. doi: 10.1074/jbc.M209256200. doi:10.1074/jbc.M209256200 [DOI] [PubMed] [Google Scholar]
- Jain R.K. Molecular regulation of vessel maturation. Nat. Med. 2003;9:685–693. doi: 10.1038/nm0603-685. doi:10.1038/nm0603-685 [DOI] [PubMed] [Google Scholar]
- Jean J.C, Eruchalu I, Cao Y.X, Joyce-Brady M. DANCE in developing and injured lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002;282:L75–L82. doi: 10.1152/ajplung.2002.282.1.L75. [DOI] [PubMed] [Google Scholar]
- Jensen S.A, Reinhardt D.P, Gibson M.A, Weiss A.S. Protein interaction studies of MAGP-1 with tropoelastin and fibrillin-1. J. Biol. Chem. 2001;276:39 661–39 666. doi: 10.1074/jbc.M104533200. doi:10.1074/jbc.M104533200 [DOI] [PubMed] [Google Scholar]
- Junger A, Kaufmann D, Scheibel T, Weberskirch R. Biosynthesis of an elastin-mimetic polypeptide with two different chemical functional groups within the repetitive elastin fragment. Macromol. Biosci. 2005;5:494–501. doi: 10.1002/mabi.200400213. doi:10.1002/mabi.200400213 [DOI] [PubMed] [Google Scholar]
- Kannan R.Y, Salacinski H.J, Butler P.E, Hamilton G, Seifalian A.M. Current status of prosthetic bypass grafts: a review. J. Biomed. Mater. Res. B Appl. Biomater. 2005;74:570–581. doi: 10.1002/jbm.b.30247. [DOI] [PubMed] [Google Scholar]
- Keeley F.W, Bellingham C.M, Woodhouse K.A. Elastin as a self-organizing biomaterial: use of recombinantly expressed human elastin polypeptides as a model for investigations of structure and self-assembly of elastin. Phil. Trans. R. Soc. B. 2002;357:185–189. doi: 10.1098/rstb.2001.1027. doi:10.1098/rstb.2001.1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielty C.M. Elastic fibres in health and disease. Expert Rev. Mol. Med. 2006;8:1–23. doi: 10.1017/S146239940600007X. doi:10.1017/S146239940600007X [DOI] [PubMed] [Google Scholar]
- Kielty C.M, Grant M.E. The collagen family: structure, assembly and organization in the extracellular matrix. In: Royce P.M, Steinmann B, editors. Connective tissue and its heritable diseases: molecular, genetic and medical aspects. Wiley-Liss; New York, NY: 2002. pp. 159–221. [Google Scholar]
- Kielty C.M, Whittaker S.P, Grant M.E, Shuttleworth C.A. Attachment of human vascular smooth muscles cells to intact microfibrillar assemblies of collagen VI and fibrillin. J. Cell Sci. 1992;103:445–451. doi: 10.1242/jcs.103.2.445. [DOI] [PubMed] [Google Scholar]
- Kielty C.M, Whittaker S.P, Shuttleworth C.A. Fibrillin: evidence that chondroitin sulphate proteoglycans are components of microfibrils and associate with newly synthesised monomers. FEBS Lett. 1996;386:169–173. doi: 10.1016/0014-5793(96)00423-1. doi:10.1016/0014-5793(96)00423-1 [DOI] [PubMed] [Google Scholar]
- Kielty C.M, Sherratt M.J, Shuttleworth C.A. Elastic fibres. J. Cell Sci. 2002;115:2817–2828. doi: 10.1242/jcs.115.14.2817. [DOI] [PubMed] [Google Scholar]
- Kielty C.M, Sherratt M.J, Marson A, Baldock C. Fibrillin microfibrils. Adv. Protein Chem. 2005a;70:405–436. doi: 10.1016/S0065-3233(05)70012-7. doi:10.1016/S0065-3233(05)70012-7 [DOI] [PubMed] [Google Scholar]
- Kielty C.M, Bax D.V, Hodson N, Sherratt M.J. Elastic fibre molecules in vascular tissue engineering. In: Vadgama P, editor. Surfaces and interfaces in biomaterials. Woodhead Publishing; Cambridge, UK: 2005b. pp. 637–665. [Google Scholar]
- Kim B.S, Nikolovski J, Bonadio J, Mooney D.J. Cyclic mechanical strain regulates the development of engineered smooth muscle tissue. Nat. Biotechnol. 1999;17:979–983. doi: 10.1038/13671. doi:10.1038/13671 [DOI] [PubMed] [Google Scholar]
- Kowal R.C, Richardson J.A, Miano J.M, Olson E.N. EVEC, a novel epidermal growth factor-like repeat-containing protein upregulated in embryonic and diseased adult vasculature. Circ. Res. 1999;84:1166–1176. doi: 10.1161/01.res.84.10.1166. [DOI] [PubMed] [Google Scholar]
- Kozel B.A, et al. Elastic fiber formation: a dynamic view of extracellular matrix assembly using timer reporters. J. Cell. Physiol. 2006;207:87–96. doi: 10.1002/jcp.20546. doi:10.1002/jcp.20546 [DOI] [PubMed] [Google Scholar]
- Krammer A, Craig D, Thomas W.E, Schulten K, Vogel V. A structural model for force regulated integrin binding to fibronectin's RGD-synergy site. Matrix Biol. 2002;21:139–147. doi: 10.1016/s0945-053x(01)00197-4. doi:10.1016/S0945-053X(01)00197-4 [DOI] [PubMed] [Google Scholar]
- Krijgsman B, Seifalian A.M, Salacinski H.J, Tai N.R, Punshon G, Fuller B.J, Hamilton G. An assessment of covalent grafting of RGD peptides to the surface of a compliant poly(carbonate-urea)urethane vascular conduit versus conventional biological coatings: its role in enhancing cellular retention. Tissue Eng. 2002;8:673–680. doi: 10.1089/107632702760240580. doi:10.1089/107632702760240580 [DOI] [PubMed] [Google Scholar]
- L'Heureux N, Paquet S, Labbe R, Germain L, Auger F.A. A completely biological tissue-engineered human blood vessel. FASEB J. 1998;12:47–56. doi: 10.1096/fasebj.12.1.47. [DOI] [PubMed] [Google Scholar]
- L'Heureux N, Stoclet J.C, Auger F.A, Lagaud G.J, Germain L, Andriantsitohaina R. A human tissue-engineered vascular media: a new model for pharmacological studies of contractile responses. FASEB J. 2001;15:515–524. doi: 10.1096/fj.00-0283com. doi:10.1096/fj.00-0283com [DOI] [PubMed] [Google Scholar]
- Lee A.A, Graham D.A, Dela Cruz S, Ratcliffe A, Karlon W.J. Fluid shear stress-induced alignment of cultured vascular smooth muscle cells. J. Biomech. Eng. 2002;124:37–43. doi: 10.1115/1.1427697. doi:10.1115/1.1427697 [DOI] [PubMed] [Google Scholar]
- Lee K.-B, Lim J.-H, Mirkin C.A. Protein nanostructures formed via direct-write dip-pen lithography. J. Am. Chem. Soc. 2003;125:5588–5589. doi: 10.1021/ja034236p. doi:10.1021/ja034236p [DOI] [PubMed] [Google Scholar]
- Lee S.S, Knott V, Jovanovic J, Harlos K, Grimes J.M, Choulier L, Mardon H.J, Stuart D.I, Handford P.A. Structure of the integrin binding fragment from fibrillin-1 gives new insights into microfibril organization. Structure. 2004;12:717–729. doi: 10.1016/j.str.2004.02.023. doi:10.1016/j.str.2004.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Mondrinos M.J, Gandhi M.R, Ko F.K, Weiss A.S, Lelkes P.I. Electrospun protein fibers as matrices for tissue engineering. Biomaterials. 2005;26:5999–6008. doi: 10.1016/j.biomaterials.2005.03.030. doi:10.1016/j.biomaterials.2005.03.030 [DOI] [PubMed] [Google Scholar]
- Liu S.Q, Tang D, Tieche C, Alkema P.K. Pattern formation of vascular smooth muscle cells subject to nonuniform fluid shear stress: mediation by gradient of cell density. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H1072–H1080. doi: 10.1152/ajpheart.01009.2002. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhao Y, Gao J, Pawlyk B, Starcher B, Spencer J.A, Yanagisawa H, Zuo J, Li T. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat. Genet. 2004;36:178–182. doi: 10.1038/ng1297. doi:10.1038/ng1297 [DOI] [PubMed] [Google Scholar]
- Loeys B.L, et al. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat. Genet. 2005;37:275–281. doi: 10.1038/ng1511. doi:10.1038/ng1511 [DOI] [PubMed] [Google Scholar]
- Lomas, A. C., Mellody, K. T., Freeman, L. J., Bax, D. V., Shuttleworth, C. A. & Kielty, C. M. In press. Fibulin-5 binds human smooth muscle cells through α5β1 and α4β1 integrins, but does not support receptor activation. Biochem. J. [DOI] [PMC free article] [PubMed]
- Long J.L, Tranquillo R.T. Elastic fiber production in cardiovascular tissue-equivalents. Matrix Biol. 2003;22:339–350. doi: 10.1016/s0945-053x(03)00052-0. doi:10.1016/S0945-053X(03)00052-0 [DOI] [PubMed] [Google Scholar]
- Maki J.M, Sormunen R, Lippo S, Kaarteenaho-Wiik R, Soininen R, Myllyharju J. Lysyl oxidase is essential for normal development and function of the respiratory system and for the integrity of elastic and collagen fibers in various tissues. Am. J. Pathol. 2005;167:927–936. doi: 10.1016/S0002-9440(10)61183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann B.K, West J.L. Cell adhesion peptides alter smooth muscle cell adhesion, proliferation, migration, and matrix protein synthesis on modified surfaces and in polymer scaffolds. J. Biomed. Mater. Res. 1999;60:86–93. doi: 10.1002/jbm.10042. doi:10.1002/jbm.10042 [DOI] [PubMed] [Google Scholar]
- Marson A, Rock M.J, Cain S.A, Freeman L.J, Morgan A, Mellody K, Shuttleworth C.A, Baldock C, Kielty C.M. Homotypic fibrillin-1 interactions in microfibril assembly. J. Biol. Chem. 2005;280:5013–5022. doi: 10.1074/jbc.M409029200. doi:10.1074/jbc.M409029200 [DOI] [PubMed] [Google Scholar]
- Martin N.D, Schaner P.J, Tulenko T.N, Shapiro I.M, Dimatteo C.A, Williams T.K, Hager E.S, DiMuzio P.J. In vivo behavior of decellularized vein allograft. J. Surg. Res. 2005;129:17–23. doi: 10.1016/j.jss.2005.06.037. doi:10.1016/j.jss.2005.06.037 [DOI] [PubMed] [Google Scholar]
- Massague J, Chen Y.G. Controlling TGF-beta signaling. Genes Dev. 2000;14:627–644. [PubMed] [Google Scholar]
- McConnell C.J, DeMont M.E, Wright G.M. Microfibrils provide non-linear elastic behaviour in the abdominal artery of the lobster Homarus americanus. J. Physiol. 1997;499:513–526. doi: 10.1113/jphysiol.1997.sp021945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcintire L.V, Wagner J.E, Papadaki M, Whitson P.A, Eskin S.G. Effect of flow on gene regulation in smooth muscle cells and macromolecular transport across endothelial cell monolayers. Biol. Bull. 1998;194:394–399. doi: 10.2307/1543123. doi:10.2307/1543123 [DOI] [PubMed] [Google Scholar]
- Mecham R.P, Davis E.C. Elastic fiber structure and assembly. In: Yurchenco P.D, Birk D.E, Mecham R.P, editors. Extracellular matrix assembly and structure. Academic Press; New York, NY: 1994. pp. 281–314. [Google Scholar]
- Megill W.M, Gosline J.M, Blake R.W. The modulus of elasticity of fibrillin-containing elastic fibres in the mesoglea of the hydromedusa Polyorchis penicillatus. J. Exp. Biol. 2005;208:3819–3834. doi: 10.1242/jeb.01765. doi:10.1242/jeb.01765 [DOI] [PubMed] [Google Scholar]
- Merrilees M.J, Lemire J.M, Fischer J.W, Kinsella M.G, Braun K.R, Clowes A.W, Wight T.N. Retrovirally mediated overexpression of versican v3 by arterial smooth muscle cells induces tropoelastin synthesis and elastic fiber formation in vitro and in neointima after vascular injury. Circ. Res. 2002;90:481–487. doi: 10.1161/hh0402.105791. doi:10.1161/hh0402.105791 [DOI] [PubMed] [Google Scholar]
- Miao M, Bellingham C.M, Stahl R, Sitarz E, Lane C, Keeley F.W. Sequence and structure determinants for the self-aggregation of recombinant polypeptides modelled after human elastin. J. Biol. Chem. 2003;278:48 553–48 562. doi: 10.1074/jbc.M308465200. doi:10.1074/jbc.M308465200 [DOI] [PubMed] [Google Scholar]
- Miao M, Cirulis J.T, Lee S, Keeley F.W. Structural determinants of cross-linking and hydrophobic domains for self-assembly of elastin-like polypeptides. Biochemistry. 2005;44:14 367–14 375. doi: 10.1021/bi0510173. doi:10.1021/bi0510173 [DOI] [PubMed] [Google Scholar]
- Michael K.E, Verekar V.N, Keselowsky B.G, Meredith J.C, Latour R.A, Garcia A.J. Adsorption-induced conformational changes in fibronectin due to interactions with well-defined surface chemistries. Langmuir. 2003;19:8033–8040. doi:10.1021/la034810a [Google Scholar]
- Mitchell S.L, Niklason L.E. Requirements for growing tissue-engineered vascular grafts. Cardiovasc. Pathol. 2003;12:59–64. doi: 10.1016/s1054-8807(02)00183-7. doi:10.1016/S1054-8807(02)00183-7 [DOI] [PubMed] [Google Scholar]
- Mithieux S.M, Weiss A.S. Elastin. Adv. Protein Chem. 2005;70:437–461. doi: 10.1016/S0065-3233(05)70013-9. doi:10.1016/S0065-3233(05)70013-9 [DOI] [PubMed] [Google Scholar]
- Mithieux S.M, Rasko J.E, Weiss A.S. Synthetic elastin hydrogels derived from massive elastic assemblies of self-organized human protein monomers. Biomaterials. 2004;25:4921–4927. doi: 10.1016/j.biomaterials.2004.01.055. doi:10.1016/j.biomaterials.2004.01.055 [DOI] [PubMed] [Google Scholar]
- Mithieux S.M, Wise S.G, Raftery M.J, Starcher B, Weiss A.S. A model two-component system for studying the architecture of elastin assembly in vitro. J. Struct. Biol. 2005;149:282–289. doi: 10.1016/j.jsb.2004.11.005. doi:10.1016/j.jsb.2004.11.005 [DOI] [PubMed] [Google Scholar]
- Mochizuki S, Brassart B, Hinek A. Signaling pathways transduced through the elastin receptor facilitate proliferation of arterial smooth muscle cells. J. Biol. Chem. 2002;277:44 854–44 863. doi: 10.1074/jbc.M205630200. doi:10.1074/jbc.M205630200 [DOI] [PubMed] [Google Scholar]
- Morbidelli L, Donnini S, Chillemi F, Giachetti A, Ziche M. Angiosuppressive and angiostimulatory effects exerted by synthetic partial sequences of endostatin. Clin. Cancer Res. 2003;9:5358–5369. [PubMed] [Google Scholar]
- Mould A.P, Humphries M.J. Regulation of integrin function through conformational complexity: not simply a knee-jerk reaction? Curr. Opin. Cell Biol. 2004;16:544–551. doi: 10.1016/j.ceb.2004.07.003. doi:10.1016/j.ceb.2004.07.003 [DOI] [PubMed] [Google Scholar]
- Muller D.J, Amrein M, Engel A. Adsorption of biological molecules to a solid support for scanning probe microscopy. J. Struct. Biol. 1997;119:172–188. doi: 10.1006/jsbi.1997.3875. [DOI] [PubMed] [Google Scholar]
- Nakamura T, et al. Fibulin-5/DANCE is essential for elastogenesis in vivo. Nature. 2002;415:171–175. doi: 10.1038/415171a. doi:10.1038/415171a [DOI] [PubMed] [Google Scholar]
- Neptune E.R, Frischmeyer P.A, Arking D.E, Myers L, Bunton T.E, Gayraud B, Ramirez F, Sakai L.Y, Dietz H.C. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat. Genet. 2003;33:407–411. doi: 10.1038/ng1116. doi:10.1038/ng1116 [DOI] [PubMed] [Google Scholar]
- Ng C.M, et al. TGF-beta-dependent pathogenesis of mitral valve prolapse in a mouse model of Marfan syndrome. J. Clin. Invest. 2004;114:1586–1592. doi: 10.1172/JCI22715. doi:10.1172/JCI200422715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen A, Itoh S, Jeney V, Yanagisawa H, Fujimoto M, Ushio-Fukai M, Fukai T. Fibulin-5 is a novel binding protein for extracellular superoxide dismutase. Circ. Res. 2004;95:1067–1074. doi: 10.1161/01.RES.0000149568.85071.FB. doi:10.1161/01.RES.0000149568.85071.FB [DOI] [PubMed] [Google Scholar]
- Niklason L.E, Gao J, Abbott W.M, Hirschi K.K, Houser S, Marini R, Langer R. Functional arteries grown in vitro. Science. 1999;284:489–493. doi: 10.1126/science.284.5413.489. doi:10.1126/science.284.5413.489 [DOI] [PubMed] [Google Scholar]
- Owens G.K. Regulation of differentiation of vascular smooth muscle cells. Physiol. Rev. 1995;75:487–517. doi: 10.1152/physrev.1995.75.3.487. [DOI] [PubMed] [Google Scholar]
- Pepe A, Guerra D, Bochicchio B, Quaglino D, Gheduzzi D, Pasquali Ronchetti I, Tamburro A.M. Dissection of human tropoelastin: supramolecular organization of polypeptide sequences coded by particular exons. Matrix Biol. 2005;24:96–109. doi: 10.1016/j.matbio.2005.01.004. doi:10.1016/j.matbio.2005.01.004 [DOI] [PubMed] [Google Scholar]
- Pereira L, D'Alessio M, Ramirez F, Lynch J.R, Sykes B, Pangilinan T, Bonadio J. Genomic organization of the sequence coding for fibrillin, the defective gene product in Marfan syndrome. Hum. Mol. Genet. 1993;2:961–968. doi: 10.1093/hmg/2.7.961. doi:10.1093/hmg/2.7.961 [DOI] [PubMed] [Google Scholar]
- Pfaff M, Reinhardt D.P, Sakai L.Y, Timpl R. Cell adhesion and integrin binding to recombinant human fibrillin-1. FEBS Lett. 1996;384:247–250. doi: 10.1016/0014-5793(96)00325-0. doi:10.1016/0014-5793(96)00325-0 [DOI] [PubMed] [Google Scholar]
- Piner R.D, Zhu J, Xu F, Hong S, Mirkin C.A. “Dip-pen” nanolithography. Science. 1999;283:661–663. doi: 10.1126/science.283.5402.661. doi:10.1126/science.283.5402.661 [DOI] [PubMed] [Google Scholar]
- Privitera S, Prody C.A, Callahan J.W, Hinek A. The 67-kDa enzymatically inactive alternatively spliced variant of beta-galactosidase is identical to the elastin/laminin-binding protein. J. Biol. Chem. 1998;273:6319–6326. doi: 10.1074/jbc.273.11.6319. doi:10.1074/jbc.273.11.6319 [DOI] [PubMed] [Google Scholar]
- Quaglino D, Pasquali-Ronchetti I. Morphology and chemical composition of connective tissue: the cardiovascular system. In: Royce P.M, Steinmann B, editors. Connective tissue and its heritable disorders. 2nd edn. Wiley; New York, NY: 2002. 121–144. [Google Scholar]
- Quondamatteo F, Reinhardt D.P, Charbonneau N.L, Pophal G, Sakai L.Y, Herken R. Fibrillin-1 and fibrillin-2 in human embryonic and early fetal development. Matrix Biol. 2002;21:637–646. doi: 10.1016/s0945-053x(02)00100-2. doi:10.1016/S0945-053X(02)00100-2 [DOI] [PubMed] [Google Scholar]
- Rashid S.T, Salacinski H.J, Fuller B.J, Hamilton G, Seifalian A.M. Engineering of bypass conduits to improve patency. Cell Prolif. 2004;37:351–366. doi: 10.1111/j.1365-2184.2004.00318.x. doi:10.1111/j.1365-2184.2004.00318.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinboth B, Hanssen E, Cleary E.G, Gibson M.A. Molecular interactions of biglycan and decorin with elastic fiber components: biglycan forms a ternary complex with tropoelastin and microfibril-associated glycoprotein 1. J. Biol. Chem. 2002;277:3950–3957. doi: 10.1074/jbc.M109540200. doi:10.1074/jbc.M109540200 [DOI] [PubMed] [Google Scholar]
- Reinhardt D.P, Keene D.R, Corson G.M, Poschl E, Bachinger H.P, Gambee J.E, Sakai L.Y. Fibrillin-1: organization in microfibrils and structural properties. J. Mol. Biol. 1996;258:104–116. doi: 10.1006/jmbi.1996.0237. doi:10.1006/jmbi.1996.0237 [DOI] [PubMed] [Google Scholar]
- Ricard-Blum S, et al. Characterization of endostatin binding to heparin and heparan sulfate by surface plasmon resonance and molecular modeling. Role of divalent cations. J. Biol. Chem. 2003;279:2927–2936. doi: 10.1074/jbc.M309868200. doi:10.1074/jbc.M309868200 [DOI] [PubMed] [Google Scholar]
- Rifkin D.B. Latent TGF-beta binding proteins: orchestrators of TGF-beta availability. J. Biol. Chem. 2005;280:7409–7412. doi: 10.1074/jbc.R400029200. doi:10.1074/jbc.R400029200 [DOI] [PubMed] [Google Scholar]
- Robinson P.N, Booms P, Katzke S, Ladewig M, Neumann L, Palz M, Pregla R, Tiecke F, Rosenberg T. Mutations of FBN1 and genotype–phenotype correlations in Marfan syndrome and related fibrillinopathies. Hum. Mutat. 2002;20:153–161. doi: 10.1002/humu.10113. doi:10.1002/humu.10113 [DOI] [PubMed] [Google Scholar]
- Rock M.J, Cain S.A, Freeman L.J, Morgan A, Mellody K, Marson A, Shuttleworth C.A, Weiss A.S, Kielty C.M. Molecular basis of elastic fiber formation. Critical interactions and a tropoelastin–fibrillin-1 cross-link. J. Biol. Chem. 2004;279:23 748–23 758. doi: 10.1074/jbc.M400212200. doi:10.1074/jbc.M400212200 [DOI] [PubMed] [Google Scholar]
- Rodgers U.R, Weiss A.S. Integrin alpha v beta 3 binds a unique non-RGD site near the C-terminus of human tropoelastin. Biochimie. 2004;86:173–178. doi: 10.1016/j.biochi.2004.03.002. doi:10.1016/j.biochi.2004.03.002 [DOI] [PubMed] [Google Scholar]
- Saharinen J, Keski-Oja J. Specific sequence motif of 8-Cys repeats of TGF-beta binding proteins, LTBPs, creates a hydrophobic interaction surface for binding of small latent TGF-beta. Mol. Biol. Cell. 2000;11:2691–2704. doi: 10.1091/mbc.11.8.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto H, Broekelmann T, Cheresh D.A, Ramirez F, Rosenbloom J, Mecham R.P. Cell-type specific recognition of RGD- and non-RGD-containing cell binding domains in fibrillin-1. J. Biol. Chem. 1996;271:4916–4922. doi:10.1074/jbc.271.9.4916 [PubMed] [Google Scholar]
- Salacinski H.J, Hamilton G, Seifalian A.M. Surface functionalisation and grafting of heparin and/or RGD in an aqueous-based process to a poly(carbonate-urea)urethane cardiovascular graft for cellular engineering applications. J. Biomed. Mater. Res. A. 2003;66:688–697. doi: 10.1002/jbm.a.10020. doi:10.1002/jbm.a.10020 [DOI] [PubMed] [Google Scholar]
- Schaner P.J, Martin N.D, Tulenko T.N, Shapiro I.M, Tarola N.A, Leichter R.F, Carabasi R.A, Dimuzio P.J. Decellularized vein as a potential scaffold for vascular tissue engineering. J. Vasc. Surg. 2004;40:146–153. doi: 10.1016/j.jvs.2004.03.033. doi:10.1016/j.jvs.2004.03.033 [DOI] [PubMed] [Google Scholar]
- Sgarbi N, Pisignano D, Di Benedetto F, Gigli G, Cingolani R, Rinaldi R. Self-assembled extracellular matrix protein networks by microcontact printing. Biomaterials. 2004;25:1349–1353. doi: 10.1016/j.biomaterials.2003.08.017. doi:10.1016/j.biomaterials.2003.08.017 [DOI] [PubMed] [Google Scholar]
- Sherratt M.J, Holmes D.F, Haston J.L, Wess T.J, Shuttleworth C.A, Kielty C.M. Fibrillin microfibrils are stiff reinforcing fibres in compliant tissues. J. Mol. Biol. 2003;332:183–193. doi: 10.1016/s0022-2836(03)00829-5. doi:10.1016/S0022-2836(03)00829-5 [DOI] [PubMed] [Google Scholar]
- Sherratt M.J, Holmes D.F, Shuttleworth C.A, Kielty C.M. Substrate-dependent morphology of supramolecular assemblies: fibrillin and type-VI collagen microfibrils. Biophys. J. 2004;86:3211–3222. doi: 10.1016/S0006-3495(04)74369-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherratt M.J, Bax D.V, Chaudhry S.S, Hodson N, Lu J.R, Saravanapavan P, Kielty C.M. Substrate chemistry influences the morphology and biological function of adsorbed extracellular matrix assemblies. Biomaterials. 2005;26:7192–7206. doi: 10.1016/j.biomaterials.2005.05.010. doi:10.1016/j.biomaterials.2005.05.010 [DOI] [PubMed] [Google Scholar]
- Shi Y, O'Brien J.E, Fard A, Mannion J.D, Wang D, Zalewski A. Adventitial myofibroblasts contribute to neointimal formation in injured porcine coronary arteries. Circulation. 1996;94:1655–1664. doi: 10.1161/01.cir.94.7.1655. [DOI] [PubMed] [Google Scholar]
- Simionescu D.T, Lu Q, Song Y, Lee J.S, Rosenbalm T.N, Kelley C, Vyavahare N.R. Biocompatibility and remodeling potential of pure arterial elastin and collagen scaffolds. Biomaterials. 2006;27:702–713. doi: 10.1016/j.biomaterials.2005.06.013. doi:10.1016/j.biomaterials.2005.06.013 [DOI] [PubMed] [Google Scholar]
- Sinha S, Kielty C.M, Heagerty A.M, Canfield A.E, Shuttleworth C.A. Upregulation of collagen VIII following porcine coronary artery angioplasty is regulated to smooth muscle cell migration not angiogenesis. Int. J. Exp. Path. 2001;82:295–302. doi: 10.1046/j.1365-2613.2001.00201.x. doi:10.1046/j.1365-2613.2001.00201.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer J.A, et al. Altered vascular remodeling in fibulin-5-deficient mice reveals a role of fibulin-5 in smooth muscle cell proliferation and migration. Proc. Natl Acad. Sci. USA. 2005;102:2946–2951. doi: 10.1073/pnas.0500058102. doi:10.1073/pnas.0500058102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitzel J, et al. Controlled fabrication of a biological vascular substitute. Biomaterials. 2006;27:1088–1094. doi: 10.1016/j.biomaterials.2005.07.048. doi:10.1016/j.biomaterials.2005.07.048 [DOI] [PubMed] [Google Scholar]
- Swartz D.D, Russell J.A, Andreadis S.T. Engineering of fibrin-based functional and implantable small-diameter blood vessels. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H1451–H1460. doi: 10.1152/ajpheart.00479.2004. doi:10.1152/ajpheart.00479.2004 [DOI] [PubMed] [Google Scholar]
- Tamburro A.M, Bochicchio B, Pepe A. The dissection of human tropoelastin: from the molecular structure to the self-assembly to the elasticity mechanism. Pathol. Biol. (Paris) 2005a;53:383–389. doi: 10.1016/j.patbio.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Tamburro A.M, Pepe A, Bochicchio B, Quaglino D, Ronchetti I.P. Supramolecular amyloid-like assembly of the polypeptide sequence coded by exon 30 of human tropoelastin. J. Biol. Chem. 2005b;280:2682–2690. doi: 10.1074/jbc.M411617200. doi:10.1074/jbc.M411617200 [DOI] [PubMed] [Google Scholar]
- Thomas A.C, Campbell G.R, Campbell J.H. Advances in vascular tissue engineering. Cardiovasc. Pathol. 2003;12:271–276. doi: 10.1016/s1054-8807(03)00086-3. doi:10.1016/S1054-8807(03)00086-3 [DOI] [PubMed] [Google Scholar]
- Thomassin L, Werneck C.C, Broekelmann T.J, Gleyzal C, Hornstra I.K, Mecham R.P, Sommer P. The pro-regions of lysyl oxidase and lysyl oxidase-like 1 are required for deposition onto elastic fibers. J. Biol. Chem. 2005;280:42 848–42 855. doi: 10.1074/jbc.M506832200. doi:10.1074/jbc.M506832200 [DOI] [PubMed] [Google Scholar]
- Thurmond F.A, Koob T.J, Bowness J.M, Trotter J.A. Partial biochemical and immunologic characterization of fibrillin microfibrils from sea cucumber dermis. Connect. Tissue Res. 1997;36:211–222. doi: 10.3109/03008209709160221. [DOI] [PubMed] [Google Scholar]
- Tiedemann K, Bätge B, Müller P.K, Reinhardt D.P. Interactions of fibrillin-1 with heparin/heparan sulphate, implications for microfibrillar assembly. J. Biol. Chem. 2001;276:36 035–36 042. doi: 10.1074/jbc.M104985200. doi:10.1074/jbc.M104985200 [DOI] [PubMed] [Google Scholar]
- Tiedemann K, et al. Microfibrils at basement membrane zones interact with perlecan via fibrillin-1. J. Biol. Chem. 2005;280:11 404–11 412. doi: 10.1074/jbc.M409882200. doi:10.1074/jbc.M409882200 [DOI] [PubMed] [Google Scholar]
- Tiwari A, Kidane A, Salacinski H.J, Punshon G, Hamilton G, Siefalian A.M. Improving endothelial cell retention for single stage seeding of prosthetic grafts: use of polymer sequences of arginine-glycine-aspartate. Eur. J. Vasc. Endovasc. Surg. 2003;25:325–329. doi: 10.1053/ejvs.2002.1854. doi:10.1053/ejvs.2002.1854 [DOI] [PubMed] [Google Scholar]
- Trask B.C, Trask T.M, Broekelmann T, Mecham R.P. The microfibrillar proteins MAGP-1 and fibrillin-1 form a ternary complex with the chondroitin sulfate proteoglycan decorin. Mol. Biol. Cell. 2000;11:1499–1507. doi: 10.1091/mbc.11.5.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueba H, Kawakami M, Yaginuma T. Shear stress as an inhibitor of vascular smooth muscle cell proliferation. Role of transforming growth factor-beta 1 and tissue-type plasminogen activator. Arterioscler. Thromb. Vasc. Biol. 1997;17:1512–1516. doi: 10.1161/01.atv.17.8.1512. [DOI] [PubMed] [Google Scholar]
- Uemura Y, Okamoto K. Elastin-derived peptide induces monocyte chemotaxis by increasing intracellular cyclic GMP level and activating cyclic GMP dependent protein kinase. Biochem. Mol. Biol. Int. 1997;41:57–64. doi: 10.1080/15216549700201061. [DOI] [PubMed] [Google Scholar]
- Vandulm P, Norde W. The adsorption of human plasma albumin on solid surfaces, with special attention to the kinetic aspects. J. Colloid Interface Sci. 1983;91:248–255. doi:10.1016/0021-9797(83)90329-6 [Google Scholar]
- Vara D.S, Salacinski H.J, Kannan R.Y, Bordenave L, Hamilton G, Seifalian A.M. Cardiovascular tissue engineering: state of the art. Pathol. Biol. (Paris) 2005;53:599–612. doi: 10.1016/j.patbio.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Vorp D.A, Maul T, Nieponice A. Molecular aspects of vascular tissue engineering. Front. Biosci. 2005;10:768–789. doi: 10.2741/1571. doi:10.2741/1571 [DOI] [PubMed] [Google Scholar]
- Walles T, Herden T, Haverich A, Mertsching H. Influence of scaffold thickness and scaffold composition on bioartificial graft survival. Biomaterials. 2003;24:1233–1299. doi: 10.1016/s0142-9612(02)00490-8. doi:10.1016/S0142-9612(02)00490-8 [DOI] [PubMed] [Google Scholar]
- Wang D.M, Tarbell J.M. Nonlinear analysis of oscillatory flow, with a nonzero mean, in an elastic tube (artery) J. Biomech. Eng. 1995;117:127–135. doi: 10.1115/1.2792260. [DOI] [PubMed] [Google Scholar]
- Weyman A.E, Scherrer-Crosbie M. Marfan syndrome and mitral valve prolapse. J. Clin. Invest. 2004;114:1543–1546. doi: 10.1172/JCI23701. doi:10.1172/JCI200423701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiberg C, Heinegard D, Wenglen C, Timpl R, Morgelin M. Biglycan organizes collagen VI into hexagonal-like networks resembling tissue structures. J. Biol. Chem. 2002;277:49 120–49 126. doi: 10.1074/jbc.M206891200. doi:10.1074/jbc.M206891200 [DOI] [PubMed] [Google Scholar]
- Williamson M.R, Black R, Kielty C.M. PCL–PU composite vascular scaffold production for vascular tissue engineering: attachment, proliferation and bioactivity of human vascular endothelial cells. Biomaterials. 2006;27:3608–3616. doi: 10.1016/j.biomaterials.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Williamson, M. R., Shuttleworth, C. A., Canfield, A. E., Black, R. & Kielty, C. M. Submitted. Fibulin-5, fibrillin-1 and elastin differentially regulate endothelial and smooth muscle cells.
- Wise S.G, Mithieux S.M, Raftery M.J, Weiss A.S. Specificity in the coacervation of tropoelastin: solvent exposed lysines. J. Struct. Biol. 2005;149:273–281. doi: 10.1016/j.jsb.2004.11.006. doi:10.1016/j.jsb.2004.11.006 [DOI] [PubMed] [Google Scholar]
- WojciakStothard B, Curtis A, Monaghan W, Macdonald K, Wilkinson C. Guidance and activation of murine macrophages by nanometric scale topography. Exp. Cell Res. 1996;223:426–435. doi: 10.1006/excr.1996.0098. doi:10.1006/excr.1996.0098 [DOI] [PubMed] [Google Scholar]
- Woodhouse K.A, Klement P, Chen V, Gorbet M.B, Keeley F.W, Stahl R, Fromstein J.D, Bellingham C.M. Investigation of recombinant human elastin polypeptides as non-thrombogenic coatings. Biomaterials. 2004;25:4543–4553. doi: 10.1016/j.biomaterials.2003.11.043. doi:10.1016/j.biomaterials.2003.11.043 [DOI] [PubMed] [Google Scholar]
- Yanagisawa H, Davis E.C, Starcher B.C, Ouchi T, Yanagisawa M, Richardson J.A, Olson E.N. Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature. 2002;415:168–171. doi: 10.1038/415168a. doi:10.1038/415168a [DOI] [PubMed] [Google Scholar]
- Yang G, Woodhouse K.A, Yip C.M. Substrate-facilitated assembly of elastin-like peptides: studies by variable-temperature in situ atomic force microscopy. J. Am. Chem. Soc. 2002;124:10 648–10 649. doi: 10.1021/ja027302g. doi:10.1021/ja027302g [DOI] [PubMed] [Google Scholar]
- Zanetti M, Braghetta P, Sabatelli P, Mura I, Doliana R, Colombatti A, Volpin D, Bonaldo P, Bressan G.M. EMILIN-1 deficiency induces elastogenesis and vascular cell defects. Mol. Cell Biol. 2004;24:638–650. doi: 10.1128/MCB.24.2.638-650.2004. doi:10.1128/MCB.24.2.638-650.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Apfelroth S.D, Hu W, Davis E.C, Sanguineti C, Bonadio J, Mecham R.P, Ramirez F. Structure and expression of fibrillin-2, a novel microfibrillar component preferentially located in elastic matrices. J. Cell Biol. 1994;124:855–863. doi: 10.1083/jcb.124.5.855. doi:10.1083/jcb.124.5.855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Bullen D, Chung S.-W, Hong S, Ryu K.S, Fan Z, Mirkin C.A, Liu C. A MEMS nanoplotter with high-density parallel dip-pen nanolithography probe arrays. Nanotechnology. 2002;13:212–217. doi:10.1088/0957-4484/13/2/315 [Google Scholar]
- Zhang Z, Wang Z, Liu S, Kodama M. Pore size, tissue ingrowth, endothelialization of small-diameter microporous polyurethane vascular prostheses. Biomaterials. 2004;25:177–187. doi: 10.1016/s0142-9612(03)00478-2. doi:10.1016/S0142-9612(03)00478-2 [DOI] [PubMed] [Google Scholar]
- Zisch A.H. Tissue engineering of angiogenesis with autologous endothelial progenitor cells. Curr. Opin. Biotechnol. 2004;15:424–429. doi: 10.1016/j.copbio.2004.08.005. doi:10.1016/j.copbio.2004.08.005 [DOI] [PubMed] [Google Scholar]