Abstract
Transposon systems are widely used for generating mutations in various model organisms. PiggyBac (PB) has recently been shown to transpose efficiently in the mouse germ line and other mammalian cell lines. To facilitate PB's application in mammalian genetics, we characterized the properties of the PB transposon in mouse embryonic stem (ES) cells. We first measured the transposition efficiencies of PB transposon in mouse embryonic stem cells. We next constructed a PB/SB hybrid transposon to compare PB and Sleeping Beauty (SB) transposon systems and demonstrated that PB transposition was inhibited by DNA methylation. The excision and reintegration rates of a single PB from two independent genomic loci were measured and its ability to mutate genes with gene trap cassettes was tested. We examined PB's integration site distribution in the mouse genome and found that PB transposition exhibited local hopping. The comprehensive information from this study should facilitate further exploration of the potential of PB and SB DNA transposons in mammalian genetics.
Keywords: ES cell, Sleeping Beauty, transposon
DNA transposons are genetic elements that can relocate between genomic sites by a “cut and paste” mechanism. Since the discovery of the first DNA transposon in maize by Barbara McClintock (1), these elements have been extensively used for genetics and functional genomics in different organisms (2–7).
Sleeping Beauty was the first DNA transposon system shown to be functional in mammalian cells. It has been tested for insertional mutagenesis in the mouse and rat germ lines (8–13), but its relatively low transposition efficiency and strong “local hopping” tendency limit its application in genome-wide screens. However, SB has successfully been used to screen for new cancer genes (14, 15).
PiggyBac was isolated from Trichoplusia ni (16) and subsequently found to transpose efficiently in many different species (5, 17–19). One important feature of the PB transposon is that it nearly always excises itself precisely and leaves no footprint behind (5, 20, 21). It was demonstrated that PB was very efficient for germ-line mutagenesis in the mouse (22, 23). It was subsequently confirmed that PB has significantly higher transposition activity in mammalian cell lines than SB and Tol2 (24). These studies suggest that PB has wide applications in dissecting gene functions (25).
To facilitate PB's application in mammals, we have characterized further the properties of the PB transposon. We measured the transposition efficiencies of PB in mouse ES cells. We also constructed a PB/SB hybrid transposon to compare the PB and SB transposon systems in ES cells. Our data demonstrated that PB transposition was inhibited by DNA methylation. We then measured the excision and reintegration rates of a single PB transposon from two independent genomic loci and tested its ability to mutate a genomic locus with gene trap cassettes. Finally, we investigated PB's integration site distribution in the mouse genome and found that at least 9% of transposition events from the Rosa26 locus were local. Our study provides fundamental information on the characteristics of PB transposition, enabling the potential of the DNA transposons to be explored for mammalian genetics.
Results
Efficient PB Transposition in Mouse ES Cells.
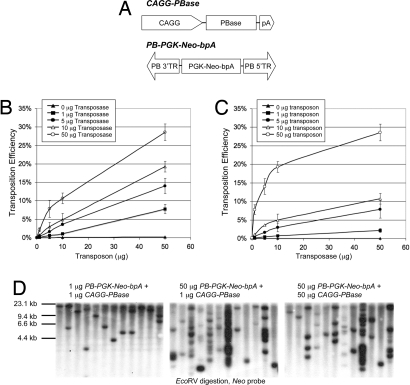
To explore the possibility of using PB for mutagenesis in mouse ES cells, we constructed a PB transposon that contained a PGK-Neo-bpA cassette, which enabled us to score random transposition events by G418 resistance because of the relative position-independent activities of the PGK promoter (Fig. 1A). The helper plasmid (CAGG-PBase) was designed to ubiquitously express PB transposase (Fig. 1A). Transposition was achieved by coelectroporation of the two plasmids into ES cells. The transposition efficiencies were calculated as the percentage of G418 resistant (G418R) cells over the total cells surviving electroporation.
Fig. 1.
PB transposition in the mouse ES cells. (A) Schematic of the PB transposase (helper) and transposon (donor) constructs. (B) PB transposition with increasing amounts of PB transposon. A fixed amount of CAGG-PBase was coelectroporated with increasing amounts of PB-PGK-Neo-bpA donor plasmid. Each number is the average obtained from three independent experiments. Error bars indicate the standard deviation from the mean. (C) PB transposition with increasing amounts of PB transposase. A fixed amount of PB-PGK-Neo-bpA transposon was coelectroporated with increasing amounts of CAGG-PBase helper plasmid. (D) Southern analysis of PB transposition events. Twelve G418R colonies were picked from each experiment. There is a unique EcoRV site in the PB-PGK-Neo-bpA transposon. Genomic DNA was digested with EcoRV and hybridized with a Neo probe. Variation of the EcoRV fragment size illustrates independent PB transposition events.
To determine the optimal condition for transposition, different amounts of the supercoiled PB-PGK-Neo-bpA donor and CAGG-PBase helper plasmids were used (Fig. 1 B and C). Using high concentration of both plasmids (50 μg of each), transposition efficiencies approached 28% of cells surviving electroporation. This result indicated that, at high DNA concentrations, essentially all of the cells receiving both the PB donor and helper plasmids would have transposition events, based on the assumption that approximately half of cells would receive one plasmid under the electroporation condition used in this study.
We noticed that the transposition efficiency differed with respect to increasing amounts of donor and helper plasmids [Fig. 1 B and C and supporting information (SI) Fig. S1]. For example, the transposition efficiencies improved 14-fold when the amount of donor DNA being increased from 1 to 50 μg. However, transposition efficiencies were only four times higher when the helper DNA was increased from 1 to 50 μg. Thus, the availability of sufficient transposon DNA was more important to increasing overall transposition rates than levels of PB transposase. Nevertheless, at least at the levels tested, there did not appear to be an inhibition of PB transposition caused by overdoses of the transposase in the mouse ES cells. This result is in agreement with two recent studies (26, 27) in human and mouse cells, but it contradicts the study in ref. 24.
Using higher concentrations of donor or helper plasmids in the coelectroporation experiments improved the overall transposition efficiencies and increased the transposition events per cell. As shown in Fig. 1D, in the experiment with 1 μg of PB-PGK-Neo-bpA and 1 μg of CAGG-PBase, one to two transposition events were observed in the G418R colonies. The average copy numbers of PB increased to five if 50 μg of each plasmid was used. In some clones, >15 integration sites were visible.
PB-SB Hybrid Transposon Can Be Mobilized by both Transposases.
To compare the SB and PB transposon systems, we constructed a hybrid transposon (PB-SB-PGK-Neo-bpA) by inserting a SB-PGK-Neo-bpA transposon cassette into an intact PB transposon (Fig. 2A). When coelectroporated with CAGG-PBase into ES cells, this new transposon functioned nearly as efficiently as the original PB-PGK-Neo-bpA transposon (data not shown).
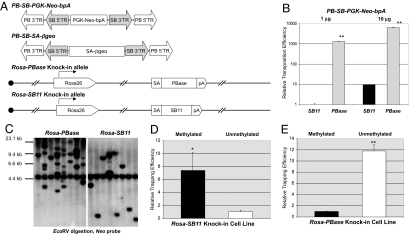
Fig. 2.
Direct comparison of PB and SB transposases, using the PB/SB hybrid transposons. (A) Schematic of PB/SB hybrid transposons, PBase, and SB11 transposase knockin cells lines. (Upper) PB-SB-PGK-Neo-bpA and PB-SB-SA-βgeo hybrid transposons. (Lower) Schematic diagram of the Rosa-PBase and Rosa-SB11 alleles. (B) Transposition efficiencies of the PB-SB-PGK-Neo-bpA hybrid transposon in Rosa-PBase and ROSA-SB11 knockin cell lines. SB transposition efficiency (1 μg of PB-SB-PGK-Neo-bpA) was arbitrarily used as the standard. There was a 1,310-fold difference between these two lines (P < 0.002) when 1 μg of the transposon DNA was used. The fold change dropped to 675-fold (P < 0.001) when 10 μg of the PB-SB-PGK-Neo-bpA transposon plasmid was used. (C) Southern blot analysis of transposition events in Rosa-PBase and Rosa-SB11 knockin lines. Eight G418 resistant colonies were picked from the PB or SB transposition experiments. Genomic DNA was digested with EcoRV and hybridized against a Neo probe. The 5-kb EcoRV fragment present in each clone comes from the insertion of a nonfunctional Neo cassette at the Hprt locus in AB2.2 cells. Other fragments represented independent transposition events. (D) Methylation of the transposon enhanced SB-mediated transposition. Methylated and unmethylated PB-SB-SA-βgeo DNA was electroporated into Rosa-SB11 cells. The trapping efficiency was calculated as the percentage of G418R cells in all of the cells surviving the electroporation. Relative trapping efficiency was normalized to the trapping efficiency of the unmethylated transposon; P < 0.02. (E) Methylation of the transposon inhibited PB-mediated transposition. The same experiment was performed in Rosa-PBase cells as in D; P < 0.002.
To compare PB and SB transposition in an identical genetic background, we constructed two ES cell lines, Rosa-PBase and Rosa-SB11, by targeting PB or SB transposase into the Rosa26 locus in AB2.2 ES cells, respectively (Fig. 2A). The transposition efficiencies were measured by transfecting different amounts of PB-SB-PGK-Neo-bpA into both cell lines (Fig. 2B and Table S1). PB transposition efficiency in Rosa-PBase cells was two to three orders of magnitude higher than that of SB in Rosa-SB11 cells (Fig. 2B).
To measure the maximum transposition rate in the presence of a constitutive PB transposase, we electroporated 50 μg of the PB-SB-PGK-Neo-bpA donor plasmid into the Rosa-PBase cells. More than half of the cells surviving electroporation became G418R, demonstrating the extremely efficient PB transposition in mouse ES cells (data not shown). In addition to the difference of transposition efficiency, we found that all of the G418R Rosa-PBase clones contained multiple integration sites, whereas most of the G418R Rosa-SB11 clones only had single transposon integrations (Fig. 2C).
To investigate whether SB and PB transposases might interfere with each other, we coelectroporated the PB-SB-PGK-Neo-bpA and CAGG-PBase plasmids into Rosa-SB11 cells or the wild-type AB2.2 ES cells respectively. No significant difference in transposition rates was noticed from these two electroporations (data not shown), demonstrating that PB and SB elements in the PB/SB transposon transposed independently.
PB Transposition Is Inhibited by DNA Methylation of the Transposon.
Methylation of the SB transposon was reported to increase its transposition efficiency (28–31). To investigate whether methylation had similar effects on the PB transposition, we constructed a PB-SB-SA-βgeo transposon (Fig. 2A). Methylation of the transposon should not affect expressing of βgeo because the expression of this gene trapping cassette is determined by endogenous promoters once it is transposed into the genome. The PB-SB-SA-βgeo hybrid transposon thus enabled us to compare DNA methylation effects on PB and SB transposon systems directly, using the same methylated DNA molecules.
PB-SB-SA-βgeo was methylated by a CpG methylase, M.SssI in vitro. We transfected an equal amount of methylated and unmethylated PB-SB-SA-βgeo DNA into Rosa-PBase or Rosa-SB11 cells, respectively (Fig. 2 D and E and Table S1). Methylation of the transposon improved the SB transposition efficiency by 9-fold (Fig. 2D), which is consistent with previous reports. In contrast, PB transposition rates were decreased 12-fold by methylation (Fig. 2E). This observation was confirmed in mouse fibroblast cells (NIH/3T3) (data not shown), suggesting that inhibition of PB transposition by DNA methylation was not specific to mouse ES cells.
Transposition of a Single PB-SB Transposon from the Rosa26 Locus.
In the previous experiments, we measured the efficiencies of transposition from plasmids to the chromosomes. To measure the transposition efficiency of a single PB transposon at a genomic locus, we constructed a PGK-[PB-SA-βgeo-PGK-Bsd]-Puro cassette and targeted it to the Rosa26 locus, using the PGK-Bsd cassette as the selection marker (Fig. 3A). This cassette was designed so that excision of the transposon would place a PGK promoter immediately in front of the Puro coding sequence, allowing the excision events to be scored by puromycin resistance (PuroR). A bovine growth hormone polyadenylation signal sequence (bpA) probe was used to detect three copies of the bpA sequence that are present both inside and outside the transposon in the targeted cassette. This probe can be used to detect the excision and the reintegration of the transposon (Fig. S2).
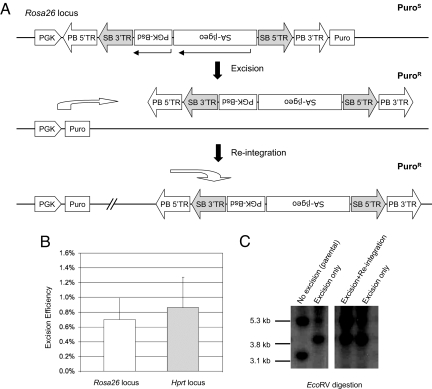
Fig. 3.
Re-mobilization of PB/SB hybrid transposon. (A) Illustration of the PGK-[PB-SB-SA-βgeo-PGK-Bsd]-Puro knockin allele at the Rosa26 locus. A SA-βgeo trapping cassette and a PGK-Bsd selection cassette were cloned into the PB/SB hybrid transposon to form the PB-SB-SA-βgeo-PGK-Bsd transposon. This complex transposon was subsequently inserted in between the PGK promoter and the Puro coding sequence in a PGK-Puro cassette and targeted to the Rosa26 locus. Transfection of the targeted ES cells with CAGG-PBase plasmid mobilized the targeted transposon. Excision reunited the PGK promoter and Puro coding sequence so that the excision events can be scored by puromycin resistance. (B) Transposon excision rates from the Rosa26 and Hprt loci. CAGG-PBase DNA were electroporated into Rosa-PGK-[PB-SB-SA-βgeo-PGK-Bsd]-Puro targeted cells to mobilize the transposon. The excision rate was adjusted to account for all of the cells surviving the electroporation. Similar experiments were carried out in the cells that the PB-SB-SA-βgeo transposon inserted into the Hprt locus (see Fig. 4). (C) Southern blot analysis of the excision events from the PGK-[PB-SB-SAβgeo-PGK-Bsd]-Puro knockin cells. The original targeted clone had both the 3.1-kb targeted EcoRV band and the 5.3-kb transposon fragment. Clones that lost PB after excision only have the 3.8-kb excision fragment. Clones in which transposon excision was followed by reintegration had both the 3.8-kb excision and 5.3-kb transposon fragments.
To mobilize the transposon, CAGG-PB transposase plasmid was transiently expressed in the PGK-[PB-SB-SAβgeo-PGK-Bsd]-Puro knockin cells. Excision events (PuroR) were detected in 0.70% of cells that survived electroporation (Fig. 3B), which were also confirmed by Southern blot analysis (Fig. 3 A and C and Fig. S2). Of the 246 PuroR ES cell clones examined, 100 (41%) retained the transposon fragment, suggesting that less than half of the excised transposon had reintegrated into the genome.
PB as a Mutagen in ES Cells.
In the last 20 years, gene trapping has been widely used to generate mutations in ES cells (32). To explore the possibility to use PB for genome-wide mutagenesis in mouse ES cells, we coelectroporated PB-SB-SA-βgeo and CAGG-PBase plasmids into AB1 ES cells. From one electroporation (50 μg of each), we typically obtained 50,000–100,000 G418R colonies, which represented ≈5% of all surviving ES cells.
To estimate the mutagenesis rate, we elected to select for disruption of the mouse Hprt gene, which spans ≈40 kb on the X chromosome (Fig. 4A). Because AB1 ES cells are male, PB-SB-SA-βgeo integration into the Hprt locus could result in loss of Hprt activity and resistance to 6-thioguanine (6-TG). We transfected AB1 cells with high concentrations of CAGG-PBase and PB-SB-SA-βgeo plasmids (50 μg of each). The transfected cells were first selected in G418 for the gene trapping events, and subsequently with 6-TG for the loss of Hprt function. The mutation rate was calculated as the percentage of 6-TGR cells vs. all of the trapped (G418R) cells. We recovered one 6-TGR colony from every 2,500 G418R clones, representing a mutation rate of 0.04% under this condition.
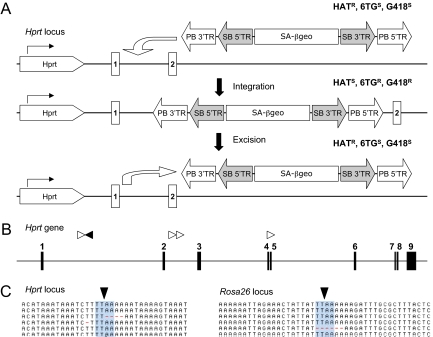
Fig. 4.
PB-mediated mutagenesis. (A) Schematic illustration of a PB-SB-SA-βgeo transposon integrated into the Hprt locus. AB1 ES cells have one copy of Hprt gene. If a PB-SB-SA-βgeo transposon integrates into Hprt locus and disrupts its function, AB1 ES cells will become HAT sensitive and 6-TG resistant. If this transposon is remobilized, the cells will become HAT resistant and 6-TG sensitive. (B) Transposon insertions in the Hprt locus. Transposon-genomic junction fragments from five 6-TG clones were mapped to the Hprt locus. Filled boxes, Hprt exons; white arrowhead, transposon integration sites of clones in which the Hprt exons were directly spliced the SA-βgeo cassette; black arrowhead, the transposon integration site of a clone in which the Hprt gene was disrupted by PB 5′TR. (C) Nearly precise excision of the PB transposon from the Hprt and Rosa26 loci. PCR products that amplify the excision sites in 30 independent excision events from the Hprt locus and 32 from the Rosa26 locus were sequenced. All but three excision events were precise. One clone had a 4-bp microdeletion, another had a 1-bp deletion, and the third had a 6-bp microdeletion.
Splinkerette PCR amplification of the flanking genomic fragments identified the transposon integration sites in introns 1, 2, and 4 of Hprt gene in five independent 6-TGR resistant clones (Fig. 4B). Hprt-LacZ fusion transcripts were identified in three independent clones by RT-PCR (Fig. S3), confirming that the resistance to 6-TG in these clones was caused by PB integration. In another 6-TGR clone, PB had integrated in the opposite orientation. RT-PCR identified a fusion transcript in which an insertion of a 74-bp sequence from PB 5′TR caused a frame shift in the Hprt coding sequence (Fig. S4). This result suggests that the PB 5′TR contains a pair of cryptic splice acceptor and donor sequences.
To determine the efficiency of remobilization from the Hprt locus, we expressed PB transposase in four independent 6-TGR cell lines, including the clone trapped by the PB 5′TR (Fig. 4A). Excision of the transposon restored the function of Hprt and HATR clones were recovered from all four cell lines transfected with PB transposase. An average excision rate of 0.86% was observed at the Hprt locus (Fig. 3B), which is comparable with that at the Rosa26 locus.
Because the PB/SB hybrid transposon integrated into the introns of Hprt gene, we tested mobilization by SB transposase (CMV-SB11) in two of the four 6-TGR lines. The excision rate was ≈10−5 (data not shown), similar to that reported for the Hprt locus (33). Therefore, the PB-SB hybrid transposon could be remobilized by the second transposase (SB) after the initial PB transposition. Interestingly, expression of SB transposase in the clone trapped by PB 5′TR did not generate HATR colonies, suggesting that the residual PB repeats could still disrupt the Hprt locus owing to the cryptic splicing donor and acceptor sequences in the PB 5′TR.
Precise Excision of PB.
PB transposition has been shown to be nearly precise in fruitfly (5, 20, 21). To confirm this observation, we examined PB excision footprints from 32 independent excision events from the Rosa26 locus and 30 from the Hprt locus. Sequence analysis of the junction fragments between PGK promoter and Puro coding sequence or the Hprt genomic fragments flanking the original PB integration site showed all but three had a precise excision (Fig. 4C). Thus, 95% of the genomic PB transposon excision events (Rosa26 and Hprt) were precise in ES cells.
Distribution of PB Integration Sites in the Genome and PB Local Hopping.
To obtain a comprehensive view of the distribution of the PB in the mouse genome, we cloned the insertion junctions and mapped these back to the genome.
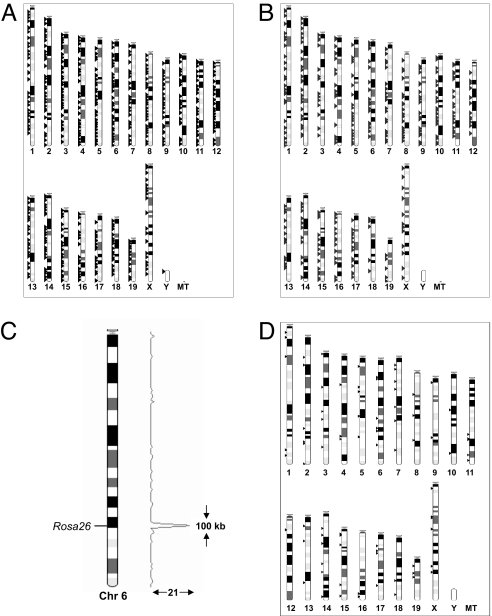
A total of 945 unique integration sites were characterized from G418R clones generated by coelectroporation of high concentrations (50 μg of each) of the CAGG-PBase and PB-SB-PGK-Neo-bpA plasmids (Fig. 5A and Dataset S1). Analysis of these random integration sites revealed that they were distributed evenly across the genome (Dataset S2). Approximately 43% of these integration sites are located in Ensembl (version 48.37a) annotated genes, which is in agreement with other observations that PB has a tendency to integrate into transcription units (22, 26).
Fig. 5.
Distribution of PB integration sites in the genome. (A) PB integration sites in the mouse genome from random transposition events. The sites were identified from transposition experiments, using CAGG-PBase and PB-SB-PGK-Neo-bpA. The transposon-genomic junction fragments were mapped to the mouse genome and displayed in Ensembl (version 48.37a). (B) PB integration sites of gene trapping clones. Splinkerette PCR was performed on G418R clones from coelectroporation of CAGG-PBase and PB-SB-SA-βgeo. Transposon-genomic junction fragments were then mapped to the mouse genome. (C) Local hopping of PB transposition from the Rosa26 locus. Nine percent of PB reintegration sites (21/264) were clustered within a 100-kb region flanking the Rosa26 locus on the donor chromosome (chr. 6). The rest of the integration sites appeared to be randomly distributed in the genome. The height of the peaks on the histograph represent the number of integration sites in a genomic region. (D) No apparent local hopping of the PB transposition from the Hprt locus. A total of 93 integration sites were cloned from PB transposition from the Hprt locus. Analysis of these sites did not show any obvious bias to either the Hprt locus or the X chromosome.
467 PB integration sites from the gene trapping of PB-SB-SA-βgeo plasmids were also identified (Fig. 5B and Dataset S3 and Dataset S4). These sites were compared with the Ensembl GeneTrap database and at least 26 genes trapped by PB were not currently trapped by the existing vectors (Dataset S5), confirming that PB transposition indeed provided an efficient alternative mutagen for mouse genetics.
Excision of a single copy of transposon from either Rosa26 or Hprt provided an opportunity to examine with a large dataset whether PB transposition exhibited any evidence of local hopping. Of 264 new insertions excised from the Rosa26 locus (Fig. 5C, Dataset S6, and Dataset S7), 18% of these sites were on the donor chromosome (chromosome 6). Interestingly, 47% of the new integration sites on chromosome 6 (21/47), or 9% (21/264) of the total reintegration sites, were clustered within a 100-kb region flanking the Rosa26 locus, with the rest of the integration sites being relatively evenly distributed across the genome. It was apparent from these data that PB transposition still had some degrees of local hopping but much less severe than SB transposition.
To investigate the local hopping property of PB transposition at another locus, we cloned 93 new integration sites from PB transposition from the Hprt locus. However, analysis of these sites did not reveal any obvious bias to either the Hprt locus or the X chromosome (Fig. 5D and Dataset S8). This can be explained by the possibility that local reintegration sites were still inside the Hprt locus because of the narrow “local hopping” window. Therefore, these reintegration events still disrupted the Hprt locus and could not be scored by HAT selection. Alternatively, the limited local hopping of PB transposition may be specific to certain genomic loci. It is also possible that during HAT selection there was more time for remobilization compared with Puro selection.
Discussion
In this report, we analyzed the basic properties of PB transposition in mouse embryonic stem cells. The extremely efficient vector-chromosome transposition make PB an excellent vehicle to efficiently deliver genetic elements into mammalian cells. Our data also argued against the existence of transposase overproduction inhibition in ES cells at the conditions tested. This unique property of PB provides a convenient avenue for further improving PB transposition in mammalian cells.
Local hopping is a property of most DNA transposons. In mouse ES cells, the frequency of local transposition of PB transposon system is much lower than that of SB (50%) (33). The local hopping interval of PB (≈100 kb) is smaller than that of SB (≈5 Mb) (13, 34). The local transposition feature of PB has not been reported either in Drosophila melanogaster (5, 35) or in the mouse (22). One explanation is that we transiently expressed PBase in ES cells, whereas the transposase in the transgenic Drosophila or mouse lines was constitutively expressed (22) or continuously induced (35) in the germ line. This explanation is supported by the elimination of local hopping in embryos and in tumors when SB transposase is constitutively expressed (15). It is possible that the lack of local hopping in PB transposition observed in previous studies was due to multiple rounds of PB transposition.
Interestingly, only 40% of the PB excision events were accompanied with reintegration of the transposon, compared with the 77% reintegration rate reported for SB in a similar setting (33). It is likely that this relatively low reintegration rate is also due to multiple rounds of excision/reintegration. Frequent transposition can help to reduce local hopping, but it also increases the possibility of leaving footprint mutations and generating chromosomal rearrangements (36). To minimize these potential side effects, tissue-specific or drug-inducible (27) PB transposase lines might be necessary for somatic and germ-line mutagenesis in the mouse.
Our study, together with those from other groups (22, 24, 26), indicate that PB transposition is more efficient than SB or other DNA transposons in mammalian cells. However, PB and SB have distinct biological properties that can be used separately or together. The PB/SB hybrid transposon, combining the unique properties of PB and SB, provides a complementary transposon tool kit for mouse geneticists, just like PB and P element in D. melanogaster (5). One possible application of the PB/SB hybrid transposon is to use PB as a vehicle to transpose to a genomic region of interest, and subsequently to employ SB to achieve regional saturation mutagenesis (13), taking advantage of SB's strong local hopping.
In summary, we have demonstrated here that the PB transposition is highly efficient in mouse ES cells, has local hopping tendency. In contrast to SB, PB transposition is inhibited by DNA methylation. PB transposons carrying gene-trapping cassettes served as excellent mutagens and can be potentially used in the large-scale mouse mutagenesis programs. Detailed analysis of PB integration sites indicated that most mouse genomic regions are accessible to PB transposition. Finally, the unique characteristics of the PB transposon make it possible to combine it with other existing mutagens, especially SB, to provide a broader and less biased coverage of the mouse genome.
Materials and Methods
Plasmid Construction and Cell Transfection.
Splinkerette PCR.
PB integration sites were determined by splinkerette PCR (37). Drug resistant colonies were pooled together, and genomic DNA was extracted from ES cells using standard protocols. Sau3AI digested genomic DNA was ligated with splinkerette and junction fragments were PCR amplified with primers HMSp1 (5′-CGA AGA GTA ACC GTT GCT AGG AGA GAC C-3′) and PB-R-Sp1 (5′-CCT CGA TAT ACA GAC CGA TAA AAC ACA TGC-3′). Nested PCR was performed by using primers HMSp2 (5′-GTG GCT GAA TGA GAC TGG TGT CGA C-3′) and PB-R-Sp2 (5′-ACG CAT GAT TAT CTT TAA CGT ACG TCA CAA-3′). PCR products were cloned into pZero2.0-TOPO (Invitrogen) and shot-gun sequenced by using M13 forward and reverse primers.
Southern Analysis.
For PB-SB-PGK-Neo-bpA and PB-SB-SA-βgeo transposition, we have used a Neo probe to detect PB or SB mediated transposition. There is a unique EcoRV site in both transposons. Variation in the sizes of the EcoRV fragments indicated different PB transposition events.
For PGK-[PB-SB-SAβgeo-PGK-Bsd]-Puro single transposon remobilization, we used a bpA probe to detect excision and reintegration at Rosa26 locus. EcoRV digestion will identify two restriction fragments, 3.1 kb and 5.3 kb, in transposon targeted clones. When the transposon is mobilized, the 3.1-kb fragment changes to 3.8 kb. The 5.3-kb EcoRV fragment will remain if excised transposon reintegrates into the ES cell genome.
Supplementary Material
Acknowledgments.
We thank Roland Rad, Juan Cadinanos, Qi Liang, Song Choon Lee, Qin Su, and Mariaestela Ortiz for critical reading of the manuscript and Nancy Holroyd, Jim Stalker, and Stephen Rice for excellent technical assistance. This work was funded by the Wellcome Trust (A.B. and P.L.), National Institutes of Health Grant 5R21GM079528 (to X.W.), and the Illinois Department of Public Health (X.W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0801017105/DCSupplemental.
References
- 1.McClintock B. The origin and behavior of mutable loci in maize. Proc Natl Acad Sci USA. 1950;36:344–355. doi: 10.1073/pnas.36.6.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayes F. Transposon-based strategies for microbial functional genomics and proteomics. Annu Rev Genet. 2003;37:3–29. doi: 10.1146/annurev.genet.37.110801.142807. [DOI] [PubMed] [Google Scholar]
- 3.Spradling AC, Rubin GM. Transposition of cloned P elements into Drosophila germ line chromosomes. Science. 1982;218:341–347. doi: 10.1126/science.6289435. [DOI] [PubMed] [Google Scholar]
- 4.Bellen HJ, et al. P-element-mediated enhancer detection: A versatile method to study development in Drosophila. Genes Dev. 1989;3:1288–1300. doi: 10.1101/gad.3.9.1288. [DOI] [PubMed] [Google Scholar]
- 5.Thibault ST, et al. A complementary transposon tool kit for Drosophila melanogaster using P and piggyBac. Nat Genet. 2004;36:283–287. doi: 10.1038/ng1314. [DOI] [PubMed] [Google Scholar]
- 6.Plasterk RH. The Tc1/mariner transposon family. Curr Top Microbiol Immunol. 1996;204:125–143. doi: 10.1007/978-3-642-79795-8_6. [DOI] [PubMed] [Google Scholar]
- 7.Osborne BI, Baker B. Movers and shakers: Maize transposons as tools for analyzing other plant genomes. Curr Opin Cell Biol. 1995;7:406–413. doi: 10.1016/0955-0674(95)80097-2. [DOI] [PubMed] [Google Scholar]
- 8.Dupuy AJ, Fritz S, Largaespada DA. Transposition and gene disruption in the male germline of the mouse. Genesis. 2001;30:82–88. doi: 10.1002/gene.1037. [DOI] [PubMed] [Google Scholar]
- 9.Fischer SE, Wienholds E, Plasterk RH. Regulated transposition of a fish transposon in the mouse germ line. Proc Natl Acad Sci USA. 2001;98:6759–6764. doi: 10.1073/pnas.121569298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horie K, et al. Efficient chromosomal transposition of a Tc1/mariner-like transposon Sleeping Beauty in mice. Proc Natl Acad Sci USA. 2001;98:9191–9196. doi: 10.1073/pnas.161071798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horie K, et al. Characterization of Sleeping Beauty transposition and its application to genetic screening in mice. Mol Cell Biol. 2003;23:9189–9207. doi: 10.1128/MCB.23.24.9189-9207.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kitada K, et al. Transposon-tagged mutagenesis in the rat. Nat Methods. 2007;4:131–133. doi: 10.1038/nmeth1002. [DOI] [PubMed] [Google Scholar]
- 13.Keng VW, et al. Region-specific saturation germline mutagenesis in mice using the Sleeping Beauty transposon system. Nat Methods. 2005;2:763–769. doi: 10.1038/nmeth795. [DOI] [PubMed] [Google Scholar]
- 14.Collier LS, et al. Cancer gene discovery in solid tumours using transposon-based somatic mutagenesis in the mouse. Nature. 2005;436:272–276. doi: 10.1038/nature03681. [DOI] [PubMed] [Google Scholar]
- 15.Dupuy AJ, et al. Mammalian mutagenesis using a highly mobile somatic Sleeping Beauty transposon system. Nature. 2005;436:221–226. doi: 10.1038/nature03691. [DOI] [PubMed] [Google Scholar]
- 16.Cary LC, et al. Transposon mutagenesis of baculoviruses: Analysis of Trichoplusia ni transposon IFP2 insertions within the FP-locus of nuclear polyhedrosis viruses. Virology. 1989;172:156–169. doi: 10.1016/0042-6822(89)90117-7. [DOI] [PubMed] [Google Scholar]
- 17.Lobo N, Li X, Fraser MJ., Jr Transposition of the piggyBac element in embryos of Drosophila melanogaster, Aedes aegypti and Trichoplusia ni. Mol Gen Genet. 1999;261:803–810. doi: 10.1007/s004380050024. [DOI] [PubMed] [Google Scholar]
- 18.Handler AM, Harrell RA., 2nd Germline transformation of Drosophila melanogaster with the piggyBac transposon vector. Insect Mol Biol. 1999;8:449–457. doi: 10.1046/j.1365-2583.1999.00139.x. [DOI] [PubMed] [Google Scholar]
- 19.Bonin CP, Mann RS. A piggyBac transposon gene trap for the analysis of gene expression and function in Drosophila. Genetics. 2004;167:1801–1811. doi: 10.1534/genetics.104.027557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fraser MJ, Ciszczon T, Elick T, Bauser C. Precise excision of TTAA-specific lepidopteran transposons piggyBac (IFP2) and tagalong (TFP3) from the baculovirus genome in cell lines from two species of Lepidoptera. Insect Mol Biol. 1996;5:141–151. doi: 10.1111/j.1365-2583.1996.tb00048.x. [DOI] [PubMed] [Google Scholar]
- 21.Elick TA, Bauser CA, Fraser MJ. Excision of the piggyBac transposable element in vitro is a precise event that is enhanced by the expression of its encoded transposase. Genetica. 1996;98:33–41. doi: 10.1007/BF00120216. [DOI] [PubMed] [Google Scholar]
- 22.Ding S, et al. Efficient transposition of the piggyBac (PB) transposon in mammalian cells and mice. Cell. 2005;122:473–483. doi: 10.1016/j.cell.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 23.Wu S, Ying G, Wu Q, Capecchi MR. Toward simpler and faster genome-wide mutagenesis in mice. Nat Genet. 2007;39:922–930. doi: 10.1038/ng2060. [DOI] [PubMed] [Google Scholar]
- 24.Wu SC, et al. piggyBac is a flexible and highly active transposon as compared to sleeping beauty, Tol2, and MosI in mammalian cells. Proc Natl Acad Sci USA. 2006;103:15008–15013. doi: 10.1073/pnas.0606979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feschotte C. The piggyBac transposon holds promise for human gene therapy. Proc Natl Acad Sci USA. 2006;103:14981–14982. doi: 10.1073/pnas.0607282103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson MH, Coates CJ, George AL., Jr PiggyBac Transposon-mediated Gene Transfer in Human Cells. Mol Ther. 2007;15:139–145. doi: 10.1038/sj.mt.6300028. [DOI] [PubMed] [Google Scholar]
- 27.Cadinanos J, Bradley A Generation of an inducible and optimized piggyBac transposon system. Nucleic Acids Res. 2007;35:e87. doi: 10.1093/nar/gkm446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ikeda R, et al. Sleeping beauty transposase has an affinity for heterochromatin conformation. Mol Cell Biol. 2007;27:1665–1676. doi: 10.1128/MCB.01500-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park CW, Kren BT, Largaespada DA, Steer CJ. DNA methylation of Sleeping Beauty with transposition into the mouse genome. Genes Cells. 2005;10:763–776. doi: 10.1111/j.1365-2443.2005.00875.x. [DOI] [PubMed] [Google Scholar]
- 30.Park CW, Park J, Kren BT, Steer CJ. Sleeping Beauty transposition in the mouse genome is associated with changes in DNA methylation at the site of insertion. Genomics. 2006;88:204–213. doi: 10.1016/j.ygeno.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 31.Yusa K, Takeda J, Horie K. Enhancement of Sleeping Beauty transposition by CpG methylation: Possible role of heterochromatin formation. Mol Cell Biol. 2004;24:4004–4018. doi: 10.1128/MCB.24.9.4004-4018.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skarnes WC, et al. A public gene trap resource for mouse functional genomics. Nat Genet. 2004;36:543–544. doi: 10.1038/ng0604-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo G, Ivics Z, Izsvak Z, Bradley A. Chromosomal transposition of a Tc1/mariner-like element in mouse embryonic stem cells. Proc Natl Acad Sci USA. 1998;95:10769–10773. doi: 10.1073/pnas.95.18.10769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carlson CM, et al. Transposon mutagenesis of the mouse germline. Genetics. 2003;165:243–256. doi: 10.1093/genetics/165.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hacker U, et al. piggyBac-based insertional mutagenesis in the presence of stably integrated P elements in Drosophila. Proc Natl Acad Sci USA. 2003;100:7720–7725. doi: 10.1073/pnas.1230526100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geurts AM, et al. Gene mutations and genomic rearrangements in the mouse as a result of transposon mobilization from chromosomal concatemers. PLoS Genet. 2006;2:e156. doi: 10.1371/journal.pgen.0020156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mikkers H, et al. High-throughput retroviral tagging to identify components of specific signaling pathways in cancer. Nat Genet. 2002;32:153–159. doi: 10.1038/ng950. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.