Abstract
The freshwater polyp Hydra has considerable regeneration capabilities. A small fragment of tissue excised from an adult animal is sufficient to regenerate an entire Hydra in the course of a few days. During the initial stages of the regeneration process, the tissue forms a hollow sphere. Then the sphere exhibits shape oscillations in the form of repeated cycles of swelling and collapse. We propose a biophysical model for the swelling mechanism. Our model takes the osmotic pressure difference between Hydra's inner and outer media and the elastic forces of the Hydra shell into account. We validate the model by a comprehensive experimental study including variations in initial medium concentrations, Hydra sphere sizes and temperatures. Numerical simulations of the model provide values for the swelling rates that are in agreement with the ones measured experimentally. Based on our results we argue that the shape oscillations are a consequence of Hydra's osmoregulation.
INTRODUCTION
The freshwater polyp Hydra (see Fig. 1 A) has captured the interest of the scientific community since J. Trembley discovered its astonishing regeneration capabilities in 1744. Since Hydra can be cultured in the laboratory and is accessible to various experimental procedures, it has become a standard model organism in developmental biology.
FIGURE 1.
(A) Adult Hydra vulgaris. (B and C) Example of the swelling-collapse oscillations during regeneration at T = 20°C. The snapshots in panel B show: 1), the initial fragment of tissue folding to form a sphere; 2), inflation stage; 3), critical inflation and rupture of the Hydra shell; and 4), collapse, healing, and beginning of a new inflation cycle.
The most remarkable feature in Hydra development is the animal's considerable regeneration potential. A small fragment excised from the body column of an adult animal (as small as 1–5% of the original tissue size) regenerates to form a complete animal in ∼48 h. Even an aggregate of previously dissociated Hydra cells regenerates successfully in the course of a few days (1,2). For a small fragment of tissue, a complex mechanism of tissue bending and healing leads to the formation of a hollow sphere made of a cell bilayer. During the subsequent development, the sphere breaks the initial spherical symmetry with the formation of a new body axis and, at later stages, the regeneration is completed with the formation of tentacles, mouth, and foot. In the case of aggregates, however, at first ectodermal and endodermal cells sort out according to their origin, then a hollow shell arises and regeneration is completed following the same process described above. If enough tissue is present, typically a unique head is formed; nevertheless, for large aggregates multiple heads and feet may appear.
During the last few decades, researchers have focused on the details of the molecular biology involved in Hydra regeneration and embryogenesis. A multitude of genes and peptides regulating development have been discovered and their interrelationship is starting to be understood (3–7). Furthermore, Hydra's unique biology has recently caught the attention of scientists from other disciplines. Among other topics, they studied body plan maintenance (8–11), minimal tissue size for regeneration (12), cavity formation (13,14), cell motility during embryogenesis (15), cell sorting (16–20) and axis formation (21) during regeneration, and regulatory networks involved in regeneration (22,23).
We are interested in a phenomenon occurring during regeneration of a small fragment of tissue and cell aggregates. After the hollow sphere has formed, the shell undergoes a series of cycles of steady inflation followed by a sudden collapse, as shown in Fig. 1, B and C.
These oscillations were first reported by Beloussov et al. (24). A more detailed experimental study was provided by Sato-Maeda and Tashiro (13). They described a sudden change in the amplitude of inflation and frequency of the oscillations during regeneration, which was followed by a change in shape toward an oblong cell ball at a later stage. Mombach et al. (14) described the formation of the hollow sphere and the subsequent oscillations with a numerical algorithm of the cellular Potts model type in two dimensions. Fütterer et al. (25) performed a Fourier analysis of the oscillating spheres and showed that a transition from elastic isotropy (where the cell ball becomes more spherical upon inflation) to elastic anisotropy (where the cell ball becomes more oblong upon inflation) starts simultaneously with the reported switch in the amplitude of inflation and frequency. Fütterer et al. (25) suggested that the elastic asymmetry could be a sign of axis formation, an idea that was later confirmed by Soriano et al. (21).
Despite these efforts, neither the mechanism nor the role of the oscillations has yet been clarified. This is even more puzzling if we consider that the oscillations consume energy at a time when Hydra cannot feed. Furthermore, it has not been explained why the sphere inflates almost linearly in time and with a remarkable periodicity, especially taking into account the significant increase in radius during swelling, which often exceeds 25% of the initial radius.
In this work, we propose that the oscillations are a by-product of Hydra osmoregulation. We will support this view by ideas from the literature, experiments with oscillating Hydra in different culture media, and a mathematical model. All relevant model parameters could be estimated from the literature. Experimental results and numerical simulations are consistent.
BIOLOGICAL BACKGROUND
Since Hydra lives in fresh water, it shares the osmotic problems of other fresh water animals (26), namely that the osmolarity of the tissue exceeds the osmolarity of the surrounding medium. Therefore, water constantly enters the tissue. This water must be removed to prevent swelling of the cells and subsequent rupture. The mechanism of water removal was studied by various authors (27–29). Benos et al. (27) found that osmoregulation is achieved by vacuoles in the cells that are connected by channels to the inner cavity of Hydra, the so-called enteron. The authors argued that Hydra constantly deposits ions in these vacuoles; consequently, water flows into the vacuoles and is transported to the enteron. If the vacuole fluid were ejected directly to the outside medium, the ions in the fluid would be lost. Since they are transported into the enteron, Hydra has the possibility to reabsorb some of the ions. This is indeed the case for potassium ions, which are present in the enteron fluid only in small amounts. Thus, the enteron fluid is hyposmotic to the tissue fluid. Sodium, on the other hand, is not reabsorbed, but constantly taken up from the outside medium, as shown by studies using radioactive sodium isotopes (29).
This mechanism leads to an ongoing flow of ions and water from the outer medium into the Hydra enteron. In a normal Hydra, the excess fluid in the enteron is removed by spontaneous contractions, ejecting the fluid through the mouth opening. It was shown in Benos et al. (27) that the frequency of these contractions decreases with increasing osmolarity of the outer medium.
In regenerating Hydra, a mouth opening and a functioning nervous system is still not present. Therefore, excess fluid in the enteron cannot easily be removed. Hydra continues to swell until rupture of the body wall tissue takes place. This way the fluid is ejected through the rupture, the Hydra ball collapses, the wound heals and the process starts anew. A similar behavior is observed in the so-called epithelial Hydra (animals that have a mouth but no nervous system) (30,31). These Hydra cannot contract and hence they also swell until the increased internal pressure is released through the mouth.
METHODS
Hydra culturing
All experiments were carried with strains of Hydra vulgaris. The animals were cultured at 18°C in Hydra medium (1.0 mM CaCl2, 1.5 mM NaHCO3, 0.1 mM MgCl2, 0.08 mM MgSO4, and 0.03 mM KNO3), fed regularly four times a week, and starved for 24 h before manipulation for experiments.
Preparation of Hydra spheres
Fragments were prepared following the procedure described in the literature (21,25). Thin disks of tissue were cut from the body column of an adult Hydra. The disks were then split into 4–8 fragments to select different sizes. The fragments closed to form hollow spheres, with initial radius R0 in the range 90–250 μm. Larger spheres were obtained from aggregates of dissociated Hydra cells, prepared as described in the literature (1,17).
To study the evolution of the spheres with different inner and outer concentrations 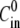 and
and 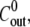 each fragment was immersed, immediately after cutting, in a chamber containing the medium at the inner concentration
each fragment was immersed, immediately after cutting, in a chamber containing the medium at the inner concentration  and left for 4 h until the hollow sphere emerged. Since the sphere forms through a mechanism of tissue bending and healing, the medium inside the shell after closure is the same as the one at its surroundings. The sphere was next transferred to the recording chamber, consisting of a 30-mm-diameter petri dish that contained the medium at the outer concentration
and left for 4 h until the hollow sphere emerged. Since the sphere forms through a mechanism of tissue bending and healing, the medium inside the shell after closure is the same as the one at its surroundings. The sphere was next transferred to the recording chamber, consisting of a 30-mm-diameter petri dish that contained the medium at the outer concentration  To ensure that variations in the outer concentration were negligible, the chamber contained 5 ml of outer medium (a volume ∼5 orders-of-magnitude larger than the typical volume of a Hydra sphere).
To ensure that variations in the outer concentration were negligible, the chamber contained 5 ml of outer medium (a volume ∼5 orders-of-magnitude larger than the typical volume of a Hydra sphere).
Experimental setup and data analysis
Between 5 and 10 spheres were placed together in the recording chamber, which was sealed to prevent evaporation. A thermal bath maintained the chamber at constant temperature T. The evolution of the spheres was monitored using a charge-coupled device camera mounted on an inverted microscope (Carl Zeiss, Oberkochen, Germany) with a 5× objective, and snapshots of the regenerating Hydra were obtained at 3 min interval with a spatial resolution of 1.8 μm/pixel. The images were later analyzed to extract the in-plane contour of the Hydra spheres, using the same procedure described in the literature (13,21).
The radius R of the sphere at time t was defined as the radius of the circle with the same area as the contour analyzed. To compare spheres of different sizes, we chose the radius at the beginning of the recording as the characteristic radius of the sphere, i.e., R0 = R(t = 0).
The R(t) plots provided the basis for the analysis of the inflation-collapse oscillations under diverse experimental conditions. We explored different Hydra sphere sizes R0, regeneration temperatures T (in the range 6–34°C), and a number of inner and outer initial concentrations  and
and  As a standard procedure, different initial concentrations were obtained by adding sucrose to Hydra medium (29). We explored concentration differences
As a standard procedure, different initial concentrations were obtained by adding sucrose to Hydra medium (29). We explored concentration differences  between −100 mM and 100 mM.
between −100 mM and 100 mM.
EXPERIMENTAL RESULTS
The R(t) plots were carefully studied and spheres that did not complete regeneration (typically those with  μm) were excluded from further analysis. The study of the inflation-collapse oscillations was restricted to the isotropic phase only, i.e., before axis formation (Fig. 1 C).
μm) were excluded from further analysis. The study of the inflation-collapse oscillations was restricted to the isotropic phase only, i.e., before axis formation (Fig. 1 C).
Examples of inflation-contraction cycles at different concentrations 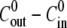 are shown in Fig. 2 A. For clarity, data is shown only from the formation of the sphere to axis formation. Spheres continue to oscillate until they complete regeneration. In general, the qualitative behavior of the oscillations is the same for all combinations of inner and outer mediums. It is characterized by periodic cycles with swelling almost linear in time. The swelling rate strongly depends on the difference between the osmolarity of the inner and outer concentrations, and
are shown in Fig. 2 A. For clarity, data is shown only from the formation of the sphere to axis formation. Spheres continue to oscillate until they complete regeneration. In general, the qualitative behavior of the oscillations is the same for all combinations of inner and outer mediums. It is characterized by periodic cycles with swelling almost linear in time. The swelling rate strongly depends on the difference between the osmolarity of the inner and outer concentrations, and 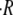 gradually decreases as the concentration difference grows.
gradually decreases as the concentration difference grows.
FIGURE 2.
(A) Examples of oscillations (from the beginning of regeneration to axis formation) at T = 20°C and for gradually higher osmotic difference  showing that the slope
showing that the slope 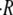 decreases with
decreases with  (B) Values of the averaged swelling rate
(B) Values of the averaged swelling rate 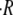 along four consecutive cycles and for three different osmotic concentration differences. Each point is an average over four spheres with initial sizes R0 in the range 150–200 μm. The cycles are limited to four since this is the maximum number of common cycles in all the spheres. The measured swelling rates for
along four consecutive cycles and for three different osmotic concentration differences. Each point is an average over four spheres with initial sizes R0 in the range 150–200 μm. The cycles are limited to four since this is the maximum number of common cycles in all the spheres. The measured swelling rates for  are approximately constant from cycle to cycle, while for
are approximately constant from cycle to cycle, while for  the swelling rate of the first cycle is much higher than the rest. (C) Linear dependence of the swelling rate
the swelling rate of the first cycle is much higher than the rest. (C) Linear dependence of the swelling rate 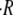 on the initial radius of the sphere R0. Each point is an average over four cycles. T = 20°C. (D) Dependence of the swelling rate
on the initial radius of the sphere R0. Each point is an average over four cycles. T = 20°C. (D) Dependence of the swelling rate 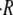 on temperature. Each point is an average over six spheres of sizes in the range 130–150 μm, and over four cycles per sphere. The curve is an exponential fit. The experiments in panels C and D were carried out with Hydra medium as initial inner and outer concentrations. HM denotes Hydra medium and Suc sucrose solution in Hydra medium.
on temperature. Each point is an average over six spheres of sizes in the range 130–150 μm, and over four cycles per sphere. The curve is an exponential fit. The experiments in panels C and D were carried out with Hydra medium as initial inner and outer concentrations. HM denotes Hydra medium and Suc sucrose solution in Hydra medium.
The swelling rate 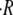 was measured by fitting the inflation stages to straight lines, as shown in Fig. 2 A. We observed that for
was measured by fitting the inflation stages to straight lines, as shown in Fig. 2 A. We observed that for 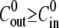 the swelling rate is constant in the course of the oscillations and therefore
the swelling rate is constant in the course of the oscillations and therefore 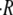 can be averaged over all cycles (Fig. 2 B). On the other hand, for
can be averaged over all cycles (Fig. 2 B). On the other hand, for 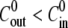 sucrose is ejected out of the sphere after the first collapse. Hence, only the first cycle is actually driven by the strong concentration difference, as shown in the top panel of Fig. 2, A and B. The subsequent cycles have similar swelling rates, with values that approximate the ones measured for
sucrose is ejected out of the sphere after the first collapse. Hence, only the first cycle is actually driven by the strong concentration difference, as shown in the top panel of Fig. 2, A and B. The subsequent cycles have similar swelling rates, with values that approximate the ones measured for 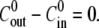
The swelling rate also depends on two other major parameters, namely the initial size of the sphere R0 and the regeneration temperature T. As shown in Fig. 2 C, the dependence of 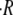 on initial radius is well described by a linear relationship. The dependence of
on initial radius is well described by a linear relationship. The dependence of 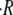 on temperature is shown in Fig. 2 D. For spheres with similar initial radius R0 = 130–150 μm and Hydra medium as inner and outer concentrations, we measured a swelling rate of 1.8 μm/h at 6°C, which increased to 6.8 μm/h at 20°C and to 30.5 μm/h at 28°C. We studied in total six different temperatures and observed that the variation of
on temperature is shown in Fig. 2 D. For spheres with similar initial radius R0 = 130–150 μm and Hydra medium as inner and outer concentrations, we measured a swelling rate of 1.8 μm/h at 6°C, which increased to 6.8 μm/h at 20°C and to 30.5 μm/h at 28°C. We studied in total six different temperatures and observed that the variation of 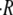 with temperature followed an exponential growth. Hydra spheres did not regenerate at T < 6°C, and most of the spheres died for
with temperature followed an exponential growth. Hydra spheres did not regenerate at T < 6°C, and most of the spheres died for 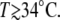
The observation that 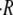 varies linearly with the sphere size R0 permits to define the relative swelling rate
varies linearly with the sphere size R0 permits to define the relative swelling rate  where 〈…〉 is average over sphere sizes. The dependence of the relative swelling rate on the concentration difference
where 〈…〉 is average over sphere sizes. The dependence of the relative swelling rate on the concentration difference  and for different sphere sizes is shown in Fig. 3 A. For
and for different sphere sizes is shown in Fig. 3 A. For 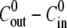 < 0 mM, the analysis is limited to the first cycle only since the effect of
< 0 mM, the analysis is limited to the first cycle only since the effect of 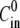 disappears afterwards. As expected,
disappears afterwards. As expected, 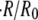 strongly decreases with the concentration difference. For
strongly decreases with the concentration difference. For 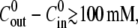 no swelling was observed.
no swelling was observed.
FIGURE 3.
(A) Relative swelling rate 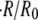 as a function of the initial radius R0 and for four different initial concentrations
as a function of the initial radius R0 and for four different initial concentrations 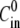 and
and 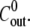 The average
The average 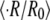 is shown with straight lines. The accompanying values indicate the concentration difference. The relative swelling rate for
is shown with straight lines. The accompanying values indicate the concentration difference. The relative swelling rate for  = −50 mM corresponds to the first cycle only. For the other concentration differences, each point is an average over 2–5 cycles of the same sphere. HM denotes Hydra medium and Suc sucrose solution in Hydra medium. (B) Dependence of the relative swelling rate
= −50 mM corresponds to the first cycle only. For the other concentration differences, each point is an average over 2–5 cycles of the same sphere. HM denotes Hydra medium and Suc sucrose solution in Hydra medium. (B) Dependence of the relative swelling rate 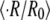 on the concentration difference
on the concentration difference  For
For 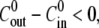 the behavior of the first cycle (triangles, each point an average over 7–12 spheres) is different from the rest (circles, each point an average over 7–12 spheres and over 2–5 cycles per sphere). All experiments were carried out at T = 20°C.
the behavior of the first cycle (triangles, each point an average over 7–12 spheres) is different from the rest (circles, each point an average over 7–12 spheres and over 2–5 cycles per sphere). All experiments were carried out at T = 20°C.
The dependence of the relative swelling rate on 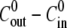 and for eight different concentration differences is summarized in Fig. 3 B. For the cases with
and for eight different concentration differences is summarized in Fig. 3 B. For the cases with 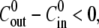 the relative swelling rate of the first cycle is clearly much larger than the rest. From the second oscillation onwards, the values of
the relative swelling rate of the first cycle is clearly much larger than the rest. From the second oscillation onwards, the values of 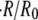 are similar to the ones for
are similar to the ones for 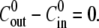 The effect of the initial high inner concentration completely disappears after the first oscillation.
The effect of the initial high inner concentration completely disappears after the first oscillation.
MODEL
Model development
We model the inflation-collapse oscillations in the simplest possible way. We assume that Hydra tissue is permeable to water and that the swelling is caused by the concentration gradient between the outside medium and the enteron fluid. Both assumptions are well supported by experimental observations. Then, the rate of change of total volume of water 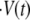 inside the sphere at time t is given by a Darcy-type law
inside the sphere at time t is given by a Darcy-type law
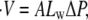 |
where Lw is the water permeability coefficient, A the tissue area, and ΔP the pressure difference between enteron and outside medium (i.e., ΔP ≡ Pin – Pout). The pressure difference is the sum of the osmotic pressure and the elastic pressure of the shell on the enteron, i.e., ΔP = ΔΠ + ΔPE. The osmotic pressure ΔΠ can be described by the van' t Hoff relation, ΔΠ(t) = kBT[Cin(t) – Cout(t)], where Cin and Cout are the inner and outer concentrations, respectively. The elastic pressure ΔPE can be easily calculated if we assume linear elasticity for the spherical shell although, as it will become clear later, this is not a significant restriction. This results in an evolution equation for the radius R of the shell, of the form
 |
(1) |
Here we have  with E Young's modulus, h the thickness of the Hydra sphere, and μ Poisson's ratio. Furthermore, R0 is the initial radius and kB Boltzmann's constant. We further assume that the outer concentration remains constant, i.e.,
with E Young's modulus, h the thickness of the Hydra sphere, and μ Poisson's ratio. Furthermore, R0 is the initial radius and kB Boltzmann's constant. We further assume that the outer concentration remains constant, i.e.,  and that there is a constant influx of new ions into the enteron. Then
and that there is a constant influx of new ions into the enteron. Then  where
where 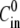 is the initial concentration of solute in the enteron, V0 and A0, are, respectively, the initial volume and area of the shell, and U is the pumping rate per initial unit area. The combination of the above expressions yields an equation for the radius R of the form
is the initial concentration of solute in the enteron, V0 and A0, are, respectively, the initial volume and area of the shell, and U is the pumping rate per initial unit area. The combination of the above expressions yields an equation for the radius R of the form
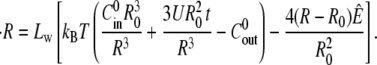 |
For convenience, a more compact expression can be obtained by introducing the relative change in radius, r = (R − R0)/R0. This gives
 |
(2) |
where the constants 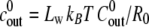 and
and 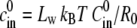 are proportional to the initial ion concentration outside and inside the shell, respectively. The quantity u = 3Lw kBT U/
are proportional to the initial ion concentration outside and inside the shell, respectively. The quantity u = 3Lw kBT U/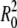 is proportional to the ion pumping rate per initial unit area, and
is proportional to the ion pumping rate per initial unit area, and 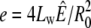 is proportional to the elastic resistance of the shell.
is proportional to the elastic resistance of the shell.
The inflation process ends with the rupture of the Hydra sphere when the radius reaches a maximal value Rrup. This leads to a sudden collapse that resets the radius to R0. The collapse results in an almost exponential decay of the relative radius change r to 0 (see Appendix A for details). Since the collapse is on the order of a few seconds, much faster that the inflation process (on the order of hours), we will replace the dynamics of the collapse by a direct resetting of r to 0 in the description of the oscillations.
It is generally accepted (32) that almost no cell divisions occur during Hydra regeneration and therefore we take R0 constant in the model. This is also supported by our experiments. As shown in Fig. 1 A, the radius of the sphere after collapse is remarkably similar from cycle to cycle.
Analysis of the model
The magnitude of the parameters of the model can be estimated using data already available in the literature (see Appendix B for details). We used Lw = 0.9 × 108 μm3 (N h)−1, R0 = 130 μm, and T = 293 K, to obtain the reduced concentrations 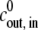 = 1.7 × 10−3
= 1.7 × 10−3 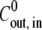 (mM h)−1, the reduced pumping rate u = 7.8 × 10−3 h−2, and the reduced elastic resistance of the shell e = 3.3 × 10−3 h−1.
(mM h)−1, the reduced pumping rate u = 7.8 × 10−3 h−2, and the reduced elastic resistance of the shell e = 3.3 × 10−3 h−1.
The rupture of the Hydra shell is simulated by setting a critical radius rrup above which the shell breaks. The value r is then set to zero and the swelling starts again. A value of rrup = 0.25 is consistent with our experiments (compare Figs. 1 C and 2 A).
A numerical simulation for r(t) using these parameters is shown in Fig. 4 A. The analysis of the simulations provides three major conclusions.
The elastic resistance of the Hydra shell, which appears in the model through the quantity e, hardly contributes to the dynamics and thus can be neglected. Simulations with e = 0 are virtually identical to the simulations with our estimated e.
The swelling process is almost linear (Fig. 4 A).
The term in Eq. 2 that accounts for the concentration of ions inside the Hydra shell cin(t) (see Eq. 2) does not change appreciably after the first few cycles, as shown in Fig. 4 B.
FIGURE 4.
Numerical simulations of the model and comparison with the experiments. (A and B) The time evolution of the relative radius r and, respectively, the inner concentration Cin is shown from a numerical integration of the model (the parameters of the simulation are described in the text, with  and
and 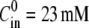 ). (B) The analytical approximation of
). (B) The analytical approximation of 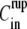 of Eq. 4
of Eq. 4 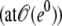 is also shown. (C) Comparison among experiment, simulation, and analytical approximation of the relative swelling rate
is also shown. (C) Comparison among experiment, simulation, and analytical approximation of the relative swelling rate 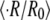 as a function of the concentration difference (for
as a function of the concentration difference (for 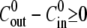 ) at T = 20°C. In this panel the experimentally measured values (circles) are compared with the ones measured in numerical simulations of the model (triangles). The solid curve shows the analytical approximation for the relative swelling rate
) at T = 20°C. In this panel the experimentally measured values (circles) are compared with the ones measured in numerical simulations of the model (triangles). The solid curve shows the analytical approximation for the relative swelling rate  (see main text and Eq. 4).
(see main text and Eq. 4).
In fact, these observations are connected to each other, and the following analysis illustrates why this is the case. Furthermore, we can estimate the swelling rate 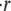 analytically.
analytically.
The observation that the inside concentration is almost constant greatly simplifies the analysis of the equations, because the time dependence and the nonlinearity of Eq. 2 are both contained in cin. Indeed, Eq. 2 tells us that the inside concentration 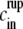 in the shell at rupture time is given by
in the shell at rupture time is given by
 |
(3) |
where only trup, the average time between ruptures, is unknown.
Assuming now that cin(t) is almost constant (i.e., cin ≈ 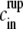 ), we can estimate trup. By solving Eq. 2 we get
), we can estimate trup. By solving Eq. 2 we get 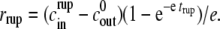 And by isolating trup, inserting it into Eq. 3, expanding the logarithm and performing standard asymptotic techniques one obtains the following estimation
And by isolating trup, inserting it into Eq. 3, expanding the logarithm and performing standard asymptotic techniques one obtains the following estimation 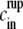 for the internal concentration
for the internal concentration 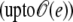
 |
(4) |
where vrup = (1 + rrup)3 – 1 is the relative volume change at rupture time. The correction term 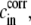 proportional to e, is given by
proportional to e, is given by  Because e is small the model equation simplifies to
Because e is small the model equation simplifies to  Its solution is trivially given by
Its solution is trivially given by 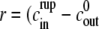 ) t.
) t.
Therefore, the analysis of the model shows that we obtain approximately linear swelling with slope  This slope depends only on the outside concentration
This slope depends only on the outside concentration  the pumping rate u, and the relative rupture radius rrup.
the pumping rate u, and the relative rupture radius rrup.
Note that this analysis only applies for cycles with a steady-state regime where the inner concentration cin does not change much. Therefore we cannot use the analysis to obtain quantitative values for the first cycle in the case 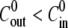 because in this case the inner concentration decreases significantly.
because in this case the inner concentration decreases significantly.
Fig. 4 C shows a direct comparison between the relative swelling rate 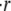 measured experimentally, the results of numerical simulations of the model, and our analytical approximation. Here we plot
measured experimentally, the results of numerical simulations of the model, and our analytical approximation. Here we plot 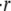 as a function of the concentration difference
as a function of the concentration difference 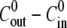 with the parameter values estimated above.
with the parameter values estimated above.
DISCUSSION
The swelling behavior of the Hydra sphere obtained from the analysis of the model is in agreement with the one observed experimentally, and is best described in terms of the relative swelling rate 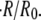 The analysis of the model shows that
The analysis of the model shows that  is independent of time and of the initial radius R0. The relative swelling rate has a weak dependence on the elasticity of the Hydra shell and a transient dependence on the initial concentration
is independent of time and of the initial radius R0. The relative swelling rate has a weak dependence on the elasticity of the Hydra shell and a transient dependence on the initial concentration  On the other hand, the relative swelling rate depends strongly on temperature through the pumping rate and on the outside concentration
On the other hand, the relative swelling rate depends strongly on temperature through the pumping rate and on the outside concentration 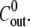
The time-independence of the swelling rate was verified experimentally for a wide range of sphere sizes, concentration differences, and temperatures (Fig. 2 A). It leads to a linear increase in radius of the Hydra shell followed by a sudden collapse, giving rise to the observed oscillations. This is in full agreement with our model.
The model also predicts that the relative swelling rate is independent of the initial radius R0. This is indeed observed experimentally (Fig. 3 A). The experiments also show that the swelling rate increases exponentially with temperature, indicating that the pumping rate u is strongly temperature-dependent.
The initial inner concentration  has a transient effect on the swelling rate. It only affects Hydra swelling for the first few oscillation cycles, after which Cin approaches an almost constant value
has a transient effect on the swelling rate. It only affects Hydra swelling for the first few oscillation cycles, after which Cin approaches an almost constant value 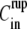 (Fig. 4 B). The outside concentration, however, has a much larger impact on the swelling dynamics. As the concentration difference
(Fig. 4 B). The outside concentration, however, has a much larger impact on the swelling dynamics. As the concentration difference 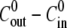 is increased, the relative swelling rate
is increased, the relative swelling rate 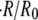 decreases. This dependence in quantitatively captured by the proposed model for a significant range of
decreases. This dependence in quantitatively captured by the proposed model for a significant range of 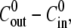 up to 50 mM (see Fig. 4 C). At concentration differences
up to 50 mM (see Fig. 4 C). At concentration differences 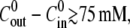 the experimental values for
the experimental values for 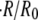 are lower than the ones obtained from the simulations, and no swelling is observed at 100 mM. We conjecture that this deviation is due to the fact that the pumping rate depends on the outside concentration. Indeed, if the outside osmolarity is high the osmotic difference to the Hydra cells is small. Consequently, little water enters the tissue and little pumping is necessary to regulate osmotic balance.
are lower than the ones obtained from the simulations, and no swelling is observed at 100 mM. We conjecture that this deviation is due to the fact that the pumping rate depends on the outside concentration. Indeed, if the outside osmolarity is high the osmotic difference to the Hydra cells is small. Consequently, little water enters the tissue and little pumping is necessary to regulate osmotic balance.
Surprisingly, the analysis of the model shows that the elastic term e r in Eq. 2 is too small to have a significant contribution to the swelling and therefore it can neglected. If e is unrealistically large, our numerical simulations show that the swelling is no longer linear.
Although the model describes the swelling mechanism during the early stages of Hydra regeneration, it can be extended to the whole regeneration process. As shown in Fig. 1 C, the inflation-collapse events continue after axis formation. The rupture radius is significantly reduced, but the oscillations are periodic and the swelling linear with time. We can conclude that the inflation mechanism is the same. The reduced rupture threshold can be understood as a weak spot in the Hydra shell, possibly related to the formation of an early mouth, as suggested by Soriano et al. (21).
Finally, there are some experimental observations that cannot be explained with the model. For instance, the Hydra shell never shrinks, even in experiments using an outer concentration much larger than the initial inner concentration. This phenomenon is not predicted by the model, but understandable from Hydra's biology: even if the outside medium has a high osmolarity, the Hydra cells are still hyperosmotic compared to it. As excess water inside the cells is always transported into the enteron, shrinking cannot take place. This phenomenon could be captured using a more elaborated model that also takes other processes in the Hydra tissue into account. A second observation bears on the swelling behavior immediately after collapse. At that time, the swelling rate is often (but not nearly always) significantly larger, typically by a factor 2, than at later times during the swelling cycle. In that case, even the biological nature of this phenomenon is unclear.
CONCLUSIONS
We have presented a biophysical model to describe the mechanical oscillations in regenerating Hydra. The model is based on the idea that Hydra swelling is driven by the osmotic difference between the inner and outer concentrations of the Hydra shell. The analysis of the model provides two major conclusions: 1), the elastic forces of the Hydra shell during the swelling are negligible compared to the osmotic ones; and 2), the swelling rate 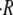 is linear with time, with a slope that essentially depends on the difference between the outside and inside initial concentrations
is linear with time, with a slope that essentially depends on the difference between the outside and inside initial concentrations 
The model is supported by experiments with regenerating Hydra spheres. The experiments show that swelling is linear with time in a wide range of concentration differences and sphere sizes, and that the relative swelling rate 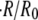 decreases with
decreases with 
Unlike most models in biology, the magnitude of the parameters in our model could be directly estimated from the literature. Numerical integrations of the model using these parameters provided values of 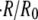 in agreement with the experiments. Hence, our model captures the physical process of the oscillations, and confirms the osmoregulatory mechanism proposed by Benos et al. (27).
in agreement with the experiments. Hence, our model captures the physical process of the oscillations, and confirms the osmoregulatory mechanism proposed by Benos et al. (27).
Acknowledgments
Prof. T. C. G. Bosch (Kiel) not only provided us with the Hydra strains used in this work but also with good advice, technical support, and encouragement. We are grateful to A. Hanold and T. Mai for assistance with the experiments at Universität Bayreuth. We are also grateful to S. Rüdiger (Berlin) for discussions and insight. The authors thank the referees for helpful suggestions on how to improve the article.
E. M. Nicola and J. Soriano acknowledge the financial support of the European Training Network PHYNECS, under grant No. HPRN-CT-2002-00312.
APPENDIX A: MODELING THE COLLAPSE OF THE SPHERE
The Hydra sphere collapses once the tension between the cells reaches a critical point and the sphere ruptures. The critical tension at the time of rupture can be estimated by the equation
 |
where μ is Poisson's ratio and h the thickness of the Hydra sphere. From the tension we can calculate the critical force on a cell with a side surface A to be
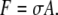 |
Using μ = 0.25, h = 20 μm, rrup = 0.25, 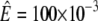 N m−1, and A = (10 μm)2 we obtain F = 190 nN. This value is on the order of magnitude of the maximum force for cadherin-mediated adhesion as measured in Chu et al. (33).
N m−1, and A = (10 μm)2 we obtain F = 190 nN. This value is on the order of magnitude of the maximum force for cadherin-mediated adhesion as measured in Chu et al. (33).
To model the collapse of the Hydra sphere that occurs after a swelling period we first observe that the rupture point of the cell ball corresponds to a circular hole with a size of the order of a Hydra cell, as described in Fütterer et al. (25). Hence, we can assume that the water flow passing through the hole is laminar. Consequently, Poiseuille's law can be applied, leading to 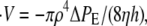 where V is the volume of the sphere, ρ and h are the radius and depth of the hole, and η is the dynamic fluid viscosity. ΔPE is the elastic pressure difference and is given by the second term of Eq. 1. Rewriting this equation for the relative radius of the sphere r we obtain
where V is the volume of the sphere, ρ and h are the radius and depth of the hole, and η is the dynamic fluid viscosity. ΔPE is the elastic pressure difference and is given by the second term of Eq. 1. Rewriting this equation for the relative radius of the sphere r we obtain 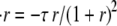 where
where  For small r this leads to an exponential decay of the radius with time, with a characteristic time constant given by 1/τ. For ρ = 5 μm, R0 = 180 μm, and
For small r this leads to an exponential decay of the radius with time, with a characteristic time constant given by 1/τ. For ρ = 5 μm, R0 = 180 μm, and 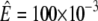 N m−1, we obtain 1/τ = 2.7 s, which is on the order of the characteristic deflation time observed in the experiments.
N m−1, we obtain 1/τ = 2.7 s, which is on the order of the characteristic deflation time observed in the experiments.
APPENDIX B: ESTIMATION OF THE PARAMETERS OF THE MODEL
The parameters of Eq. 2 can be obtained as follows. The water permeability coefficient Lw is given in Lilly (29) as Lw = 3.6 × 108 μm3 (N h)−1. This value can, however, only be seen as an order-of-magnitude estimation. The best correspondence between experiments and simulations was obtained using the value of Lw = 0.9 × 108 μm3 (N h)−1.We use R0 = 130 μm as a typical value for the initial radius of the sphere in our experiments (see Fig. 2 C). The temperature is T = 293 K. Thus, for the quantities 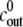 and
and  we obtain
we obtain 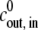 = 1.7 × 10−3
= 1.7 × 10−3 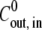 (mM h)−1.
(mM h)−1.
For the estimation of e we need  In Goidin (34) this quantity was estimated using two different methodologies giving values on the range
In Goidin (34) this quantity was estimated using two different methodologies giving values on the range  If we use the upper limit of these estimations, we get E = 11 kPa and e = 3.3 × 10−3 h−1.
If we use the upper limit of these estimations, we get E = 11 kPa and e = 3.3 × 10−3 h−1.
More difficult is the estimation of the pumping rate per unit area U. This quantity is estimated from data in the literature using two different approaches. A first approach is based on the measurement of electric currents across the body column of an adult Hydra, providing a value of A = 58 nA (35). Hence, assuming that an adult Hydra is l = 1 cm long and has a diameter of d = 1 mm, we get U = π ldA/ε = 4.6 × 107 h−1 μm−2 where E = 1.6 × 10−19 C is the elementary charge.
The second approach uses the observation that radioactive sodium is taken up from the medium by Hydra with exponential saturation (29). The measured half-time constant is γ = 2 h and the saturated concentration of sodium in the tissue is S = 17 mM. Assuming that S is constant we get a differential equation for the amount of radioactive sodium in the tissue T as follows: T ′ = K(1 – T/S). Here K is the pumping rate per Hydra volume. The equation has the solution T = S(1 − exp(−kt/S)). From this expression, we deduce K = S ln 2/γ, and finally (with h = 20 μm for the Hydra thickness) U = π ldhK = 7.1 × 107 h−1 μm−2.
Considering the very different methodologies and the high systematic errors, these estimations are surprisingly close to each other. Experimentally, we know that the swelling rate depends strongly on temperature (see Fig. 2 D). This suggests that also the pumping rate is strongly temperature-dependent. For the simulations, we used the value U = 3 × 107 h−1 μm−2, which best reproduces the swelling rate observed experimentally (for  and T = 20°C). This leads to u = 7.8 × 10−3 h−2.
and T = 20°C). This leads to u = 7.8 × 10−3 h−2.
Editor: Elliott L. Elson.
References
- 1.Gierer, A., S. Berking, H. Bode, C. David, K. Flick, G. Hansmann, H. Schaller, and E. Trenkner. 1972. Regeneration of Hydra from cell reaggregates. Nat. New Biol. 239:98–101. [DOI] [PubMed] [Google Scholar]
- 2.Technau, U., C. C. von Laue, F. Rentzsch, S. Luft, B. Hobmayer, H. Bode, and T. Holstein. 2000. Parameters of self-organization in Hydra aggregates. Proc. Natl. Acad. Sci. USA. 97:12127–12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bode, H. 2003. Head regeneration in Hydra. Dev. Dyn. 226:225–236. [DOI] [PubMed] [Google Scholar]
- 4.Steele, R. 2002. Developmental signaling in Hydra: what does it take to build a “simple” animal? Dev. Biol. 248:199–219. [DOI] [PubMed] [Google Scholar]
- 5.Holstein, T., E. Hobmayer, and U. Technau. 2003. Cnidarians: an evolutionarily conserved model system for regeneration? Dev. Dyn. 226:257–267. [DOI] [PubMed] [Google Scholar]
- 6.Bosch, T. 2003. Ancient signals: peptides and the interpretation of positional information in ancestral metazoans. Comp. Biochem. Physiol. B. 136:185–196. [DOI] [PubMed] [Google Scholar]
- 7.Genikhovich, G., U. Kürn, G. Hemmrich, and T. Bosch. 2006. Discovery of genes expressed in Hydra embryogenesis. Dev. Biol. 289:466–481. [DOI] [PubMed] [Google Scholar]
- 8.Gierer, A., and H. Meinhardt. 1972. A Theory of biological pattern formation. Kybernetik. 12:30–39. [DOI] [PubMed] [Google Scholar]
- 9.Meinhardt, H. 1993. A model for pattern formation of hypostome, tentacles and foot in Hydra: how to form structures close to each other, how to form them at a distance. Dev. Biol. 157:321–333. [DOI] [PubMed] [Google Scholar]
- 10.Sherratt, J., P. Maini, W. Jäger, and W. Müller. 1995. A receptor-based model for pattern formation in Hydra. Forma. 10:77–95. [Google Scholar]
- 11.Berking, S. 2003. A model for budding in Hydra: pattern formation in concentric rings. J. Theor. Biol. 222:37–52. [DOI] [PubMed] [Google Scholar]
- 12.Shimizu, H., Y. Sawada, and T. Sugiyama. 1993. Minimum tissue size required for Hydra regeneration. Dev. Biol. 155:287–296. [DOI] [PubMed] [Google Scholar]
- 13.Sato-Maeda, M., and H. Tashiro. 1999. Development of oriented motion in regenerating Hydra cell aggregates. Zoolog. Sci. 16:327–334. [Google Scholar]
- 14.Mombach, J., R. de Almeida, G. Thomas, A. Upadhyaya, and J. Glazier. 2001. Burst and cavity formation in Hydra cells aggregates: experiments and simulations. Physica A. 297:495–508. [Google Scholar]
- 15.Wittlieb, J., K. Khalturin, J. U. Lohmann, F. Anton-Erxleben, and T. C. G. Bosch. 2006. Transgenic Hydra allow in vivo tracking of individual stem cells during morphogenesis. Proc. Natl. Acad. Sci. USA. 103:6208–6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kataoka, N., K. Saito, and Y. Sawada. 1999. NMR microimaging of the cell sorting process. Phys. Rev. Lett. 82:1075–1078. [Google Scholar]
- 17.Rieu, J., N. Kataoka, and Y. Sawada. 1998. Quantitative analysis of cell motion during sorting in 2D aggregates of Hydra cells. Phys. Rev. E Stat. Phys. Plasmas Fluids Relat. Interdiscip. Topics. 57:924–931. [Google Scholar]
- 18.Rieu, J., A. Upadhyaya, J. Glazier, and Y. Sawada. 2000. Diffusion and deformations of single Hydra cells in cellular aggregates. Biophys. J. 79:1903–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Upadhyaya, A., J. Rieu, J. Glazier, and Y. Sawada. 2001. Anomalous diffusion and non-Gaussian velocity distribution of Hydra cells in cellular aggregates. Physica A. 293:549–558. [Google Scholar]
- 20.Rieu, J., and Y. Sawada. 2002. Hydrodynamics and cell motion during the rounding of two-dimensional Hydra cell aggregates. Eur. Phys. J. B. 27:167–172. [Google Scholar]
- 21.Soriano, J., C. Colombo, and A. Ott. 2006. Hydra molecular network reaches criticality at the symmetry-breaking axis-defining moment. Phys. Rev. Lett. 97:258102. [DOI] [PubMed] [Google Scholar]
- 22.Rohlf, T., and S. Bornholdt. 2005. Self-organized pattern formation and noise-induced control based on particle computations. J. Stat. Mech. 12:L12001. [Google Scholar]
- 23.Rohlf, T., and S. Bornholdt. 2005. Morphogenesis by coupled regulatory networks. Preprint: q-bio.MN/0401024. [DOI] [PubMed]
- 24.Beloussov, L., N. Kazakova, N. Luchinskaya, and V. Novoselov. 1997. Studies in developmental cytomechanics. Int. J. Dev. Biol. 41:793–799. [PubMed] [Google Scholar]
- 25.Fütterer, C., C. Colombo, F. Jülicher, and A. Ott. 2003. Morphogenetic oscillations during dynamical symmetry breaking of a cluster of Hydra vulgaris cells. Europhys. Lett. 64:137–143. [Google Scholar]
- 26.Rankin, J., and J. Davenport. 1981. Animal Osmoregulation. Blackie, Glasgow and London.
- 27.Benos, D., R. Kirk, W. Barba, and M. Goldner. 1977. Hyposmotic fluid formation in Hydra. Tissue Cell. 9:11–22. [DOI] [PubMed] [Google Scholar]
- 28.Zeuthen, T. 1992. From contractile vacuole to leaky epithelia. Coupling between salt and water fluxes in biological membranes. Biochim. Biophys. Acta. 1113:229–258. [DOI] [PubMed] [Google Scholar]
- 29.Lilly, S. 1956. Osmoregulation and ionic regulation in Hydra. J. Exp. Biol. 32:423–439. [Google Scholar]
- 30.Marcum, B., and R. Campbell. 1978. Development of Hydra lacking nerve and interstitial cells. J. Cell Sci. 29:17–33. [DOI] [PubMed] [Google Scholar]
- 31.Wanek, N., B. Marcum, H. Lee, M. Chow, and R. Campbell. 1980. Effect of hydrostatic pressure on morphogenesis in nerve-free Hydra. J. Exp. Zool. 211:275–280. [Google Scholar]
- 32.Park, H., A. Ortmeyer, and D. Blankenbaker. 1970. Cell division during regeneration in Hydra. Nature. 227:617–619. [DOI] [PubMed] [Google Scholar]
- 33.Chu, Y.-S., O. Eder, W. Thomas, I. Simcha, F. Pincet, A. Ben-Ze'ev, E. Perez, J. Thiery, and S. Dufour. 2006. Prototypical type I E-cadherin and type II cadherin-7 mediate very distinct adhesiveness through their extracellular domains. J. Biol. Chem. 281:2901–2910. [DOI] [PubMed] [Google Scholar]
- 34.Goidin, J. 2001. Contributions to a study of morphogenesis in fresh water Hydra. Thesis. University of Bayreuth.
- 35.Chain, B. 1980. The transepithelial potential and osmotic regulation in the green Hydra. J. Exp. Biol. 88:161–173. [Google Scholar]






