A Morphological Analysis of the Cerebellar Deficit Hypothesis of Dyslexia Developmental dyslexia has been defined as a disorder in children who fail to attain reading and spelling skills commensurate with their intellectual abilities despite conventional classroom experience1. Along with deficits in reading, children with dyslexia tend to display a variety of cognitive and visual-motor deficits in one or more of the following areas: phonological awareness, rapid phonological retrieval, information processing speed, automaticity, motor skills, and balance2. Some also present with reduced verbal short-term/working memory3, 4 and linguistic functioning5. Most causal theories of dyslexia make a satisfactory attempt at explaining the primary behavioral symptom – poor word identification; however, deficits frequently go beyond reading and may vary across individuals. Thus, the crucial test of these theories needs to address common neuropsychological deficits in addition to poor reading ability2.
Currently, there are several theories of dyslexia. Three widely researched theories include the phonological deficit hypothesis, the double deficit hypothesis, and the cerebellar deficit hypothesis. The phonological deficit hypothesis suggests that reading deficits can be attributed to a core deficit in phonological awareness, or the ability to determine the constituent sounds which comprise spoken words. This deficit in phonological awareness leads to difficulty learning grapheme-phoneme correspondence early on and to later difficulty learning decoding skills6-9. According to the double deficit hypothesis, some children with reading problems have poor phonological awareness skills while others have difficulty rapidly retrieving phonemes and words from long-term memory10 (rapid naming). Poor phonological awareness leads to reduced decoding accuracy; whereas poor rapid naming leads to slow reading rate. A double deficit occurs when children have both types of problems; these children tend to have the most severe reading deficits according to the theory and are most likely to develop dyslexia10. The cerebellar deficit hypothesis states that children with dyslexia have an automatization deficit and reduced speed of processing, along with motor and oral-motor deficits, because of a cerebellum that does not function comparably to typically developing children. These deficits lead to subsequent problems in cognitive processing and reading2,11. According to Nicolson and Fawcett, a deficit in cerebellar performance provides a complete explanation for the range of problems demonstrated by children with dyslexia, and provides a better explanation of their deficits than the phonological deficit and double deficit theories2,11,12. This manuscript seeks to examine the cerebellar deficit hypothesis through studying the morphology of the cerebellum and its relationship to cognition.
Nicolson and Fawcett have proposed two mechanisms by which the cerebellum may play a role in dyslexia2,11. One route is related to the motor theory of speech perception13, 14, which suggests recognition of the phonological units of words is based upon inferring the corresponding articulatory gestures. According to Nicolson and colleagues2, cerebellar dysfunction leads to mild motor problems in the infant, which lead to articulation difficulties. Poor quality articulatory representations lead to impaired sensitivity to the phonemic structure of language and to reduced phonological awareness2,15. In addition, decreased articulation speed can reduce verbal short-term/working memory functioning, as subvocal rehearsal is important in keeping memory traces in the store2,16. Reduced verbal working memory functioning may cause difficulties with language acquisition17, 18. The other route is related to processing speed. Cerebellar dysfunction may lead to reduced processing speed, which would affect cognitive functioning on a more global scale than merely producing deficits in phonological processing11. Based upon these two routes, the cerebellar deficit hypothesis attempts to explain the phonological deficit hypothesis and the double deficit hypothesis2. The oral-motor difficulties lead to deficits in phonological awareness whereas the processing speed deficits lead to difficulties with rapid naming. The cerebellum in particular may be involved with rapid naming given its role in speech, inner speech, and speeded processing.
Several studies have focused upon the cerebellum recently in their investigation of individuals with dyslexia. Rae and colleagues19 found the cerebellar hemispheres to be symmetric in adult males with dyslexia but asymmetric in controls. Controls had larger right cerebellar hemispheres than left when analyzing gray matter. For those with dyslexia, the more symmetric the cerebellar hemispheres were, the greater the deficits in phonological decoding. Leonard and colleagues20 found adults with phonological dyslexia to differ from controls and adults with other types of dyslexia in four key brain regions, one of which was marked leftward asymmetry of the anterior lobe of the cerebellum. When the four brain measures were normalized and summed into a single variable, it predicted phonological memory. In a follow-up study focused upon children with dyslexia, Eckert and colleagues21 found children with dyslexia to have smaller right anterior lobes of the cerebellum, pars triangularis bilaterally, and cerebral volume. Measurements of the right anterior lobe of the cerebellum and bilateral pars triangularis classified 72% of children with dyslexia and 88% of controls correctly. These measurements also were correlated with reading, spelling and language ability.
In general, there is now a considerable body of evidence that supports the role of the cerebellum in cognition from neuroimaging studies, most of which are on normal adults22-25, and studies of cerebellar patients26, 27. Much of this research has demonstrated greater right than left cerebellar hemisphere involvement in linguistic functioning, but several studies have found bilateral involvement in cognition and even vermal involvement. Fawcett and colleagues11 hypothesized that the cerebellar regions involved with dyslexia include the lateral posterior lobes of the cerebellum, as lesions here are associated with dysmetria and hypotonia. This region, along with the vermis, showed atypical activation in adults with dyslexia on tasks measuring motor skills2.
While some studies have demonstrated cerebellar involvement in dyslexia, others point to alternative explanations. For example, Ramus and colleagues28 found little support for the cerebellar deficit hypothesis in their behavioral study of adults with dyslexia, but their results did support the phonological deficit hypothesis. Zeffiro and Eden29 stated a cerebellar deficit is not likely the cause of dyslexia, as individuals with developmental dyslexia often do not display significant cerebellar signs such as ataxia and hyptonia in their research. In addition, those with acquired cerebellar damage often do not display reading problems. They suggested that the main source of dysfunction in dyslexia may lie outside of the cerebellum (such as in the perisylvian region), exerting its influence on the cerebellum through the cerebro-cerebellar connections. Thus, according to Zeffiro and Eden, while the cerebellum may appear to be mildly affected in dyslexia, the true deficit may lie outside of the cerebellum causing poor quality input to the cerebellum. The cerebellum is unable to function properly because of this poor input.
Given the controversy surrounding whether or not the cerebellum is involved with dyslexia, this study sought to test whether those with dyslexia differed from those without it in cerebellar anatomy and its relationship to cognition. It was hypothesized that children without dyslexia would present with greater rightward asymmetry compared to those with dyslexia based upon the work of Rae and colleagues19. In addition, it was hypothesized that right cerebellar hemisphere volume would predict performance on measures of phonological awareness, rapid naming, and verbal/phonological short-term memory based upon the possible routes whereby the cerebellum may be involved with dyslexia2 and the neuroimaging and lesion literature implicating greater right than left cerebellar hemisphere involvement in these functions. In general, the hypotheses on the cerebellar contribution to cognition are exploratory given the small sample size (n = 20 per cell).
This study includes children with dyslexia and ADHD. Approximately 15−40% of children with dyslexia meet criteria for ADHD30, 31, and around 10−30% of children with ADHD also have dyslexia30,31. Given this high comorbidity, findings are more generalizable to the population of children with dyslexia at large if those children with comorbid dyslexia and ADHD are included in the sample. In addition, the cerebellum has been suggested to play a role in ADHD. For example, structural MRI studies have found children with ADHD to have a smaller posterior-inferior vermis than controls32-34, with Castellanos and colleagues33 finding a significant correlation between posterior-inferior vermis morphology and several ratings of ADHD severity. Durston and colleagues35 found boys with ADHD to have reduced right cerebellar volume but not their siblings. They concluded that reduced cerebellum volume may be directly related to the pathophysiology of the disorder. In order to control for the specific effects that ADHD may have on cerebellar morphology, presence of ADHD was distributed evenly in numbers and severity across groups: those with and without dyslexia. In addition, it was hypothesized that those with and without ADHD would differ on poster-inferior vermis volume and right cerebellar hemisphere volume. Asymmetry was not expected to differ between groups, in contrast to those with and without dyslexia. Furthermore, it was hypothesized that size of the posterior-inferior vermis and right cerebellar hemisphere would correlate with measures of ADHD severity. This hypothesis also is exploratory given the small sample size.
Method
Participants
Participants were selected from consecutive referrals to a grant funded, university-based lab that also provided clinical services in the form of neuropsychological assessment. This project was part of a larger study funded by the National Institutes of Health (R01 HD26890-07), and data analysis and write-up was partially supported by another NIH grant (R03 HD048752-02). Participants included 40 children between the ages of 8−12 years (20 with dyslexia and 20 without dyslexia). 9 participants had dyslexia, 11 had dyslexia and ADHD, 11 had ADHD, and 9 were typically developing controls. This resulted in 20 children with dyslexia and 20 without it, as well as 22 children with ADHD and 18 without it. The group without dyslexia was 95% Caucasian and 70% male. The group with dyslexia was 95% Caucasian and 75% male. The group with ADHD was 96% Caucasian and 73% male; the group without ADHD was 94% Caucasian and 72% male. Exclusionary criteria required that all participants had no medical condition (except asthma or allergies), neurological impairment or psychiatric disorder (except ADHD) and that their measured intelligence was greater than 80. As per parent report, no child was on medication for ADHD at the time of evaluation.
Neuropsychological Evaluation
Measures
All participants underwent a neuropsychological evaluation as part of the study. The evaluation included measures of intelligence, academic achievement, and linguistic ability. Intelligence was assessed through the Wechsler Intelligence Scale for Children – Third Edition36. The Elision and Reversals subtests from the Comprehensive Test of Phonological Processing – Experimental Version (CTOPP; Torgesen & Wagner, unpublished test) were used to assess phonological awareness. Both subtests require analysis and synthesis of sounds and/or syllables, thus assessing more complex phonological processing. Digit Span from the WISC-III was used as a measure of verbal/phonological short-term memory. The Rapid Automatized Naming test37, 38 (RAN) was used to assess rapid phonological and lexical retrieval. Handedness was measured through the Edinburgh Handedness Inventory39.
Dyslexia
Dyslexia was diagnosed following State of Georgia criteria for a learning disability. Nonetheless, professional judgment was exercised as to the best measure of intelligence, as noted below. Participants had to have a 20-point standard score discrepancy between intelligence as assessed by the WISC-III and academic achievement in reading (i.e., word identification). FSIQ was used as the measure of intelligence unless a significant discrepancy of 12 or more points occurred between VIQ and PIQ. In this case, the higher IQ score was used as the measure of intelligence. Word identification was evaluated through the Reading subtest of the Wide Range Achievement Test –Third Edition (WRAT-3) 40. Spelling and Arithmetic also were assessed using the WRAT-3. Phonological decoding was assessed through the Word Attack subtest from the Woodcock Reading Mastery Test – Revised (WRMT-R) 41. Reading comprehension was determined through the Passage Comprehension subtest from the WRMT-R. While a word identification deficit was the defining feature for diagnosis, 70% of children with dyslexia also had a 20 point split between IQ and Word Attack and 95% of those with dyslexia had a split between IQ and Passage Comprehension.
The definition of dyslexia has become a source of great debate in the literature, with some arguing that IQ should be irrelevant to the definition based upon literature finding comparable phonological processing abilities in poor readers meeting the discrepancy definition and poor readers not meeting it42-44. Nonetheless, some studies have demonstrated a relationship between brain structure and intelligence45-47. As IQ may play a role in brain morphology, a discrepancy definition, which includes IQ, was used. The use of a discrepancy definition is consistent with other research on brain morphology in dyslexia11,21,47,48 and the World Federation of Neurology's1 definition of dyslexia.
ADHD
The diagnosis of ADHD was made through a multi-modal procedure using multiple informants, including parent and teacher feedback and examiner observations. Diagnosis was made according to the criteria outlined in the Diagnostic and Statistical Manual-Fourth Edition49. Parent and teacher input was obtained through questionnaires including the Behavior Assessment System for Children, Parent and Teacher forms50 (BASC); the Child Behavior Checklist51 (CBCL) and the Child Behavior Checklist-Teacher Report Form52 (TRF); and the SNAP checklist53. Parents also participated in a semi-structured interview using the Schedule for Affective Disorders and Schizophrenia for School-Age Children54, updated with DSM-IV criteria. This interview addressed onset, duration and setting of symptoms as well as their presence or absence. To be diagnosed with ADHD, the clinical interview findings had to be consistent with DSM-IV criteria and the child's scores on two of the three measures (CBCL, BASC, SNAP) had to be significant when the child was assessed off medication. The diagnostic procedure used in this study has been shown to be reliable in similar research using DSM-IV criteria55. Based upon clinical interview and the measures gathered, 20% had ADHD-Predominantly Inattentive Type and 35% had ADHD-Combined Type in the non-dyslexia group and 10% had Predominantly Inattentive Type and 45% had Combined Type in the dyslexia group. The two groups (dyslexia + ADHD, ADHD solely) did not differ in severity of ADHD (ps > .10 on all attention, hyperactivity, and impulsivity measures). The average severity was mild for both groups.
MRI Acquisition
Magnetic Resonance Imaging (MRI) scans were obtained on a 0.6-T Health Images scanner (Atlanta, GA). The protocol included 15 gapless, three-dimensional, 3.1mm slices [TR=51; TE=10 (prior to 9/23/95) or TE=13 (after 9/23/95)]. All children had their MRI scans completed at Athens Magnetic Imaging in Athens, Georgia. These scans were read by a board certified neurologist, and all participants were found to have scans within normal limits.
Cerebellum Measurement
Scans were measured using the public domain software Scion Image, the Windows version of NIH IMAGE56. Images were hand traced using a digitizing tablet. Measurements of the cerebellar hemispheres and vermis were based on previously published guidelines57. Specifically, the hemispheres were measured in the coronal plane on every other slice. The fourth ventricle and vermis were excluded from the hemisphere measurements. In addition, rostral measurements excluded the peduncles. When the borders of the peduncles became diffuse and were no longer distinguishable, its white matter was included in the hemisphere measurements. This diffusion coincided with the most rostral slice that showed the anterior vermis. Volumes were calculated using Cavalieri's rule to correct for overprojection58.
The vermis was measured in the sagittal plane, on the midsagital slice and one slice lateral to it on either side. The anterior lobe consisted of the lobules I-V, the superior posterior lobe consisted of the VI and VII lobules, and the inferior posterior lobe consisted of the VIII-X lobules. The anterior border of the anterior lobe was the superior medullary velum, and the primary fissure served as its posterior border. The anterior border of the superior posterior vermis lobe was the primary fissure, and the prepyramidal fissure was the posterior border. The posterior inferior vermis's anterior border was the prepyramidal fissure and the posterior border was the velum. The tonsils were excluded from the vermis measurements. Two people individually conducted the measurements blind to group membership (JBF and RM) with excellent inter-rater reliability (r =.99, p < .001). See Figures 1 and 2 for depictions of the hemisphere and vermis measurements, respectively.
Figure 1.
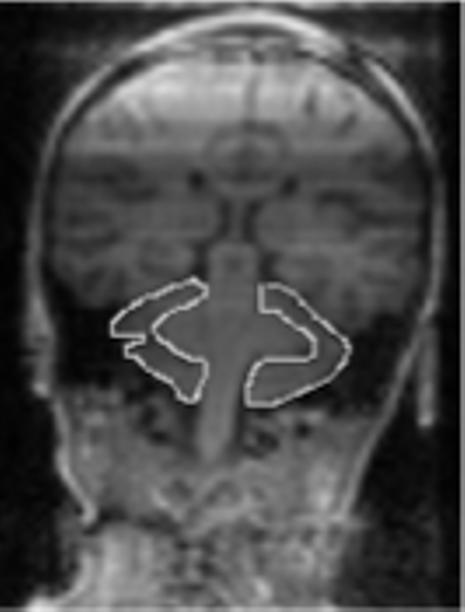
Depiction of hemisphere measurements
Figure 2.
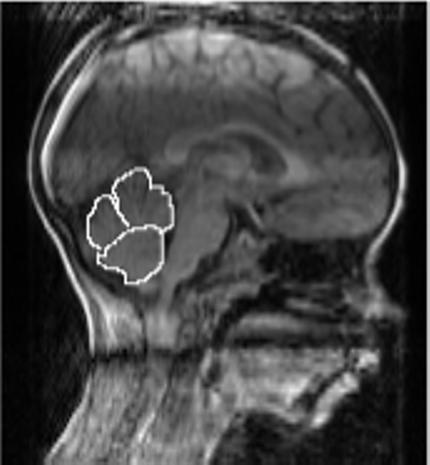
Depiction of vermis measurements
Similar to the work of Leonard and colleagues59 and Gauger, Lombardino, and Leonard60, an interhemishperic coefficient of asymmetry for the cerebellar hemispheres was derived from the following formula: (L-R)/[(L+R)*0.5)]. A negative value indicated rightward asymmetry and a positive value indicated leftward asymmetry61.
Cerebrum Measurement
Cerebral hemisphere volume was assessed to ensure any differences between groups on the cerebellar measurements were not merely due to variations in overall brain size. Measurements of the cerebral hemispheres were based on a modified approach of Leonard et al.62 and Raz et al.63. Specifically, right and left hemispheres were measured separately. Every 4th slice was measured in the coronal plane, starting at the most rostral slice in which the hemisphere was detectable and continuing until it was no longer present caudally. Measurements included all gray and white matter encompassed by the dura, but excluded the ventricles, corpus callosum, fornix, septum pallusidum, and the optic nerve, tract, and chiasm. Volume was calculated using Cavalieri's rule to correct for overprojection57.
Results
Group Descriptive Information
Children with and without dyslexia were comparable on FSIQ, cerebral hemisphere volume (right and left), age, gender, race, and handedness. Groups also were comparable on PIQ, Perceptual Organization Index (POI), Processing Speed Index (PSI), and Verbal Comprehension Index (VCI) but differed on VIQ [F(1, 37) = 4.65, p < .05] and the Freedom from Distractibility Index (FDI) [F(1, 37) = 12.30, p = .001], with VIQ and FDI being lower in the dyslexia group as would be anticipated. Groups differed on WRAT-3 Reading [F(1,38) = 23.67, p < .001], WRMT-R Word Attack [F(1, 37) = 22.72, p < .001] and WRMT-R Passage Comprehension [F(1, 37) = 17.90, p < .001]. They also differed on WRAT-3 Spelling [F(1, 38) = 21.87, p < .001] and Arithmetic [F(1,38) = 11.67, p < .01]. Descriptive statistics are presented in Table 1. In terms of those with and without ADHD, groups were comparable on all IQ measures, the academic achievement measures, age, gender, race, and handedness (ps > .10). Those with and without ADHD performed within the average range on all of the IQ and achievement measures.
Table 1.
Participant Demographic Data
| Variable | With Dyslexia | Without Dyslexia | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age | 9.55 | 1.03 | 9.89 | 1.26 |
| Edinburgh Handedness | 90.50 | 12.87 | 75.88 | 34.83 |
| WISC-III Full-Scale IQ | 103.58 | 15.39 | 110.90 | 14.49 |
| WISC-III Performance IQ | 105.47 | 14.45 | 106.25 | 10.85 |
| Perceptual Organization Index | 108.53 | 15.68 | 106.55 | 13.64 |
| Processing Speed Index | 94.67 | 12.38 | 101.48 | 11.06 |
| WISC-III Verbal IQ* | 101.68 | 16.32 | 113.50 | 17.80 |
| Verbal Comprehension Index | 103.21 | 16.61 | 112.30 | 17.92 |
| Freedom From Distractibility Index*** | 92.68 | 13.35 | 110.00 | 17.14 |
| WRAT-3 Reading*** | 82.10 | 10.62 | 103.15 | 16.17 |
| WRAT-3 Spelling*** | 84.05 | 9.09 | 102.25 | 14.85 |
| WRAT-3 Arithmetic** | 91.65 | 11.18 | 104.35 | 12.30 |
| WRMT-R Word Attack*** | 80.00 | 11.13 | 101.25 | 16.12 |
| WRMT-R Passage Comprehension*** | 80.58 | 12.35 | 100.55 | 16.68 |
Note. Age is in years. Edinburgh Handedness is measured in percent of tasks performed with the right hand. WISC-III is the Wechsler Intelligence Scale for Children — Third Edition; WRAT-3 is the Wide Range Achievement Test — Third Edition; WRMT-R is the Woodcock Reading Mastery Test — Revised.
p < .05
p < .01
p ≤ .001.
Cerebellum Measurements
FSIQ was used as a covariate, as groups differed on VIQ and FDI, and those with dyslexia tended to perform more poorly on PSI, although not significantly so. Handedness, age, and gender were not used as covariates since groups did not differ on these variables. Despite groups being comparable in right, left and total cerebral hemisphere volume (ps > .10), total cerebral hemisphere volume was used as a covariate in order to control for differences in overall brain size that could contribute to our findings.
A 2 X 2 ANOVA was used to assess for group differences in cerebellar morphology. When looking at main effects, those with dyslexia (referred to as “dyslexia”, including those with dyslexia only and those with dyslexia + ADHD) and those without dyslexia (referred to as “non-dyslexia”, including those with ADHD only and typically developing controls) were comparable on all brain measures except cerebellar hemisphere asymmetry [F(1,33) = 4.09, p < .05], with the non-dyslexia group displaying greater rightward asymmetry. Means and standard deviations of brain measurements for those with and without dyslexia are presented in Table 2. For those with and without ADHD (irrespective of presence of dyslexia), there were no significant main effects for any of the cerebellar measurements (ps > .10). The interactions between the two conditions, dyslexia or not and ADHD or not, also were not significant (ps ≥ .10). Thus, our dyslexia findings do not appear to be impacted by the presence of ADHD.
Table 2.
Descriptive Data on the Cerebrum and Cerebellum
| Variable | With Dyslexia | Without Dyslexia | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Cerebrum | ||||
| Left Hemisphere | 1576706.99 | 134079.01 | 1565855.15 | 139465.23 |
| Right Hemisphere | 1549432.29 | 129412.22 | 1538974.65 | 142320.20 |
| Cerebellum | ||||
| Left Hemisphere | 103496.89 | 11240.48 | 102124.01 | 14154.92 |
| Right Hemisphere | 104315.01 | 11113.89 | 105698.59 | 13602.97 |
| Asymmetry Ratio* | −0.026 | 0.04 | −0.051 | 0.03 |
| Anterior Vermis | 4965.01 | 622.52 | 5048.38 | 805.06 |
| Posterior-Superior Vermis | 3059.59 | 729.64 | 3140.75 | 722.13 |
| Posterior-Inferior Vermis | 3142.46 | 807.21 | 3594.10 | 752.41 |
Note. Measurements, excluding asymmetry ratio, are volumes in mm3.
p < .05
In order to determine the source of the asymmetry differences between groups, asymmetry was classified as being rightward (a negative ratio ≥ .010 in absolute value), leftward (a positive ratio ≥ .010), or symmetrical (between −.009 and .009), consistent with previous research on asymmetry61,64. Using Chi-Square, those with dyslexia differed from those without it [X2(2) = 7.93, p < .05], with reversed asymmetry only occurring in dyslexia. When looking at the four groups separately, there were no significant differences (p > .10), likely due to power, but those with ADHD only were very similar to controls in the tendency towards rightward asymmetry. See Table 3 for observed frequencies.
Table 3.
Frequencies of Cerebellar Hemisphere Asymmetry Patterns by Group
| Variable | With Dyslexia | Without Dyslexia | ||
|---|---|---|---|---|
| Rightward Asymmetry | 8 | 15 | ||
| Symmetrical | 7 | 4 | ||
| Leftward Asymmetry |
5 |
0 |
||
| Dyslexia | Co-morbid | ADHD | Controls | |
| Rightward Asymmetry | 2 | 6 | 8 | 7 |
| Symmetrical | 4 | 3 | 3 | 1 |
| Leftward Asymmetry | 3 | 2 | 0 | 0 |
Note. Those with and without dyslexia differed in cerebellar asymmetry, X2(2) = 7.93, p < .05.
Relationship between Cerebellum Morphology and Cognition
Correlations were assessed between cerebellar morphology and linguistic ability in an exploratory fashion. Given that those with and without dyslexia differed in morphology of the cerebellum and they differ in many aspects of linguistic ability such as phonological processing and digit span5-9, the relationship between morphology and cognition was assessed within group as brain-behavior relationships may differ between the groups. As can be seen in Table 4, for those without dyslexia, both cerebellar hemisphere volumes were moderately correlated with phonological awareness (Reversals) and short-term memory (Digit Span), and anterior vermis volume moderately correlated with both measures of phonological awareness (Elision and Reversals). Rapid naming was related to asymmetry.
Table 4.
Relationship between Cerebellar Morphology and Phonological Processing in Children without Dyslexia
| Variable | LH | RH | A | AV | PSV | PIV |
|---|---|---|---|---|---|---|
| CTOPP Reversals | .53* | .51* | .29 | .45* | .39 | −.11 |
| CTOPP Elision | .28 | .25 | .26 | .60** | .31 | −.01 |
| WISC-III Digit Span | .54* | .52* | .25 | .35 | .14 | .02 |
| RAN total time | −.11 | −.05 | −.36 | −.11 | −.15 | −.21 |
| RAN total errors | −.44 | −.36 | −.58* | −.08 | −.26 | .04 |
Note. RH = right hemisphere, LH = left hemisphere, A = asymmetry ratio, AV = anterior vermis, PSV = posterior superior vermis, and PIV = posterior inferior vermis. CTOPP is the Comprehensive Test of Phonological Processing — Experimental Version; WISC-III is the Wechsler Intelligence Scale for Children — Third Edition; and RAN is the Rapid Automatized Naming test.
p < .05.
p ≤ .01.
For those with dyslexia, there were few significant correlations, although left and right hemisphere volume moderately correlated with rapid naming errors. The negative correlations suggest those with larger volumes had less errors. Dyslexia results are presented in Table 5.
Table 5.
Relationship between Cerebellar Morphology and Phonological Processing in Children with Dyslexia
| Variable | LH | RH | A | AV | PSV | PIV |
|---|---|---|---|---|---|---|
| CTOPP Reversals | .18 | .07 | .33 | −.01 | .05 | .12 |
| CTOPP Elision | .19 | .06 | .38 | .05 | .25 | −.02 |
| WISC-III Digit Span | .11 | .04 | .23 | .14 | .08 | −.01 |
| RAN total time | −.42 | −.40 | −.05 | −.02 | .06 | −.22 |
| RAN total errors | −.45* | −.44 | −.04 | −.03 | −.25 | −.22 |
Note. RH = right hemisphere, LH = left hemisphere, A = asymmetry ratio, AV = anterior vermis, PSV = posterior superior vermis, and PIV = posterior inferior vermis. CTOPP is the Comprehensive Test of Phonological Processing — Experimental Version; WISC-III is the Wechsler Intelligence Scale for Children — Third Edition; and RAN is the Rapid Automatized Naming test.
p < .05.
It was of interest to determine whether the various asymmetry groups differed in linguistic functioning using ANOVA. Those with symmetry and reversed asymmetry were combined into one group due to small sample size. As can be seen in Table 6, children with and without the typical rightward asymmetry were comparable in linguistic functioning (ps > .10).
Table 6.
Relationship between Cerebellar Asymmetry and Phonological Processing
| Variable | Rightward Asymmetry | Symmetry/Leftward | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| CTOPP Reversals | 3.96 | 4.60 | 6.06 | 4.71 |
| CTOPP Elision | 16.82 | 5.20 | 17.87 | 5.10 |
| WISC-III Digit Span | 9.83 | 3.69 | 9.73 | 3.45 |
| RAN total time | 284.76 | 104.0 | 231.88 | 76.82 |
| RAN total errors | 6.10 | 13.68 | 2.56 | 1.86 |
Note. CTOPP is the Comprehensive Test of Phonological Processing — Experimental Version; WISC-III is the Wechsler Intelligence Scale for Children — Third Edition; and RAN is the Rapid Automatized Naming test. CTOPP and RAN measures are in raw scores: Digit Span is in scaled scores.
* p < .05.
Relationship between Cerebellar Morphology and Symptoms of ADHD
To assess the relationship between ADHD symptom severity and cerebellar morphology, the SNAP parent rating scale was used as it separates inattention, impulsivity, and hyperactivity into separate scales. None of the scales correlated with cerebellar morphology for those without ADHD (ps > .10). This may be related to the fact that there was limited variance as these children had normal levels of attention, activity, and impulse control. In contrast, for those with ADHD (with and without dyslexia), all three SNAP scales were significantly correlated with various aspects of cerebellum morphology (see Table 7). Those with and without dyslexia did not have any significant correlations between cerebellar morphology and the SNAP.
Table 7.
Relationship between Cerebellar Morphology and ADHD Symptoms in Children with ADHD
| Variable | LH | RH | A | AV | PSV | PIV |
|---|---|---|---|---|---|---|
| SNAP Attention | −.41 | −.50* | .16 | −.52* | −.16 | .03 |
| SNAP Hyperactivity | −.51* | −.51* | −.16 | −.47* | −.19 | −.11 |
| SNAP Impulsivity | −.37 | −.38 | −.07 | −.51* | −.10 | −.04 |
Note. RH = right hemisphere, LH = left hemisphere, A = asymmetry ratio, AV = anterior vermis, PSV = posterior superior vermis, and PIV = posterior inferior vermis. SNAP is the Swanson, Nolan, and Pelham checklist, completed by the parent. Higher scores are indicative of a greater number/severity of symptoms.
p ≤ .05.
For those with ADHD only (no dyslexia), correlations were similar, but more consistent with prior literature on ADHD. Posterior inferior vermis volume correlated with SNAP Attention (r = −.67, p < .05) and SNAP Hyperactivity (r = −.80, p < .01), and right hemisphere volume correlated with SNAP Attention (r = −.65, p < .05) and SNAP Hyperactivity (r = −.63, p < .05).
Discussion
Children with dyslexia may display a variety of deficits, including difficulties in phonological awareness, rapid naming, phonological short-term memory, information processing speed, automaticity, motor skills, and balance2. The cerebellar deficit hypothesis attempts to provide a complete explanation for these deficits by suggesting that the cerebellum is the source of dysfunction in dyslexia. This manuscript sought to examine the cerebellar deficit hypothesis through studying the morphology of the cerebellum in children with and without dyslexia and its relationship to linguistic functioning.
Cerebellar Morphology in Dyslexia
Children with and without dyslexia were comparable on all brain measurements except asymmetry, with those without dyslexia displaying greater rightward asymmetry than those with dyslexia. Given that cerebellar morphology did not differ in those with and without ADHD and there was no interaction between the two conditions, these results appear specific to the presence of dyslexia irrespective of comorbid ADHD. Hence, our hypothesis that children without dyslexia would demonstrate greater rightward asymmetry was supported. Moreover, reversed asymmetry occurred solely within the dyslexia group. In general, our findings are consistent with the work of Rae and colleagues19 who found rightward asymmetry in controls and symmetry in adult males with dyslexia.
Cerebellar Morphology in ADHD
Children with ADHD were comparable to those without ADHD on all cerebellar hemisphere and vermis measurements. Hence, our hypothesis stating children with and without ADHD would differ on posterior-inferior vermis volume and right cerebellar hemisphere volume was not supported. Thus, our findings are inconsistent with the work of Berquin and colleagues32 and Mostofsky and colleagues34 who found boys with ADHD to have reduced posterior-inferior vermis volume and with the work of Durston and colleagues35 who found boys with ADHD to have reduced right cerebellar hemisphere volume. This could be due to low power, our sample including boys and girls rather than just boys, and/or our sample being comprised primarily of children with mild ADHD when ADHD was present.
Relationship between Cerebellum Morphology and Cognition
Non-dyslexia
Cerebellar hemisphere volume was moderately correlated with phonological awareness and phonological short-term memory, and asymmetry was moderately correlated with rapid naming ability in terms of errors. This finding is partially consistent with our hypotheses in that right hemisphere volume was related to linguistic functioning. Nonetheless, the left hemisphere also was related to linguistic functioning. Our finding of both cerebellar hemispheres potentially playing a role in linguistic functioning in those without dyslexia is consistent with some functional neuroimaging research and lesion studies65.
In terms of vermis volume, anterior vermis volume was moderately correlated with both measures of phonological awareness (Elision and Reversals). This finding was not predicted in our hypotheses, as Fawcett and colleagues11 proposed more lateral, posterior involvement in the cerebellar deficit hypothesis. Nonetheless, some research on the cerebellum has demonstrated vermis involvement in various aspects of cognitive functioning65. Our results are partially consistent with those of Leonard and colleagues20 who found the anterior cerebellum to be affected in adults with phonological processing problems.
Dyslexia
Both cerebellar hemisphere volumes were moderately correlated with rapid naming errors. The rest of the linguistic variables were not significantly correlated with cerebellum morphology. Implications of these findings are discussed below.
Cerebellar Deficit Hypothesis
Overall, a few of our findings are consistent with the cerebellar deficit hypothesis of dyslexia as proposed by Nicolson and colleagues2. Cerebellar morphology was atypical in several children with dyslexia, as indexed by the asymmetry ratio, and hemisphere volume was moderately correlated with rapid naming ability in dyslexia, with smaller volume being associated with more errors. Of the three areas of phonological processing: phonological awareness, rapid naming, and phonological short-term memory, rapid naming is theorized to be the most strongly related to cerebellum functioning in dyslexia2. Nonetheless, those with symmetry/reversed asymmetry of the cerebellar hemispheres were not significantly different from those with typical rightward asymmetry on any of the linguistic measures, including rapid naming. In addition, the relationship between cerebellum morphology and linguistic functioning in dyslexia appears to be limited given our correlations. Perhaps other brain areas are helping to compensate for a dysfunctional cerebellum in dyslexia and/or other brain areas may have more substantial involvement in linguistic functions in dyslexia. Alternatively, the limited correlations may be due to subtypes, with subtypes of dyslexia having differing relationships to the cerebellum, similar to the work of Leonard and colleagues20. Through a case study approach, we will explore this possibility.
Comparison of Theories
At a behavioral level, our sample is consistent with the phonological deficit hypothesis of dyslexia at first glance, as 70% of children with dyslexia had below average performance on phonological awareness measures, consistent with much of the prior research on dyslexia5-9. However, 30% of our children with dyslexia had average phonological awareness skills, making this explanation incomplete. Our sample is more consistent with the double deficit hypothesis: 50% of the children with dyslexia in our sample had a double deficit, 20% had rapid naming problems without phonological awareness problems and 20% had phonological awareness problems without rapid naming problems (with problems being identified if participants performed below average on the measures). These findings are generally consistent with what the double deficit hypothesis would predict10. However, two children with dyslexia did not have problems with rapid naming or phonological awareness. Thus, while the double deficit hypothesis accounts for more of our cases of dyslexia than the phonological deficit hypothesis, it does not account for all of the cases of dyslexia in our sample.
Our data also is not fully consistent with the cerebellar deficit hypothesis as laid out by Nicolson and colleagues2. Based upon their theory, one would expect children with dyslexia to have a multitude of problems, including poor phonological awareness, poor rapid naming, poor digit span, poor processing speed, and poor coordination/motor functioning. However, only 14/20 children with dyslexia had rapid naming problems, 14 had phonological awareness problems, 9 had poor Digit Span, 5 had motor problems (being below average on both WISC-III Coding and the Developmental Test of Visual-Motor Integration [DTVMI]) and 3 had poor processing speed (being below average on both WISC-III Coding and Symbol Search, with a score of 84 or lower being defined as below average or “poor”). As cerebellum dysfunction should manifest in poor motor functioning and processing speed2, it is of interest that only half of the children with dyslexia performed below average on Coding and even less performed below average on the DTVMI and Symbol Search, although these are not pure measures of motor functioning and/or processing speed. As our data was collected as part of a larger study, we did not have traditional measures of cerebellar functioning with which to compare. Nonetheless, some prior research has found children with dyslexia to perform comparably to controls on cerebellar tasks as a group29. Given that only two children with dyslexia had all of the problems described in the cerebellar deficit hypothesis: poor phonological awareness, rapid naming, digit span, motor functioning, and processing speed, our data does not appear to be fully consistent with the cerebellar deficit hypothesis as described by Nicolson and colleagues2. However, one of these two children had symmetrical hemispheres while the other had reversed asymmetry, suggesting atypical cerebellar asymmetry may have been related to their host of problems. The third child with below average processing speed also had rapid naming problems, poor digit span and symmetrical hemispheres but intact phonological awareness and DTVMI performance. Thus, all children with dyslexia and slow processing speed had atypical cerebellar asymmetry and poor rapid naming, indicating a possible subtype of dyslexia. None of these children had comorbid ADHD. Nonetheless, this subset represents only 25% of the children with dyslexia who had symmetry/reversed asymmetry. Hence, having atypical asymmetry alone does not appear to be sufficient to cause poor processing speed; other etiologies also may be playing a role. When looking at those with poor motor functioning, 4/5 had a double deficit and the other had poor rapid naming. However, 3/5 had typical, rightward asymmetry and good Symbol Search performance and 2/5 had comorbid ADHD, so the subset with poor motor functioning may include different etiologies than the former subgroup. When comparing the children with poor motor functioning to the rest of the sample (poor motor functioning only occurred in dyslexia), they tended to have a smaller posterior-inferior vermis [t(38) = 2.21, p < .05]. Based upon our findings as a whole, the cerebellar deficit hypothesis may help to account for at least 6 of our cases of dyslexia, as 2 children occurred in both subsets: those with processing speed problems and those with motor problems. Given our case study analyses, the variability in relationships between structure and function in dyslexia may have influenced our cerebellar morphology-linguistic functioning correlations in the dyslexia group.
Hence, no one theory accounts for all of our cases of dyslexia given the measures we have. It may be because several of our cases of dyslexia were mild, although a comprehensive theory should account for mild cases as well. Alternately, it may be that there is too much heterogeneity in the etiology of dyslexia for one theory to account for all cases. This would be consistent with intuition and the fact that there are several different theories of dyslexia, each theory accounting for dyslexia at a group level when studied by their respective authors, but none that is universally accepted. It also is consistent with our case study data. There are some theories of dyslexia this paper was not able to examine due to insufficient data (e.g., dual route, connectionist, magnocellular), but given critical research examining these theories, they likely do not account for all cases of dyslexia either. Perhaps there are subtypes of dyslexia, each being better accounted for by one of these theories. Research has not adequately addressed this possibility as it tends to focus on dyslexia at a group level, studying one theory at a time. Clearly more research is needed in this area using a large sample size, a sample comprised of various severity levels, measures taken from each of the various theories, and measures of multiple brain regions to better examine these issues.
Relationship between Cerebellum Morphology and ADHD Symptom Severity
In those with ADHD, anterior vermis volume was moderately correlated with inattention, hyperactivity and impulsivity as measured by the parent SNAP. In addition, right hemisphere volume was moderately correlated with SNAP inattention and hyperactivity. In general, smaller volume was associated with worse symptom severity. In those with ADHD only, posterior-inferior vermis volume and right hemisphere volume were moderately to strongly correlated with inattention and hyperactivity. Thus, correlations between cerebellum morphology and ADHD symptom severity are generally consistent with our hypotheses and prior literature on ADHD and the cerebellum33,35, in that both right cerebellum hemisphere volume and posterior-inferior vermis volume were correlated with ADHD severity, particularly in those with ADHD but not dyslexia. The anterior vermis result is somewhat surprising given prior research has found primarily the posterior-inferior vermis to be affected in ADHD. However, Eckert and colleagues21 found anterior cerebellum volume to be predictive of dyslexia when in combination with other brain regions, and anterior vermis volume was correlated with inattention, hyperactivity and impulsivity when those with dyslexia were included in our ADHD sample. Hence, anterior vermis size may be correlated with ADHD symptom severity when dyslexia is comorbid with ADHD.
It is of interest to examine whether the cerebellum could be one contributor to the comorbidity between ADHD and dyslexia. Only those with and without dyslexia differed in cerebellar morphology. The ADHD* Dyslexia interaction was not significant, and none of the children with ADHD-only or controls had reversed asymmetry. Thus, reversed cerebellum asymmetry may contribute more uniquely to dyslexia. Those with ADHD versus dyslexia/ADHD may differ in their relationships between vermis volume and attention/hyperactivity, with the comorbid group showing a relationship between anterior vermis size and attention/hyperactivity but the ADHD-only group showing a relationship between posterior-inferior vermis size and attention/hyperactivity. Perhaps the anterior vermis is involved with phonological processing and attention, being one potential source of comorbidity between dyslexia and ADHD based upon our correlation results, although groups did not differ in the size of the anterior vermis. Further research should be conducted with a larger sample to look for differing and overlapping etiologies between dyslexia, ADHD, and comorbid dyslexia/ADHD, including the cerebellum.
Study Limitations
Similar to the majority of studies using MRI technology, one of the limiting factors of this study is sample size. Due to our small sample size, our power was not sufficient to test correlation differences between groups to see if true differences exist. Thus, it would be of interest to verify these findings with a larger sample in order to test how brain-behavior relationships differ between groups and to replicate these findings. In addition, it would be interesting to verify these findings using functional neuroimaging techniques.
Another limitation of this study is that data was collected on a weak scanner (0.6T) using 3.1mm slices. This limitation may lead to variability in brain measurements, although it should not lead to systematic group differences. While this limitation could not be addressed in our dataset, it should be noted that inter-rater reliability was very high (r =.99) in spite of this limitation, suggesting excellent reliability on the measurements. Replication of these findings with a better scanner and thinner slice thickness is warranted. In addition, our sample was comprised of children with mild dyslexia and/or ADHD as a group. Results may have been more conclusive and pronounced if we had a sample comprised solely of children with severe dyslexia and/or ADHD.
Conclusions
While some of our findings are supportive of the work by Nicolson and colleagues that suggests the cerebellum is atypical in dyslexia, some children with dyslexia in our sample had typical cerebellar asymmetry; asymmetry groups did not differ in linguistic functioning; and most children with dyslexia did not present with all of the key symptoms described in the cerebellar deficit hypothesis. Therefore, likely more than just the cerebellum is involved with dyslexia and/or there may be subtypes, as discussed earlier. For many children with dyslexia, dyslexia may be due to various combinations of atypical cortical and cerebellar morphology secondary to migration errors in utero20,21,59 and/or reduced numbers of large neurons in various locations66. For example, Leonard and colleagues20 found a combination of four separate brain regions, three cortical and one cerebellar, to be the most predictive of phonological dyslexia. Brown and colleagues67 and Rae and colleagues66 also found several brain regions to be affected in individuals with dyslexia, including the cerebellum. Thus, abnormal cerebellum morphology may strongly contribute to dyslexia in some individuals, mild cerebellar dysfunction when in combination with dysfunction in cortical areas may be involved with others, and some may not have cerebellar abnormalities. Given the bulk of our findings, our study partially supports the work of both Nicolson and colleagues2 and Zeffiro and Eden20. Further research into this line of questioning is indicated.
References
- 1.Neurology WFo Report of research group on dyslexia and world illiteracy. 1968 [Google Scholar]
- 2.Nicolson RI, Fawcett AJ, Dean P. Developmental dyslexia: The cerebellar deficit hypothesis. Trends in Neurosciences. 2001;24:508–511. doi: 10.1016/s0166-2236(00)01896-8. [DOI] [PubMed] [Google Scholar]
- 3.Kibby MY, Marks W, Morgan S, Long CJ. Specific Impairment in Developmental Reading Disabilities: A Working Memory Approach. Journal of Learning Disabilities. 2004;37:349–363. doi: 10.1177/00222194040370040601. [DOI] [PubMed] [Google Scholar]
- 4.Kibby MY. The contribution of phonological processing to verbal working memory span: Focus on children with reading disabilities. Dissertation Abstracts International: Section B: The Sciences and Engineering. 1999 [Google Scholar]
- 5.Pennington BF. Diagnosing Learning Disorders: A Neuropsychological Framework. Guilford; New York: 1991. [Google Scholar]
- 6.Brady S, Shankweiler DP, editors. Phonological processes in literacy: A tribute to Isabelle Y. Liberman. Lawrence Erlbaum Associates, Inc; Hillsdale, NJ: 1991. [Google Scholar]
- 7.Gottardo A, Stanovich KE, Siegel LS. The relationships between phonological sensitivity, syntactic processing, and verbal working memory in the reading performance of third-grade children. Journal of Experimental Child Psychology. 1996;63:563–582. doi: 10.1006/jecp.1996.0062. [DOI] [PubMed] [Google Scholar]
- 8.Shankweiler D, Crain S, Katz L, et al. Cognitive profiles of reading-disabled children: comparison of language skills in phonology, morphology, and syntax. Psychological Science. 1995;6:149–156. [Google Scholar]
- 9.Wagner RK, Torgesen JK, Rashotte CA, et al. Changing relations between phonological processing abilities and word-level reading as children develop from beginning to skilled readers: a 5-year longitudinal study. Dev Psychol. 1997;33:468–479. doi: 10.1037//0012-1649.33.3.468. [DOI] [PubMed] [Google Scholar]
- 10.Wolf M, Bowers PG. The double-deficit hypothesis for the developmental dyslexias. Journal of Educational Psychology. 1999;91:415–438. [Google Scholar]
- 11.Fawcett AJ, Nicolson RI, Maclagan F. Cerebellar tests differentiate between groups of poor readers with and without IQ discrepancy. Journal of Learning Disabilities. 2001;34:119–135. doi: 10.1177/002221940103400203. [DOI] [PubMed] [Google Scholar]
- 12.Fawcett AJ, Nicolson RI, Dean P. Impaired performance of children with dyslexia on a range of cerebellar tasks. Annals of Dyslexia. 1996;46:259–283. doi: 10.1007/BF02648179. [DOI] [PubMed] [Google Scholar]
- 13.Heilman KM, Voeller K, Aw A. Developmental dyslexia: a motor-articulatory feedback hypothesis. Annals of neurology. 1996;39:407–412. doi: 10.1002/ana.410390323. [DOI] [PubMed] [Google Scholar]
- 14.Liberman AM. When theories of speech meet the real world. J Psycholinguist Res. 1998;27:111–122. doi: 10.1023/a:1023289713883. [DOI] [PubMed] [Google Scholar]
- 15.Snowling M, Hulme C. The development of phonological skills. Philos Trans R Soc Lond B Biol Sci. 1994;346:21–27. doi: 10.1098/rstb.1994.0124. [DOI] [PubMed] [Google Scholar]
- 16.Baddeley A. Working memory. Oxford University Press; New York, NY: 1986. [Google Scholar]
- 17.Gathercole SE, Willis C, Baddeley AD. Differentiating phonological memory and awareness of rhyme: Reading and vocabulary development in children. British Journal of Psychology. 1991;82:387–406. [Google Scholar]
- 18.Gathercole SE, Baddeley AD. Working memory and language. Lawrence Erlbaum Associates; Hillsdale, NJ: 1993. [Google Scholar]
- 19.Rae C, Harasty JA, Dzendrowskyj TE, et al. Cerebellar morphology in developmental dyslexia. Neuropsychologia. 2002;40:1285–1292. doi: 10.1016/s0028-3932(01)00216-0. [DOI] [PubMed] [Google Scholar]
- 20.Leonard CM, Eckert MA, Lombardino LJ, et al. Anatomical risk factors for phonological dyslexia. Cerebral Cortex. 2001;11:148–157. doi: 10.1093/cercor/11.2.148. [DOI] [PubMed] [Google Scholar]
- 21.Eckert MA, Leonard CM, Richards TL, Aylward EH, Thomson J, Berninger VW. Anatomical correlates of dyslexia: Frontal and cerebellar findings. Brain. 2003;126:482–494. doi: 10.1093/brain/awg026. [DOI] [PubMed] [Google Scholar]
- 22.Akshoomoff NA, Courchesne E. A new role for the cerebellum in cognitive operations. Behavioral Neuroscience. 1992;106:731–738. doi: 10.1037//0735-7044.106.5.731. [DOI] [PubMed] [Google Scholar]
- 23.Desmond JE, Gabrieli JDE, Wagner AD, Ginier BL, Glover GH. Lobular patterns of cerebellar activation of verbal working-memory and finger-tapping tasks as revealed by functional MRI. Journal of Neuroscience. 1997;17:9675–9685. doi: 10.1523/JNEUROSCI.17-24-09675.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mechelli A, Gorno-Tempini ML, Price CJ. Neuroimaging studies of word and pseudoword reading: Consistencies, inconsistencies, and limitations. Journal of Cognitive Neuroscience. 2003;15:260–271. doi: 10.1162/089892903321208196. [DOI] [PubMed] [Google Scholar]
- 25.Paradiso S, Andreasen NC, O'Leary DS, Arndt S, et al. Cerebellar size and cognition: Correlations with IQ, verbal memory and motor dexterity. Neuropsychiatry, Neuropsychology, & Behavioral Neurology. 1997;10:1–8. [PubMed] [Google Scholar]
- 26.Fiez JA, Petersen SE, Cheney MK, Raichle ME. Impaired non-motor learning and error detection associated with cerebellar damage. A single case study. Brain. 1992;115:155–178. doi: 10.1093/brain/115.1.155. [DOI] [PubMed] [Google Scholar]
- 27.Silveri MC, Leggio MG, Molinari M. The cerebellum contributes to linguistic production: A case of agrammatic speech following a right cerebellar lesion. Neurology. 1994;44:2047–2050. doi: 10.1212/wnl.44.11.2047. [DOI] [PubMed] [Google Scholar]
- 28.Ramus F, Rosen S, Dakin SC, et al. Theories of developmental dyslexia: insights from a multiple case study of dyslexic adults. Brain. 2003;126:841–865. doi: 10.1093/brain/awg076. [DOI] [PubMed] [Google Scholar]
- 29.Zeffiro T, Eden G. The cerebellum and dyslexia: Perpetrator or innocent bystander? Comment from Thomas Zeffiro and Guinevere Eden to Nicholson et al. Trends in Neurosciences. 2001;24:512–513. doi: 10.1016/s0166-2236(00)01898-1. [DOI] [PubMed] [Google Scholar]
- 30.Holborow PL, Berry PS. Hyperactivity and learning difficulties. Journal of Learning Disabilities. 1986;19:426–431. doi: 10.1177/002221948601900713. [DOI] [PubMed] [Google Scholar]
- 31.Shaywitz SE, Fletcher JM, Shaywitz BA. Issues in the definition and classification of attention deficit disorder. Topics in Language Disorders. 1994;14:1–25. [Google Scholar]
- 32.Berquin PC, Giedd JN, Jacobsen LK, et al. Cerebellum in attention-deficit hyperactivity disorder: A morphometric MRI study. Neurology. 1998;50:1087–1093. doi: 10.1212/wnl.50.4.1087. [DOI] [PubMed] [Google Scholar]
- 33.Castellanos FX, Giedd JN, Berquin PC, et al. Quantitative brain magnetic resonance imaging in girls with attention-deficit/hyperactivity disorder. Archives of General Psychiatry. 2001;58:289–295. doi: 10.1001/archpsyc.58.3.289. [DOI] [PubMed] [Google Scholar]
- 34.Mostofsky SH, Reiss AL, Lockhart P, Denckla MB. Evaluation of cerebellar size in attention-deficit hyperactivity disorder. Journal of Child Neurology. 1998;13:434–439. doi: 10.1177/088307389801300904. [DOI] [PubMed] [Google Scholar]
- 35.Durston S, Hulshoff Pol HE, Schnack HG, et al. Magnetic resonance imaging of boys with attention-deficit/hyperactivity disorder and their unaffected siblings. Journal of the American Academy of Child & Adolescent Psychiatry. 2004;43:332–340. doi: 10.1097/00004583-200403000-00016. [DOI] [PubMed] [Google Scholar]
- 36.Wechsler D. Wechsler Intelligence Scale for Children. 3rd ed. The Psychological Corporation; San Antonio: 1991. [Google Scholar]
- 37.Denckla MB, Rudel R. Rapid automatized naming (R.A.N.): Dyslexia differentiated from other learning disabilities. Neuropsychologia. 1976;14:471–479. doi: 10.1016/0028-3932(76)90075-0. [DOI] [PubMed] [Google Scholar]
- 38.Denckla MB, Rudel R. Naming of object drawings by dyslexic children and other learning disabled children. Brain and Language. 1976;3:1–16. doi: 10.1016/0093-934x(76)90001-8. [DOI] [PubMed] [Google Scholar]
- 39.Oldfield RC. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 40.Wilkinson GS. The Wide Range Achievement Test. 3rd ed. Jastak; Wilmington, DE: 1993. [Google Scholar]
- 41.Woodcock RW. Woodcock Reading Mastery Test -- Revised. American Guidance Service; Circle Pines, MN: 1987. [Google Scholar]
- 42.Felton RH, Wood FB. A reading level match study of nonword reading skills in poor readers with varying IQ. Journal of Learning Disabilities. 1992;25:318–326. doi: 10.1177/002221949202500506. [DOI] [PubMed] [Google Scholar]
- 43.Siegel LS. IQ is irrelevant to the definition of learning disabilities. J Learn Disabil. 1989;22:469–478, 486. doi: 10.1177/002221948902200803. [DOI] [PubMed] [Google Scholar]
- 44.Vellutino FR, Scanlon DM, Lyon GR. Differentiating between difficult-to-remediate and readily remediated poor readers: More evidence against the IQ-achievement discrepancy definition of reading disability. Journal of Learning Disabilities. 2000;33:223–238. doi: 10.1177/002221940003300302. [DOI] [PubMed] [Google Scholar]
- 45.Bigler ED. Brain morphology and intelligence. Developmental Neuropsychology. 1995;11:377–403. [Google Scholar]
- 46.Lane AB, Foundas AL, Leonard CM. The evolution of neuroimaging research and developmental language disorders. Topics in Language Disorders. 2001;21:20–41. [Google Scholar]
- 47.Riccio CA, Hynd GW. Measurable biological substrates to verbal-performance differences in Wechsler scores. School Psychology Quarterly. 2000;15:386–399. [Google Scholar]
- 48.Kibby MY, Kroese JM, Morgan AE, Hiemenz JR, Cohen MJ, Hynd GW. The relationship between perisylvian morphology and verbal short-term memory functioning in children with neurodevelopmental disorders. Brain and Language. 2004;89:122–135. doi: 10.1016/S0093-934X(03)00310-9. [DOI] [PubMed] [Google Scholar]
- 49.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Author; Washington, DC: 1994. [Google Scholar]
- 50.Reynolds CR, Kampaus RW. Behavior Assessment System for Children. American Guidance Service; Circle Pines, MN: 1993. [Google Scholar]
- 51.Achenbach TM, Edelbrock C. Child Behavior Checklist. Thomas Achenbach; Burlington, VT: 1983. [Google Scholar]
- 52.Achenbach TM, Edelbrock C. Teacher's Report Form. Thomas Achenbach; Burlington, VT: 1986. [Google Scholar]
- 53.Atkins MS, Pelham WE, Licht MH. A comparison of objective classroom measures and teacher ratings of attention deficit disorder. Journal of Abnormal Child Psychology. 1985;13:155–167. doi: 10.1007/BF00918379. [DOI] [PubMed] [Google Scholar]
- 54.Puig-Antich J, Chambers W. The Schedule for Affective Disorders and Schizophrenia for School-Age Children. New York State Psychiatric Institute; New York: 1978. [Google Scholar]
- 55.Morgan AE, Hynd GW, Riccio CA, Hall J. Validity of DSM-IV ADHD Predominantly Inattentive and Combined Types: Relationship to previous DSM diagnoses/subtype differences. American Academy of Child and Adolescent Psychiatry. 1996;35:325–333. doi: 10.1097/00004583-199603000-00014. [DOI] [PubMed] [Google Scholar]
- 56.National Institutes of Health NIH Image v1.6. Public domain software developed at the U.S. National Institutes of Health. http://rsb.info.nih.gov/nih-image/
- 57.Raz N, Gunning-Dixon F, Head D, Williamson A, Acker JD. Age and sex differences in the cerebellum and the ventral pons: a prospective MR study of healthy adults. AJNR Am J Neuroradiol. 2001;22:1161–1167. [PMC free article] [PubMed] [Google Scholar]
- 58.Rosen GD, Harry JD. Brain volume estimation from serial section measurements: a comparison of methodologies. J Neurosci Methods. 1990;35:115–124. doi: 10.1016/0165-0270(90)90101-k. [DOI] [PubMed] [Google Scholar]
- 59.Leonard CM, Voeller KKS, Lombardino LJ, et al. Anomalous cerebral structure in dyslexia revealed with magnetic resonance imaging. Archives of Neurology. 1993;50:461–469. doi: 10.1001/archneur.1993.00540050013008. [DOI] [PubMed] [Google Scholar]
- 60.Gauger LM, Lombardino LJ, Leonard CM. Brain morphology in children with specific language impairment. Journal of Speech, Language, and Hearing Research. 1997;40:1272–1284. doi: 10.1044/jslhr.4006.1272. [DOI] [PubMed] [Google Scholar]
- 61.Hynd GW, Semrud-Clikeman M, Lorys AR, Novey ES, Eliopulus D. Brain morphology in developmental dyslexia and attention-deficit disorder/hyperactivity. Archives of Neurology. 1990;47:919–926. doi: 10.1001/archneur.1990.00530080107018. [DOI] [PubMed] [Google Scholar]
- 62.Leonard CM, Lombardino LJ, Walsh K, et al. Anatomical risk factors that distinguish dyslexia from SLI predict reading skill in normal children. Journal of Communication Disorders. 2002;35:501–531. doi: 10.1016/s0021-9924(02)00120-x. [DOI] [PubMed] [Google Scholar]
- 63.Raz N, Gunning FM, Head D, et al. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb Cortex. 1997;7:268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- 64.Foster LM, Hynd GW, Morgan AE, Hugdahl K. Planum temporale asymmetry and ear advantage in dichotic listening in developmental dyslexia and attention-deficit/hyperactivity disorder (ADHD). Journal of the International Neuropsychological Society. 2002;8:22–36. [PubMed] [Google Scholar]
- 65.Schmahmann JD, editor. The Cerebellum and Cognition. Academic Press; San Diego, CA: 1997. [Google Scholar]
- 66.Rae C, Lee MA, Dixon RM, et al. Metabolic abnormalities in developmental dyslexia detected by 1H magnetic resonance spectroscopy. Lancet. 1998;351:1849–1852. doi: 10.1016/S0140-6736(97)99001-2. [DOI] [PubMed] [Google Scholar]
- 67.Brown WE, Eliez S, Menon V, Rumsey JM, White CD, Reiss AL. Preliminary evidence of widespread morphological variations of the brain in dyslexia. Neurology. 2001;56:781–783. doi: 10.1212/wnl.56.6.781. [DOI] [PubMed] [Google Scholar]


