SUMMARY
Dorsal closure is a paradigm epithelial fusion episode that occurs late in Drosophila embryogenesis and leads to sealing of a midline hole by bonding of two opposing epithelial sheets. The leading edge epithelial cells express filopodia and fusion is dependent on interdigitation of these filopodia to prime formation of adhesions. Since the opposing epithelia are molecularly patterned there must exist some mechanism for accurately aligning the two sheets across this fusion seam. To address this, we generated a fly in which RFP-moesin and GFP-moesin are expressed in mutually exclusive stripes within each segment using the engrailed and patched promoters. We observe mutually exclusive interactions between the filopodia of engrailed and patched cells. Interactions between filopodia from matching cells leads to formation of tethers between them, and these tethers can pull misaligned epithelial sheets into alignment. Filopodial matching also occurs during repair of laser wounds in the ventral epithelium, and so this behaviour is not restricted to leading edge cells during dorsal closure. Finally, we characterise the behaviour of a patched expressing cell that we observe within the engrailed region of segments A1-A5, and provide evidence that this cell contributes to cell matching.
Keywords: Epithelium, Adhesion, Patterning, Embryo, Wound
INTRODUCTION
The fusion of sheets of epithelial cells is a common event during embryonic development and also occurs during the process of wound healing (Martin and Parkhurst, 2004). Failure of epithelial fusions during human embryonic development give rise to a spectrum of birth defects including spina bifida and cleft palate. A widely used model of epithelial fusion is the process of dorsal closure (DC) which occurs during Drosophila embryogenesis (Harden, 2002; Kiehart, 1999). During DC, two epithelial sheets sweep towards one another over the surface of the embryo and fuse at the dorsal midline to form a continuous epidermis. Live imaging studies have revealed that dynamic needle-like protrusions called filopodia project beyond the leading edges of the epithelial sheets during DC (Jacinto et al., 2000). When filopodia from the two epithelial sheets meet one another, they interdigitate, in a process known as ‘zippering’. Suppressing filopodia formation during DC by expressing dominant negative Cdc42 or disassembling microtubules leads to a failure of fusion, suggesting that zippering is an essential part of the fusion process (Jacinto et al., 2000; Jankovics and Brunner, 2006). Filopodial zippering has also been observed in other systems including cultured keratinocytes and in the embryonic mouse eyelid suggesting it is a universal mechanism for epithelial fusion (Vasioukhin et al., 2000; Zenz et al., 2003).
Embryonic epithelial fusions must occur in a precise fashion when the fusing sheets are patterned, as is the case for neural tube closure in vertebrates and DC in flies. The epithelium of the Drosophila embryo is finely patterned prior to DC and imprecise fusion would disrupt this patterning. Early in development, the embryo is divided into a series of repeating units called parasegments, with the boundary between parasegments forming anterior to stripes of cells expressing the transcription factor Engrailed (Lawrence and Struhl, 1996). Later in embryogenesis, visible segment boundaries form posterior to the engrailed stripes (Larsen et al., 2003). Thus by DC, the embryo is patterned into segments, with each segment being divided into an anterior (A) and a posterior (P) compartment by the parasegment boundary. Engrailed and Hedgehog are expressed exclusively in P compartments (Dahmann and Basler, 2000), while the Hedgehog receptor Patched is expressed exclusively in A compartments (Nakano et al., 1989).
DC occurs with remarkable accuracy, such that patterning is perfectly maintained across the fusion seam at single cell resolution. In order to achieve this level of accuracy, each cell in the leading edge must be able to identify, and specifically fuse with, its matching cell in the opposing epithelial sheet. Interestingly, cell-cell matching is perturbed by genetic interventions that abolish filopodia formation (dominant negative Cdc42, Ena sequentration), suggesting that, in addition to mechanical zippering, filopodia may also play a role in the cell-cell matching that occurs during epithelial fusion (Gates et al., 2007; Jacinto et al., 2000).
Filopodia are widely observed in biology and they often appear to be sensory structures, allowing a cell to explore its environment, searching for guidance cues, other cells, or suitable sites for attachment (Gerhardt et al., 2003; Ribeiro et al., 2002; Ritzenthaler et al., 2000; Zheng et al., 1996). However, while there is much circumstantial evidence that filopodia perform a sensory function, in most cases it is not possible to directly observe this occurring in the living embryo.
We have performed experiments to gain a clearer understanding of how cell-cell matching is achieved during DC. We demonstrate how filopodia contribute to matching and provide clear evidence of sensory and motile functions of filopodia in a live organism. This work also gives new insight into the organisation of the dorsal epithelium in the fly embryo.
MATERIALS AND METHODS
Plasmids
RFP-Moesin-pUASp
DNA encoding mCherry and the actin binding domain of Drosophila moesin (C-terminal 137 residues) were cloned by PCR and inserted into pUASp as KpnI-NotI and NotI-BamHI fragments respectively (Edwards et al., 1997; Rorth, 1998; Shaner et al., 2004). Expression of this construct is non-toxic and co-localisation with GFP-actin confirmed that it effectively labels the actin cytoskeleton (data not shown).
patched-GFP-Moesin expression cassette
DNA encoding the actin binding domain of Drosophila moesin and the SV40 terminator sequence were cloned by PCR and inserted into NotI-BglII and BglII-EcoRI sites of pCASPER2 respectively. 1160bp of Drosophila genomic DNA immediately upstream of the patched coding region was fused to DNA encoding eGFP by PCR and the resulting fragment was cloned into pCR4TOPO (Invitrogen) according to the manufacturer's instructions. Genomic DNA from 11200 to 1160bp upstream of ptc was then inserted upstream of this as a BamHI-NdeI fragment. Finally, the complete patched-GFP fragment was inserted into pCASPER2 upstream of moesin as a BamHI-NotI fragment.
Fly lines
UAS-RFP-moesin and patched-GFP-moesin transgenic lines were generated in a w background by p-element mediated germline transformation. UAS-RFP-moesin and patched-GFP-moesin insertions on 2nd chromosome were recombined with Engrailed-Gal4 (Bloomington stock center) (Brand and Perrimon, 1993). sGMCA (constitutively expressing GFP-moesin) (obtained from Daniel Kiehart) on 2nd chromosome was recombined with Engrailed-Gal4 and UAS-RFP-moesin.(Kiehart et al., 2000)
Imaging
Embryos were dechorionated in bleach then mounted in Voltalef oil under a coverslip. Images were collected on a Leica SP2 or SP5 confocal microscope and processed using ImageJ, Photoshop (Adobe) and Volocity (Improvision). Wounding was performed using a Spectra-Physics nitrogen laser.
RESULTS AND DISCUSSION
Expression pattern of ptc-GFP-moe and en-RFP-moesin
In order to directly observe cell matching occurring during DC we generated a fly line in which two distinct populations of epithelial cells were labelled with different fluorescent proteins enabling us to compare interactions between like and unlike cells during zippering. The strategy we used for this is illustrated in Fig 1A. P compartments were labelled red by expressing the F-actin binding domain of moesin fused to the red fluorescent protein mCherry (henceforth RFP-moesin) under the control of the engrailed (en) promoter using the UAS-Gal4 system (Brand and Perrimon, 1993; Edwards et al., 1997). Alongside this, A compartments were labelled green by expressing GFP-moesin directly under the control of 11200 bp of sequence upstream of the patched (ptc) coding region. Consistent with the known expression pattern of ptc, the ptc-GFP-moesin transgene was expressed in a 1-2 cell wide stripe at either end of A compartments during DC, thus flanking each en-RFP-moesin stripe (Fig. 1B) (Nakano et al., 1989). Expression of RFP-moesin and GFP-moesin were strictly segregated by parasegment boundaries; however, expression of both fluorophores was sporadically observed in individual cells abutting segment boundaries. The expression pattern of RFP- and GFP-moesin are maintained through DC and perfect matching of red and green stripes along the fusion seam is observed as zippering proceeds (Fig. 1Bi-iii).
Figure 1. Expression pattern of en-RFP-moesin and ptc-GFP-moesin in the dorsal epithelium.
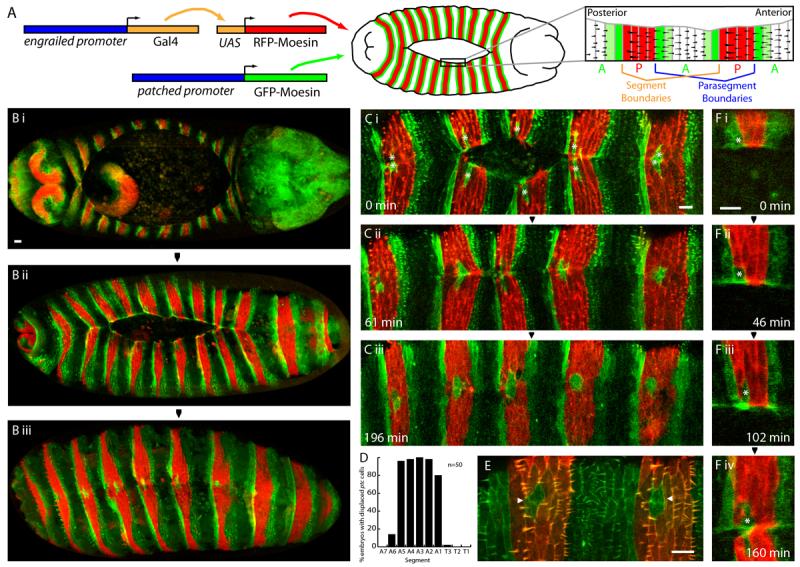
A. Schematic illustrating construction of the fly line, transgene expression pattern and dorsal trichome pattern. B,C,F. Images of embryos expressing en-RFP-moesin (red) and ptc-GFP-moesin (green). B. Dorsal view of embryos at the start (i), midway through (ii), and shortly after completion of DC (iii). C. Time course of the latter stages of DC in a live embryo. Misplaced ptc-GFP-moesin expressing cells (indicated by asterisks in Ci) are present at a conserved position at the leading edge of the en domains of all segments shown. D. Graph showing proportion of embryos with misplaced ptc-GFP-moesin expressing cells in each segment. E. Dorsal view of stage 16 embryo expressing GFP-moesin (green) constitutively and RFP-moesin (red) under control of en-Gal4. Pairs of cells in P compartment not expressing RFP-moesin are indicated by arrowheads. F. Images from movie S1 showing cell rearrangements during DC which lead to the presence of the misplaced ptc cell (indicated by asterisk) in the en domain. Scale bars 10 μm.
Complex patterning of the DC leading edge
Examination of the leading edge cells during and after DC in en-RFP-moesin/ptc-GFP-moesin embryos reveals an unexpected but reproducible aberration in patterning. Isolated cells expressing ptc-GFP-moesin rather than en-RFP-moesin are frequently present within the en domain (Fig. 1C). These misplaced ptc cells are found in a conserved location, towards the posterior end of the en domain and exclusively at the leading edge. On zippering, the misplaced ptc cells fuse with their matching counterpart in the opposing sheet, thus forming an island of two ptc-GFP-moesin expressing cells within the en domain (Fig. 1Ciii). The segmental distribution of the misplaced ptc cells is also well conserved; they are reproducibly present in segments A1-A5, but rarely in other segments (Fig. 1D). In order to identify the origin of the misplaced ptc cells, we used live imaging, commencing at the start of DC. As DC proceeds, a single ptc cell becomes isolated from the anterior edge of the A compartment by dorsal-ward migration of en cells to the leading edge (Fig. 1F, movie S1). Following DC, the pairs of misplaced ptc cells remain within the en stripe. Thus the misplaced ptc cells derive from the A compartment, but ultimately reside in the P compartment due to an epithelial rearrangement. This rearrangement is surprising, since differential adhesion characteristics should prevent mixing of A and P cells.
The arrangement of trichomes on late embryos has been widely used as a morphological readout of epithelial patterning and we were interested to establish the trichome characteristics of the misplaced ptc cells (Payre, 2004). In order to visualise the trichome pattern, GFP-moesin was expressed constitutively, alongside RFP-moesin in en stripes as a positional marker. The trichome pattern of each segment of the dorsal epithelium consists of a broad band of cells possessing trichomes alongside a narrow band with naked cuticle, corresponding to the anterior end of the A compartment (illustrated in Fig. 1A) (Bokor and DiNardo, 1996). Imaging revealed that, in common with the anterior-most cells of the A compartment, the misplaced ptc cells are naked (Fig. 1E). By contrast, the en cells which surround the misplaced ptc cells all possess trichomes. The misplaced ptc cells therefore share the morphological characteristics of the A compartment despite residing within the P compartment.
Filopodia can recognise matching cells
Having characterised the dorsal epithelium in en-RFP-moesin/ptc-GFP-moesin embryos, we then used this fly line to investigate cell matching during DC in live embryos. In these embryos we can clearly see red and green filopodia and observe their behaviour during zippering. Filopodia appear to scan the opposing epithelium and interactions are only observed between filopodia of the same colour (Fig. 2A, movie S2). When filopodia of differing colours come into close proximity with one another, no observable interaction takes place. Notably, both red to red and green to green interactions are observed, indicating that both A and P compartments are actively involved in the matching process. Thus our data suggest that at least two distinct recognition mechanisms mediate cell matching during DC. The misplaced ptc cells within the en domain described above are consistently able to recognise and specifically fuse with one another, indicating that they have recognition properties distinct from their neighbouring en cells.
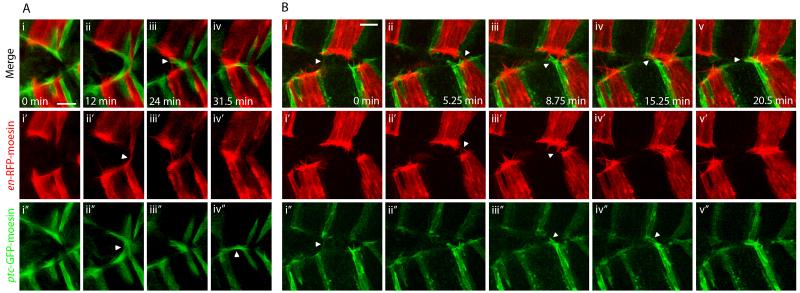
Figure 2. Cell matching and realignment during DC is mediated by filopodia.
Zippering in en-RFP-moesin (red), ptc-GFP-moesin (green) expressing embryos. A. Images from movie S2 showing filopodial matching. i. Red and green filopodia protrude from leading edge cells. ii. Contacts are made between red filopodia from opposing epithelia, while at the same time separate contacts are made between green filopodia. iii. Further contacts are made between red filopodia; however, green filopodia in close proximity to these red filopodial contacts do not interact. iii. Green filopodia transiently form contacts between ptc-GFP-moesin cells over the top of the fused red cells. B. Images from movie S3 showing realignment of misaligned epithelial sheets by filopodial searching and pulling. i. The two epithelial sheets are initially poorly aligned. A filopodial tether transiently exists between green cells but later breaks. ii. Contacts form between filopodia from en-RFP-moesin expressing cells. iii-iv. Green filopodia in the lower sheet do not interact with nearby red filopodia and ultimately form contacts with green cells some distance away in the opposing sheet. v. The tethers that result from the contacts made by red and green filopodia pull the sheets into alignment. Panels labelled ′ and ″ show en-RFP-moesin and ptc-GFP-moesin channels respectively in isolation. The described filopodial interactions are indicated by arrowheads. Scale bars 10 μm.
Filopodial tethers can realign mismatched epithelia
Often the two epithelial sheets are poorly aligned immediately prior zippering and mismatched fusion appears to be likely. Under these conditions we observe that filopodia can identify and bind to matching cells several cell diameters distant in the opposing epithelial sheet (Fig. 2B, movie S3). Having made contact with the appropriate partner, the filopodia form tethers, linking the matching cells together. Contraction of these filopodial tethers then draws matching cells towards one another, correcting the misalignment. These filopodial tethers thus appear to be able to exert sufficient contractile force to drag the entire epithelial sheet. Our data therefore suggests that, in addition to acting as sensory devices, filopodia also play an active role in motility, pulling the cell towards its point of attachment. This is consistent with recent in vitro studies demonstrating that filopodia can exert significant pulling forces (Kress et al., 2007). Realignments such as that shown in fig. 2B are common, with 44% of stripes observed (n=63) exhibiting an adjustment of one cell width or more during zippering. Notably, the filopodial interactions occurring during DC do not necessarily become permanent adhesions; we see at least one tether break on zippering of 42% of stripes observed (n=74) (see Fig. 2Bi, movie S3 for an example). These breakages occur when a tether forms in isolation, unsupported by other tethers pulling in the same direction.
Matching in embryos with leading edge asymmetries
Our data show that filopodia are able to identify matching cells and to pull misaligned epithelia into the correct alignment; however, this is under normal conditions where there is a relatively high level of symmetry in the patterning of the two opposing epithelia. We were curious to know what would happen if the patterning of the two epithelia was asymmetric. We observed that asymmetries occasionally occurred spontaneously, and we imaged DC in several such asymmetric embryos. The embryo shown in Fig. 3A has an extra misplaced ptc cell within an en domain. This extra ptc cell fuses with the neighbouring A compartment and this subsequently pushes the remainder of the segment out of alignment. This results in en cells being bought into close proximity to the opposing en stripe of the neighbouring segment and interestingly, fusion now occurs between these en cells, despite the fact that they reside in different segments. DC subsequently continues as normal but one segment out of register. We have observed a similar sequence of events occurring in three other asymmetric embryos. These observations demonstrate that while matching is specific to the compartment, it is not specific to the segment. Therefore asymmetries that are sufficiently great that filopodia can reach the equivalent compartment of a neighbouring segment have the potential to result in mismatch.
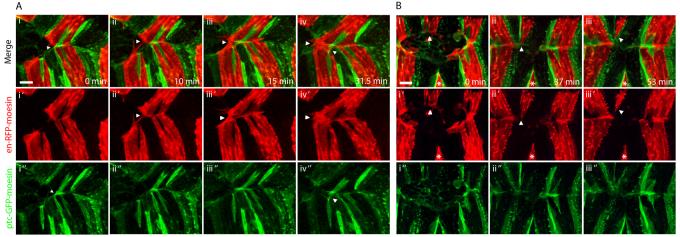
Figure 3. Matching in embryos with asymmetries between the opposing epithelial sheets.
DC zippering in en-RFP-moesin (red), ptc-GFP-moesin (green) embryos with asymmetries. A. Images from movie S4 showing zippering in an embryo with a spontaneous asymmetry. i. The upper epithelial sheet has two rather than one misplaced ptc cell in the en domain and one of these has associated with the A compartment of the lower sheet blocking matching of en cells. ii. As a result, the blocked en cells make filopodial contacts with en cells of the neighbouring segment. iii. These develop into permanent contacts. iv. Cells which are not associated with matching partners continue to produce filopodia. B. Zippering in an embryo with a laser-induced asymmetry. i. An en stripe has been removed from the leading edge of the lower epithelial sheet by laser ablation (indicated by asterisk) while the opposing en stripe (indicated by arrow) is normal. ii-iii. On contact with the opposing leading edge, the unpartnered en stripe constricts and then withdraws completely from the leading edge. Panels labelled ′ and ″ show en-RFP-moesin and ptc-GFP-moesin channels respectively in isolation. Scale bars 10 μm.
We next used laser ablation of leading edge cells to assess the effect of inducing asymmetry in symmetrically patterned embryos. We were able to specifically ablate a single en stripe of leading edge cells from one of the epithelial sheets. The corresponding en stripe of the opposing epithelial sheet no longer has matching cells with which to fuse, and we focused on the behaviour of this unpartnered en stripe on confrontation with the opposing epithelial sheet. The most common outcome (11 of 13 embryos) was that this en stripe became constricted and was effectively extruded from the leading edge, such that the en cells did not participate in zippering (Fig. 2B). In the remaining cases the unpartnered en stripe partially constricted, then fused with the opposing en stripe of the neighbouring segment, as observed in Fig. 2A.
These data demonstrate that when faced with asymmetries that cannot be resolved by filopodial searching and tethering, zippering occurs in such a way that contact between the mismatched cells is minimised.
Matching during epithelial wound healing
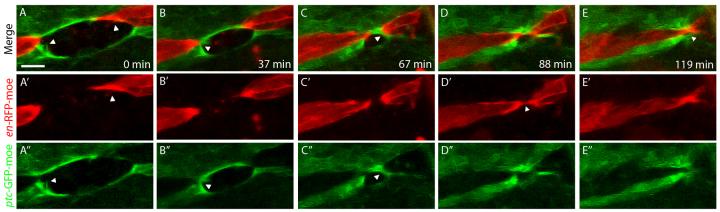
What is the molecular basis of cell matching? The data above is consistent with matching being based on just two sets of molecular interactions, one allowing A compartment cells to recognise one another and the other performing the same function for P compartment cells. An obvious possibility is that the molecules which mediate cell matching during DC are the same as those that maintain the integrity of these compartments throughout the epithelium. Alternatively, there could be a different set of recognition molecules present exclusively at the leading edge to mediate cell-cell matching. Filopodia are also observed during the healing of wounds in the ventral epithelium and we reasoned that these wound filopodia should exhibit matching behaviour if the molecules that mediate matching are present throughout the epithelium (Wood et al., 2002). Laser wounds were made to the ventral epithelium across en stripes such that the wound edge consisted of both en-RFP-moesin and ptc-GFP-moesin cells. On healing of these wounds we see repeated interactions between the filopodia of matching cells, but not between mismatching cells. In the example shown in Fig. 4, a number of filopodial tethers form between ptc cells on opposite sides of the wound as closure proceeds. Near the end of closure, a filopodial tether forms between en cells on opposite sides of the wound, leading to fusion of these cells and regeneration of the en stripe (Fig. 4, movie S5). This sequence of events was observed in 6 out of 10 similar wounds. In the remaining wounds, the tethers between ptc cells became permanent adhesions before the en cells were close enough to form tethers and hence the en stripe was not regenerated. These data suggest that both A and P compartment cells away from the leading edge can carry out filopodial matching analogous to that occurring during DC and hence the adhesion molecules that mediate the process are not leading edge specific.
Figure 4. Filopodial matching occurs during healing of a wound in the ventral epithelium.
Images from movie S5 showing healing of a wound across an en stripe in the epithelium of an en-RFP-moesin (red), ptc-GFP-moesin (green) embryo. A. Filopodia are produced by wound edge cells. B,C. Interactions occur between ptc-GFP-moesin cells across the wound. D,E. Eventually a filopodial tether forms between en-RFP-moesin cells on either side of the wound and this tether rapidly leads to fusion of the two en cells. Notably, the last part of the wound to heal is at the junction between en and ptc cells. The described filopodial interactions are indicated by arrowheads. Scale bar 10 μm.
The data presented here demonstrate that specific recognition events ensure the accuracy of fusion during DC. Filopodia facilitate matching by allowing a cell to search for its match and also to pull misaligned sheets into alignment. This explains why genetic interventions that abolish filopodia lead to an increase in mismatching (Gates et al., 2007; Jacinto et al., 2000). It appears that at least two recognition processes act during DC, one for P compartments and one for A compartment cells, but these recognition events are not segment specific since fusions can occur between matching compartments from different segments. Filopodial matching is also observed during healing of wounds in the ventral epithelium, suggesting that the molecules mediating recognition are found throughout the epithelium. These data are consistent with the notion that the adhesion molecules that mediate filopodial matching during DC are the same as those that ensure compartment integrity throughout the epithelium; however, the identity of these molecules is currently unknown. Experimental and modelling studies have shown that cells can sort based on differential levels of just one adhesion molecule, and it has been hypothesised that a single adhesion molecule may be responsible for compartmental segregation (Dahmann and Basler, 2000). Our data suggest that, at least during filopodial matching, this is not the case, since we observe specific recognition events for both A and P compartments and neither compartment is obviously dominant in the matching process. It is of course possible that multiple mechanisms contribute to cell matching and segregation, perhaps with different adhesion molecules governing the rapid, transient associations between filopodia and the long lived adhesions that hold cells together permanently. While segregation between leading edge A and P compartment cells is absolute at the parasegment boundary, as discussed above, we reproducibly see a single A compartment ptc cell move into the P compartment at the segment boundary. This may suggest that differences in adhesive properties between cells either side of the segment boundary are small, permitting a degree of mixing. However during DC, the misplaced ptc cells are consistently able to recognise and specifically fuse with matching cells in the opposing epithelial sheet, indicating adhesive properties distinct from their neighbours. When the arrangement of the misplaced ptc cells is disrupted, it can result in severe mismatches and therefore correct positioning of these cells is clearly important in epithelial sheet alignment. These cells occupy a unique and defined position in each segment and may assist the matching process by acting as a ‘keystone’ that helps to ensure precise alignment within the segment.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Phil Ingham for providing ptc DNA and Brian Stramer for helpful discussions and technical assistance. THM is funded by a Wellcome Trust advanced training fellowship.
REFERENCES
- Bokor P, DiNardo S. The roles of hedgehog, wingless and lines in patterning the dorsal epidermis in Drosophila. Development. 1996;122:1083–92. doi: 10.1242/dev.122.4.1083. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–15. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Dahmann C, Basler K. Opposing transcriptional outputs of Hedgehog signaling and engrailed control compartmental cell sorting at the Drosophila A/P boundary. Cell. 2000;100:411–22. doi: 10.1016/s0092-8674(00)80677-7. [DOI] [PubMed] [Google Scholar]
- Edwards KA, Demsky M, Montague RA, Weymouth N, Kiehart DP. GFP-moesin illuminates actin cytoskeleton dynamics in living tissue and demonstrates cell shape changes during morphogenesis in Drosophila. Dev Biol. 1997;191:103–17. doi: 10.1006/dbio.1997.8707. [DOI] [PubMed] [Google Scholar]
- Gates J, Mahaffey JP, Rogers SL, Emerson M, Rogers EM, Sottile SL, Van Vactor D, Gertler FB, Peifer M. Enabled plays key roles in embryonic epithelial morphogenesis in Drosophila. Development. 2007;134:2027–39. doi: 10.1242/dev.02849. [DOI] [PubMed] [Google Scholar]
- Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–77. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden N. Signaling pathways directing the movement and fusion of epithelial sheets: lessons from dorsal closure in Drosophila. Differentiation. 2002;70:181–203. doi: 10.1046/j.1432-0436.2002.700408.x. [DOI] [PubMed] [Google Scholar]
- Jacinto A, Wood W, Balayo T, Turmaine M, Martinez-Arias A, Martin P. Dynamic actin-based epithelial adhesion and cell matching during Drosophila dorsal closure. Curr Biol. 2000;10:1420–6. doi: 10.1016/s0960-9822(00)00796-x. [DOI] [PubMed] [Google Scholar]
- Jankovics F, Brunner D. Transiently reorganized microtubules are essential for zippering during dorsal closure in Drosophila melanogaster. Dev Cell. 2006;11:375–85. doi: 10.1016/j.devcel.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Kiehart DP. Wound healing: The power of the purse string. Curr Biol. 1999;9:R602–5. doi: 10.1016/s0960-9822(99)80384-4. [DOI] [PubMed] [Google Scholar]
- Kiehart DP, Galbraith CG, Edwards KA, Rickoll WL, Montague RA. Multiple forces contribute to cell sheet morphogenesis for dorsal closure in Drosophila. J Cell Biol. 2000;149:471–90. doi: 10.1083/jcb.149.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress H, Stelzer EH, Holzer D, Buss F, Griffiths G, Rohrbach A. Filopodia act as phagocytic tentacles and pull with discrete steps and a load-dependent velocity. Proc Natl Acad Sci U S A. 2007;104:11633–8. doi: 10.1073/pnas.0702449104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen CW, Hirst E, Alexandre C, Vincent JP. Segment boundary formation in Drosophila embryos. Development. 2003;130:5625–35. doi: 10.1242/dev.00867. [DOI] [PubMed] [Google Scholar]
- Lawrence PA, Struhl G. Morphogens, compartments, and pattern: lessons from drosophila? Cell. 1996;85:951–61. doi: 10.1016/s0092-8674(00)81297-0. [DOI] [PubMed] [Google Scholar]
- Martin P, Parkhurst SM. Parallels between tissue repair and embryo morphogenesis. Development. 2004;131:3021–34. doi: 10.1242/dev.01253. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Guerrero I, Hidalgo A, Taylor A, Whittle JR, Ingham PW. A protein with several possible membrane-spanning domains encoded by the Drosophila segment polarity gene patched. Nature. 1989;341:508–13. doi: 10.1038/341508a0. [DOI] [PubMed] [Google Scholar]
- Payre F. Genetic control of epidermis differentiation in Drosophila. Int J Dev Biol. 2004;48:207–15. [PubMed] [Google Scholar]
- Ribeiro C, Ebner A, Affolter M. In vivo imaging reveals different cellular functions for FGF and Dpp signaling in tracheal branching morphogenesis. Dev Cell. 2002;2:677–83. doi: 10.1016/s1534-5807(02)00171-5. [DOI] [PubMed] [Google Scholar]
- Ritzenthaler S, Suzuki E, Chiba A. Postsynaptic filopodia in muscle cells interact with innervating motoneuron axons. Nat Neurosci. 2000;3:1012–7. doi: 10.1038/79833. [DOI] [PubMed] [Google Scholar]
- Rorth P. Gal4 in the Drosophila female germline. Mech Dev. 1998;78:113–8. doi: 10.1016/s0925-4773(98)00157-9. [DOI] [PubMed] [Google Scholar]
- Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, Tsien RY. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol. 2004;22:1567–72. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V, Bauer C, Yin M, Fuchs E. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell. 2000;100:209–19. doi: 10.1016/s0092-8674(00)81559-7. [DOI] [PubMed] [Google Scholar]
- Wood W, Jacinto A, Grose R, Woolner S, Gale J, Wilson C, Martin P. Wound healing recapitulates morphogenesis in Drosophila embryos. Nat Cell Biol. 2002;4:907–12. doi: 10.1038/ncb875. [DOI] [PubMed] [Google Scholar]
- Zenz R, Scheuch H, Martin P, Frank C, Eferl R, Kenner L, Sibilia M, Wagner EF. c-Jun regulates eyelid closure and skin tumor development through EGFR signaling. Dev Cell. 2003;4:879–89. doi: 10.1016/s1534-5807(03)00161-8. [DOI] [PubMed] [Google Scholar]
- Zheng JQ, Wan JJ, Poo MM. Essential role of filopodia in chemotropic turning of nerve growth cone induced by a glutamate gradient. J Neurosci. 1996;16:1140–9. doi: 10.1523/JNEUROSCI.16-03-01140.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.