Abstract
Healthy men produce an enormous number of sperms, far more than necessary for conception. However, several studies suggest that semen samples where the concentration of sperms is below 40 mill/mL may be associated with longer time to pregnancy or even subfertility, and specimens where the concentration of sperms is below 15 mill/mL may carry a high risk of infertility. Historic data from the 1940s show that the bulk of young men at that time had sperm counts far above 40 mill/mL with averages higher than 100 mill/mL. However, recent surveillance studies of young men from the general populations of young men in Northern Europe show that semen quality is much poorer. In Denmark approximately 40 percent of the men have now sperm counts below 40 mill/mL. A simulation assuming that average sperm count had declined from 100 mill/mL in ‘old times’ to a current level close to 40 mill/mL indicated that the first decline in average sperm number of 20–40 mill/mL might not have had much effect on pregnancy rates, as the majority of men would still have had counts far above the threshold value. However, due to the assumed decline in semen quality, the sperm counts of the majority of 20 year old European men are now so low that we may be close to the crucial tipping point of 40 mill/mL spermatozoa. Consequently, we must face the possibility of more infertile couples and lower fertility rates in the future.
Keywords: fertility rates, male fecundity, male fertility, male reproductive health, semen quality, testicular cancer, testicular dysgenesis syndrome
Adverse trends in male reproductive health have appeared during the past half century. A world wide increase in testicular germ cell cancer has been firmly established, particularly among Caucasians (Clemmesen, 1981; Huyghe et al., 2003; Bray et al., 2006). In Denmark where the cancer registry was established already in 1943 there has been a four-fold increase in the incidence of this disease (Clemmesen, 1968; Jacobsen et al., 2006) and a young man has now almost a 1% risk of developing a testicular tumour.
Controversies regarding retrospective data on semen quality
Parallel with this development in testis cancer there has been worrying trends in semen quality. In 1992 we published a meta analysis on published data from all parts of the world (Carlsen et al., 1992) showing a decline in sperm counts, which had also been suggested by previous authors, although generally denied by prominent andrologists. The Carlsen paper received considerable attention, but in contrast to the reports on rising incidences in testis cancer, it has also caused controversy (see review by Jouannet et al., 2001).
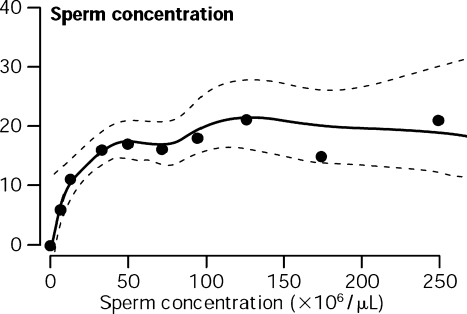
It is an interesting question, why the reports on trends in testis cancer were more or less taken ad notam, whereas the papers on adverse trends in semen quality became controversial (Jouannet et al., 2001), as both trends seem to reflect the same patho-physiological problem: a deteriorating male reproductive health. However, cancer registries are often very complete and reliable, at least in Northern European countries (Adami et al., 1994). In contrast, historic data on semen quality collected for various purposes are obviously less homogeneous mainly due to selection bias and differences in methods causing noise in the analyses (Jouannet et al., 2001). Furthermore, sperm concentration may be drastically reduced without effects on time to pregnancy (Bonde et al., 1998) and thereby fertility rates (Fig. 1). Therefore the apparent decrease in sperm counts and other semen quality variables might neither have been transmitted into a noticeable increase in time to pregnancy nor to an increase in infertility rates.
Figure 1.
From Bonde et al. (1998) Lancet: 430 couples with no previous reproductive experience, aged 20–35 years participated in a study on association between semen quality and the probability of conception in a single menstrual cycle. The couples discontinued use of contraception, and were followed up for six menstrual cycles or until a pregnancy was verified within this period. Each man was asked to provide a semen sample at enrolment. Women kept a daily record of vaginal bleeding and sexual activity. The association between semen quality and likelihood of pregnancy was assessed by logistic regression, adjusted for sexual activity and female factors associated with low fertility. There were 256 (59.5%) pregnancies among the 430 couples: 165 (65.0%) among those with a sperm concentration of 40 mill/mL or more, and 84 (51.2%) among those with lower sperm concentrations. The probability of conception increased with increasing sperm concentration up to 40 mill/mL, but any higher sperm density was not associated with additional likelihood of pregnancy.
Recent prospective studies on trends in male reproductive health
Importantly, retrospective studies on trends in semen quality have recently been followed up by controlled, prospective investigations on several male reproductive health variables in populations, including pathophysiological aspects such as cryptorchidism (Boisen et al., 2004), hypospadias (Virtanen et al., 2001; Boisen et al., 2005) as well as physiological levels of male reproductive hormones (Andersson et al., 2007; Travison et al., 2007) and semen quality among normal men (Andersen et al., 2000; Jørgensen et al., 2001, 2002, 2006; Punab et al., 2002; Richthoff et al., 2002) (Fig. 1). A clear pattern is emerging that semen quality of young men in Northern Europe is generally quite poor (Jørgensen et al., 2008; Paasch et al., 2008) and the incidence of hypospadias and cryptorchidism may be rising, at least in Denmark, where careful studies have been performed (Boisen et al., 2004, 2005). We have been surprised that we year after year have confirmed the presence of extraordinarily poor semen quality among otherwise healthy young men from the general population (Jørgensen et al., 2006). According to Bonde et al. (1998) a semen sample should ideally contain more than 40 mill/mL in order to be optimally fertile. Other recent publications are in line with this estimate. American and European studies suggested 48 mill/mL and 55 mill/mL, respectively as lowest values of the normal range for sperm counts (Guzick et al., 2001; Slama et al., 2002).
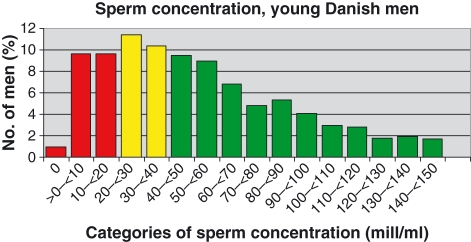
Unfortunately we have few normative data for semen quality from ‘old days’. However, there are some and it is interesting that our great nestors in andrology, Dr. Richard Hammen (Hammen, 1944) in Copenhagen and Dr. John McLeod (MacLeod, 1946) in New York in the 1940s suggested that a normal semen sample should at least contain 60 mill/mL sperms or more. McLeod wrote ‘I think that if we are to select a count level to represent the demarcation line between “poor and fair” fertility that of 60 mill/cc would be a wise choice’. He seemed to base his opinion on an investigation on 100 medical students, of which more than 65 had a sperm count that exceeded 100 mill/mL (MacLeod & Heim, 1945). For comparison we have consistently found that the median young man from the general population of Copenhagen had around 45 mill/mL (Fig. 2). Obviously we cannot control for differences in methods between studies carried out in the 1940s and current investigations. However, the haemocytometers, which were used for counting sperm cells during all years, were similar to those used for estimation of number of blood cells of which there do not appear to have been any trends since the 1940s. In addition MacLeods and Hammen were not the only researchers to report high sperm numbers; another excellent andrologist Hotchkiss in 1941 wrote a paper on semen quality among 200 fertile males with an average sperm number of 121.6 mill/mL, far higher that those of recently studied partners of pregnant women, except those in Finland (Jørgensen et al., 2001). Partners of pregnant women have now generally much lower values, among the lowest were Danish men with a mean sperm count of 76 mill/mL (Jørgensen et al., 2001; Swan et al., 2003) and men from Singapore where the average (geometric mean) was 44.7 mill/mL (Chia et al., 1998).
Figure 2.
Data from a recent surveillance project on semen quality including 3517 young men from the general population in Copenhagen (Jørgensen et al., 2006). The project was carried out during the years 1996–2005. The bars show the percentage of men according to categories of sperm concentration. The median sperm count of the population was 46 mill/mL during this period. Note that 42% of men had sperm counts below 40 mill/mL and therefore may belong to an at risk group of subfertility (Bonde et al., 1998). 20% were even below the WHO demarcation line of for subfertility (20 mill/mL).
Admittedly, McLeod in his later years changed his mind with regard to ‘normal range’ for human semen quality, and lowered the low borderline for ‘normal’ semen to 20 mill/mL (MacLeod & Gold, 1953a,b) while Dr. Alvin Paulsen (Paulsen, 1974) kept the opinion that the demarcation value between fertile and subfertile should be higher (50 mill/mL). Discussions among international andrologists about normal semen were transferred into the committees producing the WHO Guidelines for semen analysis. Since 1980 these committees have had the opinion that the cut off value for a normal semen sample should be 20 mill/mL (World Health Organization, 1980, 1987, 1992; WHO, 1999). However, our recent knowledge from the studies of Bonde et al. (1998), Guzick et al. (2001) or Slama et al. (2002) suggest that it may make more sense to use 40 mill/mL or even a slightly higher value to distinguish between an optimal semen sample and a specimen with reduced ability to conceive. In contrast to this, there are now plans among international andrologists to set a lower limit of normal to 10–15 mill/mL.
However, no matter where we would draw the line between normal and subfertile semen samples, partners of pregnant women most often have more than 40 mill/mL spermatozoa in their ejaculates, and apparently many more sperms than necessary for conception (Jørgensen et al., 2001). This redundancy in male gametes has also been shown in animal studies; spermatogenesis can be grossly disrupted without influencing fertility rates when such males were used for breeding (Ratnasooriya & Sharpe, 1989). But a crucial question is whether we have reached a threshold where the average sperm count in our current populations of young men is so low that we will begin to see an effect on fertility rates.
Are we at the tipping point?
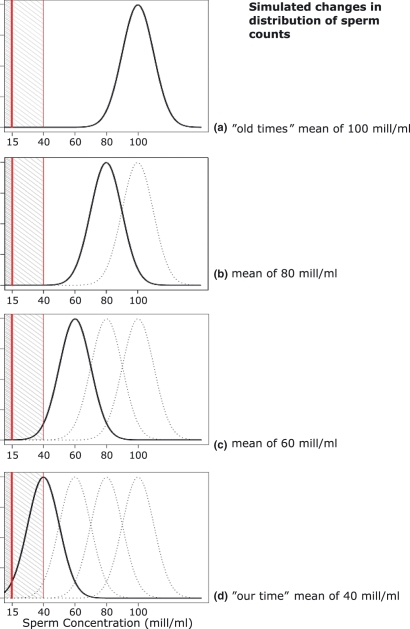
Figure 3 shows a simulation assuming that normal young men in the 1940s had an average sperm count of 100 mill/mL, which should be a plausible assumption considering the old papers by MacLeod (MacLeod & Heim, 1945) and Hotchkiss et al. (Macomber & Saunders, 1929; Hotchkiss et al., 1938). A simulated reduction in average sperm count in steps of 20 mill/mL to 80 mill/mL and even further to 60 mill/mL would not result in a noticeable increase in male subfertility. On the other hand, further reduction in average sperm count of the population to 40 mill/mL (which is rather close to where we are today) would bring many men beyond the tipping point towards subfertility, using thresholds of 15 and 40 mill/mL to demarcate substantial and moderate subfertility, respectively (Bonde et al., 1998; Guzick et al., 2001). Unfortunately, recent studies by Jørgensen et al. indicate that significant proportions of our young populations of men are below this tipping point. Even many young Finnish men, who used to show high sperm counts earlier are now below the demarcation line for good semen quality according to a recent study (Jørgensen et al., 2008).
Figure 3.
Simulation showing a gradual decrease from A to D in average sperm counts of a population from 100 mill/mL (Similar to data from studies of the 1940s) to 40 mill/mL (close to the current situation). For simplicity the simulation is based on the assumption that sperm counts of men in a population are normally distributed, although distributions of sperm counts, particularly in populations with relatively low sperm counts often show a left-skewing of the distribution curves. Note the two hatched areas between the vertical lines demarcating sperm counts of <15 mill/mL (assumed high risk of subfertility) and <40 mill/mL (assumed moderate risk of subfertility). Note that a substantial decrease in sperm count does not significantly affect fecundity until average sperm count is 60 mill/mL. As the simulation (due to lack of skewing of the curves) slightly underestimates the proportion of men with the lowest sperm counts, even more men in populations with averages of 40–60 mill/mL sperms should in reality be within the hatched areas.
Association between decreasing male fecundity and recent low pregnancy rates?
Based on the observed trends in semen quality, Jensen et al. (2007b) tested the hypothesis that an observed decreasing natural pregnancy rate among native Danes may, in fact, be partly due to decreasing male fecundity. This interpretation of the data seems to be supported by a widespread and increasing use of assisted reproductive techniques during the recent years (Andersen & Erb, 2006).
As reproductive biologists we have a great challenge to explore the observed adverse trends in human reproductive health and their causes. An important hindrance for our research is not only that we for obvious reasons cannot obtain experimental human data; we are also faced with the fact that human reproduction is – compared to most animals – a slow process. Approximately 35 years pass between two generations. The time factor is important, as there is more and more evidence that a substantial number of adult male reproductive health problems may be of fetal origin (Skakkebæk et al., 2001). Undescended testis and hypospadias are obviously of fetal origin, but there is now also overwhelming evidence to associate testis cancer with an adverse fetal environment. We have proposed that these conditions often may be part of a testicular dysgenesis syndrome (TDS), which may also include poor spermatogenesis and impaired Leydig cell function. According to our current thinking genetic as well as environmental factors must be taken into account. There is strong evidence from experimental animal studies and investigations of wildlife populations that fetal exposure to endocrine disrupters, including phthalates (Mylchreest et al., 1998; Fisher et al., 2003; Welsh et al., 2007), brominated flame retardants, bisphenol A and PFAS (perfluoralcylated chemicals), can cause TDS like conditions in animals. Humans are exposed to the very same agents through modern lifestyle. The role of these agents in reproductive health problems in humans has not been fully documented, although several recent epidemiological studies have shown associations between maternal exposures to several of these endocrine disrupters and reproductive health problems of their sons of all ages, including adulthood (Jensen et al., 2004, 2007a; Main et al., 2004, 2006, 2007; Swan et al., 2005; Damgaard et al., 2006; Damgaard et al., 2007).
Perspectives
Considering the current historically low fertility rates, which in many industrialized countries are below the levels at which populations can be sustained (Lutz et al., 2003), we find it very important to elucidate the possible contribution of male subfertility for the demographic changes (Skakkebæk et al., 2006). From a biological point of view it is more than plausible that the well documented world wide increase in testis cancer is a ‘whistleblower’ for increasing subfertility (Skakkebæk et al., 2007). Exploration of the patho-physiological mechanisms behind these trends needs much more interdisciplinary research and attention from policy makers. The rapid changes in the male reproductive variables strongly suggest that environmental factors may play a role (Toppari et al., 1996). This assumption is also in accordance with the fact that there are few known causes of low sperm counts, such as Y chromosome deletions and other genetic causes (<10%) (Krausz et al., 2003). In spite of many efforts the vast majority of men with poor semen quality remain without a known cause. This causes often a great deal of concern and anguish among young families. It is almost embarrassing for us andrologists that we can provide so poor answers to infertile young men as to what is behind their abnormal semen quality. If the hypothesis of fetal origin of these reproductive health problems is valid we may not see the full effect of preventive measures within the next 30 years, even if we knew how to instigate them now. We have no time to waste in our efforts to identify the causes of these health problems.
Acknowledgments
We are grateful to Kirsten and Freddy Johansen's Fund for financial support, and senior lecturer Joergen Holm Petersen, Department of Biostatistics for help with the completion of Fig. 3.
References
- Adami H O, Bergström R, Möhner M, Zatonski W, Storm H, Ekbom A, et al. Testicular cancer in nine Northern European countries. International Journal of Cancer. 1994;59:33–38. doi: 10.1002/ijc.2910590108. [DOI] [PubMed] [Google Scholar]
- Andersen A N, Erb K. Register data on assisted reproductive technology (ART) in Europe. Including a detailed description of ART in Denmark. International Journal of Andrology. 2006;29:12–16. doi: 10.1111/j.1365-2605.2005.00577.x. [DOI] [PubMed] [Google Scholar]
- Andersen A G, Jensen T K, Carlsen E, Jørgensen N, Andersson A M, Krarup T, Keiding N, Skakkebæk N E. High frequency of sub-optimal semen quality in an unselected population of young men. Human Reproduction. 2000;15:366–372. doi: 10.1093/humrep/15.2.366. [DOI] [PubMed] [Google Scholar]
- Andersson A M, Jensen T K, Juul A, Petersen J H, Jørgensen T, Skakkebæk N E. Secular decline in male testosterone and sex hormone binding globuline serum levels in danish population surveys. Journal of Clinical Endocrinology and Metabolism. 2007 doi: 10.1210/jc.2006-2633. in press. [DOI] [PubMed] [Google Scholar]
- Boisen K A, Kaleva M, Main K M, Virtanen H E, Haavisto A M, Schmidt I M, et al. Difference in prevalence of congenital cryptorchidism in infants between two Nordic countries. Lancet. 2004;363:1264–1269. doi: 10.1016/S0140-6736(04)15998-9. [DOI] [PubMed] [Google Scholar]
- Boisen K, Chellakooty M, Schmidt I, Kai C, Damgaard I, Suomi A M, Toppari J, Skakkebæk N E, Main K. Hypospadias in a cohort of 1072 Danish newborn boys: prevalence and relationship to placental weight, anthropometrical measurements at birth, and reproductive hormone levels at 3 months of age. Journal of Clinical Endocrinology and Metabolism. 2005;90:4041–4046. doi: 10.1210/jc.2005-0302. [DOI] [PubMed] [Google Scholar]
- Bonde J P E, Ernst E, Jensen T K, Hjollund N H I, Kolstad H, Henriksen T B, Scheike T, Giwercman A, Olsen J, Skakkebæk N E. Relation between semen quality and fertility: a population-based study of 430 first-pregnancy planners. Lancet. 1998;352:1172–1177. doi: 10.1016/S0140-6736(97)10514-1. [DOI] [PubMed] [Google Scholar]
- Bray F, Ferlay J, Devesa S S, McGlynn K A, Møller H. Interpreting the international trends in testicular seminoma and nonseminoma incidence. Nat Clin Pract Urol. 2006;3:532–543. doi: 10.1038/ncpuro0606. [DOI] [PubMed] [Google Scholar]
- Carlsen E, Giwercman A, Keiding N, Skakkebæk N E. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992;305:609–613. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia S E, Tay S K, Lim S T. What constitutes a normal seminal analysis? Semen parameters of 243 fertile men. Human Reproduction. 1998;13:3394–3398. doi: 10.1093/humrep/13.12.3394. [DOI] [PubMed] [Google Scholar]
- Clemmesen J. A doubling of morbidity from testis carcinoma in Copenhagen, 1943–1962. APMIS. 1968;72:348–349. doi: 10.1111/j.1699-0463.1968.tb01350.x. [DOI] [PubMed] [Google Scholar]
- Clemmesen J. Testis cancer incidence – suggestion of a world pattern. International Journal of Andrology. 1981;4 (Suppl):111–122. doi: 10.1111/j.1365-2605.1981.tb00660.x. [DOI] [PubMed] [Google Scholar]
- Damgaard I, Jensen T K The Nordic Cryptorchidism Study Group. Petersen J H, Skakkebæk N E, Toppari J, Main K M. Cryptorchidism and maternal alcohol consumption during pregnancy. Environmental Health Perspectives. 2007;115:272–277. doi: 10.1289/ehp.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damgaard I N, Skakkebæk N E, Toppari J, Virtanen H E, Shen H, Schramm K W, Petersen J H, Jensen T K, Main K M The Nordic Cryptorchidism Study Group. Persistent pesticides in human breast milk and cryptorchidism. Environmental Health Perspectives. 2006;114:1133–1138. doi: 10.1289/ehp.8741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher J S, Macpherson S, Marchetti N, Sharpe R M. Human “testicular dysgenesis syndrome”: a possible model using in-utero exposure of the rat to dibutyl phthalate. Human Reproduction. 2003;18:1383–1394. doi: 10.1093/humrep/deg273. [DOI] [PubMed] [Google Scholar]
- Guzick D S, Overstreet J W, Factor-Litvak P, Brazil C K, Nakajima S T, Coutifaris C, et al. Sperm morphology, motility, and concentration in fertile and infertile men. N Engl J Med JID - 0255562. 2001;345:1388–1393. doi: 10.1056/NEJMoa003005. [DOI] [PubMed] [Google Scholar]
- Hammen R. Studies on Impaired Fertility in Man with Special Reference to the Male. 1. Koebenhavn: Einar Munksgaard; 1944. pp. 1–206. [Google Scholar]
- Hotchkiss R S, Brunner E K, Grenley P. Semen analyses of two hundred fertile men. Amer J Med Sci. 1938;196:362. [Google Scholar]
- Huyghe E, Matsuda T, Thonneau P. Increasing incidence of testicular cancer worldwide: a review. Journal of Urology. 2003;170:5–11. doi: 10.1097/01.ju.0000053866.68623.da. [DOI] [PubMed] [Google Scholar]
- Jacobsen R, Møller H, Thoresen S Ø, Pukkala E, Kjær S K, Johansen C. Trends in testicular cancer incidence in the Nordic countries, focusing on the recent decrease in Denmark. International Journal of Andrology. 2006;29:199–204. doi: 10.1111/j.1365-2605.2005.00605.x. [DOI] [PubMed] [Google Scholar]
- Jensen T K, Jørgensen N, Punab M, Haugen T B, Suominen J, Zilaitiene B, et al. Association of in utero exposure to maternal smoking with reduced semen quality and testis size in adulthood: a cross-sectional study of 1,770 young men from the general population in five European countries. American Journal of Epidemiology. 2004;159:49–58. doi: 10.1093/aje/kwh002. [DOI] [PubMed] [Google Scholar]
- Jensen T K, Jørgensen N, Asklund C, Carlsen E, Holm M, Skakkebæk N E. Fertility treatment and reproductive health of male offspring: a study of 1,925 young men from the general population. American Journal of Epidemiology. 2007a;165:583–590. doi: 10.1093/aje/kwk035. [DOI] [PubMed] [Google Scholar]
- Jensen T K, Sobotka T, Hansen M A, Pedersen A T, Lutz W, Skakkebæk N E. Declining trends in conception rates in recent birth cohorts of native danish women: a possible role of deteriorating male reproductive health. International Journal of Andrology. 2007b doi: 10.1111/j.1365-2605.2007.00827.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen N, Andersen A G, Eustache F, Irvine D S, Suominen J, Petersen J H, et al. Regional differences in semen quality in Europe. Human Reproduction. 2001;16:1012–1019. doi: 10.1093/humrep/16.5.1012. [DOI] [PubMed] [Google Scholar]
- Jørgensen N, Carlsen E, Nermoen I, Punab M, Suominen J, Andersen A G, et al. East-West gradient in semen quality in the Nordic-Baltic area: a study of men from the general population in Denmark, Norway, Estonia and Finland. Human Reproduction. 2002;17:2199–2208. doi: 10.1093/humrep/17.8.2199. [DOI] [PubMed] [Google Scholar]
- Jørgensen N, Asklund C, Carlsen E, Skakkebæk N E. Coordinated European investigations of semen quality: results from studies of Scandinavian young men is a matter of concern. International Journal of Andrology. 2006;29:54–61. doi: 10.1111/j.1365-2605.2005.00635.x. [DOI] [PubMed] [Google Scholar]
- Jørgensen N, Carlsen E, Vierula M, Asklund C, Holm M, Petersen J H, et al. Recent increase in testis cancer incidence among young Finnish men in associated with a decreasing trend in semen quality. International Journal of Andrology. 2008 in press. [Google Scholar]
- Jouannet P, Wang C, Eustache F, Jensen T K, Auger J. Semen quality and male reproductive health: the controversy about human sperm concentration decline. APMIS. 2001;109:48–61. doi: 10.1034/j.1600-0463.2001.090502.x. [DOI] [PubMed] [Google Scholar]
- Krausz C, Forti G, McElreavey K. The Y chromosome and male fertility and infertility. International Journal of Andrology. 2003;26:70–75. doi: 10.1046/j.1365-2605.2003.00402.x. [DOI] [PubMed] [Google Scholar]
- Lutz W, O’Neill B C, Scherbov S. Demographics. Europe's population at a turning point. Science. 2003;299:1991–1992. doi: 10.1126/science.1080316. [DOI] [PubMed] [Google Scholar]
- MacLeod J. The semen Specimen. Laboratory examination. In: Engle E T, editor. Conference on Diagnosis in Sterility. Springfield: Charles C. Thomas; 1946. pp. 3–15. [Google Scholar]
- MacLeod J, Gold R Z. The male factor in fertility and infertility. 7. Semen quality in relation to age and sexual activity. Fertility and Sterility. 1953a;4:194–209. doi: 10.1016/s0015-0282(16)31262-6. [DOI] [PubMed] [Google Scholar]
- MacLeod J, Gold R Z. The male factor in fertility and infertility. VI. Semen quality and certain other factors in relation to ease of conception. Fertility and Sterility. 1953b;4:10–33. doi: 10.1016/s0015-0282(16)31142-6. [DOI] [PubMed] [Google Scholar]
- MacLeod J, Heim L M. Characteristics and variations in semen specimens in 100 normal young men. Journal of Urology. 1945;54:474–482. doi: 10.1016/S0022-5347(17)70101-2. [DOI] [PubMed] [Google Scholar]
- Macomber D, Saunders W B. The spermatozoa count. New Engl J Med. 1929;200:981. [Google Scholar]
- Main K M, Rajpert-De Meyts E, Toppari J, Skakkebæk N E. Endocrine disrupters and development of the reproductive system: a paediatric perspective. In: Pescovitz O H, Eugster E A, editors. Pediatric Endocrinology: Mechanisms. Philadelphia: Lippincott, Williams & Wilkins; 2004. pp. 376–390. [Google Scholar]
- Main K M, Mortensen G K, Kaleva M, Boisen K, Damgaard I, Chellakooty M, et al. Human breast milk contamination with phthalates and alterations of endogenous reproductive hormones in three months old infants. Environmental Health Perspectives. 2006;114:270–276. doi: 10.1289/ehp.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Main K M, Kiviranta H, Virtanen H E, Sundqvist E, Tuomisto J T, Tuomisto J, Vartiainen T, Skakkebæk N E, Toppari J. Flame retardants in placenta and breast milk and cryptorchidism in newborn boys. Environmental Health Perspectives. 2007;115:1519–1526. doi: 10.1289/ehp.9924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylchreest E, Cattley R C, Foster P M. Male reproductive tract malformations in rats following gestational and lactational exposure to Di(n-butyl) phthalate: an antiandrogenic mechanism? Toxicol Sci. 1998;43:47–60. doi: 10.1006/toxs.1998.2436. [DOI] [PubMed] [Google Scholar]
- Paasch U, Salzbrunn A, Glander H J, Salzbrunn H, Grunewald S, Stucke J, Skakkebæk N E, Jørgensen N. Semen quality in sub-fertile range for a significant proportion of young men from the general German population: a co-ordinated, controlled study of 791 men from Hamburg and Leipzig. International Journal of Andrology. 2008 doi: 10.1111/j.1365-2605.2007.00860.x. in press. [DOI] [PubMed] [Google Scholar]
- Paulsen C A. The testes. In: Williams R H, editor. Textbook of Endocrinology. Philadelphia: W.B. Saunders Company; 1974. pp. 323–367. [Google Scholar]
- Punab M, Zilaitiene B, Jørgensen N, Horte A, Matulevicius V, Peetsalu A, Skakkebæk N E. Regional differences in semen qualities in the Baltic region. International Journal of Andrology. 2002;25:243–252. doi: 10.1046/j.1365-2605.2002.00359.x. [DOI] [PubMed] [Google Scholar]
- Ratnasooriya W D, Sharpe R M. Evaluation of the effect of selective germ cell depletion on subsequent spermatogenesis and fertility in the rat. International Journal of Andrology. 1989;12:44–57. doi: 10.1111/j.1365-2605.1989.tb01284.x. [DOI] [PubMed] [Google Scholar]
- Richthoff J, Rylander L, Hagmar L, Malm J, Giwercman A. Higher sperm counts in Southern Sweden compared with Denmark. Human Reproduction. 2002;17:2468–2473. doi: 10.1093/humrep/17.9.2468. [DOI] [PubMed] [Google Scholar]
- Skakkebæk N E, Rajpert-De Meyts E, Main K M. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Human Reproduction. 2001;16:972–978. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- Skakkebæk N E, Jørgensen N, Main K M, Rajpert-De Meyts E, Leffers H, Andersson A M, et al. Is human fecundity declining? International Journal of Andrology. 2006;29:2–11. doi: 10.1111/j.1365-2605.2005.00573.x. [DOI] [PubMed] [Google Scholar]
- Skakkebæk N E, Rajpert-De Meyts E, Jørgensen N, Main K M, Leffers H, Andersson A M, Juul A, Jensen T K, Toppari J. Testicular cancer trends as “whistle blowers” of testicular developmental problems in populations. International Journal of Andrology. 2007;30:198–205. doi: 10.1111/j.1365-2605.2007.00776.x. [DOI] [PubMed] [Google Scholar]
- Slama R, Eustache F, Ducot B, Jensen T K, Jørgensen N, Horte A, et al. Time to pregnancy and semen parameters: a cross-sectional study among fertile couples from four European cities. Human Reproduction. 2002;17:503–515. doi: 10.1093/humrep/17.2.503. [DOI] [PubMed] [Google Scholar]
- Swan S H, Brazil C, Drobnis E Z, Liu F, Kruse R L, Hatch M, Redmon J B, Wang C, Overstreet J W. Geographic differences in semen quality of fertile U.S. males. Environmental Health Perspectives. 2003;111:414–420. doi: 10.1289/ehp.5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan S H, Main K M, Liu F, Stewart S L, Kruse R L, Calafat A M, et al. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environmental Health Perspectives. 2005;113:1056–1061. doi: 10.1289/ehp.8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toppari J, Larsen J C, Christiansen P, Giwercman A, Grandjean P, Guillette L J, Jr, et al. Male reproductive health and environmental xenoestrogens. Environ Health Perspect. 1996;104:741–803. doi: 10.1289/ehp.96104s4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travison T G, Araujo A B, O’donnell A B, Kupelian V, McKinlay J B. A population-level decline in serum testosterone levels in American men. Journal of Clinical Endocrinology and Metabolism. 2007;92:196–202. doi: 10.1210/jc.2006-1375. [DOI] [PubMed] [Google Scholar]
- Virtanen H E, Kaleva M, Haavisto A M, Schmidt I M, Chellakooty M, Main K M, Skakkebæk N E, Toppari J. The birth rate of hypospadias in the Turku area in Finland. APMIS. 2001;109:96–100. doi: 10.1034/j.1600-0463.2001.d01-109.x. [DOI] [PubMed] [Google Scholar]
- Welsh M, Saunders P T, Sharpe R M. The critical time window for androgen-dependent development of the Wolffian duct in the rat. Endocrinology. 2007;148:3185–3195. doi: 10.1210/en.2007-0028. [DOI] [PubMed] [Google Scholar]
- WHO. WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction. 4. Geneva: World Health Organization; 1999. [Google Scholar]
- World Health Organization. Laboratory Manual for the Examination of Human Semen and Semen-Cervial Mucus Interaction. 1. Geneva: World Health Organization; 1980. [Google Scholar]
- World Health Organization. WHO Laboratory Manual for the Examination of Human Semen and Semen-Cervical Mucus Interaction. 2. New York: Cambridge University Press; 1987. pp. 1–67. [Google Scholar]
- World Health Organization. WHO Laboratory Manual for the Examination of Human Semen and Semen-Cervical Mucus Interaction. 3. Cambridge: Cambridge University Press; 1992. [Google Scholar]