Abstract
Peroxisome proliferator-activated receptor-gamma (PPARγ) exerts multiple functions in determination of cell fate, tissue metabolism, and host immunity. Two synthetic PPARγ ligands (rosiglitazone and pioglitazone) were approved for the therapy of type-2 diabetes mellitus and are expected to serve as novel cures for inflammatory diseases and cancer. However, PPARγ and its ligands exhibit a janus-face behaviour as tumor modulators in various systems, resulting in either tumor suppression or tumor promotion. This may be in part due to signaling crosstalk to the mitogen-activated protein kinase (MAPK) cascades. The genomic activity of PPARγ is modulated, in addition to ligand binding, by phosphorylation of a serine residue by MAPKs, such as extracellular signal-regulated protein kinases-1/2 (ERK-1/2), or by nucleocytoplasmic compartmentalization through the ERK activators MAPK kinases-1/2 (MEK-1/2). PPARγ ligands themselves activate the ERK cascade through nongenomic and often PPARγ-independent signaling. In the current review, we discuss the molecular mechanisms and physiological implications of the crosstalk of PPARγ with MEK-ERK signaling and its potential as a novel drug target for cancer therapy in patients.
1. INTRODUCTION
1.1. The janus-face of PPARγ: tumor suppressor versus tumor promoter actions
The metabolic and cell fate regulatory functions of PPARγ place this nuclear receptor (NR) [1, 2] at the cross-road of life style and diabetic comorbidity risks, which are assumed to result from the diet and/or chronic inflammation-induced sequence of preneoplastic lesions towards manifested cancer [3]. Since decades, the association of aberrant insulin signaling in diabetics and increased cancer risk has been stated, and recently validated in patient studies with respect to colon, pancreas, breast, endometrium, prostate, liver, and bladder (see, e.g., [4–7]). Although PPARγ plays an important part in the transmission of insulin responses and physiological diet, little direct evidence exists relating these factors to PPARγ activation and the risks of the development of cancer [6–8]. One of the reasons for the lack of knowledge on the role of PPARγ is that a bona fide high-affinity natural ligand(s) for PPARγ has not been identified yet [2].
PPARγ can be activated by low-affinity ligands such as unsaturated long-chain fatty acids derived from nutrient uptake (e.g., linoleic acid) and/or inflammatory reactions (e.g., 15-deoxy-Δ(12,14)-prostaglandin J2) [9, 10]. However, those do not induce the full activity of PPARγ in most systems examined [2]. As of today, modulation of PPARγ activity is mediated by synthetics drugs, and among them the thiazolidinediones (TZDs) rosi- and pioglitazone are considered to be potent and selective PPARγ agonists [2]. These drugs were approved as insulin sensitizers for the treatment of type-2 diabetes mellitus [11] and have been proven helpful in vascular and atherogenic complications [12, 13]. However, TZD drugs can also exert protumorigenic actions in certain rodent models [14, 15]. In addition, the safety of the TZDs has been recently evaluated in clinical studies aimed to examine cancer prevalence in diabetic patients under TZD use [16–18]. One study stated a significant association of cancer risk in women under any TZD treatment (1003 patients) [17], while the other two stated no significant associations (126,971 patients [16]; 87,678 patients [18]). On the other hand, patients with long-term intake of nonsteroidal anti-inflammatory drugs (NSAIDs), cyclooxygenase (COX) inhibitors that prevent endogenous eicosanoid production and may act also as low-affinity PPARγ ligands, were reported to profit from a reduced risk for colon cancer formation [19].
These paradoxical effects resulting from PPARγ activation are derived from a complex balance of anti-versus protumor functions of PPARγ protein and its ligands in a given system. The latter are also related to the interaction of PPARγ with other oncomodulating proteins (such as MEK1 and βcatenin). In the current review, we will discuss this janus-faced role of PPARγ and its ligands in cancer with a major focus on its crosstalk with the ERK signaling cascade, which is a central signaling pathway deregulated in a majority of tumor types in humans.
1.2. The ERK cascade and cancer
The MAPK cascades are central signaling pathways that mediate the response of essentially all cellular processes stimulated by extracellular ligand, including proliferation, survival, differentiation, apoptosis, stress response, and even oncogenic transformation. Four main cascades have been identified to date, of which the Ras-Raf-MEK1/MEK2-ERK1/ERK2 cascade (ERK cascade) is the most prominent one in human cancers [20, 21]. Its multilevel organisation of kinases guarantees signal amplification and coherence, and its scaffold proteins [22] organize the pathway into a 3D module that enables crosstalk and direct interactions with other central signaling pathways such as the PPARγs.
Within the MAPK family, the ERK cascade constitutes a major signaling pathway, regulating cell proliferation and survival, as well as cell adhesion and motility, differentiation, embryonal development, and neuronal regulation [21, 23]. Its deregulation, mainly due to constitutive upregulation by receptor kinase “gain of function” mutations, contributes to cancer initiation and progression [24–26]. The majority of human carcinomas harbour increased expression or activating point mutations for the upstream components of the ERK cascade (e.g., epidermal growth factor receptor (EGFR/Her1), Her2/Neu/ErbB2, K-Ras, B-Raf) that culminate in a higher ERK activity in a large majority of human tumors. The ERK cascade currently represents the main targeted cascade (next to the angiogenic vascular endothelial growth factor/receptor (VEGF/R) system) by second-generation low molecular weight (LMW) kinase inhibitors (e.g., gefitinib, erlotinib) and monoclonal (humanized) mAbs directed against members of the EGFR family (e.g., herceptin), which are in clinical use against cancer (as reviewed in [25, 27, 28]). Therefore, inhibitors of the ERK cascade are likely to be beneficial in combating most types of cancer.
2. MECHANISMS OF CROSSTALK BETWEEN PPARγ AND THE ERK CASCADE
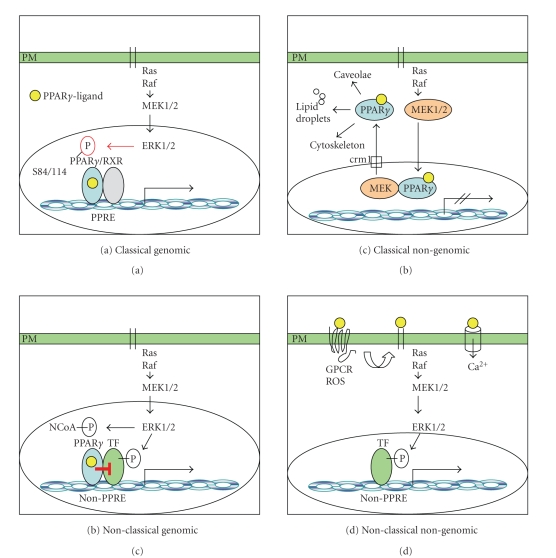
The mechanism of action and the regulation of PPARγ have attracted considerable attention over the years. Although this protein was initially shown to act as a transcription factor, studies using synthetic ligands suggested that it may exert its function via activation of signaling as well [1, 2]. According to the current knowledge, PPARγ signaling is mediated by several distinct mechanisms (Figure 1). The best known one is exerted by PPARγ protein itself, which is activated by ligand binding, heterodimerizes with the retinoic X receptor (RXR) and requires NR coregulator recruitment, events that lead to binding and transcriptional activation of PPAR-responsive elements (PPREs) in the DNA [29] (Figure 1(a)). Simultaneous activation of the ERK cascade (e.g., by mitogens) therein contributes to inhibition of this classical genomic action through serine phosphorylation of PPARγ (Figure 1(a)). Another mechanism is that PPARγ interacts with other transcription factors at the DNA level, which leads to PPRE-independent genomic actions of PPARγ protein and its ligands [9, 10] (Figure 1(c)). Activation of the ERK cascade participates in this mechanism by phosphorylation of the latter transcription factors that interact with PPARγ (Figure 1(c)). A third possibility is that nuclear export and cytoplasmic retention of PPARγ by MEK1 [30] results in “off-DNA”-interaction of PPARγ with distinct protein partners (e.g., cytoskeleton, lipid droplets, kinases), leading to alternative cytoplasmic signaling (Figure 1(b)). Finally, PPARγ ligands can function via activation of intracellular signalling (e.g., the ERK cascade) by a PPARγ-independent mechanism, which is derived from exogenous application of ligands that bind to plasma membrane-bound receptors [31] (Figure 1(d)). The latter mode of action can be “nongenomic,” that is, involving cytosolic signaling cascades, or “genomic,” that is, converging on the DNA by activation of alternative (non-PPAR) transcription factors (Figure 1(d)).
Figure 1.
Mechanisms of PPARγ-ERK signaling crosstalk: (a) serine phosphorylation of PPARγ by the ERK cascade suppresses the classical genomic action of RXR/PPARγ heterodimers on PPREs in the DNA; (b) ERK cascade phosphorylation of promitotic and proinflammatory transcription factors (TF) and NR coactivators (NCoA) modulates their interaction with PPARγ “On-DNA”; (c) nuclear export of PPARγ by MEK1 may result in “Off-DNA” interactions of PPARγ with alternative protein partners in the cytoplasm; (d) PPARγ-independent ERK cascade activation by PPARγ ligands through plasma membrane GPCRs, transactivation of the EGFR (black bars), or calcium signaling.
As apparent from the above description, interaction with the ERK cascade plays an important role in the regulation and signal transmission of PPARγ and its ligands. Overall, three main mechanisms of signaling crosstalk between the ERK cascade and PPARγ were described so far as follows: (1) phosphorylation of PPARγ (and its cofactors) by ERKs and other MAPKs (p38, JNK); (2) nongenomic activation of the ERK cascade by PPARγ ligands; and (3) compartmentalization of PPARγ by the ERK cascade component MEK1. Those are described in details in this section.
2.1. The functions of the PPARγ protein and its regulation by ERK phosphorylation
Genetic and pharmacologic studies in cells, rodent models, and human patients corroborated that the PPARγ protein serves as a master regulator of adipocyte and macrophage function in normal and pathophysiological conditions (inflammation, type-2 diabetes, obesity, atherosclerosis) [1]. Its expression in mesenchymal stem cells also associated this receptor with bone, skin, and muscle differentiation [2]. This 50-kDa protein consists of (from N- to C-terminal) the following: a transactivation function-1 (AF1) harbouring an MAPK-phosphorylation motif PXSP, a zinc-finger-type DNA-binding domain (DBD), a hinge region, the ligand-binding domain (LBD), and a flexible AF2 helix. Ligand-binding triggers the formation of the “charge clamp” between the AF2 and the core LBD, an event that enables the release of NR corepressors (NCoRs), heterodimerization with RXR, DNA-binding, NR coactivator (NCoA) recruitment, and transactivation of promoters [29] (Figure 1(a)). The LBD/AF2 interface also constitutes an important docking interface with unusual coregulators such as kinases and cell-cycle regulators (reviewed in [32]).
PPARγ positively regulates the expression of a vast spectrum of target genes involved in immunity and inflammation, differentiation, proliferation, apoptosis, cell survival, and metabolism [10]. However, PPARγ can also repress transcription by negatively interacting with several proinflammatory [9] and promitotic transcription factors [33] such as ETS, STAT, AP1, and NFκB (Figure 1(c)). Thereby, this factor promotes terminal differentiation of various normal and transformed cells of epithelial and mesenchymal origin. PPARγ(−/+) knockout mice exhibit enhanced susceptibility to chemically induced tumorigenesis [34, 35], and this enhanced susceptibility is observed also upon breeding with other strains deficient in tumor suppressors (such as APC) [36]. In patients, PPARγ protein is expressed (in varying levels) in leukemias, lipo- and osteosarcomas and in many carcinomas. Gene polymorphisms within the human population result in several “loss-of-function” PPARγ variants that are associated with metabolic diseases (insulin resistance, lipodystrophy) [37] and cancers (e.g., colon, stomach) [4, 5, 38, 39]. These data initially corroborated PPARγ as a protective transcription factor.
In line with the latter findings, ERK- (and other MAPK-) mediated phosphorylation of PPARγ reduces its genomic activity. A panel of extracellular/environmental promitotic, stress and inflammatory stimuli (growth factors, hormones, cytokines, lipid mediators/eicosanoids, UV-radiation, anisomycin, acetaldehyde, etc.) trigger the activation of the MAPK-family members: ERK, JNK, and p38 (Figure 1(a)). These MAPKs phosphorylate (in humans) Ser 84 in the PPARγ1 and Ser 114 in PPARγ2 isoform, which correspond to Ser 82/112 in mouse and are both located in the AF1 region of the molecules. This phosphorylation results in suppression of the PPARγ’s ability to transactivate target gene promoters and thereby its physiological functions (reviewed by [40, 41]). In addition, phosphorylated PPARγ is assumed to be more prone to other posttranslational modifications (sumoylation, ubiquitination) and subsequent degradation by the proteasome, an event that promotes its further downregulation upon MAPK-activation [42, 43]. But these effects are not fully characterized yet. In any event, the inhibition of PPARγ activity by MAPK phosphorylation is in accordance with the anti-inflammatory and prodifferentiation action of PPARγ and has been verified for normal (fibroblasts, adipocytes, macrophages, hepatic stellate cells) as well as cancer cell lines, various stimulating agents (as reviewed in [31, 44]) and also in vivo [45, 46]. An additional level of crosstalk is constituted by the fact that PPARγ cofactors, such as steroid receptor coactivator (SRC) family members (e.g., AIB/SRC3 in breast cancer), are phosphorylated by MAPKs and thereby are altered in their ability to coactivate transcription [47] (Figure 1(c)).
The effect of PPARγ phosphorylation by MAPKs was also supported by several in vivo studies. For example, a “knock in” of an unphosphorylable allele S112A in mice preserved their insulin sensitivity in absence of lipogenesis (weight gain) in a setting of diet-induced obesity [45]. In addition, a recent study revealed “downstream of tyrosine kinases-1” (Dok1) as an adapter protein in the insulin-signaling pathway that inhibits S112 phosphorylation of PPARγ2 in vivo [46]. Dok1 knockout mice on high fat remain lean and insulin-sensitive, and Dok1 knockout mouse embryonal fibroblasts (MEFs) show defective adipogenic differentiation, increased ERK activation and phosphorylation of PPARγ2 on S112. Mutation of S112 of PPARγ2 blocked the lean phenotype in Dok1 knockout mice, indicating that Dok1 promotes adipocyte growth and differentiation by counteracting the inhibitory effect of ERK on PPARγ. Another current intriguing example is the identification of parvinβ, a focal adhesion protein (lost in breast cancer patients), that increases the expression, S84 phosphorylation, and activity of PPARγ1 through cyclin-dependent kinase 9 (CDK) and suppressed breast cancer growth in vivo [48]. These data indicate that MAPK-mediated S84/S114 phosphorylation alters the activity of PPARγ1/2 in vitro and in vivo.
In sum, these studies initially corroborated the role of PPARγ as a tumor suppressor [2, 14], which may be shut down by MAPK-phosphorylation [44]. However, more recent evidence was collected, that PPARγ is a context-specific tumor modulator, whose effector profile is complemented and modified by PPARγ-independent effects of its ligands (e.g., TZDs and eicosanoids) and by reciprocal regulation of PPARγ through members of the ERK cascade as follows [31, 40].
2.2. PPARγ ligands influence cellular processes via a nongenomic activation of the ERK cascade
A second mechanism of crosstalk between PPARγ and the ERK cascade comprises the direct activation of ERKs by PPARγ ligands. In the past, ample data was collected on the effects of chemically distinct classes of PPARγ ligands on cells. Different ligands induce either cell growth and proliferation or growth arrest and apoptosis in various human and mouse cancer cell lines and xenografts (as extensively reviewed in [14, 31]), and also modulate angiogenesis in vitro and in vivo [49]. These effects are dose-, time-, and cell type-dependent, and manifest either in a PPARγ receptor-dependent (“genomic”) or non-PPARγ receptor-mediated (“nongenomic”) manner or in a combination of both. The mechanisms that underlie these context-dependent responses are largely unknown. One concept is based on the claim that nongenomic PPARγ ligand effects manifest at higher micromolar concentrations (>10 μM) well above the low EC50’s necessary for classical genomic actions on PPARγ/RXR heterodimers at characterized PPREs in target genes (e.g., 80 nM for rosiglitazone) [50, 51]. This assumption translated into the idea that, low doses of PPARγ ligands, for example, that correspond to the pharmacological doses prescribed for diabetic patients, exert overtly beneficial efficacy, while supra-pharmacological high doses evoke adverse effects. For example, troglitazone was retracted from the market due to hepatotoxicity, which was not a TZD-class effect but due to a drug-specific (possibly “nongenomic”) adverse action [2]. However, the literature provides examples for both pro- and antitumor actions of PPARγ ligands at similar dose ranges in similar cellular systems. Thus, an underlying principle for the separation of genomic from nongenomic PPARγ ligand effects is currently not available.
The PPARγ ligand effects are likely to be mediated either (i) through so far unknown plasma membrane-bound receptors (Figure 1(d)) or (ii) through cytoplasmatic localized PPARγ protein (Figure 1(b)). Novel G-protein coupled receptors, such as GPR30 for estradiol [52], TGR5 for bile acids [53], and GPR40 for free fatty acids [54], were identified to function as alternative signal transducers for NR-ligands. GPR40, a candidate PPAR ligand receptor, is highly expressed in the pancreas but also in monocytes and in the lower GI tract (e.g., ileum, colon) [55, 56]. Oleate, a natural PPAR ligand, increases proliferation of MCF7 human breast adenocarcinoma through binding and signaling via endogenous GPR40 [57]. TZDs were postulated as bona fide ligands for ectopic GPR40 in CHO cells and to signal via Gαi/q proteins, cAMP, calcium, and ERK activation [58]. However, in vivo proof is lacking. In addition to GPCRs, also plasma membrane-bound classical NRs interact with specific adapter or scaffold proteins in the cytoplasm and trigger the initiation of proproliferative and survival signaling [59]. For example, the estrogen receptor docks to modulator of non-genomic action of estrogen receptor (MNAR) that recruits Src and leads to activation of the p85 subunit of PI3K [60] and the ERK cascade [61]. If this situation is also relevant for PPARγ molecules remains to be shown. Many TZD effects actually target cytoplasmic proteins such as at mitochondria, the proteasome, or the translational machinery. Thus, it is possible that cytoplasmic PPARγ molecules are also involved in the transduction of “nongenomic” TZDs signals.
Downstream of the initial ligand triggering event, nongenomic responses to PPARγ ligands include transient alterations in mitochondrial functions and activation of stress (production of reactive oxygen species (ROS)) as well as kinase signaling pathways promoting proliferation and survival such as PI3K-PKB/AKT, ERK, p38, and JNK [50, 51]. Rapid signaling initiated by ligands can be mediated by membrane proximal events such as cleavage of transmembrane proteinases (ADAMs), activation of GPCRs, EGFR transactivation, calcium influx, and activation of protein tyrosine kinases (Pyk2, Src). Further downstream effects include PPARγ-independent induction of “early response genes” such as c-Fos and Egr-1. In this context, it was shown that PPARγ ligands enhance proliferation, survival and drug resistance in cancer cells, for example, by induction of the prosurvival and promitotic hormone gastrin [62]. We showed that TZDs enhance drug resistance in human colon adenocarcinoma HT29 cells in a PPRE-independent but EGFR-dependent manner, involving Src/MAPK-signaling [63]. In colon carcinoma cells, TZDs induce matrix metalloproteinase 2 (MMP2) and membrane type 1-MMP (MT1-MMP) activation and concomitantly increase tumor cell invasion through generation of ROS and activation of the ERK cascade [64]. On the other hand, ERK cascade activation by TZDs may also translate into growth inhibition and/or apoptosis [65–69]. It is currently unknown which mechanism governs the decision for pro-versus antiproliferative responses upon TZD application.
In addition to TZD drugs, also the physiological eicosanoid-type ligands for PPARγ exert tumor-modulating effects through their ability to trigger ERK cascade activation [70]. Eicosanoids are generated by cytoplasmic phospholipase A2 and cyclooxygenases (COX1/2). Some of these arachidonic acid metabolites act as endogenous PPARγ ligands ((e.g., 15-deoxy-Δ(12,14)-PGJ2) [71]), while others, like the prostaglandins of the E and D series, activate the ERK cascade through prostanoid GPCRs at the cell membrane [72]. 15-deoxy-Δ(12,14)-PGJ2 directly inhibits inhibitor-κB kinase (IKK) in an intracellular fashion and exerts various effects on inflammation, cell growth, and apoptosis independent of a prostanoid GPCR [71]. For example, in human breast MCF7 adenocarcinoma cells, 15-deoxy-Δ(12,14)-PGJ2 upregulates VEGF synthesis through induction of heme oxygenase-1, an enzyme that stimulates proliferation and angiogenesis, and triggers ERK phosphorylation in an PPARγ-independent fashion [73]. In sum, these data point out to the important role for protumor effects of PPARγ ligands of the TZD- and eicosanoid-class in the activation of ERK cascade-related proliferation and survival pathways, which stand in sharp contrast to the otherwise reported tumor suppressive effects of the latter in similar cellular systems [65–67].
In vivo preclinical and clinical data of TZDs support the concept of an overlapping profile of PPARγ receptor-dependent and independent ligand signaling. In contrast to the lessons from PPARγ(+/−) knockout mice [34, 35] and the antineoplastic action of PPARγ receptor activation in vitro [33], ample in vivo data asserted that many potent and selective PPARγ ligands actually promote tumorigenesis. Thus, PPARγ ligands induce tumor growth in rodent xenograft models [14] and enhance in vivo angiogenesis [49]. In addition, TZDs act as procancerogenic agents in wild-type and APC-deficient mouse models of colon carcinogenesis [74–77]. Importantly, clinical studies in humans failed to show a clear benefit of TZD monotherapy in cancer patients [14, 78, 79]. PPARγ ligands are procarcinogenic in human bladder, as evaluated by the PROactive study [12], and in the rodent bladder [80, 81]. As a reaction towards the safety-toxicological data collected in preclinical studies and clinical trials regarding TZD use, the US Food and Drug Administration (FDA) (http://www.fda.gov/cder/present/DIA2004/15) issued a warning of tumor-related adverse effects of novel potent PPARγ ligands that are currently in clinical trials as novel antidiabetics or obesity cures (reviewed in [82]) [83, 84]. The FDA classified all PPARγ ligands as multispecies and multiorgan carcinogens requiring strict dose finding for therapeutical use in humans. However, the full molecular mechanism of this interplay between tumor promoting versus suppressing action of PPARγ ligands is so far unknown.
2.3. Towards solving the tumor initiation/suppression paradox of PPARγ: interaction of PPARγ with the ERK cascade in cancer
Unlike the impression that is left by many articles to date, PPARγ protein does not always act as a tumor suppressor, and the PPARγ ligands are not always procancerogenic independently of the receptor. Notably, the PPARγ itself seems to be important for exacerbating mammary gland tumor formation in bitransgenic mice expressing a constitutive active PPARγ form independently of application of an exogenous ligand [85]. An interesting in vitro study corroborated the functional cooperation of the PPARγ receptor and the ERK cascade in the promotion of epithelial-mesenchymal transition (EMT) in the mouse small intestine and rat intestinal epithelial cells, which was dependent on an intact DNA-binding activity of the PPARγ receptor protein [86]. In this system, PPARγ induced ERK1/2 phosphorylation by activating PI3K, Cdc42, and p21-activated kinase (PAK), which in turn phosphorylated S298 of MEK1 that supports its activity [23]. Ectopic expression of dominant negative MEK1 blocked EMT induced by PPARγ, while constitutively active MEK1 overexpression promoted a mesenchymal morphology. However, as evident in the latter intriguing example, the exact molecular mechanisms and physiological relevance of the cooperative interactions between posttranslational regulation of NRs by kinases and rapid nongenomic kinase activation by NR-ligands are so far unknown.
Ample data supports the notion that mutual physical/allosterical associations between kinases and NRs exist that translate into reciprocal regulation of their activities [87, 88]. For example, 3-phosphoinositide-dependent protein kinase-1 (PDK1), that is the upstream activator of AKT/PKB, binds to and activates PPARγ during adipogenic differentiation [89]. Complexes of cyclins and CDKs are cofactors for and phosphorylate PPARγ in adipocytes [90, 91]. PPARγ also interacts with and is activated by ERK5 [92, 93] in order to inhibit (in conjunction with WNT signaling factors) the proliferation of lung cancer (NSCLC) cells and inflammation in endothelial cells upon flow (shear stress), indicative of a protective function of ERK5-PPARγ cooperation. These unusual NR cofactors [32], that also include retinoblastoma protein and transcriptional elongation factors, directly interact with regulatory domains in NRs and considerably add to the pleiotropic effector profile of a given NR. Several interaction partners for PPARγ protein have been identified including prominent oncogenic modulators such as βcatenin [94, 95] and MEK1 [30]. Therefore, it is likely that PPARγ interacts with or cooperates with several signaling pathways and particularly the ERK cascade in order to induce or prevent oncogenic transformation dependent on the cell type and environment.
2.3.1. Spatial regulation of PPARγ activity: MEKs export PPARγ to the cytoplasm
Next to Ser84/114 phosphorylation and the nongenomic ERK activation by PPARγ ligands, the direct interaction of PPARγ with the ERK cascade component MEK1 constitutes a third mechanism of crosstalk between PPARγ and the ERK cascade. Subcellular compartmentalization is a major mechanism in regulating cellular signaling. Interestingly, PPARγ itself can regulate the membrane translocation of other proteins such as NFκB in gut intestinal epithelial cells [96] and PKC in macrophages [97]. Several reports have demonstrated a signal-mediated translocation of PPARγ between the nucleus and the cytoplasm in vitro (as reviewed in [98]). In addition, it was shown that PPARγ is expressed predominantly in the nucleus of nonneoplastic tissues, whereas it is present in both the nucleus and the cytoplasm of tumorous tissues in squamous cell carcinoma (SCC) of the lung, indicative of a correlation of malignancy with differential PPARγ compartmentalization [99]. Moreover, a dominant negative PPARγ splice variant was described in lung SCC patients, an event that leads to the loss of apoptosis sensitivity in response to oxidative stress and cisplatin [99]. Differential compartmentalization of PPARγ was also described in gastric cancer patients [100]. The ratio of cytoplasmic/nuclear PPARγ expression decreased in the progression of intestinal metaplasia to undifferentiated cancers [100]. In salivary duct carcinoma, an aggressive tumor type, PPARγ is highly expressed (80%) and topographically located in the cytoplasm [101], indicative of an inactivation of its genomic activities in the nucleus. Cytoplasmic PPARγ was also detected in the cytoplasm (58%) of infiltrating breast carcinoma samples and was proposed as an independent prognostic factor for patients with ductal carcinoma [102]. However, the function of this subcellular distribution of PPARγ molecules are yet unknown.
The mechanism that may induce the changes in localization of PPARγ upon stimulation, or upon neoplastic transformation was only recently elucidated by us [30]. We showed that PPARγ is exported from the nucleus to the cytoplasm by MEK1/2. This is induced by a reversible interaction of PPARγ with MEK1 through association of the AF2 of the first with the N-terminal docking domain of MEK1. This export to the cytoplasm (Figure 1(b)) leads to reduction in its genomic function in the nucleus [30]. We also elucidated the molecular mechanisms of the export and the physiological implications, but the question remained is whether cytoplasmatically located PPARγ is subjected to degradation or shunted to alternative signaling compartments such as lipid droplets, ER/Golgi, cytoskeleton, or the plasma membrane. To this regard, we tend to speculate that alternative locations of PPARγ in the cell may determine the balance between tumor-suppressive and tumor-promoting functions.
2.3.2. Tumor-suppressive functions of PPARγ related to ERKs and MEKs interaction
Due to the coexpression of the ubiquitous proteins PPARγ and MEK1/2 in different organs of the body, it was interesting to identify their coregulation in various physiological and pathological processes, as described below.
Differentiation —
Due to the lethality of MEK1 knockout mice [103] and absence of phenotypes in MEK2 knockout mice [104], the major focus of interest was directed towards the role of MEK1 overexpression in vivo. Constitutively active MEK1 (S218E/S222E) has been conditionally overexpressed (among other tissues) in the skin and bone of mice [105]. All transgenic mice exhibited increased cell numbers (hyperplasia) and cell size and a defect in terminal differentiation. Interestingly, both in skin and in bone of mice, PPARγ was shown to be an important player promoting differentiation [2]. In addition, the constitutively active MEK1 overexpressing mice show dwarfism and reduced bone size due to defective ossification and impaired chondrocyte differentiation. In other systems, it was shown that osteoclast-specific PPARγ knockout mice are characterized by increased bone mass due to impaired osteoclast differentiation [106], suggesting antagonistic effects of PPARγ and MEK1 on different bone cell types: with PPARγ promoting osteoclast differentiation, and MEK1 inhibiting chondrocyte differentiation.
Skin-restricted MEK1 transgenic mice exhibit hyperproliferation, hyperkeratosis and of age papillomas at sites of wounding [105, 107]. Vice versa, epidermis-specific knockout of MEK1/2 in mice [108] resulted in hypoproliferation, apoptosis, skin barrier defects, and death, indicative of a positive role of MEK1 in skin proliferation and tissue homeostasis. PPARγ knockout mice are characterized by an increased sensitivity to experimentally-induced skin tumors [35], emphasizing the tumor suppressor and differentiation promoting activity of PPARγ in the skin. These “mirror-images” phenotypes in the organs where MEK1/2 and PPARγ are normally coexpressed may give some indication for the antagonistic regulation of the two proteins, MEK promoting proliferation and dedifferentiation, PPARγ promoting terminal differentiation. In line with this idea, it was shown that the kinase activity of MEK1 was actually dispensable for the hyperproliferative and integrin-inducing effects of the MEK1 in mouse skin [109]. Instead, a kinase-dead mutant of MEK1 elicited the same phenotype, indicative of an involvement of other MEK1-functions such as scaffolding inhibition of differentiation-promoting cellular factors.
In adipogenic differentiation systems originating from (mesenchymal) stem cells, synergistic cooperations between the MEK-ERK cascade and PPARγ have been described. In fibroblasts, differentiating towards the adipogenic lineage, a positive cooperation between PPARγ and MEK1 exists that facilitates the adipogenic program by MEK1-dependent induction of the C/EBPα gene [110]. In bone marrow-derived mesenchymal stem cells isolated from normal and streptozotocin (STZ)-induced diabetic FVB/N mice, high glucose enhanced adipogenesis, lipid accumulation, and PPARγ expression via PI3K/AKT and ERK cascade signaling, events that were all inhibited by the MEK-inhibitor PD98059 [111]. In differentiated C2C12 myocytes, the free fatty acid palmitate reduces the mRNA levels of PPARγ-coactivator-1α (PGC1α) and activated MEK, while the MEK inhibitors PD98059 and U0126 prevented such downregulation of PGC1α, indicative of a MEK-mediated inhibition of an important NR coactivator protein for PPARγ in muscle cells [112]. These findings corroborated that the MEK-ERK cascade and PPARγ signaling pathways can syn- or antagonistically cooperate to control the balance of proliferation and differentiation in an organ/cell type-specific manner.
Cell cycle —
The ERK cascade participates in the regulation of cell cycle at (i) G0/G1 and G1/S transitions in response to mitogenic stimulation (as reviewed in [24]) and (ii) in the process of Golgi fragmentation [113–115] during mitosis. This is mediated in part by the nuclear translocation of ERK upon cellular stimulation that promotes expression of “immediate early” genes such as members of the AP1 family that activate the promoters of the G1 cyclins D and E. However, the subcellular compartmentalization of ERK signaling by scaffold proteins (KSR, MP1/p14, Sef) (reviewed in [22]) indicates a novel mode of spatial separation of substrate specifities and signal translation. For example, MP1 via the adapter protein p14 tethers MEK1 to endosomes [116] and focal adhesions [117]. Sef translocates MEK1 to the Golgi apparatus, prevents nuclear translocation of ERKs, and, thereby, favours phosphorylation of cytoplasmic ERK substrates instead of nuclear ones [118]. The latter subcellular localization-determining systems may thus be as well exploited by the PPARγ-MEK1 nuclear export shuttle to regulate the cell cycle.
The PPARγ receptor has been involved in the inhibition of the G0/G1-transition by up-regulation of genes coding for the CDK-inhibitors p18(INK4C) [119] and p21(WAF1/CIP1) [120, 121], and in the inhibition of G1/S transition through upregulation of the p27(KIP1) gene [122, 123]. Upregulation of other genes implicated in cell cycle control such as PTEN or members of the BCL-gene family contributes to the growth-arresting and/or apoptosis-inducing action of PPARγ ligands [15]. The cell cycle modulatory actions of PPARγ are usually not mediated through classical PPRE binding at the DNA but rather through PPRE-independent “off-DNA” crosstalk to other transcription factors [15] and through nongenomic effects in the cytoplasm, such as inhibition of translation initiation [124, 125] and modulation of the proteasomal machinery [126–128]. The latter processes may be mediated by ligand-activated cytoplasmic PPARγ molecules or cytoplasmic alternative signal-transducers for PPARγ ligands. We therefore hypothesize that, by nuclear export and cytoplasmic retention of PPARγ-MEK1 complexes to other MEK1-scaffolding locations (e.g., at the Golgi, endosomes, focal adhesions), the genomic PPARγ functions may as well be redirected in favour of cytoplasmic signaling events. In sum, the cell cycle modulating effects of PPARγ protein and its ligands may be caused by its differential subcellular compartmentalization by MEK1.
2.3.3. Tumor-promoting functions of PPARγ, related to crosstalk with the ERK cascade
Metastasis —
In contrast to the initial assumption of PPARγ mainly acting as a tumor suppressor whose activity and/or expression is lost in cancers, PPARγ expression and activity can also be a negative predictor of cancer aggressiveness; and positive cooperation between PPARγ and components of the ERK cascade in malignant phenotypes takes place. For example, strong nuclear PPARγ expression was detected in thyroid carcinomas compared to normal tissue, and patient samples of thyroid carcinoma-associated lymph node metastasis also showed a higher percentage of PPARγ-positive staining than other case categories [129]. PPARγ expression was also elevated in human prostate cancer compared to normal prostate [130]. In patients with invasive breast carcinoma, cytoplasmic MT1-MMP and MMP9 expression positively correlated with PPARγ levels [131]. These data corroborated a positive relationship between PPARγ expression and malignancy state in certain tumor entities, a fact that was shown to be therapeutically exploitable by the use of PPARγ antagonists or siRNA. This was described in primary esophageal tumor specimen and in esophageal cancer cell lines [132], in human primary squamous cell carcinoma (SCC) and lymph node metastases [133] and in hepatocellular carcinoma (HCC) samples [134], where PPARγ expression is elevated compared to matched normal tissue. In all three cell systems, PPARγ antagonists (T0070907,GW9662) and RNAi-mediated knock-down of PPARγ levels reduced the invasiveness and adherence of cells to the extracellular matrix, triggered anoikis, or inhibited proliferation by decreasing the phosphorylation status of focal adhesion kinase (FAK), MEK, and ERK. Therefore, in tumors where elevated PPARγ and activated ERK and MEK levels contribute to the malignant phenotype, inhibition of PPARγ may be beneficial as a therapeutic strategy (see also Section 3).
Angiogenesis —
The overall vascular protective and antiatherogenic effects of PPARγ ligands provide essential add-ons for the clinical application as insulin sensitizers (reviewed in [13]). However, the proangiogenic effects of PPARγ ligands via modulation of the VEGF/VEGF-receptor system (that signals via the ERK cascade) have gained recognition (reviewed by [49]), which may be beneficial for therapy of vascular diseases (e.g., infarction) [135, 136] but detrimental in cancer tissue. For example, in rat myofibroblasts, rosiglitazone and 15-deoxy-Δ(12,14)-PGJ2 induce expression of VEGF and its receptors (Flt1 and KDR, that signal via the ERK cascade), and augment tubule formation on a matrigel, indicative of a promoting function of PPARγ and ERKs in angiogenesis [137]. In osteoblast-like MC3T3E1 cells, pioglitazone and ciglitazone augmented FGF2-induced VEGF release in a PPARγ-dependent manner and enhanced the phosphorylation of JNK [138]. In human RT4 bladder cancer cells, VEGF mRNA and protein are upregulated by PPARγ via activation of the VEGF promoter. Interestingly, the MEK inhibitor PD98059 reduced PPARγ ligand-induced expression of VEGF [139], indicative of a positive cooperation of PPARγ-ERK pathways in angiogenesis. These positive effects on angiogenesis were examined also in two clinical studies with rosiglitazone [140] and pioglitazone [136], in which it was demonstrated that chronic addition of the TZDs increased endothelial cell precursor counts and migration in diabetic patients, raising concern on the proangiogenic potential of TZDs.
Taken together, the data which revealed an antagonistic cooperation of PPARγ and ERK signaling in several cell or tissue-specific differentiation systems (skin, bone, muscle, fat) is now challenged by the findings of positive cooperation of the same components in tumor progression (metastasis, angiogenesis). Thus, the role of PPARγ as a MEK/ERK-regulated tumor suppressor seems to be of importance in normal tissue or in prevention of tumor initiation, while in advanced stages of certain tumors a synergistic cooperation between PPARγ and the ERK cascade may contribute to the malignancy of the disease. Future studies have to clarify whether PPARγ agonists, PPARγ antagonists, or PPARγ modulators/partial agonists (SPPARMs) with a selective effector profile [141] may be of interest for the therapy of certain tumor entities.
3. CLINICAL USE OF PPARγ INTERACTION WITH THE ERK CASCADE AS A DRUG TARGET
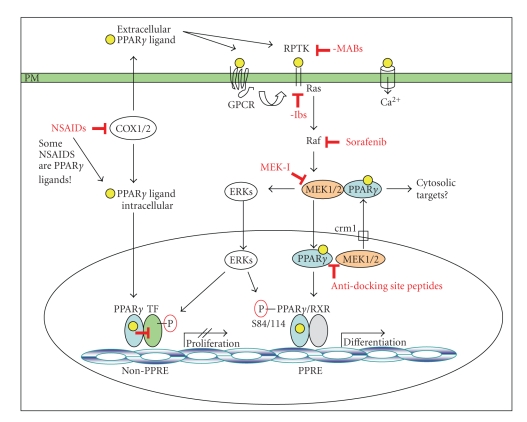
Reactivation (“differentiation”) therapy targeting functional PPARγ protein in cancer cells/tissues by exogenous application of TZD-class PPARγ ligands was lately expected to represent a novel approach to fight cancer [142]. However, differentiation-inducing monotherapy with TZDs did not show the expected clinical benefit [11]. Instead, evidence accumulated that alternative (“nongenomic”) PPARγ signaling pathways, crosstalk with the ERK cascade and elevated PPARγ expression levels in certain tumor types (where PPARγ is postulated to act as a prosurvival factor, e.g., in hepatocellular carcinoma, squamous cell carcinoma), are the cause for the observed tumor promoting effects of PPARγ ligands, and may explain the absence of clear therapeutical benefit of TZDs in cancer patients [78, 79, 143]. Therefore combination therapy of PPARγ ligands with kinase inhibitors may represent a novel strategy to circumvent the crosstalk of PPARγ and ERK cascade signaling and limit PPARγ protein activation to its classical differentiation-inducing feature (Figure 2). This dual approach is expected to avoid (a) ERK cascade-mediated downregulation of PPARγ, (b) MEK-driven nuclear export and cytoplasmic retention of PPARγ and (c) nongenomic amplification loops of PPARγ ligands towards the ERK cascade, but to promote (d) the growth-arresting and proapoptotic genomic functions of PPARγ and its ligands, and (e) the negative crosstalk of PPARγ with promitotic and proinflammatory transcription factors in the nucleus. This concept may not be suitable for tumor types with elevated “malignant” PPARγ expression/activities. However, due to the lack of clinically approved PPARγ antagonists, no statement can be currently made on the potential therapeutical benefit of PPARγ and kinase coinhibition.
Figure 2.
Model of the combination therapy using PPARγ ligand and ERK cascade inhibitors. The simultaneous inhibition of EGF receptor-initiated ERK cascade activation by specific kinase inhibitors (-ibs) or antibodies (-MABs) and supply of PPARγ ligands (in tumors that have a need for restored PPARγ activity) will avoid: (a) ERK-mediated downregulation of PPARγ through Ser84/114 phosphorylation, (b) MEK1-driven nuclear export and cytoplasmic retention of PPARγ, (c) activation of prosurvival and proproliferative ERK cascade signaling by exogenous PPARγ ligands (e.g., by TZD drugs) or endogenous eicosanoid type of PPARγ ligands (e.g., generated by COX1/2), but is expected to (d) restore the differentiation-inducing and proapoptotic functions of PPARγ and its ligands, and (e) promote the transrepressive activity of PPARγ on other promitotic and proinflammatory transcription factors (e.g., AP1, ETS, STAT, NFκB). Legend: Yellow circles = PPARγ-ligand; TF = transcription factors; ROS = reactive oxygen species; GPCR = G protein coupled receptor; RPTK = receptor protein tyrosine kinase; crm1 = exportin1; NSAID = nonsteroidal anti-inflammatory drug; COX = cyclooxygenase; -Ibs = LMW tyrosine kinase inhibitors; MABs = monoclonal tyrosine kinase antibodies.
3.1. In vitro studies
The combination of PPARγ ligands and inhibitors against receptor tyrosine kinases of the EGFR-family or cytoplasmic tyrosine kinases (e.g., Abl) revealed some promising results in leukemia and carcinoma cells. Gefitinib, an inhibitor of the EGFR/Her1 kinase, exhibits antitumor activity in only a fraction of 10–20% of patients with nonsmall cell lung cancer (NSCLC) [144]. The mechanisms underlying this resistance to gefitinib are not known. However, application of rosiglitazone reduced the growth of the NSCLC A549 cells and potentiated the antiproliferative effects of gefitinib and increased PPARγ and PTEN expression in these cells, indicative of a potential benefit of this drug combination also in cancer patients. MCF7 breast cancer cells stably transfected with ErbB2/Her2 displayed reduced differentiation and enhanced resistance to TZD-driven inhibition of anchorage-independent growth [145]. Herceptin, a monoclonal antibody against Her2 kinase, sensitized cells for the differentiation-promoting and growth-inhibitory effects of troglitazone. This concept also held true for chronic myeloid leukemia (CML) cell lines, where TZD18 (a dual PPARα/γ ligand) enhanced CDK-inhibitor p27(KIP1) expression and inhibited cyclin E, cyclin D2 and CDK2 [122]. TZD18 synergistically enhanced the antiproliferative and proapoptotic effect of imatinib, a clinically used kinase inhibitor of the Bcr-Abl fusion protein. Collectively, this work demonstrated that the targeting of receptor tyrosine kinase signaling with LMW inhibitors or monoclonal antibodies can improve the sensitivity of cancer cells to PPARγ ligand-mediated growth inhibition.
3.2. In vivo rodent and clinical studies
The clinical outcome of selective MEK inhibitors in patients studies was disappointing (CI-1040, PD0325901, AZD-6244) (reviewed in [146, 147]). On the other hand, a Raf inhibitor, sorafenib, was recently approved for clinical use; and novel selective Raf inhibitors are under development [148]. So far no clinical studies were performed using MEK or Raf inhibitors in combination with PPARγ ligands. However, successful treatment data in mouse models or patients are available for combinations of PPARγ ligands and three other types of inhibitory drugs: classical chemotherapeutics, COX-inhibitors (NSAIDs), and established tyrosine kinase inhibitors (imatinib, gefitinib, herceptin).
NSAID/COX-inhibitors have been shown to reduce the risk for colon carcinoma formation, however at the expense of gastric ulcer and cardiovascular complications [19]. Several NSAIDs are also low-affinity PPARγ ligands, a fact that led to the speculation that a part of the clinical profile of these compounds is related to low-level activation of PPARγ [19]. Therefore, clinical trials with combination therapies were initiated to exploit PPARγ activation and simultaneous blockage of the promitotic and proinflammatory COX1/2-mediated eicosanoid production, which contributes to nongenomic signaling in cancer tissues (Figure 2). Pilot clinical studies with an angiostatic triple combination of pioglitazone, rofecoxib (a selective COX2 inhibitor), and trofosfamide showed benefit in patients with angiosarcoma and hemangioendothelioma [151, 152] and advanced sarcoma [153]. A phase-II trial with the same triple combination in patients with metastatic melanoma or soft-tissue sarcoma evinced disease stabilization [152], indicative of a beneficial effect of COX2 inhibition (whose eicosanoid metabolites activate the ERK cascade) and simultaneous PPARγ activation in sensitization of tumor cells to differentiation and/or apoptosis. A recently published outcome of a phase-II trial in high-grade glioma patients (glioblastoma or anaplastic glioma) under pioglitazone and rofecoxib combined with chemotherapy (capecitabine or temozolomide) also stated some disease stabilization [157]. However, due to the severe side effects of selective COX2-inhibitors this therapeutic regimen may raise concerns.
Preclinical studies in rodents provided evidence for a therapeutic potential of combination therapy with other inhibitory agents. In mice xenografted with NSCLC A549 cells, the PI3K inhibitor PX-866 potentiated the antitumor activity of gefitinib [149]. The glucose intolerance related to PX-866 in mice was reversed by insulin and pioglitazone. PX-866 in combination with insulin sensitizers may thus be useful in facilitating the response to EGFR inhibition. The antitumoral action of rosiglitazone on experimentally induced mammary tumors induced by N-nitroso-N-methylurea (NMU) in Sprague-Dawley rats was potentiated by the selective estrogen-receptor modulator (SERM) tamoxifen with respect to the extent of tumor cell apoptosis and necrosis [150]. The PPARγ ligand RS5444 in combination with paclitaxel had additive antiproliferative effect in vitro and minimized tumor growth in nude mice xenografts of anaplastic thyroid carcinoma (ATC) cells [120]. These preclinical studies underline that the combination of PPARγ ligands and established anticancer drugs may be of clinical benefit also in cancer patients.
Interestingly, two studies provided already first-line evidence for the potential of an in vivo reactivation of PPARγ protein function by simultaneous inhibition of the COX pathway-mediated activation of the ERK cascade: LY293111, an oral PPARγ ligand, leukotriene B4 receptor antagonist and 5-lipoxygenase inhibitor, was validated for its antineoplastic efficacy in combination with chemotherapy (irinotecan, gemcitabine) in preclinical models [154] and evoked disease stabilization in patients with advanced solid tumors [155, 156]. The NSAID R-etodolac inhibits growth of prostate cancer (CWRSA6, LuCaP35) xenografts in mice by downregulation cyclin D1. However, the combination of R-etodolac with herceptin elicited an additive antitumor effect, reduced ERK phosphorylation and stabilized PPARγ protein levels [158]. These therapeutic regimens inhibited the eicosanoid-mediated activation of the ERK cascade, and in conjunction with PPARγ activation, may provide a basis for differentiation-inducing therapy in combination with classical chemotherapeutics or biologicals.
So far no clinical evidence was published on the combined use of ERK cascade inhibition and PPARγ activation (in tumors with low PPARγ expression/activity) or PPARγ inhibition (in tumors with high PPARγ expression/activity). In the future, the combination of PPARγ ligands with kinase inhibition selectively targeted by MABs against the EGFR tyrosine receptor kinase family or LMW selective inhibitors of the downstream ERK cascade, such as Raf and MEK, may constitute a possible new approach to treat cancer.
4. CONCLUSION AND PERSPECTIVES
In conclusion, PPARγ emerges as a tumor-type and tumor-stage-specific modulator that is regulated by at least three mechanisms through the ERK cascade. Downregulation is carried out through (1) MAPK-mediated Ser84/114 phosphorylation, (2) ERK cascade activation through PPARγ ligands, and (3) cooperation of PPARγ with tumor modulating proteins (such as MEK1). The overlay of these 3 mechanisms of crosstalk is likely to determine the physiological outcome of PPARγ effector functions. Consequently, interference with these interactions by LMW inhibitors, antibodies, or peptidomimetic drugs against protein docking interfaces may constitute a novel approach to redirect PPARγ effector functions from a protumorigenic towards an antitumorigenic profile. Simultaneous inhibition of ERK cascade-mediated signaling is expected to prevent adverse promitotic and prosurvival pathways triggered by PPARγ and its ligands. This therapeutic approach is assumed to be reasonable in tumors where the tumor-suppressor activities of PPARγ are lost/reduced/dysfunctional and should be restored. However, it may not be applicable for tumors where high PPARγ expression/activity levels positively correlate with the state of malignancy. Since no PPARγ antagonist or PPARγ modulator is in clinical use so far, future studies have to evaluate whether (depending on the tumor type and stage) the combination of the latter drugs with kinase inhibitors may be of therapeutical benefit in tumor entities with high PPARγ expression.
Table 1.
Combination therapy with PPARγ ligands.
| Cancer type | PPARγ ligand | Combination | Inhibitor type | Reference |
|---|---|---|---|---|
| In vitro | ||||
|
| ||||
| CML | TZD18 | Imatinib | Abl, other RPTKs | [122] |
| NSCLC A549 | Rosiglitazone | Gefitinib | EGFR/Her1 | [144] |
| Breast MCF7 | Troglitazone | Herceptin | Mab-Her2/ErbB2 | [145] |
|
| ||||
| In vivo (human xenografts or chemically-induced tumors in rodents) | ||||
|
| ||||
| NSCLC A549 | Pioglitazone | PX-866 Gefitinib | PI3K-p110α Her1/EGFR | [149] |
| Breast (by NMU) | Rosiglitazone | Tamoxifen | SERM | [150] |
| Thyroid ATC | RS5444 | Paclitaxel | Chemotherapeutic | [120] |
|
| ||||
| Clinical studies | ||||
|
| ||||
| Melanoma Sarcoma | Pioglitazone | Rofecoxib Trofosfamide | COX2 Chemotherapeutic | [151–153] |
| Advanced Solid tumors | LY293111 | Irinotecan Gemcitabine | Chemotherapeutic Chemotherapeutic | [154–156] |
| Glioblastoma Anaplastic Glioma | Pioglitazone | Rofecoxib Capecitabine Temozolomide | COX2 Chemotherapeutic Chemotherapeutic | [157] |
ABBREVIATIONS
- AF:
Activation function
- DBD:
DNA binding domain
- COX:
Cyclooxygenase
- ERK:
Extracellular signal-regulated kinase
- LBD:
Ligand binding domain
- MAPK:
Mitogen-activated protein kinase
- MEK:
MAPK/ERK kinase
- NSAID:
Nonsteroidal anti-inflammatory drug
- NCoA:
NR coactivator
- NCoR:
NR corepressor
- NR:
Nuclear receptor
- PPAR:
Peroxisome proliferator-activated receptor
- PPRE:
PPAR responsive element
- RXR:
Retinoid X receptor
- ERK cascade:
Ras-Raf-MEK1/2-ERK1/2 cascade.
References
- 1.Rosen ED, Spiegelman BM. PPARγ: a nuclear regulator of metabolism, differentiation, and cell growth. Journal of Biological Chemistry. 2001;276(41):37731–37734. doi: 10.1074/jbc.R100034200. [DOI] [PubMed] [Google Scholar]
- 2.Lehrke M, Lazar MA. The many faces of PPARγ . Cell. 2005;123(6):993–999. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 3.Lin W-W, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. The Journal of Clinical Investigation. 2007;117(5):1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slattery ML, Curtin K, Wolff R, et al. PPARγ and colon and rectal cancer: associations with specific tumor mutations, aspirin, ibuprofen and insulin-related genes (United States) Cancer Causes & Control. 2006;17(3):239–249. doi: 10.1007/s10552-005-0411-6. [DOI] [PubMed] [Google Scholar]
- 5.Zmuda JM, Modugno F, Weissfeld JL, et al. Peroxisome proliferator-activated receptor-γ polymorphism, body mass and prostate cancer risk: evidence for gene-environment interaction. Oncology. 2006;70(3):185–189. doi: 10.1159/000093805. [DOI] [PubMed] [Google Scholar]
- 6.Schiel R, Beltschikow W, Steiner T, Stein G. Diabetes, insulin, and risk of cancer. Methods and Findings in Experimental and Clinical Pharmacology. 2006;28(3):169–175. doi: 10.1358/mf.2006.28.3.985230. [DOI] [PubMed] [Google Scholar]
- 7.Khan M, Mori M, Fujino Y, et al. Site-specific cancer risk due to diabetes mellitus history: evidence from the Japan Collaborative Cohort (JACC) Study. Asian Pacific Journal of Cancer Prevention. 2006;7(2):253–259. [PubMed] [Google Scholar]
- 8.Czyżyk A, Szczepanik ZX. Diabetes mellitus and cancer. European Journal of Internal Medicine. 2000;11(5):245–252. doi: 10.1016/s0953-6205(00)00106-0. [DOI] [PubMed] [Google Scholar]
- 9.Kostadinova R, Wahli W, Michalik L. PPARs in diseases: control mechanisms of inflammation. Current Medicinal Chemistry. 2005;12(25):2995–3009. doi: 10.2174/092986705774462905. [DOI] [PubMed] [Google Scholar]
- 10.Kota BP, Huang TH-W, Roufogalis BD. An overview on biological mechanisms of PPARs. Pharmacological Research. 2005;51(2):85–94. doi: 10.1016/j.phrs.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 11.Charbonnel B. Glitazones in the treatment of diabetes mellitus: clinical outcomes in large scale clinical trials. Fundamental & Clinical Pharmacology. 2007;21(supplement 2):19–20. doi: 10.1111/j.1472-8206.2007.00533.x. [DOI] [PubMed] [Google Scholar]
- 12.Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial in macroVascular Events): a randomised controlled trial. The Lancet. 2005;366(9493):1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 13.Blaschke F, Spanheimer R, Khan M, Law RE. Vascular effects of TZDs: new implications. Vascular Pharmacology. 2006;45(1):3–18. doi: 10.1016/j.vph.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Michalik L, Desvergne B, Wahli W. Peroxisome-proliferator-activated receptors and cancers: complex stories. Nature Reviews Cancer. 2004;4(1):61–70. doi: 10.1038/nrc1254. [DOI] [PubMed] [Google Scholar]
- 15.Galli A, Mello T, Ceni E, Surrenti E, Surrenti C. The potential of antidiabetic thiazolidinediones for anticancer therapy. Expert Opinion on Investigational Drugs. 2006;15(9):1039–1049. doi: 10.1517/13543784.15.9.1039. [DOI] [PubMed] [Google Scholar]
- 16.Koro C, Barrett S, Qizilbash N. Cancer risks in thiazolidinedione users compared to other anti-diabetic agents. Pharmacoepidemiology and Drug Safety. 2007;16(5):485–492. doi: 10.1002/pds.1352. [DOI] [PubMed] [Google Scholar]
- 17.Ramos-Nino ME, MacLean CD, Littenberg B. Association between cancer prevalence and use of thiazolidinediones: results from the Vermont Diabetes Information System. BMC Medicine. 2007;5, article 17:1–7. doi: 10.1186/1741-7015-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Govindarajan R, Ratnasinghe L, Simmons DL, et al. Thiazolidinediones and the risk of lung, prostate, and colon cancer in patients with diabetes. Journal of Clinical Oncology. 2007;25(12):1476–1481. doi: 10.1200/JCO.2006.07.2777. [DOI] [PubMed] [Google Scholar]
- 19.Ulrich CM, Bigler J, Potter JD. Non-steroidal anti-inflammatory drugs for cancer prevention: promise, perils and pharmacogenetics. Nature Reviews Cancer. 2006;6(2):130–140. doi: 10.1038/nrc1801. [DOI] [PubMed] [Google Scholar]
- 20.Seger R, Krebs EG. The MAPK signaling cascade. The FASEB Journal. 1995;9(9):726–735. [PubMed] [Google Scholar]
- 21.Yoon S, Seger R. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors. 2006;24(1):21–44. doi: 10.1080/02699050500284218. [DOI] [PubMed] [Google Scholar]
- 22.Chuderland D, Seger R. Protein-protein interactions in the regulation of the extracellular signal-regulated kinase. Molecular Biotechnology. 2005;29(1):57–74. doi: 10.1385/MB:29:1:57. [DOI] [PubMed] [Google Scholar]
- 23.Shaul YD, Seger R. The MEK/ERK cascade: from signaling specificity to diverse functions. Biochimica et Biophysica Acta. 2007;1773(8):1213–1226. doi: 10.1016/j.bbamcr.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 24.Torii S, Yamamoto T, Tsuchiya Y, Nishida E. ERK MAP kinase in G1 cell cycle progression and cancer. Cancer Science. 2006;97(8):697–702. doi: 10.1111/j.1349-7006.2006.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26(22):3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 26.McCubrey JA, Steelman LS, Chappell WH, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochimica et Biophysica Acta. 2007;1773(8):1263–1284. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alvarado Y, Giles FJ. Ras as a therapeutic target in hematologic malignancies. Expert Opinion on Emerging Drugs. 2007;12(2):271–284. doi: 10.1517/14728214.12.2.271. [DOI] [PubMed] [Google Scholar]
- 28.Morgan MA, Ganser A, Reuter CWM. Targeting the RAS signaling pathway in malignant hematologic diseases. Current Drug Targets. 2007;8(2):217–235. doi: 10.2174/138945007779940043. [DOI] [PubMed] [Google Scholar]
- 29.Nolte RT, Wisely GB, Westin S, et al. Ligand binding and co-activator assembly of the peroxisome proliferator- activated receptor-γ . Nature. 1998;395(6698):137–143. doi: 10.1038/25931. [DOI] [PubMed] [Google Scholar]
- 30.Burgermeister E, Chuderland D, Hanoch T, Meyer M, Liscovitch M, Seger R. Interaction with MEK causes nuclear export and downregulation of peroxisome proliferator-activated receptor γ . Molecular and Cellular Biology. 2007;27(3):803–817. doi: 10.1128/MCB.00601-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papageorgiou E, Pitulis N, Msaouel P, Lembessis P, Koutsilieris M. The non-genomic crosstalk between PPAR-γ ligands and ERK1/2 in cancer cell lines. Expert Opinion on Therapeutic Targets. 2007;11(8):1071–1085. doi: 10.1517/14728222.11.8.1071. [DOI] [PubMed] [Google Scholar]
- 32.Miard S, Fajas L. Atypical transcriptional regulators and cofactors of PPARγ . International Journal of Obesity. 2005;29(supplement 1):S10–S12. doi: 10.1038/sj.ijo.0802906. [DOI] [PubMed] [Google Scholar]
- 33.Grommes C, Landreth GE, Heneka MT. Antineoplastic effects of peroxisome proliferator-activated receptor γ agonists. The Lancet Oncology. 2004;5(7):419–429. doi: 10.1016/S1470-2045(04)01509-8. [DOI] [PubMed] [Google Scholar]
- 34.Lu J, Imamura K, Nomura S, et al. Chemopreventive effect of peroxisome proliferator-activated receptor γ on gastric carcinogenesis in mice. Cancer Research. 2005;65(11):4769–4774. doi: 10.1158/0008-5472.CAN-04-2293. [DOI] [PubMed] [Google Scholar]
- 35.Nicol CJ, Yoon M, Ward JM, et al. PPARγ influences susceptibility to DMBA-induced mammary, ovarian and skin carcinogenesis. Carcinogenesis. 2004;25(9):1747–1755. doi: 10.1093/carcin/bgh160. [DOI] [PubMed] [Google Scholar]
- 36.Girnun GD, Smith WM, Drori S, et al. APC-dependent suppression of colon carcinogenesis by PPARγ . Proceedings of the National Academy of Sciences of the United States of America. 2002;99(21):13771–13776. doi: 10.1073/pnas.162480299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meirhaeghe A, Amouyel P. Impact of genetic variation of PPARγ in humans. Molecular Genetics and Metabolism. 2004;83(1-2):93–102. doi: 10.1016/j.ymgme.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 38.Sarraf P, Mueller E, Smith WM, et al. Loss-of-function mutations in PPARγ associated with human colon cancer. Molecular Cell. 1999;3(6):799–804. doi: 10.1016/s1097-2765(01)80012-5. [DOI] [PubMed] [Google Scholar]
- 39.Tahara T, Arisawa T, Shibata T, et al. Influence of peroxisome proliferator-activated receptor (PPAR)γ Plo12Ala polymorphism as a shared risk marker for both gastric cancer and impaired fasting glucose (IFG) in Japanese. Digestive Diseases and Sciences. 2008;53(3):614–621. doi: 10.1007/s10620-007-9944-8. [DOI] [PubMed] [Google Scholar]
- 40.Diradourian C, Girard J, Pégorier J-P. Phosphorylation of PPARs: from molecular characterization to physiological relevance. Biochimie. 2005;87(1):33–38. doi: 10.1016/j.biochi.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 41.Rochette-Egly C. Nuclear receptors: integration of multiple signalling pathways through phosphorylation. Cellular Signalling. 2003;15(4):355–366. doi: 10.1016/s0898-6568(02)00115-8. [DOI] [PubMed] [Google Scholar]
- 42.Genini D, Catapano CV. Control of peroxisome proliferator-activated receptor fate by the ubiquitin-proteasome system. Journal of Receptors and Signal Transduction. 2006;26(5-6):679–692. doi: 10.1080/10799890600928202. [DOI] [PubMed] [Google Scholar]
- 43.Floyd ZE, Stephens JM. Interferon-γ-mediated activation and ubiquitin-proteasome-dependent degradation of PPARγ in adipocytes. Journal of Biological Chemistry. 2002;277(6):4062–4068. doi: 10.1074/jbc.M108473200. [DOI] [PubMed] [Google Scholar]
- 44.Burns KA, Vanden Heuvel JP. Modulation of PPAR activity via phosphorylation. Biochimica et Biophysica Acta. 2007;1771(8):952–960. doi: 10.1016/j.bbalip.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rangwala SM, Rhoades B, Shapiro JS, et al. Genetic modulation of PPARγ phosphorylation regulates insulin sensitivity. Developmental Cell. 2003;5(4):657–663. doi: 10.1016/s1534-5807(03)00274-0. [DOI] [PubMed] [Google Scholar]
- 46.Hosooka T, Noguchi T, Kotani K, et al. Dok1 mediates high-fat diet-induced adipocyte hypertrophy and obesity through modulation of PPAR-γ phosphorylation. Nature Medicine. 2008;14(2):188–193. doi: 10.1038/nm1706. [DOI] [PubMed] [Google Scholar]
- 47.Amazit L, Pasini L, Szafran AT, et al. Regulation of SRC-3 intercompartmental dynamics by estrogen receptor and phosphorylation. Molecular and Cellular Biology. 2007;27(19):6913–6932. doi: 10.1128/MCB.01695-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnstone CN, Mongroo PS, Rich AS, et al. Parvin-β inhibits breast cancer tumorigenicity and promotes CDK9-mediated peroxisome proliferator-activated receptor gamma 1 phosphorylation. Molecular and Cellular Biology. 2008;28(2):687–704. doi: 10.1128/MCB.01617-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Giaginis C, Margeli A, Theocharis S. Peroxisome proliferator-activated receptor-γ ligands as investigational modulators of angiogenesis. Expert Opinion on Investigational Drugs. 2007;16(10):1561–1572. doi: 10.1517/13543784.16.10.1561. [DOI] [PubMed] [Google Scholar]
- 50.Feinstein DL, Spagnolo A, Akar C, et al. Receptor-independent actions of PPAR thiazolidinedione agonists: is mitochondrial function the key? Biochemical Pharmacology. 2005;70(2):177–188. doi: 10.1016/j.bcp.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 51.Gardner OS, Dewar BJ, Graves LM. Activation of mitogen-activated protein kinases by peroxisome proliferator-activated receptor ligands: an example of nongenomic signaling. Molecular Pharmacology. 2005;68(4):933–941. doi: 10.1124/mol.105.012260. [DOI] [PubMed] [Google Scholar]
- 52.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307(5715):1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 53.Watanabe M, Houten SM, Mataki C, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439(7075):484–489. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- 54.Brown AJ, Jupe S, Briscoe CP. A family of fatty acid binding receptors. DNA and Cell Biology. 2005;24(1):54–61. doi: 10.1089/dna.2005.24.54. [DOI] [PubMed] [Google Scholar]
- 55.Itoh Y, Kawamata Y, Harada M, et al. Free fatty acids regulate insulin secretion from pancreatic β cells through GPR40. Nature. 2003;422(6928):173–176. doi: 10.1038/nature01478. [DOI] [PubMed] [Google Scholar]
- 56.Itoh Y, Hinuma S. GPR40, a free fatty acid receptor on pancreatic β cells, regulates insulin secretion. Hepatology Research. 2005;33(2):171–173. doi: 10.1016/j.hepres.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 57.Hardy S, St-Onge GG, Joly É, Langelier Y, Prentki M. Oleate promotes the proliferation of breast cancer cells via the G protein-coupled receptor GPR40. Journal of Biological Chemistry. 2005;280(14):13285–13291. doi: 10.1074/jbc.M410922200. [DOI] [PubMed] [Google Scholar]
- 58.Kotarsky K, Nilsson NE, Flodgren E, Owman C, Olde B. A human cell surface receptor activated by free fatty acids and thiazolidinedione drugs. Biochemical and Biophysical Research Communications. 2003;301(2):406–410. doi: 10.1016/s0006-291x(02)03064-4. [DOI] [PubMed] [Google Scholar]
- 59.Kampa M, Castanas E. Membrane steroid receptor signaling in normal and neoplastic cells. Molecular and Cellular Endocrinology. 2006;246(1-2):76–82. doi: 10.1016/j.mce.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 60.Greger JG, Fursov N, Cooch N, et al. Phosphorylation of MNAR promotes estrogen activation of phosphatidylinositol 3-kinase. Molecular and Cellular Biology. 2007;27(5):1904–1913. doi: 10.1128/MCB.01732-06. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 61.Wong C-W, McNally C, Nickbarg E, Komm BS, Cheskis BJ. Estrogen receptor-interacting protein that modulates its nongenomic activity-crosstalk with Src/Erk phosphorylation cascade. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(23):14783–14788. doi: 10.1073/pnas.192569699. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 62.Ptak-Belowska A, Pawlik MW, Krzysiek-Mączka G, Brzozowski T, Pawlik WW. Transcriptional upregulation of gastrin in response to peroxisome proliferator-activated receptor gamma agonist triggers cell survival pathways. Journal of Physiology and Pharmacology. 2007;58(4):793–801. [PubMed] [Google Scholar]
- 63.Tencer L, Burgermeister E, Ebert MP, Liscovitch M. Rosiglitazone induces caveolin-1 by PPARγ-dependent and PPRE-independent mechanisms: the role of EGF receptor signaling and its effect on cancer cell drug resistance. Anticancer Research. 2008;28(2A):895–906. [PubMed] [Google Scholar]
- 64.Kim K-H, Cho YS, Park J-M, Yoon S-O, Kim K-W, Chung A-S. Pro-MMP-2 activation by the PPARγ agonist, ciglitazone, induces cell invasion through the generation of ROS and the activation of ERK. FEBS Letters. 2007;581(17):3303–3310. doi: 10.1016/j.febslet.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 65.Kim J-A, Park K-S, Kim H-I, et al. Troglitazone activates p21Cip/WAF1 through the ERK pathway in HCT15 human colorectal cancer cells. Cancer Letters. 2002;179(2):185–195. doi: 10.1016/s0304-3835(01)00869-2. [DOI] [PubMed] [Google Scholar]
- 66.Masamune A, Satoh K, Sakai Y, Yoshida M, Satoh A, Shimosegawa T. Ligands of peroxisome proliferator-activated receptor-gamma induce apoptosis in AR42J cells. Pancreas. 2002;24(2):130–138. doi: 10.1097/00006676-200203000-00003. [DOI] [PubMed] [Google Scholar]
- 67.Han S, Roman J. Rosiglitazone suppresses human lung carcinoma cell growth through PPARγ-dependent and PPARγ-independent signal pathways. Molecular Cancer Therapeutics. 2006;5(2):430–437. doi: 10.1158/1535-7163.MCT-05-0347. [DOI] [PubMed] [Google Scholar]
- 68.Li M, Lee TW, Yim APC, Mok TSK, Chen GG. Apoptosis induced by troglitazone is both peroxisome proliterator-activated receptor-γ- and ERK-dependent in human non-small lung cancer cells. Journal of Cellular Physiology. 2006;209(2):428–438. doi: 10.1002/jcp.20738. [DOI] [PubMed] [Google Scholar]
- 69.Hashimoto K, Farrow BJ, Evers BM. Activation and role of MAP kinases in 15d-PGJ2-induced apoptosis in the human pancreatic cancer cell line MIA PaCa-2. Pancreas. 2004;28(2):153–159. doi: 10.1097/00006676-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 70.Bos CL, Richel DJ, Ritsema T, Peppelenbosch MP, Versteeg HH. Prostanoids and prostanoid receptors in signal transduction. The International Journal of Biochemistry & Cell Biology. 2004;36(7):1187–1205. doi: 10.1016/j.biocel.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 71.Straus DS, Glass CK. Cyclopentenone prostaglandins: new insights on biological activities and cellular targets. Medicinal Research Reviews. 2001;21(3):185–210. doi: 10.1002/med.1006. [DOI] [PubMed] [Google Scholar]
- 72.Stenson WF. Prostaglandins and epithelial response to injury. Current Opinion in Gastroenterology. 2007;23(2):107–110. doi: 10.1097/MOG.0b013e3280143cb6. [DOI] [PubMed] [Google Scholar]
- 73.Kim E-H, Na H-K, Surh Y-J. Upregulation of VEGF by 15-deoxy-Δ 12,14-prostaglandin J2 via heme oxygenase-1 and ERK1/2 signaling in MCF-7 cells. Annals of the New York Academy of Sciences. 2006;1090:375–384. doi: 10.1196/annals.1378.041. [DOI] [PubMed] [Google Scholar]
- 74.Lefebvre A-M, Chen I, Desreumaux P, et al. Activation of the peroxisome proliferator-activated receptor γ promotes the development of colon tumors in C57BL/6J-APCMin/+ mice. Nature Medicine. 1998;4(9):1053–1057. doi: 10.1038/2036. [DOI] [PubMed] [Google Scholar]
- 75.Saez E, Tontonoz P, Nelson MC, et al. Activators of the nuclear receptor PPARγ enhance colon polyp formation. Nature Medicine. 1998;4(9):1058–1061. doi: 10.1038/2042. [DOI] [PubMed] [Google Scholar]
- 76.Pino MV, Kelley MF, Jayyosi Z. Promotion of colon tumors in C57BL/6J-APCmin/+ mice by thiazolidinedione PPARγ agonists and a structurally unrelated PPARγ agonist. Toxicologic Pathology. 2004;32(1):58–63. doi: 10.1080/01926230490261320. [DOI] [PubMed] [Google Scholar]
- 77.Yang K, Fan K-H, Lamprecht SA, et al. Peroxisome proliferator-activated receptor γ agonist troglitazone induces colon tumors in normal C57BL/6J mice and enhances colonic carcinogenesis in A p c 1638N/+ M I h +/− double mutant mice. International Journal of Cancer. 2005;116(4):495–499. doi: 10.1002/ijc.21018. [DOI] [PubMed] [Google Scholar]
- 78.Yee LD, Williams N, Wen P, et al. Pilot study of rosiglitazone therapy in women with breast cancer: effects of short-term therapy on tumor tissue and serum markers. Clinical Cancer Research. 2007;13(1):246–252. doi: 10.1158/1078-0432.CCR-06-1947. [DOI] [PubMed] [Google Scholar]
- 79.Kebebew E, Peng M, Reiff E, et al. A phase II trial of rosiglitazone in patients with thyroglobulin-positive and radioiodine-negative differentiated thyroid cancer. Surgery. 2006;140(6):960–967. doi: 10.1016/j.surg.2006.07.038. [DOI] [PubMed] [Google Scholar]
- 80.Egerod FL, Nielsen HS, Iversen L, Thorup I, Storgaard T, Oleksiewicz MB. Biomarkers for early effects of carcinogenic dual-acting PPAR agonists in rat urinary bladder urothelium in vivo. Biomarkers. 2005;10(4):295–309. doi: 10.1080/13547500500218682. [DOI] [PubMed] [Google Scholar]
- 81.Oleksiewicz MB, Thorup I, Nielsen HS, et al. Generalized cellular hypertrophy is induced by a dual-acting PPAR agonist in rat urinary bladder urothelium in vivo. Toxicologic Pathology. 2005;33(5):552–560. doi: 10.1080/01926230500214657. [DOI] [PubMed] [Google Scholar]
- 82.Balakumar P, Rose M, Ganti SS, Krishan P, Singh M. PPAR dual agonists: are they opening Pandora's Box? Pharmacological Research. 2007;56(2):91–98. doi: 10.1016/j.phrs.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 83.Hellmold H, Zhang H, Andersson U, et al. Tesaglitazar, a PPARα/γ agonist, induces interstitial mesenchymal cell DNA synthesis and fibrosarcomas in subcutaneous tissues in rats. Toxicological Sciences. 2007;98(1):63–74. doi: 10.1093/toxsci/kfm094. [DOI] [PubMed] [Google Scholar]
- 84.Tannehill-Gregg SH, Sanderson TP, Minnema D, et al. Rodent carcinogenicity profile of the antidiabetic dual PPAR α and γ agonist muraglitazar. Toxicological Sciences. 2007;98(1):258–270. doi: 10.1093/toxsci/kfm083. [DOI] [PubMed] [Google Scholar]
- 85.Saez E, Rosenfeld J, Livolsi A, et al. PPARγ signaling exacerbates mammary gland tumor development. Genes & Development. 2004;18(5):528–540. doi: 10.1101/gad.1167804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen L, Necela BM, Su W, et al. Peroxisome proliferator-activated receptor γ promotes epithelial to mesenchymal transformation by Rho GTPase-dependent activation of ERK1/2. Journal of Biological Chemistry. 2006;281(34):24575–24587. doi: 10.1074/jbc.M604147200. [DOI] [PubMed] [Google Scholar]
- 87.Bruna A, Nicolàs M, Muñoz A, Kyriakis JM, Caelles C. Glucocorticoid receptor-JNK interaction mediates inhibition of the JNK pathway by glucocorticoids. The EMBO Journal. 2003;22(22):6035–6044. doi: 10.1093/emboj/cdg590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Clark DE, Poteet-Smith CE, Smith JA, Lannigan DA. Rsk2 allosterically activates estrogen receptor α by docking to the hormone-binding domain. The EMBO Journal. 2001;20(13):3484–3494. doi: 10.1093/emboj/20.13.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yin Y, Yuan H, Wang C, et al. 3-phosphoinositide-dependent protein kinase-1 activates the peroxisome proliferator-activated receptor-γ and promotes adipocyte differentiation. Molecular Endocrinology. 2006;20(2):268–278. doi: 10.1210/me.2005-0197. [DOI] [PubMed] [Google Scholar]
- 90.Sarruf DA, Iankova I, Abella A, Assou S, Miard S, Fajas L. Cyclin D3 promotes adipogenesis through activation of peroxisome proliferator-activated receptor γ . Molecular and Cellular Biology. 2005;25(22):9985–9995. doi: 10.1128/MCB.25.22.9985-9995.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Abella A, Dubus P, Malumbres M, et al. Cdk4 promotes adipogenesis through PPARγ activation. Cell Metabolism. 2005;2(4):239–249. doi: 10.1016/j.cmet.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 92.Winn RA, Van Scoyk M, Hammond M, et al. Antitumorigenic effect of Wnt 7a and Fzd 9 in non-small cell lung cancer cells is mediated through ERK-5-dependent activation of peroxisome proliferator-activated receptor γ . Journal of Biological Chemistry. 2006;281(37):26943–26950. doi: 10.1074/jbc.M604145200. [DOI] [PubMed] [Google Scholar]
- 93.Akaike M, Che W, Marmarosh N-L, et al. The hinge-helix 1 region of peroxisome proliferator-activated receptor γ1 (PPARγ1) mediates interaction with extracellular signal-regulated kinase 5 and PPARγ1 transcriptional activation: involvement in flow-induced PPARγ activation in endothelial cells. Molecular and Cellular Biology. 2004;24(19):8691–8704. doi: 10.1128/MCB.24.19.8691-8704.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu J, Wang H, Zuo Y, Farmer SR. Functional interaction between peroxisome proliferator-activated receptor γ and β-catenin. Molecular and Cellular Biology. 2006;26(15):5827–5837. doi: 10.1128/MCB.00441-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Katoh M, Katoh M. WNT signaling pathway and stem cell signaling network. Clinical Cancer Research. 2007;13(14):4042–4045. doi: 10.1158/1078-0432.CCR-06-2316. [DOI] [PubMed] [Google Scholar]
- 96.Kelly D, Campbell JI, King TP, et al. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-γ and RelA. Nature Immunology. 2003;5(1):104–112. doi: 10.1038/ni1018. [DOI] [PubMed] [Google Scholar]
- 97.von Knethen A, Soller M, Tzieply N, et al. PPARγ1 attenuates cytosol to membrane translocation of PKCα to desensitize monocytes/macrophages. Journal of Cell Biology. 2007;176(5):681–694. doi: 10.1083/jcb.200605038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Burgermeister E, Seger R. MAPK kinases as nucleo-cytoplasmic shuttles for PPARγ . Cell Cycle. 2007;6(13):1539–1548. doi: 10.4161/cc.6.13.4453. [DOI] [PubMed] [Google Scholar]
- 99.Kim HJ, Hwang J-Y, Kim HJ, et al. Expression of a peroxisome proliferator-activated receptor γ1 splice variant that was identified in human lung cancers suppresses cell death induced by cisplatin and oxidative stress. Clinical Cancer Research. 2007;13(9):2577–2583. doi: 10.1158/1078-0432.CCR-06-2062. [DOI] [PubMed] [Google Scholar]
- 100.Nomura S, Nakajima A, Ishimine S, Matsuhashi N, Kadowaki T, Kaminishi M. Differential expression of peroxisome proliferator-activated receptor in histologically different human gastric cancer tissues. Journal of Experimental and Clinical Cancer Research. 2006;25(3):443–448. [PubMed] [Google Scholar]
- 101.Mukunyadzi P, Ai L, Portilla D, Barnes EL, Fan C-Y. Expression of peroxisome proliferator-activated receptor gamma in salivary duct carcinoma: immunohistochemical analysis of 15 cases. Modern Pathology. 2003;16(12):1218–1223. doi: 10.1097/01.MP.0000096042.70559.7E. [DOI] [PubMed] [Google Scholar]
- 102.Papadaki I, Mylona E, Giannopoulou I, Markaki S, Keramopoulos A, Nakopoulou L. PPARγ expression in breast cancer: clinical value and correlation with ERβ . Histopathology. 2005;46(1):37–42. doi: 10.1111/j.1365-2559.2005.02056.x. [DOI] [PubMed] [Google Scholar]
- 103.Giroux S, Tremblay M, Bernard D, et al. Embryonic death of Mek1-deficient mice reveals a role for this kinase in angiogenesis in the labyrinthine region of the placenta. Current Biology. 1999;9(7):369–372. doi: 10.1016/s0960-9822(99)80164-x. [DOI] [PubMed] [Google Scholar]
- 104.Bélanger L-F, Roy S, Tremblay M, et al. Mek2 is dispensable for mouse growth and development. Molecular and Cellular Biology. 2003;23(14):4778–4787. doi: 10.1128/MCB.23.14.4778-4787.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Scholl FA, Dumesic PA, Khavari PA. Effects of active MEK1 expression in vivo. Cancer Letters. 2005;230(1):1–5. doi: 10.1016/j.canlet.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 106.Wan Y, Chong L-W, Evans RM. PPAR-γ regulates osteoclastogenesis in mice. Nature Medicine. 2007;13(12):1496–1503. doi: 10.1038/nm1672. [DOI] [PubMed] [Google Scholar]
- 107.Hobbs RM, Silva-Vargas V, Groves R, Watt FM. Expression of activated MEK1 in differentiating epidermal cells is sufficient to generate hyperproliferative and inflammatory skin lesions. Journal of Investigative Dermatology. 2004;123(3):503–515. doi: 10.1111/j.0022-202X.2004.23225.x. [DOI] [PubMed] [Google Scholar]
- 108.Scholl FA, Dumesic PA, Barragan DI, et al. Mek1/2 MAPK kinases are essential for mammalian development, homeostasis, and Raf-induced hyperplasia. Developmental Cell. 2007;12(4):615–629. doi: 10.1016/j.devcel.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 109.Scholl FA, Dumesic PA, Khavari PA. Mek1 alters epidermal growth and differentiation. Cancer Research. 2004;64(17):6035–6040. doi: 10.1158/0008-5472.CAN-04-0017. [DOI] [PubMed] [Google Scholar]
- 110.Prusty D, Park B-H, Davis KE, Farmer SR. Activation of MEK/ERK signaling promotes adipogenesis by enhancing peroxisome proliferator-activated receptor γ (PPARγ) and C/EBPα gene expression during the differentiation of 3T3-L1 preadipocytes. Journal of Biological Chemistry. 2002;277(48):46226–46232. doi: 10.1074/jbc.M207776200. [DOI] [PubMed] [Google Scholar]
- 111.Chuang CC, Yang RS, Tsai KS, Ho FM, Liu SH. Hyperglycemia enhances adipogenic induction of lipid accumulation: involvement of extracellular signal-regulated protein kinase 1/2, phosphoinositide 3-kinase/Akt, and peroxisome proliferator-activated receptor γ signaling. Endocrinology. 2007;148(9):4267–4275. doi: 10.1210/en.2007-0179. [DOI] [PubMed] [Google Scholar]
- 112.Coll T, Jové M, Rodríguez-Calvo R, et al. Palmitate-mediated downregulation of peroxisome proliferator-activated receptor-γ coactivator 1α in skeletal muscle cells involves MEK1/2 and nuclear factor-κB activation. Diabetes. 2006;55(10):2779–2787. doi: 10.2337/db05-1494. [DOI] [PubMed] [Google Scholar]
- 113.Feinstein TN, Linstedt AD. MEK1-dependent Golgi unlinking occurs in G2 phase and promotes the G2/M cell cycle transition. Molecular Biology of the Cell. 2007;18(2):594–604. doi: 10.1091/mbc.E06-06-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shaul YD, Seger R. ERK1c regulates Golgi fragmentation during mitosis. Journal of Cell Biology. 2006;172(6):885–897. doi: 10.1083/jcb.200509063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Feinstein TN, Linstedt AD. Mitogen-activated protein kinase kinase 1-dependent Golgi unlinking occurs in G2 phase and promotes the G2/M cell cycle transition. Molecular Biology of the Cell. 2007;18(2):594–604. doi: 10.1091/mbc.E06-06-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Teis D, Taub N, Kurzbauer R, et al. p14-MP1-MEK1 signaling regulates endosomal traffic and cellular proliferation during tissue homeostasis. Journal of Cell Biology. 2006;175(6):861–868. doi: 10.1083/jcb.200607025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pullikuth A, McKinnon E, Schaeffer H-J, Catling AD. The MEK1 scaffolding protein MP1 regulates cell spreading by integrating PAK1 and Rho signals. Molecular and Cellular Biology. 2005;25(12):5119–5133. doi: 10.1128/MCB.25.12.5119-5133.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Torii S, Kusakabe M, Yamamoto T, Maekawa M, Nishida E. Sef is a spatial regulator for Ras/MAP kinase signaling. Developmental Cell. 2004;7(1):33–44. doi: 10.1016/j.devcel.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 119.Morrison RF, Farmer SR. Role of PPARγ in regulating a cascade expression of cyclin-dependent kinase inhibitors, p18(INK4c) and p21(Waf1/Cip1), during adipogenesis. Journal of Biological Chemistry. 1999;274(24):17088–17097. doi: 10.1074/jbc.274.24.17088. [DOI] [PubMed] [Google Scholar]
- 120.Copland JA, Marlow LA, Kurakata S, et al. Novel high-affinity PPARγ agonist alone and in combination with paclitaxel inhibits human anaplastic thyroid carcinoma tumor growth via p21WAF1/CIP1 . Oncogene. 2006;25(16):2304–2317. doi: 10.1038/sj.onc.1209267. [DOI] [PubMed] [Google Scholar]
- 121.Jarvis MC, Gray TJB, Palmer CNA. Both PPARγ and PPARδ influence sulindac sulfide-mediated p21WAF1/CIP1 upregulation in a human prostate epithelial cell line. Oncogene. 2005;24(55):8211–8215. doi: 10.1038/sj.onc.1208983. [DOI] [PubMed] [Google Scholar]
- 122.Zang C, Liu H, Waechter M, et al. Dual PPARα/γ ligand TZD18 either alone or in combination with imatinib inhibits proliferation and induces apoptosis of human CML cell lines. Cell Cycle. 2006;5(19):2237–2243. doi: 10.4161/cc.5.19.3259. [DOI] [PubMed] [Google Scholar]
- 123.Liu H, Zang C, Fenner MH, et al. Growth inhibition and apoptosis in human Philadelphia chromosome-positive lymphoblastic leukemia cell lines by treatment with the dual PPARα/γ ligand TZD18. Blood. 2006;107(9):3683–3692. doi: 10.1182/blood-2005-05-2103. [DOI] [PubMed] [Google Scholar]
- 124.Palakurthi SS, Aktas H, Grubissich LM, Mortensen RM, Halperin JA. Anticancer effects of thiazolidinediones are independent of peroxisome proliferator-activated receptor γ and mediated by inhibition of translation initiation. Cancer Research. 2001;61(16):6213–6218. [PubMed] [Google Scholar]
- 125.Weber SM, Chambers KT, Bensch KG, Scarim AL, Corbett JA. PPARγ ligands induce ER stress in pancreatic β-cells: ER stress activation results in attenuation of cytokine signaling. American Journal of Physiology. 2004;287(6):E1171–E1177. doi: 10.1152/ajpendo.00331.2004. [DOI] [PubMed] [Google Scholar]
- 126.Wei S, Lin L-F, Yang C-C, et al. Thiazolidinediones modulate the expression of β-catenin and other cell-cycle regulatory proteins by targeting the F-box proteins of Skp1-Cul1-F-box protein E3 ubiquitin ligase independently of peroxisome proliferator-activated receptor γ . Molecular Pharmacology. 2007;72(3):725–733. doi: 10.1124/mol.107.035287. [DOI] [PubMed] [Google Scholar]
- 127.Huang J-W, Shiau C-W, Yang Y-T, et al. Peroxisome proliferator-activated receptor γ-independent ablation of cyclin D1 by thiazolidinediones and their derivatives in breast cancer cells. Molecular Pharmacology. 2005;67(4):1342–1348. doi: 10.1124/mol.104.007732. [DOI] [PubMed] [Google Scholar]
- 128.Sharma C, Pradeep A, Wong L, Rana A, Rana B. Peroxisome proliferator-activated receptor γ activation can regulate β-catenin levels via a proteasome-mediated and adenomatous polyposis coli-independent pathway. Journal of Biological Chemistry. 2004;279(34):35583–35594. doi: 10.1074/jbc.M403143200. [DOI] [PubMed] [Google Scholar]
- 129.Galusca B, Dumollard JM, Chambonniere ML, et al. Peroxisome proliferator activated receptor gamma immunohistochemical expression in human papillary thyroid carcinoma tissues. Possible relationship to lymph node metastasis. Anticancer Research. 2004;24(3B):1993–1997. [PubMed] [Google Scholar]
- 130.Segawa Y, Yoshimura R, Hase T, et al. Expression of peroxisome proliferator-activated receptor (PPAR) in human prostate cancer. The Prostate. 2002;51(2):108–116. doi: 10.1002/pros.10058. [DOI] [PubMed] [Google Scholar]
- 131.Mylona E, Nomikos A, Magkou C, et al. The clinicopathological and prognostic significance of membrane type 1 matrix metalloproteinase (MT1-MMP) and MMP-9 according to their localization in invasive breast carcinoma. Histopathology. 2007;50(3):338–347. doi: 10.1111/j.1365-2559.2007.02615.x. [DOI] [PubMed] [Google Scholar]
- 132.Takahashi H, Fujita K, Fujisawa T, et al. Inhibition of peroxisome proliferator-activated receptor gamma activity in esophageal carcinoma cells results in a drastic decrease of invasive properties. Cancer Science. 2006;97(9):854–860. doi: 10.1111/j.1349-7006.2006.00250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Masuda T, Wada K, Nakajima A, et al. Critical role of peroxisome proliferator-activated receptor γ on anoikis and invasion of squamous cell carcinoma. Clinical Cancer Research. 2005;11(11):4012–4021. doi: 10.1158/1078-0432.CCR-05-0087. [DOI] [PubMed] [Google Scholar]
- 134.Schaefer KL, Wada K, Takahashi H, et al. Peroxisome proliferator-activated receptor γ inhibition prevents adhesion to the extracellular matrix and induces anoikis in hepatocellular carcinoma cells. Cancer Research. 2005;65(6):2251–2259. doi: 10.1158/0008-5472.CAN-04-3037. [DOI] [PubMed] [Google Scholar]
- 135.Gensch C, Clever YP, Werner C, Hanhoun M, Böhm M, Laufs U. The PPAR-γ agonist pioglitazone increases neoangiogenesis and prevents apoptosis of endothelial progenitor cells. Atherosclerosis. 2007;192(1):67–74. doi: 10.1016/j.atherosclerosis.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 136.Wang C-H, Ting M-K, Verma S, et al. Pioglitazone increases the numbers and improves the functional capacity of endothelial progenitor cells in patients with diabetes mellitus. American Heart Journal. 2006;152(6):1051.e1–1051.e8. doi: 10.1016/j.ahj.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 137.Chintalgattu V, Harris GS, Akula SM, Katwa LC. PPAR-γ agonists induce the expression of VEGF and its receptors in cultured cardiac myofibroblasts. Cardiovascular Research. 2007;74(1):140–150. doi: 10.1016/j.cardiores.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 138.Yasuda E, Tokuda H, Ishisaki A, et al. PPAR-γ ligands up-regulate basic fibroblast growth factor-induced VEGF release through amplifying SAPK/JNK activation in osteoblasts. Biochemical and Biophysical Research Communications. 2005;328(1):137–143. doi: 10.1016/j.bbrc.2004.12.163. [DOI] [PubMed] [Google Scholar]
- 139.Fauconnet S, Lascombe I, Chabannes E, et al. Differential regulation of vascular endothelial growth factor expression by peroxisome proliferator-activated receptors in bladder cancer cells. Journal of Biological Chemistry. 2002;277(26):23534–23543. doi: 10.1074/jbc.M200172200. [DOI] [PubMed] [Google Scholar]
- 140.Pistrosch F, Herbrig K, Oelschlaegel U, et al. PPARγ-agonist rosiglitazone increases number and migratory activity of cultured endothelial progenitor cells. Atherosclerosis. 2005;183(1):163–167. doi: 10.1016/j.atherosclerosis.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 141.Burgermeister E, Schnoebelen A, Flament A, et al. A novel partial agonist of peroxisome proliferator-activated receptor-γ (PPARγ) recruits PPARγ-coactivator-1α, prevents triglyceride accumulation, and potentiates insulin signaling in vitro. Molecular Endocrinology. 2006;20(4):809–830. doi: 10.1210/me.2005-0171. [DOI] [PubMed] [Google Scholar]
- 142.Kawamata H, Tachibana M, Fujimori T, Imai Y. Differentiation-inducing therapy for solid tumors. Current Pharmaceutical Design. 2006;12(3):379–385. doi: 10.2174/138161206775201947. [DOI] [PubMed] [Google Scholar]
- 143.Fenner MH, Elstner E. Peroxisome proliferator-activated receptor-γ ligands for the treatment of breast cancer. Expert Opinion on Investigational Drugs. 2005;14(6):557–568. doi: 10.1517/13543784.14.6.557. [DOI] [PubMed] [Google Scholar]
- 144.Lee SY, Hur GY, Jung KH, et al. PPAR-γ agonist increase gefitinib's antitumor activity through PTEN expression. Lung Cancer. 2006;51(3):297–301. doi: 10.1016/j.lungcan.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 145.Yang Z, Bagheri-Yarmand R, Balasenthil S, et al. HER2 regulation of peroxisome proliferator-activated receptor γ (PPARγ) expression and sensitivity of breast cancer cells to PPARγ ligand therapy. Clinical Cancer Research. 2003;9(8):3198–3203. [PubMed] [Google Scholar]
- 146.Wang D, Boerner SA, Winkler JD, LoRusso PM. Clinical experience of MEK inhibitors in cancer therapy. Biochimica et Biophysica Acta. 2007;1773(8):1248–1255. doi: 10.1016/j.bbamcr.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 147.Messersmith WA, Hidalgo M, Carducci M, Eckhardt SG. Novel targets in solid tumors: MEK inhibitors. Clinical Advances in Hematology and Oncology. 2006;4(11):831–836. [PubMed] [Google Scholar]
- 148.Madhunapantula SV, Robertson GP. Is B-Raf a good therapeutic target for melanoma and other malignancies? Cancer Research. 2008;68(1):5–8. doi: 10.1158/0008-5472.CAN-07-2038. [DOI] [PubMed] [Google Scholar]
- 149.Ihle NT, Paine-Murrieta G, Berggren MI, et al. The phosphatidylinositol-3-kinase inhibitor PX-866 overcomes resistance to the epidermal growth factor receptor inhibitor gefitinib in A-549 human non-small cell lung cancer xenografts. Molecular Cancer Therapeutics. 2005;4(9):1349–1357. doi: 10.1158/1535-7163.MCT-05-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Herbert B-S, Pearce VP, Hynan LS, et al. A peroxisome proliferator-activated receptor-γ agonist and the p53 rescue drug CP-31398 inhibit the spontaneous immortalization of breast epithelial cells. Cancer Research. 2003;63(8):1914–1919. [PubMed] [Google Scholar]
- 151.Vogt T, Hafner C, Bross K, et al. Antiangiogenetic therapy with pioglitazone, rofecoxib, and metronomic trofosfamide in patients with advanced malignant vascular tumors. Cancer. 2003;98(10):2251–2256. doi: 10.1002/cncr.11775. [DOI] [PubMed] [Google Scholar]
- 152.Reichle A, Bross K, Vogt T, et al. Pioglitazone and rofecoxib combined with angiostatically scheduled trofosfamide in the treatment of far-advanced melanoma and soft tissue sarcoma. Cancer. 2004;101(10):2247–2256. doi: 10.1002/cncr.20574. [DOI] [PubMed] [Google Scholar]
- 153.Kasper B, Ho AD, Egerer G. A new therapeutic approach in patients with advanced sarcoma. International Journal of Clinical Oncology. 2005;10(6):438–440. doi: 10.1007/s10147-005-0514-9. [DOI] [PubMed] [Google Scholar]
- 154.Hennig R, Ventura J, Segersvard R, et al. LY293111 improves efficacy of gemcitabine therapy on pancreatic cancer in a fluorescent orthotopic model in athymic mice. Neoplasia. 2005;7(4):417–425. doi: 10.1593/neo.04559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Baetz T, Eisenhauer E, Siu L, et al. A phase I study of oral LY293111 given daily in combination with irinotecan in patients with solid tumours. Investigational New Drugs. 2007;25(3):217–225. doi: 10.1007/s10637-006-9021-8. [DOI] [PubMed] [Google Scholar]
- 156.Schwartz GK, Weitzman A, O'Reilly E, et al. Phase I and pharmacokinetic study of LY293111, an orally bioavailable LTB4 receptor antagonist, in patients with advanced solid tumors. Journal of Clinical Oncology. 2005;23(23):5365–5373. doi: 10.1200/JCO.2005.02.766. [DOI] [PubMed] [Google Scholar]
- 157.Hau P, Kunz-Schughart L, Bogdahn U, et al. Low-dose chemotherapy in combination with COX-2 inhibitors and PPAR-gamma agonists in recurrent high-grade gliomas—a phase II study. Oncology. 2007;73(1-2):21–25. doi: 10.1159/000120028. [DOI] [PubMed] [Google Scholar]
- 158.Hedvat M, Jain A, Carson DA, et al. Inhibition of HER-kinase activation prevents ERK-mediated degradation of PPARγ . Cancer Cell. 2004;5(6):565–574. doi: 10.1016/j.ccr.2004.05.014. [DOI] [PubMed] [Google Scholar]