Abstract
Interfacial polyelectrolyte complexation (PEC) fiber has been proposed as a biostructural unit and biological construct for tissue engineering applications, with its ability to incorporate proteins, drug molecules, DNA nanoparticles, and cells. In this study, we evaluated the biocompatibility and blood compatibility of PEC fiber in order to assess its potential for in vivo applications in tissue engineering. Although chitosan-alginate PEC fibrous scaffold was found to be thrombogenic, the blood compatibility of the scaffold could be significantly improved by incorporating a small amount of heparin in the polyelectrolyte solution during fiber formation. The platelet microparticle production and platelet adhesion on the chitosan-alginate-heparin fibrous scaffold were comparable to those on the resting control. In vitro cytotoxicity test showed that the scaffold was not toxic to human mesenchymal stem cells (hMSCs). In the in vivo biocompatibility test in rats, no acute inflammation was observed in the subcutaneously or intramuscularly implanted specimens. Good cell in-filtration and vascularization were observed after 2 months of implantations. Enhanced extracellular matrix (ECM) deposition was observed when hMSCs were cultured in the transforming growth factor-β3 (TGF-β3)-encapsulated PEC fibrous scaffold in vitro, or when the TGF-β3-encapsulated PEC was implanted intramuscularly in vivo. The results showed that this versatile PEC fibrous scaffold could be used in various tissue engineering applications for its good biocompatible and blood compatible properties.
INTRODUCTION
Many tissue engineering applications require a bioactive and biocompatible material for building a tissue-scaffold construct. Ideally, the optimal material would provide structural support and an interactive and compatible cellular microenvironment, and also serve as a reservoir for the release of bioactive substances. Previously, we have proposed the use of interfacial polyelectrolyte complexation (PEC) to create such a bioactive and biocompatible scaffold.1 The PEC is a process of self-assembly that occurs when two oppositely charged polyelectrolytes come together. It has been applied to fabricate multilayer assemblies as antireflection coating, light-emitting diodes, and microcapsules.2–5 The interaction between two common, naturally derived polyelectrolytes, chitosan and alginate, has been exploited to produce different biomaterials in the form of microcapsule, hydrogel, film, and foam.6–8 Iwasaki et al. have produced fibers from the complexation of chitosan and alginate for cartilage tissue engineering.9 We have used the process to produce PEC fibers and demonstrated that they can be used as biostructural units for tissue engineering,1 with the possibility of encapsulating proteins,10 drug molecules, DNA,11 and cells to provide local and sustained delivery of biochemical signals. However, the biocompatibility of these fibers has not been investigated.
In addition to cytotoxicity and in vivo tissue biocompatibility, thrombogenicity is important for any new biomaterial development, particularly if these PEC fibers are likely to be in contact with blood, for example, in cardiovascular tissue engineering. Thrombogenicity is defined as the ability of a material to induce or promote the formation of thromboemboli.12 One of the major components of thrombogenicity is the platelet-material interaction. Platelets are critical to hemostasis by virtue of their ability to adhere, aggregate, and release the contents of their granules, as well as their capacity to alter their surface characteristics to support blood coagulation. It is a requirement for blood-contacting materials that platelet interactions with the surface do not lead to thrombosis. The platelet response to material surfaces in vitro, with respect to microparticle formation and platelet count, has often been used to assess hemocompatibility.13,14
In this study, we tested the cytotoxicity, blood compatibility, and tissue biocompatibility of the versatile bioactive PEC scaffolds. Cytoxicity was evaluated in terms of viability, proliferation, and differentiation, by culturing human mescenchymal stem cells (hMSCs) on the PEC scaffold. Blood compatibility was assessed with platelet activation induced by the PEC scaffold surface. Addition of a small amount of heparin into the fiber significantly improved the blood compatibility of the PEC scaffold; addition of gelatin and hyaluronic acid would improve hMSC attachment. Tissue biocompatibility was determined by histological analysis of the subcutaneous and intramuscular implantation in rats. Implantation of the PEC scaffolds induced negligible foreign body and inflammatory response. Collectively, the data suggest the potential of the tissue- and hemocompatible PEC fibrous scaffolds for various tissue engineering applications.
MATERIALS AND METHODS
PEC fiber fabrication
PEC fiber is formed by the electrostatic interaction between a polycation and a polyanion.1 Fiber formation was initiated from the interface of two oppositely charged polyelectrolyte solutions by a pair of forceps and continued to be drawn by rollers (Fig. 1). In this work, 0.5% w/v chitosan (85% degree of deacetylation, Sigma, St. Louis, MO) was used as the polycation, and 1% w/v sodium alginate solution (low molecular weight of 700,000–1,000,000, Sigma) with a viscosity of 250 cps at 25°C was used as the polyanion. The droplet volumes were fixed at 20 μL and a draw rate of 10 mm/s was typically employed. Air-dried fibers were collected and entangled with needle punching as previously described.15 Briefly, the fibers were first collected as a fibrous web and then passed through a chamber where needles with back hooks were periodically driven through the web. As the needles punched through the layers of fibers and broke them, the back hooks entangled the loose ends of the fibers to reinforce the fibers into a scaffold.
FIG. 1.

Schematic diagrams of the formation of PEC and fabrication of needle-punched scaffold.
PEC fiber with heparin was produced by drawing the fiber with 0.5% w/v chitosan against a 1:100 dilution of 2% w/v heparin (cell-culture tested heparin sodium salt from porcine intestinal mucosa, Sigma) in 1% alginate solution. PEC fiber with gelatin was formed by mixing 2% w/v gelatin solution into 1% w/v alginate and drawing against 0.5% w/v chitosan. Another type of PEC fiber, with hyaluronic acid, was formed by mixing 5% w/v hyaluronic acid in distilled water with alginate in the same fashion as gelatin, to produce fiber.
The amount of heparin released from the chitosan-alginate-heparin PEC fibrous scaffold was measured. Each scaffold, composed of approximately 100 fibers, was first washed with 1 mL of 0.9% sterile saline solution and incubated in 0.5 mL of saline solution at 37°C (n = 3). Samples were collected at 1 hour, and on days 1 and 7. The concentration of heparin in the wash and the supernatant at each time point was measured with Actichrome® Heparin (anti-fIIa) Assay kit (American Diagnostica, Stamford, CT).
Protein encapsulation
Platelet-derived growth factor-BB (PDGF-BB) has previously been incorporated into the fibers by addition into either the chitosan or alginate solution according to their molecular charge.10 In the present study, positively charged transforming growth factor-β3 (TGF-β3; human recombinant TGF-β3, Calbiochem, La Jolla, CA) was incorporated into 0.5% w/v chitosan solution to reach a final concentration of 1 mg/mL. Chitosan solution of 0.5% w/v was drawn against 1% w/v alginate solution to form chitosan-alginate fibers. The TGF-β3-encapsulated fibers were then mixed with blank chitosan-alginate fibers and the fiber mesh was entangled with needle punching. Each 5 mm×5 mm×1 mm scaffold contained approximately 200 ng of TGF-β3.
Controlled release of TGF-β3
Scaffolds containing 200 ng of TGF-β3 were immersed in DMEM/F-12 and incubated at 37°C. Samples were collected on days 2, 6, 10, 14, 19, 23, and 27. The concentration of TGF-β3 released was determined by TGF-β3 ELISA kit (R&D Systems, Minneapolis, MN) and was reported as cumulative release percentage.
Blood compatibility—in vitro evaluation of platelet response to scaffold
Platelet studies were conducted by adapting the protocol of Gemmell et al.13 Whole blood from normal healthy rabbits was collected in syringes preloaded with anticoagulant (5 Units/mL heparin). Platelet-rich plasma (PRP) was removed after centrifugation at 100 g for 10 min. All samples, including chitosan-alginate PEC scaffold, chitosan-alginate-heparin PEC scaffold, Silastic™ tubing (Dow Corning, Midland, MI), polyethylene terephthalate (PET) fibrous scaffold, cellulose acetate (Celanese Acetate, Dallas, TX) fibrous scaffold, glass microbeads (90–150 μm; Sigma), and fiber glass were weighed and washed with 1 mL of sterile 0.9% saline before addition of PRP. Since the diameters of the fibers or beads evaluated varied, a constant mass of each type of sample was used. About 200 mL of PRP was added to each of the samples in 1.7 mL polypropylene microcentrifuge tubes (n = 3). The samples were incubated for 60 min at 37°C under gentle agitation on an orbital shaker with a speed of 60 rpm. For each experiment, resting samples were also included: PRP alone and PRP with a final concentration of 5 mM ethylene diamine tetra acetate (EDTA) in micro-centrifuge tubes at 37°C. After incubation with the scaffold, approximately 180 μL of PRP from each tube was transferred to microcentrifuge tubes containing EDTA, with a final concentration of 5 mM EDTA. The material surfaces were prepared for scanning electron microscopy (SEM) by washing twice with saline and then fixing with 4% paraformaldahyde. The samples were analyzed immediately by flow cytometry or platelet counting.
Aliquots of the PRP (10 μL) were diluted in 200 μL of Hepes Tyrode buffer (HTB, pH 7.4; 137 mM sodium chloride, 2.7 mM potassium chloride, 16 mM sodium bicarbonate, 5 mM magnesium chloride, 3.5 mM HEPES, 1 g/L glucose, 2 g/L bovine serum albumin [BSA]) and incubated with 5 μL of mouse anti-CD41/CD61 antibody (Serotec, Oxford, UK) for 30 min at room temperature. Samples were washed three times with 1 mL HTB by centrifuging at 2000 rpm for 5 min. The samples were resuspended in 300 μL HTB with goat serum and Alexa-Flur 488 goat antimouse secondary antibody (1:500 dilution) for 20 min at room temperature. The excess secondary antibody was washed away with three washes of HTB. The samples were fixed with 1% paraformaldehyde and stored at 4°C prior to analysis. Platelet activation was assessed by flow cytometry (Becton-Dickinson FACScan flow cytometry) within 36 hours after fixation. Activation was monitored through the formation of platelet microparticles, which were identified by their scatter characteristics and signals for GPIIb/IIIa.
Scanning electron microscopy
Samples were incubated for 20 min in 4% paraformaldehyde (Sigma-Aldrich) in phosphate buffered saline (PBS) and then dehydrated in a graded ethanol series (30%, 50%, 70%, 95%, and two times in 100% ethanol) before critical point, dried from high purity carbon dioxide using 100% ethanol as a transitional solvent. Dried samples were supercoated with a 25 Å coat of chromium, and viewed on the top stage of a LEO FESEM (LEO 1550) (LEO Electron Microscopy, Cambridge, UK) at a 1 kV accelerating voltage.
HMSC culture
The hMSCs (Cambrex, Walkersville, MD) were cultured and expanded in MSC growth medium (MSCGM) with 5% fetal bovine serum, 2% L-glutamine, and 0.1% penicillin/streptomycin (Cambrex). MSCs used in the experiments were between passages 6 and 9.
Cell toxicity—in vitro evaluation of viability, proliferation, and differentiation
A cell suspension containing 5×105 hMSCs in 50 μL MSCGM was placed on top of a needle-punched scaffold, which was approximately 5 mm×5 mm×1 mm in size, in a 24-well plate with 6 mm transwell inserts. The cell-seeded scaffolds were cultured in MSCGM for cytotoxicity test.
Live/Dead Assay kit obtained from Molecular Probes (Eugene, OR) was used to investigate cell viability. The samples were gently washed with PBS, transferred to an 8-well cover-slip chamber, and incubated with 2 μM calcein AM and 3 μM ethidium homodimer-1 in serum-free medium for 30 min at 37°C. After incubation, the samples were washed with PBS. Medium was added to the samples before confocal imaging.
The cytotoxicity and cell proliferation in the cell-seeded fibrous scaffold over a period of 11 days were assessed by measuring the cell metabolic activity with Cell Proliferation Reagent WST-1 Assay (Roche Diagnostics, Mannheim, Germany). Briefly stated, 200 μL of WST-1 reagent was added to each of the 6-well-plates containing the sample with 2 mL of medium. The cells were incubated at 37°C for 2 hours. The cell proliferation level was determined at 450 nm. The background absorbance was measured on wells containing only the WST-1 reagent and the culture medium.
Cell-seeded scaffolds were fixed in 4% paraformaldehyde after 6 weeks of culture in either MSCGM proliferation medium or chondrogenic medium consisting of 1% BD™ ITS Premix (BD Bioscience, San Jose, CA), which contains insulin, human transferrin, and selenous acid, 100 nM dexamethasone, 40 μM proline, and 10 ng/mL TGF-β3 in DMEM/F-12 for histological analysis.
In vivo evaluation of tissue biocompatibility
For in vivo biocompatibility evaluation, two PEC scaffolds each were implanted either subcutaneously or intramuscularly in the back of adult rats. Two healthy adult female Wistar rats (Charles River, Raleigh, NC) with an approximate weight of 200–250 g were used for each time point, at 7 days and 2 months. The rats were anesthetized with an intramuscular injection of ketamine hydrochloric acid (HCl) (100 mg/kg) and xylazine (5 mg/kg). The surgical site was shaved and prepared with a solution of Betadine and alcohol. Surgery was performed under general inhalation anesthesia with 5% halothane/oxygen (O2) gas mixture and maintained with 1.5–2.5% halothane/O2 gas mixture by a nonrebreather mask. One longitudinal incision was made vertebrally through the full thickness of the skin. At both lateral sides of the incision, a subcutaneous pocket and an intramuscular pocket were created by blunt dissection with scissors. One PEC scaffold was inserted in each pocket. The intramuscular pocket was closed with vicryl® 5-o suture material, and the skin was closed with surgical staples.
For in vivo evaluation of the controlled release scaffold, blank chitosan-alginate PEC fibrous scaffold and TGF-β3-encapsulated PEC fibrous scaffold, each containing 200 ng of TGF-β3, were implanted into the intramuscular pockets, one of each scaffold into each lateral side of the back. At 2 months postimplantation, the animals were sacrificed and the implants with the surrounding tissues were retrieved and fixed in 4% paraformaldehyde for histological analysis.
Histology analysis
After being dehydrated with an alcohol gradient and paraffin-embedded, 10 μm sections of the paraformaldehyde-fixed in vivo and in vitro evaluation specimens were mounted on glass slides, washed with xylene, and rehydrated before staining with hematoxylin and eosin (H&E), Alcian blue, Safranin O/fast green, toluidine blue, or Masson trichrome.
Immunohistology chemistry
Paraffin-embedded sections were washed with xylene, rehydrated, and treated with pepsin for epitope retrieval at 1 mg/mL in Tris HCl, pH 2.0, for 15 min at room temperature. The sections were blocked with chicken serum, incubated with mouse IgG2a anticollagen antibody (Neomarkers Labvision, Fremont, CA) (1:1000 dilution) for 1 hour at room temperature. The slides were blocked with 3% peroxidase blocking solution for 10 min and incubated with HRP-conjugated chicken antimouse IgG antibody (Chemicon, Temicula, CA) at a dilution of 1:250 for 1 hour after washing. The antibody bindingsites were visualized using a DAB substrate Kit (Dako, Carpinteria, CA) and counterstained with hematoxylin. Negative control slides were incubated with nonspecific serum instead of the primary antibody.
Data analysis
All data are presented as mean ± SD. ANOVA and post hoc test were used to evaluate the statistical significance where indicated. Significance level was set at p <0.05.
RESULTS
Blood compatibility
Microparticle release and platelet count were used as the quantitative markers to evaluate the extent of bulk platelet activation and adhesion. Microparticles are small segments of activated platelets, observed as platelet dust shed from adhered platelets in SEM.16–17 Gemmell et al. reported that microparticle formation involves fibrinogen binding to the GPIIb/IIIa receptor. Percentages of microparticle release (relative to the total number of microparticles and platelets) after 1 hour incubation of materials with PRP are shown in Figure 2A. High percentages of microparticle release were observed with the chitosan-alginate PEC scaffold, as with polyethylene terepthalate (PET), glass, glass bead, and cellulose acetate fibers,18–20 all of which have been reported as platelet activating materials. However, a significantly lower percentage (p <0.01) was observed with chitosan-alginate-heparin scaffold, at a level comparable to negative resting control, compared to that of chitosan-alginate scaffold.
FIG. 2.

Blood compatibility evaluation with (A) percentage of microparticle release and (B) postincubation platelet count in bulk PRP after 1 hour of incubation with the materials. Values are shown as mean ± SD (n = 3). *p <0.01, **p <0.005.
The platelet count in the PRP supernatant after 1 hour of incubation was inversely proportional to the number of adherent platelets on the material surface. The higher degree of platelet adhesion is indicated by a lower platelet count in the PRP supernatant (Fig. 2B). Consistent with the microparticle percentage, lower platelet counts were observed on PET, glass fiber, and chitosan-alginate PEC fiber. A higher platelet count, and hence lower platelet adhesion, was observed in chitosan-alginate-heparin PEC fibers, Silastic tubing, and resting controls. The platelet count of chitosan-alginate-heparin scaffold was significantly higher than that of chitosan-alginate scaffold ( p <0.005). However, the platelet counts in the PRP samples incubated with glass bead and cellulose acetate fibers were also high, indicating that these materials have a lower degree of platelet adhesion although they yielded a high degree of platelet activation.
Platelet adhesion and morphology were also observed by SEM (Fig. 3). Very few platelets were observed on chitosan-alginate-heparin PEC fiber and Silastic™ tubing, as opposed to evidence of platelet adhesion on chitosan-alginate fiber, PET fiber, cellulose acetate fiber, glass bead, glass fiber, and glass cover slip. Adhering platelets on chitosan-alginate fiber and glass cover slip were more fully spread, possibly indicating higher levels of activation.
FIG. 3.
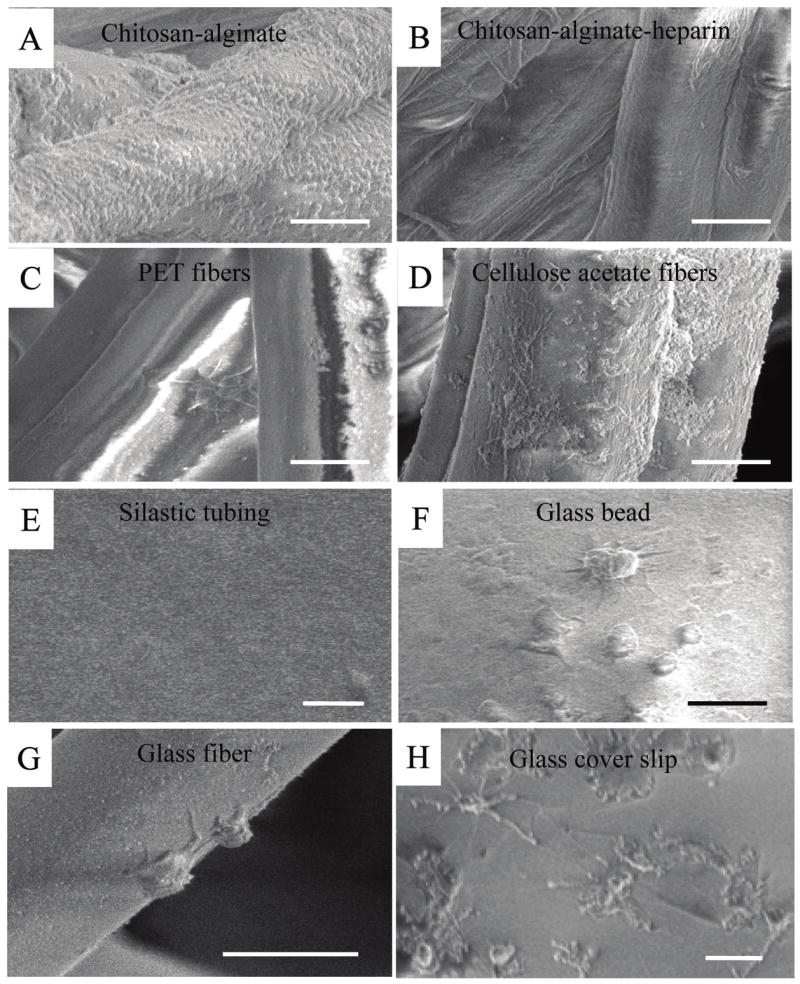
Scanning electron micrograph showing platelet adhesion on (A) chitosan-alginate PEC fibrous scaffold, (B) chitosan-alginate-heparin PEC fibrous scaffold, (C) PET fibers, (D) cellulose acetate fibers, (E) Silastic tubing, (F) glass bead, (G) glass fiber, and (H) glass cover slip. Bar = 5 μm.
Heparin release assay showed that 3.5 ± 0.2 U of uncomplexed heparin was washed out during the preincubation washing. No heparin release was detected after 1 hour of incubation. About 1.4 ± 1.0 U of heparin was detected in the supernatant after 24 hours of incubation and another 1.0 U was detected at the 7 day time point. Taken together, 2.4 U of heparin was released in 7 days, which was less than 8% of the incorporated heparin.
In vitro cytotoxicity evaluation
The results of WST-1 proliferation assay and live/dead assay of hMSCs cultured on three different formulations of PEC fibrous scaffold are shown in Figure 4A and B. Low cell adhesion was observed on the chitosan-alginate scaffold, as indicated by the low cell density in the live/dead assay and the low WST-1 absorbance throughout the culture period. Although the degree of cell attachment was low, the material was not toxic to the cells, as most of the cells attached on the scaffold remained viable after a culture of 11 days.
FIG. 4.

In vitro evaluation with (A) WST-1 cytotoxicity assay (n = 3) and (B) live/dead assay of hMSCs cultured on three different formulations of PEC fibrous scaffold. The groups in (A) are chitosan-alginate PEC fibrous scaffold (□), chitosan-alginate-gelatin PEC
Cell attachment improved significantly with the addition of gelatin or hyaluronic acid into the fibers as indicated by the Calcein AM staining (Fig. 4B). Spreading was observed in some cells on the chitosan-alginate-gelatin fibers, and significantly more on the chitosan-alginate-hyaluronic acid fibers. A slightly higher proliferation, as indicated by WST-1 assay, was also observed in the chitosan-alginate-hyaluronic acid scaffold.
Controlled release of TGF-β3 and in vitro differentiation of hMSC on TGF-β3 scaffold
The release of TGF-β3 over a period of 27 days is shown in Figure 5. As demonstrated in our previous study in the sustained release of PDGF-BB from this type of PEC fibers, interaction of the encapsulated protein with the polyelectrolytes significantly influences the release kinetics. The TGF-β3 with a net positive charge at pH 7.4 displayed a sustained release profile throughout the study period, with an average release rate of 0.64 ng/day, after an initial burst in the first 2 days. The bioactivity of the TGF-β3 released from the chitosan-alginate scaffolds was demonstrated by the differentiation of hMSC cultured on the PEC scaffold.
FIG. 5.

Cumulative release of TGF-β3 from the PEC fibrous scaffold over a period of 27 days. Data reported as mean ± SD (n = 3).
Histological staining of the samples with hMSC cultured on the TGF-β3-encapsulated scaffold and blank chitosan-alginate PEC fibrous scaffold (Fig. 6) showed an enhanced production of ECM in the TGF-β3-encapsulated scaffold. A higher intensity of toluidine blue, Alcian blue, Safranin O, Masson trichrome, and collagen II immunohistological staining indicated a higher level of expression of mucopolysaccha-rides, proteoglycans, and collagen by the cells cultured on TGF-β3-encapsulated scaffold. The enhanced ECM and collagen II expression suggests a chondrogenic-like differentiation of hMSC cultured on the TGF-β3-encapsulated scaffold.
FIG. 6.
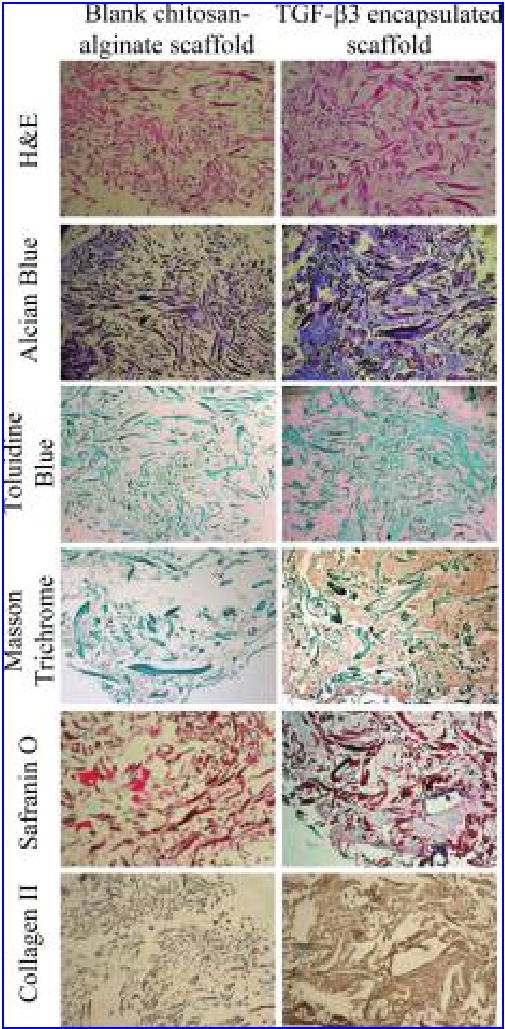
Histological staining of hMSC cultured in the blank chitosan-alginate PEC fibrous scaffold and TGF-β3-encapsulated chitosan-alginate PEC fibrous scaffold. Bar = 100 μm. Color images available online at www.liebertpub.com/ten.
In vivo biocompatibility and differentiation evaluation
Macroscopic inspection of the implantation site revealed no pathological inflammatory tissue responses to the chitosan-alginate PEC scaffolds, implanted subcutaneously or intramuscularly. There were no histological signs of inflammation in the specimens (Fig. 7A). Good cell infiltration was observed at both time points in both subcutaneous and intramuscular implantation sites. Tissue ingrowth and vascularization in the implants were noted at the later time points (Fig. 7B). The PEC scaffold did not elicit any foreign body reaction at the studied time points. Fibrotic capsule was observed after 7 days of intramuscular implantation (Fig. 7C). Capsule thickness surrounding the PEC scaffold decreased over time. The capsule exhibited high cellularity at all times, unlike the typical low cellularity in adverse foreign body response seen with most synthetic polymers.
FIG. 7.

Histological staining of in vivo biocompatibility specimens. (A) Hematoxylin and eosin (H&E) staining of the intramuscularly and subcutaneously implanted samples after 7 days and 2 months of implantation. Presence of PEC fibers is indicated by black arrows and the boundaries of the scaffolds are outlined by gray lines. (B) Vascularization (indicated by white arrows) was evidenced in the core of the implant after 2 months of implantation. (C) Fibrotic capsule formation was observed after 7 days of intramuscular implantation in Masson trichrome and toluidene blue staining, indicated by black arrows. Bar = 100 μm, except for (B), where bar = 50 μm. Color images available online at www.liebertpub.com/ten.
Figure 8 shows the histological staining of blank chitosan-alginate and TGF-(3-encapsulated PEC fibrous scaffolds implanted intramuscularly for 2 months. The host cells from the surrounding tissues migrated to the scaffolds and formed new hybrid tissue structures. A higher degree of cell infiltration was observed in the TGF-(3-encapsulated PEC scaffolds compared to the blank scaffold. Similarly, enhanced ECM production was observed on the TGF-(3-encapsulated PEC scaffold. The extracellular region in this scaffold was strongly stained by the Safranin O/fast green, indicating the presence of proteoglycan-rich matrix. Intensely stained Alcian blue also indicated the presence of highly sulfated glycosaminoglycans (GAGs). The Masson trichrome staining showed the presence of collagen in the TGF-(3-encapsulated PEC scaffold. Further immunostaining of the scaffold indicated the presence of type II collagen, the type prevalent in cartilage tissue.
FIG. 8.

Histological staining of blank chitosan-alginate PEC fibrous scaffold and TGF-β3-encapsulated chitosan-alginate PEC fibrous scaffold implanted intramuscularly for 2 months. Locations of fibers are suggested by arrows. Bar = 100 μm. Color images available online at www.liebertpub.com/ten.
DISCUSSION
This study evaluated the hemocompatibility, cytotoxicity, and in vivo biocompatibility of chitosan-alginate PEC fibrous scaffolds for potential tissue engineering applications. Although the native surface of chitosan-alginate PEC fibrous scaffold was thrombogenic, a slight modification of the polyelectrolytes formulation, by addition of a 1% v/v of 2% w/v heparin solution to the alginate solution, could significantly improve the blood compatibility of the material. The chitosan-alginate PEC fibrous scaffolds showed low cytotoxicity and good tissue biocompatibility in both in vitro and in vivo testing. The biocompatibility of the scaffolds was further inferred from the enhanced chondrogenic ECM production of hMSC both in vitro and in vivo when TGF-β3 was encapsulated in the fibers.
Although high percentage of microparticle formation and high number of adherent platelets were observed when PRP was incubated with the chitosan-alginate PEC fibrous scaffold, the blood compatible properties of the material could be significantly improved by adding 1% v/v of 2% w/v heparin solution into the alginate solution during fiber formation. Heparin is a natural component of living tissues, present in the mast cells of connective tissues.21,22 It is a polysaccharide of heterogeneous structure, belonging to the group of GAGs. The heavily sulfated polysaccharide is negatively charged and linear, consisting of repeating glucosamine and hexuronic acid residues. A highly specific interaction has been demonstrated with the plasma serpin antithrombin, causing a dramatic shift in the functional properties of this physiological coagulation inhibitor.23 Because of the negative charge, heparin molecule would theoretically complex with the polycation, chitosan, along with alginate during fiber formation. The significant improvement in blood compatibility with such small amounts of heparin was unexpected. A 2% w/v heparin solution contains around 3620 U/mL of heparin. Each fiber was produced with 10 μL of the 1:100 dilution of 2% w/v heparin in 1% w/v alginate solution, hence containing about 0.36 U of heparin per fiber. A scaffold with about 100 fibers contains 36 U of heparin. The uncomplexed heparin, which was about 10% of the incorporated heparin, on the fiber surface was washed away in the preincubation wash. No detectable heparin release was measured during the first hour of incubation, and only 2.4 U was released in 7 days. Most of the heparin molecule (90%) was complexed with the chitosan and hence not released to the environment during the PRP incubation. Therefore, we speculate that it is the surface-exposed heparin complex that contributes to the hemocompatibility.
The PEC fibrous scaffold showed no detectable in vitro cytotoxicity. However, poor cell adhesion was observed on the blank chitosan-alginate PEC fibrous scaffold. This is not unexpected since neither unmodified alginate nor unmodified chitosan is known to be good for cell attachment. For instance, while adhesion of fibroblast on chitosan film can be manipulated by changing the degree of acetylation of chitosan,24 there is no cell spreading. Similarly, cells cultured on alginate surface are known to adhere and spread poorly.25 The cell adhesiveness of the PEC fiber was significantly improved by incorporating a small quantity of gelatin or hyaluronic acid into the fiber, without grafting or surface modification.
Gelatin is not as effective as collagen I in improving cell adhesion on material surfaces.26 However, in this study, we used gelatin instead of collagen because the presence of both cationic and anionic charges in collagen would interfere with the ionic interactions of the polyelectrolytes. Collagen and gelatin coatings may be effective in improving cell adhesion, but the effect does not last because the coated collagen typically interacts poorly with the material surface.26 The gelatin incorporated into the fiber through ionic complexation should provide a more stable bioactive surface. Meanwhile, hyaluronic acid, a GAG found in ECM, can also induce cell adhesion and is used to form scaffolds for osteogenic differentiation of multipotent cells.27,28 The surface receptor on hMSCs might interact with the hyaluronic acid present on the fiber and hence enhance the cell attachment as observed in this study.
Previous studies of chitosan and alginate for implantation have shown that the purity of the polysaccharides determines their tissue biocompatibility. Some groups described a specific immunostimulatory effect for chitosan,29 while others reported a chemotactic effect on neutrophils.30–32 Intraperitoneal implantation of chitosan into BALB/c mice shows good biocompatibility.33 Similarly, purified alginates of high glucuronic acid34 or high mannuronic acid (68% mannuronate residues) content25 show good biocompatibility. Although commercially available chitosan and alginate were used as purchased in this study, without further purification, the PEC fibrous scaffold did not induce severe inflammatory or acute immunoresponse during in vivo testing. A thin fibrotic capsule was observed in the specimens implanted intramuscularly, but the capsule resolved at the 2-month time point. Cells were penetrating the fibrous scaffold from surrounding tissue, and ECM deposition was observed in the specimen at this time point. The majority of the cells at the 2-month time point were fibroblast-like. Vascularization could also be found throughout the implants. Collectively, these findings suggest a reasonably biocompatible biomaterial.
The biocompatibility of the PEC scaffolds was further concluded by the enhanced chondrogenic ECM deposition by hMSC cultured on the TGF-β3-encapsulated scaffold in vitro and in vivo, at 6 weeks and 8 weeks, respectively, which are time intervals deemed sufficient for observation of chondrogenesis in other studies.35–37 Although the emphasis of this study is on the biocompatibility issue, the fact that bioactive TGF-β3 can be delivered in a sustained manner from the fibers, without compromising the biocompatibility of the material, is an attractive feature for musculoskeletal tissue engineering. It can be expected that other bioactive growth factors can be delivered similarly for other tissue engineering applications.
CONCLUSION
In this study, we showed that PEC fibrous scaffold exhibited good biocompatibility and low in vitro cytotoxicity. Although the chitosan-alginate PEC fiber evaluated in this study was shown to be thrombogenic and exhibited low cell-adhesion surface property, by incorporating various charged polysaccharides or proteins such as heparin and gelatin into the polyelectrolyte solution during fiber formation, the blood compatibility and surface cell adhesion property of PEC fibrous scaffold could be significantly improved. We have previously demonstrated that PEC fibrous scaffold can be utilized as a biostructural unit for tissue engineering. Functional units can be built by incorporating proteins, drugs, DNA nanoparticles, surface-immobilized ligands, as well as cells into the fiber. This bioactive PEC fiber shows great potential in a wide range of tissue engineering applications with its biocompatible and nonthrombogenic properties.
Acknowledgments
The authors would like to thank Dr. Hoffmann for his help in obtaining rabbit serum, Dr. Corinne Bright for her advice and technical help in rat surgery, and Dr. Schramm for providing surgical area for the in vivo study. Support for this work is provided by the National Institutes of Health (EB003447).
References
- 1.Wan AC, Yim EK, Liao IC, Le Visage C, Leong KW. Encapsulation of biologics in self-assembled fibers as biostructural units for tissue engineering. J Biomed Mater Res A. 2004;71:586. doi: 10.1002/jbm.a.30158. [DOI] [PubMed] [Google Scholar]
- 2.Hiller J, Mendelsohn JD, Rubner MF. Reversibly erasable nanoporous anti-reflection coatings from polyelectrolyte multilayers. Nat Mater. 2002;1:59. doi: 10.1038/nmat719. [DOI] [PubMed] [Google Scholar]
- 3.Hong H, Davidov D, Tarabia M, Chayet H, Benjamin I, Faraggi EZ, Avny Y, Neumann R. Blue to red electroluminescence from self-assembled films. Synth Metals. 1997;85:1265. [Google Scholar]
- 4.Shi XY, Caruso F. Release behavior of thin-walled microcapsules composed of polyelectrolyte multilayers. Langmuir. 2001;17:2036. [Google Scholar]
- 5.Ibarz G, Dahne L, Donath E, Mohwald H. Smart micro- and nanocontainers for storage, transport, and release. Adv Mater. 2001;13:1324. [Google Scholar]
- 6.Polk A, Amsden B, De Yao K, Peng T, Goosen MF. Controlled release of albumin from chitosan-alginate microcapsules. J Pharm Sci. 1994;83:178. doi: 10.1002/jps.2600830213. [DOI] [PubMed] [Google Scholar]
- 7.Kim HJ, Lee HC, Oh JS, Shin BA, Oh CS, Park RD, Yang KS, Cho CS. Polyelectrolyte complex composed of chitosan and sodium alginate for wound dressing application. J Biomater Sci Polym Ed. 1999;10:543. doi: 10.1163/156856299x00478. [DOI] [PubMed] [Google Scholar]
- 8.Yan XL, Khor E, Lim LY. Chitosan-alginate films prepared with chitosans of different molecular weights. J Biomed Mater Res. 2001;58:358. doi: 10.1002/jbm.1029. [DOI] [PubMed] [Google Scholar]
- 9.Iwasaki N, Yamane ST, Majima T, Kasahara Y, Minami A, Harada K, Nnaka S, Maekawa N, Tamura H, Tokura S, Shiono M, Monde K, Nishimura S. Feasibility of polysaccharide hybrid materials for scaffolds in cartilage tissue engineering: evaluation of chondrocyte adhesion to polyion complex fibers prepared from alginate and chitosan. Biomacromolecules. 2004;5:828. doi: 10.1021/bm0400067. [DOI] [PubMed] [Google Scholar]
- 10.Liao IC, Wan AC, Yim EK, Leong KW. Controlled release from fibers of polyelectrolyte complexes. J Control Release. 2005;104:347. doi: 10.1016/j.jconrel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Lim SH, Liao IC, Leong KW. Nonviral gene delivery from nonwoven fibrous scaffolds fabricated by interfacial complexation of polyelectrolytes. Mol Ther. 2006;13:1163. doi: 10.1016/j.ymthe.2005.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sefton MV, Gemmell CH, Gorbet MB. What really is blood compatibility? J Biomater Sci Polym Ed. 2000;11:1165. doi: 10.1163/156856200744255. [DOI] [PubMed] [Google Scholar]
- 13.Gemmell CH. Activation of platelets by in vitro whole blood contact with materials: increases in microparticle, procoagulant activity, and soluble P-selectin blood levels. J Biomater Sci Polym Ed. 2001;12:933. doi: 10.1163/156856201753113114. [DOI] [PubMed] [Google Scholar]
- 14.Woodhouse KA, Klement P, Chen V, Gorbet MB, Keeley FW, Stahl R, Fromstein JD, Bellingham CM. Investigation of recombinant human elastin polypeptides as non-thrombogenic coatings. Biomaterials. 2004;25:4543. doi: 10.1016/j.biomaterials.2003.11.043. [DOI] [PubMed] [Google Scholar]
- 15.Hearle JWS, Purdy AT. Technique for measurement of punching force during needle-felting. Journal of the Textile Institute. 1972;63:363. [Google Scholar]
- 16.Warren BA, Vales O. The release of vesicles from platelets following adhesion to vessel walls in vitro. Br J Exp Pathol. 1972;53:206. [PMC free article] [PubMed] [Google Scholar]
- 17.Polasek J. The appearance of multivesicular structures during platelet activation as observed by scanning electron microscopy. Thromb Res. 1982;28:433. doi: 10.1016/0049-3848(82)90125-6. [DOI] [PubMed] [Google Scholar]
- 18.Wilczek K, Scheerder ID, Wang K, Verbeken E, Piessens J. Comparison of self-expanding polyethylene terephthalate and metallic stents implanted in porcine iliac arteries. Cardiovasc Intervent Radiol. 1996;19:176. doi: 10.1007/BF02577615. [DOI] [PubMed] [Google Scholar]
- 19.Wurzinger LJ, Blasberg P, van de Loecht M, Suwelack W, Schmid-Schonbein H. Model experiments on platelet adhesion in stagnation point flow. Biorheology. 1984;21:649. doi: 10.3233/bir-1984-21422. [DOI] [PubMed] [Google Scholar]
- 20.Matata BM, Yin HQ, Courtney JM, Gaylor JD, Lamba NM, Lowe GD, Suzuki K, Kimura H, Izumi K, Klinkmann H. In vitro blood compatibility evaluation of hollow fibre membrane using a controlled flow system: a comparative study. Int J Artif Organs. 1996;19:582. [PubMed] [Google Scholar]
- 21.Lindahl U, Lidholt K, Spillmann D, Kjellen L. More to “heparin” than anticoagulation. Thromb Res. 1994;75:1. doi: 10.1016/0049-3848(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 22.Olsson P, Sanchez J, Mollnes TE, Riesenfeld J. On the blood compatibility of end-point immobilized heparin. J Biomater Sci Polym Ed. 2000;11:1261. doi: 10.1163/156856200744192. [DOI] [PubMed] [Google Scholar]
- 23.Bjork I, Lindahl U. Mechanism of the anticoagulant action of heparin. Mol Cell Biochem. 1982;48:161. doi: 10.1007/BF00421226. [DOI] [PubMed] [Google Scholar]
- 24.Chatelet C, Damour O, Domard A. Influence of the degree of acetylation on some biological properties of chitosan films. Biomaterials. 2001;22:261. doi: 10.1016/s0142-9612(00)00183-6. [DOI] [PubMed] [Google Scholar]
- 25.Klock G, Pfeffermann A, Ryser C, Grohn P, Kuttler B, Hahn HJ, Zimmermann U. Biocompatibility of mannuronic acid-rich alginates. Biomaterials. 1997;18:707. doi: 10.1016/s0142-9612(96)00204-9. [DOI] [PubMed] [Google Scholar]
- 26.Jensen LT, Host NB. Collagen: scaffold for repair or execution. Cardiovasc Res. 1997;33:535. doi: 10.1016/s0008-6363(96)00247-7. [DOI] [PubMed] [Google Scholar]
- 27.Liu H, Yin Y, Yao K, Ma D, Cui L, Cao Y. Influence of the concentrations of hyaluronic acid on the properties and biocompatibility of Cs-Gel-HA membranes. Biomaterials. 2004;25:3523. doi: 10.1016/j.biomaterials.2003.09.102. [DOI] [PubMed] [Google Scholar]
- 28.Liu LS, Thompson AY, Heidaran MA, Poser JW, Spiro RC. An osteoconductive collagen/hyaluronate matrix for bone regeneration. Biomaterials. 1999;20:1097. doi: 10.1016/s0142-9612(99)00006-x. [DOI] [PubMed] [Google Scholar]
- 29.Peluso G, Petillo O, Ranieri M, Santin M, Ambrosio L, Calabro D, Avallone B, Balsamo G. Chitosan-mediated stimulation of macrophage function. Biomaterials. 1994;15:1215. doi: 10.1016/0142-9612(94)90272-0. [DOI] [PubMed] [Google Scholar]
- 30.Kosaka T, Kaneko Y, Nakada Y, Matsuura M, Tanaka S. Effect of chitosan implantation on activation of canine macrophages and polymorphonuclear cells after surgical stress. J Vet Med Sci. 1996;58:963. doi: 10.1292/jvms.58.10_963. [DOI] [PubMed] [Google Scholar]
- 31.Okamoto Y, Minami S, Matsuhashi A, Sashiwa H, Saimoto H, Shigemasa Y, Tanigawa T, Tanaka Y, Tokura S. Polymeric N-acetyl-D-glucosamine (chitin) induces histionic activation in dogs. J Vet Med Sci. 1993;55:739. doi: 10.1292/jvms.55.739. [DOI] [PubMed] [Google Scholar]
- 32.Usami Y, Okamoto Y, Minami S, Matsuhashi A, Kumazawa NH, Tanioka S, Shigemasa Y. Migration of canine neutrophils to chitin and chitosan. J Vet Med Sci. 1994;56:1215. doi: 10.1292/jvms.56.1215. [DOI] [PubMed] [Google Scholar]
- 33.VandeVord PJ, Matthew HW, DeSilva SP, Mayton L, Wu B, Wooley PH. Evaluation of the biocompatibility of a chitosan scaffold in mice. J Biomed Mater Res. 2002;59:585. doi: 10.1002/jbm.1270. [DOI] [PubMed] [Google Scholar]
- 34.Becker TA, Kipke DR, Brandon T. Calcium alginate gel: a biocompatible and mechanically stable polymer for endovascular embolization. J Biomed Mater Res. 2001;54:76. doi: 10.1002/1097-4636(200101)54:1<76::aid-jbm9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 35.Noth U, Tuli R, Osyczka AM, Danielson KG, Tuan RS. In vitro engineered cartilage constructs produced by press-coating biodegradable polymer with human mesenchymal stem cells. Tissue Eng. 2002;8:131. doi: 10.1089/107632702753503126. [DOI] [PubMed] [Google Scholar]
- 36.Vickers SM, Johnson LL, Zou LQ, Yannas IV, Gibson LJ, Spector M. Expression of alpha-smooth muscle actin by and contraction of cells derived from synovium. Tissue Eng. 2004;10:1214. doi: 10.1089/ten.2004.10.1214. [DOI] [PubMed] [Google Scholar]
- 37.Williams CG, Kim TK, Taboas A, Malik A, Manson P, Elisseeff J. In vitro chondrogenesis of bone marrow-derived mesenchymal stem cells in a photopolymerizing hydrogel. Tissue Eng. 2003;9:679. doi: 10.1089/107632703768247377. [DOI] [PubMed] [Google Scholar]


