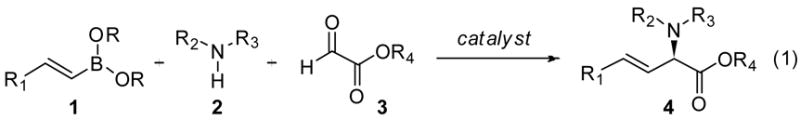
Asymmetric multicomponent reactions efficiently yield chiral compounds in a single process. 1 The Petasis reaction is the multicomponent condensation of boronic acids with amines and aldehydes.2 Accessibility of the reagents and the mild reaction conditions make the method extremely practical. The use of glyoxylates in the reaction results in the construction of α-amino acids.2b If the reaction were rendered asymmetric, the process would be an attractive approach for the synthesis of chiral amino acids.3 Asymmetric approaches have focused on the use of chiral substrates.4 Chiral amines4a,c,d and chiral boronate esters4b have been used to access enantioenriched α-amino acids. More recently, a chiral organic catalyst promoted the asymmetric addition of boronates to activated quinolines.5 Chiral biphenol-derived diols serve as proficient catalysts for asymmetric reactions involving boronates6 and we postulated their utility could be expanded to include multicomponent condensation reactions. Herein we report the development of an asymmetric Petasis reaction between alkenyl boronates, secondary amines, glyoxylates, and chiral biphenol catalysts to afford chiral α-amino acids (eq 1).
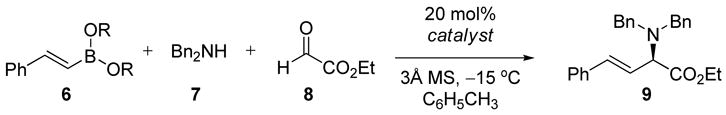
We initiated our investigation with the reaction of (E)-styrylboronates with dibenzylamine and ethyl glyoxylate (Table 1). In the absence of any catalyst, the reaction of styrylboronic acid 6a afforded the racemic α-amino ester 9 in 80% isolated yield at −15 °C (entry 1). In contrast, only trace amount of desired product was formed when (E)-diisopropyl styrylboronate 6b was subjected to the reaction (entry 2). Addition of 20 mol% (S)-BINOL to the reaction mixture resulted in a significant rate enhancement and moderate enantioselectivity (er = 60:40, entry 3). Evaluating other chiral BINOL derivatives and solvents did not provide a significant improvement in yield or enantioselectivity with the (S)-3,3′-Br2-BINOL catalyzed reaction in toluene affording the product in 65% isolated yield and 3:1 er as the best result (entry 4). Diminished yields and enantioselectivities were observed from the use of catalysts that possess large substituents at the 3,3′-positions (entries 5 & 6). Electron withdrawing substituents at these positions resulted in higher yields, but the enantioselectivity was still low (entry 7). Monosubstituted BINOL derivatives were also evaluated in the reaction (entries 8 – 10). Interestingly 3-CF3SO2-BINOL 5h catalyzed reaction resulted in higher yield and enantioselectivity (72:28 er). Finally, the use of vaulted biaryl phenols (S)-VANOL and (S)-VAPOL7 as catalysts afford the chiral α-amino ester in good yields (>77%) and good er’s (>87:13, entries 11 & 12). We next evaluated the effect of the boronate alcohol ligands on the enantioselectivity of the reaction. Dimethyl boronate resulted in higher yields and improved enantioselectivity (entry 13). However, diethyl and dibutyl boronate gave higher enantioselectivities with diethyl styrylboronate affording 9 in highest er (95.5:4.5, entry 14). The reaction of 6a in the presence of 5j resulted in almost no enantioselectivity but high yields most likely due to a high rate of uncatalyzed reaction (entry 16).
Table 1.
Asymmetric Petasis Reaction Catalyzed by Chiral Diolsa
 | |||||
|---|---|---|---|---|---|
| entry | boronate | R | catalyst | % yieldb | erc |
| 1 | 6a | H | - | 80 | - |
| 2 | 6b | i-Pr | - | <5 | - |
| 3 | 6b | i-Pr | 5a | 45 | 60:40 |
| 4 | 6b | i-Pr | 5b | 65 | 75:25 |
| 5 | 6b | i-Pr | 5c | 51 | 70:30 |
| 6 | 6b | i-Pr | 5d | 25 | 59:41 |
| 7 | 6b | i-Pr | 5e | 70 | 55:45 |
| 8 | 6b | i-Pr | 5f | 60 | 70:30 |
| 9 | 6b | i-Pr | 5g | 43 | 64:36 |
| 10 | 6b | i-Pr | 5h | 67 | 72:28 |
| 11 | 6b | i-Pr | 5i | 77 | 85:15 |
| 12 | 6b | i-Pr | 5j | 80 | 87:13 |
| 13 | 6c | CH3 | 5j | 90 | 90:10 |
| 14 | 6d | Et | 5j | 81 | 95.5:4.5 |
| 15 | 6e | n-Bu | 5j | 77 | 93:7 |
| 16 | 6a | H | 5j | 90 | 57:43 |
Reactions were run with 0.15 mmol boronate, 0.10 mmol dibenzylamine, 0.10 mmol glyoxylate, 0.020 mmol catalyst, and 3Å molecular sieves in toluene (0.1 M) for 24 h under Ar, followed by flash chromatography on silica gel.
Isolated yield.
Enantiomeric ratios determined by chiral HPLC analysis.
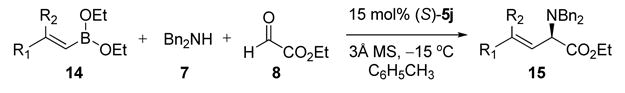
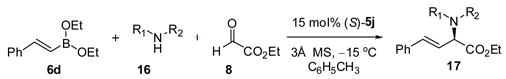
The optimized reaction conditions for dibenzylamine and ethyl glyoxylate required 15 mol% (S)-VAPOL 5j, diethyl boronate, and 3Å molecular sieves. Catalyst 5j could be recovered from the reaction and reused without lost of activity or enantioselectivity. These conditions proved to be general for a variety of alkenyl boronates (Table 2). Electron rich and electron deficient styrenyl boronates afforded corresponding α-amino ester in good yields and high er’s (entries 1 – 5). Heteroaromatic substituted alkenyl boronate 14f was also a good substrate for the reaction (entry 6). Reactions using alkyl substituted boronates displayed slower reaction rates but the selectivities remained high (entries 7 – 9). Disubstituted vinyl boronates also proved equally effective in the reaction (entries 10 & 11). We next evaluated the scope of secondary amines using the general reaction conditions (Table 3). Secondary benzyl amines afforded the corresponding α-amino esters in good yield and enantioselectivities (entries 1 –6).
Table 2.
Asymmetric Petasis Reaction with Dibenzylamine 7a
 | |||||
|---|---|---|---|---|---|
| entry | R1 | R2 | product | % yieldb | erc |
| 1 | Ph | H | 15a | 81 | 95.5:4.5 |
| 2 | p-CH3O-C6H4 | H | 15b | 84 | 96:4 |
| 3 | p-Br-C6H4 | H | 15c | 82 | 95:5 |
| 4 | m-F-C6H4 | H | 15d | 80 | 95:5 |
| 5 | m-CF3-C6H4 | H | 15e | 82 | 95:5 |
| 6 | 3-C4H3S | H | 15f | 87 | 95:5 |
| 7d | C6H11 | H | 15g | 76 | 97:3 |
| 8d | n-Bu | H | 15h | 73 | 95:5 |
| 9d | BnOCH2 | H | 15i | 74 | 95.5:4.5 |
| 10 | Ph | CH3 | 15j | 78 | 95:5 |
| 11d | n-Bu | CH3 | 15k | 71 | 93:7 |
Reactions were run with 0.25 mmol 14, 0.25 mmol amine, 0.25 mmol glyoxylate, 0.0375 mmol (S)-5j, and 3Å molecular sieves in toluene for 36 h under Ar, followed by flash chromatography on silica gel.
Isolated yield.
Determined by chiral HPLC analysis.
Reactions were run at 0 °C.
Table 3.
Asymmetric Petasis Reaction with Boronate 6da
 | ||||
|---|---|---|---|---|
| entry | amine | product | % yieldb | erc |
| 1 |
|
17a | 81 | 95:5 |
| 2 |
|
17b | 73 | 93:7 |
| 3 |
|
17c | 82 | 97:3 |
| 4 |
|
17d | 80 | 98.5:1.5 |
| 5 |
|
17e | 94 | 95:5 |
| 6 |
|
17f | 84 | 95.5:4.5 |
| 7 |
|
17g | 74 | 89:11 |
| 8 |
|
17h | 87 | 97:3 |
| 9 |
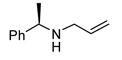
|
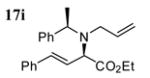
|
81 | dr 90:10 (R,R:R,S) |
| 10 |
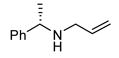
|
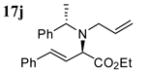
|
89 | dr 84:16 (S,R:S,S) |
Reactions were run with 0.25 mmol 6d, 0.25 mmol amine, and 15 mol % catalyst and 3Å molecular sieves in toluene for 36 h under Ar, followed by flash chromatography on silica gel.
Isolated yield.
Determined by chiral HPLC analysis.
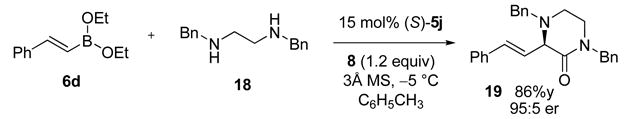
Good functional group tolerance was observed with more complex amines (entries 4 – 6). The less nucleophilic ethyl aniline (entry 7) resulted in slightly lower yield and selectivity. Diallylamine proved effective in the reaction (entry 8). Both enantiomers of allyl α-methyl-benzylamine were subjected to the (S)-5j-catalyzed reaction. The (R)-derived amine resulted in 9:1 dr with (R,R)-17i as major product (entry 9). With the (S)-amine the catalyst still appeared to control the selectivity (84:16 dr, entry 10). Diamines were also good coupling partners for the reaction. The reaction of diamine 18 with boronate 6d and ethyl glyoxylate generated piperazinone 19 in good yield er (Scheme 1).8
Scheme 1.
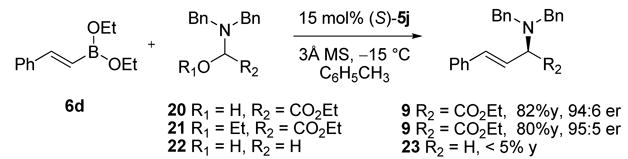
Mechanistic studies using NMR and ESI-MS analysis of reaction mixtures at room temperature indicated single ligand exchange consistent with our previous observations.6d Monitoring the reaction by 11B-NMR demonstrated conversion of a trivalent vinyl boronate to a tetravalent boronate species at 5.4 ppm consistent with previous observations.9 Also congruous with observations made by Petasis,2a aminals 20 and 21 were found to be equally reactive in the reaction to afford 9 in comparable yield and er’s whereas the use of (dibenzylamino) methanol 22 resulted in little product formation (Scheme 2). These observations highlight the possible intermediacy of an aminal and the importance of the glyoxylate ester functionality.
Scheme 2.
In summary, we have developed an enantioselective Petasis reaction catalyzed by chiral biphenols. Mechanistic studies are ongoing to facilitate expansion of the scope and utility.
Supplementary Material
Supporting Information Available: Experimental procedures and HPLC analysis for compounds 15a – 15k, 17a 17j and 19 (PDF). This material is available free of charge via the Internet at http://pubs.acs.org.
Figure 1.
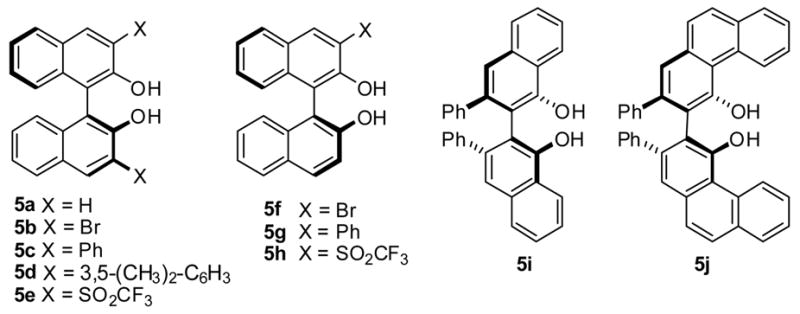
Chiral Biphenols.
Acknowledgments
This research was supported by a gift from Amgen, Inc. and the NIH (P50 GM067041 and R01 GM078240).
References
- 1.(a) Zhu J, Bienaymé H, editors. Multicomponent Reactions. Wiley-VCH: Weinheim; 2005. [Google Scholar]; (b) Yus M, Ramón DJ. Angew Chem Int Ed. 2005;44:1602–1634. doi: 10.1002/anie.200460548. [DOI] [PubMed] [Google Scholar]; (c) Dömling A. Chem Rev. 2006;106:17–89. doi: 10.1021/cr0505728. [DOI] [PubMed] [Google Scholar]; (d) Guillena G, Ramón DJ, Yus M. Tetrahedron: Asymmetry. 2007;18:693–700. [Google Scholar]
- 2.(a) Petasis NA, Akritopoulou I. Tetrahedron Lett. 1993;34:583–586. [Google Scholar]; (b) Petasis NA, Zavialov IA. J Am Chem Soc. 1997;119:445–446. [Google Scholar]; (c) Petasis NA, Goodman A, Zavialov IA. Tetrahedron. 1997;53:16463–16470. [Google Scholar]; (d) Petasis NA, Zavialov IA. J Am Chem Soc. 1998;120:11798–11799. [Google Scholar]; (e) Petasis NA. Aust J Chem. 2007;60:795–798. [Google Scholar]
- 3.(a) Maruoka K, Ooi T. Chem Rev. 2003;103:3013–3028. doi: 10.1021/cr020020e. [DOI] [PubMed] [Google Scholar]; (b) Ma JA. Angew Chem, Int Ed. 2003;42:4290–4299. doi: 10.1002/anie.200301600. [DOI] [PubMed] [Google Scholar]; (c) Najera C, Sansano JM. Chem Rev. 2007;107:4584–4671. doi: 10.1021/cr050580o. [DOI] [PubMed] [Google Scholar]
- 4.(a) Harwood LM, Currie GS, Drew MGB, Luke RWA. Chem Commun. 1996:1953–1954. [Google Scholar]; (b) Koolmeister T, Södergren M, Scobie M. Tetrahedron Lett. 2002;43:5969–5970. [Google Scholar]; (c) Nanda KK, Trotter BW. Tetrahedron Lett. 2005;46:2025–2028. [Google Scholar]; (d) Southwood TJ, Curry MC, Hutton CA. Tetrahedron. 2006;62:236–242. [Google Scholar]
- 5.Yamaoka Y, Miyabe H, Takemoto Y. J Am Chem Soc. 2007;129:6686–6687. doi: 10.1021/ja071470x. [DOI] [PubMed] [Google Scholar]
- 6.(a) Wu TR, Chong MJ. J Am Chem Soc. 2005;127:3244–3245. doi: 10.1021/ja043001q. [DOI] [PubMed] [Google Scholar]; (b) Lou S, Moquist PN, Schaus SE. J Am Chem Soc. 2006;128:12660–12661. doi: 10.1021/ja0651308. [DOI] [PubMed] [Google Scholar]; (c) Wu TR, Chong MJ. J Am Chem Soc. 2007;129:4908–4909. doi: 10.1021/ja0713734. [DOI] [PubMed] [Google Scholar]; (d) Lou S, Moquist PN, Schaus SE. J Am Chem Soc. 2007;129:15398–15404. doi: 10.1021/ja075204v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Bao J, Wulff WD, Dominy JB, Fumo MJ, Grant EB, Rob AC, Whitcomb MC, Yeung SM, Ostrander RL, Rheingold AL. J Am Chem Soc. 1996;118:3392–3405. [Google Scholar]; (b) Mitchell WD, Wulff WD. Org Lett. 2005;7:367–369. doi: 10.1021/ol047852e. [DOI] [PubMed] [Google Scholar]
- 8.Petasis NA, Patel ZD. Tetrahedron Lett. 2000;41:9607–9611. [Google Scholar]
- 9.(a) Petasis NA, Zavialov IA. Tetrahedron Lett. 1996;37:567–570. [Google Scholar]; (b) Schlienger N, Bryce MR, Hansen KT. Tetrahedron. 2000;56:10023–10030. [Google Scholar]; (c) Wang Q, Finn MG. Org Lett. 2000;2:4063–4065. doi: 10.1021/ol006710r. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Available: Experimental procedures and HPLC analysis for compounds 15a – 15k, 17a 17j and 19 (PDF). This material is available free of charge via the Internet at http://pubs.acs.org.


