Abstract
Systemic administration of dopamine D1 (SCH23390) and less so D2 (raclopride) receptor antagonists significantly reduce acquisition and expression of fructoseconditioned flavor preferences (CFP). Because dopamine in the nucleus accumbens shell (NAcS) is implicated in food reward, the present study examined whether NAcS D1 or D2 antagonists altered acquisition and/or expression of fructose-CFP. In Experiment 1, food-restricted rats with bilateral NAcS cannulae were trained to drink a fructose (8%) + saccharin (0.2%) solution mixed with one flavor (CS+/Fs) and a less-preferred 0.2% saccharin solution with mixed another flavor (CS−/s). Unlimited two-bottle tests with the two flavors in saccharin (0.2%: CS+/s, CS−/s) occurred 10 min following total bilateral NAcS doses of 0, 12, 24 or 48 nmol of SCH23390 or raclopride. Preference for CS+/s over CS−/s following vehicle treatment (76%) was significantly reduced by SCH23390 (48 nmol, 62%) and raclopride (24 nmol, 63%). In Experiment 2, rats received bilateral NAcS injections (12 nmol) of SCH23390 or raclopride on one-bottle training (16 ml) days. Yoked control rats received vehicle and were limited to the CS intakes of the D1 and D2 groups, whereas untreated controls without injections received their CS ration during training. Subsequent unlimited two-bottle tests revealed initial preferences of CS+/s over CS−/s in all groups that remained stable in untreated and yoked controls, but were lost over the 6 tests sessions in D1 and D2 groups. These data indicate that NAcS D1 and D2 antagonists significantly attenuated the expression of the fructose-CFP and did not block acquisition, but hastened extinction of fructose-CFP.
Keywords: Flavor-flavor learning, sweet taste, saccharin, SCH23390, raclopride
1. Introduction
Animals use flavor cues (taste, odor, texture) to guide their selection of nutritious foods and avoidance of toxic foods [11]. One type of learning, called flavor-flavor conditioning, occurs when a preference is acquired for an arbitrary flavor cue (e.g., banana) paired with an already liked flavor (e.g., sweet taste of saccharin) [18]. The sweet taste is considered to be an unconditioned stimulus (US) that reinforces the animal’s preference for the added flavor, which represents the conditioned stimulus (CS). One neurochemical that is implicated in the reward value of sweet taste is dopamine, primarily because sweet taste activates mesolimbic dopamine circuits that are implicated in the mediation of natural as well as drug rewards (e.g., [16,17]). Dopamine receptor antagonism suppresses the intake of sweet solutions in rats [15,21,33], potentially because of its reduction of the hedonic value of sweet taste [24,29], or other factors such as changes in incentive salience (e.g., [8,9,20,23]).
Dopamine antagonists also alter the ability of sweet solutions to reinforce the preference for other flavors. Preference for a flavored 10% sucrose solution paired with treatment with the D2 antagonist raclopride was reduced relative to a differently-flavored sucrose solution paired with vehicle treatment [19]. Sucrose can reinforce flavor preferences based on its sweet taste as well as its post-oral nutritive actions through the processes of flavor-flavor and flavor-nutrient conditioning, respectively [25]. Our laboratories have used different training procedures to separate flavor-flavor and flavornutrient conditioning [2–5,34–36]. Flavor-nutrient learning was investigated using an intragastric (IG) infusion procedure in which rats were trained to drink flavors paired with IG infusions of sucrose and water, respectively. Systemic treatment with a D1 antagonist (SCH23390) but not a D2 antagonist (raclopride) was found to block flavor conditioning by IG sucrose infusions [3]. Neither drug has much effect on the expression of a previously learned flavor preference.
Flavor-flavor learning was initially investigated using a sham-feeding procedure in which rats fitted with a gastric cannula were trained to drink a flavored 16% sucrose solution and a less preferred flavored 0.2% saccharin solution. Because gastric shamfeeding greatly reduces the post-oral actions of sucrose, a preference for the sucrosepaired flavor (the CS+) over the saccharin-paired flavor (the CS−) is assumed to result from the sugar’s more palatable taste. Rats treated systemically with D1 (SCH23390) or D2 (raclopride) receptor antagonists during sham-feeding training sessions subsequently displayed preferences for the CS+ flavor comparable to control animals [36]. However, both antagonists dose-dependently reduced the preference for the CS+ flavor when administered prior to the choice test, indicating that D1 and D2 signaling are involved in the expression of a conditioned preference [35,36]. A limitation of the sham-feeding study was that the animals consumed substantially more of the flavored sucrose solution than the flavored saccharin solution during training and therefore were more familiar with the CS+ flavor. In a subsequent study we investigated flavor-flavor conditioning by training rats to “real-feed” similar amounts of a flavored fructose+saccharin solution and a less preferred saccharin solution. Fructose rather than sucrose or glucose was used because, unlike these other sugars, fructose has minimal post-oral flavor conditioning effects [27,28]. Therefore a preference for a flavor mixed into a fructose solution is assumed to result from flavor-flavor conditioning. Using this training procedure, our laboratory [4] observed that systemic treatment with SCH23390 and, to a lesser degree, raclopride blocked acquisition of flavor-flavor conditioning induced by the fructose+saccharin solution. Both drugs also significantly reduced the expression of a CS+ preference previously conditioned by fructose.
The central anatomical sites of action for dopaminergic modulation of fructoseconditioned flavor-flavor preferences are unknown. The nucleus accumbens shell (NAcS) is a site in which sweet taste stimulates dopamine efflux (e.g., [12,16]), and where dopamine antagonists in the NAcS suppress lithium chloride-conditioned saccharin aversions [14]. Therefore, the present experiment examined whether dopamine D1 (SCH23390) or D2 (raclopride) antagonists administered bilaterally into the NAcS would dose-dependently alter the acquisition and/or expression of fructose-conditioned flavor-flavor preferences.
2. Methods
2.1. Subjects, Surgery and Histology
All experimental protocols were approved by the Queens College Institutional Animal Care and Use Committee certifying that all subjects and procedures were in compliance with the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85-23, revised 1985). Male albino Sprague-Dawley rats (260-300 g, Charles River Laboratories, Wilmington, MA) were housed individually in wire mesh cages and maintained on a 12:12 h light/dark cycle with chow (5001, PMI Nutrition International, Brentwood, MO) and water available ad libitum, except as noted below. Each rat was pretreated with chlorpromazine (3 mg/kg, i.p.) and anesthetized with Ketamine HCl (120 mg/kg, i.m.). Stainless steel guidecannulae (26-gauge, Plastics One) were aimed stereotaxically (Kopf Instruments) at bilateral placements in the NAcS using the following coordinates: incisor bar (+5 mm), 3.1 mm anterior to the bregma suture, 1.7 mm and angled 10° towards each side of the sagittal suture and 6.8 mm from the top of the skull. The cannulae were secured to the skull by four anchor screws with dental acrylic. The animals were allowed at least two weeks to recover from stereotaxic surgery before behavioral testing began. At the end of the experiment the rats were overdosed with an anesthetic (Euthasol) and were injected transcardially with potassium chloride (15 mg/ml, 0.9% saline). Transcardiac perfusions were performed with 0.9% normal saline followed by 10% buffered formalin. Coronal 40-µm sections, stained with Cresyl violet, were examined by light microscopy by an observer unfamiliar with the behavioral data.
2.2. Test Solutions
The training solutions consisted of an 8% fructose (Sigma Chemical Co., St. Louis, MO) and 0.2% sodium saccharin (Sigma) mixture or a 0.2% sodium saccharin solution; the solutions were flavored with 0.05% unsweetened grape or cherry Kool-Aid (Kraft Foods, White Plains, NY). Half of the rats in each group had the cherry flavor added to the fructose+saccharin solution, and the grape flavor added to the saccharin only solution; the flavors were reversed for the remaining rats. In the two-bottle preference tests, the cherry and grape flavors were each presented in a 0.2% saccharin solution. The fructose+saccharin-paired flavor is referred to as the CS+, and the saccharin-paired flavor as the CS- because 8% fructose is preferred to 0.2% saccharin [26]. CS+/Fs refers to the flavored fructose+saccharin solution used in training, and CS+/s refers to the same flavor presented in saccharin-only during choice testing. The CS−/s refers to the flavored saccharin solution used in training and testing. All testing took place in the rats’ home cage during the mid-light phase of the light:dark cycle. Two weeks before testing began, the rats were placed on a food restriction schedule that maintained their body weights at 85-90% of their ad libitum level. The rats were initially trained to drink unflavored 0.2% saccharin solution from calibrated sipper tubes (100 ml, 1 ml gradations) during daily 1 h sessions. The sipper tube was mounted on the front of the cage held by a taut steel spring, and was positioned 3-6 cm above the cage floor. This training procedure was repeated daily until all rats approached the sipper tubes with short (< 1 min) latency, typically within three days. The limited food rations were given 1 h after each training session.
2.3. Experiment 1
Expression Procedure
Twenty-four rats were given ten one-bottle training sessions (30 min/day) with 16 ml of the CS+/Fs solution presented on odd-numbered days, and 16 ml of the CS−/s solution presented on even-numbered days. On days 9 and 10, the rats had access to a second sipper tube containing water. This familiarized the rats to the presence of two sipper tubes used during the choice tests; water intake was negligible in these training trials. Training intakes were limited to 16 ml/session to minimize the difference between CS+/Fs and CS−/s intakes. The position of the CS and water sipper tubes varied across days using a left-right-right-left pattern. Following training, the rats were given eight two-bottle choice test sessions (30 min/day) with unlimited (50 ml) access to the CS+/s and CS−/s solutions. The positions of the two sipper tubes were counterbalanced as described above. Solution intakes during the training and testing were measured by weighing (0.1 g) the bottles before and after the 30-min sessions.
Ten min prior to the two-bottle test sessions the rats were given bilateral injections (0.5 µl/side) through a stainless steel internal cannula (33-gauge, Plastics One) that extended 1 mm past the guide cannula. This was accomplished using a Hamilton microsyringe that was connected by polyethylene tubing to the internal cannula. For the first two sessions of two-bottle tests, all rats were given a vehicle (0.9% saline) injection. Based on their CS+/s and CS−/s intakes in these tests, the rats were divided into two matched groups. The D1 group of 12 rats was treated with the D1 antagonist, SCH23390 (Sigma Chemical Co.) at total doses of 12 (6 nmol/side), 24 (12 nmol/side) and 48 (24 nmol/side) nmol administered into the NAcS. Half of the rats were tested with an ascending dose order and the remaining rats were tested in a descending dose order. The D2 group of 12 rats was similarly tested, but with microinfusions of the D2 antagonist, raclopride (Sigma Chemical Co.) at total doses of 12, 24, and 48 nmol. The rats were tested twice at each drug dose with the left-right position of the CS+ and CS- solutions counterbalanced across sessions. A one-day rest period separated each pair of drug doses for both groups.
2.4. Experiment 2
Acquisition Procedure
Four groups of rats were matched for their intakes of an unflavored 0.2% saccharin solution prior to training. The rats were given eight one-bottle training sessions (60 min/day) with the CS+/Fs solution presented on odd-numbered sessions, and the CS−/s solution presented on even-numbered sessions. A 1-day break was placed between each of the four pairs of training trials to reduce the impact of repeated NAcS injections. Rats in the D1 and D2 groups were given bilateral injections of the D1 antagonist, SCH23390 (12 nmol, 6 nmol/side, n=7) and the D2 antagonist, raclopride (12 nmol, 6 nmol/side, n=6), respectively, into the NAcS 10 min prior to each one-bottle training session. A third group (Yoked Control, n=19) received vehicle injections throughout one-bottle training, and their intakes of the CS+/Fs and CS- /s solutions were limited to the mean 60-min intakes of the D1 and D2 groups. A fourth group of unoperated rats (Control, n=15) was trained as above except without injections and with their CS+/Fs and CS−/s intakes limited to 16 ml/session; the purpose of this group was to evaluate the effectiveness of the training procedure. Following training, all groups were given six daily two-bottle choice sessions (60 min/day) with unlimited (50 ml) access to the CS+/s and CS−/s solutions; no drugs were administered prior to these sessions. The positions of the CS+/s and CS−/s solutions were counterbalanced across sessions, and the results were analyzed as mean 60-min intakes during successive pairs of sessions (referred to as Tests 1, 2, and 3).
2.5. Data analysis
In the expression study, training intakes were averaged over the five CS+/Fs and five CS+/s sessions and evaluated with a t-test. Intakes during the preference tests were averaged over the two sessions at each dose and evaluated with two-way repeated-measures analyses of variance (ANOVA, CS condition vs. Dose) for the D1 and D2 groups, respectively. Separate ANOVAs evaluated total intakes and percent CS+/s intakes as a function of dose for the two groups.
In the acquisition study, training intakes were averaged over the four CS+/Fs and 4 CS−/s sessions and were analyzed with a two-way ANOVA (CS conditions×Groups). Intakes during the preference tests were averaged over sessions 1–2, 3–4, and 5–6 (referred to as Tests 1, 2, and 3) to control for side position effects. A three-way ANOVA compared the CS intakes of D1, D2 and control groups (Group × CS × Test). Separate two-way ANOVAs evaluated total CS intakes and percent CS+/s intakes of the four groups. When main or interaction effects were found, Bonferroni corrected comparisons (p<0.05) detected significant effects.
3. Results
3.1. Histological Verification
Figure 1 is a schematic representation [22] of the bilateral cannula placements of all 56 animals in the expression (SCH23390 [SE, n=12]; raclopride [RE, n=12]) and acquisition (SCH23390 [SA, n=7]; raclopride [RA, n=6]; yoked [YA, n=19]) experiments. The bilateral cannulae were localized within the shell region of the NAcS, or bordered the core and shell regions of the NAc, or bordered the NAcS and immediately adjacent ventral diagonal band area. Multiple animals had highly similar cannula placements, and there was considerable overlap of placements for the animals involved in the five different groups, those receiving SCH23390 or raclopride in the Expression experiment, and those receiving SCH23390, raclopride or vehicle (Yoked Control) in the Acquisition experiment.
Figure 1.
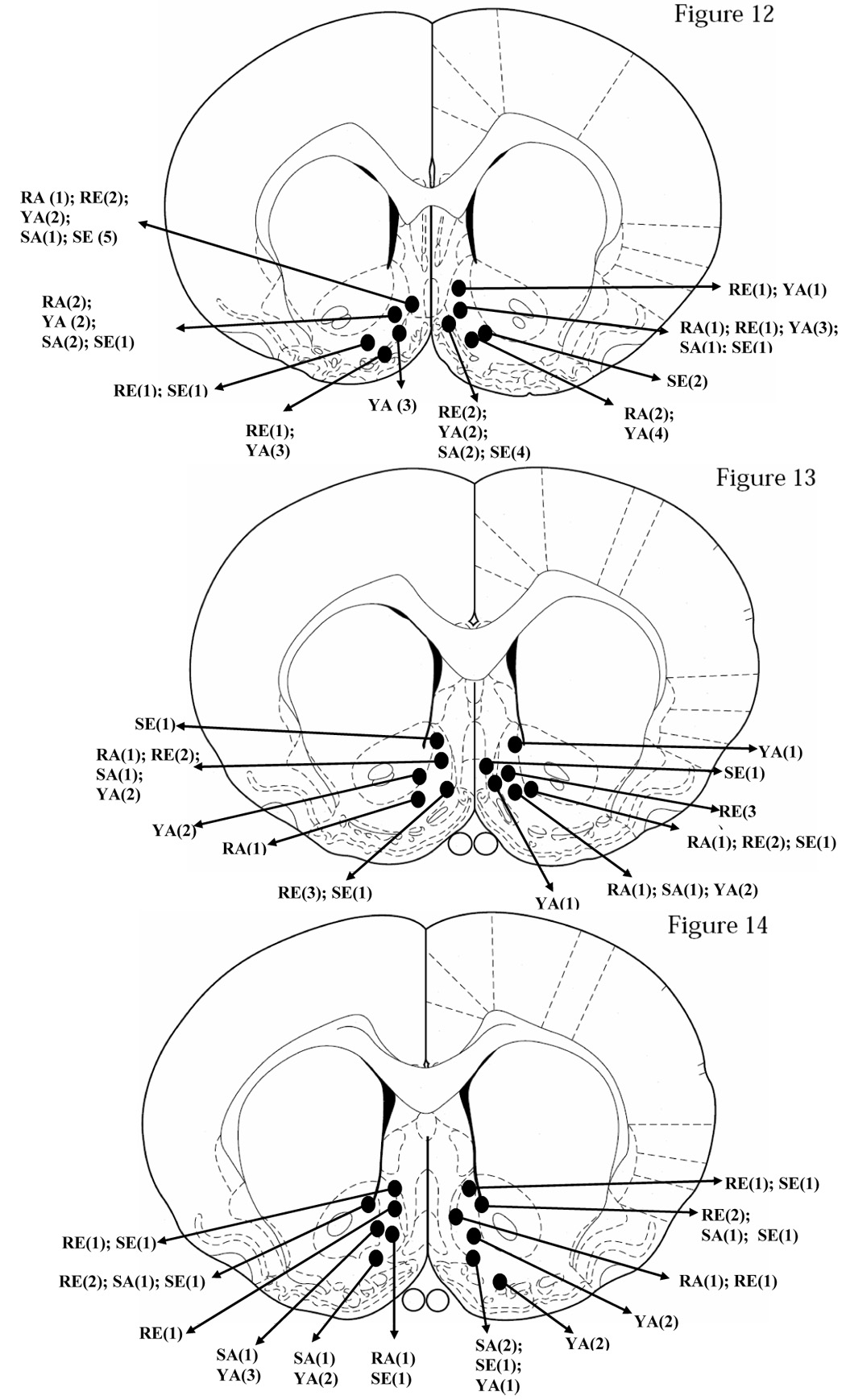
Bilateral representation of cannula sites aimed at the nucleus accumbens shell (NAcS) region of 56 rats using Figures 12, 13 and 14 of the stereotaxic atlas of Paxinos and Watson [22]. The bilateral cannulae were localized within the shell region of the NAcS, or bordered the core and shell regions of the NAc, or bordered the NAcS and immediately adjacent ventral diagonal band area. Multiple animals had highly similar cannula placements, and there was considerable overlap of placements for the animals receiving SCH23390 (SE, n=12) and raclopride (RE, n=12) in the Expression paradigm, and for the animals receiving SCH23390 (SA, n=7), raclopride (RA, n=6) and vehicle (Yoked Control, YA, n=19) in the Acquisition paradigm.
3.2. Experiment 1
Effects of DA antagonism in the NAcS on the expression of fructose-conditioned flavor preferences
The mean one-bottle training intake of the CS+/Fs solution (11.2 ml) significantly (t(23)= 4.75, p<0.0001) exceeded that of the CS−/s solution (8.4 ml). In the two-bottle preference tests, overall, the D1 rats consumed significantly more CS+/s than CS−/s (F(1,44)= 80.62, p<0.0001), but total intake significantly varied as a function of drug dose (F(3,44)= 4.55, p<0.007) and there was a significant CS×Dose interaction (F(3,44)= 5.57, p<0.003). CS+/s intakes significantly exceeded CS−/s intakes at the 0 (vehicle) and 12 nmol dose of SCH23390 but not at the 24 and 48 nmol SCH23390 doses (Figure 2A). The rats consumed significantly less CS+/s at the 24 and 48 nmol SCH23390 doses as compared to vehicle; CS−/s intakes failed to differ as a function of SCH23390 dose (Figure 2A). Significant differences in the percent CS+/s intakes were observed (F(3,33)= 4.68, p<0.008) and the preference (62%) at the 48 nmol SCH23390 dose was significantly lower than the preference (76%) following vehicle (Figure 2A). Preferences at the 12 (68%) and 24 (63%) nmol SCH23390 doses were intermediate but failed to significantly differ from the vehicle test. Differences in total CS intakes were observed across doses (F(3,33)= 7.48, p<0.0006) and the rats consumed significantly less at the 24 (11.1 ml) and 48 (10 ml), but not 12 (13.3 ml) nmol SCH23390 doses relative to vehicle (15 ml).
Figure 2. (Exp 1. Expression Procedure).

Intakes (mean ±SEM, 0.5 h) of CS+/s and CS−/s solutions in two-bottle tests in animals receiving bilateral NAcS injections of the D1 dopamine antagonist, SCH23390 (upper panel) or the D2 dopamine antagonist, raclopride (lower panel) at total doses of 0, 12, 24 or 48 nmol 10 min prior to testing. Significant differences are denoted between CS+/s and CS−/s intake within an injection condition (*) and between CS+/s intake following a drug dose relative to the vehicle treatment (+). The percentages of CS+/s intake over total intake are denoted above each pair of values with significant differences relative to vehicle treatment (#) noted.
In the two-bottle preference tests, overall, the D2 rats consumed significantly more CS+/s than CS−/s overall (F(1,42)= 111.53, p<0.0001), but total intake significantly varied as a function of drug dose (F(3,42)= 5.38, p<0.003) and there was a significant CS × Dose interaction (F(3,42)= 4.53, p<0.008). CS+/s intake was significantly higher than CS−/s intake at the 0, 12 and 48 nmol, but not the 24 nmol doses of raclopride (Figure 2B). The rats consumed significantly less CS+/s at the 24 and 48 nmol raclopride doses as compared to vehicle; CS−/s intakes failed to differ among raclopride doses (Figure 2B). There were significant differences in the percent CS+/s intakes (F(3,33)= 5.33, p<0.004) with the preference (63%) at the 24 nmol raclopride dose significantly lower than the preference (75%) following vehicle (Figure 2B). Preferences following the 12 (71%) and 48 (69%) nmol raclopride doses failed to differ significantly from vehicle. Significant differences in total intake were observed across doses (F(3,33)= 6.18, p<0.002) with total CS intakes at the 12 (11.8 ml), 24 (12.4 ml) and 48 (11.9 ml) nmol raclopride doses significantly lower than following vehicle (15.4 ml).
3.3. Experiment 2
NAcS D1 and D2 antagonist effects on the acquisition of fructoseconditioned flavor preference
In the one-bottle training intakes, overall CS+/Fs intake (11.9 ml) significantly (F(1,18)= 76.82, p<0.0001) exceeded CS−/s intake (10.1 ml) with the four groups displaying significant main (F(3,54)= 13.1, p<0.0001) and interaction (F(3,54)= 8.29, p<0.0001) effects with CS condition. Control rats displayed significantly greater CS+/Fs than CS−/s (13.4 vs. 10.6 ml) intakes; similarly, the Yoked Control rats consumed more CS+/Fs than CS−/s (10.4 vs. 7.8 ml). In contrast, significant CS+Fs vs. CS+/s differences failed to occur in D1 (11.5 vs. 9.9 ml) and D2 (12.3 vs. 12.1 ml) groups. CS+/Fs intake was significantly higher in Control rats relative to Yoked Control animals, and CS−/s intakes were significantly higher in D1, D2, and Control groups relative to the Yoked Control group. This occurred because some of the yoked control rats did not consume their entire allotted ration in every session.
Following training, the rats were given three consecutive series of preference tests (2 sessions each) without drug treatment. Significant differences in intake were observed between the CS+/s and CS−/s solutions (F(1,18)= 219.24, p<0.0001), among the four training groups (F(3,54)= 10.27, p<0.0001), and among the three tests (F(2,36)= 23.04, p<0.0001). In addition there were interactions between groups and tests (F(6,108)= 116.65, p<0.0001), between CS and tests (F(2,36)= 39.69, p<0.0001), and among groups, CS, and tests (F(6,108)= 17.82, p<0.0001). Within group comparisons revealed that the Yoked Control and Control groups (Figures 3C, 3D) consumed significantly more CS+/s than CS−/s in all three Tests. The D1 and D2 groups, in contrast, consumed significantly more CS+/s than CS−/s in Tests 1 and 2 but not in Test 3 indicating extinction of the CS+/s preference (Figures 3A, 3B). This loss of preference was due to a selective and significant reduction in CS+/s intakes from Test 1 to 3 in the D1 and D2 groups. The CS+/s intakes of the D1 and D2 groups significantly exceeded those of the Yoked Control in Tests 1 and 2, but were significantly below both control levels in Test 3. CS−/s intakes failed to change over testing in any group. Analysis of the percent CS+/s data failed to reveal any significant overall group difference but there was an interaction between group and tests (F(6,108)= 7.01, p<0.0001). Whereas percent CS+/s intakes remained stable across the Tests in both Control groups (Figures 3C, 3D), the percent intakes significantly decreased in the D1 and D2 groups with scores significantly lower in Test 3 than in Test 1. Significant differences in total CS intakes were observed among the four groups (F(3,54)= 10.27, p<0.0001), among the three tests (F(2,36)= 22.04, p<0.0001), and for the interaction between groups and tests (F(6,108)= 116.65, p<0.0001). Total CS intakes significantly increased from the first to third Tests in the Yoked (14.0 to 18.7 ml) and Control (13.3 to 15.8 ml) groups. In contrast, total CS intakes significantly decreased from the first to third Test in the D1 (20.2 to 15.1 ml) and D2 groups (19.0 to 13.4 ml). In Test 3, total CS intake in the D1 group were significantly lower than that of the Yoked group, and the total CS intake of the D2 group was significantly lower than that of the Yoked and Control groups. Finally, total CS intakes in the Yoked control group were significantly higher than those of the Control group in Tests 1 and 3.
Figure 3. (Acquisition Study).
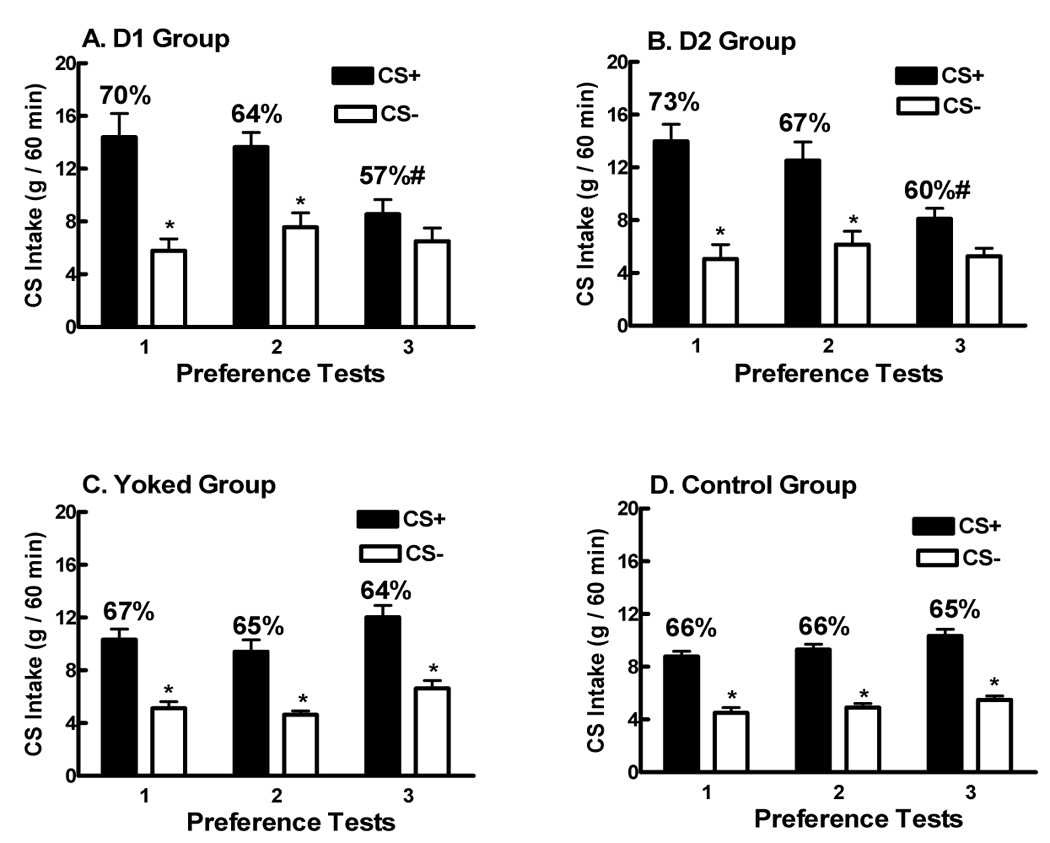
Intakes (mean ±SEM, 1 h) of three pairs of CS+/s and CS−/s solutions during three pairs of two-bottle tests. During training, the D1 group received NAcS injections of SCH23390 (12 nmol, Panel A) and the D2 group received NAcS injections of raclopride (12 nmol, Panel B); the Yoked Control group received NAcS vehicle injections (Panel C) while the Control group (Panel D) received no injections during training. Significant differences are denoted between CS+/s and CS−/s intake within each test are denoted (*) as are significant differences in the percentage of CS+/s intake over total intake (#).
3.4. Differences in the Magnitude of Fructose-Conditioned Flavor Preferences in the Acquisition and Expression Paradigms
Compared to the training procedure used in the expression paradigm (10 days of one-bottle training, 5 with the CS+/Fs and 5 with the CS−/s in 30 min sessions), training procedure in the acquisition paradigm was modified so as to reduce the impact of repeated NAcS microinjections by decreasing the number of training sessions from 10 to 8, by introducing a rest day between each of the pairs of training sessions, but also lengthening the training session from 30 to 60 min. To evaluate whether modifications in the training procedures affected fructose-conditioned preferences, combined two-day vehicle preference values of the SCH23390-tested and raclopride-tested rats in the expression paradigm were compared with the preference score (Test 1) of the untreated control group in the acquisition paradigm. Significant differences in intake were observed between acquisition and expression paradigms (F(1,23)= 4.97, p<0.036), between CS+/s and CS−/s solutions (F(1,23)= 218.49, p<0.0001), and for the interaction between paradigms and solutions (F(1,23)= 26.50, p<0.0001). Whereas the acquisition and expression training both resulted in significant respective increases in CS+/s intake (acquisition: 8.8 ml; expression: 11.7 ml) over corresponding CS−/s intake (acquisition: 4.5 ml; expression: 3.5 ml), the CS+/s intake of the expression group was significantly higher than the CS+/s intake of the acquisition group. CS−/s intakes failed to differ between the two groups. Correspondingly, the percentage of CS+/s intake over total intake was significantly (t(34)= 4.34, p<0.0002) higher in the expression paradigm (75.8%) than in the acquisition paradigm (66.4%).
4. Discussion
The present study demonstrated that direct bilateral administration into the NAcS of either the D1 receptor antagonist SCH23390 or the D2 receptor antagonist raclopride significantly attenuated the expression of a preference for a flavor paired with a frucrose/saccharin solution. These data are in agreement with the previous reports that systemic administration of D1 and D2 antagonists eliminated the expression of fructoseconditioned flavor preferences in real-feeding rats [4], and reduced the expression of sucrose-conditioned flavor preferences in sham-feeding rats [35,36]. Taken together, these findings indicate that the NAcS is a central site of action at which dopamine antagonists interfere with the expression of sugar-conditioned flavor-flavor preferences.
It is important to note, however, that the respective effects of systemic and NAcS administration differed in magnitude. SCH23390 administered into the NAcS reduced the fructose-conditioned CS+ preference from 76% (0 nmol dose) to 62% (48 nmol dose). In contrast, systemic SCH23390 eliminated the CS+ preference (39-55%) at all doses tested (50–800 nmol/kg) compared to the 77% preference observed with vehicle treatment [4]. The ability of D2 antagonism using raclopride in the NAcS to reduce the fructoseconditioned CS+ preference (75% for vehicle, 63% for the 24 nmol raclopride dose) was comparable to SCH23390, but not as dose-dependent. Similarly, systemic administration of raclopride significantly reduced the expression of the CS+ preference, but this effect was less profound and dose-dependent than that observed with systemic SCH23390 treatment. The data obtained with NAcS and systemic administration of SCH23390 suggests that the NAcS is one, but not the only site that participates in the dopaminergic mediation of the expression of fructose-conditioned flavor preferences. Consistent with this view, our laboratory [7] recently found that D1 and D2 antagonists administered into the amygdala significantly reduced the expression of fructose-conditioned flavor preferences, suggesting a regional network of sites mediating this type of flavor-flavor conditioning. Such regional networks of interacting brain sites have been proposed in other feeding paradigms (e.g., [6,10,30,32]).
In addition to attenuating the expression of the CS+ preference, injection of SCH23390 or raclopride into the NAcS during training influenced the acquisition of fructose-conditioned CS+ preference, albeit in a subtle way. Thus, D1 and D2 groups displayed a significant preference for the CS+ flavor in the initial pair of two-bottle tests (70–73%), but this preference declined to 57–60% in Test 3, and was no longer significant. In contrast, the rats in the Yoked and Control groups displayed a stable preference of about 65% from the first through the third pairs of tests. The Control group data agrees with our original study showing that fructose-conditioned flavor preferences are relatively stable (i.e., resistant to extinction) with repeated testing [1]. However, subsequent research revealed that the stability of such a preference varies as a function of the amount of CS solutions (CS+/Fs and CS−/s) the animals are given during one-bottle training sessions (Yiin et al., unpublished observations, 2005). Thus, animals trained with 10 ml rations of the CS+/Fs and CS−/s during one-bottle sessions tended to reduce their CS+ preference during repeated two-bottle testing in contrast to animals trained with CS rations of 15 ml or greater. In the present study, the D1 and D2 rats were limited to 16 ml of CS solutions during training although their average training intake was 10–12 ml/session. The Yoked Control group consumed slightly less because of the yoking procedure limiting them to a 12 ml ration at paradigm onset, and indeed consumed significantly less CS+/Fs and CS−/s solutions during training relative to the Control group. Moreover, the methodological differences in the expression and acquisition training paradigms produced significantly greater preferences for the expression (76%: ten sessions) relative to the acquisition (65%: eight sessions). However, differences in training intakes among the drug and control groups do not readily explain why the D1 and D2 rats, unlike the control rats, lost their CS+ preference with repeated two-bottle testing. It may be, however, that the salience of the CS+/Fs solution was reduced by the SCH23390 and raclopride treatment during training such that the D1 and D2 rats behaved as if they consumed less CS+/Fs than did the control rats during training. This explanation is supported by a proposed role for incentive salience in dopaminergic control of food intake and reward (e.g., [8,9]). According to this analysis, increasing the salience of the CS+/Fs solution during training (e.g., by increasing the sugar concentration) may prevent the extinction of CS+ preference observed in rats treated with DA receptor antagonists in the NAcS.
In contrast to the present results, systemic treatment with SCH23390 or raclopride (200 nmol/kg) completely prevented the acquisition of a fructose-conditioned CS+ flavor-nutrient preference [4]. Animals given systemic injections of the D1 and D2 antagonists displayed percent CS+ intakes of 46–56% (i.e., no preference) in flavor-nutrient conditioning compared to Yoked Control rats that had percent intakes of 66%. Taken together, these results indicate that the NAcS is not the critical site for the acquisition of a fructose-conditioned flavor preference. The involvement of the NAc core, which is implicated in flavor-nutrient learning ([31]; Touzani, Bodnar and Sclafani, submitted), as well as other brain sites in flavor-flavor learning, requires further investigation.
Accumbens dopamine signaling is implicated in other forms of flavor learning. As noted previously, systemic D1, but not D2 antagonism blocks the acquisition of a flavor preference conditioned by the post-oral actions of sucrose [3]. More recent findings demonstrate that injection of SCH23390 into the NAcS blocked flavor preference conditioning by IG infusions of glucose [31]. The effects of NAc injections of raclopride on flavor-nutrient learning were not investigated given the lack of effect obtained with systemic injections of the D2 antagonist. The NAc is a site at which sweet solutions in the mouth stimulate dopamine efflux (e.g., [12,16,17]). Whether IG sugar infusions also promote dopamine release in the NAc is not known. If the post-oral actions of sugar do not promote dopamine release, this could explain why D2 receptor signaling is involved in flavor-flavor but not flavor-nutrient learning. That is, the D2 antagonist may act to reduce the reward value of an oral US but not a post-oral US. Interestingly, NAcS injection of a D1, but not a D2, antagonist was observed to block LiCl-induced saccharin taste aversions [14]. These findings along with the IG sugar data suggest only D1 receptors mediate flavor learning that involves post-oral negative as well as positive unconditioned stimuli. Flavor aversions are produced by combining a neutral CS flavor with an unpalatable flavor (e.g., bitter taste; [13]). Whether flavor-flavor aversion conditioning, like flavor-flavor preference conditioning, involves both D1 and D2 receptor signaling, remains to be investigated.
ACKNOWLEDGEMENTS
This research was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases grant DK071761. We thank Karen Ackroff for her helpful comments on this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ackroff K, Sclafani A. Fructose-conditioned flavor preferences in male and female rats: effects of sweet taste and sugar concentration. Appetite. 2004;42:287–297. doi: 10.1016/j.appet.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Azzara AV, Bodnar RJ, Delamater AR, Sclafani A. Naltrexone fails to block the acquisition or expression of a flavor preference conditioned by intragastric carbohydrate infusions. Pharmacol Biochem Behav. 2000;67:545–557. doi: 10.1016/s0091-3057(00)00395-6. [DOI] [PubMed] [Google Scholar]
- 3.Azzara AV, Bodnar RJ, Delamater AR, Sclafani A. D-1 but not D-2 dopamine receptor antagonism blocks the acquisition of a flavor preference conditioned by intragastric carbohydrate infusions. Pharmacol Biochem Behav. 2001;68:709–720. doi: 10.1016/s0091-3057(01)00484-1. [DOI] [PubMed] [Google Scholar]
- 4.Baker RW, Shah MJ, Sclafani A, Bodnar RJ. Dopamine D1 and D2 antagonists reduce the acquisition and expression of flavor-preferences conditioned by fructose in rats. Pharmacol Biochem Behav. 2003;75:55–65. doi: 10.1016/s0091-3057(03)00039-x. [DOI] [PubMed] [Google Scholar]
- 5.Baker RW, Li Y, Lee MG, Sclafani A, Bodnar RJ. Naltrexone does not prevent acquisition or expression of flavor preferences conditioned by fructose in rats. Pharmacol Biochem Behav. 2004;78:239–246. doi: 10.1016/j.pbb.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 6.Baldo BA, Kelley AE. Discrete neurochemical coding of distinguishable motivational processes: insights from nucleus accumbens control of feeding. Psychopharm. 2007;191:439–459. doi: 10.1007/s00213-007-0741-z. [DOI] [PubMed] [Google Scholar]
- 7.Bernal SY, Abayev Y, Miner P, Kandova E, Pinhas A, Touzani K, Sclafani A, Bodnar RJ. Expression of fructose-conditioned flavor preferences: role of D1 and D2 dopamine receptors in the amygdala. Soc Neurosci Abstr. 2006;32 Abstract Viewer. [Google Scholar]
- 8.Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharm. 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- 9.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 10.Bodnar RJ, Levine AS. Role of opioid peptides in regulating energy balance. In: Harvey J, Withers DJ, editors. Neurobiology and Obesity. United Kingdom: Cambridge University Press; 2008. pp. 232–265. [Google Scholar]
- 11.Capaldi ED, editor. Why We Eat What We Eat. Washington, DC: American Psychological Association; 1996. [Google Scholar]
- 12.Cheng J, Feenstra MG. Individual differences in dopamine efflux in nucleus accumbens shell and core during instrumental conditioning. Learn Mem. 2006;13:168–177. doi: 10.1101/lm.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fanselow MS, Birk J. Flavor-flavor associations induce hedonic shifts in taste preference. Anim Learn Behav. 1982;10:223–228. [Google Scholar]
- 14.Fenu S, Bassareo V, Di Chiara G. A role for dopamine D1 receptors of the nucleus accumbens shell in conditioned taste aversion learning. J Neurosci. 2001;21:6897–6904. doi: 10.1523/JNEUROSCI.21-17-06897.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geary N, Smith GP. Pimozide decreases the positive reinforcing effect of sham fed sucrose in the rat. Pharmacol Biochem Behav. 1985;22:787–790. doi: 10.1016/0091-3057(85)90528-3. [DOI] [PubMed] [Google Scholar]
- 16.Genn RF, Ahn S, Phillips AG. Attenuated dopamine efflux in the rat nucleus accumbens during successive negative contrast. Behav Neurosci. 2004;118:869–873. doi: 10.1037/0735-7044.118.4.869. [DOI] [PubMed] [Google Scholar]
- 17.Hajnal A, Smith GP, Norgren R. Oral sucrose stimulation increases accumbens dopamine in the rat. Am J Physiol. 2003;286:R31–R37. doi: 10.1152/ajpregu.00282.2003. [DOI] [PubMed] [Google Scholar]
- 18.Holman EW. Immediate and delayed reinforcers for flavor preferences in the rat. Learn Motiv. 1975;6:91–100. [Google Scholar]
- 19.Hsiao S, Smith GP. Raclopride reduces sucrose preference in rats. Pharmacol Biochem Behav. 1995;50:121–125. doi: 10.1016/0091-3057(95)00315-n. [DOI] [PubMed] [Google Scholar]
- 20.Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Rev. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- 21.Muscat R, Willner P. Effects of selective dopamine receptor antagonists on sucrose consumption and preference. Psychopharm. 1989;99:98–102. doi: 10.1007/BF00634461. [DOI] [PubMed] [Google Scholar]
- 22.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Fourth Ed. Knoxville, TN: Neuroscience Associates; 1998. p. 1998. [Google Scholar]
- 23.Salamone JD, Cousins MS, Snyder BJ. Behavioral functions of nucleus accumbens dopamine: Empirical and conceptual problems with the anhedonia hypothesis. Neurosci Biobehav Rev. 1997;21:341–359. doi: 10.1016/s0149-7634(96)00017-6. [DOI] [PubMed] [Google Scholar]
- 24.Schneider LH. Orosensory self-stimulation by sucrose involves brain dopaminergic mechanisms. Ann N Y Acad Sci. 1989;575:307–319. doi: 10.1111/j.1749-6632.1989.tb53252.x. [DOI] [PubMed] [Google Scholar]
- 25.Sclafani A. How food preferences are learned: laboratory animal models. Proc Nutr Soc. 1995;54:419–427. doi: 10.1079/pns19950011. [DOI] [PubMed] [Google Scholar]
- 26.Sclafani A, Ackroff K. Glucose- and fructose-conditioned flavor preferences in rats: taste versus post-ingestive conditioning. Physiol Behav. 1994;56:399–405. doi: 10.1016/0031-9384(94)90213-5. [DOI] [PubMed] [Google Scholar]
- 27.Sclafani A, Cardieri C, Tucker K, Blusk D, Ackroff K. Intragastric glucose but not fructose conditions robust flavor preferences in rats. Am J Physiol. 1993;265:R320–R325. doi: 10.1152/ajpregu.1993.265.2.R320. [DOI] [PubMed] [Google Scholar]
- 28.Sclafani A, Fanizza LJ, Azzara AV. Conditioned flavor avoidance, preference and indifference produced by intragastric infusions of galactose, glucose and fructose in rats. Physiol Behav. 1999;67:227–234. doi: 10.1016/s0031-9384(99)00053-0. [DOI] [PubMed] [Google Scholar]
- 29.Smith GP. Dopamine and food reward. In: Fluharty S, Morrison AM, editors. Progress in Psychobiology and Physiological Psychology. vol 16. New York: Academic Press; 1995. pp. 83–144. [Google Scholar]
- 30.Stratford TR, Kelley AE. Evidence of a functional relationship between the nucleus accumbens shell and lateral hypothalamus subserving the control of feeding behavior. J Neurosci. 1999;19:11040–11048. doi: 10.1523/JNEUROSCI.19-24-11040.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Touzani K, Bodnar RJ, Sclafani A. Critical role of dopamine D1 receptors in nucleus accumbens shell in flavor preferences conditioned by intragastric glucose infusion. Appetite. 2006;388 [Google Scholar]
- 32.Will MJ, Franzblau EB, Kelley AE. Nucleus accumbens mu-opioids regulate intake of a high-fat diet via activation of a distributed brain network. J Neurosci. 2003;23:2882–2888. doi: 10.1523/JNEUROSCI.23-07-02882.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xenakis S, Sclafani A. The effects of pimozide on the consumption of a palatable saccharin-glucose solution in the rat. Pharmacol Biochem Behav. 1981;15:435–442. doi: 10.1016/0091-3057(81)90274-4. [DOI] [PubMed] [Google Scholar]
- 34.Yu W-Z, Sclafani A, Delamater AR, Bodnar RJ. Pharmacology of flavor preference conditioning in sham-feeding rats: effects of naltrexone. Pharmacol Biochem Behav. 1999;64:573–584. doi: 10.1016/s0091-3057(99)00124-0. [DOI] [PubMed] [Google Scholar]
- 35.Yu W-Z, Silva RM, Sclafani A, Delamater AR, Bodnar RJ. Pharmacology of flavor preference conditioning in sham-feeding rats: effects of dopamine receptor antagonists. Pharmacol Biochem Behav. 2000;65:635–647. doi: 10.1016/s0091-3057(99)00239-7. [DOI] [PubMed] [Google Scholar]
- 36.Yu W-Z, Silva RM, Sclafani A, Delamater AR, Bodnar RJ. Role of D1 and D2 dopamine receptors in the acquisition and expression of flavor preference conditioning in sham-feeding rats. Pharmacol Biochem Behav. 2000;67:537–544. doi: 10.1016/s0091-3057(00)00396-8. [DOI] [PubMed] [Google Scholar]


