Abstract
The present study examined behavioral sensitivity and acute tolerance to ethanol in infants with or without a moderate prenatal ethanol experience. During gestational days 17–20 dams received 0.0 or 2.0 g/kg ethanol. On postnatal day 13 pups were administered 0.0, 0.5 or 2.5 g/kg ethanol prior to assessment of locomotion. One third of the pups were evaluated at 5–10, 30–35 and 60–65 min after ethanol administration; another third was tested only during the last two post-administration periods; and the remaining third was tested only at 60–65 min. At 30–35 min blood ethanol levels were similar to those attained at 60–65 min. The main results of the study were: (a) The 2.5 g/kg ethanol dose induced biphasic motor effects: stimulation 5–10 minutes after drug administration and sedation after 30–35 or 60–65 minutes. (b) Infants exhibited acute tolerance to ethanol’s sedative effects. (c) Although pups prenatally treated with ethanol exhibited heightened locomotor activity levels, acute sensitivity and tolerance were not affected by prenatal treatment. In summary, infants are sensitive to biphasic motor consequences of ethanol and readily exhibit acute tolerance to ethanol’s sedative effects. In addition, moderate prenatal ethanol exposure was sufficient to induce hyper-reactivity in the offspring without affecting habituation.
Keyboards: Prenatal ethanol, ethanol acute sensitivity, ethanol acute tolerance, locomotor activity, habituation, infant and rat
There is a considerable body of experimental evidence indicating that prenatal exposure to ethanol can critically modulate subsequent ethanol intake. This association between prenatal ethanol exposure and later affinity for the drug has been detected in various strains of rats and mice when using a variety of modes of ethanol exposure during pregnancy (Chotro, Arias, & Laviola, 2007). Heightened ethanol consumption resulting from gestational exposure to ethanol has been observed through tests conducted during different ontogenetic periods, including infancy, adolescence and adulthood (Chotro et al., 2007; Spear & Molina, 2005). Recent epidemiologic studies have found results analogous to those reported in this preclinical research. Even when controlling genetic and environmental factors known to modulate ethanol affinity, prenatal exposure to ethanol still significantly predicts later ethanol consumption and onset of ethanol-related disorders (Alati et al., 2006; Baer, Barr, Bookstein, Sampson, & Streissguth, 1998; Baer, Sampson, Barr, Connor, & Streissguth, 2003; Yates, Cadoret, Troughton, Stewart, & Giunta, 1998).
Although mechanisms underlying the association between fetal exposure to ethanol and subsequent predisposition to accept the drug remain a matter of debate, animal models have identified factors that can mediate this association. When rats are exposed to high ethanol doses throughout the last two weeks of the gestation, long-lasting effects upon the activity of the hypothalamic-pituitary-adrenal axis (HPA) are consistently observed (Taylor, Branch, Liu, & Kokka, 1982; Taylor et al., 1981; Weinberg, Taylor, & Gianoulakis, 1996). This prenatal treatment induces heightened responsiveness to various stressors, a phenomenon that could eventually predispose the organism to use or abuse ethanol as a means of alleviating stress-related negative states (e.g. anxiety or depression). This neuroendocrinological alteration seems to be accompanied by changes in neurotransmitter systems that not only participate in stress regulation but also modulate ethanol’s positive as well as negative (anti-anxiety) reinforcing properties (Bailey, Brien, & Reynolds, 2001; Druse, Tajuddin, Kuo, & Connerty, 1990; Sari & Zhou, 2004). Furthermore, chronic exposure during gestation to large ethanol doses has also been observed to modify acute sensitivity to different ethanol’s effects. For example, this treatment enhances tolerance to hypothermic effects of relatively high ethanol doses (Abel, Bush, & Dintcheff, 1981; Anandam, Felegi, & Stern, 1980; Lancaster & Spiegel, 1989) and can sensitize the organism to the stimulant effects of ethanol (Becker, Hale, Boggan, & Randall, 1993; Rockman, Markert, & Delrizzo, 1989).
It has also been observed that prenatal exposure to moderate ethanol doses (1 or 2 g/kg, peak blood concentrations ranging between 40 and 120 mg/dl) during the last four days of gestation (gestational days 17–20; GDs 17–20) affects latter ethanol intake patterns. This moderate prenatal ethanol treatment results in high ethanol intake during infancy (Arias & Chotro, 2005a, 2005b, 2006; Chotro & Arias, 2003; Dominguez, Lopez, & Molina, 1998; Molina, Chotro, & Dominguez, 1995; Pueta, Abate, Spear, & Molina, 2005) as well as during adolescence (Chotro & Arias, 2003). In these studies, processing of ethanol-related chemosensory information by late-term fetuses seems to predispose the organism to accept ethanol odor and taste (Chotro et al., 2007; Spear & Molina, 2005). Yet, the participation of other intervenient factors in this effect can not be completely rule out. With this ethanol exposure there is no evidence of morphological alterations (Dominguez, Lopez, Chotro, & Molina, 1996) or deficits in associative learning capabilities assessed through Pavlovian conditioning procedures (Nizhnikov, Molina, Varlinskaya, & Spear, 2006; Pueta et al., 2005) attributable to the teratogenic properties of ethanol. However, these animals show a tendency towards hyper-reactivity when they are confronted with novel stimuli (Chotro & Spear, 1997; Dominguez et al., 1996). Recent experimental evidence also suggests that moderate fetal exposure to ethanol during late gestation sensitizes the neonate to positive reinforcing effects of low ethanol doses (0.25–0.75 g/kg; Nizhnikov et al., 2006) and the fetus to sedative effects of higher doses (1–2 g/kg; Chotro & Spear, 1997). It is less clear that such hyper-reactivity and heightened behavioral sensitivity to ethanol persists into subsequent stages of development, including time points where heightened ethanol affinity has been observed to be a function of prenatal ethanol experience.
It is likely that behavioral sensitivity to a given drug will vary as a function of post-administration time. This variation can occur due to pharmacokinetic processes (absorption, distribution and elimination) as well as development of acute tolerance within the process of intoxication or intervening factors such as habituation to the testing environment. Acute tolerance refers to the development of resistance to a drug’s physiological or behavioral effects within a single bout of intoxication. This particular tolerance is not explainable through metabolic adaptive mechanisms and has been detected during infancy and periadolescence in the rat (Silveri & Spear, 2001; Spear & Varlinskaya, 2005). To our knowledge, the effect of low-to-moderate ethanol during late gestation on acute tolerance has not been investigated. Altered reactivity as well as the possibility that prenatal or perinatal ethanol exposure may impair attention and non-associative learning processes (Hunt & Phillips, 2004; Westergren, Rydenhag, Bassen, Archer, & Conradi, 1996), imply experimental challenges for analyzing corresponding effects on acute sensitivity and tolerance to ethanol. As will be explained in detail, the present study is based on an experimental strategy that permits to examine the relative weights pf acute ethanol effects, learning processes such habituation, and possible interactions between these factors.
The present study examines whether behavioral sensitivity to ethanol doses known to exert biphasic (appetitive and aversive) motivational effects (Molina, Pautassi, Truxell, & Spear, 2007) would be affected by prenatal exposure to ethanol. Prior to behavioral assessment, a pharmacokinetic study determined blood ethanol concentrations in pups that differed in their prenatal history with ethanol (Experiment 1). This pharmacokinetic study pursued two goals. The first, in accord with previous metabolic studies conducted with infant rats (Kelly, Bonthius, & West, 1987), was to determine blood ethanol concentrations (BECs) at the post-administration times when the animals would be tested for spontaneous motor activity. The intention was to evaluate whether the behavioral effects of a given dose of ethanol vary despite persistence of similar levels of intoxication as operationalized through blood ethanol levels (i.e. acute tolerance). The second goal was to examine whether BECs accrued with different ethanol doses and at different post-administration times would differ as a function of prenatal ethanol treatment. This is necessary in view of recent studies reporting minimal but still significant changes in ethanol pharmacokinetics as a function of brief or chronic ethanol exposure during gestation (Bhalla, Kaur, Mahmood, & Mahmood, 2005; Nizhnikov et al., 2006).
Based on the pharmacokinetic profile obtained in Experiment 1, we conducted a second study focused on acute locomotor effects of ethanol (0.5 and 2.5 g/kg) at different post-administration times as a function of ethanol prenatal exposure. One set of animals was tested during the rising phase of blood ethanol concentrations (5–10 min), when achieving peak blood ethanol levels (30–35 min) and during a later phase of the intoxication (60–65 min, when these levels nevertheless remained at peak levels). A second group of animals was evaluated only during the last two post-administration times while the remaining group was evaluated during only the last period. This design, which we will term “inverted ladder” (see Figure 2 and Experiment 2: Methods), should allow determination of the weight of the acute effects of varying levels of intoxication (as a function of dose and post-administration time) while controlling for non-associative learning processes (e.g. sensitization or habituation) that might occur during test. In turn, these learning processes might be modulated not only by state of intoxication, but also by prenatal ethanol exposure to the drug.
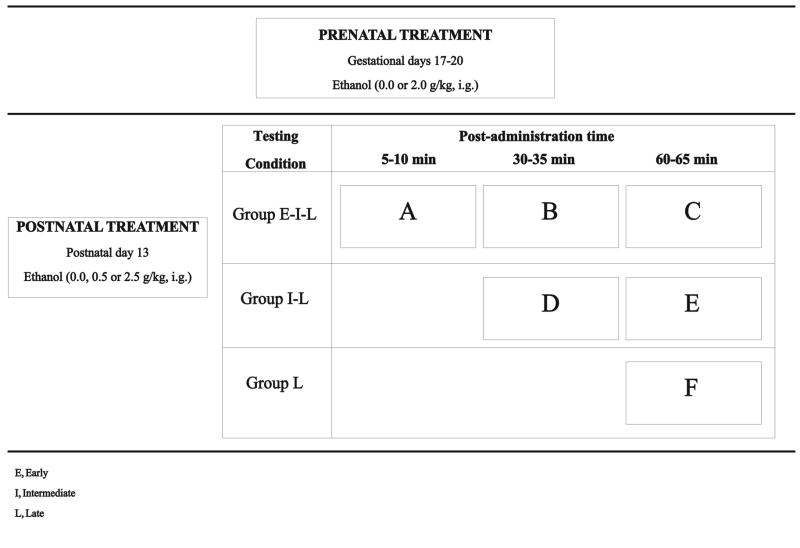
Figure 2.
Prenatal (0.0 or 2.0 g/kg) and postnatal (0.0, 0.5 or 2.5 g/kg) ethanol treatments and testing conditions that define the design under consideration. Given the structure of the design we will refer to it as “inverted ladder”. One third of the animals corresponding to each prenatal treatment were sequentially evaluated at 5–10, 30–35 and 60–65 min [group early-intermediate-late (E–I–L)]. Another third were tested only during the last two post-administration periods [30–35 and 60–65 min; group intermediate-late (I-L)] while the remaining subjects were tested only at post-administration time 60–65 min. [group late (L)].
EXPERIMENT 1
This experiment was conducted to determine infantile BECs resulting from the intragastric (i.g.) administration of 0.5 and 2.5 g/kg ethanol. Blood ethanol levels were determined at 7.5, 32.5 and 62.5 min post-administration time. According to prior studies it could be expected that after i.g. administration of 2.5 g/kg ethanol, infantile BECs at 7.5 min post-administration time will be well below peak blood ethanol levels (Kelly et al., 1987; Molina, Pautassi et al., 2007; Pautassi, Sanders, Miller, Spear, & Molina, 2006). Peak blood ethanol levels in these circumstances are encountered between 30 and 90 min post-administration. In the case of the 0.5 g/kg ethanol dose, BECs were expected to remain relatively stable across the selected time points. The present study also pursued the explicit intention of contrasting BECs of pups prenatally exposed to ethanol and BECs of pups with no prior ethanol experience.
Material and Methods
Subjects
One hundred and eight Sprague-Dawley pups (56 females and 52 males), representative of 12 litters were utilized. Animals were born and reared at the vivarium of the Center for Developmental Psychobiology (Binghamton University, NY) under conditions of constant room temperature (22 ± 1.0 ºC), on a 12-hour light 12-hour dark cycle. Births were examined daily and the day of parturition was considered as postnatal day 0 (PD0). All litters were culled to 10 pups (5 females and 5 males whenever possible) within 48 hours after birth. All procedures were in accordance with the Guide for Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996) and the guidelines indicated by the Binghamton University handling review committee.
Procedures
Maternal Ethanol Administration
From GD 17 through GD 20 pregnant dams received one daily intragastric (i.g.) administration of either 0.0 or 2.0 g/kg ethanol. The ethanol dose (2.0 g/kg) resulted from the administration of a volume equivalent to 0.015 ml per gram of body weight of a 16.8% v/v ethanol solution. Control dams (0.0 g/kg) were administered an equivalent volume of vehicle (water). All administrations were performed during the morning (10:00 – 11:00 hrs).
Determination of Blood Ethanol Concentrations (BECs)
On PD 13 pups were separated from their mothers and quasi randomly assigned to a given ethanol treatment (i.g. administration of either 0.5 or 2.5 g/kg ethanol). Two pups (1 male and 1 female), representative of the same litter and ethanol treatment, were placed in a holding maternity cage (45 x 20 x 20 cm) partially filled with clean wood shavings. The floor of the cage was maintained at 31 C (± 1 C) through the use of a heating pad. One hour latter, body weights were individually recorded (± 0.01 g) and pups received an i.g. administration of 0.5 or 2.5 g/kg ethanol dose (volume administered was 0.015 ml per gram of body weight of a 4.2 % v/v or 21 % ethanol solution, respectively). Intragastric administrations were performed using a 10-cm length of polyethylene tubing (PE-10 Clay Adams, Parsippany, New Jersey) attached to a 1 ml disposable syringe equipped with a 27 G x 1/2 needle. The tubing was gently introduced through the mouth and slowly pushed into the stomach. The entire procedure took less than 20 seconds per pup.
Pups were sacrificed at 7.5, 32.5 or 62.5 minutes after receiving the corresponding ethanol dose. Trunk blood was obtained following decapitation. Blood samples were collected using a heparinized capillary tube. They were immediately centrifuged (6.000 rpm; Micro-Haematocrit Centrifuge, Hawksley & Sons LTD, Sussex, England) and stored at −70 ºC. BECs were determined using an AM1 Alcohol Analyzer (Analox Instruments, Lunenburg, MA). Calculation of BECs was made by oxidating ethanol to acetaldehyde in the presence of ethanol oxidase. The apparatus measures the rate of oxygen required by this process, which is proportional to ethanol concentration. BECs were expressed as milligrams of ethanol per deciliter of body fluid (mg/dl = mg%).
Data analysis
BECs were analyzed by means of a 2 [prenatal ethanol treatment (0.0 or 2.0 g/kg)] x 2 [postnatal ethanol treatment (0.5 or 2.5 g/kg)] x 3 [post-administration time (7.5, 32.5 or 62.5 minutes)] x 2 [gender (male or female)] between factor ANOVA. Significant main effects or interactions were further analyzed through post-hoc tests (Newman-Keuls test with a Type I error set at 0.05).
Results
Figure 1 depicts the pharmacokinetic profiles defined by pre- and postnatal ethanol treatment and post-administration time. The corresponding ANOVA revealed significant main effects of post-administration time [F(2,84) = 22.19, p < 0.001] and postnatal ethanol treatment [F(1,84) = 747.54, p < 0.001]. The interaction between these factors also exerted a significant effect, F(2,84) = 27.80, p < 0.001. As could be expected, at all post-administration times, the 2.5 g/kg ethanol dose resulted in significantly higher BECs relative to those obtained when employing 0.5 g/kg ethanol. In addition, BECs corresponding to the higher ethanol dose significantly varied as a function of post-administration time. BECs at 7.5 min were significantly lower than those encountered at 32.5 and 62.5 min. The values recorded during these last two time points (32.5 and 62.5 min) were very similar. BECs derived from the administration of 0.5 g/kg ethanol were low and stable across all post-administration times. Prenatal ethanol treatment did not exert a significant main effect nor did it interact with any of the remaining factors. No significant effect of gender or interaction with the remaining factors was found in terms of BECs.
Figure 1.
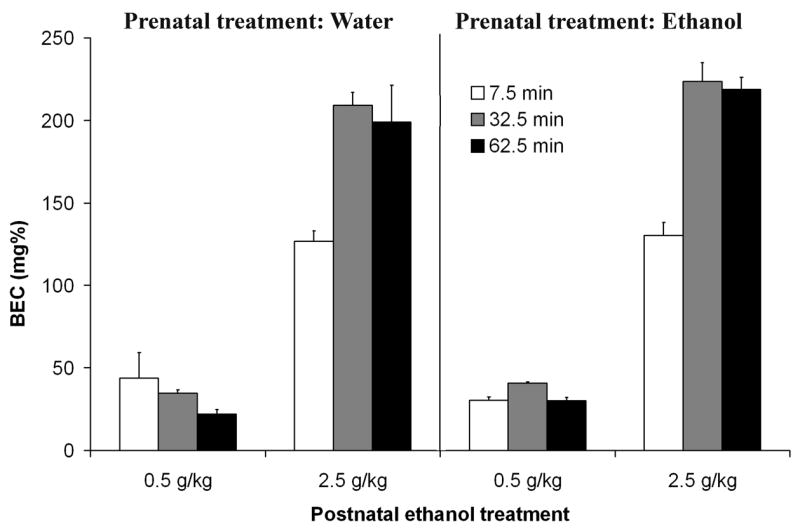
Blood ethanol concentration (mg %) as a function of prenatal (0.0 or 2.0 g/kg) and postnatal (0.5 or 2.5 g/kg) ethanol treatments, and post-administration time (7.5, 32.5 or 62.5 min). Vertical lines illustrate standard errors of the means.
In summary, infantile blood ethanol levels did not vary significantly as a function of prenatal exposure to the drug. BECs differed only as a function of ethanol dose, post-administration time and the interaction between these factors. With 0.5 g/kg ethanol, BECs were stable across time and always lower than those observed with 2.5 g/kg ethanol. With 2.5 g/kg BECs at 32.5 and 62.5 min were equivalent and higher than those recorded soon after administration (7.5 min). In accord with previous studies, the levels attained 30–60 min following administration of 2.5 g/kg ethanol represent peak values (Kelly et al., 1987; Lopez, Spear, & Molina, 1996; Molina, Pautassi et al., 2007; Pautassi et al., 2006).
EXPERIMENT 2
The pharmacokinetic profile established in Experiment 1 enabled us to conduct a behavioral study meant to determine infantile acute sensitivity to ethanol (0.5 or 2.5 g/kg) in terms of motor activity patterns as a function of prenatal experience with the drug. To our knowledge, there are no previous studies analyzing locomotor effects of ethanol in preweanling heterogeneous rats. In adult heterogeneous rats, ethanol’s stimulant effects have been rarely reported and what seems to predominate in the intoxicated adult rat is the drug’s motor suppressing effect, even when employing ethanol doses below 2.0 g/kg (Chuck, McLaughlin, Arizzi-LaFrance, Salamone, & Correa, 2006; Erickson & Kochhar, 1985; Salamone et al., 2006). In rat strains selectively bred for ethanol ingestion the activating effect of ethanol has been consistently observed when employing ethanol doses below 1.0 g/kg (Agabio et al., 2001; Krimmer, 1991; Paivarinta & Korpi, 1993; Quintanilla, 1999; Rodd et al., 2004; Waller, Murphy, McBride, Lumeng, & Li, 1986). As mentioned, preweanling rats show a marked sensitivity to the biphasic reinforcing effects of ethanol during the course of an acute intoxication (Molina et al., 2007), and also, they seem to be more predisposed to display heightened ethanol intake patterns than in later stages of development (Sanders and Spear, 2007). If sensitivity to ethanol’s effects upon locomotor activity is associated with the sensitivity to the reinforcing properties of the drug, it is plausible that ethanol exerts biphasic locomotor effects in preweanling rats.
Locomotion was evaluated during the rising phase of the blood ethanol curve or when BECs reached peak values. Beyond estimating the impact of prenatal ethanol experiences upon locomotor effects induced by ethanol, the present experiment was intended to: (a) determine whether the ethanol doses administered during infancy exert primarily activating, sedative or biphasic locomotive effects, (b) analyze these effects independently of the effects of progressive familiarization with the testing context and (c) test the development of acute tolerance at post-administration times where BECs remain high and stable.
Material and Methods
Subjects
One hundred and ninety nine preweanlings (96 females and 103 males), representative of 27 litters were tested. Breeding and housing conditions of these animals were the same as those described in Experiment 1.
Procedures
Maternal Ethanol Administration
Pregnant dams (GDs 17–20) were either treated with vehicle (0.0 g/kg ethanol) or ethanol (2.0 g/kg), with the same i.g. procedures used in Experiment 1.
Locomotor activity assessment
Locomotor activity was tested on postnatal day 13 (PD13) after pups were intragastrically administered with 0.0, 0.5 or 2.5 g/kg ethanol. Behavioral activity was evaluated in square Plexiglas containers (10 x 10 x 12 cm). The floor of these containers was lined with sandpaper (coarse: 60, Gatorgrid, USA). A new sheet of sandpaper was employed for each animal. A circuit board (2-cm in width) surrounded the four sides of each chamber. This board had six infrared photo emitters and six infrared photo-receptors. The photo beams crossed the chamber generating a matrix of nine cells that allowed measurement of overall amount of activity. Custom-made software served to analyze the number of beams crossed by each subject every 10th of a second. Each activity test had a total duration of 5 min. Data was collapsed using 1-min bins.
Motor activity was registered at different post-administration times: 5–10 min (Early, E), 30–35 min (Intermediate, I) and/or 60–65 min (Late, L). The experimental groups are named in terms of the post-administration intervals at which they were tested. One third of the animals corresponding to each prenatal treatment were sequentially evaluated during all post-administration intervals (5–10, 30–35 and 60–65 min; group E–I–L). Another third were tested only during the last two post-administration periods (30–35 and 60–65 min; group I-L) while the remaining subjects were tested only at post-administration time 60–65 min. (group L). Figure 2 depicts treatment and testing conditions that define the experimental design, which we will refer to as “inverted ladder design”. This design allows tests of the effects of post-administration time with or without prior habituation to the testing apparatus.
One hour prior to the behavioral test, pups representative of each prenatal treatment (0/0 or 2.0 g/kg ethanol) were separated from their mothers and quasi-randomly assigned to one of the nine independent groups defined by the following between factors: postnatal ethanol treatment (0.0, 0.5 or 2.5 g/kg) and testing condition (E–I–L, I-L or L). The quasi-random distribution allowed us to avoid litter and gender overrepresentation in any given group (Holson & Pearce, 1992). Table 1 summarizes the number of pups assigned to each treatment condition and the body weights of the animals that were tested.
Table 1.
Body weights and number of pups assigned to each group as a function of prenatal (0.0 or 2.0 g/kg) and postnatal (0.0, 0.5 or 2.5 g/kg) ethanol treatments and testing conditions that define the design of the Experiment 2.
| Prenatal ethanol treatment | Postnatal ethanol treatment | Testing condition | Body weight PD 13 (grams) | N |
|---|---|---|---|---|
| 0.0 g/kg | 0.0 g/kg | E–I–L | 36.76 | 12 |
| I–L | 35.77 | 9 | ||
| L | 36.51 | 10 | ||
| 0.5 g/kg | E–I–L | 36.95 | 11 | |
| I–L | 36.63 | 9 | ||
| L | 37.25 | 10 | ||
| 2.5 g/kg | E–I–L | 36.73 | 11 | |
| I–L | 36.64 | 11 | ||
| L | 36.82 | 9 | ||
| 2.0 g/kg | 0.0 g/kg | E–I–L | 36.40 | 13 |
| I–L | 36.16 | 13 | ||
| L | 36.23 | 11 | ||
| 0.5 g/kg | E–I–L | 35.82 | 13 | |
| I–L | 35.91 | 11 | ||
| L | 36.07 | 11 | ||
| 2.5 g/kg | E–I–L | 36.99 | 12 | |
| I–L | 37.02 | 13 | ||
| L | 36.42 | 10 |
Pups assigned to a given postnatal ethanol treatment were kept in a holding maternity cage (45 x 20 x 20 cm) partially filled with clean wood shavings. The floor of the cage was maintained at 27 C (± 1 C) through the use of a heating pad. One hour later body weights were individually recorded (± 0.01 g) and pups were given 0.0, 0.5 or 2.5 g/kg ethanol.
Behavioral evaluations started by gently placing each pup in the center of the activity chamber. After completion of each 5-min test, pups were returned to the holding cages. Once all testing procedures were completed, infants were returned to their biological mothers.
Design and Data analysis
As previously stated, not all pups were tested at each specific post-administration time. Group E–I–L was tested 5–10, 30–35 and 60–65 min following postnatal drug treatment. Group I-L was tested only during the two last time intervals while group L was tested only during the last post-administration time. This obviously implies that when considering this independent variable and the categories that define it, we are not dealing with a simple factorial design. Taking this into consideration, different statistical approaches were utilized to answer the specific questions under examination. In each subsection of the Results we will specify the phenomenon under analysis and how the data were processed for inferential purposes. The general statistical strategy consisted in the use of mixed ANOVAs that included the most pertinent groups in order to answer a specific question.
Preliminary analysis did not find significant effect of gender or interaction with the remaining factors under consideration. In order to simplify the statistical processing of the data, activity scores were collapsed across gender. Prenatal (0.0 or 2.0 g/kg) and postnatal (0.0, 0.5 or 2.5 g/kg) ethanol treatments were included as between factors in all statistical analyses. Testing condition (E–I–L, I-L or L) also was a between factor when comparing motor activity scores of animals tested for the first time. Hence, the scores under consideration for group E–I–L are those corresponding to the first post-administration interval (E: 5–10 min), the values under consideration for group I-L are those obtained during post-administration time 30–35 min, and scores of group I are those from the last testing interval (60–65 min). Testing condition also was a between factor for analysis of locomotor activity scores at 60–65 min post-administration time. In this case, the “between factor” alludes to amount of experience with the test environment prior to being tested at 60–65 min. In other words, group E–I–L had two prior exposures to the testing environment, group I-L had only one exposure and group L had no prior exposure. Post-administration time was treated as a within factor only when examining performance of the same group of animals across tests, centered in the performance of pups assigned to group E–I–L (i.e., the only group evaluated at each of 5–10, 30–35 and 60–65 min).
The dependent variable under examination in all cases was general motor activity as operationalized through the number of infrared beams interrupted by each pup per minute. In all the ANOVA’s, each of the 5 minutes of the test served as a repeated measure. Significant main effects or interactions indicated by the ANOVAs were further analyzed through post-hoc tests (Newman-Keuls test with a Type I error set at 0.05).
Results
Body weights
A 3-way between ANOVA (prenatal ethanol treatment x postnatal ethanol treatment x testing condition) was used to process body weights of the animals prior to behavioral testing procedures. This ANOVA did not detect significant main effects or interactions (Table 1).
Locomotor activity
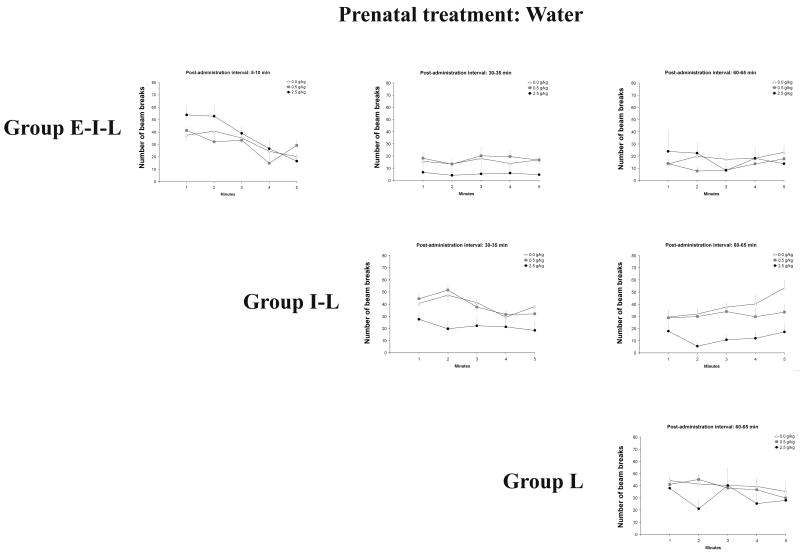
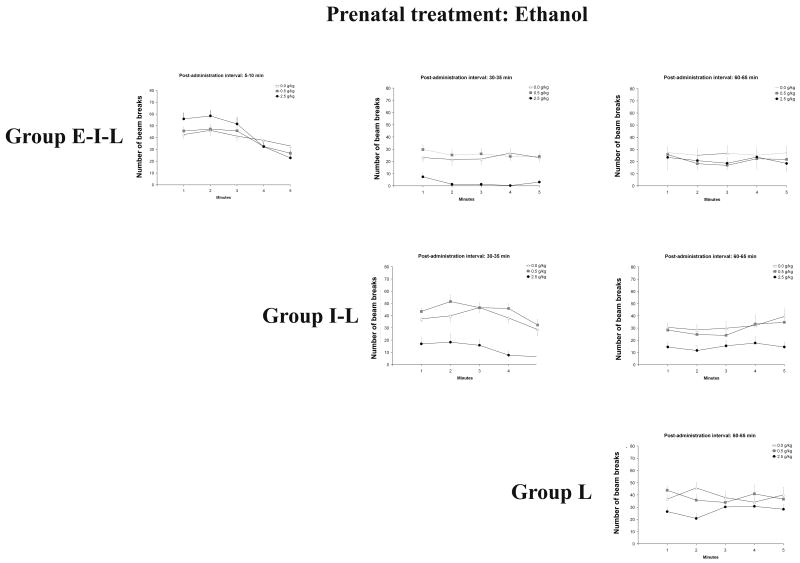
Figures 3a and 3b depict the behavioral profiles of all groups as a function of prenatal ethanol exposure. In accordance with specific questions under examination, additional graphs will be provided to illustrate significant main effects or interactions corresponding to each result’s subsection.
Figure 3.
Figure 3a and 3b: Overall behavioral profiles as a function of prenatal (0.0 or 2.0 g/kg) and postnatal (0.0, 0.5 or 2.5 g/kg) ethanol treatments, and testing conditions [early-intermediate-late (E–I–L), intermediate-late (I-L) or late (L)]. Group E–I–L was evaluated at 5–10, 30–35 and 60–65, while group I-L was evaluated at 30–35 and 60–65 min. Finally, group L was tested at 60–65 min. Vertical lines illustrate standard errors of the means.
Effects of ethanol upon motor activity at different post-administration times: Within-group analysis (Statistical comparisons of cells A, B and C, see Figure 2)
In this analysis we included only those subjects that were sequentially evaluated during the course of the toxic state (group E–I–L). This provides an overall perspective of ethanol’s effects on motor activity at the different post-administration times without specifically controlling for non-associative learning processes (e.g habituation) that may occur due to repeated exposure to the testing environment.
A 4-way mixed ANOVA was employed. Prenatal (0.0 or 2.0 g/kg ethanol) and postnatal (0, 0.5 or 2.5 g/kg ethanol) ethanol treatments represented the between factors under consideration while post-administration interval (5–10, 30–35 and 60–65 min) and minutes in each test (1–5 min) were included as within factors. This analysis indicated significant main effects of post-administration interval [F(2,132) = 58.43, p < 0.001], minutes [F(4,264) = 11.35, p < 0.001] and prenatal ethanol treatment [F(1,66) = 7.77, p < 0.01]. Pups prenatally exposed to ethanol exhibited higher levels of activity than those given only water during late gestation (see Figure 4). Prenatal treatment did not interact with any of the remaining factors under consideration. The overall ANOVA also indicated that the following interactions were significant: postnatal ethanol treatment x post-administration interval [F(4,132) = 6.55, p < 0.0005], postnatal ethanol treatment x minute [F(8,264) = 2.75, p < 0.01] and post-administration interval x minute [F(8.528) = 14.99, p < 0.001]. Post-hoc tests indicated that in the early interval (5–10 mins) activity levels during the first 2 minutes were significantly higher than those observed during the following 3 minutes. This difference was not observed during subsequent post-administration intervals. It is also notable that levels of activity during the first 4 minutes of the initial testing interval (5–10 min) were significantly higher than those recorded during the first 4 minutes of the subsequent tests (30–35 and 60–65 min). These results appear to indicate some level of habituation occurring during the course of the first test and its persistence during the last two evaluations (see Figures 3a and 3b).
Figure 4.
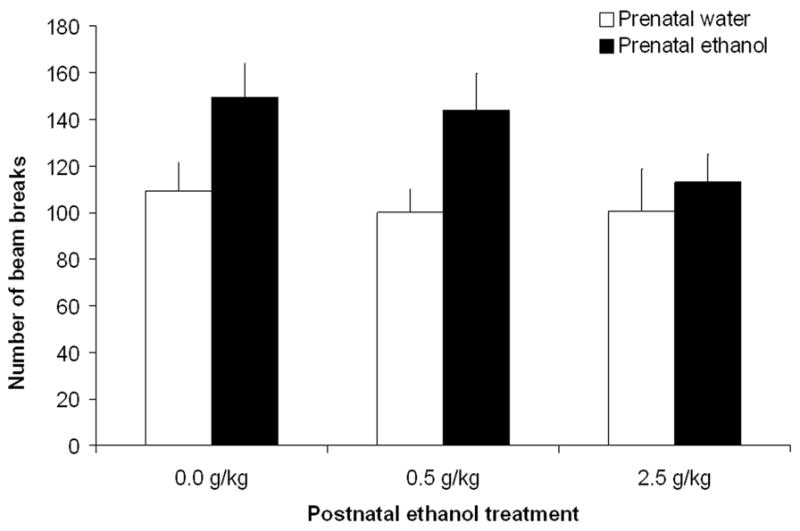
Locomotor activity as a function of prenatal (0.0 or 2.0 g/kg) and postnatal (0.0, 0.5 or 2.5 g/kg) ethanol treatments in pups from group early-intermediate-late (E–I–L). Data are collapsed across minutes and post-administration interval. The ANOVA revealed a significant effect of prenatal ethanol treatment. Vertical lines illustrate standard errors of the means.
The remaining significant interactions were further analyzed through follow-up ANOVAs since there is no unambiguous way to determine the error term for post-hoc comparisons involving between-within interactions. In the case of the interaction between postnatal ethanol treatment and post-administration interval we conducted one-way between-group ANOVAs to examine the effects of ethanol dose at each post-administration time. These ANOVAs indicated no effects of postnatal ethanol treatment during the first (5-10 mins) and last (60–65 mins) testing sessions. In the intermediate post-administration interval (30–35 mins) there was a significant effect of dose [F(2,135) = 25.75, p < 0.001]: pups receiving 2.5 g/kg ethanol had less activity than infants treated with vehicle or a lower ethanol dose (see Figure 5).
Figure 5.
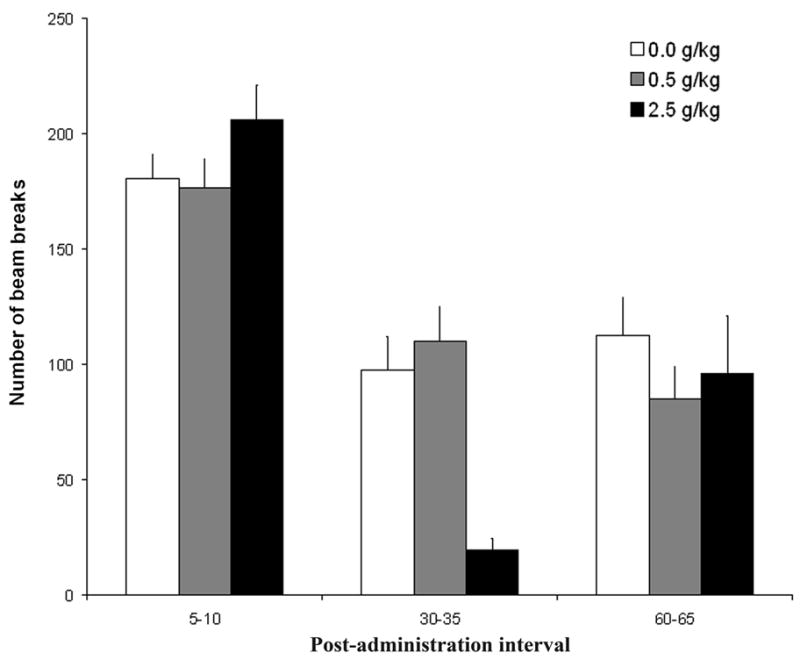
Locomotor activity as a function of postnatal ethanol administration (0.5 or 2.5 g/kg) and post-administration interval (5–10, 30–35 and 60–65). In this figure we included only data from group E–I–L. Data are collapsed across minutes and prenatal ethanol treatment. The ANOVA revealed a significant interaction between postnatal ethanol treatment and post-administration time. Vertical lines illustrate standard errors of the means.
Additional one-way ANOVAs were conducted to further clarify the effect of each particular ethanol dose (0.0, 0.5 or 2.5 g/kg) across post-administration intervals. These post-hoc tests among pups treated with any of the three doses showed activity scores higher during the first post-administration interval than in the last two. Furthermore, with 2.5 g/kg ethanol, levels of activity at 60–65 mins were significantly higher than those recorded in the preceding test (30–35 min; see also Figure 5). This result suggests recovery of activity levels within the state of intoxication despite the fact that BECs remain high and stable, implying acute tolerance.
The basis of the interaction between postnatal ethanol treatment and minute was also analyzed through subsequent one-way ANOVAs. A significant effect of postnatal ethanol treatment was observed only during the last minute of the test. Pups treated with the highest ethanol dose exhibited lower levels of activity than subjects treated with vehicle or 0.5 g/kg ethanol, suggesting the onset of sedation.
In summary, prenatal ethanol exerted a significant effect on infantile motor activity independent of postnatal administration of the drug. This effect suggests a tendency for hyperactivity among pups prenatally exposed to ethanol. The results also indicated the development of habituation across tests and a marked sedative effect of the higher ethanol dose (2.5 g/kg) at 30–35 min. It is important to mention that at 60–65 mins., pups treated with this relatively high ethanol dose showed higher activity levels than those registered in the earlier testing trial. This effect suggests development of acute behavioral tolerance to ethanol’s motor suppressing effects, since BECs at 30–35 and 60–65 min were found to be equivalent (see Experiment 1). Behavioral habituation also occurred in all groups. So it is difficult to be certain whether the absence of a dose effect during the last test (60–65 min) is due to acute tolerance in the 2.5 g/kg ethanol group or to lower levels of activity at this post-administration interval due to habituation.
Effects of ethanol upon motor activity at different post-administration times: Between-group analysis (Statistical comparisons of cells A, D and F; see Figure 2)
The experimental design allows comparison of behavioral effects of different ethanol doses as the toxic states proceed temporally, independently of habituation to the test that can otherwise confound these effects. The present statistical approach focuses on initial responsiveness to a novel context while systematically varying post-administration time. Hence, the data in this analysis are from the first test of E–I–L, I-L and L pups, which were tested following ethanol administration at 5–10, 30–35 or 60–65 min, respectively. A mixed ANOVA served to process this information. The between factors were: prenatal ethanol treatment (0.0 or 2.0 g/kg ethanol), postnatal ethanol treatment (0.0, 0.5 or 2.5 g/kg), and testing condition (E–I–L, I-L or L). Minutes of test served as a repeated measure.
As can be observed in Figures 3a and 3b, motor activity patterns in pups treated with 0.0 and 0.5 g/kg were very similar. In these animals it appears that habituation occurred within each testing trial; i.e. pups were more active during the first minutes of the test and gradually declined in activity with passage of time. Pups treated with the highest ethanol dose (2.5 g/kg) seemed to differ from both vehicle controls and pups treated with 0.5 g/kg ethanol, with significantly higher levels of activity immediately after drug administration (5–10 mins post-administration time). This effect appears to be particularly evident during the first few minutes of this test period. In contrast, when ethanol reached peak levels (30–35 mins) the 2.5 g/kg ethanol dose induced markedly lower activity levels than the other groups. This sedation-like effect seems to be of a lesser magnitude during a subsequent post-administration time (60–65 mins) at which BECs remained at peak levels.
These observations were confirmed by the corresponding inferential analysis. The ANOVA revealed significant main effects of the following factors: postnatal ethanol treatment [F(2,181) = 12.70, p < 0.001] and minute within each test [F(4,724) = 29.92, p< 0.001].. The following two-way interactions also achieved statistical significance: postnatal ethanol treatment x testing condition, [F(4,181) = 11.09, p < 0.001], testing condition x minute [F(8,724) = 6.27, p < 0.001] and prenatal ethanol treatment x testing condition F(2,181) = 3.09, p < 0.05. The 3-way interaction comprising postnatal ethanol treatment, testing condition and minute also attained significance, [F(16,724) = 2.33, p < 0.005]. Post-hoc tests indicated that, independently of the postnatal ethanol treatment, pups prenatally exposed to ethanol (condition E–I–L when tested in the early phase) exhibited significantly higher levels of motor activity than pups prenatally treated with only water (see Figure 6). In addition, pups prenatally treated with ethanol showed higher activity scores when they were tested 5–10 min after receiving the i.g. administration (Group E–I–L) than when they were evaluated for the first time at 30–35 or 60–65 min (Groups I-L and L), whereas prenatal water treated controls did not differ in activity between testing conditions (see also Figure 6).
Figure 6.
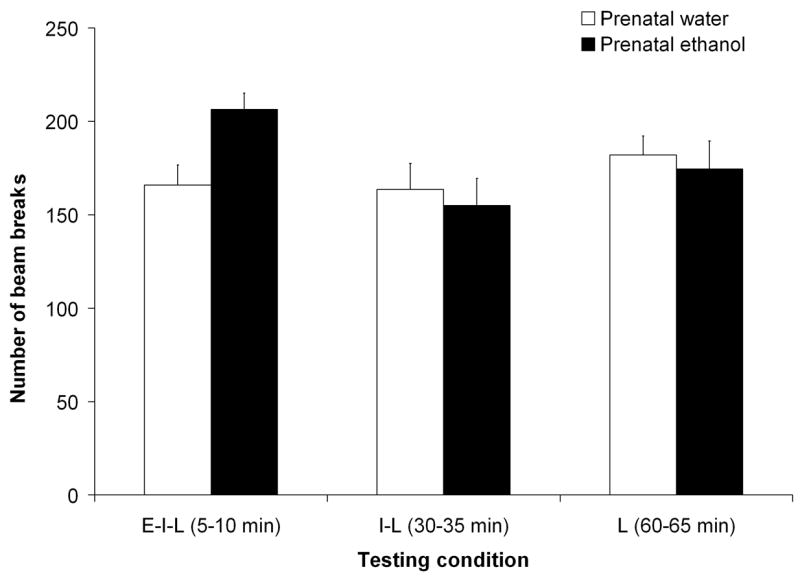
Locomotor activity as a function of prenatal ethanol treatment (0.0 or 2.0 g/kg) and testing condition [early-intermediate-late (E–I–L), intermediate-late (I-L) or late (L)]. Data are collapsed across minutes and postnatal ethanol administration. The data included in this picture are from the first test of E–I–L, I-L and L groups. The ANOVA revealed a significant interaction prenatal ethanol treatment by testing condition. Vertical lines illustrate standard errors of the means.
To determine the loci of the significant 3-way interaction, follow-up 2-way ANOVAs (postnatal ethanol treatment x minute) were performed for each post-administration time. Immediately following drug administration (5–10 mins) there was a significant main effect of minute tempered by a significant effect of the interaction comprising this factor and postnatal ethanol treatment [F(4,276) = 39.28, and F(8,276) = 3.78, both p < 0.0005; respectively]. In additional one-way follow-up ANOVAs, the effect of ethanol dose was tested across minutes. Independently of ethanol dose, motor activity was significantly higher (p < 0.001 in each dose) during the first 2 min than during the last 2 min. In other words, motor activity progressively decreased during the course of the test. One-way ANOVAs comparing the effects of ethanol dose within each minute revealed a significant effect of postnatal treatment only during the first 2 min of the test (p < 0.05). According to the corresponding post-hoc tests, pups treated with 2.5 g/kg ethanol exhibited significantly more activity 5–10 min after ethanol administration than counterparts treated with vehicle or a lower ethanol dose (0.5 g/kg; see Figure 7).
Figure 7.
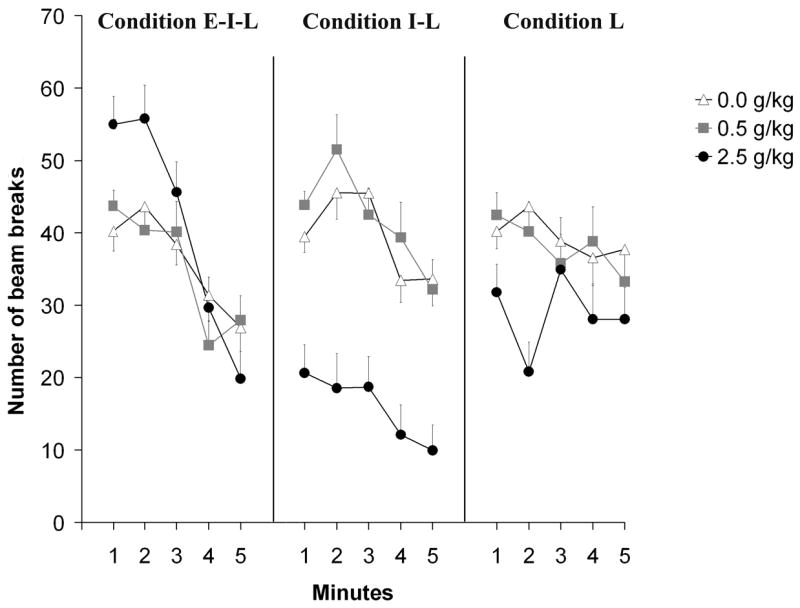
Locomotor activity as a function of postnatal ethanol administration (0.5 or 2.5 g/kg) and testing condition [early-intermediate-late (E–I–L), intermediate-late (I–L) or late (L)]. The activity scores included in this figure are from the first test of E–I–L, I–L and L groups. Data are collapsed across prenatal ethanol treatment. The ANOVA revealed a significant interaction between postnatal ethanol treatment, testing condition and minutes. Vertical lines illustrate standard errors of the means.
The 2-way ANOVA corresponding to the second post-administration interval (30–35 mins) indicated significant main effects of postnatal drug treatment and minutes, [F(2,63) = 32.49 and F(4,252) = 8.72, respectively, both p’s < 0.001]. Post-hoc tests showed that pups treated with 2.5 g/kg ethanol displayed significantly lower activity levels than pups receiving the alternative doses. Activity during the last 2 minutes of the test was lower that of preceding minutes. During the 60–65 min test, the 2-way ANOVA revealed only a main effect of dose, F(2,58) = 3.76, p < 0.05. Post-hoc tests showed that pups treated with 2.5 g/kg ethanol had significantly lower activity scores than pups given 0.5 g/kg ethanol or water (see Figure 7).
To further examine the 3-way interaction comprising postnatal ethanol dose, testing condition and minute we conducted follow-up 2-way ANOVAs for each postnatal ethanol treatment. This strategy should also allow a test of acute tolerance to ethanol by contrasting motor performance at 30–35 and 60–65 mins, time intervals at which BECs were equivalent. In the case of pups treated with vehicle or 0.5 g/kg ethanol, only minutes exerted significant effects, F(4,260) = 9.19, p < 0.001 and F(4,248) = 11.77, p < 0.001, respectively (see Figure 7). Activity was higher during the first 3 minutes of the test than during the last 2 minutes. The statistical profile obtained in the case of infants treated with 2.5 g/kg ethanol was different. In this case, the main factors minutes as well as testing condition exerted significant effects, F(4,252) = 11.28 and F(2,63) = 18.79, respectively, both p’s < 0.001. Post-hoc tests showed that within each post-administration interval, motor activity scores varied in a similar way as when examining the effects of vehicle or 0.5 g/kg ethanol, i.e. activity scores were higher during the first 3 minutes of the test than during the last 2 minutes. Furthermore, post-hoc comparisons indicated that activity levels were significantly higher 5–10 min after ethanol administration (group E–I–L) than after 30–35 min (group I-L) or 60-65 min (group L). Interestingly, activity scores in group I-L (30–35 mins) were significantly lower than those in group L (60–65 mins).
In summary, the present statistical strategy was meant to examine effects of different ethanol doses at different post-administration times while avoiding confounding by differential duration of exposure to the testing environment. Prenatal ethanol exposure was found to generate high levels of activity soon after postnatal administration procedures. This effect was independent of postnatal ethanol dose and was not observed during later stages of the acute state of intoxication. It also became clear that pups treated with 2.5 g/kg ethanol exhibited higher levels of activity during the early stage of the intoxication than pups treated with vehicle or a lower ethanol dose (0.5 g/kg). This effect rapidly subsided and during later stages of the toxic state, the highest ethanol dose induced motor sedative effects. In turn, this sedative effect was more pronounced at 30–35 mins than at 60–65 mins post-administration time. Because BECs were similar across these post-administration intervals (see Experiment 1), the significant activity differences between these intervals probably reflect the process of acute tolerance.
Effects of infantile ethanol intoxication and varying levels of experience with the testing environment (Statistical comparison of cells C, E and F; see Figure 2)
The goal of the present statistical approach is to compare ethanol’s psychomotor effects as a function of prenatal treatment and differential levels of experience with the testing chamber. We explicitly compared motor activity levels during the last post-administration time (60–65 mins) as a function of prenatal and postnatal ethanol treatment and testing condition. In this analysis, testing condition provides the opportunity to weigh the role of varying levels of testing experience in psychomotor activity patterns. The ANOVA was defined by 3 between factors (prenatal and postnatal ethanol treatments, and testing condition) and by a within factor represented by minutes during the test. This analysis indicated significant main effects of postnatal ethanol treatment and testing condition, [F(2,181) = 9.41, p < 0.0005, and F(2,181) = 16.86, p < 0.001, respectively]. The interaction between postnatal ethanol treatment and testing condition also achieved significance, F(4,181) = 2.78, p < 0.05. Post-hoc tests revealed that the number of prior experiences with the testing environment was a critical factor determining activity rates (see Figure 8). Pups administered 0.0 or 0.5 g/kg ethanol that had being tested twice before (group E–I–L) exhibited significantly lower activity scores than pups treated with the same dose but never tested before (group L) or tested only once before (group I-L). In addition, pups administered 2.5 g/kg ethanol, whether tested for the first or second time exhibited significantly lower scores than those given 0.5 g/kg ethanol or water. These dose-related differences were not observed when infants had two prior testing experiences.
Figure 8.
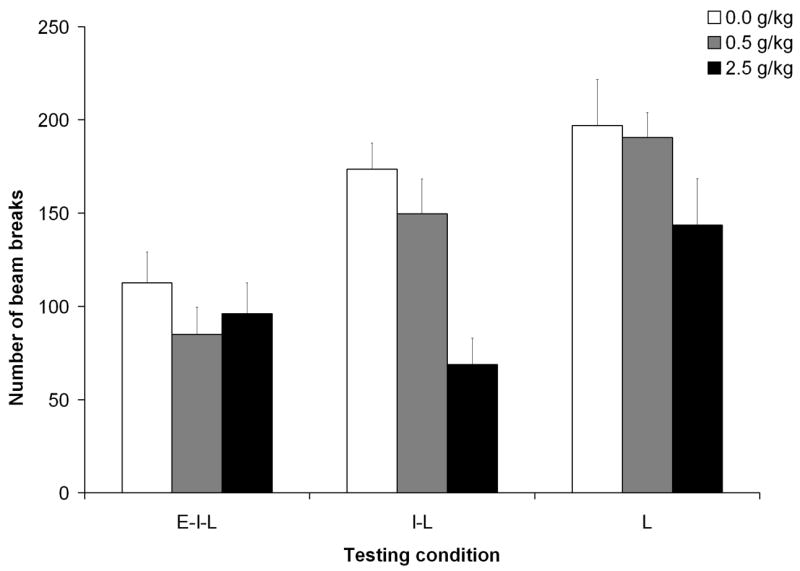
Locomotor activity as a function of postnatal ethanol administration (0.5 or 2.5 g/kg) and testing condition [early-intermediate-late (E–I–L), intermediate-late (I–L) or late (L)]. In this picture has been taking into account activity data collected at 60–65 min. Data are collapsed across minute and prenatal ethanol treatment. The ANOVA revealed a significant interaction between postnatal ethanol treatment and testing condition. Vertical lines illustrate standard errors of the means.
In summary, the results confirm the development of behavioral habituation as a function of progressive experience with the testing environment, as well as the importance of exerting stimulus control over habituation in testing behavior at different points following ethanol administration. The sedative effects of the highest ethanol dose (2.5 g/kg) were clearly detectable 60 min after ethanol administration when the testing environment is completely or relatively novel for the organism. Behavioral habituation resulting from additional experience with the testing environment (in this case, two prior tests) also produced a marked decrement in locomotion that impeded conclusions regarding the sedative effects of the drug. Finally, the results suggest that ethanol exposure during late gestation does not affect rate of behavioral habituation or sensitivity to the sedative effects of a relatively high ethanol dose.
General Discussion
The present study was conceived to analyze the impact of moderate ethanol exposure (2.0 g/kg) during late gestation (GDs 17 to 20) upon postnatal sensitivity to acute ethanol. We tested whether motor activity patterns induced by low and high ethanol doses known to exert biphasic motivational effects in preweanling rats (Molina, Pautassi et al., 2007) would be affected by this moderate prenatal ethanol treatment. The lower dose employed in the present study (0.5 g/kg) did not exert sedation or stimulation in any time point within the course of intoxication. In contrast, the higher ethanol dose employed (2.5 g/kg) exerted biphasic locomotor effects. Soon after ethanol administration this relatively high ethanol dose increased motor activity, whereas in latter stages of the intoxication process (30–35 and 60–65 min) the same ethanol dose decreased locomotion. Prenatal ethanol treatment did not affect sensitivity to the acute motor activity effects induced by this relatively high ethanol dose at any post-administration interval.
Late gestational exposure to ethanol is sufficient to increase subsequent affinity for ethanol ingestion and responsiveness to stimuli that predict postabsorptive effects of the drug. These effects are partially regulated by fetal learning comprising ethanol’s chemosensory cues and the contingency between these cues and ethanol’s postaborptive effects (Chotro et al., 2007; Molina, Spear, Spear, Mennella, & Lewis, 2007; Spear and Molina, 2005). In animal models of Fetal Alcohol Syndrome, in which animals are chronically exposed to high ethanol doses in utero, marked behavioral and physiological changes in response to an ethanol challenge have been observed. These changes may also affect ethanol intake patterns. For example, prenatal ethanol exposure results in heightened behavioral sensitivity to the stimulatory effects of ethanol in mice (Becker et al., 1993) and resistance to the drug’s thermoregulatory disruptions in rats (Abel et al., 1981; Molina, Hoffmann, Spear, & Spear, 1987). According to the present study, brief exposure to a moderate ethanol dose during late gestation does not affect infantile sensitivity to either the drug’s stimulatory or sedative effects or the development of acute tolerance. This modality of ethanol administration during late pregnancy results in increased perinatal sensitivity to ethanol’s sedative effects (Chotro & Spear, 1997). According to the present results this effect is no longer present in a later stage of ontogenetic development.
The only effect attributable to ethanol exposure during late gestation observed in this study was heightened behavioral activity at testing. This effect was observed only in infants prenatally exposed to ethanol that were evaluated soon after being intragastrically administered with either vehicle or ethanol (see Figures 4 and 6). When testing occurred 30 or 60 mins after administration, prenatal ethanol treatment did not affect locomotion patterns (see Figures 4 and 7). These results argue in favor of heightened reactivity to a stressor as a function of prior late gestational exposure to ethanol. Recent research indicates that preweanlings show behavioral signs of distress (increased ultrasound emissions) after being intragastrically or intraperitoneally administered with an apparently innocuous substance such as physiological saline (Pautassi, Nizhnikov, Molina, Boehm, & Spear, 2007). In prior studies we have observed similar phenomena. This effect was encountered soon after birth when animals were stimulated with a distinct olfactory cue (Chotro & Spear, 1997; Dominguez et al., 1996). Interestingly, and also in accordance with the present results, this interaction between prenatal ethanol and postnatal stress did not affect habituation: prenatally exposed animals in the present study were capable of exhibiting progressive decrements in locomotion as a function of repeated experiences with a novel environment.
Hyper-reactivity to various stressors as a function of prenatal ethanol exposure has been documented in both human and preclinical research (for a recent review see Zhang, Sliwowska, & Weinberg, 2005). Ethanol prenatal treatment effectively disrupts the physiological balance of the hypothalamic-pituitary-adrenal axis yielding a particular sensitivity to a variety of environmental stressors; among others, novel events and experimental manipulations such as handling and administration procedures. Apparently, the moderate prenatal ethanol treatment employed here is sufficient to induce hyper-reactivity. It remains to be determined if the neuroendocrinological bases of this phenomenon are homologous to those reported in the case of chronic exposure to relatively high ethanol doses during the course of pregnancy.
As mentioned, independently of prenatal treatment, the highest ethanol dose employed here (2.5 g/kg) exerted biphasic motor effects. During the rising phase of the blood ethanol curve, almost immediately after drug administration, pups receiving 2.5 g/kg ethanol exhibited significantly higher levels of activity than pharmacological controls. This effect rapidly subsided (Figure 7). This rapid drop in activity rates may be explained by rapid habituation occurring during the test session or emergence of sedative effects induced by this relatively high ethanol dose. The sedative effects were clearly observed 30 min following administration, when BECs reach peak levels. In the case of rat strains genetically selected for ethanol affinity or sensitivity to the drug’s reinforcing properties [Alcohol-preferring (P), High-alcohol-drinking (HAD), Sardinian-alcohol-preferring (sP), Alcohol-preferring (AA) and UChA rat strains], stimulating motor effects of ethanol have been consistently observed (see Agabio et al., 2001; Krimmer, 1991; Paivarinta & Korpi, 1993; Quintanilla, 1999; Rodd et al., 2004; Waller, Murphy, McBride, Lumeng, & Li, 1986).These activating effects have been primarily detected with low ethanol doses (normally below 1.0 g/kg). In genetically heterogenous rats ethanol’s activating effects have been rarely reported, and what seems to predominate is motor suppression (Chuck et al., 2006; Erickson & Kochhar, 1985; Salamone et al., 2006). When focusing on the ontogeny of genetically heterogenous rats, recent studies consistently indicate heightened affinity for ethanol ingestion and marked sensitivity to ethanol’s reinforcing properties during early stages in development (Chotro et al., 2007; Molina, Spear et al., 2007; Spear and Molina, 2005).
It has been recently reported that Sprague-Dawley infants similar to those in the present study consume, without the need of initiation procedures, high amounts of ethanol (Sanders & Spear, 2007; Truxell & Spear, 2004). These animals have also been observed to readily manifest biphasic motivational properties of ethanol. When given a relatively high ethanol dose (2.0 g/kg) pups detect ethanol’s reinforcing properties soon after its intragastric administration. Thirty minutes later aversive effects are predominant (Molina, Pautassi et al., 2007; Pautassi, Godoy, Spear, & Molina, 2002). This time course of ethanol’s motivational properties coincides with the biphasic (activating and sedative) effects obtained with 2.5 g/kg ethanol in the present study. The temporal coincidence between biphasic motivational and locomotor effects seems to argue in favor of the hypothesis that similar mechanisms underlie these processes (Risinger & Cunningham, 1992; Wise & Bozarth, 1987). Nevertheless, it is important to note that the lower dose here utilized (0.5 g/kg) was not observed to exert motor activating effects even though it is sufficient to act as a positive or negative (anti-anxiety) reinforcer in the developing infant (Molina, Ponce, Truxell, & Spear, 2006; Pautassi, Melloni, Ponce, & Molina, 2005; Pautassi et al., 2007). As a function of these considerations it appears that the results of the present study and those derived from the analysis of ethanol’s early motivational effects partially support the hypothesis for common mechanisms underlying ethanol’s reinforcement and activating effects. These considerations also imply that specific stages in ontogeny may represent an appropriate experimental niche, alternative to genetic and phylogenetic approaches, for the analysis of mechanisms regulating drug-related motor and motivational effects.
The design of Experiment 2 also permitted the observation of apparent acute tolerance within the process of intoxication. This effect was observed with 2.5 g/kg ethanol, in terms of explicitly contrasting motor activity patterns at two time points where BECs were high and stable. It was clear that 30 min after administration, this ethanol dose significantly reduced locomotion. At 60 min pups exhibited a partial recovery from this sedative effect. This behavioral profile was observed when methodologically minimizing the impact of habituation to the circumstances of testing. This result appears to be in strong agreement with the observations of Silveri and Spear (2001) relative to the emergence of this phenomenon during early ontogeny. These authors reported development of acute tolerance in preweanlings that received an intraperitoneal administration of 3.2 g/kg ethanol. Acute tolerance was revealed in terms of motor impairments in a swim task at post-administration times comparable to those here examined. It could be argued that ethanol is metabolized faster in the brain than in the periphery of the central nervous system. If this was the case, brain ethanol levels at 60 min could be lower than in previous post-administration intervals. This hypothesis can not be completely dismissed. Yet, it is necessary to observe that in preweanling rats, but not in adolescent or adult rats, brain ethanol levels attained with a high ethanol dose (3.2 g/kg) remain at peak levels even 105 minutes after ethanol administration (Silvery and Spear, 2001).
Independent of drug treatment, the behavioral data suggested rapid infantile behavioral habituation to a novel environment. This habituation seems to be manifested within the course of a testing interval or when animals are repeatedly tested in this particular context (see e.g. Figures 5 and 8). There were no indications supporting an effect of prenatal treatment upon this apparent learning process. The null effect of relatively low-to-moderate ethanol exposure during late gestation is in accordance with prior studies examining behavioral reactivity to repeated stimulation with salient chemosensory stimuli (Abate, Pepino, Dominguez, Spear, & Molina, 2000; Arias & Chotro, 2005a, 2005b). Deficits in the capability of the developing organism to habituate to novel stimuli as a function of ethanol prenatal exposure have been reported. Apparently, these detrimental effects are obtained when ethanol is chronically administered during gestation or when relatively high ethanol doses are employed during a temporal window of brain vulnerability to the teratogenic properties of this drug (e.g. Hofmann, Patyk, & Weinberg, 2005; Hunt & Phillips, 2004).
Beyond the analysis of ethanol-related responsiveness as a function of prenatal exposure to the drug, the results of the present study indicate that the “inverted ladder” design may represent a simple and powerful methodological tool for examining neurobehavioral outcomes of exposure to teratogens or postnatal sensitivity to drugs of abuse. This approach appears to allow an integrated evaluation of unconditioned reactivity to the drug or environment, adaptive processes linked to each of these factors (e.g. acute drug tolerance and habituation) and the possible interaction between them.
Acknowledgments
This work was supported by Supported by grants from the NIAAA (AA11960, AA013098, AA015992) and the NIMH (MH035219) to NES and the Agencia Nacional de Promocion Cientifica y Tecnologica to JCM, Postdoctoral fellowship from Ministerio de Educacion y Ciencia from Spain to CA, as well as Fundacion Antorchas, Argentina, CONICET (PIP 6485) and FONCyT (PICT 05-38084) to E.C.M (this study has been conducted during the period corresponding to her Doctorate Program in Biological Sciences, Cordoba University). The authors wish to express their gratitude to Teri Tanehaus and Heather Murphy for their technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abate P, Pepino MY, Dominguez HD, Spear NE, Molina JC. Fetal associative learning mediated through maternal alcohol intoxication. Alcohol Clin Exp Res. 2000;24(1):39–47. [PubMed] [Google Scholar]
- Abel EL, Bush R, Dintcheff BA. Exposure of rats to alcohol in utero alters drug sensitivity in adulthood. Science. 1981;212(4502):1531–1533. doi: 10.1126/science.7233243. [DOI] [PubMed] [Google Scholar]
- Agabio R, Carai MA, Lobina C, Pani M, Reali R, Vacca G, et al. Alcohol stimulates motor activity in selectively bred Sardinian alcohol-preferring (sP), but not in Sardinian alcohol-nonpreferring (sNP), rats. Alcohol. 2001;23(2):123–126. doi: 10.1016/s0741-8329(00)00144-0. [DOI] [PubMed] [Google Scholar]
- Alati R, Al Mamun A, Williams GM, O'Callaghan M, Najman JM, Bor W. In utero alcohol exposure and prediction of alcohol disorders in early adulthood: a birth cohort study. Arch Gen Psychiatry. 2006;63(9):1009–1016. doi: 10.1001/archpsyc.63.9.1009. [DOI] [PubMed] [Google Scholar]
- Anandam N, Felegi W, Stern JM. In utero alcohol heightens juvenile reactivity. Pharmacol Biochem Behav. 1980;13(4):531–535. doi: 10.1016/0091-3057(80)90276-2. [DOI] [PubMed] [Google Scholar]
- Arias C, Chotro MG. Increased palatability of ethanol after prenatal ethanol exposure is mediated by the opioid system. Pharmacol Biochem Behav. 2005a;82(3):434–442. doi: 10.1016/j.pbb.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Arias C, Chotro MG. Increased preference for ethanol in the infant rat after prenatal ethanol exposure, expressed on intake and taste reactivity tests. Alcohol Clin Exp Res. 2005b;29(3):337–346. doi: 10.1097/01.alc.0000156115.35817.21. [DOI] [PubMed] [Google Scholar]
- Arias C, Chotro MG. Interactions between prenatal ethanol exposure and postnatal learning about ethanol in rat pups. Alcohol. 2006;40(1):51–59. doi: 10.1016/j.alcohol.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Baer JS, Barr HM, Bookstein FL, Sampson PD, Streissguth AP. Prenatal alcohol exposure and family history of alcoholism in the etiology of adolescent alcohol problems. J Stud Alcohol. 1998;59(5):533–543. doi: 10.15288/jsa.1998.59.533. [DOI] [PubMed] [Google Scholar]
- Baer JS, Sampson PD, Barr HM, Connor PD, Streissguth AP. A 21-year longitudinal analysis of the effects of prenatal alcohol exposure on young adult drinking. Arch Gen Psychiatry. 2003;60(4):377–385. doi: 10.1001/archpsyc.60.4.377. [DOI] [PubMed] [Google Scholar]
- Bailey CD, Brien JF, Reynolds JN. Chronic prenatal ethanol exposure increases GABA(A) receptor subunit protein expression in the adult guinea pig cerebral cortex. J Neurosci. 2001;21(12):4381–4389. doi: 10.1523/JNEUROSCI.21-12-04381.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC, Hale RL, Boggan WO, Randall CL. Effects of prenatal ethanol exposure on later sensitivity to the low-dose stimulant actions of ethanol in mouse offspring: possible role of catecholamines. Alcohol Clin Exp Res. 1993;17(6):1325–1336. doi: 10.1111/j.1530-0277.1993.tb05249.x. [DOI] [PubMed] [Google Scholar]
- Bhalla S, Kaur K, Mahmood A, Mahmood S. Postnatal development of alcohol dehydrogenase in liver and intestine of rats exposed to ethanol in utero. Indian J Med Res. 2005;121(1):39–45. [PubMed] [Google Scholar]
- Chotro MG, Arias C. Prenatal exposure to ethanol increases ethanol consumption: a conditioned response? Alcohol. 2003;30(1):19–28. doi: 10.1016/s0741-8329(03)00037-5. [DOI] [PubMed] [Google Scholar]
- Chotro MG, Arias C, Laviola G. Increased ethanol intake after prenatal ethanol exposure: studies with animals. Neurosci Biobehav Rev. 2007;31(2):181–191. doi: 10.1016/j.neubiorev.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Chotro MG, Spear NE. Repeated exposure to moderate doses of alcohol in the rat fetus: evidence of sensitization to toxic and chemosensory aspects of alcohol. Alcohol Clin Exp Res. 1997;21(2):360–367. [PubMed] [Google Scholar]
- Chuck TL, McLaughlin PJ, Arizzi-LaFrance MN, Salamone JD, Correa M. Comparison between multiple behavioral effects of peripheral ethanol administration in rats: sedation, ataxia, and bradykinesia. Life Sci. 2006;79(2):154–161. doi: 10.1016/j.lfs.2005.12.045. [DOI] [PubMed] [Google Scholar]
- Dominguez HD, Lopez MF, Chotro MG, Molina JC. Perinatal responsiveness to alcohol's chemosensory cues as a function of prenatal alcohol administration during gestational days 17–20 in the rat. Neurobiol Learn Mem. 1996;65(2):103–112. doi: 10.1006/nlme.1996.0012. [DOI] [PubMed] [Google Scholar]
- Dominguez HD, Lopez MF, Molina JC. Neonatal responsiveness to alcohol odor and infant alcohol intake as a function of alcohol experience during late gestation. Alcohol. 1998;16(2):109–117. doi: 10.1016/s0741-8329(97)00169-9. [DOI] [PubMed] [Google Scholar]
- Druse MJ, Tajuddin N, Kuo A, Connerty M. Effects of in utero ethanol exposure on the developing dopaminergic system in rats. J Neurosci Res. 1990;27(2):233–240. doi: 10.1002/jnr.490270214. [DOI] [PubMed] [Google Scholar]
- Erickson CK, Kochhar A. An animal model for low dose ethanol-induced locomotor stimulation: behavioral characteristics. Alcohol Clin Exp Res. 1985;9(4):310–314. doi: 10.1111/j.1530-0277.1985.tb05550.x. [DOI] [PubMed] [Google Scholar]
- Hofmann CE, Patyk IA, Weinberg J. Prenatal ethanol exposure: sex differences in anxiety and anxiolytic response to a 5-HT1A agonist. Pharmacol Biochem Behav. 2005;82(3):549–558. doi: 10.1016/j.pbb.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14(3):221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Hunt PS, Phillips JS. Postnatal binge ethanol exposure affects habituation of the cardiac orienting response to an olfactory stimulus in preweanling rats. Alcohol Clin Exp Res. 2004;28(1):123–130. doi: 10.1097/01.ALC.0000108650.02216.1A. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources, C. o. L. S. National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academic Press; 1996. [Google Scholar]
- Kelly SJ, Bonthius DJ, West JR. Developmental changes in alcohol pharmacokinetics in rats. Alcohol Clin Exp Res. 1987;11(3):281–286. doi: 10.1111/j.1530-0277.1987.tb01308.x. [DOI] [PubMed] [Google Scholar]
- Krimmer EC. HAS and LAS rats respond differentially to behavioral effects of ethanol, pentobarbital, chlorpromazine and chlordiazepoxide. Pharmacol Biochem Behav. 1991;39(1):5–13. doi: 10.1016/0091-3057(91)90389-j. [DOI] [PubMed] [Google Scholar]
- Lancaster FE, Spiegel KS. Voluntary beer drinking by pregnant rats: offspring sensitivity to ethanol and preference for beer. Alcohol. 1989;6(3):207–217. doi: 10.1016/0741-8329(89)90020-7. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Spear NE, Molina JC. Ontogenetic differences in the expression of olfactory-conditioned aversions resulting from a state of acute alcohol intoxication in the rat. Alcohol. 1996;13(5):473–481. doi: 10.1016/0741-8329(96)00037-7. [DOI] [PubMed] [Google Scholar]
- Molina JC, Chotro MG, Dominguez HD. Fetal alcohol learning derived from ethanol contamination of the prenatal environment. In: Lecanuet JP, Fifer WP, Krasnegor N, Smotherman WP, editors. Fetal development: A psychobiological perspective. Hillsdale, NJ: Lawrence Erlbaum Associates; 1995. pp. 419–438. [Google Scholar]
- Molina JC, Hoffmann H, Spear LP, Spear NE. Sensorimotor maturation and alcohol responsiveness in rats prenatally exposed to alcohol during gestational day 8. Neurotoxicol Teratol. 1987;9(2):121–128. doi: 10.1016/0892-0362(87)90088-2. [DOI] [PubMed] [Google Scholar]
- Molina JC, Pautassi RM, Truxell E, Spear N. Differential motivational properties of ethanol during early ontogeny as a function of dose and postadministration time. Alcohol. 2007;41(1):41–55. doi: 10.1016/j.alcohol.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina JC, Ponce LF, Truxell E, Spear NE. Infantile sensitivity to ethanol's motivational effects: Ethanol reinforcement during the third postnatal week. Alcohol Clin Exp Res. 2006;30(9):1506–1519. doi: 10.1111/j.1530-0277.2006.00182.x. [DOI] [PubMed] [Google Scholar]
- Molina JC, Spear NE, Spear LP, Mennella JA, Lewis MJ. The International society for developmental psychobiology 39th annual meeting symposium: Alcohol and development: beyond fetal alcohol syndrome. Dev Psychobiol. 2007;49(3):227–242. doi: 10.1002/dev.20224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizhnikov ME, Molina JC, Varlinskaya EI, Spear NE. Prenatal ethanol exposure increases ethanol reinforcement in neonatal rats. Alcohol Clin Exp Res. 2006;30(1):34–45. doi: 10.1111/j.1530-0277.2006.00009.x. [DOI] [PubMed] [Google Scholar]
- Paivarinta P, Korpi ER. Voluntary ethanol drinking increases locomotor activity in alcohol-preferring AA rats. Pharmacol Biochem Behav. 1993;44(1):127–132. doi: 10.1016/0091-3057(93)90289-6. [DOI] [PubMed] [Google Scholar]
- Pautassi RM, Godoy JC, Spear NE, Molina JC. Early responsiveness to stimuli paired with different stages within the state of alcohol intoxication. Alcohol Clin Exp Res. 2002;26(5):644–654. [PubMed] [Google Scholar]
- Pautassi RM, Melloni C, Ponce LF, Molina JC. Acute ethanol counteracts the acquisition of aversive olfactory learning in infant rats. Alcohol. 2005;36(2):99–105. doi: 10.1016/j.alcohol.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Pautassi RM, Nizhnikov M, Molina JC, Boehm SL, 2nd, Spear N. Differential effects of ethanol and midazolam upon the devaluation of an aversive memory in infant rats. Alcohol. 2007;41(6):421–431. doi: 10.1016/j.alcohol.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautassi RM, Sanders S, Miller S, Spear N, Molina JC. Early ethanol's anxiolytic effects assessed through an unconditional stimulus revaluation procedure. Alcohol Clin Exp Res. 2006;30(3):448–459. doi: 10.1111/j.1530-0277.2006.00049.x. [DOI] [PubMed] [Google Scholar]
- Pueta M, Abate P, Spear N, Molina JC. Interactions between ethanol experiences during late gestation and nursing: Effects upon infantile and maternal responsiveness to ethanol. International journal of comparative psuchology. 2005;18:207–224. [Google Scholar]
- Quintanilla ME. Effect of low doses of ethanol on spontaneous locomotor activity in UChB and UChA rats. Addiction Biology. 1999;4(4):443–448. doi: 10.1080/13556219971434. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Cunningham CL. Ethanol produces rapid biphasic hedonic effects. Ann N Y Acad Sci. 1992;654:506–508. doi: 10.1111/j.1749-6632.1992.tb26014.x. [DOI] [PubMed] [Google Scholar]
- Rockman GE, Markert LE, Delrizzo M. Effects of prenatal ethanol exposure on ethanol-induced locomotor activity in rats. Alcohol. 1989;6(5):353–356. doi: 10.1016/0741-8329(89)90003-7. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, McKinzie DL, Webster AA, Murphy JM, Lumeng L, et al. Low-dose stimulatory effects of ethanol during adolescence in rat lines selectively bred for high alcohol intake. Alcohol Clin Exp Res. 2004;28(4):535–543. doi: 10.1097/01.alc.0000122107.08417.d0. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, McLaughlin P, Chuck TL, Arizzi-LaFrance MN, Betz AJ. Central vs. peripheral administration of ethanol, acetaldehyde and acetate: Stimulatory and suppressive effects on locomotion, lever pressing and response initiation. Alcoholism-Clinical and Experimental Research. 2006;30(6):84A–84A. [Google Scholar]
- Sanders S, Spear NE. Ethanol acceptance is high during early infancy and becomes still higher after previous ethanol ingestion. Alcohol Clin Exp Res. 2007;31(7):1148–1158. doi: 10.1111/j.1530-0277.2007.00400.x. [DOI] [PubMed] [Google Scholar]
- Sari Y, Zhou FC. Prenatal alcohol exposure causes long-term serotonin neuron deficit in mice. Alcohol Clin Exp Res. 2004;28(6):941–948. doi: 10.1097/01.alc.0000128228.08472.39. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Acute, rapid, and chronic tolerance during ontogeny: observations when equating ethanol perturbation across age. Alcohol Clin Exp Res. 2001;25(9):1301–1308. [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Adolescence. Alcohol sensitivity, tolerance, and intake. Recent Dev Alcohol. 2005;17:143–159. [PubMed] [Google Scholar]
- Spear NE, Molina JC. Fetal or infantile exposure to ethanol promotes ethanol ingestion in adolescence and adulthood: a theoretical review. Alcohol Clin Exp Res. 2005;29(6):909–929. doi: 10.1097/01.alc.0000171046.78556.66. [DOI] [PubMed] [Google Scholar]
- Taylor AN, Branch BJ, Liu SH, Kokka N. Long-term effects of fetal ethanol exposure on pituitary-adrenal response to stress. Pharmacol Biochem Behav. 1982;16(4):585–589. doi: 10.1016/0091-3057(82)90420-8. [DOI] [PubMed] [Google Scholar]
- Taylor AN, Branch BJ, Liu SH, Wiechmann AF, Hill MA, Kokka N. Fetal exposure to ethanol enhances pituitary-adrenal and temperature responses to ethanol in adult rats. Alcohol Clin Exp Res. 1981;5(2):237–246. doi: 10.1111/j.1530-0277.1981.tb04895.x. [DOI] [PubMed] [Google Scholar]
- Truxell E, Spear NE. Immediate acceptance of ethanol in infant rats: ontogenetic differences with moderate but not high ethanol concentration. Alcohol Clin Exp Res. 2004;28(8):1200–1211. doi: 10.1097/01.alc.0000134220.34842.18. [DOI] [PubMed] [Google Scholar]
- Waller MB, Murphy JM, McBride WJ, Lumeng L, Li TK. Effect of low dose ethanol on spontaneous motor activity in alcohol-preferring and -nonpreferring lines of rats. Pharmacol Biochem Behav. 1986;24(3):617–623. doi: 10.1016/0091-3057(86)90567-8. [DOI] [PubMed] [Google Scholar]
- Weinberg J, Taylor AN, Gianoulakis C. Fetal ethanol exposure: hypothalamic-pituitary-adrenal and beta-endorphin responses to repeated stress. Alcohol Clin Exp Res. 1996;20(1):122–131. doi: 10.1111/j.1530-0277.1996.tb01054.x. [DOI] [PubMed] [Google Scholar]
- Westergren S, Rydenhag B, Bassen M, Archer T, Conradi NG. Effects of prenatal alcohol exposure on activity and learning in Sprague-Dawley rats. Pharmacol Biochem Behav. 1996;55(4):515–520. doi: 10.1016/s0091-3057(96)00277-8. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94(4):469–492. [PubMed] [Google Scholar]
- Yates WR, Cadoret RJ, Troughton EP, Stewart M, Giunta TS. Effect of fetal alcohol exposure on adult symptoms of nicotine, alcohol, and drug dependence. Alcohol Clin Exp Res. 1998;22(4):914–920. [PubMed] [Google Scholar]
- Zhang X, Sliwowska JH, Weinberg J. Prenatal alcohol exposure and fetal programming: effects on neuroendocrine and immune function. Exp Biol Med (Maywood) 2005;230(6):376–388. doi: 10.1177/15353702-0323006-05. [DOI] [PubMed] [Google Scholar]