Abstract
Background
Serum carotenoid concentrations relate inversely to cardiovascular disease incidence. To clarify the effect of carotenoids on atherosclerotic risk factors, we examined the association of circulating carotenoids with inflammation, oxidative stress, endothelial dysfunction, and smoking.
Methods
Black and white men and women in the Coronary Artery Risk Development in Young Adults study, ages 18 to 30 years at recruitment (1985–1986) from 4 US cities, were investigated over 15 years. We included 2048 to 4580 participants in analyses of the sum of serum α-carotene, β-carotene, zeaxanthin/lutein, and β-cryptoxanthin concentrations and of lycopene at year 0 and at year 7.
Results
The year 0 sum of 4 carotenoids was inversely associated (all P <0.05) with year 0 leukocyte count (slope per sum carotenoid SD, −0.17); year 7 fibrinogen (slope, −0.10); year 7 and year 15 C-reactive protein (slope, −0.12 and −0.09); and year 15 F2-isoprostanes (slope, −13.0), soluble P-selectin (slope, −0.48), and soluble intercellular adhesion molecule-1 (sICAM1; slope, −5.1). Leukocyte counts and sICAM1 and F2-isoprostane concentrations had stronger associations in smokers than in nonsmokers, and sICAM1 concentrations were higher in the highest carotenoid quartile in smokers than in the lowest carotenoid quartile in nonsmokers. Superoxide dismutase was positively associated with the sum of 4 carotenoids (slope, 0.12; P <0.01). Lycopene was inversely associated only with sICAM1. The year 7 carotenoid associations with these markers were mostly similar to those at year 0.
Conclusions
Circulating serum carotenoids were associated, some interactively with smoking, in apparently beneficial directions with markers of inflammation, oxidative stress, and endothelial dysfunction.
Several epidemiologic studies have proposed an inverse relationship between serum carotenoids and cardiovascular disease (1–4). Inflammation, oxidative stress, and endothelial dysfunction are known to be associated with atherosclerosis and cardiovascular diseases (5–11). Although several cross-sectional studies reported that serum carotenoids are related inversely to these markers of the atherosclerosis pathway (12–15), we know of no studies that investigated whether carotenoid concentrations predict the future values of these markers.
We conducted cross-sectional and longitudinal analyses to clarify whether circulating carotenoids are associated with markers of oxidative stress, inflammation, and endothelial dysfunction. In each case we also investigated whether the relation of serum carotenoid concentrations with these variables differed according to smoking status, following our prior findings of an interaction of smoking with circulating carotenoids and several other variables (16, 17).
Materials and Methods
THE CORONARY ARTERY RISK DEVELOPMENT IN YOUNG ADULTS AND YOUNG ADULT LONGITUDINAL TRENDS IN ANTIOXIDANTS STUDIES
The Coronary Artery Risk Development in Young Adults (CARDIA) 6 study, a biethnic, prospective, multicenter epidemiologic study of the evolution of risk factors in young adults, has been described (18). Briefly, from 1985 to 1986, 5115 African American and white individuals (ages 18 to 30 years) were examined in Birmingham, AL; Chicago, IL; Minneapolis, MN; and Oakland, CA. At the Birmingham, Minneapolis, and Chicago sites, participants were randomly selected from total communities or from specific census tracts. In Oakland, random selection was from members of the Kaiser Permanente Medical Care Program. Recruitment achieved nearly equal numbers at each site of race (African American, white), sex, education (high school or less, more than high school), and age (18–24 years, 25–30 years). Fifty percent of invited individuals contacted were examined (47% of African Americans and 60% of whites) and became the CARDIA cohort. Young Adult Longitudinal Trends in Antioxidants (YALTA) is a CARDIA ancillary study in which serum carotenoid concentrations were measured in most CARDIA participants at years 0 and 7, and circulating extracellular superoxide dismutase (SOD), F2-isoprostanes, soluble P-selectin, and soluble intercellular adhesion molecule-1 (sICAM1) were measured at year 15.
We excluded individuals with missing data on smoking status (n = 20) or serum carotenoids (n = 302), confounding factors (n = 80), or who were not fasting for at least 8 h (n = 113) at year 0, leaving 4600 (89.9%) participants for analysis. After excluding the participants for whom the given measurement data were not available (not measured or participant nonfasting) or [in C-reactive protein (CRP) analyses only] who had CRP concentrations ≥10 mg/dL (possible acute inflammation), the number of participants for study of year 0 carotenoids and year 0 leukocyte counts was 4580; numbers associated with other analytes are provided in Table 1–Table 4.
Table 1.
Year of measurement of serum carotenoids and blood measures investigated in relation to them.a
| Year 0 | Year 5 | Year 7 | Year 15 | |
|---|---|---|---|---|
| Carotenoids | • | • | ||
| Leukocyte count | A | |||
| C-reactive protein | A, B | A, B, C | ||
| Fibrinogen | A | A, B | ||
| Superoxide dismutase | A, B | |||
| F2-isoprostanes | A, B | |||
| P-selectin | A, B | |||
| Soluble ICAM1 | A, B |
">A, Variable investigated in relation to year 0 carotenoids. B, Variable investigated in relation to year 7 carotenoids. C, Change in the variable investigated in relation to year 7 carotenoids.
Table 4.
Relation of year 7 sum of 4 carotenoids with markers of inflammation, oxidative stress, and endothelial dysfunction measured at years 7 and 15.a
| Biochemical marker | Slopeb | P for slope |
|---|---|---|
| Year 7 CRP (n = 3044), mg/Lc | −0.17 | <0.01 |
| Year 15 CRP (n =2250), mg/Lc | −0.12 | <0.01 |
| Year 7 fibrinogen (n = 3220), mmol/Ld | −0.14 | <0.01 |
| Year 15 Superoxide dismutase (n = 2041), U/mLd | 0.04 | 0.45 |
| Year 15 Plasma F2-isoprostanes (n = 2078), pmol/Ld | −15.6 | <0.01 |
| Year 15 P-selectin (n = 2078), ng/Ld | −0.75 | <0.01 |
| Year 15 Soluble ICAM1 (n = 2048), ng/Ld | −5.09 | <0.01 |
CARDIA 1992–1993 to 2000–2001. Values adjusted for race; sex; center; age; education; hypertension; diabetes; ethanol intake; LDL cholesterol; total energy intake; BMI; physical activity; use of vitamin A, C, E, and carotenoid supplements; and smoking status. Sum of 4 carotenoids: sum of α -carotene, β -carotene, zeaxanthin/lutein, and β -cryptoxanthin. Participants with CRP concentrations >10 mg/dL were excluded from CRP analyses to avoid any effect of acute inflammation.
β -Coefficient per SD of carotenoid.
Adjusted geometric mean.
Adjusted mean.
SELF-REPORT AND ANTHROPOMETRIC MEASUREMENTS
Sex, race, date of birth, medication use, and weekly alcohol consumption were determined at each examination by structured interview or self-administered questionnaire. Participants were classified at year 0 as never, former, or current smokers (interviewer-administered questionnaire) as of year 0; the 186 “nonsmoking” participants and the 23 with missing smoking status whose year 0 cotinine concentration was ≥79.5 nmol/L (14 ng/mL) were reclassified as current smokers. A physical activity score was derived from the CARDIA Physical Activity History, a simplified version of the Minnesota Leisure Time Physical Activity Questionnaire (19). Alcohol intake (mL/day) was computed from the self-reported frequency of beer, wine, and liquor consumed per week. The interviewer-administered CARDIA diet history was obtained at years 0 and 7 and included 1609 unique food codes at either year. Body weight with light clothing was measured to the nearest 0.1 kg (0.2 pounds), and height without shoes was measured to the nearest 0.5 cm. Body mass index (BMI) was weight/height squared (kg/m2).
COLLECTION, PROCESSING, AND WHOLE BLOOD MEASUREMENT
All participants were fasting for ≥8 h and asked to avoid smoking and heavy physical activity for at least 2 h before blood collection at each examination. Leukocyte counts at CARDIA year 0 were measured as cells (×109) per liter whole blood using a leukocyte counter in the local center hospital laboratory. After plasma or serum separation from whole blood, aliquots were stored at −70 °C until they were shipped on dry ice to a central laboratory.
YALTA used sera obtained at CARDIA years 0 and 7 to assay the carotenoids α- and β-carotene, lycopene, zeaxanthin/lutein, and β-cryptoxanthin (Molecular Epidemiology and Biomarker Research Laboratory, University of Minnesota), with an HPLC-based assay modified from the method of Bieri et al. (20) to optimize detection of carotenoids with calibration as described by Craft et al. (21) and sample handling as described by Gross et al. (22). Calibration was performed with pure compounds [Hoffmann-La Roche; Sigma Chemical Co. (now Sigma-Aldrich)]. Quality-control procedures included routine analysis of plasma and serum control pools containing high and low concentrations of each analyte. In addition, the laboratory routinely analyzed NIST reference sera and was a participant in the NIST Fat-Soluble Vitamin Quality Assurance Group. The CVs were <10% for all analytes and control pools. The intraclass correlation coefficients (between-person variance/between-person plus within-person variance) were 0.93 for α-carotene, 0.98 for β-carotene, 0.73 for zeaxanthin/lutein, 0.97 for β-cryptoxanthin, and 0.73 for lycopene (23).
Year 0 and year 7 lipid concentrations were measured by the University of Washington Northwest Lipid Research Clinic Laboratory. Triglycerides and HDL-cholesterol were measured by enzymatic procedures, with HDL-cholesterol measured after dextran sulfate–magnesium precipitation and LDL-cholesterol calculated using the Friedewald equation.
Year 5 fibrinogen was assessed by the Clauss method using reagents from the Dade Division, Baxter Healthcare Corp. (24). Year 7 fibrinogen antigen was measured using the BN-II nephelometer (N Antiserum to Human Fibrinogen; Dade Behring Inc.), calibrated with standard normal plasma (SNP reagent, Dade). A high-sensitivity ELISA measured year 7 and year 15 serum CRP at the Department of Pathology, University of Vermont, using a BN-II analyzer (25). Year 15 extracellular SOD activity (U/mL) was assayed according to Cayman Chemical kit procedures. The protocol was followed exactly, and the samples were diluted 1:2 with sample buffer. This analysis was automated with a Beckman Biomek. A human soluble P-selectin immunoassay was performed by ELISA with reagent sets supplied by R&D Systems, Inc, modified by pipetting by Biomek-robotic automation (Beckman) and a 1:5 dilution rather than the recommended 1:20 dilution. A human sICAM1 immunoassay was measured by ELISA with reagent sets supplied by R&D systems, Inc. Year 15 plasma free F2-isoprostanes were measured by gas chromatography–mass spectrometry at the Molecular Epidemiology and Biomarker Research Laboratory at the University of Minnesota (Minneapolis, MN) (8). Year 10 γ-glutamyltransferase (GGT) was measured with a SMAC II continuous-flow analyzer (Technicon Instruments Corp.) at American Bio-science Laboratories (now Smith-Kline Beecham). At year 10, GGT was measured colorimetrically with nitroanilide methodology on a Roche Cobas Mira Plus chemistry instrument at Linco Research Inc. (17).
STATISTICAL ANALYSIS
The CARDIA and YALTA studies did not measure each analyte at each examination. Timing of measurements included in this report is provided in Table 1. In these analyses, we often related other variables to the sum of α-carotene, β-carotene, zeaxanthin/lutein, and β-cryptoxanthin; we omitted lycopene from this sum because its associations with many variables were strikingly different from those of the other 4 carotenoids. Most baseline characteristics are presented as prevalence and means of dependent variables across quartiles of year 0 serum carotenoid–independent variables with adjustment for age, sex, race, center, and education. We used multiple linear regression analyses to calculate the adjusted means of markers of inflammation, oxidative stress, and endothelial dysfunction, adjusting for center, race, sex, and year 0 values of education (years), age (years), total energy intake, alcohol consumption (mL/day), BMI (kg/m2), physical activity (continuous), LDL-cholesterol (continuous), systolic blood pressure, use of vitamin supplements (A, C, or E), and cigarette smoking (never, former, or current; further separation of current smokers by dose did not affect findings). Further adjustment for HDL-cholesterol (continuous) and triglycerides (continuous), as we have recommended (26), did not basically alter the findings. For year 7 carotenoid analyses, we used year 7 covariates. We further separated the year 7 carotenoids into 2 components: the year 0 sum of 4 carotenoids and the difference (year 7 sum of 4 carotenoids − year 0 sum of 4 carotenoids). We included these 2 components in a joint model. In addition, we analyzed the relationships of the year 0 sum of 4 carotenoids with year 15 markers of inflammation, oxidative stress, and endothelial dysfunction, adjusted for each other.
We present analyses based on baseline smoking data; excluding participants who had changed smoking status at the time of measurement of each biochemical marker did not greatly alter findings. In all analyses except those presented in Table 1, we combined former and never smokers as nonsmokers after confirming that oxidative stress was lower in former smokers than in current smokers. Interaction models included a product term.
Results
YEAR 0 BASELINE CHARACTERISTICS
Among 4580 participants with measured year 0 leukocyte counts, 55.0% were women, 49.4% were white, and 60.5% had more than high school education. Mean (SD) age was 24.8 (3.6) years at year 0. At year 0, 34.3%, 11.6%, and 54.1% were current, former, and never smokers, respectively. The mean sum of 4 serum carotenoids was 80.3 (43.5) nmol/L, and that of serum lycopene was 55.6 (27.0) nmol/L.
Study participants with a higher year 0 sum of 4 serum carotenoids tended to be older, white, female, more highly educated, and less likely to be current smokers (Table 2). Year 0 BMI and total energy intake correlated inversely with the sum of 4 serum carotenoids. Vegetable or fruit intake and use of vitamin A, C, or E supplements (all of which may increase serum carotenoid concentrations) were more frequent in higher serum carotenoid groups, whereas with meat or alcohol intake the carotenoid concentrations were lower. These results suggest that those with a higher sum of 4 serum carotenoids tended to have healthier lifestyles. Total and HDL cholesterol were higher in higher serum carotenoid groups, as anticipated given that the carotenoids are fat-soluble (26). This same pattern emerged individually for α- and β-carotene, zeaxanthin/lutein, and β-cryptoxanthin (data not shown). However, those with higher lycopene tended to have less healthy lifestyles. Mean intakes of meat and alcohol and concentrations of triglycerides were higher across lycopene quartiles, whereas intake of fruit and use of vitamin supplements were lower. In more specific analysis of food groups, lycopene concentration was positively related to tomatoes, ketchup, pickles, dairy desserts, low-fat cheese, red meat obtained at fast food restaurants, other red meat, and onions and inversely related to energy intake, tofu, and canned fish (data not shown). Lycopene was unrelated to cigarette smoking.
Table 2.
Year 0 characteristics (mean or percentage) according to quartile of serum carotenoid concentration.a
| Year 0 sum of 4 carotenoid quartile, nmol/L | Year 0 lycopene quartile, nmol/L | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Year 0 characteristic | Overall | 10.0–51.3 | 51.3–71.6 | 71.6–97.9 | 97.9–574.9 | 1.2–35.0 | 35.1–52.5 | 52.6–72.0 | 72.1–177.9 |
| n | 4580 | 1145 | 1142 | 1146 | 1147 | 1144 | 1144 | 1144 | 1148 |
| Age (mean), year | 24.8 | 24.3 | 24.6 | 24.8 | 25.7 | 25.1 | 24.8 | 24.6 | 24.8 |
| Sex, % female | 55.0 | 50.0 | 52.5 | 54.1 | 63.4 | 60.7 | 58.0 | 53.5 | 48.0 |
| Race, % whites | 49.4 | 47.3 | 43.3 | 48.3 | 58.6 | 50.5 | 49.4 | 49.8 | 47.7 |
| Center | |||||||||
| Birmingham | 23.4 | 27.7 | 27.8 | 22.4 | 15.7 | 19.3 | 24.0 | 25.3 | 25.0 |
| Chicago | 21.6 | 18.4 | 21.1 | 21.3 | 25.4 | 14.2 | 22.3 | 24.7 | 25.0 |
| Minneapolis | 27.8 | 29.4 | 28.7 | 28.7 | 24.3 | 23.0 | 24.7 | 28.4 | 35.0 |
| Oakland | 27.3 | 24.5 | 22.3 | 27.6 | 34.6 | 43.5 | 29.0 | 21.5 | 15.0 |
| Education | |||||||||
| <12 years, % | 9.9 | 15.7 | 10.9 | 8.7 | 4.2 | 8.9 | 11.1 | 10.8 | 8.7 |
| 12 years, % | 29.6 | 37.8 | 32.6 | 27.7 | 20.5 | 28.6 | 31.2 | 28.8 | 29.9 |
| ≥13 years, % | 60.5 | 46.5 | 56.5 | 63.6 | 75.3 | 62.5 | 57.7 | 60.3 | 61.4 |
| Smoking status | |||||||||
| Current, %b | 34.3 | 46 | 39 | 31 | 21 | 34 | 34 | 34 | 35 |
| Former, %b | 11.6 | 9 | 10 | 14 | 14 | 12 | 11 | 12 | 11 |
| Never, %b | 54.1 | 45 | 51 | 56 | 65 | 54 | 55 | 54 | 54 |
| Total energy intake (mean), kcal/dayb | 2957 | 3050 | 3017 | 2947 | 2815 | 2959 | 2955 | 2960 | 2954 |
| Fish intake (mean), times/weekb | 1.7 | 1.5 | 1.6 | 1.7 | 1.8 | 1.8 | 1.7 | 1.6 | 1.6 |
| Meat intake (mean), times/weekb | 10.8 | 11.4 | 11.1 | 11.0 | 9.7 | 10.2 | 10.8 | 11.0 | 11.2 |
| Vegetable intake (mean), times/weekb | 15.8 | 13.8 | 15.4 | 16.1 | 18.0 | 15.8 | 15.4 | 16.1 | 16.1 |
| Fruit intake (mean), times/weekb | 7.0 | 5.4 | 6.6 | 7.2 | 8.7 | 7.3 | 7.0 | 7.0 | 6.6 |
| Ethanol intake (mean), g/weekb | 12.1 | 16.5 | 12.7 | 10.8 | 8.6 | 11.1 | 12.1 | 12.2 | 13.1 |
| Systolic BP (mean), mmHgb | 110 | 112 | 111 | 110 | 109 | 110 | 111 | 110 | 111 |
| BMI (mean), kg/m2b | 24.5 | 25.9 | 24.5 | 24.2 | 23.3 | 24.8 | 24.5 | 24.1 | 24.5 |
| Physical activity (mean), exercise unitsb | 419 | 395 | 411 | 432 | 437 | 418 | 422 | 422 | 414 |
| Use of vitamin supplements (yes), %b,c | 30 | 25 | 29 | 31 | 36 | 31 | 31 | 32 | 28 |
| Total cholesterol (mean), mmol/Lb | 4.57 | 4.32 | 4.46 | 4.67 | 4.81 | 4.29 | 4.44 | 4.58 | 4.96 |
| LDL cholesterol (mean), mmol/Lb | 2.82 | 2.61 | 2.73 | 2.91 | 3.01 | 2.59 | 2.70 | 2.81 | 3.16 |
| HDL cholesterol (mean), mmol/Lb | 1.38 | 1.29 | 1.35 | 1.38 | 1.47 | 1.34 | 1.35 | 1.39 | 1.41 |
| Triglycerides (mean), mmol/Lb | 0.82 | 0.92 | 0.81 | 0.82 | 0.72 | 0.78 | 0.83 | 0.82 | 0.84 |
| Fasting blood glucose (mean), mg/dLb | 4.58 | 4.61 | 4.58 | 4.58 | 4.54 | 4.57 | 4.57 | 4.59 | 4.58 |
| α -Carotene (mean), nmol/Lb | 5.0 | 2.1 | 3.3 | 4.6 | 10.0 | 5.1 | 4.9 | 5.0 | 5.0 |
| β -Carotene (mean), nmol/Lb | 28.2 | 11.8 | 19.4 | 27.7 | 53.8 | 25.4 | 127.2 | 28.9 | 31.3 |
| Zeaxanthin/lutein (mean), nmol/Lb | 32.3 | 18.9 | 27.6 | 35.2 | 47.3 | 25.4 | 31.5 | 34.6 | 37.6 |
| β -Cryptoxanthin (mean), nmol/Lb | 14.8 | 7.2 | 11.6 | 15.6 | 24.7 | 13.3 | 14.3 | 15.2 | 16.3 |
| Sum of 4 carotenoids (mean), nmol/Lb,d | 80.3 | 40.1 | 61.9 | 83.1 | 135.8 | 69.2 | 77.9 | 83.6 | 90.2 |
| Lycopene (mean), nmol/Lb | 55.6 | 42.8 | 55.1 | 61.0 | 63.3 | 24.2 | 44.1 | 62.0 | 91.8 |
CARDIA 1985–2001. Restricted to 4580 participants in whom leukocyte count was assessed at year 0. n, number of participants; BP: blood pressure.
Adjusted for age, sex, race, race-sex, center, and education.
Vitamin supplements: vitamins A, C, and E and carotenoids.
Sum of 4 serum carotenoids: α-carotene, β-carotene, zeaxanthin/lutein, and β-cryptoxanthin.
YEAR 0 SERUM CAROTENOID AND MARKERS OF INFLAMMATION
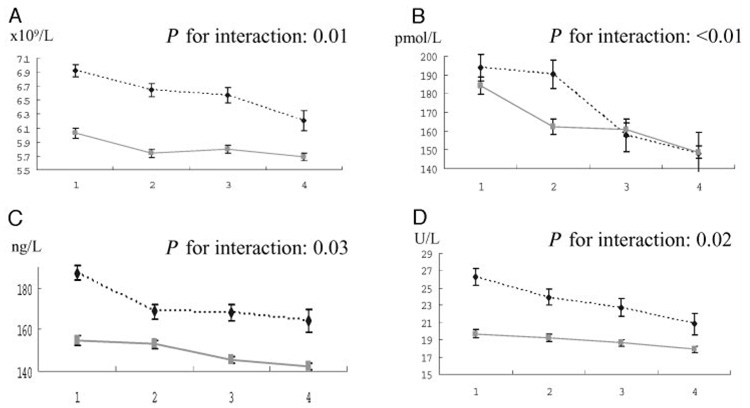
There were inverse relationships of the sum of 4 carotenoids (Table 2) and of α-carotene and β-carotene, zeaxanthin/lutein, and β-cryptoxanthin individually (see Table 1 in the Data Supplement that accompanies the online version of this article at http://www.clinchem.org/content/vol53/issue3) with year 0 leukocyte counts, a relationship that was stronger in smokers than in nonsmokers (slope of leukocyte counts per SD of the sum of 4 carotenoids, −0.37 in smokers and −0.11 in nonsmokers; P for interaction 0.01; Fig. 1A). However, year 0 leukocyte count was unrelated to circulating lycopene (Table 3).
Fig. 1.
The relationships of the year 0 sum of 4 serum carotenoid concentrations with year 0 leukocyte count (A), year 15 F2-isoprostanes (B), year 15 sICAM1 (C), and year 10 geometric means of GGT according to year 0 smoking status (current vs nonsmoker) (D).
Solid line, data in nonsmokers; broken line, current smokers. Error bars represent 1 standard error.
Table 3.
Relation of year 0 serum carotenoids with markers of inflammation, oxidative stress, and endothelial dysfunction measured at year 0, 5, 7, and 15.a
| Quartile of serum carotenoid |
|||||||
|---|---|---|---|---|---|---|---|
| Biochemical marker | Year 0 carotenoids | Q1 | Q2 | Q3 | Q4 | Slopeb | P for slope |
| Year 0 leukocyte count, ×109/L | Sum of 4 carotenoidsc | 6.36 | 6.06 | 6.07 | 5.89 | −0.17 | <0.01 |
| (n = 4580, SD = 1.79)d,e | Lycopene | 6.05 | 6.13 | 6.05 | 6.16 | 0.03 | 0.30 |
| Year 7 CRP (mg/L) | Sum of 4 carotenoids | 1.20 | 1.16 | 1.04 | 0.88 | −0.12 | <0.01 |
| (n = 3039, SD (ln scale) = 1.02)f | Lycopene | 1.04 | 1.11 | 0.99 | 1.11 | 0.00 | 0.92 |
| Year 15 CRP, mg/L | Sum of 4 carotenoids | 1.37 | 1.31 | 1.28 | 1.05 | −0.09 | <0.01 |
| (n = 2581, SD (ln scale) = 1.03)f | Lycopene | 1.23 | 1.28 | 1.28 | 1.19 | −0.03 | 0.21 |
| Year 5 Fibrinogen (mmol/L)e | Sum of 4 carotenoids | 7.77 | 7.73 | 7.78 | 7.72 | −0.01 | 0.65 |
| (n = 3515, SD = 1.48) | Lycopene | 7.74 | 7.72 | 7.73 | 7.81 | 0.02 | 0.41 |
| Year 7 Fibrinogen, mmol/Le | Sum of 4 carotenoids | 9.94 | 10.00 | 9.94 | 9.75 | −0.10 | <0.01 |
| (n = 3250, SD = 1.94) | Lycopene | 9.89 | 9.90 | 9.87 | 9.97 | 0.04 | 0.25 |
| Year 15 Superoxide dismutase (U/mL)e | Sum of 4 carotenoids | 4.55 | 4.59 | 4.57 | 4.86 | 0.12 | 0.02 |
| (n = 2346, SD = 2.22) | Lycopene | 4.77 | 4.62 | 4.62 | 4.58 | −0.06 | 0.27 |
| Year 15 Plasma F2-isoprostanes, pmol/Le | Sum of 4 carotenoids | 188 | 172 | 161 | 150 | −13.0 | <0.01 |
| (n = 2395, SD = 84) | Lycopene | 172 | 165 | 165 | 167 | −1.9 | 0.34 |
| Year 15 P-selectin (ng/L)e | Sum of 4 carotenoids | 37.6 | 36.6 | 37.5 | 36.5 | −0.48 | 0.04 |
| (n = 2385, SD = 10.6) | Lycopene | 37.0 | 37.4 | 37.0 | 36.7 | −0.14 | 0.56 |
| Year 15 Soluble ICAM1, ng/Le | Sum of 4 carotenoids | 167 | 158 | 154 | 150 | −5.1 | <0.01 |
| (n = 2357, SD = 41) | Lycopene | 162 | 157 | 157 | 152 | −3.6 | <0.01 |
CARDIA 1985–1986 to 2000–2001. Values adjusted for race; sex; center; age; education; hypertension; diabetes; ethanol intake; LDL cholesterol; total energy intake; BMI; physical activity; use of vitamin A, C, E, and carotenoid supplements; and smoking status. Participants with CRP concentrations >10 mg/dL were excluded from CRP analyses to avoid any effect of acute inflammation.
β -Coefficient per SD of carotenoid.
Sum of 4 carotenoids: sum of α -carotene, β -carotene, zeaxanthin/lutein, and β -cryptoxanthin.
SD of the given variable in the adjusted model.
Adjusted mean.
Adjusted geometric mean (SD is given on the ln scale because it is not well-defined on the arithmetic scale for a skewed variable).
The year 0 serum sum of 4 carotenoids was also inversely related to year 7 and year 15 CRP concentrations; each year 0 carotenoid, except for lycopene, was inversely related to these 2 variables. Furthermore, there was no interaction between year 0 serum carotenoids and year 0 smoking status in estimating CRP concentrations (range of P for interaction: year 7, 0.04–0.97; year 15, 0.16–0.98).
The year 0 sum of 4 carotenoid concentrations was not related to year 5 fibrinogen but was inversely related to year 7 fibrinogen concentrations, as measured with the more precise nephelometric method. No interactions between serum carotenoids and year 0 smoking were observed in estimating fibrinogen concentration (range of P for interaction, 0.10–0.91).
YEAR 0 CAROTENOIDS AND YEAR 15 OXIDATIVE STRESS AND ENDOTHELIAL DYSFUNCTION
The sum of 4 carotenoids and each individual carotenoid except lycopene were positively related to year 15 SOD (Table 3). No interactions were observed between carotenoids and smoking in predicting SOD activity (range of P for interaction, 0.17–0.95).
Plasma F2-isoprostane concentration was inversely related to the sum of 4 carotenoids and to each carotenoid except lycopene. This inverse relationship of the sum of 4 carotenoids with plasma F2-isoprostanes was stronger in current smokers than in nonsmokers (Fig. 1B; slope of F2-isoprostanes per SD increase of sum of 4 carotenoids, −26.8 in smokers and −10.5 in nonsmokers; P for interaction <0.01).
Soluble P-selectin concentration was significantly inversely related to the sum of 4 carotenoids and β-carotene, with nonsignificant, shallow inverse relationships with each of the other carotenoids. No interactions of carotenoids and smoking in estimating P-selectin were observed (range of P for interaction, 0.35–0.94).
sICAM1 was inversely related to the sum of 4 carotenoids and to each individual carotenoid, including lycopene. The inverse relationship of the sum of 4 carotenoids with sICAM1 was more evident in current smokers than nonsmokers (Fig. 1C; slope of sICAM1 per SD increase of sum of 4 carotenoids in current smokers, −10.7; in nonsmokers, −4.1; P for interaction = 0.03).
We previously reported that the inverse relationship of serum carotenoids with GGT was stronger in current or former smokers than in never smokers (17). We recomputed geometric mean year 10 GGT for consistency with this paper (comparing nonsmokers with current smokers); the resulting inverse associations, stronger in smokers than in nonsmokers, are shown in Fig. 1D.
YEAR 7 CAROTENOIDS AS BASELINE
Findings in relation to year 7 serum carotenoids mostly corroborated those reported above for year 0 carotenoids (Table 4; and Table 2 in the online Data Supplement).
We also studied year 7 carotenoid concentrations and change in CRP concentration between years 7 and 15 (n = 2142). The slope of log-transformed year 15 CRP adjusted for year 7 CRP per SD of sum of 4 carotenoids for all participants was −0.02; P for slope = 0.41; with no difference between nonsmokers and smokers.
COMPARISONS AMONG SEVERAL CARDIOVASCULAR RISK FACTORS AND CAROTENOIDS
Adjustment among the year 15 markers attenuated their associations with the year 0 sum of 4 carotenoid concentrations compared with those reported in Table 2 and Table 3, but all remained statistically significant except for P-selectin. The slopes of the year 15 measures regressed on the year 0 sum of 4 carotenoid concentrations and adjusted for each other (n = 2096) were SOD, β = 0.11, P = 0.04; F2-isoprostanes, β = −10.8, P <0.01; sICAM1, β = −3.3, P <0.01; CRP, β = −0.07, P <0.01; and P-selectin, β= −0.14, P = 0.58.
Discussion
Serum total and individual carotenoids, with the exception of lycopene, were inversely associated with markers of inflammation, oxidative stress, and endothelial dysfunction even after adjustment in multiple regression analyses. These associations support the inverse relation of carotenoids with cardiovascular diseases that has been reported by others (1–4), in that they provide numerous pathways that might mediate the carotenoid and atherosclerotic disease relationship.
Consistent with some previous cross-sectional studies that reported an inverse association between carotenoids and markers of systemic inflammation (12–15), we observed significant inverse associations between carotenoids and leukocyte counts, CRP, and fibrinogen. A recent experimental study reported that intervention with a high intake of carotenoid-rich fruit and vegetables reduced plasma CRP in healthy, nonsmoking men (27).
Serum carotenoids may also beneficially affect the oxidative stress pathway to atherosclerosis, consistent with our findings for 2 biochemical variables in this pathway: positive correlations with SOD, an important antioxidant enzyme, and negative relations with F2-isoprostanes, formed in vivo from systemic oxidative damage (28), with the reaction of free radicals and arachidonic acid (29). Our findings are in line with some clinical trials (30, 31) on SOD and our previous report that most year 0 circulating antioxidants measured in YALTA, except for lycopene and γ-tocopherol, related inversely to GGT activity, which we believe indicates oxidative stress (32).
One cross-sectional study reported an inverse association of sICAM1 with lutein and lycopene (12). Our findings were similar: i.e., sICAM1 related inversely to all carotenoids, including lycopene. Our results were also consistent with an in vitro study of confluent human aortic endothelial cell cultures incubated for 24 h with each of the 5 most prevalent carotenoids in human plasma (α-carotene, β-carotene, β-cryptoxanthin, lutein, and lycopene); pretreatment with β-carotene, lutein, and lycopene significantly reduced the expression of sICAM-1 (33).
There was evidence that smoking modified relations of circulating carotenoids with some of these biomarkers. GGT (17) and, as shown here, F2-isoprostanes tend to be higher in smokers than nonsmokers. Plasma sICAM1 is also known to be higher in chronic smokers and is dose-related to daily cigarette consumption (34). Furthermore, the inverse associations of year 0 leukocyte counts, year 15 F2-isoprostanes, year 15 sICAM1, and year 10 GGT with carotenoids were stronger in current smokers than in nonsmokers (all P for interaction ≤0.02). Although mean F2-isoprostanes were similar in smokers and nonsmokers in the highest carotenoid quartile, for GGT, leukocyte counts, and sICAM1, smokers in the highest quartile of the sum of 4 carotenoids actually showed higher mean concentrations than nonsmokers in the lowest quartile (Fig. 1). Because cigarette smoking leads to increased cumulative exposure to both endogenous and exogenous reactive oxygen species, cigarette smokers are exposed to higher oxidative stress than are nonsmokers (35). Smoking can alter β-carotene metabolism and may alter the metabolism of other carotenoids, particularly when they are present in high concentrations, and especially with the use of supplements (36). For example, ferrets living for 6 months in a smoky environment (urinary cotinine equivalent to 30 cigarettes/day in humans) had altered β-carotene metabolism compared with ferrets living in a smoke-free environment (37, 38). Post-mortem in vitro study of lung tissue showed evidence of altered β-carotene metabolism. Specifically, metabolites formed from excentric cleavage (e.g., β-apo-carotenals) were much more abundant in the ferrets living in the smoky environment than those in the smoke-free environment (37, 38). Simultaneously, there were decreased concentrations of β-carotene, retinol, and retinoic acid in the ferrets exposed to smoke. Lower concentrations of retinol and retinoic acid are known to inhibit cell differentiation; consequent increased cell proliferation in the smoky environment may therefore promote cancer (39, 40). It is possible that the alterations in β-carotene metabolism may also have an impact on atherosclerosis. Exposure of rats to smoke demonstrated the complex interaction of smoking and carotenoids (41). The short-term beneficial effect of passive smoke on experimental myocardial infarction (by inuring the myocardium to small doses of oxidative stress) was negated by simultaneous administration of carotenoids, presumably by neutralizing the effects of free radicals. Our findings are consistent with the supposition that smoking changes metabolism of carotenoids, differently in different pathways. Thus, although higher serum carotenoids might partially neutralize the harmful oxidative stress due to smoking, such benefit did not completely negate the smoking effect on many endpoints demonstrated herein and did not reduce risk for incident diabetes or insulin resistance (16).
We cannot discern whether the apparently beneficial effects were directly attributable to higher serum carotenoid concentrations or to other mechanisms strongly correlated with the carotenoids. For example, circulating carotenoids may reflect only a healthy diet and intake of dietary constituents that are highly correlated with intake of carotenoids. The absence of benefit of isolated β-carotene supplements in clinical trials of clinical outcomes, especially in smokers (42, 43), may indicate that the circulating carotenoids are markers of other important physiologic processes, rather than acting directly on the physiology. The consistently weak associations with lycopene, a strong antioxidant, further support the notion that the circulating carotenoids mark lifestyle and other physiologic processes. If antioxidant properties were the only basis for carotenoid associations, lycopene should have associations similar to the other carotenoids. Another study has reported that lycopene was not related to any of the food groups they examined (44). The present study showed that participants with higher lycopene tended to have less healthy lifestyles; mean intakes of meat and alcohol and concentrations of triglycerides were higher across lycopene quartiles, whereas intake of fruit and use of vitamin supplements were lower. The CARDIA sample includes many younger adults who frequent fast-food restaurants. Ketchup in fast foods may be a major source of lycopene for them; fast food consumption has been shown in this cohort to result in weight gain and insulin resistance (45).
Limitations of the study included lack of measurement of several biochemical variables at year 0, and the study could not confirm whether the associations with higher concentrations of carotenoids were already present at baseline or had evolved during the study. Whether any of the observed interrelationships is causative remains unknown from studies like ours. An interpretive limitation subsumes the large number of comparisons inherent in this project, so that some apparent findings may actually have arisen by chance. However, our analyses were hypothesis-driven, and considerably more consistency was seen across biological pathways than would be expected if multiple comparisons were a large problem.
In conclusion, carotenoids were inversely associated with future oxidative stress, inflammation, and endothelial dysfunction. Although many of these relationships were not associated with smoking status, some were, consistent with the hypothesis that protective mechanisms are working harder but less effectively in smokers. Therefore, these findings offer additional insight into pathways by which higher concentrations of most serum carotenoids, or their specific beneficial components, might help to prevent cardiovascular diseases.
Acknowledgments
This research was supported by contracts N01-HC-95095, N01-HC-48047, N01-HC-48048, and N01-HC-48049 and grant R01-HL-53560, all from the National Heart, Lung, and Blood Institute, National Institutes of Health. A.H. was supported by the Banyu Fellowship Program (Banyu Life Science Foundation International, Tokyo, Japan).
Footnotes
Nonstandard abbreviations: CARDIA, Coronary Artery Risk Development in Young Adults; YALTA, Young Adult Longitudinal Trends in Antioxidants; SOD, superoxide dismutase; sICAM1, soluble intercellular adhesion molecule-1; BMI, body mass index; CRP, C-reactive protein; GGT, γ-glutamyltransferase.
References
- 1.De Waart FG, Schouten EG, Stalenhoef AF, Kok FJ. Serum carotenoids, α-tocopherol and mortality risk in a prospective study among Dutch elderly. Int J Epidemiol. 2001;30:136–143. doi: 10.1093/ije/30.1.136. [DOI] [PubMed] [Google Scholar]
- 2.Kohlmeier L, Kark JD, Gomez-Gracia E, Martin BC, Steck SE, Kardinaal AF, et al. Lycopene and myocardial infarction risk in the EURAMIC study. Am J Epidemiol. 1997;146:618–626. doi: 10.1093/oxfordjournals.aje.a009327. [DOI] [PubMed] [Google Scholar]
- 3.Evans RW, Shaten BJ, Day BW, Kuller LH. Prospective association between lipid soluble antioxidants and coronary heart disease in men: the Multiple Risk Factor Intervention Trial. Am J Epidemiol. 1998;147:180–186. doi: 10.1093/oxfordjournals.aje.a009432. [DOI] [PubMed] [Google Scholar]
- 4.Buijsse B, Feskens EJ, Schlettwein-Gsell D, Ferry M, Kok FJ, Kromhout D, et al. Plasma carotene and α-tocopherol in relation to 10-y all-cause and cause-specific mortality in European elderly: the Survey in Europe on Nutrition and the Elderly, a Concerted Action (SENECA) Am J Clin Nut. 2005;82:879–886. doi: 10.1093/ajcn/82.4.879. [DOI] [PubMed] [Google Scholar]
- 5.Fortmann SP, Ford E, Criqui MH, Folsom AR, Harris TB, Hong Y, et al. CDC/AHA Workshop on Markers of Inflammation and Cardiovascular Disease: Application to Clinical and Public Health Practice: report from the population science discussion group. Circulation. 2004;110:e554–e559. doi: 10.1161/01.CIR.0000148982.95775.BF. [DOI] [PubMed] [Google Scholar]
- 6.Ridker PM, Hennekes CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 7.Blankenberg S, Rupprecht HJ, Bickel C, Peetz D, Hafner G, Tiret L, et al. Circulating cell adhesion molecules and death in patients with coronary artery disease. Circulation. 2001;104:1336–1342. doi: 10.1161/hc3701.095949. [DOI] [PubMed] [Google Scholar]
- 8.Gross M, Steffes M, Jacobs DR, Yu X, Lewis L, Lewis CE, et al. Plasma F2-isoprostanes and coronary artery calcification: the CARDIA study. Clin Chem. 2005;51:125–131. doi: 10.1373/clinchem.2004.037630. [DOI] [PubMed] [Google Scholar]
- 9.Wang B, Pan J, Wang L, Zhu H, Yu R, Zou Y. Associations of plasma 8-isoprostane levels with the presence and extent of coronary stenosis in patients with coronary artery disease. Atherosclerosis. 2006;184:425–430. doi: 10.1016/j.atherosclerosis.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Blake GJ, Ridker PM. Inflammatory bio-markers and cardiovascular risk prediction. J Intern Med. 2002;252:283–294. doi: 10.1046/j.1365-2796.2002.01019.x. [DOI] [PubMed] [Google Scholar]
- 11.Danesh J, Lewington S, Thompson SG, Lowe GD, Collins R, Kostis JB, et al. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis. JAMA. 2005;294:1799–1809. doi: 10.1001/jama.294.14.1799. [DOI] [PubMed] [Google Scholar]
- 12.van Herpen-Broekmans WM, Klöpping-Ketelaars IA, Bots ML, Kluft C, Princen H, Hendriks HF, et al. Serum carotenoids and vitamins in relation to markers of endothelial function and inflammation. Eur J Epidemiol. 2004;19:915–921. doi: 10.1007/s10654-004-5760-z. [DOI] [PubMed] [Google Scholar]
- 13.Kritchevsky SB, Bush AJ, Pahor M, Gross MD. Serum carotenoids and markers of inflammation in nonsmokers. Am J Epidemiol. 2000;152:1065–1071. doi: 10.1093/aje/152.11.1065. [DOI] [PubMed] [Google Scholar]
- 14.Erlinger TP, Guallar E, Miller ER, Stolzenberg-Solomon R, Appel LJ. Relationship between systemic markers of inflammation and serum β-carotene levels. Arch Intern Med. 2001;161:1903–1908. doi: 10.1001/archinte.161.15.1903. [DOI] [PubMed] [Google Scholar]
- 15.Seljeflot I, Arnesen H, Brude IR, Nenseter MS, Drevon CA, Hjermann I. Effects of omega-3 fatty acids and/or antioxidants on endothelial cell markers. Eur J Clin Invest. 1998;28:629–635. doi: 10.1046/j.1365-2362.1998.00336.x. [DOI] [PubMed] [Google Scholar]
- 16.Hozawa A, Jacobs DR, Jr, Steffes MW, Gross MD, Steffen LM, Lee DH. Associations of serum carotenoid concentrations with the development of diabetes and with insulin concentration: interaction with smoking: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Am J Epidemiol. 2006;163:929–937. doi: 10.1093/aje/kwj136. [DOI] [PubMed] [Google Scholar]
- 17.Lee DH, Gross MD, Jacobs DR. Association of serum carotenoids and tocopherols with gamma-glutamyltransferase: the Cardiovascular Risk Development in Young Adults (CARDIA) study. Clin Chem. 2004;50:582–588. doi: 10.1373/clinchem.2003.028852. [DOI] [PubMed] [Google Scholar]
- 18.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Jr, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs DR, Jr, Hahn LP, Haskell WL, Pirie P, Sidney S. Validity and reliability of a short physical activity history: CARDIA and the Minnesota Heart Health Program. J Cardiopulm Rehabil. 1989;9:448–459. doi: 10.1097/00008483-198911000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bieri J, Brown ED, Smith JC., Jr Determination of individual carotenoids in human plasma by high performance chromatography. J Liq Chromatogr. 1985;8:473–484. [Google Scholar]
- 21.Craft NE, Brown ED, Smith JC., Jr Effects of storage and handling conditions on concentrations of individual carotenoids, retinol, and tocopherol in plasma. Clin Chem. 1988;34:44–48. [PubMed] [Google Scholar]
- 22.Gross MD, Prouty CB, Jacobs DR., Jr Stability of carotenoids and α-tocopherol during blood collection and processing procedures. Clin Chem. 1995;41:943–944. [PubMed] [Google Scholar]
- 23.Iribarren C, Folsom AR, Jacobs DR, Gross MD, Belcher JD, Eckfeld JH. Association of serum vitamin levels, LDL susceptibility to oxidation and autoantibodies against MDA-LDL with carotid atherosclerosis: a case-control study: the ARIC Study Investigators: Atherosclerosis Risk in Communities. Arterioscler Thromb Vasc Biol. 1997;17:1171–1177. doi: 10.1161/01.atv.17.6.1171. [DOI] [PubMed] [Google Scholar]
- 24.Green D, Ruth KJ, Folsom AR, Liu K. Hemostatic factors in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Arterioscler Thromb. 1994;14:686–693. doi: 10.1161/01.atv.14.5.686. [DOI] [PubMed] [Google Scholar]
- 25.Rifai N, Tracy RP, Ridker PM. Clinical efficacy of an automated high-sensitivity C-reactive protein assay. Clin Chem. 1999;45:2136–2141. [PubMed] [Google Scholar]
- 26.Gross M, Yu X, Hannan P, Prouty C, Jacobs DR. Lipid standardization of serum fat-soluble antioxidant concentrations: the YALTA study. Am J Clin Nut. 2003;77:458–466. doi: 10.1093/ajcn/77.2.458. [DOI] [PubMed] [Google Scholar]
- 27.Watzl B, Kulling SE, Moseneder J, Barth SW, Bub A. A 4-wk intervention with high intake of carotenoid-rich vegetables and fruit reduces plasma C-reactive protein in healthy, nonsmoking men. Am J Clin Nutr. 2005;82:1052–1058. doi: 10.1093/ajcn/82.5.1052. [DOI] [PubMed] [Google Scholar]
- 28.Morrow JD, Chen Y, Brame CJ, Yang J, Sanchez SC, Xu J, et al. The isoprostanes: unique prostaglandin-like products of free-radical-initiated lipid peroxidation. Drug Metab Rev. 1999;31:117–139. doi: 10.1081/dmr-100101910. [DOI] [PubMed] [Google Scholar]
- 29.Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, Roberts LJ., 2nd A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc Natl Acad Sci U S A. 1990;87:9383–9387. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dixon ZR, Burri BJ, Clifford A, Frankel EN, Schneeman BO, Parks E, et al. Effects of a carotene-deficient diet on measures of oxidative susceptibility and superoxide dismutase activity in adult women. Free Radic Biol Med. 1994;17:537–544. doi: 10.1016/0891-5849(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 31.Hininger I, Chopra M, Thurnham DI, Laporte F, Richard MJ, Favier A, et al. Effect of increased fruit and vegetable intake on the susceptibility of lipoprotein to oxidation in smokers. Eur J Clin Nutr. 1997;51:601–606. doi: 10.1038/sj.ejcn.1600451. [DOI] [PubMed] [Google Scholar]
- 32.Lee DH, Blomhoff R, Jacobs DR. Is serum gamma glutamyltransferase a marker of oxidative stress? Free Radic Res. 2004;38:535–539. doi: 10.1080/10715760410001694026. [DOI] [PubMed] [Google Scholar]
- 33.Martin KR, Wu D, Meydani M. The effect of carotenoids on the expression of cell surface adhesion molecules and binding of monocytes to human aortic endothelial cells. Atherosclerosis. 2000;150:265–274. doi: 10.1016/s0021-9150(99)00375-5. [DOI] [PubMed] [Google Scholar]
- 34.Witkowska AM. Soluble ICAM-1: a marker of vascular inflammation and lifestyle. Cytokine. 2005;31:127–134. doi: 10.1016/j.cyto.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 35.Krinsky NI. Antioxidant functions of carotenoids. Free Radic Biol Med. 1989;7:617–635. doi: 10.1016/0891-5849(89)90143-3. [DOI] [PubMed] [Google Scholar]
- 36.Russell RM. The enigma of β-carotene in carcinogenesis: what can be learned from animal studies. J Nutr. 2004;134:262S–268S. doi: 10.1093/jn/134.1.262S. [DOI] [PubMed] [Google Scholar]
- 37.Wang XD, Liu C, Bronson RT, Smith DE, Krinsky NI, Russell RM. Retinoid signaling and activator protein-1 expression in ferrets given β-carotene supplements and exposed to tobacco smoke. J Natl Cancer Inst. 1999;91:60–66. doi: 10.1093/jnci/91.1.60. [DOI] [PubMed] [Google Scholar]
- 38.Liu C, Russell RM, Wang XD. Low dose β-carotene supplementation of ferrets attenuates smoke-induced lung phosphorylation of JNK, p38 MAPK, and p53 proteins. J Nutr. 2004;134:2705–2710. doi: 10.1093/jn/134.10.2705. [DOI] [PubMed] [Google Scholar]
- 39.Sporn MB, Roberts AB, Roche NS, Kagechika H, Shudo K. Mechanism of action of retinoids. J Am Acad Dermatol. 1986;15:756–764. doi: 10.1016/s0190-9622(86)70231-4. [DOI] [PubMed] [Google Scholar]
- 40.Byers S, Pishvaian M, Crockett C, Peer C, Tozeren A, Sporn M, et al. Retinoids increase cell-cell adhesion strength, β-catenin protein stability, and localization to the cell membrane in a breast cancer cell line: a role for serine kinase activity. Endocrinology. 1996;137:3265–3273. doi: 10.1210/endo.137.8.8754749. [DOI] [PubMed] [Google Scholar]
- 41.Paiva SA, Novo R, Matsubara BB, Matsubara LS, Azevedo PS, Minicucci MF, et al. Beta-carotene attenuates the paradoxical effect of tobacco smoke on the mortality of rats after experimental myocardial infarction. J Nutr. 2005;135:2109–2013. doi: 10.1093/jn/135.9.2109. [DOI] [PubMed] [Google Scholar]
- 42.The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and β carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994;330:1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 43.Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, et al. Effects of a combination of β carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150–1155. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 44.Jansen MC, Van Kappel AL, Ocke MC, Van ’t Veer P, Boshuizen HC, Riboli E, et al. Plasma carotenoid levels in Dutch men and women, and the relation with vegetable and fruit consumption. Eur J Clin Nutr. 2004;58:1386–1395. doi: 10.1038/sj.ejcn.1601981. [DOI] [PubMed] [Google Scholar]
- 45.Pereira MA, Kartashov AI, Ebbeling CB, Van Horn L, Slattery ML, Jacobs DR, et al. Fast-food habits, weight gain, and insulin resistance (the CARDIA study): 15-year prospective analysis. Lancet. 2005;365:36–42. doi: 10.1016/S0140-6736(04)17663-0. [DOI] [PubMed] [Google Scholar]