Abstract
Purpose
To develop and validate a new parental questionnaire addressing symptoms and health related quality of life (HRQL) in congenital nasolacrimal duct obstruction (NLDO).
Design
Cross-sectional study.
Participants
Children aged 6 to <48 months with and without clinical signs of NLDO.
Methods
A new questionnaire was developed using semi-structured interviews with parents of children with NLDO and through discussions with expert clinicians. Questionnaires were completed by parents of children with NLDO and without NLDO. Cronbach’s alpha was calculated as a measure of internal-consistency reliability. Factor analysis was used to evaluate a priori subscales; symptoms and HRQL. Discriminant construct validity was assessed by comparing questionnaire scores between children with and without NLDO and between affected and unaffected eyes of children with unilateral NLDO. Instrument responsiveness was determined by comparing pre- and post-surgical intervention scores in a subset of NLDO subjects who underwent surgical treatment.
Main outcome measure
NLDO questionnaire score.
Results
87 children were enrolled, 56 with NLDO and 31 without. All but two questions on the questionnaire showed a good distribution of responses, a high correlation with the rest of the questionnaire and excellent discrimination between patients with and without NLDO. Cronbach’s alpha values were good for the overall questionnaire (0.95), and for two predetermined subscales; symptoms (0.95) and HRQL (0.85). On a 0 to 4 scale, NLDO patients had worse scores compared to non-NLDO patients for both symptoms (mean difference = 2.1; 95% confidence interval (CI): 1.8 to 2.4) and HRQL (mean difference 1.2; 95% CI: 0.8 to 1.5) subscales. NLDO patients had worse scores pre-intervention compared to post-intervention for both the symptoms (mean difference = 2.2; 95% CI: 1.6 to 2.9) and HRQL (mean difference = 1.4; 95% CI: 0.8 to 2.1) subscales. Finally, NLDO patients had worse symptom scores for affected eyes compared to unaffected eyes (mean difference = 2.3; 95% CI: 1.9 to 2.6).
Conclusions
This novel NLDO questionnaire is useful in quantifying parental perception of symptoms and HRQL in childhood NLDO. The questionnaire may have a role in future clinical studies of NLDO
INTRODUCTION
Congenital nasolacrimal duct obstruction (NLDO) has been estimated to affect up to 20% of newborns,1 with spontaneous resolution occurring in over 90%.1 For unresolved cases, surgical intervention is commonly performed. There is no agreement on the best age for intervention or the best treatment modality. Research to address these issues should include both randomized clinical trials and prospective observational studies, but the design of such studies is limited by the lack of standardized outcome measures.
In previous studies of interventions for congenital NLDO, investigators have relied on the clinical exam, dye disappearance test, parental opinion, or a composite of all three measures in the assessment of success or failure.2–11 Questionnaires have been used infrequently, and then in only simple form, to assess NLDO outcomes.12–18 We are unaware of any previous attempt to prospectively use a comprehensive questionnaire to assess severity of symptoms and health related quality of life (HRQL) in congenital NLDO.
The present study was designed to test the validity of a newly-developed symptoms and HRQL questionnaire. We determined whether each question contributed to the overall instrument, and whether two a priori subscales (symptoms and HRQL) showed appropriated loading and internal-consistency reliability. We also studied whether parental questionnaire scores differed between children with and without NLDO, between affected and unaffected eyes of children with unilateral NLDO and whether scores improved post-intervention among children with NLDO.
METHODS
Informed consent
Institutional Review Board/Ethics Committee approval was obtained and written informed consent was obtained from a parent or guardian of each subject. The study was conducted in a HIPAA-compliant manner.
Development of questionnaire
The content area for the questionnaire was identified through semi-structured interviews of parents of children with NLDO and through discussions with pediatric ophthalmologists and oculo-plastic surgeons. Parental interviews solicited NLDO symptoms and elicited issues of worry or concern. 28 unique items were identified through the parental interviews and clinician discussions (Table 1).
Table 1.
Questionnaire responses
| NLDO Patients (n=56) |
Non-NLDO Patients (n=31) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Item (abbreviated text) | Always (4) | Often (3) | Sometimes (2) | Rarely (1) | Never (0) | Mean Response (0&–4) | Always (4) | Often (3) | Sometimes (2) | Rarely (1) | Never (0) | Mean Response (0&–4) |
| 1. Tears “well-up” in my child’s eye | ||||||||||||
| a. when indoors | 25 | 36 | 21 | 9 | 9 | 2.59 | 0 | 0 | 16 | 16 | 68 | 0.48 |
| b. when outdoors and the temperature is cold | 39 | 27 | 23 | 4 | 7 | 2.88 | 0 | 6 | 16 | 16 | 61 | 0.68 |
| c. when outdoors and it is windy | 43 | 23 | 23 | 4 | 7 | 2.91 | 3 | 6 | 32 | 3 | 55 | 1.00 |
| d. when outdoors even if it is not cold or windy | 34 | 25 | 27 | 7 | 7 | 2.71 | 0 | 0 | 16 | 13 | 71 | 0.45 |
| e. when has an upper respiratory tract infection (cold) | 55 | 20 | 18 | 2 | 5 | 3.18 | 3 | 3 | 32 | 3 | 58 | 0.90 |
| 2. Tears rundown my child’s cheek | ||||||||||||
| a. when indoors | 11 | 36 | 23 | 21 | 9 | 2.18 | 0 | 0 | 10 | 13 | 77 | 0.32 |
| b. when outdoors and the temperature is cold | 23 | 32 | 20 | 13 | 13 | 2.41 | 0 | 3 | 10 | 10 | 77 | 0.39 |
| c. when outdoors and it is windy | 25 | 32 | 18 | 13 | 13 | 2.45 | 0 | 3 | 10 | 13 | 74 | 0.42 |
| d. when outdoors even if it is not cold or windy | 20 | 27 | 27 | 14 | 13 | 2.27 | 0 | 0 | 6 | 13 | 81 | 0.26 |
| e. when has an upper respiratory tract infection (cold | 25 | 29 | 27 | 9 | 11 | 2.48 | 0 | 0 | 16 | 13 | 71 | 0.45 |
| 3. My child has “gunk” in the corner of the eye | ||||||||||||
| a. when he or she wakes up | 41 | 25 | 14 | 5 | 14 | 2.73 | 3 | 0 | 32 | 19 | 45 | 0.97 |
| b. during the day even after cleaning eye in the morning | 25 | 39 | 9 | 7 | 20 | 2.43 | 0 | 0 | 3 | 19 | 77 | 0.26 |
| c. when has an upper respiratory tract infection (cold) | 38 | 29 | 14 | 5 | 14 | 2.70 | 0 | 3 | 16 | 23 | 58 | 0.65 |
| d. during the day even when continually cleaning the eye | 25 | 36 | 9 | 13 | 18 | 2.38 | 0 | 0 | 3 | 10 | 87 | 0.16 |
| 4. My child’s eye looks glassy | 18 | 32 | 18 | 18 | 14 | 2.21 | 0 | 3 | 3 | 23 | 71 | 0.39 |
| 5. The skin around my child’s eye is red | 13 | 34 | 25 | 5 | 23 | 2.07 | 3 | 3 | 10 | 10 | 74 | 0.52 |
| 6. My child’s eyeball is red | 0 | 4 | 20 | 29 | 48 | 0.79 | 0 | 0 | 3 | 6 | 90 | 0.13 |
| 7. My child rubs their eye | 4 | 45 | 25 | 21 | 5 | 2.20 | 0 | 6 | 42 | 29 | 23 | 1.32 |
| 8. The appearance of one or both of my child’s eyeballs bothers me | 13 | 18 | 16 | 11 | 43 | 1.46 | 3 | 10 | 13 | 3 | 71 | 0.71 |
| 9. The appearance of one or both of my child’s eyelids bothers me | 13 | 29 | 14 | 11 | 34 | 1.75 | 0 | 6 | 6 | 3 | 84 | 0.35 |
| 10. Child is bothered by their eye(s) | 9 | 29 | 27 | 23 | 13 | 1.98 | 0 | 3 | 13 | 16 | 68 | 0.52 |
| 11. Child’s eye condition interferes with his/her daily activities | 2 | 5 | 21 | 32 | 39 | 0.98 | 0 | 3 | 10 | 16 | 71 | 0.45 |
| 12. Child’s eye condition interferes with my (parent) daily activities | 2 | 7 | 13 | 30 | 48 | 0.84 | 3 | 0 | 6 | 10 | 81 | 0.35 |
| 13. I feel fine about my child’s eye(s)* | 14 | 30 | 34 | 13 | 9 | 2.29 | 13 | 3 | 13 | 29 | 42 | 1.16 |
| 14. I worry about my child’s eye(s) | 23 | 38 | 27 | 5 | 7 | 2.64 | 10 | 6 | 42 | 16 | 26 | 1.58 |
| 15. Other people comment about my child’s eye(s) | 29 | 27 | 18 | 11 | 16 | 2.41 | 6 | 10 | 10 | 16 | 58 | 0.90 |
| 16. I feel fine about the way my child’s eye(s) appear in photos* | 9 | 7 | 21 | 41 | 21 | 1.41 | 26 | 3 | 19 | 29 | 23 | 1.81 |
| 17. Other children tease my child about his/her eye(s) | 0 | 0 | 2 | 4 | 95 | 0.07 | 0 | 0 | 0 | 0 | 100 | 0.00 |
Data for questions 13 and 16 have been transformed so that a higher score implies a negative response. All values except means are percentages. Percentages may not sum to 100% due to rounding.
Each question was structured as a statement to be answered on a Likert-type scale with 5 ordered response choices; always, often, sometimes, rarely or never (Table 1). Symptom-related questions were answered separately for the right and left eye. HRQL-related questions pertained to the child and were only answered once in each administration of the questionnaire. The Likert-type scale allowed easy transformation of each question’s response to a numerical score (ranging from 0 to 4) for each question, and for scoring of subscales or the entire questionnaire.
Based on the topic of the individual questions, two subscales were defined prior to the start of the study; symptoms, (questions 1 to 7), and HRQL (question 8 and beyond).
At each clinic visit the questionnaire was completed on paper by the parent or guardian and faxed to the data coordinating center. For 19 of 106 (18%) subjects the questionnaire was incomplete and their data were not used in the analysis. After observing the initial high frequency of incomplete questionnaires, the questionnaire formatting was modified to minimize the number of incomplete questionnaires.
Patient population
For NLDO patients, eligibility criteria include age 6 to <48 months and the presence of NLDO as judged by the investigator upon enrollment into the study. We chose this age range because our Pediatric Eye Disease Investigator Group (PEDIG) was actively planning both an observational study and a randomized clinical trial in children of these ages with NLDO.
Fifty-six children (mean age 16 months ± 10 months) were enrolled with NLDO and had a complete questionnaire. Forty-six of these children had at least one of three pre-defined signs of NLDO: increased tearing (epiphora), increased tear lake and/or mucus discharge. Twenty-eight had unilateral signs and 18 had bilateral signs. Ten patients did not have explicit signs on the day of examination, but were judged by the investigator to have NLDO on the basis of history.
Sites were also asked to enroll approximately the same number of subjects of the same age without NLDO. Children were excluded if they had conditions that might present with symptoms similar to those found in NLDO (e.g. allergic conjunctivitis). Thirty-one non-NLDO subjects were enrolled and had a complete questionnaire (mean age 25 months ± 13 months). The clinical diagnoses of these were as follows: 11 strabismus, 5 pseudo strabismus, 8 oculoplastic conditions not involving the nasolacrimal system or affecting tearing, 4 resolved eye conditions, and one each with amblyopia, refractive error, and post-cataract surgery.
Follow-up examinations
Investigators were asked to administer the questionnaire again if the child returned for a subsequent follow-up visit as part of his or her routine care. The parents of 18 patients completed the repeated questionnaire, 11 NLDO patients after surgical intervention (7 simple probings, 2 simple probings with inferior turbinate infracture and 2 silicone tube placements), 2 NLDO patients who did not undergo surgery, and 5 non-NLDO patients who returned for follow-up for their non-NLDO diagnosis. The time between the first and second questionnaire administration was 16 to 76 days in the NLDO patients undergoing surgery (median 49 days) and 8 to 41 days following surgery (median 20 days). All but one of the 18 follow-up questionnaires was completed by the same parent completing the initial questionnaire.
Statistical analysis
Responses for the right eye were used in all subjects except for those with unilateral NLDO affecting the left eye.
The distribution of responses for each item for NLDO and non-NLDO patients was compared. To determine internal-consistency reliability of the questionnaire, Cronbach’s alpha19 was calculated overall and for each of two a priori subscales (symptoms, questions 1 to 7; and HRQL, questions 8 and following). Principal factor analysis with orthogonal varimax rotation was performed for patients with NLDO (n=56), constraining the analysis to two factors, to assess item loadings on the predefined subscales; item loadings greater than 0.5 were considered noteworthy. Item loadings can be thought of as the correlation coefficient between the individual item and the unmeasured latent factor (e.g., HRQL).
Individual subscales scores were scaled between 0 (never) and 4 (always), after summing across appropriate items with equal weight across items. Subscale scores were compared between NLDO and non-NLDO patients, and the results reported as a difference in means with 95% confidence intervals. For patients with unilateral signs (n=28), subscale scores were compared between affected and unaffected eyes. For patients who completed a follow-up exam with administration of the questionnaire on a second occasion, the scores for the first and second administration were similarly compared.
RESULTS
Performance of the individual items on the questionnaire
All 28 items showed a good distribution of responses (Table 1) with the exception of question 17, “Other children tease my child about his/her eye(s).” This question was answered “never” by all but 3 of 87 parents. In addition, inspection of the distribution of responses for question 16 revealed poor discrimination between patients with and without NLDO. These findings lead us to exclude questions 16 and 17 from further analysis and from our final recommendations for implementation of the questionnaire. Excluding these poorly-functioning items yielded a Cronbach’s alpha of 0.95 for the overall questionnaire.
Factor analysis
Analysis of factor loadings indicated that questions 1a, 1b, 1c, 1d, 1e, 2a, 2b, 2c, 2d, 2e, and 4) loaded on the symptoms factor (Table 2), whereas questions 3a, 3b, 3c, 3d, 5, 6, 8, 9, 10, 11, 12, 14, and 15 loaded on the HRQL factor (Table 2). Cronbach’s alpha was 0.95 for the symptoms subscale and 0.85 for the HRQL subscale, indicating good internal-consistency reliability.
Table 2.
Correlation between items and factors from factor analysis in the 56 NLDO patients
| Factor labeled as subscale |
||
|---|---|---|
| Items composing symptoms subscale | Symptoms | HRQL |
| 1. Tears “well-up” in my child’s eye | ||
| a. when indoors | 0.7 | 0.4 |
| b. when outdoors and the temperature is cold | 0.9 | 0.3 |
| c. when outdoors and it is windy | 0.9 | 0.3 |
| d. when outdoors even if it is not cold or windy | 0.8 | 0.4 |
| e. when has an upper respiratory tract infection (cold) | 0.7 | 0.4 |
| 2. Tears rundown my child’s cheek | ||
| a. when indoors | 0.8 | 0.2 |
| b. when outdoors and the temperature is cold | 0.9 | 0.1 |
| c. when outdoors and it is windy | 0.9 | 0.0 |
| d. when outdoors even if it is not cold or windy | 0.9 | 0.1 |
| e. when has an upper respiratory tract infection (cold | 0.8 | 0.3 |
| 3. My child has “gunk” in the corner of the eye | ||
| a. when he or she wakes up | 0.4 | 0.7 |
| b. during the day even after cleaning eye in the morning | 0.4 | 0.7 |
| c. when has an upper respiratory tract infection (cold) | 0.4 | 0.7 |
| d. during the day even when continually cleaning the eye | 0.4 | 0.7 |
| 4. My child’s eye looks glassy | 0.5 | 0.3 |
| 5. The skin around my child’s eye is red | 0.3 | 0.6 |
| 6. My child’s eyeball is red | 0.2 | 0.5 |
| 7. My child rubs their eye | 0.2 | 0.2 |
| Items composing health-related quality of life (HRQL) subscale | ||
| 8. The appearance of one or both of my child’s eyeballs bothers me | 0.1 | 0.5 |
| 9. The appearance of one or both of my child’s eyelids bothers me | 0.2 | 0.7 |
| 10. My child is bothered by their eye(s) | 0.2 | 0.6 |
| 11. My child’s eye condition interferes with his/her daily activities | 0.0 | 0.6 |
| 12. My child’s eye condition interferes with my (parent) daily activities | 0.0 | 0.6 |
| 13. I feel fine about my child’s eye(s) | 0.1 | 0.4 |
| 14. I worry about my child’s eye(s) | 0.3 | 0.7 |
| 15. Other people comment about my child’s eye(s) | 0.3 | 0.7 |
Factor loadings ≥ 0.5 appear in bold.
Comparison of NLDO and non-NLDO patients
The distribution of responses to each question (Table 1) demonstrates separation between the 56 NLDO patients and the 31 non-NLDO patients.
The overall questionnaire score was worse in NLDO (mean ± sd = 2.45 ± 0.64) than in non-NLDO patients (mean ± sd = 0.61 ± 0.45; mean difference 1.84, 95% CI 1.57 to 2.10).
The symptom subscale score was worse in NLDO (mean ± sd = 2.67 ± 0.71) than in non-NLDO patients (mean ± sd = 0.54 ± 0.53; mean difference 2.13, 95% CI 1.83 to 2.43, Figure 1). The HRQL score was also worse in NLDO (mean ± sd = 1.93 ± 0.82) than in non-NLDO patients (mean ± sd = 0.75 ± 0.71; mean difference 1.18, 95% CI 0.82 to 1.54, Figure 1), indicating a worse HRQL in NLDO patients. Caution should be exercised in interpreting the HRQL results, because parental perception of HRQL in non-NLDO patients is dependent on the specific conditions in the study population.
Figure 1.
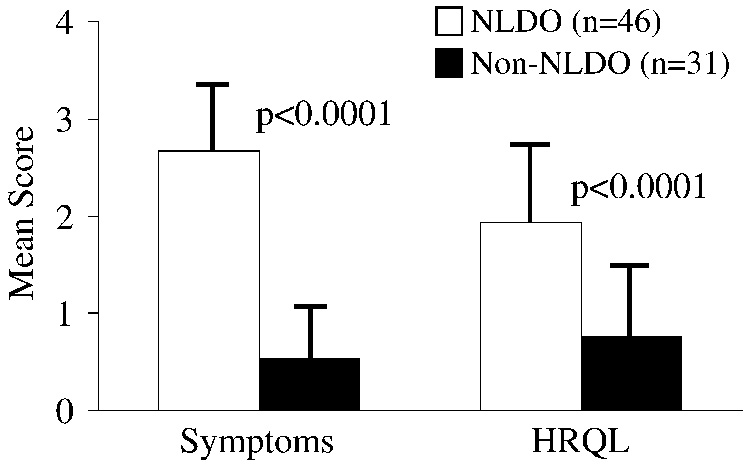
Comparison of symptom and HRQL scores between NLDO patients with clinical signs of NLDO (n=46) and patients without NLDO (n=31). Symptom and HRQL scores were higher in patients with NLDO (p<0.0001), indicating more severe NLDO symptoms and worse HRQL.
Comparison of affected versus unaffected eyes in unilateral NLDO patients
Twenty-eight of 56 patients with NLDO had only unilateral signs. For these 28 unilateral cases of NLDO, the symptoms subscale score was worse in the affected eyes (mean ± sd = 2.73 ± 0.61) than in the unaffected eyes (mean ± sd = 0.46 ± 0.59; mean difference 2.26, 95% CI 1.94 to 2.58).
Responsiveness of the questionnaire to treatment
In the 11 NLDO patients who underwent surgery, the symptoms subscale score was worse preoperatively (mean ± sd = 2.66 ± 0.66) than postoperatively (mean ± sd = 0.41 ± 0.72; mean difference 2.25, 95% CI 1.63 to 2.86). The HRQL score was also worse preoperatively (mean ± sd = 1.83 ± 0.85) than postoperatively (mean ± sd = 0.41 ± 0.56; mean difference 1.42, 95% CI 0.78 to 2.06), indicating an improvement in HRQL following surgery.
DISCUSSION
We have developed a parental questionnaire for use in children with NLDO that discriminates between patients with and without this diagnosis and between affected and unaffected eyes. Our two a priori subscales of symptoms and HRQL had appropriate item loading and high internal-consistency reliability. Our new questionnaire also appears responsive to treatment, and therefore may be useful as an outcome measure in studies of interventions for childhood NLDO.
We constrained the factor analysis to two underlying factors, based on our expectation that there would be two subscales (i.e., symptoms and HRQL). Future refinements of the questionnaire, based on larger sample sizes, may explore whether the underlying covariance structure supports more than two factors. Indeed, items 3, 5 and 6, originally designed as symptom questions, load more strongly on the HRQL subscale than the symptoms subscale (Table 2), and items 7 and 13 do not load strongly on either subscale. These items may contribute to other, yet to be determined, subscales.
Although we did find a difference in the HRQL subscale between patients with and without NLDO, this analysis is limited by the specific conditions present in the non-NLDO patients. Given a different set of diagnoses in non-NLDO patients, it is possible that little difference in HRQL would be evident. Nevertheless, we designed the current questionnaire to assess HRQL issues that had been identified by parents and ophthalmologists as specifically important to NLDO patients.
Previous questionnaires for congenital and childhood NLDO have been of limited scope. Some questionnaires appear to have included only two or three questions with yes or no responses.12, 14 Some have used questions which combine different symptoms, for example, “symptoms of watering and/or discharge”12 but with only a single yes or no response. Such simplifications tend to reduce a questionnaire’s sensitivity to subtle differences between patients and over time. Such lack of questionnaire sensitivity, might be one explanation for the findings of Sturrock et al,12 who described little difference in symptoms reported by congenital NLDO patients who had undergone probing, 4 to 13 years previously, compared with a control group of non-NLDO patients who had undergone strabismus surgery (30% vs. 26% reporting symptoms, respectively).12 The questionnaire we describe in the current report includes multiple questions, each limited to a single symptom in a single environmental condition. Our questionnaire also incorporates a 5 point Likert-type scale for all responses, which we believe is more likely to distinguish between different severities of NLDO.
There are limitations to our study. We did not perform a formal evaluation of test-retest reliability. Nevertheless, 7 patients returning 34 to 140 days later without treatment (i.e., 2 with NLDO and 5 without NLDO) had similar scores on the first and second administration of the questionnaire. In contrast, 11 patients treated surgically for their NLDO, showed marked improvement in their symptom and HRQL scores on the follow-up exam, suggesting responsiveness of the questionnaire to treatment.
In conclusion, we have developed a new questionnaire for NLDO in children. The questionnaire has two distinct subscales of symptoms and HRQL, and appears responsive to treatment. This PEDIG NLDO questionnaire (available at http://public.pedig.jaeb.org) will be useful for conducting clinical research in this condition, and is currently being used as a secondary outcome measure in two PEDIG studies of NLDO in children.
Acknowledgments
Supported in part by National Institutes of Health Grants EY15799 (JMH) and EY11751 (RWB), Research to Prevent Blindness, Inc., New York.
Footnotes
Presented in part at the Annual Meeting of the Association for Research in Vision and Ophthalmology, Fort Lauderdale, FL, May 2005.
No conflicting relationships exist
Precis Symptoms and health-related quality of life in childhood nasolacrimal duct obstruction can be assessed by a 26-item parental questionnaire.
References
- 1.MacEwen CJ, Young JD. Epiphora during the first year of life. Eye. 1991;5:596–600. doi: 10.1038/eye.1991.103. [DOI] [PubMed] [Google Scholar]
- 2.MacEwen CJ, Young JD. The fluorescein disappearance test (FDT): an evaluation of its use in infants. J Pediatr Ophthalmol Strabismus. 1991;28:302–305. doi: 10.3928/0191-3913-19911101-04. [DOI] [PubMed] [Google Scholar]
- 3.Becker BB, Berry FD, Koller H. Balloon catheter dilatation for treatment of congenital nasolacrimal duct obstruction. Am J Ophthalmol. 1996;121:304–309. doi: 10.1016/s0002-9394(14)70279-x. [DOI] [PubMed] [Google Scholar]
- 4.Tao S, Meyer DR, Simon JW, Zobal-Ratner J. Success of balloon catheter dilatation as a primary or secondary procedure for congenital nasolacrimal duct obstruction. Ophthalmology. 2002;109:2108–2111. doi: 10.1016/s0161-6420(02)01216-2. [DOI] [PubMed] [Google Scholar]
- 5.Lim CS, Martin F, Beckenham T, Cumming RC. Nasolacrimal duct obstruction in children: outcome of intubation. J AAPOS. 2004;8:466–472. doi: 10.1016/j.jaapos.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Kashkouli MB, Beigi B, Parvaresh MM, et al. Late and very late initial probing for congenital nasolacrimal duct obstruction: what is the cause of failure? Br J Ophthalmol. 2003;87:1151–1153. doi: 10.1136/bjo.87.9.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gunton KB, Chung CW, Schnall BM, et al. Comparison of balloon dacryocystoplasty to probing as the primary treatment of congenital nasolacrimal duct obstruction. J AAPOS. 2001;5:139–142. doi: 10.1067/mpa.2001.115218. [DOI] [PubMed] [Google Scholar]
- 8.Welsh MG, Katowitz JA. Timing of Silastic tubing removal after intubation for congenital nasolacrimal duct obstruction. Ophthal Plast Reconstr Surg. 1989;5:43–48. doi: 10.1097/00002341-198903000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Katowitz JA, Welsh MG. Timing of initial probing and irrigation in congenital nasolacrimal duct obstruction. Ophthalmology. 1987;94:698–705. doi: 10.1016/s0161-6420(87)33392-5. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein SM, Goldstein JB, Katowitz JA. Comparison of monocanalicular stenting and balloon dacryoplasty in secondary treatment of congenital nasolacrimal duct obstruction after failed primary probing. Ophthal Plast Reconstr Surg. 2004;20:352–357. doi: 10.1097/01.iop.0000134271.25794.96. [DOI] [PubMed] [Google Scholar]
- 11.MacEwen CJ, Young JD, Barras CW, et al. Value of nasal endoscopy and probing in the diagnosis and management of children with congenital epiphora. Br J Ophthalmol. 2001;85:314–318. doi: 10.1136/bjo.85.3.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sturrock SM, MacEwen CJ, Young JDH. Long term results after probing for congenital nasolacrimal duct obstruction. Br J Ophthalmol. 1994;78:892–894. doi: 10.1136/bjo.78.12.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burns SJ, Kipioti A. Follow-up after probing for congenital nasolacrimal duct obstruction. J Pediatr Ophthalmol Strabismus. 2001;38:163–165. doi: 10.3928/0191-3913-20010501-10. [DOI] [PubMed] [Google Scholar]
- 14.Lee DH, Fudemberg SJ, Davitt BV, Cruz OA. Success of simple probing and irrigation in patients with nasolacrimal duct obstruction and otitis media. J AAPOS. 2005;9:192–194. doi: 10.1016/j.jaapos.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Guinot-Saera A, Koay P. Efficacy of probing as treatment of epiphora in adults with blocked nasolacrimal ducts. Br J Ophthalmol. 1998;82:389–391. doi: 10.1136/bjo.82.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozturk S, Konuk O, Ilgit ET, et al. Outcome of patients with nasolacrimal polyurethane stent implantation: do they keep tearing? Ophthal Plast Reconstr Surg. 2004;20:130–135. doi: 10.1097/01.iop.0000115597.92546.d5. [DOI] [PubMed] [Google Scholar]
- 17.McCullough KM. Naso-lacrimal duct balloon dilatation: medium to long term follow-up. Clin Radiol. 2001;56:13–16. doi: 10.1053/crad.2000.0549. [DOI] [PubMed] [Google Scholar]
- 18.Delaney YM, Khooshabeh R. Fluorescein transit test time and symptomatic outcomes after external dacryocystorhinostomy. Ophthal Plast Reconstr Surg. 2002;18:281–284. doi: 10.1097/00002341-200207000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Bland JM, Altman DG. Cronbach's alpha. Br Med J. 1997;314:572. doi: 10.1136/bmj.314.7080.572. [DOI] [PMC free article] [PubMed] [Google Scholar]


