Abstract
Secretion of neurotransmitters is initiated by voltage-gated calcium influx through presynaptic, voltage-gated N-type calcium channels. These channels interact with the SNARE proteins, which are core components of the exocytosis process, via the synaptic protein interaction (synprint) site in the intracellular loop connecting domains II and III of their α1B subunit. Interruption of this interaction by competing synprint peptides inhibits fast, synchronous transmitter release. Here we identify a voltage-dependent, but calcium-independent, enhancement of transmitter release that is elicited by trains of action potentials in the presence of a hyperosmotic extracellular concentration of sucrose. This enhancement of transmitter release requires interaction of SNARE proteins with the synprint site. Our results provide evidence for a voltage-dependent signal that is transmitted by protein–protein interactions from the N-type calcium channel to the SNARE proteins and enhances neurotransmitter release by altering SNARE protein function.
Release of neurotransmitters from presynaptic nerve terminals is initiated by Ca2+ influx through presynaptic Ca2+ channels (1). N-type Ca2+ channels (2) are located in the nerve terminals of many neurons (3, 4) and mediate the Ca2+ influx, which triggers transmitter release (5–9). They are composed of pore-forming α1B subunits in association with β and α2δ subunits (10–13). Recent experiments have shown that N-type calcium channels also bind directly to the SNARE proteins involved in neurotransmitter release (14–16) through a synaptic protein-interaction (synprint) site in the large intracellular loop connecting domains II and III of their α1B subunits (LII-III) (17–19). Disruption of this interaction by synprint peptides reduces the efficacy of Ca2+ entry in stimulating exocytosis (20, 21). These experiments support the hypothesis that interaction of SNARE proteins with the synprint site of N-type calcium channels is required to dock synaptic vesicles near the source of calcium to ensure fast, efficient transmitter release.
Interaction of N-type calcium channels with SNARE proteins through the synprint site also may have direct functional effects on the release process. For example, although the release of neurotransmitters is triggered by calcium influx, changes in presynaptic membrane potential may serve as a secondary or parallel control of the release process. Given the direct interaction between presynaptic Ca2+ channels and the SNARE complex, voltage-dependent signals transmitted from the Ca2+ channel to the SNARE protein complex may serve to regulate its function. By analogy, in skeletal muscle, the α1S subunit of L-type Ca2+ channels serves as a voltage sensor for excitation–contraction coupling via its LII-III, which activates intracellular Ca2+ release in response to membrane depolarization through interaction with ryanodine-sensitive Ca2+ release channels in the sarcoplasmic reticulum (22, 23). Ca2+-independent neurotransmitter release induced by membrane depolarization has been proposed previously (24), but this suggestion has been disputed by measurements showing that intracellular Ca2+ increased during the membrane-depolarization protocol used (25). In the experiments presented here, we have measured Ca2+-independent neurotransmitter release stimulated by hyperosmotic solution and tested the effects of depolarization and presynaptic injection of synprint peptides on it. Acetylcholine release triggered by local application of a hypertonic solution in Ca2+-free medium was increased by simultaneous tetanic stimulation by a train of action potentials, even when changes in intracellular Ca2+ were prevented by a chelator or depletion of intracellular Ca2+ stores. Introduction of the synprint peptide from N-type Ca2+ channels into presynaptic neurons reversibly decreased the voltage-dependent enhancement of Ca2+-independent transmitter release. Our results suggest that the N-type Ca2+ channel serves as a voltage sensor that enhances docking and/or exocytosis of synaptic vesicles through interaction of the synprint site with SNARE complexes in addition to mediating Ca2+ entry, which triggers transmitter release.
EXPERIMENTAL PROCEDURES
Superior cervical ganglion (SCG) neurons from 7-day postnatal rats were prepared as described (10). Conventional intracellular recordings were made from two neighboring neurons, cultured for 6–8 weeks, using microelectrodes filled with 1 M potassium acetate (70–80 MΩ). Neuron pairs were selected by the proximity of their cell bodies. Excitatory postsynaptic potentials (EPSPs) were recorded from one cell of the neuron pairs when action potentials were generated in the other neuron by passage of current through an intracellular recording electrode in modified Krebs solution consisting of 136 mM NaCl/5.9 mM KCl/1 mM CaCl2/1.2 mM MgCl2/11 mM glucose/3 mM Na-Hepes, pH 7.4, and neurons then were superfused with the solution containing 0 mM Ca2+ and 10 mM Mg2+. After disappearance of evoked EPSPs, 0.5 M sucrose was applied to neuron pairs by using a puffer pipette with a pressure pulse of 3.5 psi for 2 s. Intracellular injection of 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate (BAPTA) and fusion proteins was performed as described (20). BAPTA and fusion proteins were dissolved in the suction pipette solution: 150 mM potassium acetate/5 mM Mg2+-ATP/10 mM Hepes, pH 7.4, and were introduced into the presynaptic cell body by diffusion from a glass suction pipette (12–13 MΩ tip resistance for BAPTA; 16–18 MΩ for fusion proteins) at t = 0. Fast Green FCF (5%, Sigma) was included in the pipette solution to confirm entry into the presynaptic cell body. Electrophysiological data were collected and analyzed by using software written by L. Tauc (Centre National de la Recherche Scientifique, Gif-sur-Yvette, France).
RESULTS AND DISCUSSION
Voltage-Dependent Enhancement of Neurotransmitter Release.
Ca2+-independent exocytotic release of acetylcholine from the readily releasable vesicle pool in SCG neurons in cell culture (20, 26) was stimulated by focal application of hypertonic solution (27), a procedure that causes an increase in the rate of miniature EPSPs (mEPSPs) (28, 29). Fig. 1a shows mEPSPs induced by 0.5 M sucrose puff-applied for 2 s onto synaptic pairs of cultured SCG neurons (20, 26) in modified Krebs solution containing 0 mM Ca2+ and 10 mM Mg2+. The mEPSPs were increased markedly by simultaneous tetanic stimulation of the presynaptic neurons (Fig. 1d). The increase in the 0.5 M sucrose-induced transmitter release depended on the frequency but not on the number of stimulation pulses; a tetanic stimulation at 100 Hz for 2 s enhanced the sucrose responses, but stimulation at 10 Hz for 30 s did not have a detectable effect (data not shown). The integral of the mEPSPs was increased by 1.62 ± 0.11-fold (n = 32, mean ± SEM) with a tetanic stimulation at 100 Hz for 4 s. We use the term “voltage-dependent enhancement of neurotransmitter release” to describe this increase in mEPSPs during trains of action potentials, because the results presented below show that it requires membrane depolarization by action potentials and is Ca2+-independent. We use the ratio of the mEPSP integral with and without tetanic stimulation or the percentage increase in mEPSP integral during trains of action potentials as parameters to quantitate voltage-dependent enhancement of transmitter release. This measure of neurotransmitter release would detect changes in the size of the readily releasable pool of synaptic vesicles and in the rate of release of synaptic vesicles from the readily releasable pool.
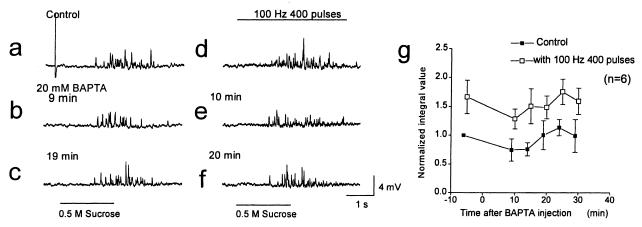
Figure 1.
Voltage-dependent enhancement of neurotransmitter release. mEPSPs in Krebs solution containing 0 mM Ca2+ and 10 mM Mg2+ induced by a puff-application of 0.5 M sucrose for 2 s onto synaptic pairs of sympathetic neurons in culture, at 5 min before injection (a) and 9 min (b) and 19 min (c) after disruption of the membrane patch for injection of BAPTA from a suction pipette containing 20 mM BAPTA. mEPSPs triggered by 0.5 M sucrose were facilitated by simultaneously applied presynaptic current pulses (4 nA) for 5 ms at 100 Hz for 4 s (a–c vs. d–f). mEPSPs 4 min before injection (d), and 10 min (e) and 20 min (f) after BAPTA injection are shown. (a–f) mEPSPs from one representative experiment are illustrated. The large deflection before the application of sucrose in a is an action potential. (g) Normalized average integral values of mEPSPs with (□) or without (■) presynaptic action potentials induced by 400 pulses at 100 Hz are plotted from six experiments like the one illustrated in a–f.
Voltage-Dependent Enhancement of Transmitter Release Is Ca2+-Independent.
Measurement of a voltage-dependent component of transmitter release requires prevention of rises in intracellular Ca2+ that might induce Ca2+-dependent release. To prevent Ca2+ entry, the tetanic stimulation in our experiments was applied in medium containing 0 mM Ca2+ and 10 mM Mg2+ after complete disappearance of EPSPs evoked by single presynaptic action potentials or tetanic trains of action potentials (see Experimental Procedures). However, trains of action potentials might cause changes in cytosolic free Ca2+ levels in Ca2+-free medium. To further exclude Ca2+ entry during the action potential train as a source of the increase in transmitter release, the fast Ca2+ chelator BAPTA was introduced into the presynaptic cell bodies through an injection pipette (10) containing 20 mM BAPTA for ≥7 min. After removal of the injection pipette, the BAPTA concentration inside the SCG neuron cell bodies was ≥1 mM (≥5% of the concentration in the pipette), as estimated from the color intensity of the coinjected dye Fast Green FCF and correction for the effect of molecular mass on diffusion (30). This concentration of BAPTA was sufficient to completely block transmitter release evoked by action potentials in the presence of 1 mM extracellular Ca2+. Under control conditions in medium with 0 mM Ca2+ and 10 mM Mg2+, introduction of BAPTA caused a small decrease in mEPSPs induced by 0.5 M sucrose application for 2 s (Fig. 1b), which reversed by 19 min after injection (Fig. 1c). Integration of these mEPSPs and plotting as a function of time revealed a variable, reversible reduction of 25 ± 19% (Fig. 1g; n = 6). Before introduction of BAPTA, tetanic stimulation increased the mEPSPs by 1.67 ± 0.29-fold (Fig. 1 a and d). Even though BAPTA completely blocked synaptic transmission in the presence of 1 mM extracellular Ca2+, tetanic stimulation at 100 Hz for 4 s in medium with 0 mM Ca2+/10 mM Mg2+ increased the mEPSP integral by a similar factor (Fig. 1 e and f), ranging from 1.48-fold to 1.98-fold from 10 to 30 min after injection of BAPTA when compared with the control value at the closest time point (Fig. 1g). Because chelating intracellular Ca2+ with BAPTA does not prevent the action potential-dependent increase in mEPSPs, a general rise in cytosolic Ca2+ can be excluded as a mechanism for the increase of Ca2+-independent transmitter release with membrane depolarization.
Because we replaced extracellular Ca2+ with Mg2+, it was possible that a low level of Mg2+ entry through Ca2+ channels caused the increase in transmitter release during trains of action potentials (31). We tested this in two ways. When Mg2+ concentration was reduced 10-fold to 1 mM, the increase in mEPSP integral was 61 ± 11% (SEM, n = 6), comparable to the average increase of 62 ± 12% in the presence of 10 mM Mg2+ in other experiments. When 5 μM ω-conotoxin GVIA was added to completely block ion permeation through N-type Ca2+ channels, the increase in mEPSP integral was 58 ± 20% (SEM, n = 6) before addition of toxin and 55 ± 18% (SEM) 20 min after addition of toxin when normal Ca2+-dependent synaptic transmission was completely blocked (20). In two cells, this high concentration of ω-conotoxin GVIA caused a steady rundown of the control response to 0.5 M sucrose, so these cells were not included in our quantitative comparison. Considered together, these two types of control experiments provide strong evidence that the increase in mEPSP integral during trains of action potentials does not result from Mg2+ influx through Ca2+ channels.
If the increase in mEPSP integral is caused by an increase in vesicular release, it would be accompanied by an increase in the frequency of mEPSPs, reflecting an increase in the rate of exocytosis of individual vesicles. To test this point, we compared the increment in mEPSP integral caused by a train of action potentials to the increment in mEPSP frequency recorded simultaneously. In six synaptic pairs, we found an increase of 36 ± 10% (SEM) for mEPSP frequency compared with 58 ± 20% (SEM) for mEPSP integral, demonstrating that most of the increase in mEPSP integral is caused by an increase in mEPSP frequency. Because multiple quanta are more likely to be released together during trains of action potentials in the presence of 0.5 M sucrose, it is likely that our measurements underestimate the true increase in mEPSP frequency and that nearly all of the increase in mEPSP integral is attributable to increased vesicular release. Evidently, action potentials in the absence of Ca2+ influx are sufficient to increase mEPSPs under these conditions. These results suggest that a voltage-dependent signal can be transmitted from a membrane voltage sensor to the docking and release machinery.
Injection of the Synprint Peptide Blocks Voltage-Dependent Enhancement of Neurotransmitter Release.
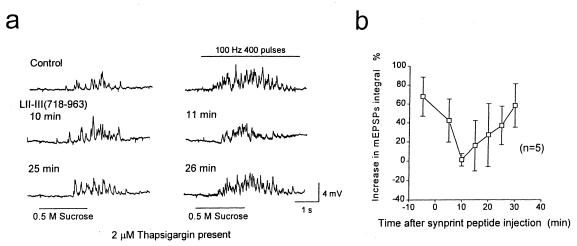
Depolarization causes outward movement of gating charges in voltage-gated ion channels, leading to their activation (32, 33). Gating-charge movement itself is sufficient for activation of excitation–contraction coupling by a voltage-dependent signal transmitted through LII-III of skeletal muscle L-type Ca2+ channels to the Ca2+ release channels in the sarcoplasmic reticulum (22, 23). As the N-type Ca2+ channel is the only known voltage-sensitive protein that interacts directly with the SNARE proteins in sympathetic nerve terminals, it is a likely source of the voltage-dependent signal that enhances transmitter release. To examine the functional significance of LII-III of the α1B subunit of N-type Ca2+ channels in voltage-dependent enhancement of neurotransmitter release, we used the recombinant peptide LII-III(718–963), which blocks the interaction of Ca2+ channels with SNARE proteins and inhibits neurotransmitter release when injected into presynaptic neurons (20, 21). This peptide was introduced into the presynaptic SCG neurons, and 0.5 M sucrose was puff-applied for 2 s with or without a tetanic stimulation at 100 Hz for 4 s. The hypertonic solution was applied in medium containing 0 mM Ca2+ and 10 mM Mg2+ after detecting a synaptic pair by recording evoked EPSPs in Krebs solution containing 1 mM Ca2+. Injection of LII-III(718–963) from α1B at t = 0 gradually decreased voltage-dependent enhancement of transmitter release (Fig. 2a). Before injection (Fig. 2a, Control), a robust increase in transmitter release was observed during the action potential train. By 15 min after injection, the increase in transmitter release during the train of action potentials was much reduced (Fig. 2a). With a pipette containing 65 μM LII-III(718–963), which produced a concentration of approximately 3.2 μM in the cell, the decrease in voltage-dependent enhancement of release was rapidly reversible, recovering to nearly the control level by 25–30 min after injection (Fig. 2a). The mean increase in mEPSPs during tetanic stimulation decreased from 1.46 ± 0.11-fold before injection to 0.88 ± 0.09-fold (i.e., less than the starting level) at 10 min after starting injection (Fig. 2b; SEM, n = 9). By 30 min, the mean mEPSP integral recovered to the control value before injection (Fig. 2b). The time course of decrease and recovery of the voltage-dependent enhancement of release is similar to the time course of inhibition of synaptic transmission by this synprint peptide observed previously (20). Reversal of the effects of the synprint peptide is likely to result from proteolysis, as this peptide is highly susceptible to proteolytic degradation. The reversible inhibition of the voltage-dependent enhancement of transmitter release by the synprint peptide, in parallel with the reversible inhibition of synaptic transmission observed previously (20), implicates LII-III of the N-type calcium channel in transmission of a voltage-dependent signal to the docking and release machinery that enhances neurotransmitter release.
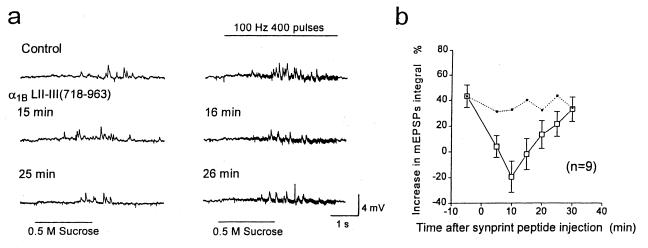
Figure 2.
Effects of the N-type Ca2+ channel synprint peptide on voltage-dependent enhancement of transmitter release. (a) mEPSPs induced by 0.5 M sucrose (Left) or 0.5 M sucrose plus a presynaptic tetanic stimulation at 100 Hz for 4 s (Right) are shown. LII-III(718–963) of α1B was introduced into the presynaptic cell bodies at t = 0 from a pipette containing 65 μM peptide in Krebs solution containing 0 mM Ca2+ and 10 mM Mg2+. mEPSPs from one representative experiment recorded 5 and 4 min before injection and 15, 16, 25, and 26 min after injection are illustrated. (b) Normalized average mEPSP integral values with and without presynaptic action potentials, 400 pulses at 100 Hz, are plotted from nine experiments like the one illustrated in a. Dotted line shows the mEPSP integral values from control experiments shown in Fig. 3b.
In contrast to the decrease in mEPSPs during tetanic stimulation, injection of LII-III(718–963) slightly increased (24 ± 18%, SEM, n = 9) the transmitter release triggered by 0.5 M sucrose without tetanic stimulation at 10 min after starting injection, indicating that the inhibition of voltage-dependent enhancement of release is specific for the increment in release during trains of action potentials. Thus, the synprint peptide does not inhibit the functions of the SNARE proteins or other components of the release machinery required for transmitter release induced by hyperosmotic solution.
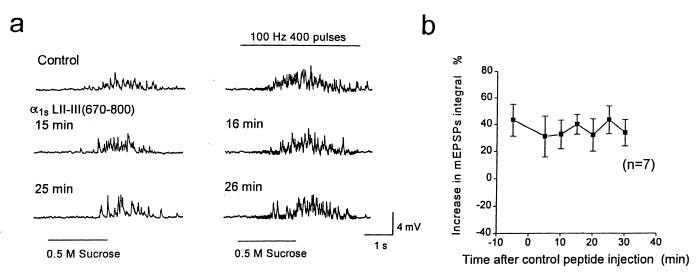
As a further control for nonspecific effects, injection of a recombinant fusion protein containing LII-III(670–800) from the α1S subunit of skeletal muscle Ca2+ channels at a concentration of 140 μM in the injection pipette produced no significant change in the increase in mEPSPs during tetanic stimulation at 100 Hz for 4 s (Fig. 3 a and b; n = 7), compared with the marked effect of LII-III(718–963) of α1B (Fig. 2b). The lack of effect of this peptide from α1S, which does not bind SNARE proteins (17), confirms that the inhibitory effect of the synprint-site peptide from the α1B subunit of N-type Ca2+ channels on the voltage-dependent enhancement of transmitter release is specific. These results support the conclusion that direct interaction of LII-III of the α1B subunit of N-type Ca2+ channels transmits a voltage-dependent signal to the SNARE complex and enhances synaptic vesicle docking and/or exocytosis.
Figure 3.
Effects of L-type Ca2+ channel peptide on voltage-dependent enhancement of neurotransmitter release. (a) mEPSPs induced by 0.5 M sucrose (Left) or 0.5 M sucrose plus a presynaptic tetanic stimulation at 100 Hz for 4 s (Right). As a control, 140 μM LII-III(670–800) of α1S from the skeletal muscle L-type Ca2+ channel was injected in Krebs solution containing 0 mM Ca2+ and 10 mM Mg2+. mEPSPs from one representative experiment recorded 5 and 4 min before injection and 15, 16, 25, and 26 min after injection are illustrated. (b) Difference between normalized average integral values of the mEPSPs with and without presynaptic action potentials is plotted from seven experiments like the one illustrated in a.
Effect of Depletion of Intracellular Stores of Ca2+ on Voltage-Dependent Neurotransmitter Release.
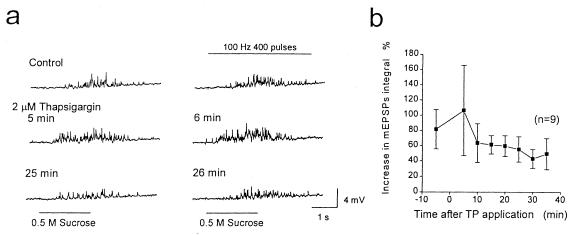
Exocytosis can be influenced by release of Ca2+ from intracellular stores (35, 36), and it is conceivable that intracellular Ca2+ release in a restricted space near docked vesicles could increase transmitter release in response to action potentials and local sodium influx, even in the presence of BAPTA. Moreover, hypertonic solution has been reported to decrease voltage-dependent Ca2+ current (27, 34) but to produce a rise in [Ca2+]i because of efflux from intracellular stores (35). To examine the possible role of intracellular stores, the SERCA Ca2+-ATPase inhibitor thapsigargin (37) was applied to deplete Ca2+ from intracellular stores of the endoplasmic reticulum of presynaptic neurons. Thapsigargin was drop-applied extracellularly in the Krebs solution containing 0 mM Ca2+ and 10 mM Mg2+ to produce a final concentration of 2 μM. Neuron pairs were selected by recording EPSPs in Krebs solution containing 1 mM Ca2+. Thapsigargin caused an increase in the basal neurotransmitter release induced by 0.5 M sucrose application by 41 ± 14% (SEM, n = 9) at 5 min after bath application (Fig. 4a), presumably because of a leak of Ca2+ from the intracellular stores into the cytosol. The mEPSP integral returned to control levels by 25 min after treatment with thapsigargin, consistent with depletion of the intracellular stores of Ca2+ to a level that no longer can stimulate transmitter release (Fig. 4a). During treatment with 2 μM thapsigargin, the voltage-dependent enhancement of transmitter release also appeared to increase. For example, the increase in mEPSP integral during action potential trains was 1.80 ± 0.26-fold before bath application of thapsigargin and 2.49 ± 0.69-fold at 6 min after bath application in the experiment illustrated in Fig. 4a. However, the mean increase in the integral values of mEPSPs recorded from nine cells during action potential stimulation was 81 ± 25% before thapsigargin and 106 ± 59% at 6 min after thapsigargin application, a difference that is not statistically significant (Fig. 4b). Although the voltage-dependent enhancement of transmitter release in the presence of thapsigargin is highly variable by this measure, the variability reflects primarily high variability of the basal release induced by 0.5 M sucrose in the presence of thapsigargin. Comparing total mEPSP integral before and after thapsigargin treatment shows an increase in 9 of 10 synaptic pairs (mean ratio before and after thapsigargin = 1.32 ± 0.11, SEM), confirming a significant effect of thapsigargin on total (e.g., basal plus voltage-dependent) neurotransmitter release.
Figure 4.
Effects of the SERCA Ca2+-pump inhibitor thapsigargin on voltage-dependent enhancement of transmitter release. (a) mEPSPs induced by 0.5 M sucrose (Left) or 0.5 M sucrose plus a presynaptic tetanic stimulation at 100 Hz for 4 s (Right). At t = 0, thapsigargin was drop-applied into the Krebs bathing solution containing 0 mM Ca2+ and 10 mM Mg2+, producing a concentration of 2 μM. mEPSPs from one representative experiment recorded 5 and 4 min before injection and 5, 6, 25, and 26 min after bath application are illustrated. (b) Difference between normalized average integral values of the mEPSPs with and without presynaptic action potentials, 400 pulses at 100 Hz, is plotted from nine experiments like the one illustrated in a.
After depletion of the intracellular Ca2+ stores by incubation for 30 min in the presence of thapsigargin, voltage-dependent enhancement of release was retained (Fig. 4b). The mean values were slightly less than control values before thapsigargin (Fig. 4b), but remained within the range of values observed in our complete series of experiments (compare with Fig. 1). These results indicate that a rise in cytosolic Ca2+ discharged from intracellular stores is able to increase neurotransmitter release induced by 0.5 M sucrose, but depletion of Ca2+ from the intracellular stores does not prevent the voltage-dependent enhancement of release. Thus, Ca2+ release from intracellular stores cannot be responsible for the voltage-dependent enhancement of neurotransmitter release as measured in our experiments.
Block of Voltage-Dependent Enhancement of Transmitter Release in Neurons with Depleted Intracellular Ca2+ Stores.
To further confirm the role of the synprint site in voltage-dependent enhancement of transmitter release, LII-III(718–863) was introduced into the presynaptic neurons in the presence of 2 μM thapsigargin in Krebs solution containing 0 mM Ca2+ and 10 mM Mg2+. Sucrose (0.5 M) was applied 50–60 min after starting treatment of synaptically coupled neuron pairs with 2 μM thapsigargin, so the intracellular stores would be completely empty. By 10 min after injection of the synprint peptide, the voltage-dependent enhancement of neurotransmitter release was nearly completely inhibited (Fig. 5a). This decrease was reversible, recovering to nearly control values by 25 min after injection (Fig. 5a). The mean increase in mEPSPs resulting from the voltage-dependent enhancement of release was decreased from 1.68 ± 0.21-fold (SEM, n = 5) before injection of synprint peptide to 1.03 ± 0.12-fold at 10 min after starting injection of LII-III(718–863) at 65 μM in the pipette and reversed to control values by 30 min after injection (Fig. 5b). The maximum decrease in the voltage-dependent enhancement of transmitter release was similar to that observed in the absence of 2 μM thapsigargin.
Figure 5.
Inhibition of the voltage-dependent enhancement of neurotransmitter release by synprint peptide in the presence of thapsigargin. (a) mEPSPs induced by 0.5 M sucrose (Left) or 0.5 M sucrose plus a presynaptic tetanic stimulation at 100 Hz for 4 s (Right). Sixty-five micrometers of LII-III(718–963) was injected at t = 0 in the presence of 2 μM thapsigargin in Krebs solution containing 0 mM Ca2+ and 10 mM Mg2+. mEPSPs from one representative experiment recorded 5 and 4 min before injection and 10, 11, 25, and 26 min after injection are illustrated. (b) Difference between normalized average integral values of the mEPSPs with and without presynaptic action potentials is plotted from five experiments like the one illustrated in a.
Evidence for a Voltage-Dependent Enhancement of Neurotransmitter Release via Interaction of SNARE Proteins and N-Type Ca2+ Channels.
Our experiments provide strong evidence that membrane potential is the primary effector of the stimulus-dependent enhancement of transmitter release observed in our experiments. The enhancement of transmitter release is caused by trains of conducted action potentials that briefly depolarize the nerve terminals. Influx of Ca2+ and Mg2+ is not required because changes in the extracellular concentrations of these divalent cations, chelation of intracellular Ca2+, and depletion of intracellular stores of Ca2+ do not affect the enhancement of transmitter release. Moreover, the enhancement of transmitter release is reversibly blocked by the synprint peptide, which prevents interaction of N-type Ca2+ channels with SNARE proteins but does not alter action-potential generation or Ca2+ channel activity (20). Therefore, the fluxes of Na+ or K+ during the action potentials cannot be primarily responsible for the enhanced transmitter release because these ion currents are not affected by the synprint peptide. Altogether, our results show that the action potential-dependent enhancement of transmitter release is independent of influx of Ca2+ but dependent on direct interactions of the N-type Ca2+ channels with SNARE proteins. Because N-type Ca2+ channels are activated by membrane depolarization, our results suggest that the activated state of the N-type Ca2+ channel has a direct stimulatory effect on the actions of the SNARE proteins in neurotransmitter release. As SNARE proteins are thought to function in docking and preparation of synaptic vesicles to enter the readily releasable pool as well as in release of vesicles from that pool, the effects of interaction of N-type Ca2+ channels with SNARE proteins through the synprint region may result from changes in the size of the readily releasable pool, the rate of release of vesicles in that pool, or both.
Functional Interactions of N-Type Ca2+ Channels with SNARE Proteins in Neurotransmitter Release.
The synprint site of the α1B subunit of N-type Ca2+ channels interacts directly with the synaptic membrane proteins syntaxin and SNAP-25 (17, 18), which are cleaved by botulinum neurotoxin types A (37), E (38), and C1 (39, 40). Neurotransmitter release induced by high sucrose solution is blocked by botulinum neurotoxin types A (41, 42) and C1 (42), indicating that syntaxin and SNAP-25 are involved in Ca2+-independent transmitter release as well as Ca2+-dependent secretion. Ca2+-independent exocytotic neurotransmitter release from the readily releasable vesicle pool is stimulated by focal application of hypertonic solution (27). Thus, the same vesicle pool is shared by action potential-evoked and hypertonic solution-induced transmitter release (27), and both forms of release are mediated by syntaxin and SNAP-25. The voltage-dependent enhancement of Ca2+-independent exocytotic neurotransmitter release observed here is likely to reflect an underlying voltage-dependent signal that also enhances Ca2+-dependent transmitter release during synaptic transmission.
Direct interactions between presynaptic N-type Ca2+ channels and SNARE proteins evidently are required for efficient Ca2+-dependent transmitter release and for voltage-dependent enhancement of transmitter release. Previous functional studies of the synprint site of α1B revealed that binding to syntaxin and SNAP-25 is important for synchronous transmitter release triggered by action potentials (20, 21). Our present results indicate an additional important role for the synprint site of the α1B subunit of N-type Ca2+ channels—transmission of a voltage-dependent signal to the SNARE proteins that enhances transmitter release. This signal is transmitted by direct protein–protein interaction and likely reflects a conformational response of the SNARE protein complex to the transmembrane movement of the gating charges of the Ca2+ channel upon depolarization. Blocking this signal would reduce neurotransmitter release and contribute to the inhibition of synaptic transmission by synprint peptides observed previously (20, 21). Thus, our results support the conclusion that the N-type Ca2+ channel generates a voltage-dependent signal that is transmitted to the release machinery through interaction of the synprint site with SNARE proteins and that this voltage-dependent signal enhances docking and priming of synaptic vesicles in the readily releasable pool and/or exocytosis of docked synaptic vesicles from the readily releasable pool by the rapid influx of Ca2+ mediated by the open channel.
Analogous Function of Ca2+ Channel–Effector Interactions in Synaptic Transmission and Excitation–Contraction Coupling.
Our results reveal clear analogies between the function of the N-type Ca2+ channel in synaptic transmission and the function of the skeletal muscle L-type Ca2+ channel in excitation–contraction coupling. Ca2+ sparks, the unitary events in excitation–contraction coupling (43, 44), are synchronized to yield a coherent physiological signal by depolarization-dependent activation of the skeletal muscle L-type Ca2+ channel in the transverse tubule, which interacts directly with the ryanodine-sensitive Ca2+ release channels in the sarcoplasmic reticulum to induce rapid and synchronous Ca2+ release (22, 23). The voltage-dependent signal is transmitted to the ryanodine-sensitive Ca2+ release channel by the LII-III segment of the α1S subunit of the skeletal muscle Ca2+ channels in the transverse tubules (22, 23). In analogy, we find that release of single synaptic vesicles to induce mEPSPs, the unitary events in synaptic transmission (28), is enhanced by a voltage-dependent signal from the N-type Ca2+ channel transmitted by interaction of the LII-III segment of its α1B subunit with SNARE proteins. Block of this interaction reduces fast, synchronous synaptic transmission (20). Thus, the LII-III segments of both N-type Ca2+ channels in presynaptic terminals and L-type Ca2+ channels in the triad junctions of skeletal muscle function as effector-interaction domains and transmit a voltage-dependent signal to their interacting partners. Ca2+ channels in other compartments of neurons also may generate intracellular signals by direct interaction with effector proteins that respond to Ca2+ influx.
Acknowledgments
We thank Dr. Sandra Bajjalieh and Dr. Kenneth Mackie (University of Washington) for comments on the manuscript and Dr. Ladislav Tauc (Laboratoire de Neurobiologie Cellulaire et Moleculaire, Centre National de la Recherche Scientifique, Gif-sur-Yvette, France) for a gift of data analysis software. This work was supported by grants from The Japanese Ministry of Education, Science and Culture (S.M.), the Human Frontier Science Program (S.M.), and the National Institutes of Health (W.A.C., D.K.K., and C.T.Y.).
ABBREVIATIONS
- EPSP
excitatory postsynaptic potential
- mEPSP
miniature EPSP
- SCG
superior cervical ganglion
- BAPTA
1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate
References
- 1.Augustine G J, Neher E. Curr Opin Neurobiol. 1992;2:302–307. doi: 10.1016/0959-4388(92)90119-6. [DOI] [PubMed] [Google Scholar]
- 2.Nowycky M C, Fox A P, Tsien R W. Nature (London) 1985;316:440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- 3.Robitaille R, Adler E M, Charlton M P. Neuron. 1990;5:773–779. doi: 10.1016/0896-6273(90)90336-e. [DOI] [PubMed] [Google Scholar]
- 4.Westenbroek R E, Hell J W, Warner C, Dubel S J, Snutch T P, Catterall W A. Neuron. 1992;9:1099–1115. doi: 10.1016/0896-6273(92)90069-p. [DOI] [PubMed] [Google Scholar]
- 5.Hirning L D, Fox A P, McCleskey E W, Olivera B M, Thayer S A, Miller R J, Tsien R W. Science. 1988;239:57–61. doi: 10.1126/science.2447647. [DOI] [PubMed] [Google Scholar]
- 6.Stanley E F. Neuron. 1993;11:1007–1011. doi: 10.1016/0896-6273(93)90214-c. [DOI] [PubMed] [Google Scholar]
- 7.Wu L-G, Saggau P. J Neurosci. 1994;14:5613–5822. doi: 10.1523/JNEUROSCI.14-09-05613.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Regehr W G, Mintz I M. Neuron. 1995;16:131–139. [Google Scholar]
- 9.Luebke J I, Dunlap K, Turner T J. Neuron. 1993;11:895–902. doi: 10.1016/0896-6273(93)90119-c. [DOI] [PubMed] [Google Scholar]
- 10.Dubel S J, Starr T V B, Hell J, Ahlijanian M K, Enyeart J J, Catterall W A, Snutch T P. Proc Natl Acad Sci USA. 1992;89:5058–5062. doi: 10.1073/pnas.89.11.5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams M E, Brust P F, Feldman D H, Patthi S, Simerson S, Maroufi A, McCue A F, Velicelebi G, Ellis S B, Harpold M M. Science. 1992;257:389–395. doi: 10.1126/science.1321501. [DOI] [PubMed] [Google Scholar]
- 12.McEnery M W, Snowman A M, Sharp A H, Adams M E, Snyder S H. Proc Natl Acad Sci USA. 1991;88:11095–11099. doi: 10.1073/pnas.88.24.11095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Witcher D R, De Waard M, Sakamoto J, Franzini-Armstrong C, Pragnell M, Kahl S D, Campbell K P. Science. 1993;261:486–489. doi: 10.1126/science.8392754. [DOI] [PubMed] [Google Scholar]
- 14.Bajjalieh S M, Scheller R H. J Biol Chem. 1995;270:1971–1974. doi: 10.1074/jbc.270.5.1971. [DOI] [PubMed] [Google Scholar]
- 15.Südhof T. Nature (London) 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- 16.Söllner T, Whiteheart S W, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman J E. Nature (London) 1993;362:318–312. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- 17.Sheng Z-H, Rettig J, Takahashi M, Catterall W A. Neuron. 1994;13:1303–1313. doi: 10.1016/0896-6273(94)90417-0. [DOI] [PubMed] [Google Scholar]
- 18.Sheng Z-H, Rettig J, Cook T, Catterall W A. Nature (London) 1996;379:451–454. doi: 10.1038/379451a0. [DOI] [PubMed] [Google Scholar]
- 19.Rettig J, Sheng Z H, Kim D K, Hodson C D, Snutch T P, Catterall W A. Proc Natl Acad Sci USA. 1996;93:7363–7368. doi: 10.1073/pnas.93.14.7363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mochida S, Sheng Z-H, Baker C, Kobayashi H, Catterall W A. Neuron. 1996;17:781–788. doi: 10.1016/s0896-6273(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 21.Rettig J, Heinemann C, Ashery U, Sheng Z-H, Yokoyama C T, Catterall W A, Neher E. J Neurosci. 1997;17:6647–6656. doi: 10.1523/JNEUROSCI.17-17-06647.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanabe T, Beam K G, Powell J A, Numa S. Nature (London) 1988;336:134–139. doi: 10.1038/336134a0. [DOI] [PubMed] [Google Scholar]
- 23.Tanabe T, Beam K G, Adams B A, Niidome T, Numa S. Nature (London) 1990;346:567–569. doi: 10.1038/346567a0. [DOI] [PubMed] [Google Scholar]
- 24.Hochner B, Parnas H, Parnas I. Nature (London) 1989;342:433–435. doi: 10.1038/342433a0. [DOI] [PubMed] [Google Scholar]
- 25.Mulkey R M, Zucker R S. Nature (London) 1991;350:153–155. doi: 10.1038/350153a0. [DOI] [PubMed] [Google Scholar]
- 26.O’Lague P H, Obata K, Claude P, Furschpan F F, Potter D. Proc Natl Acad Sci USA. 1974;71:3602–3606. doi: 10.1073/pnas.71.9.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenmund C, Stevens C F. Neuron. 1996;16:1197–1207. doi: 10.1016/s0896-6273(00)80146-4. [DOI] [PubMed] [Google Scholar]
- 28.Fatt P, Katz B. J Physiol (London) 1952;117:109–128. [PMC free article] [PubMed] [Google Scholar]
- 29.Hubbard J I, Jones S F, Landau E M. J Physiol (London) 1968;197:639–657. doi: 10.1113/jphysiol.1968.sp008579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pusch M, Neher E. Pflügers Arch. 1988;411:204–211. doi: 10.1007/BF00582316. [DOI] [PubMed] [Google Scholar]
- 31.Hubbard J I, Jones S F, Landau E M. J Physiol (London) 1968;194:355–380. doi: 10.1113/jphysiol.1968.sp008413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Armstrong C M. Physiol Rev. 1981;61:644–682. doi: 10.1152/physrev.1981.61.3.644. [DOI] [PubMed] [Google Scholar]
- 33.Yang N, Horn R. Neuron. 1995;15:213–218. doi: 10.1016/0896-6273(95)90078-0. [DOI] [PubMed] [Google Scholar]
- 34.Brosius D C, Hackett J T, Tuttle J B. J Neurophysiol. 1992;68:1229–1234. doi: 10.1152/jn.1992.68.4.1229. [DOI] [PubMed] [Google Scholar]
- 35.Reyes M, Stanton P K. J Neurosci. 1996;16:5951–5960. doi: 10.1523/JNEUROSCI.16-19-05951.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thastrup O, Cullen P J, Drobak B K, Hanley M R, Dawson A P. Proc Natl Acad Sci USA. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blasi J, Chapman E R, Link E, Binz T, Yamasaki S, De Camilli P, Sudhof T C, Niemann H, Jahn R. Nature (London) 1993;365:160–163. doi: 10.1038/365160a0. [DOI] [PubMed] [Google Scholar]
- 38.Schiavo G, Rossetto O, Catsicas S, Polverino de Laureto P, DasGupta B R, Benfenati F, Montecucco C. J Biol Chem. 1993;268:23784–23787. [PubMed] [Google Scholar]
- 39.Blasi J, Chapman E R, Yamasaki S, Binz T, Niemann H, Jahn R. EMBO J. 1993;12:4821–4828. doi: 10.1002/j.1460-2075.1993.tb06171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foran P, Lawrence G W, Shone C C, Foster K A, Dolly J O. Biochemistry. 1996;35:2630–2636. doi: 10.1021/bi9519009. [DOI] [PubMed] [Google Scholar]
- 41.Dreyer F, Rosenberg F, Becker C, Bigalke H, Penner R. Naunyn-Schmiedeberg’s Arch Pharmacol. 1987;335:1–7. doi: 10.1007/BF00165027. [DOI] [PubMed] [Google Scholar]
- 42.Capogna M, McKinney R A, O’Connor V, Gahwiler B H, Thompson S M. J Neurosci. 1997;17:7190–7202. doi: 10.1523/JNEUROSCI.17-19-07190.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng H, Lederer W J, Cannell M B. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- 44.Santana L F, Kranias E G, Lederer W J. J Physiol (London) 1997;503:21–29. doi: 10.1111/j.1469-7793.1997.021bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]