Abstract
Increased synthesis of NO during airway inflammation, caused by induction of nitric-oxide synthase 2 in several lung cell types, may contribute to epithelial injury and permeability. To investigate the consequence of elevated NO production on epithelial function, we exposed cultured monolayers of human bronchial epithelial cells to the NO donor diethylenetriaamine NONOate. At concentrations generating high nanomolar levels of NO, representative of inflammatory conditions, diethylenetriaamine NONOate markedly reduced wound closure in an in vitro scratch injury model, primarily by inhibiting epithelial cell migration. Analysis of signaling pathways and gene expression profiles indicated a rapid induction of the mitogen-activated protein kinase phosphatase (MPK)-1 and decrease in extracellular signal-regulated kinase (ERK)1/2 activation, as well as marked stabilization of hypoxia-inducible factor (HIF)-1α and activation of hypoxia-responsive genes, under these conditions. Inhibition of ERK1/2 signaling using U0126 enhanced HIF-1α stabilization, implicating ERK1/2 dephosphorylation as a contributing mechanism in NO-mediated HIF-1α activation. Activation of HIF-1α by the hypoxia mimic cobalt chloride, or cell transfection with a degradation-resistant HIF-1α mutant construct inhibited epithelial wound repair, implicating HIF-1α in NO-mediated inhibition of cell migration. Conversely, NO-mediated inhibition of epithelial wound closure was largely prevented after small interfering RNA suppression of HIF-1α. Finally, NO-mediated inhibition of cell migration was associated with HIF-1α-dependent induction of PAI-1 and activation of p53, both negative regulators of epithelial cell migration. Collectively, our results demonstrate that inflammatory levels of NO inhibit epithelial cell migration, because of suppression of ERK1/2 signaling, and activation of HIF-1α and p53, with potential consequences for epithelial repair and remodeling during airway inflammation.
Inflammatory diseases of the respiratory tract are commonly associated with increased production of NO, because of induction and activation of the inducible isoform of nitric-oxide synthase (NOS2),2 within inflammatory immune cells as well as within the respiratory epithelium (1, 2). Indeed, elevated concentrations of exhaled NO and increased epithelial expression of NOS2 are characteristic features of chronic inflammatory airway diseases such as asthma (1, 2). The biological roles of NOS2 within the airway epithelium include contribution to innate host defense (3, 4), regulation of epithelial ion transport (5), and maintenance of epithelial barrier integrity (1, 6, 7). In addition, our recent studies showed that physiological concentrations of NO can promote airway epithelial cell migration and repair in response to in vitro injury, which was associated with increased expression and activation of gelatinase B (matrix metalloproteinase-9; MMP-9) (8). In contrast to these salutary properties of constitutive airway NO production, increased epithelial NOS2 expression has been associated with production of pro-inflammatory mediators and with increased epithelial injury and permeability (9–12). Also, whereas NO can promote wound repair, inappropriate overproduction of NO has also been associated with impaired wound healing (13) and reduced epithelial wound repair (8, 14) and may thereby contribute to prolonged epithelial injury and remodeling during chronic inflammatory conditions associated with increased NO production.
Because the airway epithelium is routinely subjected to environmental stresses, it experiences continuous injury, which is rapidly restored by wound healing processes, involving rapid migration of neighboring cells to denuded regions and proliferative responses to restore overall epithelial integrity. Studies of in vitro epithelial cell migration and wound repair have revealed the involvement of a complex set of cell signaling pathways and changes in gene expression. Among these, activation of p44/42 MAPK (ERK1/2) and stress-activated protein kinases such as p38 appear to be critical in controlling cell migration (15–17), either by direct actions on the motility machinery (15, 16) or by regulating the expression of genes that mediate cell adhesion, spreading, and migration, including members of the matrix metalloproteinase family, such as MMP-9 (8, 18). The ability of NO to modulate cell signaling pathways and gene expression has been intensively studied and involves various diverse mechanisms (19–22). Although NO is able to regulate some of these pathways by binding to its “receptor,” the heme protein guanylyl cyclase (GC), and production of the second messenger cGMP, other mechanisms include oxidation or nitrosation of susceptible targets such as reduced cysteine residues. Indeed, accumulating evidence indicates that NO modulates cell signaling by S-nitrosation of critical protein cysteines, especially under conditions of elevated NO production (23). However, the implications for NOS2-mediated actions on airway epithelial signaling and function have not been fully characterized.
A number of studies have indicated that NO can also impact on the activation of the transcription factor, hypoxia-inducible factor-1 (HIF-1) (22, 24), a critical regulator of cellular adaptive responses to hypoxia that regulates cell growth and survival, as well as cell adhesion and migration (25, 26). HIF is a heterodimeric transcription factor composed of one of three α-subunits (HIF-1α, HIF-2α, and HIF-3α) and a HIF-1β-subunit (also known as aryl hydrocarbon nuclear translocator). The HIF-1α subunit is actively degraded under normoxic conditions, because of oxygen-dependent hydroxylation of proline residues 402 and 564 by prolyl-4-hydroxylases and interaction with pVHL (von Hippel-Lindau protein) but is stabilized under hypoxic conditions, allowing HIF-1α accumulation and translocation to the nucleus, association with HIF-1β and activation of transcriptional activity (25). Although some studies have shown that NO can destabilize HIF-1α under hypoxic conditions (27), NO is also capable of stabilizing HIF-1α under normoxic conditions and can stimulate HIF-1-mediated gene expression, thereby mimicking a hypoxic response (27–30). HIF-1 not only mediates adaptive responses to hypoxia but is also recognized to play important roles in inflammatory processes (31), and recent studies have demonstrated HIF-1α activation during chronic airway inflammation (32, 33), conditions typically associated with increased NOS2 expression. Although it is well appreciated that HIF-1 can be activated under normoxic conditions by inflammatory cytokines (34–36) or bacterial or viral stimuli (37, 38), which was in some cases attributed to intermediate NO production (38), the significance of HIF-1 in mediating airway inflammation and/or injury is only beginning to be appreciated.
The present studies were undertaken to determine the impact of inflammatory concentrations of NO on bronchial epithelial wound repair and cell migration in vitro and to identify the major signaling pathways involved in these responses. Our results suggest that elevated concentrations of NO inhibit epithelial cell migration and wound repair in large part by two interdependent mechanisms, including induction of the MAPK phosphatase MKP-1 and consequent inhibition of ERK1/2 activation, as well as stabilization and activation of the transcription factors HIF-1α and p53. Moreover, in addition to potential direct effects of these signaling changes on actin cytoskeletal organization, they were also associated with NO-dependent changes in expression of a number of genes involved in epithelial migration and remodeling, such as MMP-9 and PAI-1.
EXPERIMENTAL PROCEDURES
Cell Culture and Treatments—Experiments were performed using a human bronchial epithelial cell line (HBE1) (kindly provided by Drs. R. Wu and J. Yankaskas). The cells were grown and maintained as previously described (8) and seeded in 12- or 24-well plates (Corning, Corning, NY) or in 8-well chamber slides (Nunc, Rochester, NY) to reach confluence before experimentation. Before cell treatments, the medium was replaced, and the cells were treated with the NO donor, diethylenetriamine NONOate (DETA NONOate; Cayman Chemical, Ann Arbor, MI; t½ = 20 h at 37 °C), or the hypoxia mimic CoCl2, for up to 24 h. Where noted, pharmacological inhibitors of MEK1/2 (U0126; 10 μm), PI3K/AKT (LY294002; 10 μm), GC (1H-[1,2,4]oxadiazolo-[4,3-a]quinoxalin-1-one; ODQ, 10 μm), or protein kinase G (KT5823, 10 μm) were added 15 min before cell stimulation. None of the agents used significantly affected cell morphology or viability under these conditions. Unless indicated otherwise, all of the reagents used were obtained from Sigma-Aldrich.
In Vitro Wound Repair Assay—To create in vitro epithelial injury, confluent HBE1 cell monolayers were mechanically wounded by creating a linear scratch of ∼0.2–0.3-mm width using a sterile P-200 pipette tip. The cells were washed with medium to remove cell debris, fresh medium was added to each well, and the appropriate reagents were added. Wound closure was followed over 24 h by light microscopy, and the wound areas were imaged using a digital camera (Canon Powershot A620) for calculation of the percentage of wound closure using National Institutes of Health ImageJ software (8).
Analysis of Cell Viability and Proliferation—Effects of DETA-NO on cell viability was evaluated by analysis of lactate dehydrogenase release (Sigma) following the manufacturer's instructions. For analysis of cell proliferation, HBE1 cells were plated at 105 cells/well in 6-well plates and cultured in the absence or presence of DETA-NO, and total cell numbers were counted after 24 and 48 h.
Transwell Cell Migration—For quantitative analysis of cell migration, HBE1 cells were seeded at 5 × 104 cells/well on 8-μm polycarbonate fibronectin-coated membrane inserts (Nunc). The ability of cells to migrate into these membrane pores by haplotaxis was determined after 24 h, by removal of nonmigrated cells with a cotton swab and staining of remaining migrated cells with 0.1% crystal violet in 0.1 m borate (pH 9.0), 2% ethanol. The stained cells were extracted with 10% acetic acid for quantitative analysis of absorbance at 562 nm (8).
Semiquantitative RT-PCR—Total RNA was extracted using TRIzol (Invitrogen) and reverse transcription (RT) was performed using 1–3 μg of total RNA, and PCRs were performed using a GeneAmp® PCR system 9700 (Applied Biosystems, Foster City, CA), as detailed previously (8) with oligonucleotides recognizing HIF-1α, MMP-9, PAI-1, carbonic anhydrase IX, and lysyl oxidase-like 2 (Operon). PCR products were resolved by 1% agarose gel electrophoresis and visualized by ethidium bromide staining. Expression of genes of interest was normalized to glyceraldehyde-3-phosphate dehydrogenase by band densitometry analysis using National Institutes of Health ImageJ software.
Western Blot Analysis—The cells were extracted in lysis buffer (50 mm Hepes, pH 7.4, 250 mm NaCl, 10% glycerol, 1% Triton X-100, 1.5 mm MgCl2, 1 mm phenylmethylsulfonyl fluoride, 1 mm EGTA, 2 mm Na3VO4, 10 μg/ml aprotinin, and 10 μg/ml leupeptin) on ice for 15 min, and the cell lysates were collected by scraping and centrifuged (14,500 rpm, 5 min) to remove cell debris. The samples containing equivalent amounts of protein (10–20 μg; determined using BCA method; Pierce) were separated on 10% SDS-PAGE gels, transferred to polyvinylidene difluoride membranes, and blotted with primary antibodies against HIF-1α (1:250; Santa Cruz), pERK1/2 (1:500; Santa Cruz), ERK1/2 (1:500; Cell Signaling), pAkt and Akt (1:1000; Cell Signaling), MKP-1 (H66; 1:1000; Santa Cruz), Ras (1:1000; Transduction Labs), p53 or p-p53(Ser15) (1:1000; Cell Signaling). Primary antibodies were detected with horseradish peroxidase-conjugated secondary antibodies (Sigma) and visualized by enhanced chemiluminescence (ECL; Pierce).
Immunofluorescence Analysis—Confluent HBE1 cells on 8-well chamber slides (Nunc) were subjected to the appropriate treatments and subsequently fixed in 4% paraformaldehyde for 15 min, and permeabilized using 100% methanol. After blocking in 2% dry milk in PBS, 0.1% Triton X-100 for 1 h, the cells were probed either with a HIF-1α mouse monoclonal antibody (BD Biosciences; 1:200) or with a pERK1/2 rabbit polyclonal antibody (Santa Cruz; 1:200) in 2% bovine serum albumin/PBS/Triton X-100 for 1 h at 37 °C, washed twice with PBS, and then incubated with a secondary antibody (Alexa-Fluor 568 goat anti mouse/rabbit; 1:250; Molecular Probes Inc., Eugene, OR) in 2% bovine serum albumin/PBS for 1 h at 37 °C in the dark. After two washes with PBS, the nuclei were stained with Sytox® green (1:10,000 in PBS) for 5 min, and the slides were mounted and imaged using an Olympus BX50 confocal laser scanning microscope and Lasersharp 2000 software (Bio-Rad).
Transfection with Mutant HIF-1α—A plasmid containing full-length hemagglutinin-tagged constitutively stabilized HIF-1α (containing the mutations P402A and P564A; a kind gift from Dr. Eric Huang) was transiently transfected into HBE1 cells, 48 h prior to experimentation. Briefly, the cells were incubated with the mutant HIF-1α-containing plasmid or control plasmid (pcDNA, ∼1 μg) mixed with Lipofectamine Plus (Invitrogen) and incubated at 37 °C for 6 h.
siRNA Silencing of HIF-1α Expression—HIF-1α expression was silenced using predesigned HIF-1α siRNA sequence primers (Santa Cruz) that were transfected into HBE1 cells according to the manufacturer's instructions. Briefly, HIF-1α-targeted or control siRNA sequences were mixed with transfection reagent (Santa Cruz) and added to HBE1 cells at a final concentration of 100 nm, for 6 h at 37 °C. The cells were incubated for an additional 48 h before experimentation.
In Situ Analysis of Gelatinase Activity—Confluent HBE1 cells in 8-well chamber slides were subjected to wounding and treatment with DETA-NO and subsequently overlaid with DQ™ gelatin (Invitrogen; 100 μg/ml) for 2 h at 37 °C. After washing with PBS and staining of nuclei using 4′,6′-diamino-2-phenylindole, slides were fixed with 4% paraformaldehyde and analyzed using laser scanning confocal microscopy. As a positive control, the cells were incubated with the MMP activator p-chloromercuribenzoic acid (p-CMB; 200 μm, 2 h) prior to incubation with DQ™ gelatin.
Microarray Analysis—HBE1 cells were incubated for 24 h in the absence or presence of 500 μm DETA-NO, and after RNA isolation and cDNA synthesis, in vitro transcription and microarray analysis was performed in accordance with Affymetrix protocols (Affymetrix, Santa Clara, CA), at the DNA Microarray Core Facility at the University of Vermont. Equal amounts of pooled RNA from triplicate treatments were hybridized on Affymetrix human genome U133A2.0 arrays, which were analyzed using Affymetrix GeneChip software, and the changes in gene expression profiles were subsequently evaluated using DAVID (National Institutes of Health) for functional annotation clustering.
Data Analysis and Statistics—The quantitative data are presented as the means ± S.D. from at least three separate experiments, and statistical significance was evaluated using Student's t test.
RESULTS
Nitric Oxide Reduces Epithelial Wound Closure by Inhibiting Cell Migration—We recently reported that NO, at low nanomolar concentrations, is capable of promoting epithelial cell migration and wound closure in injured airway epithelial cell monolayers, indicating a potential role of constitutive airway epithelial NO production in maintenance and restoration of epithelial integrity (8). The present studies were designed to address the potential impact of elevated NO production, as observed during airway inflammation, on epithelial cell migration and wound closure. For this purpose, HBE1 cells were exposed to 100–500 μm of the NO donor DETA-NO, which slowly decomposes at neutral pH to releases NO (t½ = ∼20 h at 37 °C) to generate persistent NO concentrations for several hours. Measurements of actual NO concentrations during cell exposure to 500 μm DETA-NO, using an NO-specific electrode (Harvard Apparatus), indicated a relatively constant NO concentration of ∼400 nm, consistent with earlier findings and comparable with reported NO concentrations at sites of active inflammation (1, 39).
As illustrated in Fig. 1A, exposure of linearly wounded HBE1 cell monolayers to >100 μm DETA-NO resulted in a dose-dependent decrease in the rate of wound closure. Because these concentrations of DETA-NO did not induce significant cytotoxicity (as determined by analysis of lactate dehydrogenase release; not shown) and only modestly reduced HBE1 cell proliferation (Fig. 1B), these inhibitory effects of NO are most likely due to impaired cell migration. This was more directly evaluated in a Transwell migration assay, which showed that epithelial cell migration was significantly decreased in the presence of DETA-NO (500 μm) (Fig. 1C). Thus, our results indicate that elevated production of NO during airway inflammation may have detrimental effects on epithelial injury and repair by inhibiting epithelial cell migration. Whereas the ability of NO to stimulate cell migration was found to depend on cGMP-mediated pathways (8), these inhibitory effects of NO were not prevented by the sGC inhibitor ODQ (not shown) and were therefore independent of cGMP.
FIGURE 1.
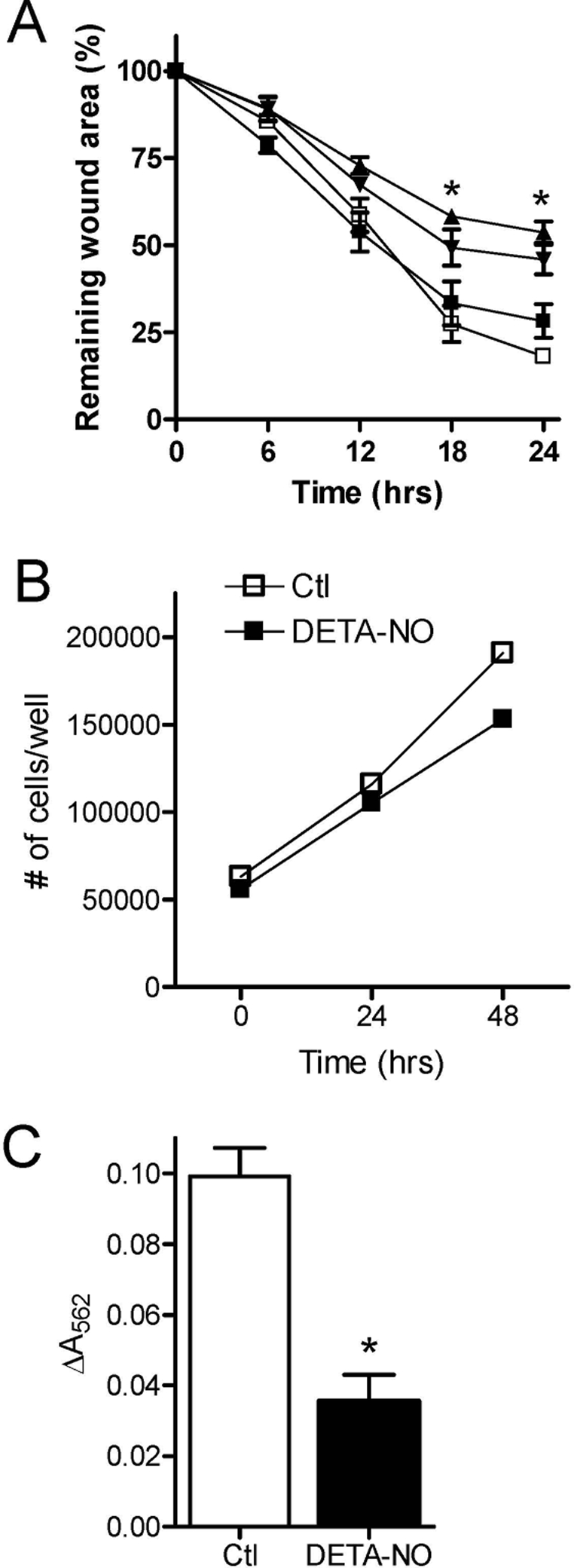
NO inhibits airway epithelial wound closure by suppressing cell migration. A, confluent HBE1 cells were wounded by creating a linear scratch, and the rate of wound closure was monitored in the absence (open squares) or presence of 100 (filled squares), 250 (inverted triangles), or 500 μm (triangles) DETA NONOate. Wound closure was calculated by digital imaging and the percentage of initial wound area (means ± S.D.; n = 4; *, p < 0.001 compared with control (Ctl)). B, effect of DETA-NO of HBE1 cell proliferation. HBE1 cells (105) were incubated in the absence (open squares) or presence (filled squares) of 500 DETA-NO, and proliferation was monitored by cell counting (n = 2). C, for analysis of cell migration, HBE1 cells were plated on fibronectin-coated porous polycarbonate transwell membranes and incubated in the absence or presence of DETA NONOate (500 μm). The migrated cells were stained and extracted for quantitation by optical density (means ± S.D.; n = 5; *, p < 0.001).
NO Inhibits ERK1/2 and Akt Phosphorylation—Because epithelial cell migration is mediated by a complex sequence of various cell signaling events, including the activation of the protein kinases ERK1/2, c-Jun N-terminal kinase, p38, and PI3K/ Akt (16, 17), we evaluated the effects of NO on these various pathways under our experimental conditions. As illustrated in Fig. 2A, HBE1 cell exposure to DETA-NO (500 μm) resulted in a time-dependent dephosphorylation of ERK1/2, starting at ∼2 h, suggesting inhibition of this signaling pathway. Exposure of HBE1 cells to DETA NONOate also inhibited Akt phosphorylation after 24 h, but not at earlier time points (Fig. 2A). Analysis of phosphorylated forms of c-Jun N-terminal kinase or p38 MAPK under these conditions did not reveal significant changes under these conditions (results not shown).
FIGURE 2.

Effect of NO on ERK1/2 and Akt. A, HBE1 cells were treated with DETA-NO (500 μm) and phosphorylated and unphosphorylated forms of ERK1/2 and Akt, as well as MKP-1 and Ras, were detected by Western blotting (representative blots of three replicates are shown). B, HBE1 cells were exposed to DETA-NO in the absence or presence of the sGC inhibitor ODQ (10 μm) or the PKG inhibitor KT5823 (10 μm), for 4 h. ERK phosphorylation was determined by Western blot analysis of pERK and ERK and quantified by densitometry (means ± S.D., n = 4; *, p < 0.005 versus non-NO-treated cells; #, p < 0.005 versus untreated control (Ctl)). C, involvement of ERK1/2 and PI3K in HBE1 wound closure was determined by incubating scratched cells in the absence or presence of the MEK1/2 inhibitor U0126 (10 μm) or the PI3K inhibitor LY294002 (10 μm). Wound closure (%) was determined after 24 h (means ± S.D., n = 4; *, p < 0.005 versus control).
Recent studies in other cell systems have shown that NO is capable of reducing ERK1/2 phosphorylation, which was related to down-regulation of Ras expression (40) or to induction of the MAPK phosphatase MKP-1 (41, 42). We therefore explored changes in either Ras or MKP-1 expression in association with NO-mediated ERK1/2 dephosphorylation. As shown in Fig. 2A, ERK1/2 dephosphorylation by DETA-NO was not associated with significant changes in Ras expression but corresponded closely with induction of MKP-1.
To test whether NO reduces ERK1/2 phosphorylation by activating GC and cGMP-dependent protein kinases, we evaluated the effects of DETA-NO on ERK phosphorylation in the presence of GC inhibitor ODQ (10 μm) or the protein kinase G inhibitor KT5823 (10 μm). As shown in Fig. 2B, these inhibitors slightly reduced ERK1/2 phosphorylation but did not prevent the inhibitory effects of DETA-NO, indicating that NO-induced ERK dephosphorylation occurred by a mechanism independent of cGMP.
The importance of ERK1/2 and PI3K/Akt in HBE1 cell wound closure was confirmed using pharmacological inhibitors of these pathways. Indeed, the addition of the MEK1/2 inhibitor U0126 (10 μm) or the PI3K inhibitor LY294002 (10 μm) dramatically inhibited wound closure of injured HBE1 monolayers (Fig. 2C), illustrating the involvement of these signaling pathways in wound closure. Overall, these results suggest that NO-mediated inhibition of epithelial wound closure is mediated by inhibitory effects on ERK1/2 and Akt signaling pathways, because of cGMP-independent induction of the ERK phosphatase MKP-1.
NO Promotes HIF-1α Stabilization and Activation—To further explore the mechanism(s) by which NO may effect airway epithelial cell migration and wound closure, we performed DNA microarray analysis of transcriptional changes in HBE1 cells following incubation with 500 μm DETA-NO for 24 h. The results of this analysis indicated that over 250 genes were either up- or down-regulated by more than 2-fold in response to DETA-NO. Many of these regulated genes are involved in cell cycle regulation and proliferation, redox regulation and metabolism, and control of cell motility and migration (supplemental Table S1).
The most prominently up-regulated genes in response to DETA-NO were noted to be characteristic of cell responses to hypoxia and subject to regulation by the transcription factor HIF-1 (supplemental Table S1 and Fig. S1). We therefore verified the activation of HIF-1 in HBE1 cells during exposure to DETA-NO (500 μm), by analysis of HIF-1α stabilization and nuclear translocation. Indeed, HBE1 cell exposure to DETA-NO resulted in rapid HIF-1α accumulation, which peaked after 4–8 h, and then declined to near basal levels after 24 h (Fig. 3A). Similar stabilization of HIF-1α was also observed in response to the hypoxia-mimetic CoCl2 (100 μm) (Fig. 3A). Moreover, both DETA-NO and CoCl2 promoted nuclear translocation of HIF-1α, as analyzed by immunofluorescence, consistent with its activation (Fig. 3B). Finally, RT-PCR analysis of selected HIF-regulated genes, PAI-1, carbonic anhydrase IX, and lysyl oxidase-like 2 confirmed enhanced expression of these genes after HBE1 treatment with DETA-NO (Fig. 3C).
FIGURE 3.
NO induces HIF-1α stabilization and activation. A, HBE1 cells were exposed to DETA-NO (500 μm) for indicated times or to the hypoxia mimic CoCl2 (100 μm; 4 h), and whole cell lysates were prepared for Western blot analysis of HIF-1α protein (representative of two experiments). B, immunofluorescence analysis of HIF-1α nuclear translocation in HBE1 cells in response to CoCl2 (100 μm; 4 h) or DETA-NO (500 μm; 4 h). C, RT-PCR analysis of hypoxia-responsive genes, PAI-1, carbonic anhydrase IX, and lysyl oxidase-like 2, in untreated HBE1 cells and cells treated with 500 μm DETA-NO for 24 h. D, HBE1 cells were exposed to DETA-NO (4 h) in the absence or presence of the sGC inhibitor ODQ (10 μm) or the PKG inhibitor KT5823 (10 μm), and HIF-1α protein was determined by Western blot analysis and quantified by densitometry (means ± S.D., n = 4; *, p < 0.005 versus non-NO-treated cells; #, p < 0.005 versus corresponding control (Ctl or CTL)). E, HBE1 cells were treated with either CoCl2 (100 μm; 4 h) or the MEK1 inhibitor, U0126 (10 μm; 4 h), and the effects on HIF-1α stabilization and ERK1/2 phosphorylation were determined by Western blot. F, immunofluorescence analysis of nuclear translocation of HIF-1α in HBE1 cells after incubation with U0126 (10 μm; 4 h). G, HIF-1α stabilization was determined after a 4-h incubation of HBE1 cells with U0126 (10 μm), DETA-NO (500 μm), or both.
As expected (27), NO-dependent HIF-1α stabilization was not prevented by either ODQ or KT5823 (Fig. 3D), suggesting a mechanism independent of cGMP, such as through S-nitrosation of HIF-1α or alternative targets within this pathway (29, 30). Accordingly, analysis of cell lysates for S-nitrosothiols using ozone-enhanced chemiluminescence, as described previously (43), revealed marked increases in overall cell S-nitrosothiol levels, from <10 pmol/mg protein in untreated HBE1 cells to 252 ± 81 pmol/mg protein, 24 h after exposure to 500 μm DETA-NO (n = 4).
Inhibition of ERK1/2 Promotes HIF-1α Activation—We noted that stabilization of HIF-1α and ERK1/2 dephosphorylation by DETA-NO occurred with relatively similar kinetics (Figs. 2A and 3A), suggesting that these signaling events might be interrelated. Although several studies have shown that ERK1/2 may positively regulate HIF-1α-mediated transactivation (44–46), it is not known whether ERK1/2 also regulates HIF-1α stabilization or whether HIF-1 activation could also regulate ERK signaling. As shown in Fig. 3E, HIF-1 activation by CoCl2 (100 μm) did not significantly affect ERK1/2 phosphorylation, but inhibition of ERK1/2 phosphorylation using the MEK1/2 inhibitor U0126 (10 μm) slightly increased HIF-1α protein levels after 4 h, as shown by Western blot analysis. Moreover, immunofluorescence analysis indicated a modest increase in HIF-1α after treatment with U0126, although most HIF-1α immunopositive signal appeared in the cytoplasm (Fig. 3F), consistent with previous observations that ERK inhibition blocks HIF-1α nuclear localization (45, 46). Finally, the stimulatory effects of DETA-NO and U0126 on HIF-1α stabilization were found to be additive (Fig. 3G), suggesting that NO-mediated HIF-1α stabilization involved inhibition of ERK activation as well as mechanisms independent of ERK.
HIF-1α Activation Inhibits Epithelial Wound Closure—Although HIF-1 was previously shown to enhance hypoxia-induced cell migration (47, 48), HIF-1α stabilization under normoxic conditions by CoCl2 was recently reported to reduce vascular smooth muscle cell attachment and migration (26). Therefore, we addressed the possible involvement of HIF-1 activation in NO-mediated inhibition of epithelial wound closure in HBE1 cells. As shown in Fig. 4A, exposure of injured HBE1 cell monolayers to CoCl2 (100 μm) was found to markedly inhibit HBE1 wound closure, suggesting that HIF-1 activation inhibits wound repair. Because CoCl2 is a nonspecific activator of HIF-1 and could potentially inhibit cell migration by alternative mechanisms, we used an alternative approach to enhance HIF-1 by transient transfection of HBE1 cells with a stable HIF-1α construct in which the two proline hydroxylation targets were replaced by alanine (P402A/P564A). Whereas transfection of HBE1 cells with the control plasmid did not significantly affect wound closure of linearly scratched monolayers, wound closure was significantly reduced in HBE1 cells that were transfected with a stable HIF-1α mutant (Fig. 4A), consistent with an inhibitory effect of HIF-1 activation on epithelial wound closure.
FIGURE 4.

HIF-1α activation inhibits epithelial wound closure. A, HBE1 cell monolayers were injured and wound closure was followed in the absence or presence of CoCl2 (100 μm). Alternatively, HBE1 cells were transiently transfected with a degradation-resistant HIF-1α construct (P402A/P564A) or with control vector (pcDNA), 48 h prior to scratch injury, and wound repair was measured after 24 h (means ± S.D., n = 4–5; *, p < 0.005 versus control (Ctl); #, p < 0.05 versus pcDNA). B, HBE1 cells were transfected with negative control (Scr.) or HIF-1α-targeted siRNA, and HIF-1α expression was analyzed 48 h by semi-quantitative RT-PCR (upper panel). HBE1 cells were transfected with negative control (scramble) or HIF-1α-targeted siRNA, 48 h prior to scratch injury, and the effects on wound closure were determined in the absence (white bars) or presence (black bars) of 500 μm DETA-NO over 24 h (lower panel). The values are the means ± S.D., n = 4–5; *, p < 0.05 versus non-NO-treated cells; #, p < 0.05 versus scrambled siRNA.
To verify whether HIF-1α activation also contributes to the inhibitory effects of NO, we silenced HIF-1α expression using siRNA prior to HBE1 cell wounding and DETA-NO exposure. As illustrated in Fig. 4B, HIF-1α mRNA expression was markedly suppressed 48 h after transfection with HIF-1α-targeted siRNA. Moreover, NO-mediated inhibition of epithelial wound closure was largely reversed after transfection with HIF-1α siRNA (Fig. 4B), suggesting the importance of HIF-1α stabilization and activation in NO-mediated inhibition of epithelial wound repair.
Role of ERK1/2 and HIF-1 in NO-regulated Expression of MMP-9 and PAI-1—Recently, we and others have demonstrated the importance of MMP-9 expression and activation in airway epithelial cell migration and wound repair (8). Therefore, we investigated whether NO-mediated inhibition of epithelial wound closure was associated with decreased MMP-9 expression and/or activation. Indeed, exposure of wounded HBE1 cells to 500 μm DETA-NO markedly decreased MMP-9 mRNA expression, as determined by semi-quantitative RT-PCR (Fig. 5A), and reduced gelatinase activity, analyzed using an in situ zymography assay (Fig. 5B). Consistent with earlier reports (49), MMP-9 expression by HBE1 cells was found to depend on ERK1/2 and PI3K/Akt and was dramatically attenuated after cell treatment with the MEK1/2 inhibitor U0126 (10 μm) or the PI3K inhibitor LY294002 (10 μm) (Fig. 5C). In contrast, neither U0126 nor LY294002 significantly affected expression of PAI-1 (Fig. 5C), a negative regulator of uPA/MMP-9-mediated cell migration (50, 51) that was induced by NO. Conversely, siRNA silencing of HIF-1α did not significantly prevent NO-mediated suppression of MMP-9 but attenuated NO-mediated induction of PAI-1 (Fig. 5D), confirming the importance of HIF-1 in NO-mediated induction of PAI-1.
FIGURE 5.
NO-mediated regulation of MMP-9 and PAI-1: role of ERK and HIF-1α. Exposure of linearly scratched HBE1 cells to DETA-NO (500 μm) for 24 h reduces MMP-9 mRNA expression (A), and gelatinase activity, measured using in situ zymography. B, the MMP activator, p-CMB (200 μm), was used as a positive control (Ctl). C, effect of the ERK1/2 (MEK1/2) inhibitor U0126 (10 μm) and the PI3K/Akt inhibitor LY294002 (10 μm) (24 h) on MMP-9 or PAI-1 mRNA expression in HBE1 cells. D, effects of HIF-1α siRNA on NO-mediated suppression of MMP-9 and NO-mediated induction of PAI-1, measured after 24 h. Representative gels of two to three experiments are shown. E, activation of p53 phosphorylation on Ser15 by DETA-NO and effects of HIF-1α siRNA. The cells were transfected with HIF-1α siRNA or control siRNA and stimulated with DETA-NO for 4 h, before Western blot analysis of HIF-1α, phospho-p53(Ser15) or p53. Representative gels of two to three experiments are shown.
HIF-1α Contributes to NO-mediated Activation of p53—Stabilization of HIF-1α typically results in increased HIF-1 transcriptional activity, but HIF-1α also exerts alternative functions by interaction with p53, resulting in stabilization and activation of this transcription factor (52, 53). Activation of p53 not only exerts anti-proliferative effects but is also capable of inhibiting cell migration by cytosolic regulation of the actin cytoskeleton (54). We therefore explored whether NO-mediated HIF-1α stabilization in HBE1 cells contributes to p53 activation. As shown in Fig. 5E, exposure of HBE1 to 500 μm DETA-NO for 4 h resulted in HIF-1α stabilization and increased levels of p-p53(Ser15), although total p53 levels were not significantly enhanced at this time point, consistent with earlier observations (39). Moreover, cell transfection with HIF-1α-targeted siRNA markedly reduced NO-mediated HIF-1α stabilization and largely prevented accumulation of p-p53(Ser15) (Fig. 5E), consistent with a role for HIF-1α in p53 stabilization and activation.
DISCUSSION
The major finding of our present study is that NO, at concentrations representative of acute inflammation (39, 40), can suppress airway epithelial wound repair, primarily by inhibiting epithelial cell migration. Moreover, our studies reveal two inter-related mechanisms by which NO inhibits in vitro epithelial wound repair that involve induction of MKP-1 and suppression of ERK1/2 signaling and stabilization and activation of the transcription factors HIF-1α and p53. Although our studies are based on in vitro studies with an exogenous NO donor and do not directly address the importance of endogenously produced NO within inflamed airways, a number of studies have demonstrated that the use of 500 μm DETA-NO adequately mimics NO production and NO-mediated effects by e.g. activated macrophages (36, 40), suggesting that our studies are relevant for in vivo inflammatory conditions. Therefore, our data demonstrate a pathophysiological role for increased airway NO under conditions of inflammation and highlight mechanisms by which increased expression and activity of NOS2 could contribute to airway epithelial injury and disrepair/remodeling during chronic airway inflammatory disease.
Our findings extend our previously observed paradox (8), indicating that low nanomolar concentrations of NO promote epithelial cell migration and would repair, whereas higher concentrations attenuate epithelial wound repair by inhibiting cell migration. Although several previous studies have shown that NO can inhibit epithelial cell migration in vitro (14, 55) and retard cutaneous wound healing and collagen biosynthesis (56, 57), the mechanisms involved in these effects are incompletely understood. Our overall findings are also qualitatively consistent with those recently reported by Ridnour et al. (42, 58), who observed similar biphasic effects of NO on cell migration and proliferation in other cell types that are of importance in wound healing in vivo. Therefore, the adverse effect of NO on cell migration or proliferation and on overall wound healing may present a general phenomenon, even though the molecular mechanisms may be different depending on the involved cell type.
Consistent with earlier studies (14), NO-mediated inhibition of cell migration of HBE1 cells is independent of sGC-cGMP signaling and is most likely due to oxidative or nitrosative signaling. Accordingly, we detected marked increases in S-nitrosylated proteins in NO-exposed cells, and analysis of global gene expression profiles indicated a number of up-regulated genes that are characteristic of cell responses to oxidative/nitrosative stress (supplemental Table S1). Analysis of various protein kinase signaling pathways in response to DETA-NO revealed that prolonged exposure to NO resulted in decreased phosphorylation of ERK1/2 and PI3K/Akt, both critically involved in controlling cell migration (15, 16). Moreover, consistent with several recent studies (41, 42), ERK1/2 dephosphorylation was temporally associated with induction of MAPK phosphatase-1 (MKP-1), a dual specificity phosphatase that is encoded by an immediate-early gene that is induced in conditions of inflammation and stress (59). Inhibition of ERK1/2 and PI3K/Akt signaling may impact on cell migration by direct effects on the cytoskeleton, for example by reduced phosphorylation of myosin light chain kinase within pseudopodia (15) or by suppressing the expression and activation of critical proteins such as MMP-9, a critical mediator of epithelial cell migration (8, 60).
In addition to inhibition of ERK1/2 and Akt signaling, our studies also revealed an important role for HIF-1α activation in cell responses to DETA-NO that result in reduced cell migration and wound repair. HIF-1α activation was demonstrated by stabilization of HIF-1α protein, its nuclear localization, and increased expression of a number of hypoxia-responsive genes (Fig. 3 and supplemental Fig. S1). Several previous studies have demonstrated that NO is capable of stabilizing HIF-1α under normoxic conditions by mechanisms that are unrelated to sGC activation and synthesis of the second messenger cGMP. These include direct inhibition of prolyl-4-hydroxylase activity (61), S-nitrosylation of critical cysteine residues within HIF-1α (29) or pVHL (30), or activation of upstream signaling pathways that control HIF-1α expression or HIF-1 transcriptional activity, such as p44/42 MAPK or PI-3K/Akt (27, 37, 44, 46). Although we did not observe significant activation of ERK1/2 or Akt under our conditions, overall protein S-nitrosation in HBE1 cells was markedly enhanced in response to DETA-NO. However, the precise role of specific protein S-nitrosation in HIF-1 regulation is still unclear (27, 62), and we therefore did not attempt to further dissect the critical protein targets that mediate HIF-1 activation under these conditions.
HIF-1 activation has been associated with enhanced emigration of circulating blood cells in response to hypoxia (31, 63) and was found to contribute to hypoxia-induced glioma cell migration and invasion (47) and to renal epithelial cell migration during hypoxia (48). However, forced expression of HIF-1α or normoxic HIF-1 activation with CoCl2 in vascular smooth muscle cells HIF-1α stabilization were found to reduce cell attachment and migration (26). Consistent with these latter findings, we observed that activation of HIF-1 using CoCl2 or cell transfection with a stable HIF-1α mutant attenuated epithelial wound closure of HBE1 cells. Moreover, NO-mediated inhibition of wound closure was largely prevented after siRNA knockdown of HIF-1α, confirming the negative impact of HIF-1α activation on cell migration and wound closure under normoxic conditions. Thus, our studies suggest that HIF-1 may participate in airway epithelial remodeling under inflammatory conditions associated with enhanced NO production, by impeding epithelial cell migration.
The apparent opposing contribution of HIF-1α to cell migration during hypoxia or during normoxic conditions suggests that HIF-1α may possess alternative properties during these various conditions. Activation of HIF-1α during hypoxia typically results in the enhanced expression of genes that promote cell survival, proliferation, or migration, such as vascular endothelial growth factor (VEGF) and lysyl oxidases (25, 48). In addition, our results also indicate that HIF-1α activation by DETA-NO also contributed to induction of PAI-1, which is capable of inhibiting uPA/MMP-9-mediated cell migration (50, 51). This would suggest that HIF-1α-mediated inhibition of HBE1 cell migration observed in our studies may, at least in part, be due to up-regulation of PAI-1. Moreover, HIF-1 and MMP-9 are typically both enhanced or activated in malignant tumors, although it is unclear to what extent these events are causally related (64). Our studies indicate that siRNA knockdown of HIF-1α suppressed MMP-9 expression in HBE1 cells, consistent with recent studies in glioma cell lines (47), but did not prevent NO-mediated down-regulation of MMP-9. Therefore, NO-mediated MMP-9 suppression occurred by a mechanism independent of HIF-1α activation and most likely involved inhibition of ERK1/2 (and to a lesser extent) PI3K/Akt signaling.
In addition to regulating gene expression, activation of HIF-1α can also result in increased activation of the tumor suppressor p53, by interaction with Hdm2 (52, 53, 65). Consistent with earlier findings (39, 66), we observed that cell exposure to NO results in enhanced p53 phosphorylation on serine 15, which leads to p53 accumulation by decreasing its binding activity with Hdm2 and enhances its transcriptional activity. Our current findings indicate that NO-dependent p53 Ser15 phosphorylation was attenuated after siRNA silencing of HIF-1α, indicating the importance of HIF-1α activation in this process. In addition to its well known anti-proliferative properties, p53 also affects various features of migrating cells, such as cell spreading and polarization, and stimulation of p53 activity has been associated with delayed wound repair and re-epithelialization in vivo (54). Hence, activation of p53 may represent an additional mechanism by which NO, through HIF-1α stabilization, may reduce cell migration and epithelial wound repair.
The ability of HIF-1α to activate target genes (through dimerization with HIF-1β) and promote cell survival or proliferation requires its phosphorylation by ERK1/2 (45, 46), whereas dephosphorylated HIF-1α appears to mediate opposing functions due to interactions with p53 (53). Accordingly, suppression of MPK1 was recently found to enhance HIF-1α transcriptional activity during hypoxia, by enhancing ERK1/2-mediated phosphorylation (67). This implies that MPK-1 induction and ERK1/2 dephosphorylation by NO may impinge on HIF-1α activity by preventing excessive HIF-1α nuclear localization and transactivation and promoting cytosolic interactions with Hdm2/p53. Accordingly, although inhibition of ERK1/2 using U0126 actually mildly enhanced HIF-1α stabilization under normoxic conditions, this was not associated with significant nuclear localization (Fig. 3). Moreover, inhibition of ERK1/2 was found to enhance NO-mediated stabilization of HIF-1α (Fig. 3).
Overall, our findings indicate that NO can inhibit epithelial cell migration and wound repair by concerted mechanisms, involving the induction of MKP-1 and consequent ERK-1/2 dephosphorylation, as well as stabilization and activation of HIF-1α. These events are linked to altered expression of various genes that control epithelial repair and remodeling, including MMP-9 and PAI-1. Moreover, inhibition of ERK1/2 signaling also regulates HIF-1α to mitigate its proliferative and pro-migratory properties of HIF-1α in favor of anti-proliferative effects and inhibition of cell migration, which are associated with p53 activation. Fig. 6 illustrates the various interrelated mechanisms addressed in this study by which NO inhibits cell migration.
FIGURE 6.
Proposed mechanisms by which NO mediates epithelial cell migration through regulation of ERK1/2 and HIF-1α pathways. VEGF, vascular endothelial growth factor.
Although recent studies suggest the involvement of HIF-1 in airway epithelial responses to viral infection (38) and in inflammatory signaling or subepithelial remodeling during allergic airway inflammation (32), chronic obstructive pulmonary disease (33), or idiopathic pulmonary fibrosis (68), the importance of HIF-1α activation and the involvement of NO in these conditions is not clear at present. Analogous to previous studies showing biphasic regulation of cell migration by TNF-α, with initial activation of filopodia and increased cell migration followed by inhibition of cell migration and up-regulation of p53 at later stages (54), our results may suggest that the induction of NO production during inflammation may similarly be involved in such biphasic regulation of epithelial cell migration to assure coordinated wound repair. Some studies have reported the presence of activated p53 during chronic inflammation (66), but very little is known to date about the contribution of p53 to inflammation and/or remodeling. Our studies suggest that activation of HIF-1α, and perhaps p53, may play important roles in epithelial repair and/or remodeling under conditions of chronic airway inflammation associated with increased production of NO.
Supplementary Material
Acknowledgments
We thank Jeffrey Bond for assistance with analysis of the microarray data and Yvonne Janssen-Heininger for use of the Sievers NO analyzer (43).
This work was supported, in whole or in part, by National Institutes of Health Grants HL074295 and HL068865. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1 and Fig. S1.
Footnotes
The abbreviations used are: NOS, nitric-oxide synthase; DETA-NO, diethylenetriaamine NONOate; ERK, extracellular signal-regulated kinase; GC, guanylyl cyclase; HIF, hypoxia inducible factor; MAPK, mitogen-activated protein kinase; MKP, MAPK phosphatase; MMP, matrix metalloproteinase; ODQ, 1H-[1,2,4]oxadiazolo-[4,3-a]quinoxalin-1-one; PAI, plasminogen activator inhibitor; PI3K, phosphatidylinositol 3-kinase; siRNA, small interfering RNA; RT, reverse transcription; PBS, phosphate-buffered saline.
References
- 1.Bove, P. F., and van der Vliet, A. (2006) Free Radic. Biol. Med. 41 515–527 [DOI] [PubMed] [Google Scholar]
- 2.Xu, W., Zheng, S., Dweik, R. A., and Erzurum, S. C. (2006) Free Radic. Biol. Med. 41 19–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Darling, K. E., and Evans, T. J. (2003) Infect. Immun. 71 2341–2349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng, S., De, B. P., Choudhary, S., Comhair, S. A., Goggans, T., Slee, R., Williams, B. R., Pilewski, J., Haque, S. J., and Erzurum, S. C. (2003) Immunity 18 619–630 [DOI] [PubMed] [Google Scholar]
- 5.Hardiman, K. M., McNicholas-Bevensee, C. M., Fortenberry, J., Myles, C. T., Malik, B., Eaton, D. C., and Matalon, S. (2004) Am. J. Respir. Cell Mol. Biol. 30 720–728 [DOI] [PubMed] [Google Scholar]
- 6.Rose, F., Guthmann, B., Tenenbaum, T., Fink, L., Ghofrani, A., Weissmann, N., Konig, P., Ermert, L., Dahlem, G., Haenze, J., Kummer, W., Seeger, W., and Grimminger, F. (2002) J. Immunol. 169 1474–1481 [DOI] [PubMed] [Google Scholar]
- 7.Vyas-Read, S., Shaul, P. W., Yuhanna, I. S., and Willis, B. C. (2007) Am. J. Physiol. 293 L212–L221 [DOI] [PubMed] [Google Scholar]
- 8.Bove, P. F., Wesley, U. V., Greul, A. K., Hristova, M., Dostmann, W. R., and van der Vliet, A. (2007) Am. J. Respir. Cell Mol. Biol. 36 138–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eriksson, U., Egermann, U., Bihl, M. P., Gambazzi, F., Tamm, M., Holt, P. G., and Bingisser, R. M. (2005) J. Immunol. 175 2715–2720 [DOI] [PubMed] [Google Scholar]
- 10.Watkins, D. N., Garlepp, M. J., and Thompson, P. J. (1997) Br. J. Pharmacol. 121 1482–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sparkman, L., and Boggaram, V. (2004) Am. J. Physiol. 287 L764–L773 [DOI] [PubMed] [Google Scholar]
- 12.Han, X., Fink, M. P., Uchiyama, T., Yang, R., and Delude, R. L. (2004) Am. J. Physiol. 286 G126–G136 [DOI] [PubMed] [Google Scholar]
- 13.Schwentker, A., Vodovotz, Y., Weller, R., and Billiar, T. R. (2002) Nitric Oxide 7 1–10 [DOI] [PubMed] [Google Scholar]
- 14.Kiviluoto, T., Watanabe, S., Hirose, M., Sato, N., Mustonen, H., Puolakkainen, P., Ronty, M., Ranta-Knuuttila, T., and Kivilaakso, E. (2001) Am. J. Physiol. 281 G1151–G1157 [DOI] [PubMed] [Google Scholar]
- 15.Chodniewicz, D., and Klemke, R. L. (2004) Exp. Cell Res. 301 31–37 [DOI] [PubMed] [Google Scholar]
- 16.Fitsialos, G., Chassot, A. A., Turchi, L., Dayem, M. A., LeBrigand, K., Moreilhon, C., Meneguzzi, G., Busca, R., Mari, B., Barbry, P., and Ponzio, G. (2007) J. Biol. Chem. 282 15090–15102 [DOI] [PubMed] [Google Scholar]
- 17.White, S. R., Tse, R., and Marroquin, B. A. (2005) Am. J. Respir. Cell Mol. Biol. 32 301–310 [DOI] [PubMed] [Google Scholar]
- 18.Liang, K. C., Lee, C. W., Lin, W. N., Lin, C. C., Wu, C. B., Luo, S. F., and Yang, C. M. (2007) J. Cell Physiol. 211 759–770 [DOI] [PubMed] [Google Scholar]
- 19.Gow, A. J., and Ischiropoulos, H. (2001) J. Cell Physiol. 187 277–282 [DOI] [PubMed] [Google Scholar]
- 20.Bogdan, C. (2001) Trends Cell Biol. 11 66–75 [DOI] [PubMed] [Google Scholar]
- 21.Hemish, J., Nakaya, N., Mittal, V., and Enikolopov, G. (2003) J. Biol. Chem. 278 42321–42329 [DOI] [PubMed] [Google Scholar]
- 22.Sandau, K. B., Zhou, J., Kietzmann, T., and Brune, B. (2001) J. Biol. Chem. 276 39805–39811 [DOI] [PubMed] [Google Scholar]
- 23.Hess, D. T., Matsumoto, A., Kim, S. O., Marshall, H. E., and Stamler, J. S. (2005) Nat. Rev. Mol. Cell Biol. 6 150–166 [DOI] [PubMed] [Google Scholar]
- 24.Kimura, H., Weisz, A., Kurashima, Y., Hashimoto, K., Ogura, T., D'Acquisto, F., Addeo, R., Makuuchi, M., and Esumi, H. (2000) Blood 95 189–197 [PubMed] [Google Scholar]
- 25.Semenza, G. L. (2000) J. Appl. Physiol. 88 1474–1480 [DOI] [PubMed] [Google Scholar]
- 26.Corley, K. M., Taylor, C. J., and Lilly, B. (2005) J. Cell Biochem. 96 971–985 [DOI] [PubMed] [Google Scholar]
- 27.Brune, B., and Zhou, J. (2007) Cardiovasc. Res. 75 275–282 [DOI] [PubMed] [Google Scholar]
- 28.Berchner-Pfannschmidt, U., Yamac, H., Trinidad, B., and Fandrey, J. (2007) J. Biol. Chem. 282 1788–1796 [DOI] [PubMed] [Google Scholar]
- 29.Li, F., Sonveaux, P., Rabbani, Z. N., Liu, S., Yan, B., Huang, Q., Vujaskovic, Z., Dewhirst, M. W., and Li, C. Y. (2007) Mol. Cell 26 63–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palmer, L. A., Doctor, A., Chhabra, P., Sheram, M. L., Laubach, V. E., Karlinsey, M. Z., Forbes, M. S., Macdonald, T., and Gaston, B. (2007) J. Clin. Investig. 117 2592–2601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cramer, T., Yamanishi, Y., Clausen, B. E., Forster, I., Pawlinski, R., Mackman, N., Haase, V. H., Jaenisch, R., Corr, M., Nizet, V., Firestein, G. S., Gerber, H. P., Ferrara, N., and Johnson, R. S. (2003) Cell 112 645–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee, K. S., Kim, S. R., Park, S. J., Park, H. S., Min, K. H., Jin, S. M., Lee, M. K., Kim, U. H., and Lee, Y. C. (2006) J. Allergy Clin. Immunol. 118 120–127 [DOI] [PubMed] [Google Scholar]
- 33.Polosukhin, V. V., Lawson, W. E., Milstone, A. P., Egunova, S. M., Kulipanov, A. G., Tchuvakin, S. G., Massion, P. P., and Blackwell, T. S. (2007) Virchows Arch. 451 793–803 [DOI] [PubMed] [Google Scholar]
- 34.Hellwig-Burgel, T., Rutkowski, K., Metzen, E., Fandrey, J., and Jelkmann, W. (1999) Blood 94 1561–1567 [PubMed] [Google Scholar]
- 35.Jung, Y. J., Isaacs, J. S., Lee, S., Trepel, J., and Neckers, L. (2003) FASEB J. 17 2115–2117 [DOI] [PubMed] [Google Scholar]
- 36.Zhou, J., Fandrey, J., Schumann, J., Tiegs, G., and Brune, B. (2003) Am. J. Physiol. 284 C439–C446 [DOI] [PubMed] [Google Scholar]
- 37.Frede, S., Stockmann, C., Freitag, P., and Fandrey, J. (2006) Biochem. J. 396 517–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kilani, M. M., Mohammed, K. A., Nasreen, N., Tepper, R. S., and Antony, V. B. (2004) Inflammation 28 245–251 [DOI] [PubMed] [Google Scholar]
- 39.Thomas, D. D., Espey, M. G., Ridnour, L. A., Hofseth, L. J., Mancardi, D., Harris, C. C., and Wink, D. A. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 8894–8899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferlito, M., Irani, K., Faraday, N., and Lowenstein, C. J. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 11689–11694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pervin, S., Singh, R., Freije, W. A., and Chaudhuri, G. (2003) Cancer Res. 63 8853–8860 [PubMed] [Google Scholar]
- 42.Ridnour, L. A., Isenberg, J. S., Espey, M. G., Thomas, D. D., Roberts, D. D., and Wink, D. A. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 13147–13152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reynaert, N. L., Ckless, K., Korn, S. H., Vos, N., Guala, A. S., Wouters, E. F., van der Vliet, A., and Janssen-Heininger, Y. M. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 8945–8950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kasuno, K., Takabuchi, S., Fukuda, K., Kizaka-Kondoh, S., Yodoi, J., Adachi, T., Semenza, G. L., and Hirota, K. (2004) J. Biol. Chem. 279 2550–2558 [DOI] [PubMed] [Google Scholar]
- 45.Mylonis, I., Chachami, G., Samiotaki, M., Panayotou, G., Paraskeva, E., Kalousi, A., Georgatsou, E., Bonanou, S., and Simos, G. (2006) J. Biol. Chem. 281 33095–33106 [DOI] [PubMed] [Google Scholar]
- 46.Richard, D. E., Berra, E., Gothie, E., Roux, D., and Pouyssegur, J. (1999) J. Biol. Chem. 274 32631–32637 [DOI] [PubMed] [Google Scholar]
- 47.Fujiwara, S., Nakagawa, K., Harada, H., Nagato, S., Furukawa, K., Teraoka, M., Seno, T., Oka, K., Iwata, S., and Ohnishi, T. (2007) Int. J. Oncol. 30 793–802 [PubMed] [Google Scholar]
- 48.Higgins, D. F., Kimura, K., Bernhardt, W. M., Shrimanker, N., Akai, Y., Hohenstein, B., Saito, Y., Johnson, R. S., Kretzler, M., Cohen, C. D., Eckardt, K. U., Iwano, M., and Haase, V. H. (2007) J. Clin. Investig. 117 3810–3820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wesley, U. V., Bove, P. F., Hristova, M., McCarthy, S., and van der Vliet, A. (2007) J. Biol. Chem. 282 3213–3220 [DOI] [PubMed] [Google Scholar]
- 50.Lazar, M. H., Christensen, P. J., Du, M., Yu, B., Subbotina, N. M., Hanson, K. E., Hansen, J. M., White, E. S., Simon, R. H., and Sisson, T. H. (2004) Am. J. Respir. Cell Mol. Biol. 31 672–678 [DOI] [PubMed] [Google Scholar]
- 51.Legrand, C., Polette, M., Tournier, J. M., de Bentzmann, S., Huet, E., Monteau, M., and Birembaut, P. (2001) Exp. Cell Res. 264 326–336 [DOI] [PubMed] [Google Scholar]
- 52.An, W. G., Kanekal, M., Simon, M. C., Maltepe, E., Blagosklonny, M. V., and Neckers, L. M. (1998) Nature 392 405–408 [DOI] [PubMed] [Google Scholar]
- 53.Suzuki, H., Tomida, A., and Tsuruo, T. (2001) Oncogene 20 5779–5788 [DOI] [PubMed] [Google Scholar]
- 54.Roger, L., Gadea, G., and Roux, P. (2006) Biol. Cell 98 141–152 [DOI] [PubMed] [Google Scholar]
- 55.Cetin, S., Leaphart, C. L., Li, J., Ischenko, I., Hayman, M., Upperman, J., Zamora, R., Watkins, S., Ford, H. R., Wang, J., and Hackam, D. J. (2007) Am. J. Physiol. 292 G1347–G1358 [DOI] [PubMed] [Google Scholar]
- 56.Shukla, A., Rasik, A. M., and Shankar, R. (1999) Mol. Cell Biochem. 200 27–33 [DOI] [PubMed] [Google Scholar]
- 57.Amadeu, T. P., and Costa, A. M. (2006) J. Cutan. Pathol. 33 465–473 [DOI] [PubMed] [Google Scholar]
- 58.Ridnour, L. A., Windhausen, A. N., Isenberg, J. S., Yeung, N., Thomas, D. D., Vitek, M. P., Roberts, D. D., and Wink, D. A. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 16898–16903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang, X., and Liu, Y. (2007) Cell Signal. 19 1372–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Legrand, C., Gilles, C., Zahm, J. M., Polette, M., Buisson, A. C., Kaplan, H., Birembaut, P., and Tournier, J. M. (1999) J. Cell Biol. 146 517–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Metzen, E., Zhou, J., Jelkmann, W., Fandrey, J., and Brune, B. (2003) Mol. Biol. Cell 14 3470–3481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lisy, K., and Peet, D. J. (2008) Cell Death Differ. 15 642–649 [DOI] [PubMed] [Google Scholar]
- 63.Weis, M., Schlichting, C. L., Engleman, E. G., and Cooke, J. P. (2002) Arterioscler. Thromb. Vasc. Biol. 22 1817–1823 [DOI] [PubMed] [Google Scholar]
- 64.Kim, S. J., Rabbani, Z. N., Dewhirst, M. W., Vujaskovic, Z., Vollmer, R. T., Schreiber, E. G., Oosterwijk, E., and Kelley, M. J. (2005) Lung Cancer 49 325–335 [DOI] [PubMed] [Google Scholar]
- 65.Chen, D., Li, M., Luo, J., and Gu, W. (2003) J. Biol. Chem. 278 13595–13598 [DOI] [PubMed] [Google Scholar]
- 66.Hofseth, L. J., Saito, S., Hussain, S. P., Espey, M. G., Miranda, K. M., Araki, Y., Jhappan, C., Higashimoto, Y., He, P., Linke, S. P., Quezado, M. M., Zurer, I., Rotter, V., Wink, D. A., Appella, E., and Harris, C. C. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 143–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu, C., Shi, Y., Du, Y., Ning, X., Liu, N., Huang, D., Liang, J., Xue, Y., and Fan, D. (2005) Exp. Cell Res. 309 410–418 [DOI] [PubMed] [Google Scholar]
- 68.Tzouvelekis, A., Harokopos, V., Paparountas, T., Oikonomou, N., Chatziioannou, A., Vilaras, G., Tsiambas, E., Karameris, A., Bouros, D., and Aidinis, V. (2007) Am. J. Respir. Crit. Care Med. 176 1108–1119 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





