Abstract
Type VII collagen (C7) is a major component of anchoring fibrils, structures that mediate epidermal-dermal adherence. Mutations in gene COL7A1 encoding for C7 cause dystrophic epidermolysis bullosa (DEB), a genetic mechano-bullous disease. The biological consequences of specific COL7A1 mutations and the molecular mechanisms leading to DEB clinical phenotypes are unknown. In an attempt to establish genotype-phenotype relationships, we generated four individual substitution mutations that have been associated with recessive DEB, G2049E, R2063W, G2569R, and G2575R, and purified the recombinant mutant proteins. All mutant proteins were synthesized and secreted as a 290-kDa mutant C7 α chain at levels similar to wild type C7. The G2569R and G2575R glycine substitution mutations resulted in mutant C7 with increased sensitivity to protease degradation and decreased ability to form trimers. Limited proteolytic digestion of mutant G2049E and R2063W proteins yielded aberrant fragments and a triple helix with reduced stability. These two mutations next to the 39-amino acid helical interruption hinge region caused local destabilization of the triple-helix that exposed an additional highly sensitive proteolytic site within the region of the mutation. Our functional studies demonstrated that C7 is a potent pro-motility matrix for skin human keratinocyte migration and that this activity resides within the triple helical domain. Furthermore, G2049E and R2063W mutations reduced the ability of C7 to support fibroblast adhesion and keratinocyte migration. We conclude that known recessive DEB C7 mutations perturb critical functions of the C7 molecule and likely contribute to the clinical phenotypes of DEB patients.
Type VII collagen (C7)3 resides within the basement membrane zone beneath stratified squamous epithelium (1, 2). C7 is a major component of anchoring fibrils, structures that attach the epidermal basement membrane of the skin to the underlying papillary dermis (3, 4). In hereditary dystrophic epidermolysis bullosa (DEB), anchoring fibrils are diminutive and/or reduced in number (5–7). DEB is caused by mutations in the COL7A1 gene encoding C7 (8–11).
C7 is a homotrimer composed of three identical α chains. Each α chain has a 145-kDa central collagenous triple-helical segment characterized by repeating Gly-X-Y amino acid sequences. The helical central domain is flanked by a large 145-kDa amino-terminal, noncollagenous domain (NC1) and a small 34-kDa carboxyl-terminal non-collagenous domain (NC2) (4, 12). Within the papillary dermis, C7 molecules form antiparallel, tail-to-tail dimers stabilized by disulfide bonding through a small carboxyl-terminal NC2 overlap between two C7 molecules. A portion of the NC2 domain is then proteolytically removed (13, 14). The antiparallel dimers then aggregate laterally to form anchoring fibrils which interact with microfibrils, collagen fibrils, and micro-thread-like fibers within the papillary dermis. These interactions are thought to secure the epidermis and its underlying basement membrane zone to the underlying papillary dermis.
We have expressed and purified large quantities of recombinant full-length C7 as well as various domains including the NC1 and NC2 domains in human 293 cells (15–17). Using these recombinant proteins, we carried out extensive structural and functional studies and showed that recombinant C7 is identical to authentic C7 purified from skin cells (15). These parameters include the ability to assemble into correctly folded helical trimers resistant to protease degradation, to bind to various extracellular matrix (ECM) components, and to support the matrix attachment of dermal fibroblasts. Our studies also demonstrated that NC1 interacts with various ECM components including fibronectin, laminin 5, type I collagen, and type IV collagen (16). Therefore, structural alterations in C7 may result in functional disruption of its interactions with ECM components, leading to the epidermal-dermal separation seen in DEB. We also showed that the NC2 domain and its adjacent triplehelical domain initiate the triple-helical assembly of C7 and catalyze the antiparallel dimer formation of the procollagen C7 (17).
It is important for the integrity of the basement membrane zone that anchoring fibrils have the correct structure. This is underscored by the occurrence of pathological changes within the dermal-epidermal junction caused by mutations in the COL7A1 gene encoding human C7. The full-length cDNA for C7 contains 8833 nucleotides encoding 2944 amino acids (8, 9). More than 300 distinct mutations, occurring within NC1, NC2, and the helical domains of COL7A1, have been identified in DEB patients (18–21).
DEB is a clinically heterogeneous disorder with different degrees of blistering, scarring, and extracutaneous involvement. It is inherited in either an autosomal dominant or recessive (RDEB) fashion (18). The phenotypical variability depends on the type of mutations in DEB alleles and their position within the gene. The identification of an increasing number of mutations has revealed some general genotype-phenotype correlations (22, 23). Dominant DEB mainly results from glycine substitution mutations within the collagenous domain of one COL7A1 allele. RDEB results from a combination of mutations such as premature stop codons, missense, and splice-site mutations on both alleles (24).
Gene defects identified in some RDEB patients were either homozygous for a missense mutation or were compound heterozygous for a premature stop codon and a missense mutation (25, 26). It has been suggested that these mutations result in the synthesis of a C7 polypeptide with decreased stability and/or altered function. Despite a growing number of known collagen mutations, however, the biological consequences of specific COL7A1 mutations and the molecular mechanisms leading to DEB clinical phenotypes are unknown.
In the current study, we used an efficient eukaryotic recombinant approach to engineer and generate four C7 mutant molecules (G2049E, R2063W, G2569R, and G2575R) in sufficient quantities for biological characterization and functional analysis. These four mutations are associated with severe, mutilating Hallopeau-Siemens type of recessive DEB and result in significant pathological changes in the number and morphology of anchoring fibrils (25–28). However, the molecular basis underlying these changes is unknown. We demonstrated that two mutations, G2049E and R2063W, next to the 39-amino acid helical interruption hinge region caused local destabilization of the triple helix and reduced the C7 ability to support cell adhesion and migration, whereas the other two G2569R and G2575R glycine substitution mutations interfered with molecular stability and triple helical assembly of C7.
EXPERIMENTAL PROCEDURES
Cell Culture—The human embryonic kidney cell line 293 (ATCC, Manassas, VA) was routinely cultured in Dulbecco's modified essential medium/Ham's F-12 (1:1) supplemented with 10% fetal bovine serum. Human fibroblasts from neonatal foreskin were initiated into culture as described previously (15). The cells were subcultured and passaged in Dulbecco's modified essential medium supplemented with 10% fetal bovine serum and used in cell attachment assays between passages 3–6.
Primary human keratinocytes were purchased from Cascade Biologics (Portland, OR) and cultured in low calcium, serum-free keratinocyte growth medium supplemented with bovine pituitary extract and epidermal growth factor (SFM; Invitrogen) as described by Boyce and Ham (29) and modified by O'Keefe and Chiu (30). Third- or fourth-passage keratinocytes were used for cell migration studies.
Site-directed Mutagenesis and Transfection—Site-directed mutagenesis was performed on C7 cDNA in the pRC/CMV vector using a commercial kit (QuikChange™ site-directed mutagenesis kit, Stratagene, Inc., La Jolla, CA) according to the manufacturer's instructions as described previously (15). Briefly, a pair of complementary primers with 39 bases was designed, and a mutation to change glycine to arginine (G2569R and G2575R), glycine to aspartic acid (G2049E), or arginine to tryptophan (R2063W) was placed in the middle of the primers. Parental cDNA inserted in pRC/CMV was amplified using Pyrococcus furiosus DNA polymerase with these primers for 16 cycles in a DNA thermal cycler (PerkinElmer Life Sciences). After digestion of the parental DNA with DpnI, the amplified DNA with nucleotide substitution incorporated was transformed into Escherichia coli (XL1-Blue). The mutations were confirmed by automated DNA sequencing.
The expression vector encoding for wild type or mutant C7 cDNA was used to transfect the human embryonal kidney cell line 293 (ATCC) using Lipofectin (Invitrogen) as previously described (18). Stable clones were selected using 500 μg of G418/ml (15).
Protein Purification and Analysis—For immunoblot analysis, clonal cell lines resistant to G418 were grown to confluence, after which the medium was changed to serum-free medium, and the cultures were maintained for an additional 24 h. The media were collected, equilibrated to 5 mm EDTA, 50 μm N-ethylmaleimide, and 50 μm phenylmethylsulfonyl fluoride, concentrated 10–15-fold (Centricon-100, Amicon, Beverly, MA), and subjected to 6% SDS-PAGE. Proteins were then electrotransferred onto a nitrocellulose membrane. The presence of recombinant C7 was detected with polyclonal antibodies to the NC1 or NC2 domains of C7 (31) followed by a horseradish peroxidase-conjugated goat anti-rabbit IgG and enhanced chemiluminescence detection reagent (Amersham Biosciences).
For large-scale purification of recombinant wild type or mutant C7, serum-free media were equilibrated to 5 mm EDTA, 50 μm phenylmethylsulfonyl fluoride, and 50 μm N-ethylmaleimide and precipitated with 300 mg/ml ammonium sulfate at 4 °C overnight with stirring (4, 15). Precipitated proteins were collected by centrifuging at 1.2 × 106 g/min for 1 h and then resuspended and dialyzed in buffer A (65 mm NaCl, 25 mm Tris-HCl, pH 7.8). After dialysis, insoluble material was collected by centrifugation at 8600 × g for 20 min, and the pellet was redissolved in buffer B (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 5 mm EDTA, 2 mm N-ethylmaleimide, 2 mm phenylmethylsulfonyl fluoride). The solution was clarified as above, and the supernatant, S1, was passed over a Q-Sepharose column (GE Healthcare) equilibrated in the same buffer. Elution was then carried out with a linear gradient from 0.2 to 1.0 m NaCl of appropriate volume size. The wild type C7 or mutant C7s eluted between 0.7 and 1 m NaCl (15).
Cell Adhesion Assay—Evaluation of cell attachment to ECM proteins was performed as previously described (32). Briefly, 96-well microtiter plates were coated with 100 μl of various ECM proteins (20 μg/ml) or purified recombinant NC1, wild type, and mutant C7 (20 μg/ml) in 20 mm carbonate buffer, pH 9.3, at 4 °C overnight and then blocked with phosphate-buffered saline containing 0.5 μg/ml heat-inactivated bovine serum albumin at 37 °C for 1 h. 2 × 104 fibroblasts suspended in Dulbecco's modified essential medium without serum were added to each well. The cells were allowed to attach for 1.5 h at 37 °C and, after removal of the unattached cells, washed and fixed with 70% ethanol for 10 min. Adherent cells were then stained for 15 min with 0.5% crystal violet and washed extensively with distilled water, solubilized in 100 μl of 1% SDS, and quantified by measuring the absorbance at 540 nm.
Cell Migration Assay—Keratinocyte migration was assayed by the method of Albrecht-Buehler (33), as modified by Woodley et al. (34). Briefly, colloidal gold salts were immobilized on coverslips and covered with type I collagen (30 μg/ml), type IV collagen (40 μg/ml), fibronectin (40 μg/ml), laminin 1 (80 μg/ml), and purified recombinant C7 (3–20 μg/ml). Keratinocyte cultures were suspended, plated on the coverslips, and allowed to migrate for 16–20 h. The cells were fixed in 0.1% formaldehyde in phosphate-buffered saline and examined under dark field optics with a video camera attached to a computer equipped with a frame grabber. The computer analyzes 15 nonoverlapping fields in each experimental condition with NIH Image 1.6 and determines the percentage area of each field consumed by cell migration tracks, a so-called migration index.
The methodology to determine statistical differences in migration indexes between experiments has previously been published (35). In brief, statistical analyses of the migration indexes between triplicate sets of experimental conditions were performed using Microsoft Excel. All data were expressed as the means ± S.E. of separate experiments. Differences between means were determined by Student's t test for unpaired samples. Only those at p < 0.05 were considered significant, and differences at p > 0.05 were insignificant.
Proteases Digestion—Purified wild type or mutant C7 was incubated with trypsin (Sigma) in 50 mm Tris-HCl, pH 7.4, 150 mm NaCl at 14 °C for 2 h or with pepsin in 0.1 m acetic acid at 4°C for 2 h at an enzyme-to-substrate ratio of 1:10 by weight as described previously (2, 15). The digestion products were then analyzed by SDS-PAGE followed by immunoblot analysis with a polyclonal antibody against the collagenous domain of C7.
RESULTS
Recombinant Production of C7 Mutants—Previous studies had identified four homozygous missense mutations (G2569R, G2575R, G2049E, and R2063W) within the triple helical domain of C7 in patients with recessive DEB (25–28). To investigate the molecular defects underlying these mutations, site-directed mutagenesis was used to generate the same mutation in C7 expression constructs (Fig. 1). We wished to compare wild type C7 and the four mutated C7 proteins for several essential biochemical and functional properties. As shown in the immunoblot analysis in Fig. 2A, transfection of these constructs into 293 cells resulted in the secretion of a 290-kDa mutant C7 at a similar level as the wild type (compare lanes 2 and 3–6). This protein was absent in the control vector-transfected 293 cells (Fig. 2A, lane 1). These recombinant C7 mutant proteins were then purified from serum-free culture medium of stably transfected 293 cells using our previously established procedure (15). Analysis of the purified mutant collagens by SDS-PAGE revealed the presence of a single band corresponding to the α chain of C7, indicating that the mutant collagens were secreted from cells as intact proteins (Fig. 2B). There was no significant difference in the protein yield (2–5 μg/ml) between wild type and mutant C7.
FIGURE 1.
Domain organization and cDNA construct for expression of C7 mutants. The 2944-amino acid sequence of C7 consists of a central triplehelical domain (TH) flanked by a large amino-terminal noncollagenous domain, NC1, and a smaller carboxyl-terminal noncollagenous domain, NC2. The triple-helical domain contains a non-collagenous 39-amino acid hinge region (HR) which is more sensitive to protease digestion. Four mutant C7 proteins (G2569R, G2575R, G2049E, and R2063W) generated by site-directed mutagenesis are shown in the schematic with the approximate positions of mutation indicated by the arrow. WT, wild type.
FIGURE 2.
Expression and purification of recombinant C7 mutant proteins. A, conditioned media from control vector cytomegalovirus (Con), wild type C7 (WT), or C7 mutant (G2569R, G2575R, G2049E, or R2063W)-transfected 293 cells were concentrated and subjected to 6% SDS-PAGE followed by immunoblot analysis with an affinity-purified polyclonal antibody to NC1 domain. The positions of the molecular weight marker and 290-kDa C7 are indicated. B, 6% SDS-PAGE and Coomassie Blue staining of wild type C7 (WT) and mutant C7 proteins purified from conditioned media of stably transfected 293 cells.
Characterization of Structural Properties of Recombinant C7 Mutants—We showed previously that C7 assembled to form S-S bonded trimers in vitro (15). Under nonreducing conditions, the G2569R and G2575R C7 proteins ran as a 290-kDa monomer and a 900-kDa trimer (Fig. 3, lanes 2 and 3). In contrast, wild type C7 as well as the two other mutant proteins (G2049E and R2063W) ran purely as 900-kDa trimers (lanes 1, 4, and 5). These data indicated that a glycine substitution at either 2569 or 2575 residues reduces the ability of C7 to form disulfide-bonded trimers.
FIGURE 3.
Formation of trimers by C7 mutants. 293 cells were transfected with either wild type C7 (WT) or with the indicated C7 mutant, and conditioned media were concentrated and subjected to 4–12% SDS-PAGE under non-reducing conditions followed by immunoblot analysis with a polyclonal antibody to the NC2 domain. The positions of molecular weight markers, monomer (M) and trimer (T), of C7 are indicated.
To further examine if these trimers are stable and have a proper triple helical conformation in their collagenous domain, purified wild type and mutant C7s were subjected to partial digestion with either trypsin or pepsin, and the digestion products were detected by immunoblot analysis. Fig. 4A is a schematic of C7 showing where protease (P) cleaves the molecule. Under these conditions, the noncollagenous NC1 and NC2 domain of native, wild type C7 was removed. The triple helical domain of wild type C7 resisted digestion, except for partial cleavage into P1 and P2 fragments (Fig. 4A) at the site of the hinge region in the center of the helical domain, as described by Burgeson (2). The antibody (α-TH) used for the immunoblot can only recognize the P1 fragment. As shown in Figs. 4, B and C, both pepsin and trypsin treatment of all 4 mutant C7s resulted in complete digestion of the TH fragment and produced a weak P1 fragment compared with wild type C7 (Fig. 4, B and C, compare lane 1 with lanes 2–5). Trypsin and pepsin digestion of the G2049E and R2063W mutant C7s also revealed an additional band below the P1 fragment.
FIGURE 4.
A schematic representation and characterization of the triplehelical domain of recombinant C7 mutants. A, a schematic diagram of the C7 α chain. The triple helical domain (TH) of C7 contains a 39-amino acid of non-helical interruption (hinge region). This site of C7 is sensitive to pepsin or trypsin digestion, and the helix can be cleaved into carboxyl-terminal P1 and amino-terminal P2 fragments. Each fragment represents approximately one-half of the TH domain. The protease cleavage sites (P) are indicated by arrows. The recognition region for a polyclonal antibody to the TH domain (α-TH) is also indicated. The antibody (α-TH) can only recognize the P1 fragment. B and C, purified wild type C7 (WT) or mutant C7 protein, as indicated, were treated with pepsin (B) and trypsin (C) and analyzed by 6% SDS-PAGE followed by immunoblot analysis with a polyclonal antibody to the triple-helical domain. The positions of molecular mass markers, the 200-kDa intact triple helical domain (TH), and the 120-kDa carboxyl-terminal half of the TH fragment (P1) are indicated.
The identities of P1 and the new band lower than P1 released by trypsin digestion (Fig. 4C) were determined by mass spectrometry analysis. We found that the amino terminus of the P1 fragment starts at amino acid residue 1953, which is exactly within the triple helical interruption “hinge” region (amino acid residues from 1940 to 1978). Furthermore, the amino terminus of the lower new band starts at residue 2159, which is 206 amino acids downstream from the P1 protease-sensitive site for wild type C7. These results demonstrated that the triple helical domain of all four mutant C7s is less stable against proteolysis than wild type C7. This suggests that a glycine or arginine substitution causes abnormal and incomplete folding of the C7 molecule.
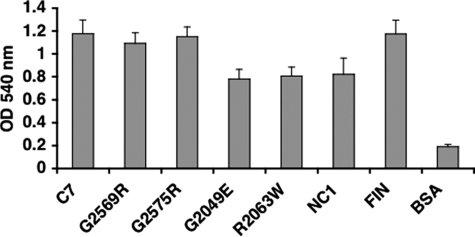
Cell Adhesion Activity of C7 Mutants—We previously demonstrated that wild type C7 promotes the adhesion of human dermal fibroblasts and identified two cell binding sites, one within the NC1 domain and the other within the triple helical domain (32). Therefore, we evaluated the ability of the four mutant C7s to support fibroblast adhesion. Purified recombinant wild type or mutant C7 and NC1 were immobilized in plastic wells and assayed for their ability to mediate the attachment of human fibroblasts (Fig. 5). As expected, recombinant wild type C7 as well as NC1 promoted vigorous attachment of human dermal fibroblasts. The G2569R and G2575R mutant C7s supported fibroblast attachment as well as wild type C7. In contrast, the G2049E and G2063W mutations generated C7s with reduced ability to support human fibroblast adhesion compared with wild type C7. They supported cell adhesion to levels similar to that generated by NC1 domain alone.
FIGURE 5.
Adhesion of human fibroblasts to C7 mutants. Human fibroblasts were plated onto wells coated with purified wild type C7 (C7), indicated mutant C7s, NC1, fibronectin (FIN), and bovine serum albumin (BSA) at a concentration of 20 μg/ml overnight at 4 °C. Attached cells were fixed and stained as described under “Experimental Procedures,” and the optical density at 540 nm was measured as an index of cell adhesion. These data represent the mean ± S.D. of triplicate determinations in one representative experiment. Similar results were obtained in two other independent experiments.
Migration Activity of Wild Type and Mutant C7s—Previous studies using supernatants from cultured keratinocytes expressing C7 suggested that C7 might promote keratinocyte migration (36). However, direct evidence that purified C7 alone promotes keratinocyte motility was lacking. Our recent studies showed that there was a dramatic enhancement of skin wound closure in both murine and human skin wounds when wild type C7 was delivered early to healing wounds (37). These data suggest that C7 may possess properties that enhance cell migration. To examine this possibility, we compared purified wild type C7 with other purified ECM molecules including type I and IV collagens and fibronectin (ECMs known to promote human keratinocyte motility) for their ability to support keratinocyte migration (Fig. 6). Fig. 6A shows representative microscopic fields of keratinocytes migrating on various purified ECMs. Consistent with previous studies, human keratinocytes were highly migratory on type I collagen, type IV collagen, and fibronectin but not on laminin 1 or no matrix. Purified recombinant C7 strongly promoted human keratinocyte migration to levels similar to that generated by type I collagen. The migration index values were 31.2 for C7, 27.22 for type I collagen, 21.22 for type IV collagen, 15.8 for fibronectin, 1.29 for laminin 1, and 1.69 without matrix. Purified recombinant C7 promoted cell migration in a dose-dependent and saturable manner (Fig. 7). Maximum levels of migration were obtained when the wells were coated with 15 μg/ml C7 with the migration index calculated to be 29.6. These data indicated that C7 is a potent promotility matrix for human keratinocytes.
FIGURE 6.
Migration of human keratinocytes on various ECMs. A, coverslips were coated with colloidal gold salts, then keratinocytes were plated on no matrix (NO), type I collagen (C1, 30 μg/ml), type IV collagen (C4, 40 μg/ml), fibronectin (FIN, 40 μg/ml), laminin 1 (L1, 80 μg/ml), or purified recombinant C7 (20 μg/ml) and incubated for 18 h. Representative fields were photographed at 40× under dark field optics. B, migration index expresses the percentage of the total field area consumed by the migration tracks. Error bars, S.E. of three different experiments.
FIGURE 7.
Dose-dependent migration of keratinocyte to C7. Coverslips were coated with colloidal gold, and keratinocytes were plated on the indicated concentrations of C7 and incubated for 18 h. Migration indices were determined by the percentage of the total field area consumed by the migration tracks. Error bars, S.E. of three different experiments.
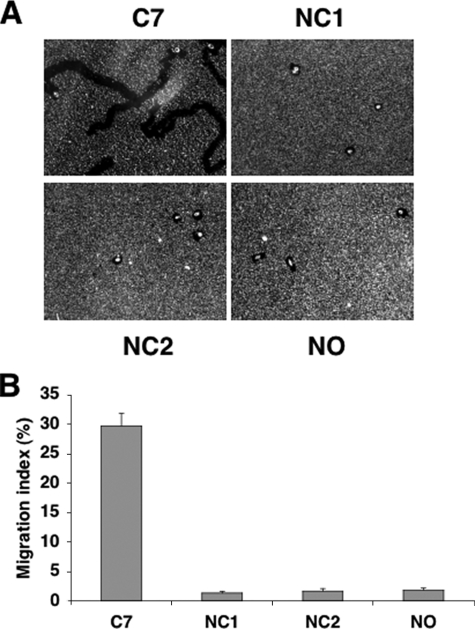
To further identify which domain of C7 is responsible for the C7 pro-motility activity, we subjected our purified noncollagenous domains NC1 and NC2 along with full-length C7 to the migration assays. As shown in Fig. 8, neither NC1 nor NC2 promoted cell migration. Their migration indices (1.34 and 1.68, respectively) were the same as no matrix (1.82). This is in contrast to full-length C7 which produced a migration index of 29.7. These results suggest that the pro-motility activity of C7 resides within the triple helical domain.
FIGURE 8.
Migration of human keratinocytes on various C7 domains. A, coverslips were coated with colloidal gold, and keratinocytes were plated on C7 (20 μg/ml), NC1 (40 μg/ml), NC2 (40 μg/ml), or no matrix (NO) and incubated for 18 h. Representative fields were photographed at 40× under dark field optics. B, migration index expresses the percentage of the total field area consumed by the migration tracks. Error bars, S.E. of three different experiments.
Because all four missense mutations generated in this study occurred within the triple helical domain, there is the possibility that these mutations may localize within the domain that is critical for C7 pro-motility activity. To determine whether any of these mutations had an effect on the ability of wild type C7 to support keratinocyte migration, we subjected wild type C7 and the four mutant C7s to the migration assays. As shown in Fig. 9, G2049E and R2063W mutant C7s had significantly reduced ability to promote keratinocyte migration, producing migration indices of 8.92 and 4.89, respectively. In contrast, G2569R and G2575R mutant C7s supported keratinocyte migration similar to that of wild type C7 with migration indices of 28.2 and 27.8, respectively.
FIGURE 9.
Migration of human keratinocytes on various C7 mutants. A, coverslips were coated with colloidal gold, and keratinocytes were plated on wild type C7 or purified mutant C7 proteins (20 μg/ml) and incubated for 18 h. Representative fields were photographed at 40× under dark field optics. NO, no matrix. B, migration index expresses the percentage of the total field area consumed by the migration tracks. Error bars, S.E. of three different experiments.
DISCUSSION
The efficient production and secretion of human wild type C7 and four RDEB mutant C7s was achieved using a eukaryotic expression vector in a human cell line that has no endogenous C7. The expression system used here allowed for the production of native, post-translationally modified C7 in quantities sufficient for comprehensive functional and structural studies and for the elucidation of the effects of human mutations on the structure and function of C7 by site-directed mutagenesis. By employing this approach, we generated four C7 mutant molecules (G2049E, R2063W, G2569R, and G2575R) in sufficient amounts and studied how single amino acid substitutions affect C7 homotrimer formation, folding, stability, cell attachment, and cell migration.
Homozygous glycine substitution mutations (G2569R or G2575R) are associated with severe RDEB (25, 26). Labeling of the dermal-epidermal junction in these patients revealed normal or near normal intensity of C7 staining compared with normal control skin, as expected for most missense mutations within the triple helical domain. However, immunoelectron microscopy showed substantially reduced numbers of anchoring fibrils with an altered wisp-like morphology (25, 26). In a previous study utilizing an optical biosensor to study intermolecular interactions, it was demonstrated that the G2575R substitution increased affinity between mutant molecules (38). It was hypothesized that the rapid rate of binding between the G2575R mutant molecules was due to the formation of an additional binding site within the mutated region, thereby causing abnormal aggregates.
The biological consequences of glycine substitutions in C7 can be numerous and perturb the formation and function of anchoring fibrils by different mechanisms. In general, the tight packing of collagen triple helices makes them relatively resistant to protease degradation. Glycine substitutions within the collagen tripeptide repeat can alter this conformation and, as a consequence, its function and/or stability. A local disruption in the triple helical structure can be detected by measuring the susceptibility of the collagen to proteolytic cleavage in vitro. In the present study, the G2569R or G2575R mutant C7s had an increased sensitivity to proteases and a decreased ability to form trimers. These mutations occur within a helical region of C7 consisting of 74 contiguous Gly-X-Y repeat sequences. It is interesting to note that these two mutations are only five amino acid residues apart and disrupt the repetitive Gly-X-Y sequence that is characteristic of collagens by replacing a glycine residue with a charged residue. The substitution of a glycine residue in this helical segment may lead to destabilization and local unraveling of the triple-helix. This destabilization is manifested as an increased sensitivity to protease digestion in vitro. Because of this local structural alteration, it is likely that peptide bonds near the G2569R or G2575R mutations are more susceptible to proteolysis by extracellular proteinases present at the epidermal basement membrane zone. Similarly, our previous studies disclosed another glycine substitution mutation, G2749R, that caused incomplete folding of recombinant C7 and rendered it sensitive to proteases (15). Our finding that G2569R or G2575R mutation leads to increased sensitivity to proteolysis and a corresponding decreased steady-state level of C7 in vivo may contribute to the markedly reduced number of normal anchoring fibrils in RDEB patients. Alternatively, the mutation-associated structural alteration could also have a direct negative impact on C7 function. In this regard it is interesting to note that the G2569R or G2575R mutations both occur within the domain that contains a C7 fibronectin binding site (39).
The G2569R or G2575R mutant C7s had reduced ability to form disulfide-bonded trimers. The reasons why a single glycine substitution can exert such an effect is unclear. However, this mutation occurs near the domain which initiates triplehelical assembly of C7 and directs antiparallel dimer formation (17). Therefore, it is possible that these amino acids are located within motifs required for triple-helical assembly of C7.
In a study by Hovnanian et al. (25), a RDEB family was described in which a gene defect in C7 was identified as a single amino acid substitution from arginine to tryptophan at residue R2063W within the triple-helical domain. Specifically, this substitution was in exon 73, in close vicinity to a non-collagenous 39-amino acid helical interruption, the so-called hinge region. Arginine is an aliphatic, hydrophilic, and positively charged amino acid present at the third position of a Gly-X-Y motif in the triple helical domain of C7. Tryptophan is a larger aromatic and hydrophobic amino acid absent in the collagenous domain. Because the 2063 arginine residue is conserved in human, mouse, and hamster C7, it likely lies in a critical functional region of C7 (28). Our data show that this mutation leads to reduced stability of the triple helix and an increased sensitivity to protease degradation. The Arg-2063 arginine lies in the middle of the collagenous domain, next to the unraveled hinge region. It is, therefore, conceivable that replacement of arginine with tryptophan significantly alters the conformation of the C7 molecule and induces more unraveling in the area nearby the hinge region, which makes it even more accessible to protease degradation. Consistent with this observation, protease digestion of the R2063W mutant C7 revealed an additional fragment, suggesting the mutation exposes an additional protease site.
The G2049E mutation was identified in a number of RDEB patients who were compound heterozygotes for this mutation and a nonsense mutation leading to a premature stop codon (26). These patients had reduced C7 staining at the dermal-epidermal junction and complete absence of anchoring fibrils. This glycine substitution also occurs within exon 73 close to the hinge region in the center of the triple helix. The segment encoded by exon 73 is a hot spot for glycine substitution mutations and contains amino acid residues as a part of a 35-triplet stretch of Gly-X-Y that is flanked by noncollagenous sequences of 39 and 6 amino acid residues (18). Therefore, it is possible that substitutions of glycine residues in this short, imperfect collagenous segment may lead to greater destabilization than glycine substitutions within a long uninterrupted collagenous segment. Consistent with this notion, our studies demonstrated that this mutation, similar to R2063W, also leads to destabilization of the triple helix and makes the mutant C7 more susceptible to protease digestion. It is interesting to note that four other C7 mutations, G2006D, G2034R, G2015E, and R2008G, within exon 73 next to the hinge region have also been shown to interfere with protein folding in a dominant negative manner and cause intracellular accumulation of the mutant molecules (15, 40). However, we did not detect any difference in the secretion of these two mutant proteins when compared with wild type C7.
We previously showed that recombinant full-length C7 is a better adhesive ligand than NC1 for human fibroblast attachment. We also demonstrated that a truncated type VII minicollagen, containing the intact noncollagenous NC1 and NC2 and half of the central helical collagenous domain, supported fibroblast attachment at levels similar to those of NC1 alone. These results indicate that the deleted 678 amino acids (residues 1920–2603) within the central collagenous domain contain the additional fibroblast binding site(s). Consistent with these data, we showed in the present study that G2049E and R2063W mutant C7s demonstrated reduced ability to support fibroblast adhesion. These data indicate that amino acids near the hinge region likely contain a critical domain for dermal fibroblast adhesion.
In previous studies we observed that the wounds of mice IV injected with gene-corrected RDEB fibroblasts overexpressing C7 demonstrated accelerated wound healing compared with control mice injected with uncorrected RDEB cells (37). Similarly, C7 delivered to the wound bed via intradermal or intravenous injection of the recombinant protein also promoted accelerated closure of skin wounds.4 Wound healing consists of various steps including clotting, inflammation, fibroplasia, angiogenesis, and the migration of human keratinocytes over the wound bed, so-called re-epithelialization. Our data suggest that C7 may promote keratinocyte migration. In our present studies we showed that recombinant C7 is a potent pro-motility matrix for human keratinocyte migration. The pro-motility activity of C7 was localized to the triple helical domain as purified recombinant noncollagenous domains, NC1 or NC2, failed to promote keratinocyte migration. Interestingly, two mutations, G2049E and R2063W, next to the 39-amino acid helical interruption hinge region, generated mutated C7s with significantly reduced ability to promote keratinocyte migration. Two other mutants, G2569R and G2575R, supported keratinocyte migration at levels similar to those of wild type C7. It is, therefore, possible that exon 73 may contain amino acid residues critical for the pro-motility function of C7.
Until now, we have already produced six RDEB missense mutant C7s and carried out extensive structure and function analysis. Table 1 summarizes the results from our past and present studies. The G2569R, G2575R, and G2749R glycine substitution mutations resulted in mutant C7s with increased sensitivity to protease degradation and decreased ability to form disulfide-bonded trimers. G2049E and R2063W mutations not only decreased C7 stability from protease digestion but also reduced the ability of C7 to support fibroblast matrix attachment and keratinocyte migration. Finally, the R2008G mutation caused an intracellular accumulation of C7. It appears that mutations in the same locus of C7 sequences alter the same C7 function.
TABLE 1.
Structural and functional properties of all the RDEB C7 mutants generated in our laboratory
ND, not determined; T, trimer; M, monomer.
| C7 mutants | Trimer formation | Protease stability | Cellular secretion | Cell binding | Cell migration |
|---|---|---|---|---|---|
| Wild type | T | Resistant | + | + | + |
| R2008G | T | Resistant | Reduced | Reduced | ND |
| R2049E | T | Sensitive | + | Reduced | Reduced |
| R2063W | T | Sensitive | + | Reduced | Reduced |
| G2569R | T + M | Sensitive | + | + | + |
| G2575R | T + M | Sensitive | + | + | + |
| G2749R | T + M | Sensitive | + | + | + |
The recombinant expression system combined with site-directed mutagenesis is a useful system for elucidating the effects of human mutations on the structure and function of C7. Using this approach, we demonstrated that C7 consists of domains that play critical roles in folding, molecular stability, cell attachment, and cell motility. These data demonstrate that specific mutations result in specific perturbations of C7 function that are translated into a clinical DEB phenotype.
Acknowledgments
We thank Tim Gallaher for technical support of mass spectrometry analyzes.
This work was supported, in whole or in part, by National Institutes of Health Grant Grants RO1 AR33625 (to D. T. W.) and RO1 AR47981 (M. C.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: C7, type VII collagen; NC1 and NC2, amino- and carboxyl-terminal noncollagenous domain, respectively, of type VII collagen; DEB, dystrophic epidermolysis bullosa; RDEB, recessive DEB; TH, triple helical; ECM, extracellular matrix; P, protease.
D. T. Woodley, Y. Hou, X. Y. Wang, W. Li, and M. Chen, unpublished observation.
References
- 1.Uitto, J., Chung-Honet, L. C., and Christiano, A. M. (1992) Exp. Dermatol. 1 2–11 [DOI] [PubMed] [Google Scholar]
- 2.Burgeson, R. E. (1993) J. Investig. Dermatol. 101 252–255 [DOI] [PubMed] [Google Scholar]
- 3.Keene, D. R., Sakai, L. Y., Lundstrum, G. P., Morris, N. P., and Burgeson, R. E. (1987) J. Cell Biol. 104 611–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lunstrum, G. P., Sakai, L. Y., Keene, D. R., Morris, N. P., and Burgeson, R. E. (1986) J. Biol. Chem. 261 9042–9048 [PubMed] [Google Scholar]
- 5.Uitto, J., and Christiano, A. M. (1993) Semin. Dermatol. 12 191–201 [PubMed] [Google Scholar]
- 6.Tidman, M. J., and Eady, R. A. J. (1985) J. Investig. Dermatol. 84 373–377 [DOI] [PubMed] [Google Scholar]
- 7.Lin, A. N., and Carter, D. M. (eds) (1992) Epidermolysis Bullosa: Basic and Clinical Aspects, Springer-Verlag, New York
- 8.Parente, M. G., Chung, L. C., Ryynanen, J., Woodley, D. T., Wynn, K. C., Bauer, E. A., Mattei, M.-G., Chu, M.-L, and Uitto, J. (1991) Proc. Natl. Acad. Sci. U. S. A. 88 6931–6935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christiano, A. M., Greenspan, D. S., Lee, S., and Uitto, J. (1994) J. Biol. Chem. 269 20256–20262 [PubMed] [Google Scholar]
- 10.Uitto, J., Bauer, E. A., and Moshell, A. N. (1992) J. Investig. Dermatol. 38 391–395 [DOI] [PubMed] [Google Scholar]
- 11.Hovnanian, A., Duquesnoy, P., Blanchet-Bardon, C., Knowlton, R. G., Amselm, S., Lathrop, M., Dubertret, L., Uitto, J., and Goosens, M. (1992) J. Clin. Investig. 90 1032–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lunstrum, G. P., Kuo, H.-J., Rosenbaum, L. M., Keene, D. R., Glanville, R. W., Sakai, L. Y., and Burgeson, R. E. (1987) J. Biol. Chem. 262 13706–13712 [PubMed] [Google Scholar]
- 13.Morris, N. P., Keene, D. R., Glanville, R. W., Bentz, H., and Burgeson, R. E. (1986) J. Biol. Chem. 261 5638–5644 [PubMed] [Google Scholar]
- 14.Bruckner-Tuderman, L., Nilssen, O., Zimmermann, D. R., Dours-Zimmermann, M. T., Ulrike-Kalinke, D., Gedde-Dahl, T., and Jan-Olof, W. (1995) J. Cell Biol. 131 551–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen, M., Costa, F. K., Lindvay, C. R., Han, Y.-P., and Woodley, D. T. (2002) J. Biol. Chem. 277 2118–2124 [DOI] [PubMed] [Google Scholar]
- 16.Chen, M., Marinkovich, M. P., Veis, A., O'Toole, E. A., Rao, C. N., Cai, X.-Y., and Woodley, D. T. (1997) J. Biol. Chem. 272 14516–14522 [DOI] [PubMed] [Google Scholar]
- 17.Chen, M., Keene, D. R., Costa, F. K., Tahk, S. H., and Woodley, D. T. (2001) J. Biol. Chem. 276 21649–21655 [DOI] [PubMed] [Google Scholar]
- 18.Varki, R., Sadowski, S., Uitto, J., and Pfendner, E. (2007) J. Med. Genet. 44 181–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uitto, J., and Christiano, A. M. (1994) Arch. Dermatol. Res. 287 16–22 [DOI] [PubMed] [Google Scholar]
- 20.Christiano, A. M., and Uitto, J. (1996) Curr. Opin. Dermatol. 3 225–232 [Google Scholar]
- 21.Uitto, J., Hovnanian, A., and Christiano, A. M. (1995) Proc. Assoc. Am. Physicians 107 245–252 [PubMed] [Google Scholar]
- 22.Jarvikallio, A., Pulkkinen, L., and Uitto, J. (1997) Hum. Mutat. 10 338–347 [DOI] [PubMed] [Google Scholar]
- 23.Pukkinen, L., and Uitto, J. (1999) Matrix Biol. 18 29–42 [DOI] [PubMed] [Google Scholar]
- 24.Christiano, A. M., Anhalt, G., Gibbons, S., Bauer, E. A., and Uitto, J. (1994) Genomics 21 160–168 [DOI] [PubMed] [Google Scholar]
- 25.Hovnanian, A., Rochat, A., Bodemer, C., Petit, E., Rivers, C. A., Prost, C., Fraitag, S., Christiano, A. M., Uitto, J., Lathrop, M., Barrandon, Y., and Prost, Y. (1997) Am. J. Hum. Genet. 61 599–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christiano, A. M., McGrath, J. A., Tan, K. C., and Uitto, J. (1996) Am. J. Hum. Genet. 58 671–681 [PMC free article] [PubMed] [Google Scholar]
- 27.Shimizu, H., McGrath, J. A., Christiano, A. M., Nishikawa, T., and Uitto, J. (1996) J. Investig. Dermatol. 96 119–124 [DOI] [PubMed] [Google Scholar]
- 28.Gardella, R., Castiglia, D., Posteraro, P., Bernardini, S., Zoppi, N., Paradisi, M., Tadini, G., Barlati, S., McGrath, J. A., Zambruno, G., and Colombi, M. (2002) J. Investig. Dermatol. 119 1456–1462 [DOI] [PubMed] [Google Scholar]
- 29.Boyce, S. T., and Ham, R. G. (1983) J. Investig. Dermatol. 81 (suppl.) 33–44 [DOI] [PubMed] [Google Scholar]
- 30.O'Keefe, E. J., and Chiu, M. L. (1988) J. Investig. Dermatol. 90 2–7 [DOI] [PubMed] [Google Scholar]
- 31.Chen, M., Petersen, M. J., Li, H.-L., Cai, X.-Y., O'Toole, E. A., and Woodley, D. T. (1997) J. Investig. Dermatol. 108 125–128 [DOI] [PubMed] [Google Scholar]
- 32.Chen, M., O'Toole, E. A., Li, Y.-Y., and Woodley, D. T. (1999) Exp. Cell Res. 249 231–239 [DOI] [PubMed] [Google Scholar]
- 33.Albrecht-Buehler, G. (1977) Cell 11 395–404 [DOI] [PubMed] [Google Scholar]
- 34.Woodley, D. T., Bachmann, P. M., and O'Keefe, E. J. (1988) J. Cell. Physiol. 136 140–146 [DOI] [PubMed] [Google Scholar]
- 35.Chen, J. D., Kim, J. P., Zhang, K., Sarret, Y., Wynn, K. C., Kramer, R. H., and Woodley, D. T. (1993) Exp. Cell Res. 209 216–223 [DOI] [PubMed] [Google Scholar]
- 36.Goto, M., Sawamura, D., Nishie, W., Sakai, K., McMilian, J. R., Akiyama, M., and Shimizu, H. (2006) J. Investig. Dermatol. 126 2614–2620 [DOI] [PubMed] [Google Scholar]
- 37.Woodley, D. T., Remington, J., Huang, Y., Hou, Y. P., Li, W., Keene, D. R., and Chen, M. (2007) Mol. Ther. 15 628–635 [DOI] [PubMed] [Google Scholar]
- 38.Brittingham, R., Colombo, M., Ito, H., Steplewski, A., Birk, D. E., Uitto, J., and Fertala, A. (2005) J. Biol. Chem. 280 191–198 [DOI] [PubMed] [Google Scholar]
- 39.Lapiere, J. C., Chen, J. D., Iwasaki, T., Hu, L.-R., Uitto, J., and Woodley, D. T. (1994) J. Investig. Dermatol. 94 637–641 [DOI] [PubMed] [Google Scholar]
- 40.Hammami-Hauasli, N., Schumann, H., Raghunath, M., Kilgus, O., Luthi, U., Luger, T., and Bruckner-Tuderman, L. (1998) J. Biol. Chem. 273 19228–19234 [DOI] [PubMed] [Google Scholar]