Abstract
G-protein-coupled receptors play a key step in cellular signal transduction cascades by transducing various extracellular signals via G-proteins. Rhodopsin is a prototypical G-protein-coupled receptor involved in the retinal visual signaling cascade. We determined the structure of squid rhodopsin at 3.7Å resolution, which transduces signals through the Gq protein to the phosphoinositol cascade. The structure showed seven transmembrane helices and an amphipathic helix H8 has similar geometry to structures from bovine rhodopsin, coupling to Gt, and humanβ2-adrenergic receptor, coupling to Gs. Notably, squid rhodopsin contains a well structured cytoplasmic region involved in the interaction with G-proteins, and this region is flexible or disordered in bovine rhodopsin and humanβ2-adrenergic receptor. The transmembrane helices 5 and 6 are longer and extrude into the cytoplasm. The distal C-terminal tail contains a short hydrophilic α-helix CH after the palmitoylated cysteine residues. The residues in the distal C-terminal tail interact with the neighboring residues in the second cytoplasmic loop, the extruded transmembrane helices 5 and 6, and the short helix H8. Additionally, the Tyr-111, Asn-87, and Asn-185 residues are located within hydrogen-bonding distances from the nitrogen atom of the Schiff base.
G-protein-coupled receptors (GPCRs)4 transduce a diverse class of extracellular signals such as photons, hormones, and glycoproteins to a small number of G-proteins (1, 2). Thousands of GPCRs mediate signals through a limited number of G-protein subtypes, such as Gs, Gi/o/t/z, or Gq/11, whose activation in cellular signaling pathways result in up- or down-regulation of cAMP or activation of the phosphoinositol cascade, respectively (2, 3). Several GPCR subtypes recognize the same intrinsic ligand such as adrenaline, histamine, or serotonin, and each subtype transduces the same extracellular stimulus to different G subtypes. Thus, the cytoplasmic portion of each GPCR should have characteristic regions that can discriminate between and activate the correct coupling G-protein. Using software based on hidden Markov models, such as 3D-Coffee and PRED-COUPLE, phylogenetic analyses can distinguish the G-protein specificity of each GPCR, and the G-protein subtype coupled to each GPCR can be classified (3, 4).
Despite intensive structural studies of GPCRs including rhodopsins from diverse organisms, there are only a few available atomic structures of the rhodopsin-type (class A) GPCRs (5–10). Bovine rhodopsin couples to transducin Gt, a pertussis toxin-sensitive G-protein in the Gi/o/t/z subtype, and down-regulates cGMP but not cAMP (31). Bovine rhodopsin was previously the only structure-determined GPCR at high resolution in the tetragonal and trigonal crystal forms (5, 6). Recently, the structures of the human β2-adrenergic receptor (β2AR) coupling to Gs were determined in a complex with a monoclonal antibody Fab fragment to the third cytoplasmic loop (CL3) and a chimeric form whose entire CL3 region was replaced by T4 lysozyme (7–9).
GPCRs have seven structurally conserved transmembrane helices (TH1–TH7) with various post-translational modifications including N-terminal acetylation, N-glycosylation, palmitoylation, and phosphorylation. Both the N-terminal and the C-terminal tails as well as the extracellular and cytoplasmic loop regions (EL1–EL3 and CL1–CL3, respectively) have low sequence homology. Although the extracellular ligand-binding regions are quite distinct, there is the same amphipathic short helix H8 in both bovine rhodopsin and β2AR (5, 8). The GPCR cytoplasmic region, including the C-terminal tail, should have a structure capable of simultaneously discriminating between G-protein subtypes and activating conserved G-proteins. Some activated GPCRs such as BLT1 can promiscuously activate more than two G subtypes, Gi and Gq (3, 10). Squid rhodopsin couples to bovine Gt as well as the intrinsic Gq (11, 12). Thus, structural information on novel GPCR recognition and coupling to G subtypes would be indispensable for elucidating the recognition and activation mechanisms for each G-protein.
Here, we report the unique crystal structure of Gq coupling rhodopsin from the Japanese flying squid at 3.7 Å resolution in an orthorhombic crystal, as well as confirmation of its chemical structure and post-translational modifications.
EXPERIMENTAL PROCEDURES
Purification of Squid Rhodopsin—All procedures were carried out at room temperature in the dark or under dim red light unless otherwise indicated. Squid rhodopsin was prepared from Todarodes pacificus caught in the Japan Sea, using previously described methods (13). Briefly, the rhabdomeric membranes were isolated from squid retina by repetitive sucrose flotation. The membranes were treated with V8 protease (Pierce, 50:1 w/w of rhodopsin:V8 protease) at room temperature for 1 h to remove the unique C-terminal proline-rich extension of the squid rhodopsin. The reaction was terminated by extensive washing with HEPES buffer (5 mm HEPES, pH 7.0, 1 mm EDTA, 1 mm dithiothreitol). The membranes were solubilized with 2% (w/v) dodecyl maltoside (DDM, Anatrace) for 1 h at 4 °C. After centrifugation, the supernatant was loaded onto a DEAE-cellulose column (Whatman) equilibrated with buffer A (50 mm HEPES, pH 7.0, 0.05% (w/v) DDM). The unbound fraction was collected and applied to a concanavalin A-Sepharose 4B column (Amersham Biosciences) equilibrated with buffer A. The rhodopsin was eluted with 0.2 m α-methyl mannoside solution. Fractions containing squid rhodopsin were pooled and dialyzed against buffer A and then concentrated by ultrafiltration (Amicon Ultra, Millipore).
N-terminal Sequencing and Mass Analyses—The identity and integrity of the purified protein were assessed by N-terminal amino acid sequencing by Edman degradation and various mass spectrometric analyses, including MALDI-TOF/MS, MALDI-TOF/TOF-MS/MS, nano-liquid chromatographyquadrapole TOF-MS/MS, and high performance liquid chromatography-electrospray ionization-iontrap-MS/MS as described in the supplemental materials (14).
Crystallization—Crystals were grown by the hanging-drop vapor diffusion method. One microliter of protein sample (10 mg/ml) in a solution of 10 mm HEPES, pH 7.0, 200 mm NaCl, 2 mm dodecyldimethylamine oxide, 0.03% (w/v) DDM was mixed with 1 μl of reservoir solution (0.1 m HEPES, pH 7.0, 8% (v/v) ethylene glycol, 28% (w/v) polyethylene glycol 400) and left to equilibrate at 20 °C. Crystals appeared after 5 days and stopped growing within 2 weeks.
Structure Determination and Refinement—X-ray diffraction data were collected at 100 K on beam line BL45XU at SPring-8. Data were reduced using the program HKL2000 (15). The structure was determined by molecular replacement with the program MOLREP in the CCP4 program suite (16) using a monomer of the trigonal crystal structure of the bovine rhodopsin (PDB code: 1GZM) (17) as a search model. Refinement and model building were performed iteratively with the programs CNS (18), REFMAC5 in CCP4 (16), and O (19). During refinement, we used grouped, unrestrained B-factor refinement with a single group for the entire molecule. All refinements were carried out with 10% of the reflections for cross validation. Despite the low resolution data and the low sequence homology between squid and bovine rhodopsins (24%), the structure was well refined thanks to the structural similarity of the transmembrane helices and the positions for a disulfide bridge, an 11-cis-retinal chromophore in the Schiff base linkage, and the conserved residues. B-factor sharpening was used to generate detailed maps using the CNS program with Bsharp values ranging from –50 to –150 Å2 (20). Data collection and refinement statistics are shown in Table 1. All figures including electrostatic potential surfaces were prepared using PyMOL (DeLano Scientific LLC). The coordinates have been deposited in the Protein Data Bank (PDB) with the accession code 2ZIY.
TABLE 1.
Data collection and refinement statistics
| Data collection | |
| Wavelength (Å) | 0.97950 |
| Resolution (Å) | 43.2-3.7 |
| Measured reflections | 43,571 |
| Unique reflections | 6,680 |
| Completeness (%)a | 93.3 (74.3) |
| Rmerge (%)b | 6.4 (77.5)c |
| Space group | C2221 |
| Unit cell (Å) | a = 84.3, b = 108.7, c = 142.2 |
| Refinement | |
| Resolution (Å) | 43.2-3.7 |
| Reflections used | 6,647 |
| Rwork/Rfree (%)d,e | 30.2/33.0 (41.4/43.2) |
| r.m.s.f deviation | |
| bond (Å) | 0.014 |
| angle (°) | 2.01 |
| Ramachandran statistics | |
| Most favored region (%) | 70.4 |
| Additional allowed region (%) | 27.1 |
| Generously allowed region (%) | 2.1 |
| Disallowed region (%) | 0.3 |
Values in parentheses are for the highest-resolution shell (3.83-3.70 Å).
Rmerge = ∑i|I(h)i — 〈I(h)〉|/∑i|I(h)i|, where 〈I(h)〉 is the mean intensity of equivalent reflections.
The last shell Rmerge is rather high as a result of strong anisotropy.
Rwork = ∑|Fo — Fc|/∑|Fo|, where Fo and Fc are the observed and calculated structure factor amplitudes, respectively.
Rfree = ∑|Fo — Fc|/∑|Fo|, calculated using a test data set, 10% of total data randomly selected from the observed reflections.
r.m.s., root mean square.
RESULTS AND DISCUSSION
Chemical Structure of Squid Rhodopsin—We first confirmed the chemical structure of V8 protease-treated squid rhodopsin. N-terminal sequencing and various MS analyses showed that the squid rhodopsin was cleaved at Glu-373 by the V8 protease, and the polypeptide chain was from Gly-2 to Glu-373 containing a Val to Ile substitution at position 18 with all the expected post-translational modifications. These included N-glycosylation and two palmitoylations, as well as a Schiff-based retinal. The molecular mass of squid rhodopsin was 43,842 Da (calculated molecular mass 43,883) by MALDI-TOF MS analysis (supplemental Fig. 1) (14, 21, 22).
Overall Structure—The overall structure of C-terminal truncated squid rhodopsin is a class A GPCR, with seven transmembrane helices, including the amphipathic short helix H8 and a prominent, well ordered cytoplasmic region (see Fig. 2A). All amino acid residues except the first 2 residues were defined in the structure, with a disulfide bridge between Cys-108 and Cys-186 in the extracellular region, the 11-cis-retinal connected to Lys-305 by the Schiff base, and the two palmitoyl thioesters of Cys-336 and Cys-337. We did not model the N-glycan of Asn-8 in the structure due to vague maps, although there were substantial electron densities in 2Fo – Fc and Fo – Fc maps where the glycan would be located (supplemental Fig. 2) (22).
FIGURE 2.
Crystal structure of squid rhodopsin. A, schematic model of squid rhodopsin with multicolored cylindrical helices. Transmembrane helices are indicated as TH1–TH7, and amphipathic short helix H8 is indicated as H8. 11-cis-Retinal at Lys-305 and palmitoylated cysteines Cys-336 and Cys-337 are indicated by the yellow sphere-and-stick models. A hydrophilic short helix in each N- and C-terminal tail is indicated as NH and CH, respectively. N and C termini are indicated by the letters N and C, and the cytoplasmic loop 3 is indicated as CL3. Putative transmembrane-spanning regions are indicated by yellow belts. B, superimposed schematic models and electrostatic surfaces of known GPCR structures. Superimposed schematic structures of squid and bovine rhodopsins, and β2AR are shown. Squid rhodopsin in this study is shown in orange, bovine rhodopsin in the trigonal crystal (1GZM) (17) is blue, and β2AR (2RH1) excluding the T4 lysozyme part of CL3 (8) is sky blue. The electrostatic surfaces of squid rhodopsin (Squ Rhod), bovine rhodopsin in the trigonal crystal (Bov Rhod), and β2AR (β2AR) are represented in blue (positive) to red (negative) with the squid rhodopsin structure in an orange schematic. To clarify the contribution of the distal C-terminal tail, the electrostatic surface of squid rhodopsin without the C-terminal tail after Glu-343 was also calculated (Squ C-trc). Different TH5 regions are indicated by the red line.
Squid rhodopsin has seven transmembrane helices (TH1–TH7) connected by three extracellular (EL1–EL3) and three cytoplasmic (CL1–CL3) loops with N- and C-terminal tails on the extracellular and cytoplasmic sides of the membrane (Fig. 2A). The transmembrane helices bend around the conserved proline or glycine residues, especially those with adjacent bulky aromatic residues (Fig. 1) (5, 8). The amphipathic H8 is located just after TH7 at the putative cytoplasmic surface of the membrane and is a conserved structure in rhodopsin-like GPCRs (5, 8, 23). The transmembrane helices of squid rhodopsin can be superimposed on those of bovine rhodopsin and human β2AR with the root mean square deviations of 1.5 and 2.2 Å, respectively, indicating that the transmembrane helical architecture of the three GPCRs are well conserved, despite many bends in the helices.
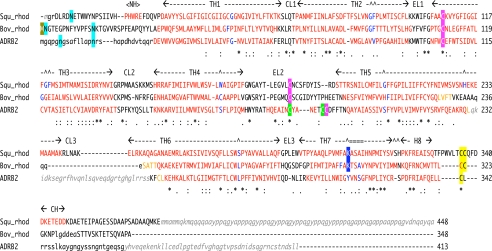
FIGURE 1.
Structural sequence alignment of squid rhodopsin, bovine rhodopsin and β2AR. The structural alignment was based on the 3D-Coffee alignment (4). Residues on helix regions are colored red, and residue(s) of helix bending are colored in blue. Transmembrane helical regions (TH1–TH7) and helix H8 with extracellular (EL1–EL3) and cytoplasmic (CL1–CL3) loops, and each helix in N- and C-terminal tails (NH and CH) are indicated. Posttranslational modifications are shaded by the following colors: cyan, N-glycosylation; a pair of pink or green, disulfide bridge(s); yellow, palmitoylated cysteine; blue, Schiff-based lysine with 11-cis-retinal; gold, N-terminal methionine acetylation. Residues indicated by small letters are not in models but in crystal protein samples, and residues indicated by small letters in italic gray do not exist in the crystal sample proteins due to expression processing, protease digestion, or protein engineering. Squ_rhod, squid rhodopsin (PDB code: 2ZIY in this study); Bov_rhod, bovine rhodopsin (1F88 (5) or 1GZM (17)); and ADRB2 (2RH1 (8)).
The conformation of the N- and C-terminal tails and the CL2, CL3, and EL3 loops differs significantly between bovine and squid rhodopsins, whereas the EL2 conformation is similar to bovine rhodopsin to accommodate the same 11-cis-retinal ligand found in visual chromophores. In the proximal C-terminal tail, squid rhodopsin has a 5-residue insertion between H8 and the palmitoylated cysteines, whereas bovine rhodopsin and β2AR have a palmitoylated cysteine immediately after H8 (Fig. 1) (6, 8).
In the large helix bundle cavity on the extracellular side, 11-cis-retinal bonds to Lys-305 with a putative protonated Schiff base, which is countered by the hydroxyl group of Tyr-111 as well as sandwiched by the amides of Asn-87 and Asn-185 at both sides of the retinal within hydrogen bond distances from the Schiff base nitrogen atom (21). The architecture surrounding the Schiff base is consistent with a smaller blue shift upon photoactivation than that of bovine rhodopsin, whose Schiff base is directly countered by the charged Glu-113 (5, 24).
The extended TH5 and TH6 and the cytoplasmic loops fold into the compact structure (Fig. 2), although squid rhodopsin has longer helices than bovine rhodopsin and β2AR on the cytoplasmic side (5, 8). Squid rhodopsin has a 12-amino-acid insertion in the CL3 region when compared with bovine rhodopsin (Fig. 1) (21). TH5 elongates to Lys-239 with a bend at His-230 located at the putative cytoplasmic surface of the membrane, in addition to a small bend at Gly-208. TH6 extends from Glu-245 with a bend at Ser-275 before Pro-276 and Tyr-277 (Fig. 2A). In the distal C-terminal tail, the residues from Asp-341 to Asp-347 form an additional short hydrophilic C-terminal helix CH. After helix CH, the C-terminal tail from Lys-348 to Glu-373 (which is the C terminus in the V8-treated sample) interacts closely with the extended TH5/TH6 region. The C-terminal region returns to the putative membrane surface in an extended structure via polar interactions with CL2 (Fig. 2). Therefore, the truncated residual polyproline region may align along the surface of the plasma membrane to form the ordered rhabdomeric structure in squid photoreceptor cells (21).
Interaction with G-protein—The cytoplasmic region is folded compactly in squid rhodopsin as described and is thought to interact with the coupling of Gq. The folded region consists of CL2, the helix bundle end of TH5 and TH6 extruded into the cytoplasm as the extended CL3 region, and H8 as well as the distal C-terminal tail including the CH helix. Except for the distal C-terminal tail, these regions interact directly with the respective G-protein in bovine rhodopsin, as shown by peptide competition studies (32). The electrostatic potentials of the cytoplasmic surfaces have a profile characteristic of intracellular GPCR domains (Fig. 2B). The distinct electrostatic profiles between these structures are located around the TH5 intracellular surface region of the putative plasma membrane. Interestingly, the corresponding TH5 region was important to Gi coupling but less so to Gq in BLT1 (28). Chemical cross-linking studies of the active form of bovine rhodopsin showed that the CL3 center residues are located close to the Gt-coupled site (29, 30). In the two different crystal forms of bovine rhodopsin, CL3 showed prominent, different conformations (5, 6, 17), whereas the CL3 region was disordered or substituted in both β2AR structures (7, 8). In the trigonal bovine rhodopsin crystal, CL3 adopts an extended helix-like conformation similar to that of squid rhodopsin (Fig. 2B) (17) but not in the tetragonal crystal due to hindrance in the crystal packing by contact with neighboring molecules (5, 6).
The compact hydrophilic intracellular domain of squid rhodopsin on the plasma membrane should participate in recognizing the G-protein during activation. Like other class A GPCRs (26, 27), squid rhodopsin with H8 anchored by the cysteine palmitoylations (Fig. 2A) effectively encounters the G-protein anchored by lipid modification(s) on the lateral membrane surface (25). However, the mechanism of G-protein activation by GPCR remains to be further elucidated.
Supplementary Material
Author's Choice—Final version full access.
The atomic coordinates and structure factors (code 2ZIY) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental text and two supplemental figures.
Footnotes
The abbreviations used are: GPCR, G-protein coupled receptor; PDB, Protein Data Bank; TH, transmembrane helix; CL, cytoplasmic loop; EL, extracellular loop; CH, C-terminal tail helix; NH, N-terminal tail helix; DDM, dodecyl maltoside; β2AR, β2-adrenergic receptor; MALDI-TOF, matrix-assisted laser desorption/ionization-time of flight; TOF/TOF, tandem time of flight; MS, mass spectrometry; MS/MS, tandem mass spectrometry.
References
- 1.Pierce, K. L., Premont, R. T., and Lefkowitz, R. J. (2002) Nat. Rev. Mol. Cell Biol. 3 639–650 [DOI] [PubMed] [Google Scholar]
- 2.Elefsinioti, A. L., Bagos, P. G., Spyropoulos, I. C., and Hamodrakas, S. J. (2004) BMC Bioinformatics 5 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sgourakis, N. G., Bagos, P. G., and Hamodrakas, S. J. (2005) Bioinformatics 21 4101–4106 [DOI] [PubMed] [Google Scholar]
- 4.Poirot, O., Suhre, K., Abergel, C., O'Toole, E., and Notredame, C. (2004) Nucleic Acids Res. 32 W37–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palczewski, K., Kumasaka, T., Hori., T., Behnke, C. A., Motoshima, H., Fox, B. A., Le Trong, I., Teller, D. C., Okada, T., Stenkamp, R. E., Yamamoto, M., and Miyano, M. (2000) Science 289 739–745 [DOI] [PubMed] [Google Scholar]
- 6.Okada, T., Sugihara, M., Bondar, A. N., Elstner, M., Entel, P., and Buss, V. (2004) J. Mol. Biol. 342 571–583 [DOI] [PubMed] [Google Scholar]
- 7.Rasmussen, S. G., Choi, H. J., Rosenbaum, D. M., Kobilka, T. S., Thian, F. S., Edwards, P. C., Burghammer, M., Ratnala, V. R., Sanishvili, R., Fischetti, R. F., Schertler, G. F., Weis, W. I., and Kobilka, B. K. (2007) Nature 450 383–387 [DOI] [PubMed] [Google Scholar]
- 8.Cherezov, V., Rosenbaum, D. M., Hanson, M. A., Rasmussen, S. G., Thian, F. S., Kobilka, T. S., Choi, H. J., Kuhn, P., Weis, W. I., Kobilka, B. K., and Stevens, R. C. (2007) Science 318 1258–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenbaum, D. M., Cherezov, V., Hanson, M. A., Rasmussen, S. G., Thian, F. S., Kobilka, T. S., Choi, H. J., Yao, X. J., Weis, W. I., Stevens, R. C., and Kobilka, B. K. (2007) Science 318 1266–1273 [DOI] [PubMed] [Google Scholar]
- 10.Murakami, M., Kitahara, R., Gotoh, T., and Kouyama, T. (2007) Acta Crystallogr. F Struct. Biol. Crystalliz. Comm. 63 475–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sail, H. R., and Michel-Villaz, M. (1984) Proc. Natl. Acad. Sci. U. S. A. 81 5111–5115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terakita, A., Yamashita, T., Tachibanaki, S., and Shichida, Y. (1998) FEBS Lett. 439 110–114 [DOI] [PubMed] [Google Scholar]
- 13.Kito, Y., Seki, T., and Hagins, F. M. (1982) Methods Enzymol. 81 44–48 [DOI] [PubMed] [Google Scholar]
- 14.Nakamura, T., Dohmae, N., and Takio, K. (2004) Proteomics 4 2558–2566 [DOI] [PubMed] [Google Scholar]
- 15.Otwinowski, Z., and Minor, W. (1997) Methods Enzymol. 276 307–326 [DOI] [PubMed] [Google Scholar]
- 16.Collaborative Computational Project, Number 4. (1994) Acta Crystallogr. Sect. D Biol. Crystallogr. 50 760–76315299374 [Google Scholar]
- 17.Li, J., Edwards, P. C., Burghammer, M., Villa, C., and Schertler, G. F. (2003) J. Mol. Biol. 343 1409–1438 [DOI] [PubMed] [Google Scholar]
- 18.Brünger, A. T., Adams, P. D., Clore, G. M., Gros, P., Grosse-Kunstleve, R. W., Jiang, J.-S., Kuszewski, J., Nilges, N., Pannu, N. S., Read, R. J., Rice, L. M., Simonson, T., and Warren, G. L. (1998) Acta Crystallogr. Sect. D Biol. Crystallogr. 54 905–921 [DOI] [PubMed] [Google Scholar]
- 19.Jones, T. A., Zou, J. Y., Cowan, S. W., and Kjeldgaard, M. (1991). Acta Crystallogr. Sect. A 47 110–119 [DOI] [PubMed] [Google Scholar]
- 20.Brünger, A. T. (2007) Nature Protocols 2 2728–2733 [DOI] [PubMed] [Google Scholar]
- 21.Hara-Nishimura, I., Kondo, M., Nishimura, M., Hara, R., and Hara, T. (1993) FEBS Lett. 317 5–11 [DOI] [PubMed] [Google Scholar]
- 22.Takahashi, N., Masuda, K., Hiraki, K., Yoshihara, K., Huang, H. H., Khoo, K. H., and Kato, K. (2003) Eur. J. Biochem. 270 2627–2632 [DOI] [PubMed] [Google Scholar]
- 23.Okuno, T., Yokomizo, T., Hori, T., Miyano, M., and Shimizu, T. (2005) J. Biol. Chem. 280 32049–32052 [DOI] [PubMed] [Google Scholar]
- 24.Terakita, A., Koyanagi, M., Tsukamoto, H., Yamashita, T., Miyata, T., and Shichida, Y. (2004) Nat. Struct. Mol. Biol. 11 284–289 [DOI] [PubMed] [Google Scholar]
- 25.Go, L., and Mitchell, J. (2003) Comp. Biochem. Physiol. B135 601–609 [Google Scholar]
- 26.Delos Santos, N. M., Gardner, L. A., White, S. W., and Bahouth, S. W. (2006) J. Biol. Chem. 281 12896–12907 [DOI] [PubMed] [Google Scholar]
- 27.Okuno, T., Ago, H., Terawaki, K., Miyano, M., Shimizu, T., and Yokomizo, T. (2003) J. Biol. Chem. 278 41500–41509 [DOI] [PubMed] [Google Scholar]
- 28.Kuniyeda, K., Okuno, T., Terawaki, K., Miyano, M., Yokomizo, T., and Shimizu, T. (2007), J. Biol. Chem. 282 3998–4006 [DOI] [PubMed] [Google Scholar]
- 29.Cai, K., Itoh, Y., and Khorana, H. G. (2001) Proc. Natl. Acad. Sci. U. S. A., 98 4877–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Itoh, Y., Cai, K., and Khorana, H. G. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 4883–4887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McLauhlin, S. K., McKinnon, P. J., and Margolskee, R. F. (1992) Nature 357 563–569 [DOI] [PubMed] [Google Scholar]
- 32.König, B., Arendt, A., McDowell, J. H., Kalert, M., Hargrave, P. A., and Hofmann, K. P. (1989) Proc. Natl. Acad. Sci. U. S. A. 86 6878–6882 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.