Abstract
Leptin, an adipose-derived hormone, has been implicated in several physiological processes involving the hippocampus. However, the role of leptin in adult hippocampal neurogenesis remains unknown. Here we show that leptin regulates neurogenesis in the dentate gyrus of adult mice as well as in cultured adult hippocampal progenitor cells. Chronic administration of leptin to adult mice increased cell proliferation without significant effects on the differentiation and the survival of newly proliferated cells in the dentate gyrus. The expression of the long form leptin receptor, LepRb, was detected in hippocampal progenitor cells by reverse transcription-PCR and immunohistochemistry. Leptin treatment also increased proliferation of cultured adult hippocampal progenitor cells. Analysis of signal transduction pathways revealed that leptin stimulated phosphorylation of Akt and STAT3 but not ERK1/2. Furthermore, pre-treating the cells with specific inhibitors of Akt or STAT3 attenuated leptin-induced cell proliferation in a dose-dependent manner. Taken together, our results support a role for leptin in adult hippocampal neurogenesis and suggest the involvement of the Akt and STAT3 signaling pathways in mediating the actions of leptin on neurogenesis.
The dentate gyrus of the hippocampus is one of the two brain regions where adult neurogenesis persists throughout life. Adult neurogenesis is regulated by physiological and pathological events and modulated by pharmacological manipulations at any of three primary stages: cell proliferation, differentiation, and survival. Neurogenesis in the dentate gyrus has been found to be negatively influenced by stress and is suppressed in various animal models of depression (1-3). Conversely, new neuron generation in the dentate gyrus is stimulated by treatment with antidepressants (4, 5). Also, neurotrophins, growth factors, and cytokines have been shown to be capable of modulating neurogenesis of the adult dentate gyrus (6-8).
Leptin is an adipocyte-derived cytokine encoded by the obese (ob) gene. It circulates as a 16-kDa peptide and is transported into the brain via a saturable transport system (9-11). Leptin signals in the brain by binding to the long form leptin receptor (LepRb),2 a Type I cytokine receptor, which results in Janus kinase 2 (Jak2)-mediated phosphorylation of two tyrosine residues (Tyr985 and Tyr1138) in the cytoplasmic tail of the receptor (12). Phosphorylated Tyr985 of LepRb recruits SH2-containing protein-tyrosine phosphatase-2, leading to activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase (ERK) pathway (13, 14). Phosphorylated Tyr1138 of LepRb activates signal transducer and activator of transcription 3 (STAT3), which dimerizes and is translocated to the nucleus where it acts as a transcription factor (12). Jak2 also phosphorylates insulin receptor substrate-1 and -2, resulting in activation of the phosphatidylinositol 3-kinase (PI3K)-Akt pathway (15, 16).
Leptin is well known for its role in the control of food intake and body weight, which is believed to be mediated via interaction with LepRb in the hypothalamus (17). Recent findings demonstrate that leptin facilitates spatial learning and memory (18-20) and produces antidepressant-like effects (21, 22). Adult neurogenesis has been proposed to mediate hippocampal-dependent learning and therapeutic actions of antidepressants (23-26). However, whether leptin regulates adult hippocampal neurogenesis remains unknown. In the present study, we investigated the impact of leptin on cell proliferation, differentiation, and survival in the dentate gyrus of adult mice. We also characterized the possible signaling mechanisms by which leptin exerts its effects on neurogenesis in cultured adult hippocampal stem/progenitor cells.
MATERIALS AND METHODS
Animals—Male C57BL/6J mice, weighing 20-25 g, were purchased from Jackson Laboratories (Bar Harbor, ME). Animals were housed in groups of five, and maintained on a 12-h light/dark cycle (lights on at 07:00 h) with ad libitum access to food and water. Animals were habituated to the housing conditions for 7-10 days prior to the beginning of the experimental procedures. All procedures were carried out in accordance with the National Institutes of Health Guide.
Drugs—Recombinant rat leptin (R&D Systems, Minneapolis, MN) was dissolved in sterile saline (for in vivo experiments) or in cell culture medium (for in vitro experiments). Akt inhibitor VIII (1,3-dihydro-1-(1-((4-(6-phenyl-1H-imidazo[4,5-g]quinoxalin-7-yl)phenyl)methyl)-4-piperidinyl)-2H-benzimidazol-2-one), an isozyme-selective Akt inhibitor specific for Akt1/2, and STAT3 inhibitor cucurbitacin II (27, 28) were dissolved in DMSO in a stock solution of 5 mg/ml (Calbiochem, La Jolla, CA).
Effect of Leptin on Cell Proliferation and Differentiation in the Dentate Gyrus of Adult Mice—Leptin at a dose of 1 mg/kg was used to study its effect on cell proliferation in the adult dentate gyrus of the hippocampus. This dose of leptin was chosen based upon its effectiveness in inducing antidepressant-like effects in rats (21) and mice (29). To study the time course effects of leptin, 36 mice were used. Animals received intraperitoneal injection of saline (n = 18) or leptin (1 mg/kg, n = 18), twice daily at the beginning and end of the light cycle. Body weight was measured daily during the period of treatment. Saline-treated and leptin-treated animals were assigned to three groups for 1, 5, or 14 days of treatment (n = 6/group). Subsequently, mice were injected with bromodeoxyuridine (BrdUrd), a thymidine analog that can be incorporated into the DNA during the S-phase of cell division, to label proliferating cells and determine the fate of newly proliferated cells. Animals received three intraperitoneal injections of BrdUrd in total (50 mg/kg body weight in 0.1 m PBS buffer, pH 7.2), spacing at 8-h intervals over 24 h. The time interval corresponds with the length of S-phase of cell division reported in mice (∼6-9 h) (30, 31). Animals were transcardially perfused 2 h after the last BrdUrd injection for immunohistochemical evaluation of BrdUrd-labeled cells.
To evaluate the effect of leptin on cell differentiation, ten mice received intraperitoneal injection with saline (n = 5) or leptin (1 mg/kg, n = 5) for 14 days followed by BrdUrd labeling as described above. To track the fate of newly proliferated cells, animals were sacrificed 28 days after the last BrdUrd injection.
Effect of Leptin on Cell Survival in the Adult Dentate Gyrus—To determine the effect of leptin on the survival of newly proliferated cells, two groups of mice (n = 6/group) were administered with BrdUrd as described above (3 × 50 mg/kg, 8-h intervals) before treatment. The two groups of mice were treated with either saline or leptin (1 mg/kg, intraperitoneal) for 14 days before transcardiac perfusion.
Immunohistochemistry Tissue Preparation—Animals were anesthetized with an intramuscular injection of rabbit ketamine cocktail (43 mg/kg ketamine, 9 mg/kg xylazine, and 1.4 mg/kg acepromazine in saline) and perfused through the ascending aorta using 0.1 m PBS followed by 4% paraformaldehyde (PFA) in PBS. The brain was removed and fixed overnight in 4% PFA, and then transferred to 30% sucrose in PBS. Brains were cut into 40-μm coronal sections on a cryostat and stored in cryoprotectant (30% sucrose, 30% ethylene glycol, 1% polyvinyl pyrrolidone, 0.05 m sodium phosphate buffer) until processing for immunohistochemistry.
BrdUrd Immunostaining—The brain sections from the same experiment were processed for BrdUrd immunostaining simultaneously to minimize staining variations. Because BrdUrd is incorporated into the DNA, the sections were first pretreated to denature DNA to make it assessable to BrdUrd antibody. Brain sections were incubated in a solution containing 50% formamide and 50% 2× SSC for 2 h at 65 °C. Then, the sections were briefly rinsed in PBS buffer, treated with 1% hydrogen peroxide for 10 min, and incubated in 2 n HCl solution with 0.3% Triton X-100 for 30 min followed by incubation with 0.1 m boric acid for 10 min. Subsequently, the sections were blocked in a solution containing 0.3% Triton X-100 and 3% normal goat serum in PBS for 1 h, and then incubated with mouse monoclonal anti-BrdUrd antibody (1:200, Roche Applied Science) in the blocking solution overnight at 4 °C. After rinsing in PBS buffer, the sections were then incubated with biotinylated goat anti-mouse antibody in blocking solution (1:400) for 1 h at room temperature, followed by incubation with avidin-biotin complex (1:100, Vector Laboratories, Burlingame, CA) for 1 h. BrdUrd immunoreactivity was revealed using 3′-diaminobenzidine (Vector Laboratories).
Double Fluorescence Immunolabeling—To determine the phenotypes of BrdUrd-labeled cells, brain sections were processed for BrdUrd, neuronal nuclei (NeuN), or glial fibrillary acidic protein (GFAP) fluorescent double labeling. Briefly, sections were first pretreated for DNA denaturation as described above, and then incubated with rat-anti-BrdUrd antibody (1:200, Accurate Chemical, Westburg, NY) and mouse anti-NeuN (1:400, Chemicon, Temecula, CA) or rabbit anti-GFAP (1:1000, Chemicon). After washing in PBS (0.1 m) buffer, sections were incubated for 4 h with fluorescent secondary antibodies: Alexa Fluor 488 goat anti-rat IgG to reveal immunoreactivity of BrdUrd and Alexa Fluor 546 goat anti-mouse IgG or Alexa Fluor 546 goat anti-rabbit IgG to reveal immunoreactivity of NeuN or GFAP, respectively (1:400 for all three antibodies, Molecular Probes, Eugene, Ontario, Canada). Then, the sections were washed in PBS and mounted onto coated glass slides, and coversliped with fluorescent mounting medium.
Cell Quantification and Analyses—The coronal tissue sections were mounted on polylysine-coated glass slides and were numerically coded, and stereological counts were performed by experimenters blinded to the experimental condition of each sample. The brain was sectioned at 40 μm, and every sixth section was spaced 240 μm apart throughout the entire rostral/caudal extent of the hippocampus (from Bregma -1.06 mm to Bregma -3.80 mm) was used to assess the number of BrdUrd-labeled cells within the subgranular zone along the superior and inferior blades of the dentate gyrus. This strategy makes certain that the same BrdUrd-labeled cell will not be counted twice on adjacent sections and guarantees that the area of the dentate gyrus counted for each animal is constant. Counting was accomplished at 400× magnification using an Olympus BX51 microscope equipped with a 40× objective lens. This allowed clear visualization of each cell and enabled us to distinguish single cells from clusters. Cells were counted throughout each section by focusing through each focal plane of the section to ensure that all BrdUrd-labeled cells were visible. The number of BrdUrd-labeled cells was multiplied by 6 to obtain the total number of cells per dentate gyrus.
Double labeled cells for BrdUrd and NeuN or GFAP were visualized and counted with a 60× oil immersion objective using a Zeiss 510 confocal and multiphoton microscope. The number of BrdUrd-labeled cells that expressed NeuN or GFAP was determined by counting a minimum of 35 BrdUrd-labeled cells on the sections spanning the entire dentate gyrus as described previously. Images were captured with a 60× oil immersion lens on an Olympus FV500 confocal microscope using Fluoview Imaging Software (Olympus, Tokyo, Japan). The extent of colocalization was validated by viewing cells on three planes (X, Y, and Z) using Z-plane sectioning. Cells single labeled for BrdUrd or double labeled for BrdUrd/NeuN or BrdUrd/GFAP were counted. The percentage of BrdUrd cells double labeled for NeuN or GFAP was calculated by dividing the number of double labeled cells by the total number of BrdUrd cells and multiplying 100.
Cell Culture—Adult rat hippocampal stem/progenitor cells (Chemicon) were grown in neural expansion media containing Dulbecco's modified Eagle's medium, and F-12 nutrient mixture, 1× B-27 supplement, 2 mm l-glutamine, 1× solution of penicillin and streptomycin, and FGF-2 (20 ng/ml) in 100-mm polystyrene tissue culture dishes or 8-well chamber slides coated with poly-l-ornithine (10 mg/ml) and mouse laminin (5 μg/ml). The cultured cells were maintained in a 37 °C humidified incubator with 5% CO2 and complete change of media containing fresh FGF-2 every other day and passaged once every 5-6 days.
Expression of LepRb in Adult Rat Hippocampal Stem/Progenitor Cells—To determine whether LepRb transcript is expressed in adult rat hippocampal stem/progenitor cells, reverse transcription PCR (RT-PCR) was performed. Briefly, adult hippocampal stem cells were collected, and total RNA was extracted. The extracted RNA was subsequently used to generate cDNA using SuperScript™ First Strand Synthesis System for RT-PCR. A 572-bp fragment corresponding to the C-terminal region of LepRb was amplified using the primers LepRb Forward (5′-GTGACCAGTGTAACAGTGCTAAC-3′) and LepRb Reverse (5′-GGGGCATGTAAGGTACAAAG-3′). The RT-PCR reaction conditions consisted of an initial denaturing stage at 94 °C for 4 min followed by 35 cycles at 94 °C for 30 s, 50 °C for 45 s, and 72 °C for 1 min. A final elongation step at 72 °C for 10 min was added to the end of the program. The PCR product was visualized by electrophoresis on a 1% agarose gel.
Immunocytochemistry was employed to detect protein expression of LepRb in adult hippocampal stem/progenitor cells. Cells were plated on glass coverslips in 24-well plates and grown in neural expansion media containing FGF-2. After 24 h the cells were fixed in 4% PFA, rinsed in PBS, and processed for immunocytochemistry for LepRb. Blocking solution (0.3% Triton X-100, 1% bovine serum albumin, and 3% normal goat serum in PBS) was added to each well for 1 h after which rabbit anti-LepRb (1:100, Linco, St. Charles, MO) and mouse anti-Nestin (1:1000, Chemicon) in blocking solution were added, and the mixture was incubated overnight at 4 °C. After rinsing in PBS buffer, the cells were then incubated with Alexa Fluor 546 goat anti-rabbit and Alexa Fluor 488 goat anti-mouse (1:400 for both antibodies, Molecular Probes, Eugene, OR) secondary antibody for 4 h. The coverslips were washed and mounted on glass slides with ProLong® Gold Antifade reagent containing 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen, Carlsbad, CA).
Effect of Leptin on Cell Proliferation and Differentiation in Adult Hippocampal Stem/Progenitor Cell Culture—Cell proliferation and differentiation experiments were carried out in 8-well chamber slides. For cell proliferation studies, each well of the chamber slide was seeded with 10,000 cells in 500 μl of neural expansion media containing FGF-2. After 24 h, the media was replaced with fresh media with or without leptin and incubated for 48 h. Then, BrdUrd (10 μm) was added 4 h before the cells were fixed in 4% PFA. Cell proliferation was determined by performing immunocytochemistry for BrdUrd and counting the number of BrdUrd-labeled cells in each well. For each well, eight random fields were chosen under the 20× objective, and the total number of BrdUrd-labeled cells was counted.
Cell differentiation experiments were carried out similarly with some modifications. Cells were seeded in 8-well culture slides with neural expansion media containing FGF-2. The media was replaced 24 h later with either fresh neural expansion media without FGF-2 or fresh neural expansion media without FGF-2 containing leptin and incubated for 48 h. Subsequently, the cells were incubated in media containing BrdUrd (10 μm) for 4 h. FGF-2 was removed from the media to allow differentiation of neural precursors. Cells were maintained with fresh changes of media every other day for 8 additional days and then fixed with 4% PFA. Neural differentiation was determined by performing double labeling immunocytochemistry for BrdUrd and the neuronal marker TuJ1 or the glial marker GFAP.
For BrdUrd detection, the fixed cells were incubated in 2 n HCl with 0.3% Triton X-100 for 30 min followed by 0.1 m boric acid (pH 8.0) for 10 min and three rinses with PBS prior to incubation blocking solution (0.3% Triton X-100, 1% bovine serum albumin, and 3% normal goat serum in PBS) for 1 h. Next, the cells were incubated with a mouse monoclonal anti-BrdUrd antibody (1:400) in the blocking solution overnight at 4 °C. After three PBS rinses, the Alexa Fluor 488 goat anti-mouse secondary antibody (1:400) was applied in blocking solution for 4 h at room temperature. The cells were rinsed in PBS three times and visualized using a fluorescent microscope. For double immunocytochemical detection with antibodies against BrdUrd, TuJ1, or GFAP, the procedure was performed as described for BrdUrd detection except two primary antibodies were added simultaneously for incubation overnight.
Effect of Leptin on Phosphorylation of Akt, STAT3, and ERK1/2 in Adult Hippocampal Stem/Progenitor Cells—Cells were plated on polyornithine and laminin-coated 100-mm polystyrene plates at a density of 1 × 106 cells per plate and grown in neural expansion media for 24 h. The media was replaced with neural expansion media without FGF-2 for 24 h after which fresh media containing 0, 0.3 nm, or 3.0 nm leptin was added. The cells were collected after 15 min, and total protein was extracted using lysis buffer (50 mm Hepes, pH 7.6, 1% Triton X-100, 150 mm NaCl, 20 mm sodium pyrophosphate, 20 mm β-glycerophosphate, 10 mm NaF) containing a mixture of phosphatase inhibitors (leupeptin, aprotinin, sodium orthovanadate, phenylmethylsulfonyl fluoride, Ser/Thr phosphatase inhibitor mixture, Tyr phosphatase inhibitor mixture). The total protein concentration was assessed by the Bradford assay, and 40 μg of total protein was run on an SDS-PAGE gel. The proteins were then transferred to a nitrocellulose membrane and blocked in a solution of 1% dry milk and 0.1% Tween 20 in 1× Tris-buffered saline and subsequently incubated in primary antibody (anti-Akt, anti-phosphorylated Akt on Thr308, anti-STAT3, anti-phosphorylated STAT3 on Tyr705, and anti-ERK1/2 and anti-phosphorylated ERK1/2 on Thr202/Tyr204) (all antibodies from Cell Signaling, 1:1000) in a solution of 1% bovine serum albumin and 0.1% Tween 20 in Tris-buffered saline overnight at 4 °C. After washing the membrane was incubated in secondary antibody conjugated to horseradish peroxidase (1:10,000) in blocking solution for 1 h. Next, the membrane was washed and the electrogenerated chemiluminescence (ECL) reaction solutions were added (solution 1: 0.1 m Tris-HCl, luminol, p-coumaric acid; solution 2: 0.1 m Tris-HCl, hydrogen peroxide).
Effect of Signal Transduction Inhibition on Actions of Leptin on Adult Hippocampal Stem/Progenitor Cell Proliferation—Adult hippocampal stem/progenitor cells were seeded in 8-well chamber slides as described for proliferation studies. Twenty-four hours after plating, the cells were incubated with fresh media containing various concentrations of Akt inhibitor VIII or STAT3 inhibitor cucurbitacin I for 4 h before the treatment with leptin or vehicle. Cells were grown for 48 h, and then BrdUrd was added, and the mixture was incubated for 4 h.
Statistical Analyses—The results were expressed as mean ± S.E. Statistical analyses were performed with a two-tailed Student's t test for cell proliferation, differentiation, and survival studies in vivo, one-factor ANOVA with repeated measures for body weight, and two-factor ANOVA for the in vitro studies. Student-Newman-Keuls post hoc comparisons followed the ANOVAs. p < 0.05 was considered statistically significant.
RESULTS
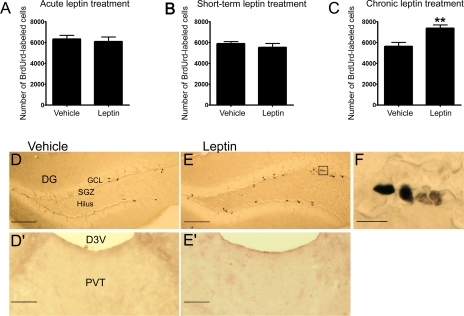
Effect of Leptin on New Cell Proliferation in the Dentate Gyrus of Adult Mice—The time course effect of leptin on adult hippocampal cell proliferation was determined in mice at a dose (1 mg/kg body weight) that has been shown to be effective in inducing antidepressant-like effects (21, 29). Cell proliferation was assessed by administration of BrdUrd at a relatively low dose (3 × 50 mg/kg over 24 h) to minimize toxic effects. This dose of BrdUrd was selected based on several previous studies that have demonstrated that repeated intraperitoneal injections of 50 mg/kg is sufficient to label proliferating cells in mice without having toxic effects on proliferating cells in the dentate gyrus (30, 35-37). BrdUrd-labeled cells, often found in clusters, were distributed in the inner layer of the granular cell layer and the hilus of the dentate gyrus (Fig. 1). The BrdUrd-labeled cells were counted in both subgranular cell layer and hilus of the dentate gyrus. The total number of BrdUrd-labeled cells in the dentate gyrus was not significantly affected by leptin treatment for 1 or 5 days as compared with their respective vehicle-treated control groups (Fig. 1). After 14 days of treatment, however, leptin significantly increased the number of BrdUrd-labeled cells in the dentate gyrus (Fig. 1, p < 0.01), indicating enhanced cell proliferation. No BrdUrd-positive cells were observed in the paraventricular thalamus, a brain area adjacent to the dentate gyrus, where neurogenesis has not been reported to occur (Fig. 1).
FIGURE 1.
Effect of leptin administration on cell proliferation in the dentate gyrus of adult mice. BrdUrd labeling was used to assess cell proliferation. Mice were injected intraperitoneally with leptin (1 mg/kg) or vehicle twice daily for 1, 5, or 14 days followed by BrdUrd labeling. The number of BrdUrd-labeled cells was counted in the subgranular zone and hilus of the dentate gyrus. A, acute treatment (1 day) with vehicle or leptin. B, short term treatment (5 days) with vehicle or leptin. C, chronic treatment (14 days) with vehicle or leptin. Images in D and E showing BrdUrd-labeled cells in the adult dentate gyrus of mice treated with vehicle (left panel) or leptin (right panel) for 14 days. Images in D′ and E′ showing BrdUrd-labeled cells in the paraventricular thalamus treated with vehicle (left panel) or leptin (right panel) for 14 days. The image in F indicates a high magnification view of proliferating cells. D3V, dorsal third ventricle; DG, dentate gyrus; GCL, granule cell layer; PVT, paraventricular thalamus; SGZ, subgranular zone. Scale bar = 100 μmin D and E, 50 μmin D′ and E′, and 10 μmin F. Data are expressed as the mean number of BrdUrd-labeled cells per dentate gyrus ± S.E. (n = 6 per group). **, p < 0.01.
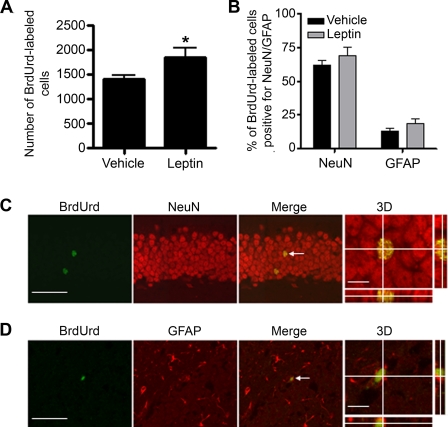
Effect of Leptin on Cell Differentiation and Survival in the Dentate Gyrus of Adult Mice—To determine if leptin treatment could affect the fate of newborn cells, BrdUrd-labeled cells were analyzed 28 days after BrdUrd administration in mice treated with leptin or vehicle. This length of time is known to be sufficient for newly proliferated cells to differentiate into their mature phenotypes (38). The total number of BrdUrd-labeled cells remained significantly higher in the leptin-treated group than the vehicle-treated group 28 days after BrdUrd administration (Fig. 2A, p < 0.05). The extent of differentiation of survived BrdUrd-labeled cells was determined by double labeling immunohistochemistry with antibodies against BrdUrd and the neuronal marker, NeuN, or the glial marker, GFAP (Fig. 2, B-D). Analysis of colocalization of BrdUrd with NeuN or GFAP using confocal microscopy indicated that the majority of BrdUrd-labeled cells possessed the neuronal phenotype, a low percentage of BrdUrd-labeled cells were labeled for GFAP (Fig. 2B). The percentages of BrdUrd-labeled cells that were double labeled for either NeuN or GFAP were not significantly altered by leptin treatment (p = 0.49 for NeuN, and p = 0.86 for GFAP).
FIGURE 2.
Effect of leptin administration on cell differentiation. Mice were injected with leptin (1 mg/kg) or vehicle twice daily for 14 days followed by BrdUrd labeling and were perfused 28 days later. A, a significantly higher number of BrdUrd-positive cells remained in the leptin-treated group than in the control group 28 days after BrdUrd labeling. B, quantitative analysis showing the effect of leptin on the percentage of BrdUrd-labeled cells double labeled for NeuN or GFAP. Results are expressed as mean ± S.E. (n = 5 per group). *, p < 0.05, compared with the vehicle-treated control group. C, confocal microscopic images showing colocalization of BrdUrd with NeuN. D, confocal microscopic images showing colocalization of BrdUrd with GFAP. White arrows indicating double labeled cells. Scale bars in C and D = 50 μm for the images showing BrdUrd and NeuN/GFAP and 10 μm for the three-dimensional (3D) images.
To determine the effect of leptin on the survival of newly proliferated cells, BrdUrd was administered to mice to label proliferating cells before leptin treatment began. The survival of newly proliferated cells was evaluated by quantifying BrdUrd-labeled cells after 14 days of leptin or vehicle treatment. There was no significant difference in the number of survived BrdUrd-labeled cells between vehicle (100 ± 18.9%)- and leptin (131 ± 28%)-treated groups (p = 0.89).
Effect of Leptin on Body Weight—Body weight of the mice was measured throughout the course of leptin treatment. Body weight gain was significantly decreased by leptin treatment from day 3 after the initiation of leptin treatment (F(1,130) = 8.742, p = 0.01), and this effect persisted until the end of the experiment (Fig. 3), suggesting that chronic intermittent administration of leptin did not develop tachyphylaxis to the actions of leptin.
FIGURE 3.
Effect of leptin administration on body weight. Body weight was measured for 14 days during the treatment with leptin (1 mg/kg, intraperitoneal). Data are expressed as mean ± S.E. **, p < 0.01; n = 6 per group.
Expression of LepRb in Adult Hippocampal Stem/Progenitor Cells—To determine whether adult hippocampal progenitor cells express LepRb, we examined LepRb gene and protein expression in cultured adult hippocampal stem/progenitor cells by RT-PCR and immunocytochemistry. A 572-bp band corresponding to the C-terminal region of the LepRb was detected by RT-PCR (Fig. 4A), indicating the presence of LepRb transcripts in adult hippocampal progenitor cells. The presence of LepRb in cultured hippocampal stem/progenitor cells was further confirmed by immunocytochemical staining with antibodies against LepRb and Nestin, an intermediate filament family member that has been used as a marker for progenitor cells (32-34) (Fig. 4B). The immunoreactivity of LepRb was observed in the cytoplasm. The nucleus showed punctuate LepRb immunoreactivity. The LepRb-positive cells were also labeled for Nestin (Fig. 4B). No immunostaining was seen when primary antibody was omitted. Taken together, these results indicate the presence of both mRNA and protein of LepRb in adult hippocampal progenitor cells.
FIGURE 4.
Expression of the long form leptin receptor (LepRb) in adult hippocampal stem/progenitor cells. A, RT-PCR using cDNA extracted from adult stem/hippocampal stem cells produced a 572-bp band corresponding to the C-terminal region of LepRb. B, confocal microscopic images showing that adult hippocampal stem/progenitor cells stained with Nestin (green) are positive for immunoreactivity of the leptin receptor (red). 4′,6-Diamidino-2-phenylindole staining (blue) revealing nuclei. Scale bar = 10 μm.
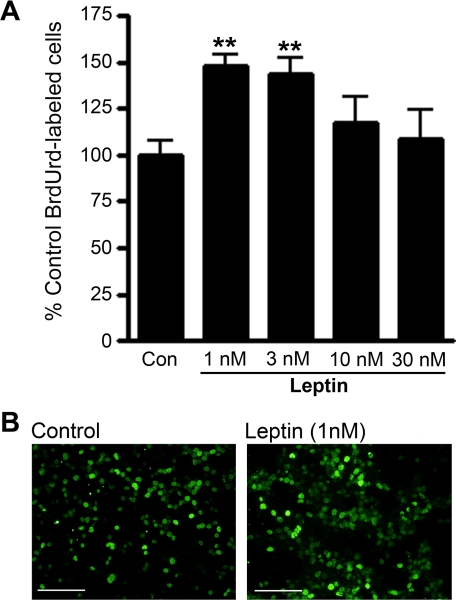
Effect of Leptin on Cell Proliferation and Differentiation in Adult Hippocampal Stem/Progenitor Culture—To determine whether leptin has a direct role in cell proliferation, cultured adult hippocampal stem/progenitor cells were incubated with different concentrations of leptin (1-30 nm) prior to BrdUrd labeling. Analyses of the number of BrdUrd-labeled cells revealed an inverted U shape of the dose-response relationship between leptin and cell proliferation (Fig. 5A). The maximal effect of leptin was observed with the dose of 1 nm (148.3 ± 5.4% relative to vehicle control). ANOVA indicated a significant effect of the treatment (F(4,18) = 4.5, p < 0.01). Post hoc analyses showed that the number of BrdUrd-labeled cells was significantly increased following 1 nm and 3 nm leptin, but higher concentrations of leptin at 10 nm or 30 nm elicited no significant effects. It is possible that leptin at high concentrations is cytotoxic or binds to other receptors nonspecifically, which may consequently counteract the actions of leptin mediated through LepRb.
FIGURE 5.
Effects of leptin treatment on proliferation of adult hippocampal stem/progenitor cells. Cells were treated with various concentrations of leptin (1-30 nm) for 48 h and labeled with BrdUrd (10 μm) in the last 4 h of incubation. A, quantitative analysis revealed that the number of BrdUrd-labeled cells was increased by leptin treatment at concentrations of 1 nm and 3 nm when compared with control. Data are expressed as mean ± S.E. **, p < 0.01. B, representative microscopic images showing BrdUrd-labeled adult hippocampal progenitor cells in control (left panel) and leptin-treated groups (right panel). Scale bar = 100 μm.
To determine whether leptin affects cell differentiation in adult hippocampal stem/progenitor cells, cells were grown for 8 days following BrdUrd labeling without FGF-2 in the media to allow differentiation. Leptin treatment showed no significant effect on the percentages of BrdUrd-labeled cells that were positive for neuronal marker TuJ1 or the glial marker GFAP (Fig. 6). These in vitro findings are consistent with the results from in vivo studies showing the effects of leptin on cell proliferation and differentiation in the dentate gyrus of mice (as shown in Figs. 1 and 2).
FIGURE 6.
Effect of leptin treatment on differentiation of cultured adult hippocampal stem/progenitor cells. Cells that were treated with leptin (1 nm) for 48 h and labeled with BrdUrd (10 μm) in the last 4 h of incubation were allowed to differentiate for 8 days before fixation for immunohistochemical processing. A, representative microscopic images showing that BrdUrd-labeled cells differentiated into neuronal (TuJ1-positive in red) or glial (GFAP-positive in green) cells. B, quantitative analysis indicating that the percentage of BrdUrd-labeled cells that were positive for TuJ1 or GFAP was not altered by leptin treatment. Data are expressed as mean ± S.E. Scale bar = 10 μm.
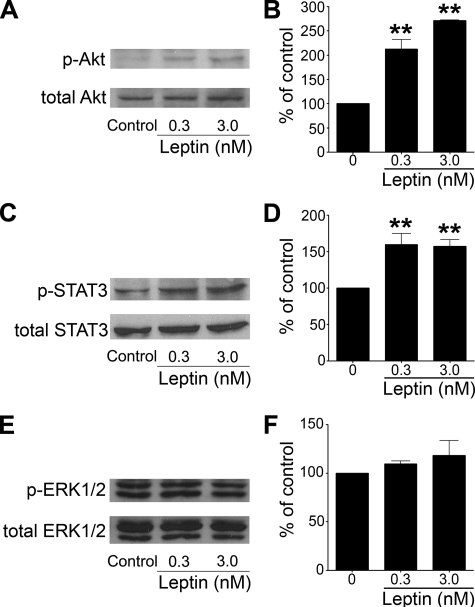
Activation of Intracellular Signal Transduction Pathways by Leptin in Adult Hippocampal Stem/Progenitor Culture—Leptin has been demonstrated to activate multiple signal transduction pathways, which requires phosphorylation. To determine the signaling pathways that can be activated by leptin in adult hippocampal stem/progenitor cells, phosphorylation of Akt on Thr308, STAT3 on Tyr705, and ERK1/2 on Thr202/Tyr204 was evaluated by Western blot following leptin treatment at different doses. Basal phosphorylation of Akt in cultured hippocampal progenitor cells was low. Leptin treatment for 15 min dose-dependently elevated the level of phosphorylated Akt (Fig. 7, A and B). STAT3 showed a moderate level of basal phosphorylation in cultured hippocampal progenitor cells, and leptin treatment for 15 min further increased STAT3 phosphorylation (Fig. 7, C and D). In contrast, phosphorylated ERK1/2 was not significantly altered after leptin treatment at either dose (Fig. 7, E and F). These results suggest the PI3K/Akt and Jak2/STAT3 signaling pathways are recruited by leptin in adult hippocampal progenitor cells.
FIGURE 7.
Effect of leptin on phosphorylation of Akt, STAT3, and ERK1/2 in cultured adult hippocampal stem/progenitor cells. Cells were stimulated with leptin (0.3 nm and 3 nm) or vehicle for 15 min. The phosphorylation of Akt, STAT3, and ERK1/2 was detected using phospho-specific antibodies. A, representative immunoblot showing phosphorylated Akt and total Akt. B, the ratio of densitometric measurements of phospho-Akt and total Akt expressed as percent of control. C, representative immunoblot showing phosphorylated STAT3 and total STAT3. D, the ratio of densitometric measurements of phospho-STAT3 and total STAT3 expressed as percent of control. E, representative immunoblot showing phosphorylated ERK1/2 and total ERK1/2. F, the ratio of densitometric measurements of phospho-ERK1/2 and total ERK1/2 expressed as percent control. **, p < 0.01 as compared with control. The data are representative of two individual experiments.
Effect of Blockade of PI3K/Akt and Jak2/STAT3 Signaling Pathways on Leptin-stimulated Adult Hippocampal Cell Proliferation—To further determine whether activation of the PI3K/Akt signaling pathway mediates the action of leptin on cell proliferation, adult hippocampal stem/progenitor cells were exposed to different concentrations of the Akt inhibitor VIII followed by leptin treatment. The pretreatment with the Akt inhibitor VIII significantly reduced the number of BrdUrd-labeled cells in a dose-dependent manner when compared with the leptin-treated group (F(4,15) = 6.001; p < 0.01) (Fig. 8). Treating the cells with different concentrations of STAT3 inhibitor cucurbitacin I also reduced the number of BrdUrd-labeled cells when compared with leptin-treated cells as well as the control group (F(4,13) = 25.203; p < 0.0001) (Fig. 9). These results suggest that both the PI3K/Akt and the Jak2/STAT3 pathways are involved in the stimulatory effect of leptin on proliferation of neural progenitor cells.
FIGURE 8.
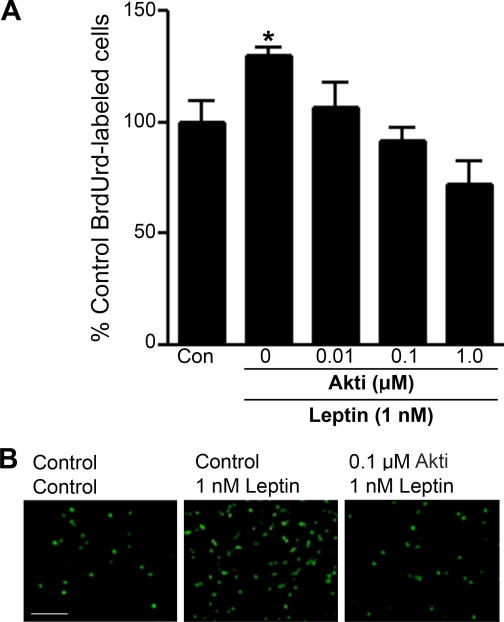
Effect of inhibition of Akt on leptin-induced cell proliferation. Cultured adult hippocampal stem/progenitor cells were incubated with various concentrations of Akt inhibitor VIII (0.01-1.0 μm) for 4 h followed by treatment with leptin (1 nm) for 48 h and labeling with BrdUrd (10 μm) during the last 4 h of incubation. A, quantitative analysis revealed that pretreatment with Akt inhibitor VIII attenuates the leptin-induced increase in the number of BrdUrd-labeled cells in a dose-dependent manner. B, representative microscopic images of BrdUrd-labeled cells from three treatment conditions: control-control (left), control-leptin (center), and Akt inhibitor VIII-leptin (right). *, p < 0.05; scale bar = 100 μm.
FIGURE 9.
Effect of inhibition of STAT3 on leptin-induced cell proliferation. Cultured adult hippocampal stem/progenitor cells were incubated with various concentrations of cucurbitacin I (0.1-10 nm) for 4 h followed by treatment with leptin (1 nm) for 48 h and labeling with BrdUrd (10 μm) during the last 4 h of incubation. A, quantitative analysis revealed that pretreatment with cucurbitacin I attenuated the leptin-induced increase in the number of BrdUrd-labeled cells. B, representative microscopic images of BrdUrd-labeled cells from three treatment conditions: control-control (left), control-leptin (center), and cucurbitacin I-leptin (right). *, p < 0.05; scale bar = 100 μm.
DISCUSSION
In the present study, we have demonstrated that leptin increases adult hippocampal neurogenesis both in vitro and in vivo. The leptin-stimulated neurogenesis mainly resulted from increased cell proliferation, because leptin showed no significant effect on cell differentiation and survival. Leptin signaling recruited the PI3K/Akt and Jak2/STAT3 pathways in adult hippocampal stem/progenitor cells, and inhibition of these signal transduction pathways led to the attenuation of the actions of leptin on proliferation of adult hippocampal progenitor cells, suggesting that a mechanism dependent on Akt and STAT3 activation mediates leptin action.
Cell proliferation in the dentate gyrus was increased by chronic but not short term or acute administration of leptin, as indicated by the differential changes in total number of BrdUrd-labeled cells after 1, 5, or 14 days of leptin treatment. This temporal profile of the actions of leptin on cell proliferation is consistent with the findings with chronic treatment using different classes of existing antidepressants (26, 39, 40). Interestingly, we observed a tendency toward a decrease in the number of BrdUrd-positive cells in the dentate gyrus over treatment time in control animals. A similar finding was also shown in the study by Nakagawa et al. (40), in which the number of BrdUrd-positive cells in the dentate gyrus in control animals appeared to be reduced after chronic (14 days) vehicle injection in comparison with acute (1 day) injection. It is possible that the repeated injection procedure is stressful to the animals, which could cause suppression of cell proliferation. In support of this, stress hormones and stress have been demonstrated to decrease cell proliferation in the dentate gyrus (41-44).
Although many newly proliferated cells in the dentate gyrus die shortly after birth, some of them survive and differentiate (38). Confocal microscopic analysis demonstrated that the majority of newly proliferated cells in the dentate gyrus differentiate into neurons, and a small percentage of new cells differentiate into glia 28 days after BrdUrd labeling. However, the percentage of neurons and glial cells out of the total BrdUrd-labeled cells did not differ between leptin-treated and vehicle-treated groups, indicating that differentiation of new cells in the dentate gyrus was not influenced by leptin treatment. Another stage of neurogenesis is survival of newborn cells. Recently, it has been reported that chronic intracerebroventricular infusion of leptin protects hippocampal neurons in the CA1 and CA3 from cell death induced by neuronal insults (45). To determine whether leptin affects the survival of newly proliferated cells in the dentate gyrus, BrdUrd was administered to animals prior to the onset of 2 weeks of leptin injections. Leptin exhibited no significant effect on the survival of newly proliferated cells in the dentate gyrus. The differential effects of leptin on survival of mature neurons in CA1 and CA3 versus newborn neurons in the dentate gyrus of the hippocampus may imply that the actions of leptin on cell survival may be dependent on the developmental status of the neuron.
Leptin is known to suppress appetite, increase energy expenditure, and reduce body weight gain (46-48). Given that dietary restriction and physical activity have been shown to enhance hippocampal neurogenesis (49-52), one possibility is that the effects of leptin on neurogenesis could be secondary to the changes in energy homeostasis. If this is the case, a temporal correlation between weight loss and neurogenic activity would be predicted. However, body weight gain was seen to be decreased without significant effect on hippocampal neurogenesis after 5 days of leptin treatment. Also, dietary restriction promotes neurogenesis by increasing the survival of newly generated neuronal cells rather than increasing the proliferation (49, 50), which is in contrast to the stimulatory effect of leptin on cell proliferation. Moreover, it has been demonstrated that locomotor activity was not altered by leptin following either acute or chronic administration (21, 53). These findings support that the effect of leptin on neurogenesis is dissociated from its impact on energy homeostasis. Nonetheless, the possibility of a contribution of leptin-induced negative energy balance following chronic administration (14 days) to its neurogenic effect in vivo cannot be ruled out completely.
Our in vivo results from cell proliferation, differentiation, and survival studies in mice suggest that leptin-stimulated hippocampal neurogenesis is likely to be the consequence of increasing cell proliferation rather than modifying the fate of cells after they are generated. A similar neuroproliferative effect without changing cell differentiation was observed in cultured adult hippocampal progenitor cells. The detection of mRNA and protein of LepRb in these cells suggests that leptin may regulate neurogenesis via direct interaction with LepRb on hippocampal progenitor cells. Activation of LepRb has been reported to stimulate multiple intracellular signal transduction pathways, including PI3K-Akt, Jak2-STAT3, and ERK1/2 signaling pathways (54). To determine the signaling mechanisms responsible for the neuroproliferative effect of leptin, activation of the PI3K-Akt, Jak2-STAT3, and ERK1/2 pathways were examined by assessing phosphorylation of Akt, STAT3, and ERK1/2 in hippocampal stem/progenitor cell culture. We found that leptin dose-dependently increased phosphorylation of Akt and STAT3 but not ERK1/2. Based upon these results, we hypothesized that the PI3K-Akt and Jak2-STAT3 signaling pathways may mediate leptin-induced cell proliferation. Consistent with this, inhibition of Akt activity attenuated leptin-induced cell proliferation. Our results are in agreement with previous reports that PI3K-Akt transduces intracellular signals that control adult hippocampal neural progenitor cell proliferation (55, 56). Overexpression of Akt increases cell proliferation, whereas expression of a dominant negative Akt inhibits cell proliferation (55, 56). A question that remains to be addressed is how Akt mediates the actions of leptin on neurogenesis. It has been shown that Akt inhibits glycogen synthase kinase-3β activation by inducing its phosphorylation (57). Glycogen synthase kinase-3β is known to negatively regulate Wnt/β-catenin signaling via phosphorylating β-catenin, which mediates β-catenin degradation (58). Both in vitro and in vivo studies have recently identified Wnt/β-catenin signaling as a important pathway in adult hippocampal neurogenesis involved in the control of proliferation of progenitor cells and neuronal differentiation (59, 60). Thus, we speculate that Akt may mediate the effect of leptin on hippocampal progenitor cell proliferation through interacting with the Wnt-β-catenin signaling pathway.
The observation that inhibition of STAT3 by cucurbitacin reversed leptin-induced increase in proliferation of hippocampal progenitor cells suggests a potential role of Jak2-STAT3 signaling. Studies have demonstrated that STAT3 is important for the maintenance of neural progenitors. Expression of a constitutively active form of STAT3 in neural progenitors derived from the embryonic mouse neocortex increases the number of neurospheres formed by progenitor cells (61). Conversely, deletion of STAT3 results in a decrease in the number of primary neurospheres (61). These findings support that STAT3 enhances proliferation of neural progenitor cells. However, the effect of STAT3 on differentiation fate of neural progenitor cells remains controversial. On one hand, it has been shown that neuronal differentiation is promoted by suppression of STAT3 (62) and inhibited by overexpression of this signaling molecule (61). On the other hand, activation of STAT3 has been reported to be associated with stimulated neurogenesis (63) and required for the neurotrophic effects of ciliary neurotrophic factor and leukemia inhibitory factor on developing sensory neurons (64). Therefore, further investigation is needed to clarify the role of STAT3 in neurogenesis.
The ERK1/2 pathway has also been shown to play an important role in regulating proliferation of neural progenitors (65, 66). However, ERK1/2 phosphorylation in cultured adult hippocampal progenitor cells was not altered by leptin treatment. This is somewhat unexpected, because ERK1/2 has been shown to be phosphorylated by the synthetic cannabinoid HU210, a stimulator of hippocampal neural progenitor cell proliferation (67). The lack of leptin-induced activation of ERK1/2 could be due to a dephosphorylation mechanism that negatively regulates ERK1/2 phosphorylation. Alternatively, our results may suggest that the ERK1/2 pathway is not recruited by leptin signaling in mediating hippocampal neurogenesis.
In summary, our results indicate that leptin increases the production of new neurons in the adult dentate gyrus. Analysis of signaling pathways in vitro suggests that leptin may stimulate proliferation of adult hippocampal neural progenitor cells through a mechanism that is dependent on Akt and STAT3 activation. These results support a novel role of leptin in the processes of adult hippocampal neurogenesis, providing new insights into the mechanisms of neurogenic regulation. Future work will be needed to determine whether the same signaling mechanisms operate in vivo and whether hippocampal neurogenesis is required for the actions of leptin on hippocampal-related processing such as learning, memory and emotional responses.
Acknowledgments
We thank Margaret Wey for technical assistance in tissue preparation. Confocal microscopic images were generated in the Core Optical Imaging Facility, which is supported by Grants NIH-NCI P30 CA54174, NIH-NIA P30 AG013319, and NIH-NIA P01AG19316.
This work was supported, in whole or in part, by National Institutes of Health Grants MH073844 and MH076929 (to X.-Y. L.). This work was also supported by Scientist Development Award AHA0530345N (to X.-Y. L.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: LepRb, long form leptin receptor; Jak2, Janus kinase 2; ERK, extracellular signal-regulated kinase; STAT3, signal transducer and activator of transcription 3; PI3K, phosphatidylinositol 3-kinase; BrdUrd, bromodeoxyuridine; PBS, phosphate-buffered saline; PFA, paraformaldehyde; NeuN, neuronal nuclei; GFAP, glial fibrillary acidic protein; FGF-2, fibroblast growth factor-2; RT, reverse transcription; ANOVA, analysis of variance.
References
- 1.Duman, R. S., Malberg, J., and Nakagawa, S. (2001) J. Pharmacol. Exp. Ther. 299 401-407 [PubMed] [Google Scholar]
- 2.Gould, E., Tanapat, P., McEwen, B. S., Flugge, G., and Fuchs, E. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 3168-3171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McEwen, B. S. (1999) Annu. Rev. Neurosci. 22 105-122 [DOI] [PubMed] [Google Scholar]
- 4.Duman, R. S., Nakagawa, S., and Malberg, J. (2001) Neuropsychopharmacology 25 836-844 [DOI] [PubMed] [Google Scholar]
- 5.Jacobs, B. L. (2002) Brain Behav. Immun. 16 602-609 [DOI] [PubMed] [Google Scholar]
- 6.Emsley, J. G., and Hagg, T. (2003) Exp. Neurol. 183 298-310 [DOI] [PubMed] [Google Scholar]
- 7.Lu, B., and Chow, A. (1999) J. Neurosci. Res. 58 76-87 [PubMed] [Google Scholar]
- 8.Ransome, M. I., and Turnley, A. M. (2007) J. Neurochem. 102 1953-1965 [DOI] [PubMed] [Google Scholar]
- 9.Banks, W. A., Kastin, A. J., Huang, W., Jaspan, J. B., and Maness, L. M. (1996) Peptides 17 305-311 [DOI] [PubMed] [Google Scholar]
- 10.Karonen, S. L., Koistinen, H. A., Nikkinen, P., and Koivisto, V. A. (1998) Eur. J. Nucl. Med. 25 607-612 [DOI] [PubMed] [Google Scholar]
- 11.Burguera, B., Couce, M. E., Curran, G. L., Jensen, M. D., Lloyd, R. V., Cleary, M. P., and Poduslo, J. F. (2000) Diabetes 49 1219-1223 [DOI] [PubMed] [Google Scholar]
- 12.Munzberg, H., and Myers, M. G., Jr. (2005) Nat. Neurosci. 8 566-570 [DOI] [PubMed] [Google Scholar]
- 13.Bjorbaek, C., Buchholz, R. M., Davis, S. M., Bates, S. H., Pierroz, D. D., Gu, H., Neel, B. G., Myers, M. G., Jr., and Flier, J. S. (2001) J. Biol. Chem. 276 4747-4755 [DOI] [PubMed] [Google Scholar]
- 14.Zhang, E. E., Chapeau, E., Hagihara, K., and Feng, G. S. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 16064-16069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niswender, K. D., Morton, G. J., Stearns, W. H., Rhodes, C. J., Myers, M. G., Jr., and Schwartz, M. W. (2001) Nature 413 794-795 [DOI] [PubMed] [Google Scholar]
- 16.Xu, A. W., Kaelin, C. B., Takeda, K., Akira, S., Schwartz, M. W., and Barsh, G. S. (2005) J. Clin. Invest. 115 951-958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedman, J. M., and Halaas, J. L. (1998) Nature 395 763-770 [DOI] [PubMed] [Google Scholar]
- 18.Li, X. L., Aou, S., Oomura, Y., Hori, N., Fukunaga, K., and Hori, T. (2002) Neuroscience 113 607-615 [DOI] [PubMed] [Google Scholar]
- 19.Oomura, Y., Hori, N., Shiraishi, T., Fukunaga, K., Takeda, H., Tsuji, M., Matsumiya, T., Ishibashi, M., Aou, S., Li, X. L., Kohno, D., Uramura, K., Sougawa, H., Yada, T., Wayner, M. J., and Sasaki, K. (2006) Peptides 27 2738-2749 [DOI] [PubMed] [Google Scholar]
- 20.Farr, S. A., Banks, W. A., and Morley, J. E. (2006) Peptides 27 1420-1425 [DOI] [PubMed] [Google Scholar]
- 21.Lu, X. Y., Kim, C. S., Frazer, A., and Zhang, W. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 1593-1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu, X.-Y. (2007) Curr. Opin. Pharmacol. 7 648-652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raber, J., Rola, R., LeFevour, A., Morhardt, D., Curley, J., Mizumatsu, S., VandenBerg, S. R., and Fike, J. R. (2004) Radiat. Res. 162 39-47 [DOI] [PubMed] [Google Scholar]
- 24.Shors, T. J., Townsend, D. A., Zhao, M., Kozorovitskiy, Y., and Gould, E. (2002) Hippocampus 12 578-584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shors, T. J., Miesegaes, G., Beylin, A., Zhao, M., Rydel, T., and Gould, E. (2001) Nature 410 372-376 [DOI] [PubMed] [Google Scholar]
- 26.Santarelli, L., Saxe, M., Gross, C., Surget, A., Battaglia, F., Dulawa, S., Weisstaub, N., Lee, J., Duman, R., Arancio, O., Belzung, C., and Hen, R. (2003) Science 301 805-809 [DOI] [PubMed] [Google Scholar]
- 27.Blaskovich, M. A., Sun, J., Cantor, A., Turkson, J., Jove, R., and Sebti, S. M. (2003) Cancer Res. 63 1270-1279 [PubMed] [Google Scholar]
- 28.Sun, J., Blaskovich, M. A., Jove, R., Livingston, S. K., Coppola, D., and Sebti, S. M. (2005) Oncogene 24 3236-3245 [DOI] [PubMed] [Google Scholar]
- 29.Kim, C., Huang, T., Garza, J., Ramos, F., Frazer, A., Liu, F., and Lu, X.-Y. (2006) Neuropsychopharmacology 31 S237-S238 [Google Scholar]
- 30.Burns, K. A., and Kuan, C. Y. (2005) Eur. J. Neurosci. 21 803-807 [DOI] [PubMed] [Google Scholar]
- 31.Hayes, N. L., and Nowakowski, R. S. (2002) Brain Res. Dev. Brain Res. 134 77-85 [DOI] [PubMed] [Google Scholar]
- 32.Kempermann, G., Gast, D., Kronenberg, G., Yamaguchi, M., and Gage, F. H. (2003) Development 130 391-399 [DOI] [PubMed] [Google Scholar]
- 33.Lendahl, U., Zimmerman, L. B., and McKay, R. D. (1990) Cell 60 585-595 [DOI] [PubMed] [Google Scholar]
- 34.Reynolds, B. A., Tetzlaff, W., and Weiss, S. (1992) J. Neurosci. 12 4565-4574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taupin, P. (2007) Brain Res. Rev. 53 198-214 [DOI] [PubMed] [Google Scholar]
- 36.Cameron, H. A., and McKay, R. D. (2001) J. Comp. Neurol. 435 406-417 [DOI] [PubMed] [Google Scholar]
- 37.Cooper-Kuhn, C. M., and Kuhn, H. G. (2002) Brain Res. Dev. Brain Res. 134 13-21 [DOI] [PubMed] [Google Scholar]
- 38.Cameron, H. A., Woolley, C. S., McEwen, B. S., and Gould, E. (1993) Neuroscience 56 337-344 [DOI] [PubMed] [Google Scholar]
- 39.Malberg, J. E., Eisch, A. J., Nestler, E. J., and Duman, R. S. (2000) J. Neurosci. 20 9104-9110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakagawa, S., Kim, J. E., Lee, R., Malberg, J. E., Chen, J., Steffen, C., Zhang, Y. J., Nestler, E. J., and Duman, R. S. (2002) J. Neurosci. 22 3673-3682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Czeh, B., Welt, T., Fischer, A. K., Erhardt, A., Schmitt, W., Muller, M. B., Toschi, N., Fuchs, E., and Keck, M. E. (2002) Biol. Psychiatry 52 1057-1065 [DOI] [PubMed] [Google Scholar]
- 42.Pham, K., Nacher, J., Hof, P. R., and McEwen, B. S. (2003) Eur. J. Neurosci. 17 879-886 [DOI] [PubMed] [Google Scholar]
- 43.Kim, J. B., Ju, J. Y., Kim, J. H., Kim, T. Y., Yang, B. H., Lee, Y. S., and Son, H. (2004) Brain Res. 1027 1-10 [DOI] [PubMed] [Google Scholar]
- 44.Wong, E. Y., and Herbert, J. (2006) Neuroscience 137 83-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo, Z., Jiang, H., Xu, X., Duan, W., and Mattson, M. P. (2008) J. Biol. Chem. 283 1754-1763 [DOI] [PubMed] [Google Scholar]
- 46.Halaas, J. L., Gajiwala, K. S., Maffei, M., Cohen, S. L., Chait, B. T., Rabinowitz, D., Lallone, R. L., Burley, S. K., and Friedman, J. M. (1995) Science 269 543-546 [DOI] [PubMed] [Google Scholar]
- 47.Pelleymounter, M. A., Cullen, M. J., Baker, M. B., Hecht, R., Winters, D., Boone, T., and Collins, F. (1995) Science 269 540-543 [DOI] [PubMed] [Google Scholar]
- 48.Zhang, Y., Proenca, R., Maffei, M., Barone, M., Leopold, L., and Friedman, J. M. (1994) Nature 372 425-432 [DOI] [PubMed] [Google Scholar]
- 49.Lee, J., Duan, W., Long, J. M., Ingram, D. K., and Mattson, M. P. (2000) J. Mol. Neurosci. 15 99-108 [DOI] [PubMed] [Google Scholar]
- 50.Lee, J., Seroogy, K. B., and Mattson, M. P. (2002) J. Neurochem. 80 539-547 [DOI] [PubMed] [Google Scholar]
- 51.Trejo, J. L., Carro, E., and Torres-Aleman, I. (2001) J. Neurosci. 21 1628-1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Praag, H., Kempermann, G., and Gage, F. H. (1999) Nat. Neurosci. 2 266-270 [DOI] [PubMed] [Google Scholar]
- 53.Overton, J. M., Williams, T. D., Chambers, J. B., and Rashotte, M. E. (2001) Hypertension 37 663-669 [DOI] [PubMed] [Google Scholar]
- 54.Bjorbaek, C., and Kahn, B. B. (2004) Recent Prog. Horm. Res. 59 305-331 [DOI] [PubMed] [Google Scholar]
- 55.Wang, L., Zhang, Z. G., Zhang, R. L., Jiao, Z. X., Wang, Y., Pourabdollah-Nejad, D. S., LeTourneau, Y., Gregg, S. R., and Chopp, M. (2006) J. Cereb. Blood Flow Metab. 26 556-564 [DOI] [PubMed] [Google Scholar]
- 56.Peltier, J., O'Neill, A., and Schaffer, D. V. (2007) Dev. Neurobiol. 67 1348-1361 [DOI] [PubMed] [Google Scholar]
- 57.Naito, A. T., Akazawa, H., Takano, H., Minamino, T., Nagai, T., Aburatani, H., and Komuro, I. (2005) Circ. Res. 97 144-151 [DOI] [PubMed] [Google Scholar]
- 58.Miller, J. R., and Moon, R. T. (1996) Genes Dev. 10 2527-2539 [DOI] [PubMed] [Google Scholar]
- 59.Adachi, K., Mirzadeh, Z., Sakaguchi, M., Yamashita, T., Nikolcheva, T., Gotoh, Y., Peltz, G., Gong, L., Kawase, T., Alvarez-Buylla, A., Okano, H., and Sawamoto, K. (2007) Stem Cells 25 2827-2836 [DOI] [PubMed] [Google Scholar]
- 60.Hirabayashi, Y., and Gotoh, Y. (2005) Neurosci. Res. 51 331-336 [DOI] [PubMed] [Google Scholar]
- 61.Yoshimatsu, T., Kawaguchi, D., Oishi, K., Takeda, K., Akira, S., Masuyama, N., and Gotoh, Y. (2006) Development 133 2553-2563 [DOI] [PubMed] [Google Scholar]
- 62.Gu, F., Hata, R., Ma, Y. J., Tanaka, J., Mitsuda, N., Kumon, Y., Hanakawa, Y., Hashimoto, K., Nakajima, K., and Sakanaka, M. (2005) J. Neurosci. Res. 81 163-171 [DOI] [PubMed] [Google Scholar]
- 63.Jung, K. H., Chu, K., Lee, S. T., Kim, S. J., Sinn, D. I., Kim, S. U., Kim, M., and Roh, J. K. (2006) Brain Res. 1073-1074, 190-201 [DOI] [PubMed] [Google Scholar]
- 64.Alonzi, T., Middleton, G., Wyatt, S., Buchman, V., Betz, U. A., Muller, W., Musiani, P., Poli, V., and Davies, A. M. (2001) Mol. Cell Neurosci. 18 270-282 [DOI] [PubMed] [Google Scholar]
- 65.Learish, R. D., Bruss, M. D., and Haak-Frendscho, M. (2000) Brain Res. Dev. Brain Res. 122 97-109 [DOI] [PubMed] [Google Scholar]
- 66.Zhou, L., Del Villar, K., Dong, Z., and Miller, C. A. (2004) Brain Res. 1021 8-19 [DOI] [PubMed] [Google Scholar]
- 67.Jiang, W., Zhang, Y., Xiao, L., Van Cleemput, J., Ji, S. P., Bai, G., and Zhang, X. (2005) J. Clin. Invest. 115 3104-3116 [DOI] [PMC free article] [PubMed] [Google Scholar]