Abstract
The mechanisms that allow Mycobacterium tuberculosis (Mtb) to persist in human tissue for decades and to then abruptly cause disease are not clearly understood. Regulatory elements thought to assist Mtb to enter such a state include the heme two-component sensor kinases DosS and DosT and the cognate response regulator DosR. We have demonstrated previously that O2, nitric oxide (NO), and carbon monoxide (CO) are regulatory ligands of DosS and DosT. Here, we show that in addition to O2 and NO, CO induces the complete Mtb dormancy (Dos) regulon. Notably, we demonstrate that CO is primarily sensed through DosS to induce the Dos regulon, whereas DosT plays a less prominent role. We also show that Mtb infection of macrophage cells significantly increases the expression, protein levels, and enzymatic activity of heme oxygenase-1 (HO-1, the enzyme that produces CO), in an NO-independent manner. Furthermore, exploiting HO-1+/+ and HO-1-/- bone marrow-derived macrophages, we demonstrate that physiologically relevant levels of CO induce the Dos regulon. Finally, we demonstrate that increased HO-1 mRNA and protein levels are produced in the lungs of Mtb-infected mice. Our data suggest that during infection, O2, NO, and CO are being sensed concurrently rather than independently via DosS and DosT. We conclude that CO, a previously unrecognized host factor, is a physiologically relevant Mtb signal capable of inducing the Dos regulon, which introduces a new paradigm for understanding the molecular basis of Mtb persistence.
Mycobacterium tuberculosis (Mtb)3 causes approximately two million deaths annually, and it is estimated that one third of the world's population is latently infected with Mtb (1). This clinically latent state of disease can last for decades during which Mtb remains unresponsive to drug therapy. Evidence suggests roles for at least two signals, oxygen tension and nitric oxide (NO), in assisting Mtb to enter and maintain a latent state (2–4). Using the murine model for latent tuberculosis (TB), interferon γ (IFN-γ), tumor necrosis factor-α, inducible nitric-oxide synthase (iNOS), and, thus, NO were shown to be continuously expressed and necessary to prevent reactivation of TB (5).
Several studies have demonstrated a dramatic overlap between gene expression profiles of Mtb cultured under hypoxic conditions and upon treatment with NO (6–8). These findings led to the identification of the 48-member Mtb dormancy (Dos) regulon, which is under the control of the two heme sensor kinases DosS and DosT, and the response regulator DosR (9). Notably, mycobacterial dosR mutants were shown to be impaired for survival during hypoxia (8, 10), whereas the Mtb Beijing lineage was shown to constitutively express the Dos regulon 50-fold higher than non-Beijing strains (11). Intriguingly, the Mtb dosR mutant is attenuated in guinea pigs (12), but not mice (13).
Recently, we (14) and others (15–17) have shown that DosS and DosT are heme proteins that in addition to binding O2 and NO are able to bind CO. The latter findings led us to hypothesize that CO could be a third physiologically relevant ligand whose binding might induce the Dos regulon. CO is produced in vivo by heme oxygenase (HO), which is a cytoprotective enzyme that degrades heme to generate CO (18). Notably, HO-1 confers protection against oxidative cellular stress through the anti-oxidative, anti-apoptotic, and anti-inflammatory actions of its byproducts (18–20). In addition, HO-1 has been shown to inhibit key components of innate immunity, the production of tumor necrosis factor-α and NO (18–20). Thus, HO activity in the context of inflammatory lesions caused by live bacteria may have important implications for both the host and the pathogen. Importantly, a recent seminal study described a protective role for HO-1-generated CO in the progression of experimental cerebral malaria (21).
CO has become the focus of a great deal of interest, especially when the therapeutic potential of CO and CO-generating molecules as novel gaseous therapy was recognized (20). However, prior to this study the role of HO-1, and therefore CO, had not been considered in Mtb pathogenesis. Herein, we hypothesize that host HO-1 generates CO, which induces the Mtb Dos regulon. To test this hypothesis, we examined the induction of the Mtb Dos regulon in response to CO exposure and assessed the independent contribution of DosS and DosT in relaying this signal to the Mtb Dos regulon. We also studied the expression of the Mtb Dos regulon in response to physiological levels of CO generated within macrophages. Lastly, we examined HO-1 levels in macrophages and in the lungs of Mtb-infected and uninfected mice.
EXPERIMENTAL PROCEDURES
Mice—C3HeB/FeJ mice were purchased from the Jackson Laboratory. HO-1-/- mice and age-matched HO-1+/+ littermates (C57BL/6 × FVB background) and iNOS-/- (C57BL6 background) were used. The animal protocols were approved by the Institutional Care and Use Committees at the University of Alabama at Birmingham and Harvard School of Public Health. The mice were regularly tested and found to be free of common mouse pathogens and were used for experiments at 6–12 weeks of age.
Mtb Mutant Strains—Mtb dos mutant strains were kind gifts from Dr. David Sherman (Seattle Biomedical Research Institute, WA). Construction of MtbΔdosS and MtbΔdosR is described by Sherman et al. (6), and MtbΔdosT and MtbΔΔdosSΔdosT are described by Roberts et al. (9). MtbΔdosR was found to be attenuated for growth in the Wayne model for in vitro dormancy (8, 13) and is not capable of inducing the Mtb Dos regulon under hypoxic conditions or in response to NO (8). The expression of hspX was reduced 40–45% in MtbΔdosS or MtbΔdosT, whereas MtbΔdosSΔdosT abolishes expression of hspX in response to hypoxia (9).
Bacterial Infection—Virulent Mtb (strain Erdman, TMC 107) was obtained from the Trudeau Mycobacterial Culture Collection (Trudeau Institute). Mice were intravenously infected with 1 × 105 colony-forming units of Mtb in 100 μl of phosphate-buffered saline via tail vein. Mtb H37Rv was used in all other experiments.
Microarray Hybridization and Data Analysis—Microarrays used in this study were produced and processed at the Center for Applied Genomics at the Public Health Research Institute as described earlier (22). See supplemental information for details.
Expression Analysis of the Mtb Dos Regulon in Bone Marrow-derived Macrophages (BMM)—Four independent experiments were performed using BMM collected from 8–12-week-old HO-1-/- mice and HO-1+/+ littermates (C57BL/6 × FVB background). 1–2 × 106 macrophages were infected with a multiplicity of infection of 5–10. RNA was isolated and utilized in real-time PCR analysis according to previously described protocols (14, 23). The -fold expression of a specified gene transcript of bacilli residing in HO-1+/+ BMM was measured relative to the gene transcript of bacilli residing in HO-1-/- BMM. Expression was normalized using Mtb 16 S rRNA as internal control.
Expression Analysis of HO-1—Experiments were performed using BMM collected from 8–12-week-old HO-1-/- mice and HO-1+/+ littermates (C57BL/6 × FVB background). 1–2 × 106 macrophages were infected with a multiplicity of infection of 5–10. Extracellular bacteria were removed by washing with cell culture medium prewarmed to 37 °C after 12–14 h of infection. Macrophages were lysed with guanidine thiocyanate, and bacteria were harvested by centrifugation. Approximately 1–2 μg of bacterial RNA was isolated from four independent biological samples and analyzed via quantitative PCR (Q-PCR).
Western Blot Analysis—Approximately 2 × 106 cells (murine macrophage cell line or BMM) were grown in a six-well plate and infected with Mtb (multiplicity of infection 5–10). For Western blot analysis, cells were harvested and lysed in radioimmune precipitation buffer, samples were sonicated and centrifuged, and the protein concentration of the lysate was determined using the BCA protein assay. Western blot analysis was performed as described earlier (24).
HO Enzymatic Activity Assay—RAW cells grown in 175-cm2 tissue culture flasks (5 × 107 cells) were infected with Mtb (multiplicity of infection 5–10). Uninfected cells were used to serve as controls. HO activity was determined as described earlier (25).
Immunohistochemistry—Tissue sections from infected animals (n = 4) of each group were treated with 10% formalin in neutral buffer and cut into sections to 5 μm. These sections were deparaffinated and rehydrated in graded ethanol. Sections were washed in phosphate-buffered saline followed by endogenous peroxidase inactivation in 0.3% H2O2, labeled with primary antibody against HO-1 (Stressgen, SPA-896), stained with anti-rabbit peroxidase (Jackson Laboratories), and developed with the 3,3′-diaminobenzidine (DAB) substrate kit (Vector Laboratories) to produce a characteristic brown color. Control reactions were performed using rabbit IgG instead of the primary antibody, and the secondary antibody alone. Sections were also stained with hematoxylin and counterstained with eosin according to previously described protocols (26).
Statistical Analysis—Results are expressed as mean ± S.E. and are derived from at least three independent experiments. Student's t-test or analysis of variance with Student-Newman-Keuls post-test was used for comparisons.
RESULTS
CO Elicits the Induction of the Complete Mtb Dos Regulon— We have recently reported that the redox state of the DosS and DosT heme irons, as well as the ligands O2, NO, and CO, modulates DosS and DosT autokinase activity (14). Because the effect of CO on the complete Dos regulon is not yet established, we sought to determine whether CO could selectively induce the Mtb Dos “fingerprint,” consisting of ∼48 genes. We used microarray expression profiling and captured the transcriptional response of Mtb cells exposed to 50 μm CO. The results demonstrate that CO rapidly induced the complete Dos regulon (Fig. 1). We repeated the microarray experiment using the MtbΔdosR mutant strain and found that induction of the Dos regulon by CO was mediated via DosR (Fig. 1). Thus, the induction of the Dos regulon by hypoxia (6), NO (7, 8), and now CO is mediated by the DosR/S/T system. We next tested a series of CO concentrations (5 nm-100 μm) and examined fdxA expression via Q-PCR as an indicator (9) for Dos regulon expression. We observed that full induction of fdxA occurred at ≥5 μm CO (Fig. 2A). Similarly, we analyzed the time course for the induction of fdxA expression after exposure to 50 μm CO and found that maximal expression occurred within 30 min (results not shown). By 24 h post treatment, expression of fdxA declined to basal levels but rapidly increased upon treatment with a second dose of CO (results not shown).
FIGURE 1.
Up-regulation of the complete Mtb Dos regulon upon exposure to CO. Microarray expression analysis was performed using RNA isolated from wild-type Mtb and MtbΔdosR cells treated/untreated with 50 μm CO for 3 h. Arrows indicate the direction of transcription according to TubercuList. CHP, conserved hypothetical protein; HP, hypothetical protein.
FIGURE 2.
Regulation of the Mtb Dos regulon in response to CO. A, using Q-PCR, fdxA expression was examined after exposing Mtb cells to different concentrations of CO. B, Mtb DosS is the preferred sensor of CO. Wild-type Mtb, MtbΔdosS, MtbΔdosT, and MtbΔdosSΔdosT cells were independently exposed to CO followed by dosR, hspX, and fdxA expression analysis using Q-PCR. Results are expressed as mean ± S.D. (n = 3 in triplicate).
In sum, these data demonstrate that, similar to hypoxia and NO, exposure to low, non-toxic concentrations of CO specifically induces the complete Mtb Dos regulon in a rapid and coordinated fashion. Thus, we have identified CO as a third signal that induces the Mtb Dos regulon.
Mtb DosS Is the Primary Sensor of CO—Because dosT, as opposed to dosS, is not up-regulated in response to any condition tested to date, including hypoxia (6), NO (7, 8), or CO (Fig. 1), we sought to examine the independent contributions of DosS and DosT in modulating the Dos regulon. First, we exposed MtbΔdosSΔdosT cells to CO and found that similar to the MtbΔdosR strain, this strain was impaired in its ability to induce the Dos regulon (Fig. 2B). We also utilized the single knock-out strains, MtbΔdosS and MtbΔdosT, and found that MtbΔdosS was severely attenuated in its ability to induce the Dos regulon compared with wild-type Mtb, whereas MtbΔdosT was moderately affected (Fig. 2B). We conclude that CO is sensed through the Mtb DosR/S/T system and that DosS is the preferred, but not the sole, sensor of CO.
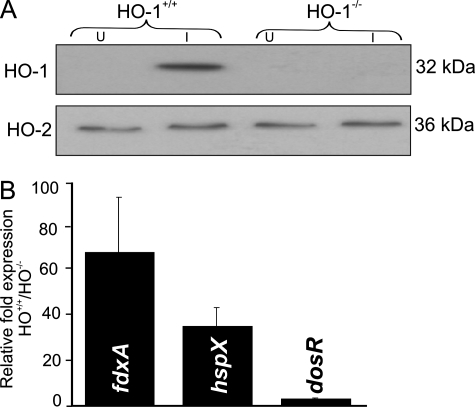
Induction of the Mtb Dos Regulon in Macrophages Is Modulated by HO-1—We next sought to determine whether HO-1-generated CO could be a physiologically relevant ligand of Mtb DosS and DosT to ultimately modulate the Dos regulon. We utilized BMM isolated from HO-1+/+ and HO-1-/- mouse strains rather than chemical inhibitors or inducers of HO, which have nonspecific effects, including inhibition of guanylate cyclase activity (27), inhibition (28) or stimulation of NO synthase activity (29), and inactivation of caspase 3 and 8 (30). To analyze the effect of Mtb infection on HO-1 expression, we first infected BMM from 8–12-week-old HO-1+/+ mice with Mtb and subsequently used Western blot analysis to examine HO-1 levels. Mtb-infected BMM showed significantly increased HO-1 levels compared with uninfected BMM (Fig. 3A), whereas the constitutive isoform of HO-2 was unchanged. Similar to previous iNOS studies (31), we infected BMM from HO-1+/+ and HO-1-/- mice with Mtb and performed Q-PCR using primers specific for fdxA, hspX, and dosR. Prior studies have shown that these genes are highly responsive to hypoxia and NO (6–8). We observed a 3- to 68-fold up-regulation of these genes in bacilli recovered from HO-1+/+ cells relative to HO-1-/- cells (Fig. 3B), supporting a role for HO-1 in the induction of the Dos regulon. In sum, these data suggest that Mtb-infected macrophages specifically up-regulate HO-1 to increase CO, which is sensed by the Mtb sensor kinases DosS and DosT and then relayed to DosR to induce the Dos regulon.
FIGURE 3.
Induction of the Mtb Dos regulon in macrophages is modulated by HO-1. A, HO-1 expression in HO-1+/+ and HO-1-/- BMM. BMM were collected from HO-1-/- and HO-1+/+ littermates and infected with Mtb. HO-1 protein levels were analyzed using Western blotting. Note that levels of HO-2, the constitutive isoform of HO, are unchanged. U, uninfected; I, infected. B, HO-1-generated CO induces the Mtb Dos regulon. HO-1-/- and HO-1+/+ BMM were independently infected with Mtb and RNA isolated from intracellular bacilli. Q-PCR was used to analyze the expression of dosR, hspX, and fdxA (an established “fingerprint” of the Dos regulon). Results are expressed as mean ± S.D. (n = 3 in triplicate).
Up-regulation of HO-1 Is Independent of the NO Pathway—It is well known that during Mtb infection iNOS is induced via IFN-γ to generate NO (32). Because NO is an inducer of HO-1 (see supplemental note 1 and supplemental Fig. S1) and because the above results demonstrate up-regulation of HO-1 in response to Mtb infection (Fig. 3A), we sought to examine the contribution of the NO signaling pathway in modulating HO-1 induction. We infected RAW 264.7 macrophage cells with Mtb and exploited Q-PCR to examine HO-1 expression 24 h post-infection. We found that Mtb significantly up-regulates HO-1 expression (Fig. 4A) compared with uninfected RAW 264.7 cells and the induction of HO-1 mRNA was directly associated with increased HO-1 protein (Fig. 4B) after infection with Mtb. Note that HO-1 can exist in a 32-kDa wild type and a cleaved 28-kDa form in immunoblot blots (33). Furthermore, time course experiments demonstrated that HO-1 expression was induced within 2 h of Mtb infection and was maintained for at least 24 h (data not shown). To specifically examine the potential link between HO-1 and the NO signaling pathway, we also infected the RAW 264.7 γ-NO(-) cell line, which is defective in the IFN-γ-mediated induction of iNOS (34). Our Q-PCR analysis showed that HO-1 expression following Mtb infection (Fig. 4A) is significantly increased in the RAW 264.7 γ-NO(-) cell line at levels comparable with that of the parental cell line (Fig. 4A). Corroborating these data is the Western blot analysis showing increased HO-1 protein levels in Mtb-infected RAW 264.7 γ-NO(-) cells (Fig. 4B). We also utilized the highly specific NOS inhibitor N-(3-{aminomethyl} benzyl) acetamidine (1400W) and found that HO-1 mRNA and protein levels were significantly increased (Fig. 4, A and B) in the presence of the inhibitor during Mtb infection. Finally, we infected iNOS2-/- BMM with Mtb and again observed a significant increase in HO-1 expression upon Mtb exposure (Fig. 4A).
FIGURE 4.
Induction of HO-1 in response to Mtb infection is independent of the NO signaling pathway. A, increase in HO-1 mRNA in response to Mtb infection. RAW cells and BMM from iNOS2-/- mice were infected with Mtb, and HO-1 expression was analyzed using Q-PCR. Results are expressed as mean ± S.D. (n = 3 in triplicate). B, increase in HO-1 protein levels in response to Mtb infection. HO-1 protein is increased in RAW cells 24 h post infection and in the presence and absence of 1400W. Note that HO-1 can migrate as two bands (see “Results” for details).
HO Enzymatic Activity Is Increased upon Infection of BMM with Mtb—Having observed that HO-1 mRNA and protein levels increase in macrophages upon infection with Mtb (Figs. 3A and 4, A and B), we now sought to examine (i) whether increased HO-1 expression reflects an increase in HO enzymatic activity leading to increased CO levels, and (ii) the role of the NO pathway in modulating HO enzymatic activity upon infection. RAW 264.7 cells were infected with Mtb and used in the HO enzymatic activity assay. In this widely used assay, HO stoichiometrically releases CO, molecular iron, and biliverdin. The latter is then converted to bilirubin by biliverdin reductase and is therefore an exact indicator of the amount of released CO. Subsequently, it was observed that macrophages infected with Mtb displayed ∼8-fold higher HO enzymatic activity compared with uninfected control cells (Fig. 5A). Importantly, identical experiments were conducted with RAW 264.7 γ-NO(-) and RAW 264.7 cells treated with 1400W. Again, the results demonstrate that HO activity was not affected by a defective IFN-γ signaling pathway or a specific iNOS inhibitor (Fig. 5, B and C). Collectively, these results suggest that the induction of HO-1, and therefore CO, upon Mtb infection is independent of IFN-γ-mediated NO production and point toward an alternative pathway by which Mtb induces HO-1.
FIGURE 5.
HO enzymatic activity is increased in response to Mtb infection. Biliverdin is a precise indicator of the amount of released CO (see “Results” for details). HO enzymatic activity was measured in RAW 264.7 (A), RAW 264.7 γ-NO(-) (B), and RAW 264.7 (C) cells with 1400W 24 h post-infection with Mtb. U, uninfected; I, infected. Results are expressed as mean ± S.E.*, p <0.001 (n = 3–6/gp).
HO-1 Is Induced in Lesions of Mtb-infected Mice—Using a systematic series of in vitro culture and macrophage infection experiments described above, we made the observation that Mtb specifically senses and responds to physiological levels of CO to induce the Dos regulon. To determine whether CO was produced at the site of infection, as is the case for NO (3), we assessed whether HO-1 mRNA is up-regulated in mouse lungs following Mtb challenge. Upon infection with Mtb, HO-1 expression was significantly increased (19.5-fold) (Fig. 6A). Staining of lung lesions with HO-1-specific antibodies demonstrated that HO-1-expressing cells were associated with TB lesions. Also, a high proportion of HO-1-positive cells were found within extensive inflammatory lesions containing necrotic areas in the lungs of the mice (Fig. 6B, I and II). The staining was localized predominantly in alveolar epithelial cells and infiltrating macrophages (Fig. 6B, I, inset).
FIGURE 6.
HO-1 is significantly induced in the lungs of Mtb-infected mice. A, relative HO-1 mRNA abundance measured in the lungs of uninfected (U) or Mtb-infected (I) (4 weeks post infection) C3HeB/FeJ mice. Results are expressed as mean ± S.D. (n = 4 in triplicate). B, immunohistochemistry of Mtb-infected lungs (4 weeks post infection). Expression of HO-1 protein within TB lung lesions was demonstrated using HO-1-specific antibodies. Panel I, staining with HO-1-specific antibodies; panel II, hematoxylin and eosin staining. TB lung lesions of C3HeB/FeJ mice (panels I and II) contained numerous HO-1-positive cells (brown color in panel I and inset) within cell wall surrounding areas of necrosis.
In sum, we have shown that increased levels of HO-1 mRNA, protein, and therefore CO are produced in the lungs of Mtb-infected mice. The data suggest both diverging and complementary roles for NO and CO in Mtb pathogenesis and identify CO as a signaling ligand for DosS and DosT to modulate the Mtb Dos regulon.
DISCUSSION
The Mtb DosR/S/T two-component system responds to at least two physiologically relevant dormancy signals, O2 (6) and NO (7, 8). In this study, we discovered for the first time that CO is a third physiologically relevant signal capable of inducing the complete 48-member Mtb Dos regulon. We have also shown that CO is primarily sensed through DosS. Importantly, we have demonstrated that the levels of CO produced within macrophages via HO-1 were sensed by Mtb and resulted in the induction of key members of the Dos regulon. We further demonstrated that HO-1 expression and protein levels, as well as HO enzymatic activity in macrophages, are significantly increased upon Mtb infection and are independent of the NO signaling pathway. Lastly, we have demonstrated that HO-1 is significantly increased in the lungs of Mtb-infected mice. These findings demonstrate an important and previously unknown function for HO-1 and, thus, CO. Notably, the ability of three diatomic gases, CO, NO, and O2, to induce an identical set of Mtb genes is an unparalleled finding and presents a useful paradigm for redox signal transduction in prokaryotic cells.
Recently, our laboratory (14) as well as others (15–17) has shown that DosS and DosT are GAF-containing heme proteins. We proposed a sense-and-lock model that suggests that the ligation state of Mtb DosS and DosT can be “locked” by gradients of NO and CO throughout progression of disease (14). In a recent study demonstrating a protective role for CO in experimental cerebral malaria (21), it was proposed that CO “locks” cell-free hemoglobin in the Fe(II) state, thereby preventing oxidation to the unstable met Fe(III) state, which can react with reactive oxygen species to disrupt the blood brain barrier (21). The overlap between this model and the sense-and-lock model for the Mtb Dos regulon (14) illustrates one of the most important properties of CO: the ability to react only with Fe(II) and not Fe(III). NO, on the other hand, can react with both Fe(II) and Fe(III) species.
In this study, we have utilized microarray technology and Q-PCR and conclusively demonstrated that similar to hypoxia and NO (6–8), low concentrations of exogenously provided CO induce the complete Dos regulon within minutes of CO exposure. Earlier studies have suggested that M. bovis BCG, M. gordonae, M. smegmatis, and M. tuberculosis H37Ra oxidize high concentrations of CO (35) via CO dehydrogenase (Rv0373c/Rv0375c). However, earlier microarray studies as well as this study have shown that neither hypoxia (6), NO (7, 8), nor CO (Fig. 1) differentially regulates expression of these genes.
Importantly, our findings demonstrating that CO is sensed primarily through DosS, with DosT playing a less prominent role, agree with recent kinetic data reporting that DosS (Kd = 36 nm) has a higher affinity for CO than DosT (Kd = 940 nm) (17). Nonetheless, this should be viewed with caution, because an earlier study has shown that DosS and DosT contributed essentially equally toward regulating fdxA (9) in response to hypoxia despite differences in binding of O2 to DosS (Kd = 3 μm) or DosT (Kd = 26 μm) (9). Also, because dosT expression is not responsive to either O2, NO, or CO (or any other condition according to our knowledge) and appears to be constitutively expressed, DosS or DosT protein levels may also influence regulation of the Dos regulon. Thus, the biological significance of the highly regulated nature of dosS, as opposed to constitutively expressed dosT, is an important, albeit unexplained, finding.
Because HO generates equimolar amounts of CO, Fe(II), and biliverdin using heme as a substrate in vivo, it was important to demonstrate that not only exogenously provided CO but also physiological levels of CO are capable of inducing the Mtb Dos regulon upon infection. Using mouse HO-1+/+ and HO-1-/- BMM cells, we demonstrated that CO produced within macrophages significantly induces expression of the Mtb Dos regulon. Because HO generates not only CO, but also Fe(II) and biliverdin, it could be argued that these two components (or even heme) rather than CO induce the Dos regulon. However, neither heme (see supplemental text note 2) nor iron (36) was shown to induce the Dos regulon, and there is no evidence to suggest that biliverdin is capable either. Rather, data from several independent studies (14, 15, 17) as well as our in vitro cell-based studies (Fig. 1) provide convincing evidence that DosS and DosT are exclusively sensing CO and not the other heme breakdown products.
HO-1 is the most important endogenous source of CO and is highly induced upon oxidative stress (37–39), whereas HO-2 is the low level, constitutive HO that contributes minimally to overall CO levels (40). Heme-independent CO arises primarily from photo-oxidation, peroxidation of lipids, and xenobiotic compounds and represents a fraction of total endogenous CO production (41).
Because continuous production of IFN-γ and tumor necrosis factor α, which synergistically regulate iNOS expression, were previously shown to be essential to prevent reactivation of persistent Mtb in infected mice (5), we examined the contribution of the NO signaling pathway in modulating HO-1 pathway. We independently infected three different cell types, two of which had defects in the IFN-γ/NO response pathway, and demonstrated that the induction of HO-1 in response to Mtb infection occurs via an unknown mechanism that is independent of NO, and possibly IFN-γ, signaling. Notably, we documented a significant increase of HO-1 in the lungs of Mtb-infected mice. This is not unusual, as HO-1 is rapidly induced in response to oxidative stress and inflammatory reactions, which are the major underlying causes of many diseases. However, a surprising finding was that this response appears to be independent of the NO pathway.
The combined production of NO and CO at the site of infection now provides insight into how gradients of NO and CO (and O2) may shape disease progression. For example, “optimal” levels of O2, NO, and CO could maintain Mtb in a metabolically quiescent state. On the other hand, because of the strong anti-apoptotic and anti-inflammatory properties of CO (18, 19, 42), excessive amounts of CO and also NO (5) could cause destructive immunopathology. Regardless, these findings provide mechanistic insight into how the presence of multiple gases (Fig. 7 and supplemental note 3) generates a “double-edged sword” that can either be beneficial or detrimental. Furthermore, differences in the on- and off-rates of the gases for DosS and DosT (17) and differential host and bacillary responses are evidence of a dynamic mechanism that Mtb has evolved to modulate disease during discrete disease states. Mtb may also exploit other strategies to sense O2 or NO. For example, Mtb WhiB3 was shown to bind NO and react with O2 via its 4Fe-4S cluster, which might initiate a metabolic switchover to a preferred in vivo carbon source, fatty acids (43). Lastly, the recent link between TB and CO air pollution (44), as well as the role of HO-1 in suppressing human immunodeficiency virus replication (45), suggests that the findings in this study may have important socioeconomic and environmental health implications.
FIGURE 7.
Hypothetical model depicting a role for CO in Mtb pathogenesis. Because numerous studies reported striking differences in local O2, NO, and CO concentrations in organs, tissues, and single cells (see supplemental note 3), the model predicts that gradients of O2, NO, or CO are sensed in conjunction by DosS and DosT, rather than independently. DosS is the preferred sensor of CO (thick blue arrow), whereas DosT is less capable (thin blue arrow) of inducing the Dos regulon. This contrasts with O2, which inhibits expression of the Dos regulon (blocked arrow), albeit not during hypoxia. The model predicts that a combination of microenvironmental O2, NO, or CO (overlapping circles) is crucial in modulating the state of the disease. Although modeling the above events in the context of chronic TB is technically difficult, the well established role of HO-1 in modulating apoptosis/necrosis, its anti-inflammatory properties (specifically the effect on tumor necrosis factor α), and the release of Fe(II) have significant implications for understanding the mechanism of Mtb persistence.
In sum, although the exact role of HO-1 and CO in Mtb pathogenesis remains to be established, the findings in this study significantly advance our understanding of the Mtb Dos dormancy response, conventionally viewed as only being induced by two in vivo relevant signals, hypoxia and NO, and therefore represent a hitherto unexplored area of TB research. We have shown that CO generates expression profiles identical to that of Mtb cells exposed to hypoxia or NO and that DosS is the preferred sensor of CO. We have also shown that physiological levels of CO specifically induce the Mtb Dos regulon. Furthermore, we have demonstrated that HO-1 expression, protein, and enzymatic activity significantly increase in response to Mtb infection and that these events are independent of the NO signaling pathway. Lastly, we have demonstrated that HO-1 is produced at the site of infection, the lungs of Mtb-infected mice. These findings have broad implications for understanding the mechanistic basis for Mtb persistence.
Supplementary Material
Acknowledgments
We thank members of the Steyn laboratory and Mary Hondalus for critical review of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants AI058131 (to A. J. C. S.), DK59600, DK75532, and HL068157 (to A. A.), and HL059836 (to I. K.). This work was also supported by the University of Alabama at Birmingham (UAB) Center for AIDS Research (to A. J. C. S.), UAB Center for Free Radical Biology (to A. J. C. S.), and UAB Center for Emerging Infections and Emergency Preparedness (to A. J. C. S.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1 and supplemental information.
Footnotes
The abbreviations used are: Mtb, Mycobacterium tuberculosis; NO, nitric oxide; TB, tuberculosis; IFN-γ, interferon γ; iNOS, inducible nitric-oxide synthase; CO, carbon monoxide; HO-1, heme oxygenase-1; BMM, bone marrow-derived macrophage; Q-PCR, quantitative PCR.
References
- 1.Dye, C., Scheele, S., Dolin, P., Pathania, V., and Raviglione, M. C. (1999) J. Am. Med. Soc. 282 677-686 [DOI] [PubMed] [Google Scholar]
- 2.Cosma, C. L., Sherman, D. R., and Ramakrishnan, L. (2003) Annu. Rev. Microbiol. 57 641-676 [DOI] [PubMed] [Google Scholar]
- 3.MacMicking, J. D., North, R. J., LaCourse, R., Mudgett, J. S., Shah, S. K., and Nathan, C. F. (1997) Proc. Natl. Acad. Sci. U. S. A. 94 5243-5248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wayne, L. G., and Sohaskey, C. D. (2001) Annu. Rev. Microbiol. 55 139-163 [DOI] [PubMed] [Google Scholar]
- 5.Flynn, J. L., Scanga, C. A., Tanaka, K. E., and Chan, J. (1998) J. Immunol. 160 1796-1803 [PubMed] [Google Scholar]
- 6.Sherman, D. R., Voskuil, M., Schnappinger, D., Liao, R., Harrell, M. I., and Schoolnik, G. K. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 7534-7539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohno, H., Zhu, G., Mohan, V. P., Chu, D., Kohno, S., Jacobs, W. R., J., and Chan, J. (2003) Cell. Microbiol. 5 637-648 [DOI] [PubMed] [Google Scholar]
- 8.Voskuil, M. I., Schnappinger, D., Visconti, K. C., Harrell, M. I., Dolganov, G. M., Sherman, D. R., and Schoolnik, G. K. (2003) J. Exp. Med. 198 705-713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts, D. M., Liao, R. P., Wisedchaisri, G., Hol, W. G., and Sherman, D. R. (2004) J. Biol. Chem. 279 23082-23087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boon, C., and Dick, T. (2002) J. Bacteriol. 184 6760-6767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reed, M. B., Gagneux, S., Deriemer, K., Small, P. M., and Barry, C. E., III (2007) J. Bacteriol. 189 2583-2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malhotra, V., Sharma, D., Ramanathan, V. D., Shakila, H., Saini, D. K., Chakravorty, S., Das, T. K., Li, Q., Silver, R. F., Narayanan, P. R., and Tyagi, J. S. (2004) FEMS Microbiol. Lett. 231 237-245 [DOI] [PubMed] [Google Scholar]
- 13.Rustad, T. R., Harrell, M. I., Liao, R., and Sherman, D. R. (2008) PLoS ONE 3 e1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar, A., Toledo, J. C., Patel, R. P., Lancaster, J. R., Jr., and Steyn, A. J. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 11568-11573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ioanoviciu, A., Yukl, E. T., Moenne-Loccoz, P., and de Montellano, P. R. (2007) Biochemistry 46 4250-4260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sardiwal, S., Kendall, S. L., Movahedzadeh, F., Rison, S. C., Stoker, N. G., and Djordjevic, S. (2005) J. Mol. Biol. 353 929-936 [DOI] [PubMed] [Google Scholar]
- 17.Sousa, E. H., Tuckerman, J. R., Gonzalez, G., and Gilles-Gonzalez, M. A. (2007) Protein Sci. 16 1708-1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maines, M. D. (1997) Annu. Rev. Pharmacol. Toxicol. 37 517-554 [DOI] [PubMed] [Google Scholar]
- 19.Tracz, M. J., Alam, J., and Nath, K. A. (2007) J. Am. Soc. Nephrol. 18 414-420 [DOI] [PubMed] [Google Scholar]
- 20.Abraham, N. G., and Kappas, A. (2005) Free Radic. Biol. Med. 39 1-25 [DOI] [PubMed] [Google Scholar]
- 21.Pamplona, A., Ferreira, A., Balla, J., Jeney, V., Balla, G., Epiphanio, S., Chora, A., Rodrigues, C. D., Gregoire, I. P., Cunha-Rodrigues, M., Portugal, S., Soares, M. P., and Mota, M. M. (2007) Nat. Med. 13 703-710 [DOI] [PubMed] [Google Scholar]
- 22.Pang, X., Vu, P., Byrd, T. F., Ghanny, S., Soteropoulos, P., Mukamolova, G. V., Wu, S., Samten, B., and Howard, S. T. (2007) Microbiology 153 1229-1242 [DOI] [PubMed] [Google Scholar]
- 23.Butcher, P. D., Mangan, J. A., and Monahan, I. M. (1998) Methods Mol. Biol. 101 285-306 [DOI] [PubMed] [Google Scholar]
- 24.Deshane, J., Chen, S., Caballero, S., Grochot-Przeczek, A., Was, H., Li Calzi, S., Lach, R., Hock, T. D., Chen, B., Hill-Kapturczak, N., Siegal, G. P., Dulak, J., Jozkowicz, A., Grant, M. B., and Agarwal, A. (2007) J. Exp. Med. 204 605-618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balla, G., Jacob, H. S., Balla, J., Rosenberg, M., Nath, K., Apple, F., Eaton, J. W., and Vercellotti, G. M. (1992) J. Biol. Chem. 267 18148-18153 [PubMed] [Google Scholar]
- 26.Pan, H., Yan, B. S., Rojas, M., Shebzukhov, Y. V., Zhou, H., Kobzik, L., Higgins, D. E., Daly, M. J., Bloom, B. R., and Kramnik, I. (2005) Nature 434 767-772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ignarro, L. J., Ballot, B., and Wood, K. S. (1984) J. Biol. Chem. 259 6201-6207 [PubMed] [Google Scholar]
- 28.Meffert, M. K., Haley, J. E., Schuman, E. M., Schulman, H., and Madison, D. V. (1994) Neuron 13 1225-1233 [DOI] [PubMed] [Google Scholar]
- 29.Morse, D., and Choi, A. M. (2002) Am. J. Respir. Cell Mol. Biol. 27 8-16 [DOI] [PubMed] [Google Scholar]
- 30.Blumenthal, S. B., Kiemer, A. K., Tiegs, G., Seyfried, S., Holtje, M., Brandt, B., Holtje, H. D., Zahler, S., and Vollmar, A. M. (2005) FASEB J. 19 1272-1279 [DOI] [PubMed] [Google Scholar]
- 31.Schnappinger, D., Ehrt, S., Voskuil, M. I., Liu, Y., Mangan, J. A., Monahan, I. M., Dolganov, G., Efron, B., Butcher, P. D., Nathan, C., and Schoolnik, G. K. (2003) J. Exp. Med. 198 693-704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fenton, M. J., Vermeulen, M. W., Kim, S., Burdick, M., Strieter, R. M., and Kornfeld, H. (1997) Infect. Immun. 65 5149-5156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin, Q., Weis, S., Yang, G., Weng, Y. H., Helston, R., Rish, K., Smith, A., Bordner, J., Polte, T., Gaunitz, F., and Dennery, P. A. (2007) J. Biol. Chem. 282 20621-20633 [DOI] [PubMed] [Google Scholar]
- 34.Alley, E. W., Murphy, W. J., and Russell, S. W. (1995) Gene 158 247-251 [DOI] [PubMed] [Google Scholar]
- 35.King, G. M. (2003) Appl. Environ. Microbiol. 69 7257-7265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez, G. M., Voskuil, M. I., Gold, B., Schoolnik, G. K., and Smith, I. (2002) Infect Immun. 70 3371-3381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dulak, J., and Jozkowicz, A. (2003) Acta Biochim. Pol. 50 31-47 [PubMed] [Google Scholar]
- 38.Tenhunen, R., Marver, H. S., and Schmid, R. (1968) Proc. Natl. Acad. Sci. U. S. A. 61 748-755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tenhunen, R., Marver, H. S., and Schmid, R. (1969) J. Biol. Chem. 244 6388-6394 [PubMed] [Google Scholar]
- 40.Maines, M. D. (1988) FASEB J. 2 2557-2568 [PubMed] [Google Scholar]
- 41.Piantadosi, C. A. (2002) Antioxid. Redox. Signal 4 259-270 [DOI] [PubMed] [Google Scholar]
- 42.Alcaraz, M. J., Fernandez, P., and Guillen, M. I. (2003) Curr. Pharm. Des. 9 2541-2551 [DOI] [PubMed] [Google Scholar]
- 43.Singh, A., Guidry, L., Narasimhulu, K. V., Mai, D., Trombley, J., Redding, K. E., Giles, G. I., Lancaster, J. R., Jr., and Steyn, A. J. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 11562-11567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tremblay, G. A. (2007) Int. J. Tuberc. Lung Dis. 11 722-732 [PubMed] [Google Scholar]
- 45.Devadas, K., and Dhawan, S. (2006) J. Immunol. 176 4252-4257 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.