Abstract
The HIV-1 accessory protein Nef enhances virus infectivity by facilitating an early post-entry step of infection. Nef acts in the virus producer cell, leading to a beneficial modification to HIV-1 particles. Nef itself is incorporated into HIV-1 particles, where it is cleaved by the viral protease during virion maturation. To probe the role of virion-associated Nef in HIV-1 infection, we generated a fusion protein consisting of the host protein cyclophilin A (CypA) linked to the amino terminus of Nef. The resulting CypA-Nef protein enhanced the infectivity of Nef-defective HIV-1 particles and was specifically incorporated into the virions via association with Gag during particle assembly. Pharmacologic or genetic inhibition of CypA-Nef binding to Gag prevented incorporation of CypA-Nef into virions and inhibited infectivity enhancement. Our results indicate that infectivity enhancement by Nef requires its association with a component of the assembling HIV-1 particle.
Introduction
Human immunodeficiency virus type 1 (HIV-1) and other lentiviruses encode accessory proteins in addition to the prototypic gag, pol and env open reading frames. One of these, Nef, is found only in primate lentiviruses and is required for efficient HIV-1 replication in primary CD4+ T cells, macrophages, and some T cell lines (Miller et al., 1994; Spina et al., 1994). Inactivation of Nef in HIV-1 and the related simian immunodeficiency virus (SIV) leads to attenuated replication and delayed onset of disease in the infected host (Kestler III et al., 1991; Learmont et al., 1999). Expression of Nef also has profound effects on cells, including downmodulation of cell surface CD4 and MHC class I expression as well as enhancement of T cell activation (Baur et al., 1994; Garcia and Miller, 1991; Kestler III et al., 1991; Schwartz et al., 1996).
Nef-defective viral particles are 4–40 fold less infectious than wild type HIV-1 in single cycle infection assays (Aiken and Trono, 1995; Chowers et al., 1994; Miller et al., 1994). Despite intensive studies, the molecular mechanism by which Nef enhances HIV-1 infectivity remains unclear. Nef does not markedly enhance the efficiency of HIV-1 entry into cells, nor does it appear to modulate the intrinsic stability of the HIV-1 capsid shell that surrounds the viral ribonucleoprotein complex (Cavrois et al., 2004; Day, Munk, and Guatelli, 2004; Forshey and Aiken, 2003; Tobiume et al., 2003). Nef-defective HIV-1 particles contain normal quantities of reverse transcriptase and enter cells efficiently but are impaired for reverse transcription in target cells (Aiken and Trono, 1995; Chowers et al., 1995; Schwartz et al., 1995). Wild type and Nef-defective virions exhibit similar protein compositions (Miller et al., 1995; Schwartz et al., 1995), except when produced in cells expressing CD4, where Nef-induced CD4 down regulation promotes incorporation of the viral envelope glycoproteins into HIV-1 particles (Lama, Mangasarian, and Trono, 1999; Lundquist, Zhou, and Aiken, 2004; Ross, Oran, and Cullen, 1999). Expression of Nef in trans in the virus-producing cell, but not in the target cell, complements the impaired infectivity of Nef-defective HIV-1 particles (Aiken and Trono, 1995; Miller et al., 1995). A recent study shows that the host protein dynamin 2 is required for HIV-1 infectivity enhancement by Nef (Pizzato et al., 2007), and Nef has been reported to promote incorporation of cholesterol into HIV-1 particles (Zheng et al., 2003). A notable difference between wild type and Nef-defective virions is the presence of Nef itself in HIV-1 particles (Bukovsky et al., 1997; Pandori et al., 1996; Welker et al., 1996), where it is cleaved by the viral protease. Collectively, these observations suggest that Nef may promote HIV-1 infection by modulating the budding virion to facilitate uncoating, intracellular transport, or reverse transcription in target cells.
Incorporation of Nef into HIV-1 particles has been correlated with infectivity enhancement. Incorporation of Nef into HIV-1 particles depends on its association with cell membranes: mutations that prevent its myristoylation, as well as those in its amino terminal basic domain, result in impaired virion incorporation as well as reduced HIV-1 infectivity (Aiken and Trono, 1995; Welker et al., 1998). Mutations in a C-terminal region of Nef were also found to inhibit virion incorporation of Nef and HIV-1 infectivity (Zheng et al., 2003). However, a limitation of genetic loss-of-function studies is the possibility of pleiotropic effects of the mutations; such studies must therefore be interpreted with caution. Thus, a genetic correlation of infectivity enhancement with the presence of Nef in HIV-1 particles does not necessarily establish a role of virion-associated Nef in HIV-1 infection.
The host cell protein cyclophilin A (CypA) also promotes HIV-1 infection. CypA is a peptidylprolyl isomerase and the target of the immunosuppressive drug cyclosporine A (CsA). CsA inhibits HIV-1 replication in primary T cells and established T cell lines (Bartz et al., 1995; Karpas et al., 1992; Wainberg et al., 1988). CypA is incorporated into HIV-1 particles via association with the CA region of Gag (Franke, Yuan, and Luban, 1994; Thali et al., 1994a). Mutations in Gag that inhibit CypA binding also reduce HIV-1 infectivity, as does production of HIV-1 particles in the presence of CsA (Braaten, Franke, and Luban, 1996; Franke, Yuan, and Luban, 1994; Thali et al., 1994a). These findings suggested that incorporation of CypA into HIV-1 particles is necessary of optimal infectivity. However, recent studies using CypA-deficient cells have shown that the decrease in HIV-1 infectivity observed when virions are produced in the presence of CsA is independent of the CypA-Gag interaction (Hatziioannou et al., 2005; Sokolskaja, Sayah, and Luban, 2004). Thus, it appears that the engagement of the viral capsid by target cell CypA following HIV-1 entry promotes HIV-1 infection.
In the present study, we exploited the ability of CypA to be specifically incorporated into HIV-1 particles to examine the role of producer cell Nef in HIV-1 infectivity. By analyzing the effects of an engineered CypA-Nef fusion protein, we obtained novel evidence that association of Nef with the assembling virion is necessary for enhancement of HIV-1 infectivity by the viral protein.
Results
Expression of a CypA-Nef fusion protein
To regulate incorporation of Nef into HIV-1 particles, we constructed a cDNA encoding a fusion protein consisting of CypA fused to the amino terminus of HIV-1 Nef (Fig. 1A). Because Nef is myristylated at its amino terminus and this modification is required for incorporation into HIV-1 particles during their assembly, we expected that virion incorporation of the resulting CypA-Nef fusion protein (CypA-Nef) would require interaction of the CypA region of the molecule with Gag during assembly. Both Nef and CypA-Nef were efficiently expressed in transfected 293T cells, as detected by immunoblotting using a Nef-specific antiserum (Fig. 1B). As a control for CypA-Nef, we also generated CypA expression plasmid. To circumvent the interfering immunoblotting signal from the endogenous CypA, HA-tagged CypA and HA-tagged CypA-Nef were also constructed. These proteins were expressed under control of the CMV promoter and were produced at similar levels (Fig. 1B). Immunoblot analysis of endogenous CypA and β-actin levels demonstrated that overexpression of Nef, CypA-Nef, HA-CypA, or HA-CypA-Nef did not significantly alter the endogenous level of CypA in 293T cells (data not shown).
Fig. 1.
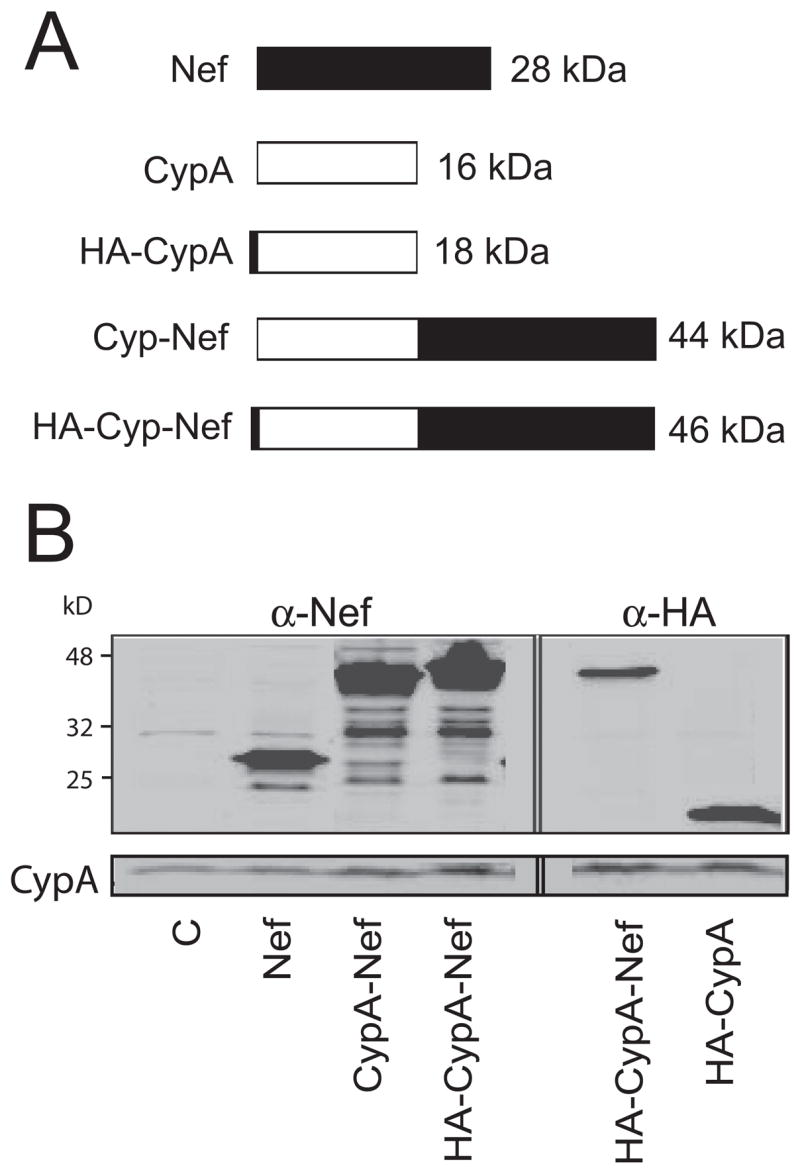
Protein constructs used in this study and their expression in 293T cells. (A) Schematic of the five proteins used in this study. (B) Expression of the proteins in 293T cells. Cell lysates were analyzed by immunoblotting. Proteins in the left panel were detected with rabbit antibody for Nef, and proteins in the right panel were detected with rat antibody for HA tag. Abbreviations are as follows: C, control vector; WT, wild type HIV-1; CypA, cyclophilin A; HA: hemagglutinin epitope tag; CA, viral capsid protein.
CypA-Nef enhances the infectivity of Nef-defective HIV-1
To determine whether CypA-Nef can functionally complement the reduced infectivity of Nef-defective particles, we cotransfected 293T cells with a Nef-defective HIV-1 provirus and plasmids encoding CypA-Nef or Nef and analyzed the infectivity of the resulting virus stocks by titration on P4 reporter cells. The P4 cell line is a Hela cell derivative that has been modified to express human CD4 and contains a stably-transfected Tat-responsive lacZ reporter. Infection of these cells with HIV results in expression of Tat and expression of β-galactosidase, thus allowing quantitation of infected cells following visualization by staining with X-gal. The infectivity of Nef-defective HIV-1 particles was enhanced by CypA-Nef by approximately three-fold, to a level approximately one-half that of wild type HIV-1 but similar to the infectivity of Nef-defective HIV-1 particles complemented by a Nef-expression plasmid (Fig. 2A). To determine whether the Nef portion of CypA-Nef is necessary for infectivity enhancement, we produced Nef-defective HIV-1 particles by cotransfection of 293T cells with a vector encoding only CypA. The results indicated that exogenous CypA alone did not enhance the infectivity of Nef-defective virions (Fig. 2B). Viruses were also produced by cotransfection of Nef-defective HIV-1 provirus with plasmids encoding the HA-tagged proteins. The resulting viruses were then assayed for infectivity. HA-CypA-Nef enhanced the infectivity of Nef-defective HIV-1 while HA-CypA did not (Fig. 2B). Together, these data suggest that CypA-Nef enhances infectivity by a mechanism that depends on the Nef portion of the fusion protein. Therefore, the strategy could be useful for testing the requirement for association of Nef with the assembling virion in HIV-1 infectivity enhancement.
Fig. 2.
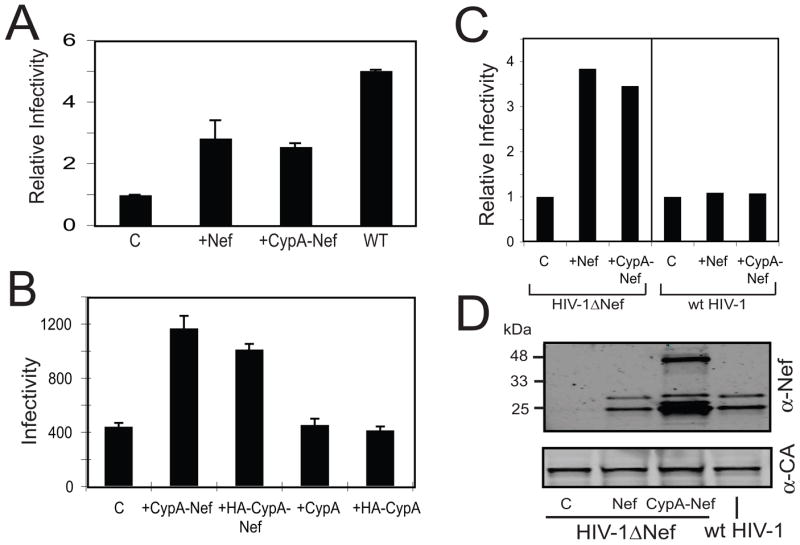
CypA-Nef is incorporated into Nef-defective HIV-1 particles and enhances their infectivity. Viruses were produced in 293T cells by cotransfection with a Nef-defective HIV-1 provirus together with plasmids encoding the indicated proteins. Wild type (WT) HIV-1 was tested as a control. Virus supernatants were harvested and assayed for p24 and titrated on P4 indicator target cells. Infectivity was determined as the number of infected cells per ng of p24 in the respective inocula. (A) Shown are the relative infectivity values obtained from 3 independent experiments, with error bars representing one standard deviation. Infectivity values were normalized to that of the control Nef-defective virus. (B) The infectivity results from a representative single experiment. Error bars represent the standard deviation of triplicate assays of each virus. (C) Wild type and Nef-defective virions were produced by cotransfection with constructs encoding Nef or CypA-Nef. The resulting supernatants were assayed for infectivity on P4 indicator cells. Infectivity is shown relative to the corresponding Nef-defective and wild type control viruses to illustrate the differential effects of expression of Nef and CypA-Nef on the two viruses. (D) Immunoblot analysis of pelleted virus particles with antibodies specific for Nef and CA. The results shown in this figure are representative of 2 independent experiments.
To examine whether CypA-Nef functionally substitutes for Nef in HIV-1 infection, we asked whether this fusion protein could also enhance the infectivity of wild type HIV-1 particles. To test this, cells were cotransfected with wild type and Nef-defective HIV-1 proviruses together with Nef or CypA-Nef expression vectors, and the resulting virus stocks were assayed for infectivity. CypA-Nef and Nef enhanced the infectivity of wild type HIV-1 only slightly, while both Nef and CypA-Nef enhanced the infectivity of Nef-defective HIV-1 by three-fold (Fig. 2C). These results indicate that CypA-Nef expression during HIV-1 particle production specifically complements the infectivity impairment resulting from the lack of Nef.
CypA-Nef is incorporated into the virion and associates with the viral core
To test whether CypA-Nef is incorporated into HIV-1 particles, we purified the virions from the culture supernatants and prepared lysates of the transfected 293T cells. Analysis of protein immunoblots revealed the presence of Nef and CypA-Nef in viral particles (Fig. 2D). Two major bands were observed in the particles containing CypA-Nef, corresponding in size to the full-length fusion protein and a product equivalent in mobility with the large fragment of Nef resulting from cleavage of Nef by the viral protease. Thus both cleaved and uncleaved forms of CypA-Nef were detected in HIV-1 particles, consistent with the known ability of virion-associated Nef to be cleaved by the viral protease (Bukovsky et al., 1997; Pandori et al., 1996; Welker et al., 1996). We have not yet determined whether cleavage of CypA-Nef is mediated by the viral protease or is cleaved at the same position as Nef itself. In some experiments, the level of virion-associated CypA-Nef was significantly greater than that of Nef when provided in trans by cotransfection, suggesting that the fusion protein may be more efficiently incorporated into HIV-1 particles than Nef itself. We conclude that CypA-Nef is efficiently incorporated into HIV-1 particles.
In previous studies, our laboratory reported that Nef, though associated with the HIV-1 core, does not modulate the intrinsic stability of the viral core (Forshey and Aiken, 2003; Kotov et al., 1999). To determine whether CypA-Nef had an effect on the yield of cores, which is a measure of their intrinsic stability (Forshey et al., 2002), we purified viral cores from Nef-defective HIV-1 particles produced by cotransfection with Nef or CypA-Nef expression constructs. The CA protein was detected at similar levels in association with cores recovered from the two viruses (Fig. 3A), suggesting that CypA-Nef does not alter the intrinsic stability of the HIV-1 core. Samples of purified cores were then analyzed by immunoblotting for the presence of Nef and CypA-Nef. Both proteins were detected in the purified cores at comparable levels, suggesting that CypA-Nef, like Nef, associates with the mature HIV-1 core (Fig. 3B). This intravirion association is likely dependent on the Nef region of CypA-Nef, since both full-length CypA-Nef and the cleaved fragment of Nef were copurified with the core structures, and the bulk of virion-associated CypA is removed upon purification of HIV-1 cores ((Welker et al., 2000); our unpublished observations).
Fig. 3.
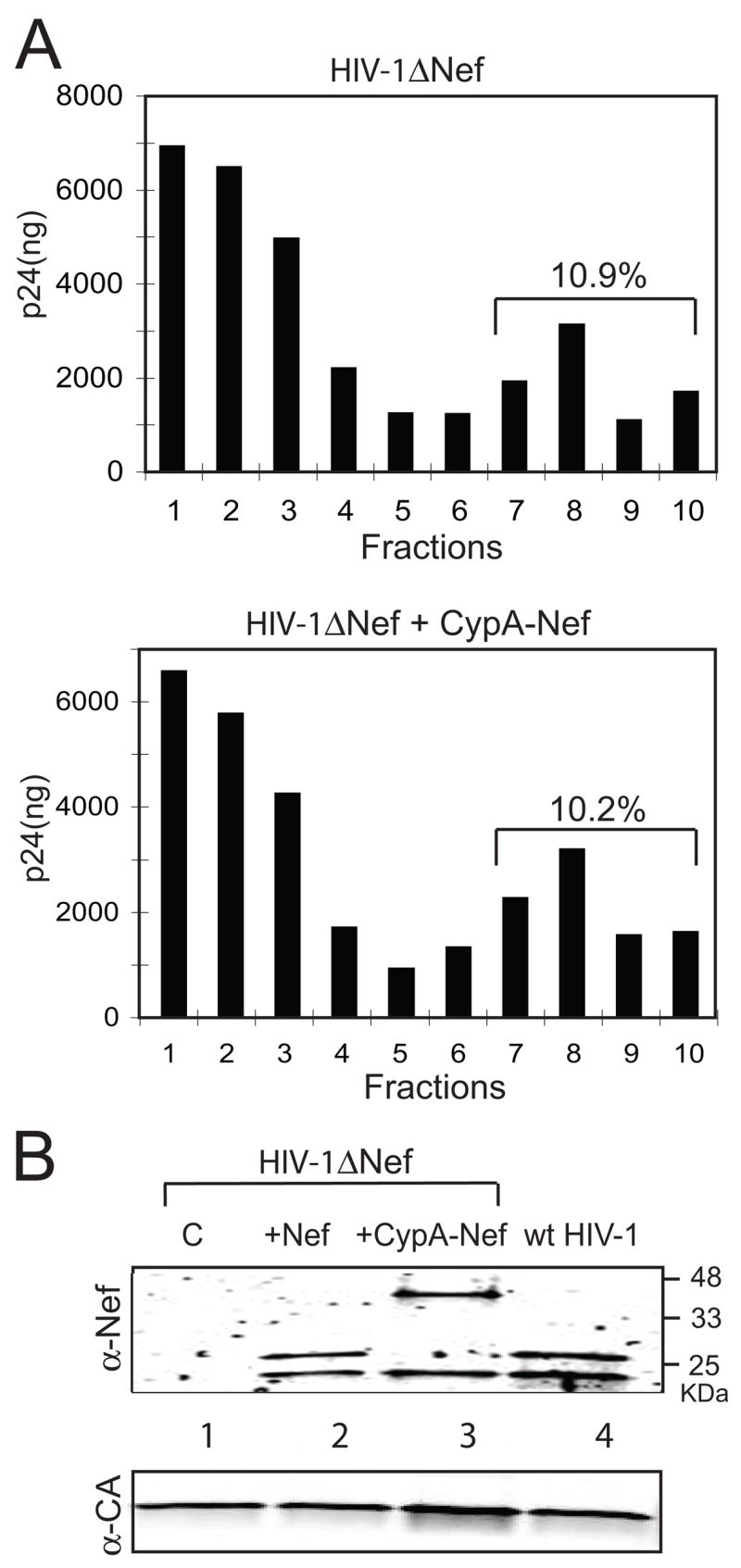
CypA-Nef copurifies with HIV-1 cores. Concentrated HIV-1 particles were sedimented through a layer containing 0.5% Triton X-100 into a linear sucrose density gradient. (A) Fractions were collected from the top and p24 levels were quantified by ELISA. Top panel: Nef-defective HIV-1; bottom panel: Nef-defective HIV-1 produced by contransfection with the CypA-Nef expression construct. (B) Immunoblot analysis of purified HIV-1 cores. Cores present in the peak fraction (fraction 8) from each gradient were pelleted and analyzed by immunoblotting using antibodies specific for Nef and CA. Lane 1: Nef-defective HIV-1 cores; lane 2: cores isolated from Nef-defective HIV-1 particles produced in cells expressing wild type Nef; lane 3: cores isolated from Nef-defective HIV-1 particles produced in cells expressing CypA-Nef; lane 4: wild type HIV-1 cores. The upper and lower panels show the proteins detected with antibodies to Nef and CA, respectively.
Genetic correlation of Nef and CypA-Nef function in HIV-1 infectivity enhancement
Point mutations in Nef have been shown to impair HIV-1 infectivity enhancement (Craig, Pandori, and Guatelli, 1998; Fackler et al., 2006; Goldsmith et al., 1995; Lundquist et al., 2002). To further probe the issue of whether CypA-Nef enhances the infectivity of Nef-defective HIV-1 particle by a Nef-related mechanism, we constructed four CypA-Nef mutants encoding specific substitutions in the Nef portion of the protein. Nef.4E-4Q and Nef.EKH40AAA were competent for enhancement of HIV-1 infectivity, while HIV-1 encoding Nef.69A/72A and Nef.KEK96AAA were poorly infectious (Fig. 4A). When we cotransfected CypA-Nef plasmids that contain the same mutations together with Nef-defective provirus, we observed that the mutant proteins enhanced the infectivity of Nef-defective virions differently. CypA-Nef.4E-4Q and CypA-Nef.EKH40 enhanced the infectivity of Nef-defective virions by 2.5-fold, while CypA-Nef.69A/72A and CypA-Nef.KEK96AAA failed to enhance the infectivity of Nef-defective virions (Fig. 4B). We observed a statistically significant correlation of the infectivity enhancement by CypA-Nef mutants in trans and Nef mutants in cis (R2=0.9991; p<0.05 by Spearman’s rank correlation test; Fig. 4C). Immunoblot analysis confirmed that the mutant CypA-Nef proteins were efficiently incorporated into HIV-1 particles (Fig. 4D). These results suggest that Nef and CypA-Nef employ a common mechanism to enhance the infectivity of Nef-defective virions.
Fig. 4.
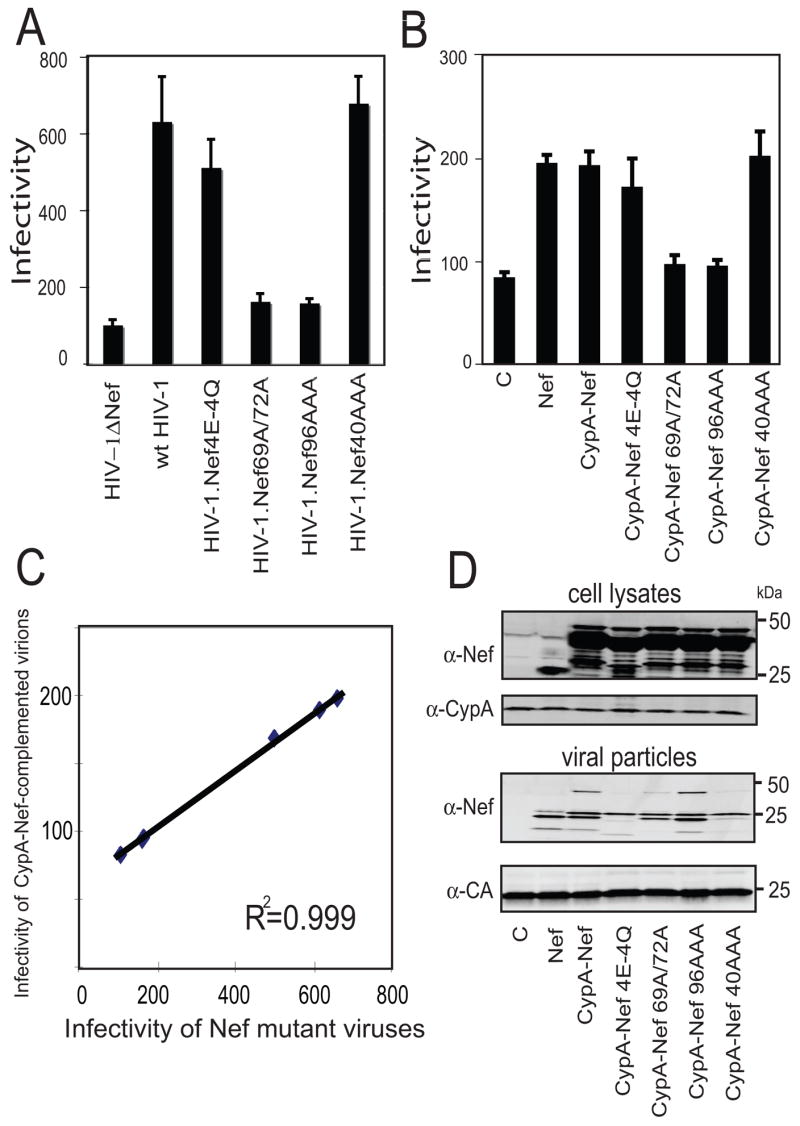
Point mutations in the Nef portion of CypA-Nef abolish infectivity enhancement. Viral supernatants were harvested and assayed for infectivity on P4 cells. The results shown are representative of 3 independent experiments. (A) Infectivity of HIV-1 mutants encoding substitutions in nef. (B) Infectivity of virions generated by cotransfection of the CypA-Nef mutants and nef-defective provirus. (C) Plot of infectivity of Nef mutant viruses (abscissa) vs. viruses complemented with the corresponding mutant CypA-Nef proteins (ordinate). (D) Immunoblot analysis of CypA-Nef expression in 293T cells (upper panel) and incorporation into Nef-defective HIV-1 particles (lower panel).
CsA treatment inhibits virion incorporation of CypA-Nef and infectivity enhancement
To determine whether enhancement of HIV-1 infectivity by CypA-Nef requires its association with Gag in the virus-producing cell, we produced viruses by culturing the transfected 293T cells in the presence and absence of CsA to inhibit the binding of CypA to Gag. This approach has been used previously to prevent the incorporation of endogenous CypA into HIV-1 particles. Viruses were harvested and assayed for infectivity and assayed by immunoblotting for levels of CypA-Nef. CsA treatment reduced the infectivity of control Nef-defective virions by approximately three-fold (Fig. 5A), consistent with the well documented ability of CsA to inhibit HIV-1 infectivity when present at the time of virus production (Aiken, 1997; Braaten, Franke, and Luban, 1996; Steinkasserer et al., 1995; Thali et al., 1994b). By contrast, CsA reduced the infectivity of the virions produced by cotransfection of CypA-Nef by about 9-fold, effectively abolishing the positive effect of CypA-Nef on HIV-1 infectivity (Fig. 5A). As a control, we also tested the effects of CsA on Nef-defective virions produced by coexpression of Nef. CsA inhibited the infectivity of this virus by 5-fold (Fig. 5A), consistent with the reported greater sensitivity of wild type vs. Nef-defective HIV-1 to CsA (Aiken, 1998). Immunoblot analysis of the pelleted virions revealed that CsA reduced the level of virion-associated CypA-Nef but not Nef itself (Fig. 5B). Analysis of cell lysates indicated that CsA treatment did not significantly alter the expression of CypA-Nef or Nef (Fig. 5B). Thus, treatment of virus-producer cells inhibited HIV-1 infectivity enhancement by CypA-Nef and its incorporation of the protein into virions. Collectively, these results suggest that enhancement of HIV-1 infectivity by CypA-Nef is dependent on its interaction with the assembling virion and possibly its incorporation into virions.
Fig. 5.
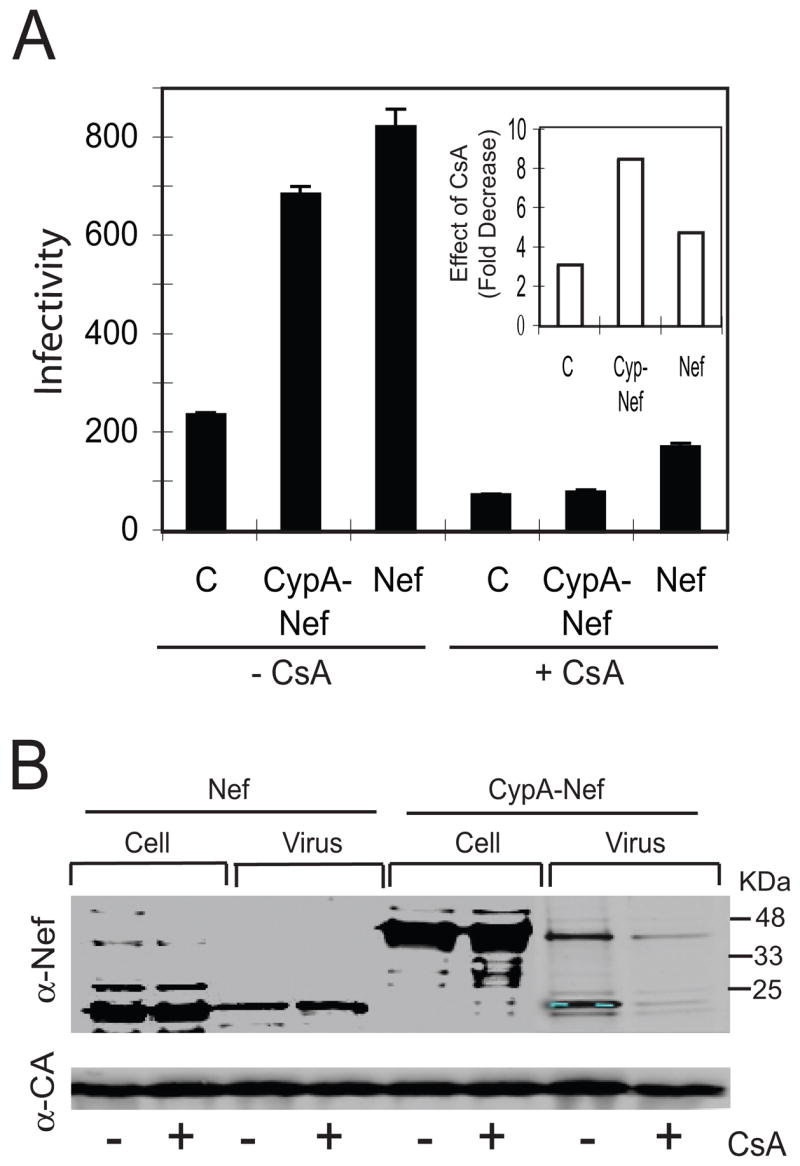
CsA inhibits HIV-1 incorporation of CypA-Nef and its ability to enhance viral infectivity. Viruses were produced in transfected cells cultured in the presence and absence of 5 μM CsA. (A) Infectivity of viruses on P4 indicator cells. Inset: fold inhibition of infectivity by CsA. Shown are the mean values of triplicate infections; results are representative of two independent experiments. (B) Immunoblot analysis of cell lysates and pelleted viruses using antibodies specific for Nef and CA.
Mutations in the CypA binding site of Gag binding site diminish both HIV-1 incorporation of CypA-Nef and infectivity enhancement
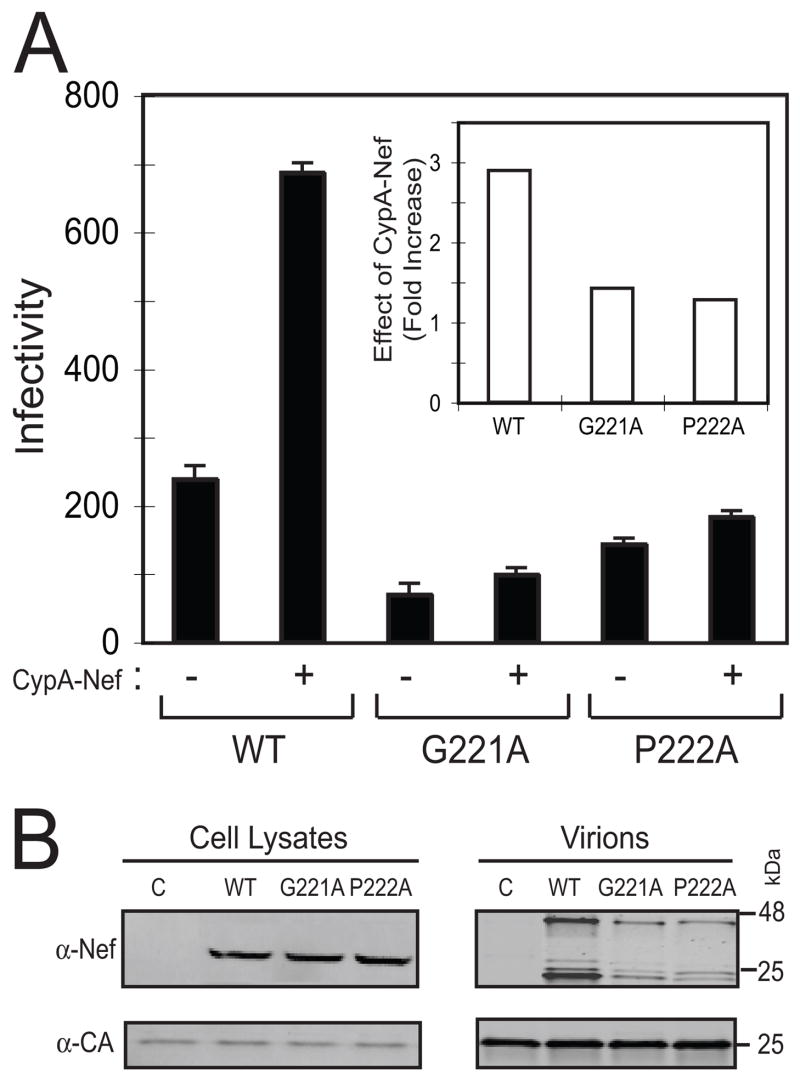
Incorporation of cellular CypA into HIV-1 particles is also dependent on the sequence of specific loop on the surface of the CA protein (Franke, Yuan, and Luban, 1994; Gamble et al., 1996; Thali et al., 1994a). To further test whether HIV-1 incorporation of CypA-Nef is correlated with infectivity enhancement, we used two well-characterized HIV-1 Gag mutants, G221A and P222A, that inhibit CypA binding. Importantly, the ability of Nef to enhance HIV-1 infectivity is unaffected by either of these Gag mutations (Aiken, 1998). Additional control studies confirmed that the mutations do not impair the ability of nef-defective HIV-1 infectivity to be is enhanced by expression of Nef in trans (Supplementary Fig. 1). The mutant viruses were produced by cotransfection with the CypA-Nef expression construct, and the resulting virus particles were assayed for infectivity and analyzed by immunoblotting to determine the levels of CypA-Nef incorporation. By contrast to the control virus lacking these mutations, CypA-Nef enhanced the infectivity of the G221A and P222A mutant viruses only slightly (Fig. 6A). The levels of virion-associated CypA-Nef were also decreased in the Gag mutants despite equivalent cellular expression (Fig. 6B).
Fig. 6.
Mutations in the CypA-binding site in Gag inhibit HIV-1 incorporation of CypA-Nef and infectivity enhancement. Viruses were produced by cotransfection of Nef-defective proviruses containing the indicated mutations in Gag with plasmids encoding Nef or CypA-Nef. (A) Infectivity of the viruses. Inset: ratio of infectivity of viruses produced with and without coexpression of CypA-Nef. (B) Immunoblot analysis of cell lysates and pelleted viruses using antibodies specific for Nef and CA.
To further test the requirement for CypA-Nef binding to Gag in HIV-1 infectivity enhancement, we analyzed the effects of a substitution (R55A) in the CypA region of CypA-Nef that interferes with HIV-1 incorporation of CypA (Dorfman et al., 1997). This protein, CypA.R55A-Nef, was expressed in cells at levels similar to wild type CypA-Nef, but its incorporation into HIV-1 particles was markedly reduced and it failed to enhance the infectivity of Nef-defective HIV-1 (Supplemental Fig. 2). Thus, a mutation in the CypA region of CypA-Nef prevented incorporation of the fusion protein into HIV-1 particles and suppressed its ability to enhance HIV-1 infectivity. We conclude that association of CypA-Nef with the assembling viral particle is necessary for infectivity enhancement.
Discussion
Despite significant efforts on the part of several laboratories, the mechanism by which Nef enhances HIV-1 infectivity remains poorly defined. Nef modifies HIV-1 particles in such a way as to facilitate an early post-penetration step in infection. One consistently observed Nef-specific virion modification is incorporation of Nef itself into HIV-1 particles. In this study, we fused CypA to Nef to allowed controlled incorporation of Nef into HIV-1 particles via association with Gag during particle assembly. CypA-Nef specifically enhanced the infectivity of Nef-defective HIV-1 particles. CypA-Nef was incorporated into the newly formed viral particles; genetic or pharmacologic inhibition of the interaction Gag-CypA-Nef interaction resulted in reduced the ability of CypA-Nef to be incorporated into HIV-1 particles and to enhance virus infectivity. Furthermore, mutations in the Nef that inhibit HIV-1 infectivity also inactivated CypA-Nef, thus demonstrating that the infectivity enhancement by CypA-Nef mutants is correlated with the infectivity enhancement by the same Nef mutants. CypA-Nef was detected in purfied HIV-1 cores, suggesting that the Nef component determines the subviral localization. Collectively, these results indicate that CypA-Nef and Nef enhance the infectivity of Nef-defective virions by a common mechanism.
Our data suggest that interaction of Nef with the assembling HIV-1 particle is required for infectivity enhancement. Mutations in the CypA segment of CypA-Nef, or in Gag, that prevented the interaction of the two proteins also inhibited infectivity enhancement, as did addition of CsA during virus assembly. It is possible that Nef must be incorporated into HIV-1 particles to enhance infectivity, but our data do not definitively establish this as Nef may act prior to virus budding and maturation and incorporation into virions may be a by-product of Nef’s accumulation at the site of virus assembly. In a previous study, Fackler and coworkers reported that the 4E4Q Nef mutant enhances HIV-1 infectivity yet is poorly incorporated into HIV-1 particles (Fackler et al., 2006). In attempts to confirm their observations, we have found that the 4E4Q mutant Nef protein is readily detectable in pelleted HIV-1 particles rigorously purified by velocity gradient sedimentation (our unpublished data). In additional studies, we attributed the discrepancy with the Fackler data to the antibody used for detection. Therefore, virion incorporation of Nef is correlated with infectivity enhancement, and a role for virion-associated Nef in HIV-1 infection remains a viable hypothesis.
In this study, we exploited the specific binding of CypA to Gag to target Nef the assembling virion as a fusion with CypA. However, the specific mechanism by which Nef itself engages the virus assembly complex remains unclear. Incorporation of Nef into HIV-1 particles depends on its membrane-binding ability, suggesting that Nef may be incorporated into particles passively by being present at the membrane where the virus buds. A fraction of cellular Nef protein copurifies with detergent-resistant membrane domains known as lipid rafts (Giese et al., 2006; Wang et al., 2000; Zheng et al., 2001). These and other distinct membrane structures have been implicated in HIV-1 assembly (Ding et al., 2003; Holm et al., 2003; Ono and Freed, 2001). It is therefore plausible that Nef localizes to membranes on which HIV-1 assembly occurs. Additional evidence suggests that Nef interacts specifically with internal components of the virion. Nef binds the transframe protein p6* in Gag-Pol, and can interact with both reverse transcriptase and integrase proteins (Ciuffi et al., 2004). Nef also copurifies with isolated HIV-1 cores, suggesting that it relocalizes from the viral membrane to the core during maturation (Kotov et al., 1999). Uncoating of HIV-1 cores in vitro results in dissociation of CA and RT, releasing an uncoated ribonucleoprotein complex with which Nef remains associated (Forshey and Aiken, 2003). Thus, while membrane binding is essential for incorporation of Nef into HIV-1 particles, Nef may perform its function by specifically targeting an internal component of the HIV-1 core.
How would a Nef-mediated virion modification lead to enhanced infectivity? Several biochemical virion modifications have been attributed to Nef, including phosphorylation of MA (Swingler et al., 1997), stimulation of Env incorporation (Schiavoni et al., 2004), and elevation of virion-associated cholesterol and ganglioside GM1 (Zheng et al., 2003; Zheng et al., 2001). Thus, Nef may promote infectivity via multiple effects on virion composition. Inhibition of proteasome activity enhances cellular permissiveness to HIV-1 infection (Schwartz et al., 1998; Wei et al., 2005), and we recently showed that addition of proteasome inhibitors during virus inoculation preferentially enhances the infectivity of Nef-defective particles, effectively reducing HIV-1 dependence on Nef (Qi and Aiken, 2007). This finding suggests that Nef-defective virions are hypersusceptible to proteasomal interference in the target cell. We hypothesize that a Nef-dependent modification of the assembling virion renders it less susceptible to proteasome-dependent restriction in target cells. Despite the attractiveness of this model, Dueck and coworkers have recently reported evidence suggesting that the stimulatory effect of proteasome inhibitors on HIV-1 infection results from an indirect effect on the cell cycle rather than an antiviral effect of the proteasome (Dueck and Guatelli, 2007). They were also unable to confirm our observation that the Nef-defective HIV-1 infection impairment is relieved by proteasome inhibitors (Qi and Aiken, 2007). We suspect that the latter discrepancy is due to differences in experimental conditions. Though the extent of rescue of the Nef-defective phenotype by proteasome inhibitors varies between individual experiments, we have consistently observed this effect and stand by the conclusions of our previous study. It remains unclear whether the preferential enhancement of Nef-defective HIV-1 by proteasome inhibitors is due to elevated susceptibility of Nef-defective HIV-1 to cytoplasmic degradation or a cell-cycle-dependent selective block to Nef- particles, and both are possibilities.
Nef may promote proteasomal evasion directly by tethering the viral core it to a cellular trafficking pathway, thereby facilitating intracytoplasmic transport. Nef contains several motifs that are involved in trafficking in the endocytic pathway, and associates with known cellular trafficking molecules and pathways (reviewed in (Bresnahan et al., 1998; Craig, Pandori, and Guatelli, 1998; Erdtmann et al., 2000; Fackler et al., 2006; Greenberg, Iafrate, and Skowronski, 1998; Janvier et al., 2001; Lacaille and Androlewicz, 2000; Mandic et al., 2001). Thus, Nef could promote association of the core with components of the endocytic machinery. Several observations are consistent with this model. First, the impaired infectivity of Nef-defective HIV-1 particles is relieved by pseudotyping by heterologous viral envelopes which target viral entry to an endocytic pathway, such as those of vesicular stomatitis virus and Ebola virus (Aiken, 1997; Chazal et al., 2001; Luo et al., 1998). This not the case for other heterologous Env proteins that do not render HIV-1 infection dependent on endosomal acidification, such as the amphotropic murine leukemia virus (Aiken and Trono, 1995; Miller et al., 1995). Thus, endocytic entry of HIV-1 may physically bypass an intracellular restriction to the Nef-defective viral core. Second, mutations in a dileucine motif in Nef inhibit both CD4 downregulation and infectivity enhancement (Craig, Pandori, and Guatelli, 1998), suggesting that Nef may bind a single cellular factor required for both of these activities. Finally, the infectivity of Nef-defective HIV-1 particle is preferentially enhanced by treatment of target cells with chemicals that depolymerize the cortical actin cytoskeleton (Campbell, Nunez, and Hope, 2004), suggesting that this network forms a barrier to intracytoplamic transport of Nef-defective HIV-1 cores. Despite the attractiveness of the model, the evidence in support of a role of Nef in facilitating intracellular trafficking of the HIV-1 cores is largely circumstantial.
The ability of CypA-Nef to enhance HIV-1 infectivity may facilitate identification of the essential functional regions of Nef. Nef is composed of two discrete domains, a flexible linker domain and a globular core domain (Barnham et al., 1997; Grzesiek et al., 1996; Lee et al., 1996a; Lee et al., 1996b); reviewed in (Geyer, Fackler, and Peterlin, 2001), and thus far it has not been possible to segregate the membrane-binding function of Nef from infectivity enhancement. By targeting Nef to the virion via CypA, it should be possible to identify the minimal Nef determinants of infectivity enhancement from those necessary for its association with the assembling virus.
Materials and Methods
Cells and viruses
293T and HeLa-CD4/LTR-lacZ (P4) cells were cultured in Dulbecco’s modified Eagle medium (Cellgro) supplemented with 10% fetal bovine serum, penicillin (50 IU/ml), and streptomycin (50 μg/ml) at 37°C and 5% CO2. The HIV-1 proviral DNA constructs R7 and R7 Nef (Aiken and Trono, 1995) were used for these studies. This viral clone encodes a CXCR4-dependent HIV-1 envelope protein. The Gag mutants G221A and P222A have been described (Aiken, 1998). VSV-G-pseudotyped HIV-1 particles were produced as previously described (Aiken, 1997). Viruses encoding amino acid substitutions in Nef were based on the X4-tropic R9 viral clone and were previously described (Lundquist et al., 2002). Viruses were produced by polyethyleneimine (PEI) transfection of 293T cells (10 μg of plasmid DNA per 5 × 106 cells) (Durocher, Perret, and Kamen, 2002). Where indicated, CsA (Sandoz, Inc.) was added to a final concentration of 5 μg/ml 12h after transfection. One day later, the culture supernatants were harvested and clarified by passing through 0.45-μm pore size syringe filters, and aliquots were frozen at −80°C. The CA contents of the virus stocks were quantified by p24 enzyme-linked immunosorbent assay (ELISA), as previously described. The P4 cell line, a Hela clone engineered to express CD4 and an integrated long terminal repeat (LTR)-lacZ reporter cassette, was used to quantify HIV-1 infectivity (Charneau, Alizon, and Clavel, 1992). HIV-1 stocks were serially diluted in culture medium, and samples (0.125 ml) were used to inoculate P4 target cells seeded the day before (20,000 cells per well in 48-well plates). Two hours after inoculation, the cultures were fed with additional medium (0.5 ml) and cultured for another 48 h prior to being stained with 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-gal) to detect infected cells. To determine the number of infected cells per well, individual wells were visualized using a charge-coupled device camera equipped with a macro lens, and blue cells were counted using NIH Image software. Infections were performed in triplicate, and only values within the linear range of the infection assay (50–500 blue cells per well) were used to calculate infectivity. Infectivity was calculated as the number of blue cells per ng of p24 added to the well.
Expression plasmids
All expression plasmids in this study were generated in the CMX-PL1 expression vector. Fusion proteins were produced by PCR splice overlap extension. The CypA-Nef cDNA was generated by PCR overlap fusion using the primers 5′-actgtggacaactcgagggtggcaagtggtcaaaaa-3′ (sense) and 5′-tttttgaccacttgccaccctcgagttgtccacagt-3′ (antisense). The HA tags and R55A mutation were also introduced by PCR. The primer for HA-CypA was: 5′-aaggatccaccatgtacgatgttccagattacgctcttgtcaaccccaccgtgttc-3′. The primers for generating the R55A mutation in CypA were: 5′-tcctgctttcacgcaattatcccggggtttatg-3′ (sense) and 5′-cataaaccccgggataattgcgtgaaagcagga-3′ (antisense). PCR fragments were inserted into the CMV promoter-based expression plasmid CMX-PL1 using the BamHI and PstI restriction sites. The constructs were sequenced to confirm the presence of the desired open reading frame and the absence of undesired mutations.
Isolation of HIV-1 cores
Native HIV-1 cores were isolated from concentrated virions as previously described (Forshey et al., 2002; Kotov et al., 1999). Fractions containing HIV-1 cores were identified by quantifying CA by p24 ELISA and by determining the densities of the gradient fractions by refractometric analysis. Cores present in the peak fractions were concentrated by ultracentrifugation at 100,000 ×g for 30 min at 4°C following dilution into STE buffer (10 mM Tris-HCl pH 8.0, 0.1 M NaCl, 1 mM EDTA) to reduce the solution density.
Protein analyses
Cell lysates, viral pellets, and pelleted HIV-1 cores were subjected to electrophoresis on 4–20% polyacrylamide gradient gels containing SDS (Bio-Rad Laboratories), and proteins were transferred electrophoretically to nitrocellulose membranes. Protein blots were probed with antibodies to individual HIV-1 proteins, including rabbit polyclonal anti-CA and anti-Nef (from D. Trono), anti-CypA (Upstate Biotechnology, Inc.), and rat monoclonal to HA (Roche Applied Science). Following incubation with infrared dye-conjugated secondary antibodies, protein bands were detected using a LI-COR Odyssey imaging system.
Supplementary Material
Supplementary Fig. 1. Nef, but not CypA-Nef, enhances the infectivity of CA mutants impaired for Gag-CypA association. Nef- proviruses encoding wild type, G221A, and P222A Gag proteins were cotransfected with the Nef or CypA-Nef expressin plasmids in trans. (A) The infectivity of these viruses was measured on P4 indicator cells. The relative infectivity of each virus is indicated in panel (B).
Supplementary Fig. 2. Mutation of R55A in the CypA region of CypA-Nef reduces incorporation into HIV-1 particles and enhancement of infectivity. (A) Infectivity of Nef-defective viruses produced by coexpression of CypA-Nef and CypAR55A-Nef. (B) Immunoblot of cell lysates and pelleted virions probed with Nef-specific antiserum. For the lower panels, the blot was reprobed with antibodies specific for CypA (left panel) and CA (right panel) to determine the levels of CypA-Nef in the cell lysates and the relative quantities of virions that were analyzed.
Fig. 7.
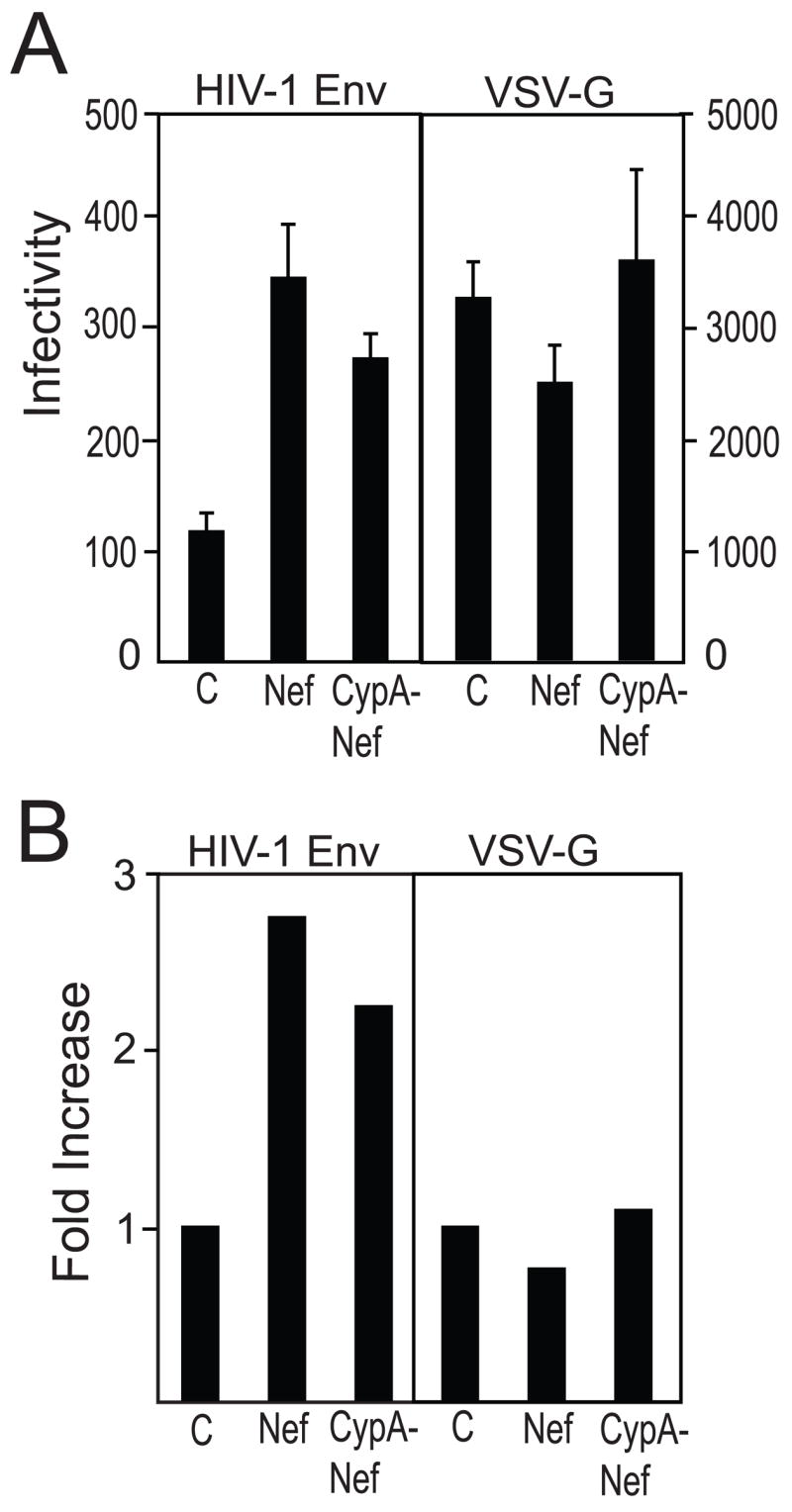
CypA-Nef enhances the infectivity of Nef-defective virions bearing native HIV-1 Env but not VSV-G. Viral particles were produced by transfecting 293T cells with R7.nef- provirus and Nef or CypA-Nef expression plasmids in trans (VC, vector control). For HIV-1(VSV-G) pseudotyped particles, the R7.nef-env- proviral construct was cotransfected with a VSV-G expression plasmid and the other indicated expression constructs. (A) Analysis of infectivity. Shown are the mean values of triplicate determinations, with error bars representing one standard deviation. The infectivity of each virus relative to its respective vector control is shown in panel (B).
Acknowledgments
We thank members of the Aiken lab for helpful suggestions and Chisu Song for assistance with the artwork. This work was supported by NIH R01 AI40364 "Mechanism of Nef Action in HIV-1 Infection".
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aiken C. Pseudotyping human immunodeficiency virus type 1 (HIV-1) by the glycoprotein of vesicular stomatitis virus targets HIV-1 entry to an endocytic pathway and suppresses both the requirement for Nef and the sensitivity to cyclosporin A. J Virol. 1997;71(8):5871–5877. doi: 10.1128/jvi.71.8.5871-5877.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiken C. Mechanistic independence of Nef and cyclophilin A enhancement of human immunodeficiency virus type 1 infectivity. Virology. 1998;248:139–147. doi: 10.1006/viro.1998.9254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiken C, Trono D. Nef stimulates human immunodeficiency virus type 1 proviral DNA synthesis. J Virol. 1995;69:5048–5056. doi: 10.1128/jvi.69.8.5048-5056.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnham KJ, Monks SA, Hinds MG, Azad AA, Norton RS. Solution structure of a polypeptide from the N terminus of the HIV protein Nef. Biochemistry. 1997;36(20):5970–80. doi: 10.1021/bi9629945. [DOI] [PubMed] [Google Scholar]
- Bartz SR, Hohenwalter E, Hu MK, Rich DH, Malkovsky M. Inhibition of human immunodeficiency virus replication by nonimmunosuppressive analogs of cyclosporin A. Proc Natl Acad Sci U S A. 1995;92(12):5381–5. doi: 10.1073/pnas.92.12.5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur AS, Sawai ET, Dazin P, Fantl WJ, Cheng-Mayer C, Peterlin BM. HIV-1 Nef leads to inhibition or activation of T cells depending on its intracellular localization. Immunity. 1994;1:373–384. doi: 10.1016/1074-7613(94)90068-x. [DOI] [PubMed] [Google Scholar]
- Braaten D, Franke EK, Luban J. Cyclophilin A is required for an early step in the life cycle of human immunodeficiency virus type 1 before the initiation of reverse transcription. J Virol. 1996;70(6):3551–3560. doi: 10.1128/jvi.70.6.3551-3560.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresnahan PA, Yonemoto W, Ferrell S, Williams-Herman D, Geleziunas R, Greene WC. A dileucine motif in HIV-1 Nef acts as an internalization signal for CD4 downregulation and binds the AP-1 clathrin adaptor. Curr Biol. 1998;8(22):1235–8. doi: 10.1016/s0960-9822(07)00517-9. [DOI] [PubMed] [Google Scholar]
- Bukovsky A, Dorfman T, Weimann A, Gottlinger HG. Nef association with human immunodeficiency virus type 1 virions and cleavage by the viral protease. J Virol. 1997;71(2):1013–1018. doi: 10.1128/jvi.71.2.1013-1018.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell EM, Nunez R, Hope TJ. Disruption of the actin cytoskeleton can complement the ability of Nef to enhance human immunodeficiency virus type 1 infectivity. J Virol. 2004;78(11):5745–55. doi: 10.1128/JVI.78.11.5745-5755.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavrois M, Neidleman J, Yonemoto W, Fenard D, Greene WC. HIV-1 virion fusion assay: uncoating not required and no effect of Nef on fusion. Virology. 2004;328(1):36–44. doi: 10.1016/j.virol.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Charneau P, Alizon M, Clavel F. A second origin of DNA plus-strand synthesis is required for optimal human immunodeficiency virus replication. J Virol. 1992;66:2814–2820. doi: 10.1128/jvi.66.5.2814-2820.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazal N, Singer G, Aiken C, Hammarskjold M-L, Rekosh D. Human immunodeficiency virus type 1 particles pseudotyped by envelope proteins that fuse at low pH no longer require Nef for optimal infectivity. J Virol. 2001;75:4014–4018. doi: 10.1128/JVI.75.8.4014-4018.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowers MY, Pandori MW, Spina CA, Richman DD, Guatelli JC. The growth advantage conferred by HIV-1 nef is determined at the level of viral DNA formation and is independent of CD4 downregulation. Virology. 1995;212:451–457. doi: 10.1006/viro.1995.1502. [DOI] [PubMed] [Google Scholar]
- Chowers MY, Spina CA, Kwoh TJ, Fitch NJS, Richman DD, Guatelli JC. Optimal infectivity in vitro of human immunodeficiency virus type 1 requires an intact nef gene. J Virol. 1994;68:2906–2914. doi: 10.1128/jvi.68.5.2906-2914.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciuffi A, Munoz M, Bleiber G, Favre M, Stutz F, Telenti A, Meylan PR. Interactions of processed Nef (58–206) with virion proteins of HIV type 1. AIDS Res Hum Retroviruses. 2004;20(4):399–407. doi: 10.1089/088922204323048140. [DOI] [PubMed] [Google Scholar]
- Craig HM, Pandori MW, Guatelli JC. Interaction of HIV-1 Nef with the cellular dileucine-based sorting pathway is required for CD4 down-regulation and optimal viral infectivity. Proc Natl Acad Sci USA. 1998;95:11229–11234. doi: 10.1073/pnas.95.19.11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JR, Munk C, Guatelli JC. The membrane-proximal tyrosine-based sorting signal of human immunodeficiency virus type 1 gp41 is required for optimal viral infectivity. J Virol. 2004;78(3):1069–79. doi: 10.1128/JVI.78.3.1069-1079.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Derdowski A, Wang JJ, Spearman P. Independent segregation of human immunodeficiency virus type 1 Gag protein complexes and lipid rafts. J Virol. 2003;77(3):1916–26. doi: 10.1128/JVI.77.3.1916-1926.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfman T, Weimann A, Borsetti A, Walsh CT, Gottlinger HG. Active-site residues of cyclophilin A are crucial for its incorporation into human immunodeficiency virus type 1 virions. J Virol. 1997;71(9):7110–3. doi: 10.1128/jvi.71.9.7110-7113.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dueck M, Guatelli J. Evidence against a direct antiviral activity of the proteasome during the early steps of HIV-1 replication. Virology. 2007;361(1):1–8. doi: 10.1016/j.virol.2007.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durocher Y, Perret S, Kamen A. High-level and high-throughput recombinant protein production by transient transfection of suspension-growing human 293-EBNA1 cells. Nucleic Acids Res. 2002;30(2):E9. doi: 10.1093/nar/30.2.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdtmann L, Janvier K, Raposo G, Craig HM, Benaroch P, Berlioz-Torrent C, Guatelli JC, Benarous R, Benichou S. Two Independent Regions of HIV-1 Nef are Required for Connection with the Endocytic Pathway Through Binding to the ?1 Chain of AP1 Complex. Traffic. 2000;1(11):871–883. doi: 10.1034/j.1600-0854.2000.011106.x. [DOI] [PubMed] [Google Scholar]
- Fackler OT, Moris A, Tibroni N, Giese SI, Glass B, Schwartz O, Krausslich HG. Functional characterization of HIV-1 Nef mutants in the context of viral infection. Virology. 2006;351(2):322–39. doi: 10.1016/j.virol.2006.03.044. [DOI] [PubMed] [Google Scholar]
- Forshey BM, Aiken C. Disassembly of human immunodeficiency virus type 1 cores in vitro reveals association of nef with the subviral ribonucleoprotein complex. J Virol. 2003;77(7):4409–14. doi: 10.1128/JVI.77.7.4409-4414.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forshey BM, von Schwedler U, Sundquist WI, Aiken C. Formation of a human immunodeficiency virus type 1 core of optimal stability is crucial for viral replication. J Virol. 2002;76(11):5667–77. doi: 10.1128/JVI.76.11.5667-5677.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke EK, Yuan HEH, Luban J. Specific incorporation of cyclophilin A into HIV-1 virions. Nature. 1994;372:359–362. doi: 10.1038/372359a0. [DOI] [PubMed] [Google Scholar]
- Gamble TR, Vajdos FF, Yoo S, Worthylake DK, Houseweart M, Sundquist WI, Hill CP. Crystal structure of human cyclophilin A bound to the amino-terminal domain of HIV-1 capsid. Cell. 1996;87(7):1285–1294. doi: 10.1016/s0092-8674(00)81823-1. [DOI] [PubMed] [Google Scholar]
- Garcia JV, Miller AD. Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nature. 1991;350:508–511. doi: 10.1038/350508a0. [DOI] [PubMed] [Google Scholar]
- Geyer M, Fackler OT, Peterlin BM. Structure--function relationships in HIV-1 Nef. EMBO Rep. 2001;2(7):580–5. doi: 10.1093/embo-reports/kve141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese SI, Woerz I, Homann S, Tibroni N, Geyer M, Fackler OT. Specific and distinct determinants mediate membrane binding and lipid raft incorporation of HIV-1(SF2) Nef. Virology. 2006;355(2):175–91. doi: 10.1016/j.virol.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Goldsmith MA, Warmerdam MT, Atchison RE, Miller MD, Greene WC. Dissociation of the CD4 downregulation and viral infectivity enhancement functions of human immunodeficiency virus type 1 Nef. J Virol. 1995;69:4112–4121. doi: 10.1128/jvi.69.7.4112-4121.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg ME, Iafrate AJ, Skowronski J. The SH3 domain-binding surface and an acidic motif in HIV-1 Nef regulate trafficking of class I MHC molecules. EMBO J. 1998;17(10):2777–2789. doi: 10.1093/emboj/17.10.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzesiek S, Bax A, Clore GM, Gronenborn AM, Hu JS, Kaufman J, Palmer I, Stahl SJ, Wingfield PT. The solution structure of HIV-1 Nef reveals an unexpected fold and permits delineation of the binding surface for the SH3 domain of Hck tyrosine protein kinase. Nat Struct Biol. 1996;3(4):340–5. doi: 10.1038/nsb0496-340. [DOI] [PubMed] [Google Scholar]
- Hatziioannou T, Perez-Caballero D, Cowan S, Bieniasz PD. Cyclophilin interactions with incoming human immunodeficiency virus type 1 capsids with opposing effects on infectivity in human cells. J Virol. 2005;79(1):176–83. doi: 10.1128/JVI.79.1.176-183.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm K, Weclewicz K, Hewson R, Suomalainen M. Human immunodeficiency virus type 1 assembly and lipid rafts: Pr55(gag) associates with membrane domains that are largely resistant to Brij98 but sensitive to Triton X-100. J Virol. 2003;77(8):4805–17. doi: 10.1128/JVI.77.8.4805-4817.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janvier K, Craig H, Le Gall S, Benarous R, Guatelli J, Schwartz O, Benichou S. Nef-induced CD4 downregulation: a diacidic sequence in human immunodeficiency virus type 1 Nef does not function as a protein sorting motif through direct binding to beta-COP. Journal of Virology. 2001;75(8):3971–6. doi: 10.1128/JVI.75.8.3971-3976.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpas A, Lowdell M, Jacobson S, Hill F. Inhibition of human immunodeficiency virus and growth of infected T cells by the immunosuppressive drugs cyclosporin A and FK506. Proc Natl Acad Sci USA. 1992;89:8351–8355. doi: 10.1073/pnas.89.17.8351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kestler HW, III, Ringler DJ, Mori K, Panicali DL, Sehgal PK, Daniel MD, Desrosiers RC. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- Kotov A, Zhou J, Flicker P, Aiken C. Association of Nef with the human immunodeficiency virus type 1 core. J Virol. 1999;73(10):8824–8830. doi: 10.1128/jvi.73.10.8824-8830.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacaille VG, Androlewicz MJ. Targeting of HIV-1 Nef to the centrosome: implications for antigen processing. Traffic. 2000;1(11):884–91. doi: 10.1034/j.1600-0854.2000.011107.x. [DOI] [PubMed] [Google Scholar]
- Lama J, Mangasarian A, Trono D. Cell-surface expression of CD4 reduces HIV-1 infectivity by blocking Env incorporation in a Nef- and Vpu-inhibitable manner. Curr Biol. 1999;9(12):622–31. doi: 10.1016/s0960-9822(99)80284-x. [DOI] [PubMed] [Google Scholar]
- Learmont JC, Geczy AF, Mills J, Ashton LJ, Raynes-Greenow CH, Garsia RJ, Dyer WB, McIntyre L, Oelrichs RB, Rhodes DI, Deacon NJ, Sullivan JS. Immunologic and virologic status after 14 to 18 years of infection with an attenuated strain of HIV-1. A report from the Sydney Blood Bank Cohort. N Engl J Med. 1999;340(22):1715–22. doi: 10.1056/NEJM199906033402203. [DOI] [PubMed] [Google Scholar]
- Lee CH, Saksela K, Mirza UA, Chait BT, Kuriyan J. Crystal structure of the conserved core of HIV-1 Nef complexed with a Src family SH3 domain. Cell. 1996a;85:931–942. doi: 10.1016/s0092-8674(00)81276-3. [DOI] [PubMed] [Google Scholar]
- Lee CH, Saksela K, Mirza UA, Chait BT, Kuriyan J. Crystal structure of the conserved core of HIV-1 Nef complexed with a Src family SH3 domain. Cell. 1996b;85(6):931–42. doi: 10.1016/s0092-8674(00)81276-3. [DOI] [PubMed] [Google Scholar]
- Lundquist CA, Tobiume M, Zhou J, Unutmaz D, Aiken C. Nef-mediated downregulation of CD4 enhances human immunodeficiency virus type 1 replication in primary T lymphocytes. J Virol. 2002;76(9):4625–33. doi: 10.1128/JVI.76.9.4625-4633.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundquist CA, Zhou J, Aiken C. Nef stimulates human immunodeficiency virus type 1 replication in primary T cells by enhancing virion-associated gp120 levels: coreceptor-dependent requirement for Nef in viral replication. J Virol. 2004;78(12):6287–96. doi: 10.1128/JVI.78.12.6287-6296.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo T, Douglas JL, Livingston RL, Garcia JV. Infectivity enhancement by HIV-1 Nef is dependent on the pathway of virus entry: implications for HIV-based gene transfer systems. Virology. 1998;241:224–233. doi: 10.1006/viro.1997.8966. [DOI] [PubMed] [Google Scholar]
- Mandic R, Fackler OT, Geyer M, Linnemann T, Zheng YH, Peterlin BM. Negative factor from SIV binds to the catalytic subunit of the V-ATPase to internalize CD4 and to increase viral infectivity. Molecular Biology of the Cell. 2001;12(2):463–73. doi: 10.1091/mbc.12.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MD, Warmerdam MT, Gaston I, Greene WC, Feinberg MB. The human immunodeficiency virus-1 nef gene product: a positive factor for viral infection and replication in primary lymphocytes and macrophages. J Exp Med. 1994;179:101–113. doi: 10.1084/jem.179.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MD, Warmerdam MT, Page KA, Feinberg MB, Greene WC. Expression of the human immunodeficiency virus type 1 (HIV-1) nef gene during HIV-1 production increases progeny particle infectivity independently of gp160 or viral entry. J Virol. 1995;69:570–584. doi: 10.1128/jvi.69.1.579-584.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono A, Freed EO. Plasma membrane rafts play a critical role in HIV-1 assembly and release. Proc Natl Acad Sci U S A. 2001;98(24):13925–30. doi: 10.1073/pnas.241320298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandori MW, Fitch NJS, Craig HM, Richman DD, Spina CA, Guatelli JC. Producer-cell modification of human immunodeficiency virus type 1: Nef is a virion protein. J Virol. 1996;70(7):4283–4290. doi: 10.1128/jvi.70.7.4283-4290.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzato M, Helander A, Popova E, Calistri A, Zamborlini A, Palu G, Gottlinger HG. Dynamin 2 is required for the enhancement of HIV-1 infectivity by Nef. Proc Natl Acad Sci U S A. 2007;104(16):6812–7. doi: 10.1073/pnas.0607622104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi M, Aiken C. Selective restriction of Nef-defective human immunodeficiency virus type 1 by a proteasome-dependent mechanism. J Virol. 2007;81(3):1534–6. doi: 10.1128/JVI.02099-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross TM, Oran AE, Cullen BR. Inhibition of HIV-1 progeny virion release by cell-surface CD4 is relieved by expression of the viral Nef protein. Current Biol. 1999;9:613–621. doi: 10.1016/s0960-9822(99)80283-8. [DOI] [PubMed] [Google Scholar]
- Schiavoni I, Trapp S, Santarcangelo AC, Piacentini V, Pugliese K, Baur A, Federico M. HIV-1 Nef enhances both membrane expression and virion incorporation of Env products. A model for the Nef-dependent increase of HIV-1 infectivity. J Biol Chem. 2004;279(22):22996–3006. doi: 10.1074/jbc.M312453200. [DOI] [PubMed] [Google Scholar]
- Schwartz O, Marechal V, Danos O, Heard J-M. Human immunodeficiency virus type 1 Nef increases the efficiency of reverse transcription in the infected cell. J Virol. 1995;69(7):4053–4059. doi: 10.1128/jvi.69.7.4053-4059.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz O, Marechal V, Friguet B, Arenzana-Seisdedos F, Heard JM. Antiviral activity of the proteasome on incoming human immunodeficiency virus type 1. J Virol. 1998;72(5):3845–50. doi: 10.1128/jvi.72.5.3845-3850.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz O, Marechal V, Le Gall S, Lemonnier F, Heard JM. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nature Medicine. 1996;2(3):338–342. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- Sokolskaja E, Sayah DM, Luban J. Target cell cyclophilin A modulates human immunodeficiency virus type 1 infectivity. J Virol. 2004;78(23):12800–8. doi: 10.1128/JVI.78.23.12800-12808.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spina CA, Kwoh TJ, Chowers MY, Guatelli JC, Richman DD. The importance of nef in the induction of human immunodeficiency virus type 1 replication from primary quiescent CD4 lymphocytes. J Exp Med. 1994;179:115–123. doi: 10.1084/jem.179.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinkasserer A, Harrison R, Billich A, Hammerschmid F, Werner G, Wolff B, Peichl P, Palfi G, Schnitzel W, Mlynar E, et al. Mode of action of SDZ NIM 811, a nonimmunosuppressive cyclosporin A analog with activity against human immunodeficiency virus type 1 (HIV-1): interference with early and late events in HIV-1 replication. J Virol. 1995;69(2):814–24. doi: 10.1128/jvi.69.2.814-824.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swingler S, Gallay P, Camaur D, Song J, Abo A, Trono D. The Nef protein of human immunodeficiency virus type 1 enhances serine phosphorylation of the viral matrix. Journal of Virology. 1997;71(6):4372–7. doi: 10.1128/jvi.71.6.4372-4377.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thali M, Bukovsky A, Kondo E, Rosenwirth B, Walsh CT, Sodroski J, Gottlinger HG. Functional association of cyclophilin A with HIV-1 virions. Nature. 1994a;372:363–365. doi: 10.1038/372363a0. [DOI] [PubMed] [Google Scholar]
- Thali M, Bukovsky A, Kondo E, Rosenwirth B, Walsh CT, Sodroski J, Gottlinger HG. Functional association of cyclophilin A with HIV-1 virions. Nature. 1994b;372(6504):363–5. doi: 10.1038/372363a0. [DOI] [PubMed] [Google Scholar]
- Tobiume M, Lineberger JE, Lundquist CA, Miller MD, Aiken C. Nef does not affect the efficiency of human immunodeficiency virus type 1 fusion with target cells. J Virol. 2003;77(19):10645–50. doi: 10.1128/JVI.77.19.10645-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainberg MA, Dascal A, Blain N, Fitz-Gibbon L, Boulerice F, Numazaki K, Tremblay M. The effect of cyclosporine A on infection of susceptible cells by human immunodeficiency virus type 1. Blood. 1988;72(6):1904–10. [PubMed] [Google Scholar]
- Wang JK, Kiyokawa E, Verdin E, Trono D. The Nef protein of HIV-1 associates with rafts and primes T cells for activation. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(1):394–9. doi: 10.1073/pnas.97.1.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei BL, Denton PW, O’Neill E, Luo T, Foster JL, Garcia JV. Inhibition of lysosome and proteasome function enhances human immunodeficiency virus type 1 infection. J Virol. 2005;79(9):5705–12. doi: 10.1128/JVI.79.9.5705-5712.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker R, Harris M, Cardel B, Krausslich H-G. Virion incorporation of human immunodeficiency virus type 1 Nef is mediated by a bipartite membrane-targeting signal: analysis of its role in enhancement of viral infectivity. J Virol. 1998;72(11):8833–8840. doi: 10.1128/jvi.72.11.8833-8840.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker R, Hohenberg H, Tessmer U, Huckhagel C, Krausslich HG. Biochemical and structural analysis of isolated mature cores of human immunodeficiency virus type 1. J Virol. 2000;74(3):1168–77. doi: 10.1128/jvi.74.3.1168-1177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker R, Kottler H, Kalbitzer HR, Krausslich HG. Human immunodeficiency virus type 1 Nef protein is incorporated into virus particles and specifically cleaved by the viral proteinase. Virology. 1996;219:228–236. doi: 10.1006/viro.1996.0240. [DOI] [PubMed] [Google Scholar]
- Zheng YH, Plemenitas A, Fielding CJ, Peterlin BM. Nef increases the synthesis of and transports cholesterol to lipid rafts and HIV-1 progeny virions. Proc Natl Acad Sci U S A. 2003;100(14):8460–5. doi: 10.1073/pnas.1437453100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng YH, Plemenitas A, Linnemann T, Fackler OT, Peterlin BM. Nef increases infectivity of HIV via lipid rafts. Curr Biol. 2001;11(11):875–9. doi: 10.1016/s0960-9822(01)00237-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. Nef, but not CypA-Nef, enhances the infectivity of CA mutants impaired for Gag-CypA association. Nef- proviruses encoding wild type, G221A, and P222A Gag proteins were cotransfected with the Nef or CypA-Nef expressin plasmids in trans. (A) The infectivity of these viruses was measured on P4 indicator cells. The relative infectivity of each virus is indicated in panel (B).
Supplementary Fig. 2. Mutation of R55A in the CypA region of CypA-Nef reduces incorporation into HIV-1 particles and enhancement of infectivity. (A) Infectivity of Nef-defective viruses produced by coexpression of CypA-Nef and CypAR55A-Nef. (B) Immunoblot of cell lysates and pelleted virions probed with Nef-specific antiserum. For the lower panels, the blot was reprobed with antibodies specific for CypA (left panel) and CA (right panel) to determine the levels of CypA-Nef in the cell lysates and the relative quantities of virions that were analyzed.