Abstract
Tyrosine hydroxylase (TH), the rate limiting enzyme in catecholamine synthesis, is frequently used as a marker of dopaminergic neuronal loss in animal models of Parkinson’s disease (PD). We have been exploring the normal function of the PD-related protein α-synuclein (α-Syn) with regard to dopamine synthesis. TH is activated by the phosphorylation of key seryl residues in the TH-regulatory-domain. Using in vitro models, our laboratory discovered that α-Syn inhibits TH by acting to reduce TH phosphorylation, which then reduces dopamine synthesis [31, 33]. We recently began exploring the impact of α-Syn on TH in vivo, by transducing dopaminergic neurons in α-Syn knockout mouse (ASKO) olfactory bulb using wild type human α-Syn lentivirus. At 3.5 – 21 days after viral delivery, α-Syn expression was transduced in periglomerular dopaminergic neurons. Cells with modest levels of α-Syn consistently co-labeled for Total-TH. However, cells bearing aggregated α-Syn, as revealed by proteinase K or Thioflavin-S treatment had significantly reduced Total-TH immunoreactivity, but high phosphoserine-TH labeling. On immunoblots, we noted that Total-TH immunoreactivity was equivalent in all conditions, although tissues with α-Syn aggregates again had higher phosphoserine-TH levels. This suggests that aggregated α-Syn is no longer able to inhibit TH. Although the reason(s) underlying reduced Total-TH immunoreactivity on tissue sections await(s) confirmation, the dopaminergic phenotype was easily verified using phosphorylation-state-specific TH antibodies. These findings have implications not only for normal α-Syn function in TH regulation, but also for measuring cell loss that is associated with synucleinopathy.
Keywords: Parkinson’s disease, lentivirus, knockout mice, transduction
α-Synuclein (α-Syn) is a presynaptic protein [25] implicated in normal brain function [11] and in Parkinson’s disease (PD) [32]. α-Syn functions as a chaperone protein based, in part, on its homology to the 14-3-3 proteins [30], which bind to and regulate the activity of many cellular proteins. Our laboratory discovered a functional interaction between α-Syn and TH [31, 33], the major dopamine biosynthetic enzyme that is modulated by 14-3-3. While 14-3-3 is known to bind to and stimulate TH activity [18], we discovered that α-Syn binding to TH, inhibits its activity by reducing TH phosphorylation [31, 33], likely acting as a 14-3-3 antagonist to modulate 3,4-dihydroxy-L-phenylalanine (DOPA) generation.
Short term regulation of TH depends on the phosphorylation of seryl residues, Ser19, Ser31, and Ser40, in the TH regulatory domain [5, 14]. We previously demonstrated significant reduction in TH phosphorylation in dopaminergic cells that overexpress α-Syn [31, 33]. The serine that regulates 14-3-3 binding to TH is phospho-Ser19 (PSer19), a site that is highly phosphorylated in dopaminergic neurons throughout the brain [36]. To evaluate the impact of α-Syn on TH in vivo, in the absence of endogenous α-Syn, we obtained ASKO mice [1] and generated wild type human α-Syn lentivirus using established methodologies [16]. Herein, we share our novel findings revealing that when α-Syn becomes aggregated, immunoreactivity (ir) for Total-TH appears to be reduced in dopaminergic neurons. However, TH neurons were readily labeled using well-characterized phospho-TH antibodies [15, 21, 23, 36, 37]. While further analyses are needed to define the mechanism(s) underlying reduced Total-TH-ir as α-Syn becomes aggregated, the data provide the first evidence for an inhibitory role of α-Syn in dopamine synthesis in vivo.
We explored the impact of α-Syn on TH in vivo using male ASKO mice, (strain B6:129X1-Snca, Tm1ROSL/J; Jackson Labs) [1] weighing 23 – 30g, housed on 12 hr light/dark cycles, with food and water ad libitum. Mice were handled according to NIH guidelines on protocols approved by the University of Pittsburgh Animal Care and Use Committee.
Lentivirus was generated using a vector system modified from human immunodeficiency virus, for transducing non-dividing cells [27], kindly provided by Prof. Didier Trono (University of Geneva, Switzerland). Plasmids included pCMVΔR8.91 (packaging), pHR’CMVGFP (transfer), and pVSVG (viral envelope). Wild type human α-Syn in pcDNA3.1 (gift of Yong-Jian Liu, University of Pittsburgh) was KpnI/XbaI digested, then inserted in pcDNA3 at KpnI/EcoRV after ligation of KpnI/ApaI (blunt-ended). α-Syn-pcDNA was digested with HinDIII (blunt-ended) and XhoI, and ligated into pHR’CMVGFP. For co-transfections, HEK293T cells were seeded at 9 × 106cells per 10 cm plate. The next day, media were replaced with OptiMEM (Gibco-Invitrogen, Foster City, CA) followed by triple transfection using Lipofectamine 2000 (Invitrogen). Lentiviral particles were purified from culture media at 72 hr [16]. Titers ranged from ~ 1 × 108 − 1 × 1010 TU/ml. Transduction efficiency was confirmed in HEK293T cells after 6 - 20 ul of virus in Hexadimethrine bromide (H-9268, Sigma-Aldrich), which yielded a dose dependent increase in α-Syn on immunoblots reacted with α-Syn antibody (610786, BD-Transduction Labs) (not shown). Virus was handled according to EHS and University of Pittsburgh Institutional Biosafety Committee for rDNA Research guidelines.
For in vivo transduction, mice were anesthetized with ketamine, 80 mg/kg, and xylazine, 12 mg/kg (Sigma-Aldrich), prior to bilateral delivery of α-Syn or GFP lentivirus into olfactory bulbs. Coordinates were: ± Bregma (AP +4.28, ML ±0.375, DV −2.75 to −1.75). Mice, immobilized in a stereotaxic frame, received solutions delivered by infusion pump (Stoelting, Wood Dale, IL) as the needle was extracted (1.0 μl per injection, at 0.2μl/min). Mice (N = 33; 22 α-Syn transduced, 4 PBS injected, and 7 GFP transduced) were sacrificed at 3.5, 7, 14, and 21 days by anesthesia overdose. These time points were selected to ensure ample α-Syn transduction in a pool of neurons that turns over. All time points yielded similar results. Brains were extracted, bisected longitudinally, and one hemisphere was post fixed in 4% formaldehyde at 4°C, then cryoprotected in 15–30% sucrose-PBS. The opposite hemisphere was frozen for biochemistry for immunoblots, prepared using established methods [22, 31, 33, 39, 41], and quantified using Odyssey Infrared Imaging according to the manufacturer (Li-Cor, Lincoln, NB).
Cryomicrotome (Microm HM550, Waldorf, Germany) sections (8 – 30 μm) were thaw-mounted onto slides or collected in −20°C cryoprotectant. Thick sections were processed free-floating. Sections were blocked in 10% goat serum, 5% BSA, 0.1 % glycine, 0.3% Triton-X-100, in 1x PBS, then in three primary antibodies at 4°C for 15 hr, followed by sequential incubations in secondary antibodies, 1 hr at RT. TH antibodies included: (1) chicken polyclonal (TH-S, 1:250, Aves Labs, Tigard, OR) against two peptides in the N- and C-termini but not overlapping TH phosphorylation sites, (2) mouse monoclonal (MAB318, 1:100, Chemicon; Temecula, CA) against TH purified from PC12 cells (epitope is outside the N-terminus); (3) rabbit polyclonal (AB152, 1:100, Chemicon; Temecula, CA) against denatured TH from rat pheochromocytoma, (4) rabbit polyclonal (P40101-0, 1:500, Pel-Freeze, Rogers, AK) against SDS-denatured purified recombinant rat or bovine TH; (5) sheep polyclonal (AB1542, 1:1000, Chemicon) against native TH from rat pheochromocytoma. Other antibodies included phospho-specific TH antibodies: rabbit anti-PSer19 (AB5425, 1:1000, Chemicon), rabbit anti-PSer31 (AB5423, 1:1000, Chemicon), rabbit anti-PSer40 (AB5423, 1:100, Chemicon); or chicken PSer19 and PSer40 TH polyclonal antibodies custom made for us by Aves Labs. GFP transduced neurons were immunolabeled using chicken anti-GFP (AB1690, 1:500, Chemicon). Secondary antibodies included affinity-purified: donkey-anti-goat Alexa-546 (1:700; Invitrogen/Molecular Probes; Foster City, CA), goat-anti-chicken Alexa-488 (1:700; Invitrogen/Molecular Probes), goat-anti-rabbit Alexa-647 (1:700; Invitrogen/Molecular Probes), donkey-anti-sheep Alexa-488 (1:700, Invitrogen/Molecular Probes), or CY3-conjugated goat-anti-chicken (#103-165-155, 1:700, Jackson ImmunoResearch). Nuclei were DAPI stained when mounted on slides using Vectashield Hardset (Vector Labs, Burlingame, CA).
Three-color-laser confocal microscopy (Olympus IX81) for detecting wavelengths from UV for DAPI, to green (Alexa-488), red (Alexa-546 and CY-3), to farred (Alexa-647). Images were captured and data quantified by Fluoview10 (Olympus), with all settings being equal for all conditions. Data from intensity profiles were accumulated in Excel (Microsoft) and evaluated using GraphPad (Instat, San Diego), with statistical significance set at 0.05.
Protein aggregation was measured by proteinase K (PK, Invitrogen) treatment [8, 28] on thaw-mounted sections that were air dried 20 min at RT, circled with Pap-Pen (Polysciences, Warrington, PA), then rewet in 1 x PBS. Tissues were digested in 2.5μg/ml PK in 0.1 M-Tris-HCL buffer, 0.005 mM EDTA, pH 7.5 for 15–30 min, RT. Sections were then immunostained as described above. For Thioflavin-S we utilized floating sections (30 μm) that were first immunolabled then reacted with 1% aqueous Thioflavin-S (Sigma-Aldrich, St Louis) 30 min RT, followed by ethanol washes. All sections were light protected at 4°C prior to evaluation.
Normal morphology was apparent in α-Syn transduced mouse olfactory bulb as can be appreciated by DAPI staining (Fig 1A). As expected, many periglomerular neurons (Fig 1A, arrows) were dopaminergic as confirmed by Total-TH immunoreactivity (ir) (Fig 1B). Because the lentiviral particles we used are packaged with vesicular stomatitis virus glycoprotein (VSV-G) envelope protein, we anticipated that most olfactory neurons would be transduced. However, to our surprise, α-Syn expression was almost exclusively noted in dopaminergic neurons, seen here at 7 days post-transduction (Fig 1C). The reason for this relatively selective tropism is unknown, however, α-Syn-ir colocalized with TH as noted in this higher magnification image (Fig 1D, arrow). As anticipated, untransduced olfactory bulbs lacked α-Syn-ir (Fig 1E).
Figure 1. α-Syn is co-expressed with Total-TH in periglomerular olfactory neurons of lentivirus transduced α-Syn knockout mice.
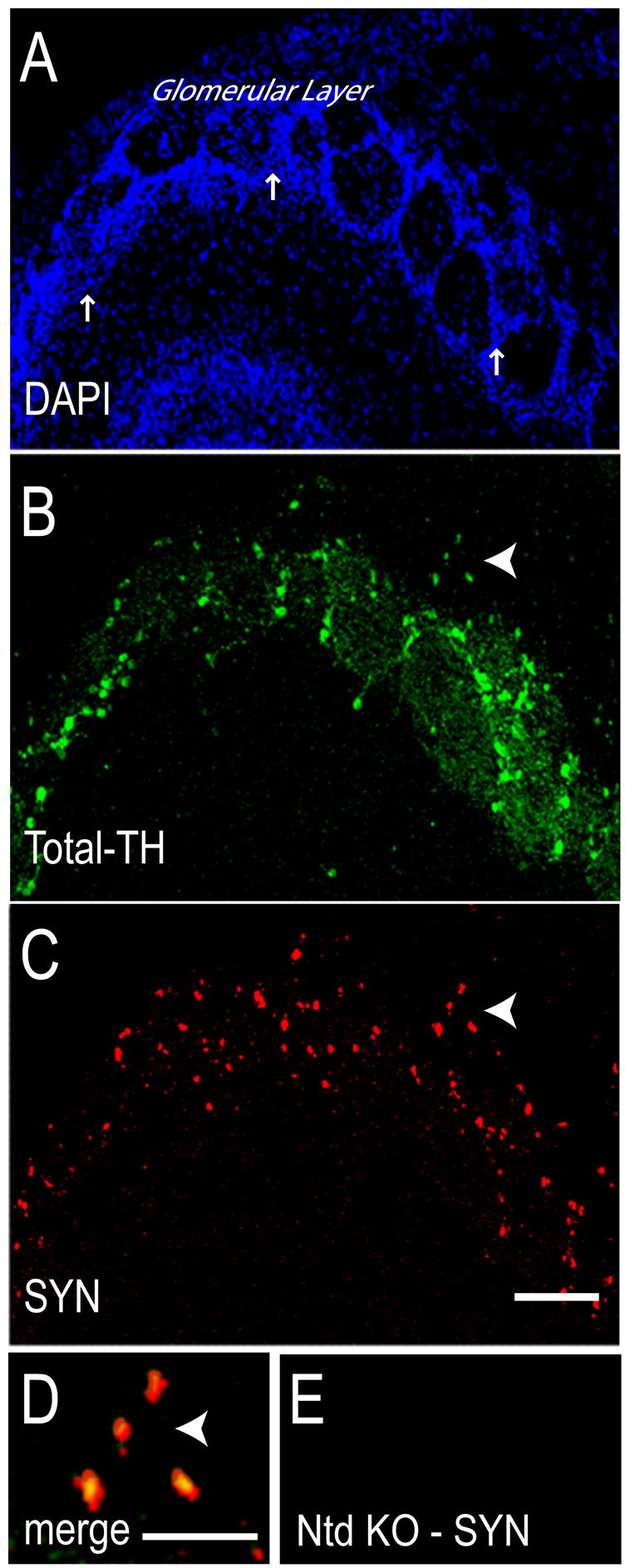
At 7 days after stereotaxic delivery of α-Syn lentivirus (A) DAPI stained nuclei reveal normal olfactory bulb morphology. Arrows point to periglomerular regions. (B) Many periglomerular neurons were labeled for Total-TH, confirming their dopaminergic phenotype. Arrowhead points to a group of four TH-ir neurons at the edge of a glomerulus. (C) Many neurons express wild type human α-Syn after transduction. Arrowhead points to the same four neurons as in B. (D) At high magnification the merged Total-TH-ir and α-Syn-ir appears yellow, confirming α-Syn expression in DA neurons (arrowhead). (E) Non-transduced (Ntd) olfactory bulb of a PBS-injected ASKO mouse lacks α-Syn-ir. Total-TH antibody used was TH-S. Size bars, 100 μm (A–C), 25 μm (D, E).
Previously, we noted that α-Syn acts to inhibit TH phosphorylation and dopamine synthesis in vitro [31, 33]. Under normal conditions, functional α-Syn and TH are soluble proteins, but both accumulate in Lewy bodies [20, 26, 38]. We have long hypothesized that TH phosphorylation levels will increase when α-Syn aggregates [32, 33]. To begin evaluating this possibility, we triple immunolabeled ASKO olfactory bulbs at 3.5 – 21 days post-transduction with antibodies for α-Syn, Total-TH, or TH phosphorylated at Ser19, Ser31, or Ser40. Because our data were nearly identical for all three phospho-Ser-specific-TH antibodies, we present only the PSer19 results herein. At 3.5 days after PBS injection olfactory bulbs had overlapped Total-TH and TH PSer19 signals and no α-Syn-ir, as expected (Fig 2A). In ASKO mice transduced with wild type human α-Syn there was extensive overlap of α-Syn-ir with Total-TH-ir (Fig 2B). However, cells having stronger α-Syn-ir showed reduced Total-TH-ir (Fig 2B, cells at arrowheads), although their dopaminergic phenotype was confirmed by PSer19 staining (Fig 2B). When we quantified signal intensities for cells 1, 2, and 3 in Figure 2B, we noted a significant inverse correlation between Total-TH-ir and α-Syn-ir and a positive correlation between α-Syn-ir and TH PSer19-ir (Fig 2C) (N = 85 cells, P < 0.0001). In contrast, GFP transduction did not reduce Total-TH-ir or increase PSer19-ir, as can be appreciated in the merged image where GFP-transduced dopaminergic neurons appear yellow (Fig 2D). Total-TH levels were equivalent on tissue homogenate immunoblots from α-Syn- and GFP-transduced mice at 3.5 and 7 days (Fig 2E), although PSer19 levels were significantly higher in α-Syn transduced tissues compared to GFP-transduced controls (Fig 2E, ANOVA, P < 0.02).
Figure 2. α-Syn transduction, but not GFP transduction, reduces Total-TH immunoreactivity in dopaminergic neurons.
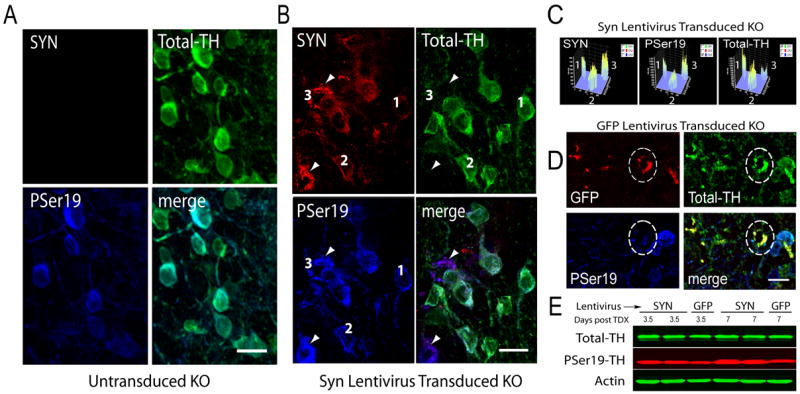
(A) Untransduced periglomerular dopaminergic neurons in ASKO mice at 3.5 days post-surgery have no α-Syn-ir, abundant Total-TH-ir that overlaps with TH PSer19-ir. B) Many α-Syn transduced neurons contain Total-TH-ir, confirming efficient α-Syn transduction of dopaminergic neurons. Cells with strong α-Syn-ir, also have strong TH-PSer19-ir but Total-TH-ir is reduced, which appear purple in the merged image (at arrowheads). (C) Intensity profiles of cells 1, 2, and 3, from Figure 2B reveal that cell 1 has low α-Syn-ir, low PSer19-ir, and high Total-TH-ir; cell 2 has moderate levels of α-Syn-ir, PSer19-ir, and Total-TH-ir; while cell 3 has high α-Syn-ir, high Pser19-ir, but low Total-TH-ir. (D) GFP-transduced neurons were immunostained for GFP using CY-3 immunofluorescence at 14 days post-surgery (red). The circled GFP-ir neuron has strong Total-TH-ir (green circled cell) and low levels of PSer19-ir (blue circled cell). GFP-transduced dopaminergic neurons appear yellow in the merged image including the circled cell and other cells in the same microscopic field. (E) Tissues from α-Syn-transduced olfactory bulbs have equivalent Total-TH levels but elevated PSer19 compared to GFP-transduced tissues at 3.5 and 7 days post-transduction (post-TDX). Actin serves as a loading control. Total-TH antibodies used were TH-S in A and B, AB1542 in D, and MAB318 in E. Size bars, 20 μm.
We verified that α-Syn aggregation was associated with reduced Total-TH-ir, using proteinase K (PK) and Thioflavin-S labeling of tissue sections from α-Syn- and GFP-transduced tissues at 3.5 and 21 days post-transduction using well-established methods [2, 6, 12, 13, 24, 28, 35]. After PK, tissues triple-labeled for α-Syn, PSer19, and Total-TH revealed that PK-resistant α-Syn colocalized with PSer19-ir, however, Total-TH labeling was absent (Fig 3A) as verified by Fluoview analysis (N = 50, t = 41.75, P < 0.0001), suggesting that Total-TH was soluble and digested away. Triple labeling for Thioflavin-S, α-Syn, and Total-TH at 14 days post-transduction revealed coincident α-Syn and Thioflavin-S staining confirming aggregation in cells with reduced, but perceptible, Total-TH-ir (Fig 3B, at arrowhead). Untransduced neurons, at the border of the injection site, maintained robust Total-TH-ir (Fig 3B, at arrows) much like labeling noted in cells of Figure 2A. Similar findings were noted at all time points. Importantly, GFP aggregation sometimes occurred as confirmed by Thioflavin-S staining; however Total-TH-ir was not reduced nor was PSer19-ir increased under those conditions (not shown). This suggests that α-Syn aggregation may have unique effects on TH phosphorylation and the reduction in Total-TH-ir.
Figure 3. α-Syn aggregation, confirmed by proteinase K or Thioflavin-S labeling, correlates with TH PSer19 immunoreactivity.
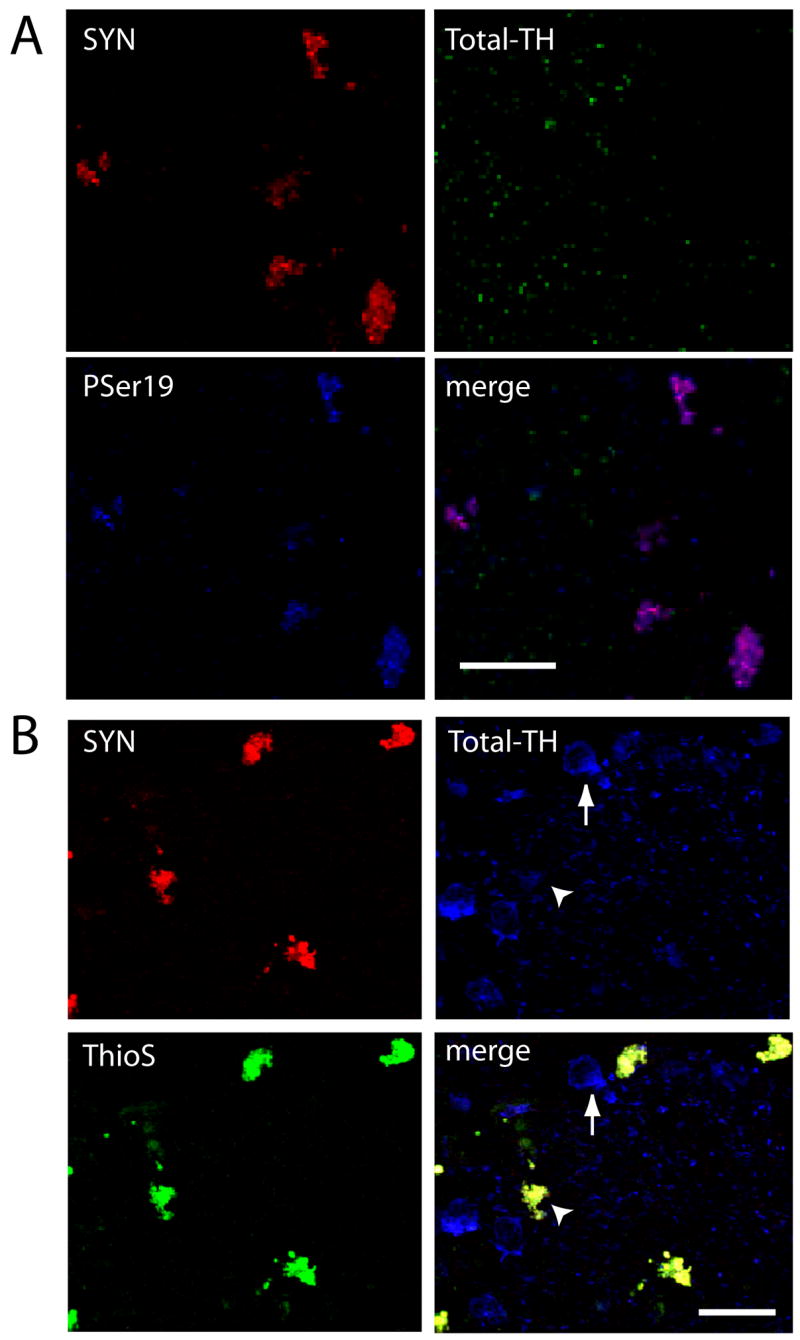
(A) Proteinase K-resistant α-Syn-ir in isolated neurons (red) that lack Total-TH-ir (green) but have high levels of PSer19-ir (blue), which appear purple in the merged image of cells at 7 days post-transduction. (B) Aggregated α-Syn (red) colocalizes with Thioflavin-S (green) in neurons at 14 days post-transduction. Total-TH-ir is reduced in these cells (arrowhead) compared to untransduced dopaminergic neurons at the edge of the injection, which lack α-Syn and have strong Total-TH-ir (arrow). Merged signal appears yellow due to strong α-Syn-ir and Thioflavin-S signal. Total-TH antibodies used were TH-S in A, and P40101-0 in B. Size bars, 25 μm.
α-Syn has a tendency to aggregate both in vitro [3, 9, 10] and in vivo [7, 17, 29]. Furthermore, α-Syn levels increase with age in nigral dopaminergic neurons [8], and toxins can further increase α-Syn levels which may enhance aggregation [24]. The presence of extra copies of the α-Syn gene can induce pathology in rare PD families [7, 17, 29]. Our previous data show that α-Syn inhibits TH phosphorylation and activity [31, 33], likely working in counterpoint to 14-3-3 to modulate DA synthesis. Because phosphorylation of TH Ser19 potentiates Ser40 phosphorylation [4, 19, 21, 40], leading to increased DA synthesis [34], our finding of high levels of phosphorylated TH in neurons bearing α-Syn aggregates (Fig 3A) suggests that TH activity and DA synthesis may be increased in the cells [32, 33]. Because our earlier work revealed that α-Syn also inhibits aromatic amino acid decarboxylase (AADC), the enzyme that converts DOPA to dopamine [39], unbridled DA synthesis may occur as soluble α-Syn levels drop below an acceptable threshold, although this awaits confirmation.
Cumulatively, our findings suggest that increasing α-Syn to levels that promote its aggregation may have the capacity to overactivate TH and AADC, which may ultimately contribute to nigral cell loss over time [32]. Our findings further suggest that immunostaining of Total-TH may underestimate dopaminergic loss in cells with insoluble α-Syn, as noted using five different Total-TH antibodies. In contrast, cells were strongly labeled for TH PSer19, not only pointing to important effects of α-Syn on TH regulation, but also providing a novel tool for confirming dopaminergic phenotypes. Additionally, our findings reveal the usefulness of lentiviral α-Syn transduction of ASKO mice as a model system in which to further elucidate the impact of α-Syn on TH regulation, dopamine synthesis, and cell viability.
Acknowledgments
We thank NINDS (NS42094) and the Fox Foundation for support, D. Trono and Y.J. Liu for plasmids, H.M. Na, S.M. Reid and C.J. Pedersen for technical assistance, and J. Wang for critical reading of the manuscript. We dedicate our work to M.J. Fox, R. Beyer, and J. Cordy, and in memory of L. “Rusty” Lanelli.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–52. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- 2.Alves da Costa C, Paitel E, Vincent B, Checler F. Alpha-synuclein lowers p53-dependent apoptotic response of neuronal cells. Abolishment by 6-hydroxydopamine and implication for Parkinson’s disease. J Biol Chem. 2002;277:50980–4. doi: 10.1074/jbc.M207825200. [DOI] [PubMed] [Google Scholar]
- 3.Biere AL, Wood SJ, Wypych J, Steavenson S, Jiang Y, Anafi D, Jacobsen FW, Jarosinski MA, Wu GM, Louis JC, Martin F, Narhi LO, Citron M. Parkinson’s disease-associated alpha-synuclein is more fibrillogenic than beta- and gamma-synuclein and cannot cross-seed its homologs. J Biol Chem. 2000;275:34574–9. doi: 10.1074/jbc.M005514200. [DOI] [PubMed] [Google Scholar]
- 4.Bobrovskaya L, Dunkley PR, Dicksosteron PW. Phosphorylation of Ser19 increases both Ser40 phosphorylation and enzyme activity of tyrosine hydroxylase in intact cells. J Neurochem. 2004;90:857–64. doi: 10.1111/j.1471-4159.2004.02550.x. [DOI] [PubMed] [Google Scholar]
- 5.Campbell DG, Hardie DG, Vulliet PR. Identification of four phosphorylation sites in the N-terminal region of tyrosine hydroxylase. J Biol Chem. 1986;261:10489–92. [PubMed] [Google Scholar]
- 6.Cappai R, Leck SL, Tew DJ, Williamson NA, Smith DP, Galatis D, Sharples RA, Curtain CC, Ali FE, Cherny RA, Culvenor JG, Bottomley SP, Masters CL, Barnham KJ, Hill AF. Dopamine promotes alpha-synuclein aggregation into SDS-resistant soluble oligomers via a distinct folding pathway. FASEB J. 2005:04–3437fje. doi: 10.1096/fj.04-3437fje. [DOI] [PubMed] [Google Scholar]
- 7.Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M. alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet. 2004;364:1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- 8.Chu Y, Kordower JH. Age-associated increases of alpha-synuclein in monkeys and humans are associated with nigrostriatal dopamine depletion: Is this the target for Parkinson’s disease? Neurobiol Dis. 2007;25:134–49. doi: 10.1016/j.nbd.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 9.Conway KA, Harper JD, Lansbury PT. Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat Med. 1998;4:1318–20. doi: 10.1038/3311. [DOI] [PubMed] [Google Scholar]
- 10.Conway KA, Lee SJ, Rochet JC, Ding TT, Harper JD, Williamson RE, Lansbury PT., Jr Accelerated oligomerization by Parkinson’s disease linked alpha-synuclein mutants. Ann N Y Acad Sci. 2000;920:42–5. doi: 10.1111/j.1749-6632.2000.tb06903.x. [DOI] [PubMed] [Google Scholar]
- 11.George RJ, Haycock JW, Johnston JP, Craviso GL, Waymire JC. In vitro phosphorylation of bovine adrenal chromaffin cell tyrosine hydroxylase by endogenous protein kinases. J Neurochem. 1989;52:274–84. doi: 10.1111/j.1471-4159.1989.tb10928.x. [DOI] [PubMed] [Google Scholar]
- 12.Golts N, Snyder H, Frasier M, Theisler C, Choi P, Wolozin B. Magnesium inhibits spontaneous and iron-induced aggregation of alpha-synuclein. J Biol Chem. 2002;277:16116–23. doi: 10.1074/jbc.M107866200. [DOI] [PubMed] [Google Scholar]
- 13.Hashimoto M, Hsu LJ, Sisk A, Xia Y, Takeda A, Sundsmo M, Masliah E. Human recombinant NACP/alpha-synuclein is aggregated and fibrillated in vitro: relevance for Lewy body disease. Brain Res. 1998;799:301–6. doi: 10.1016/s0006-8993(98)00514-9. [DOI] [PubMed] [Google Scholar]
- 14.Haycock JW. Phosphorylation of tyrosine hydroxylase in situ at serine 8, 19, 31, and 40. J Biol Chem. 1990;265:11682–91. [PubMed] [Google Scholar]
- 15.Haycock JW, Lew JY, Garcia-Espana A, Lee KY, Harada K, Meller E, Goldstein M. Role of serine-19 phosphorylation in regulating tyrosine hydroxylase studied with site- and phosphospecific antibodies and site-directed mutagenesis. J Neurochem. 1998;71:1670–5. doi: 10.1046/j.1471-4159.1998.71041670.x. [DOI] [PubMed] [Google Scholar]
- 16.Hong CS, Goins WF, Goss JR, Burton EA, Glorioso JC. Herpes simplex virus RNAi and neprilysin gene transfer vectors reduce accumulation of Alzheimer’s disease-related amyloid-beta peptide in vivo. Gene Ther. 2006 doi: 10.1038/sj.gt.3302719. [DOI] [PubMed] [Google Scholar]
- 17.Ibanez P, Bonnet AM, Debarges B, Lohmann E, Tison F, Pollak P, Agid Y, Durr A, Brice A. Causal relation between alpha-synuclein gene duplication and familial Parkinson’s disease. Lancet. 2004;364:1169–71. doi: 10.1016/S0140-6736(04)17104-3. [DOI] [PubMed] [Google Scholar]
- 18.Ichimura T, Isobe T, Okuyama T, Takahashi N, Araki K, Kuwano R, Takahashi Y. Molecular cloning of cDNA coding for brain-specific 14-3-3 protein, a protein kinase-dependent activator of tyrosine and tryptophan hydroxylases. Proc Natl Acad Sci U S A. 1988;85:7084–8. doi: 10.1073/pnas.85.19.7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kleppe R, Toska K, Haavik J. Interaction of phosphorylated tyrosine hydroxylase with 14-3-3 proteins: evidence for a phosphoserine 40-dependent association. J Neurochem. 2001;77:1097–107. doi: 10.1046/j.1471-4159.2001.00318.x. [DOI] [PubMed] [Google Scholar]
- 20.Kuljis RO, Martin-Vasallo P, Peress NS. Lewy bodies in tyrosine hydroxylase-synthesizing neurons of the human cerebral cortex. Neurosci Lett. 1989;106:49–54. doi: 10.1016/0304-3940(89)90200-0. [DOI] [PubMed] [Google Scholar]
- 21.Lew JY, Garcia-Espana A, Lee KY, Carr KD, Goldstein M, Haycock JW, Meller E. Increased Site-Specific Phosphorylation of Tyrosine Hydroxylase Accompanies Stimulation of Enzymatic Activity Induced by Cessation of Dopamine Neuronal Activity. Mol Pharmacol. 1999;55:202–209. doi: 10.1124/mol.55.2.202. [DOI] [PubMed] [Google Scholar]
- 22.Lin E, Cavanaugh JE, Leak RK, Perez RG, Zigmond MJ. Rapid activation of ERK by 6-hydroxydopamine promotes survival of dopaminergic cells. J Neurosci Res. 2007;86:108–117. doi: 10.1002/jnr.21478. [DOI] [PubMed] [Google Scholar]
- 23.Lindgren N, Goiny M, Herrera-Marschitz M, Haycock JW, Hokfelt T, Fisone G. Activation of extracellular signal-regulated kinases 1 and 2 by depolarization stimulates tyrosine hydroxylase phosphorylation and dopamine synthesis in rat brain. Eur J Neurosci. 2002;15:769–773. doi: 10.1046/j.1460-9568.2002.01901.x. [DOI] [PubMed] [Google Scholar]
- 24.Manning-Bog AB, McCormack AL, Li J, Uversky VN, Fink AL, Di Monte DA. The herbicide paraquat causes up-regulation and aggregation of alpha-synuclein in mice: paraquat and alpha-synuclein. J Biol Chem. 2002;277:1641–4. doi: 10.1074/jbc.C100560200. [DOI] [PubMed] [Google Scholar]
- 25.Maroteaux L, Campanelli JT, Scheller RH. Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J Neurosci. 1988;8:2804–15. doi: 10.1523/JNEUROSCI.08-08-02804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakashima S, Ikuta F. Tyrosine hydroxylase protein in Lewy bodies of parkinsonian and senile brains. J Neurol Sci. 1984;66:91–6. doi: 10.1016/0022-510x(84)90144-8. [DOI] [PubMed] [Google Scholar]
- 27.Naldini L, Blomer U, Gage FH, Trono D, Verma IM. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci U S A. 1996;93:11382–8. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neumann M, Kahle PJ, Giasson BI, Ozmen L, Borroni E, Spooren W, Muller V, Odoy S, Fujiwara H, Hasegawa M, Iwatsubo T, Trojanowski JQ, Kretzschmar HA, Haass C. Misfolded proteinase K-resistant hyperphosphorylated alpha-synuclein in aged transgenic mice with locomotor deterioration and in human alpha-synucleinopathies. J Clin Invest. 2002;110:1429–39. doi: 10.1172/JCI15777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishioka K, Hayashi S, Farrer MJ, Singleton AB, Yoshino H, Imai H, Kitami T, Sato K, Kuroda R, Tomiyama H, Mizoguchi K, Murata M, Toda T, Imoto I, Inazawa J, Mizuno Y, Hattori N. Clinical heterogeneity of alpha-synuclein gene duplication in Parkinson’s disease. Ann Neurol. 2006;59:298–309. doi: 10.1002/ana.20753. [DOI] [PubMed] [Google Scholar]
- 30.Ostrerova N, Petrucelli L, Farrer M, Mehta N, Choi P, Hardy J, Wolozin B. alpha-Synuclein shares physical and functional homology with 14-3-3 proteins. J Neurosci. 1999;19:5782–91. doi: 10.1523/JNEUROSCI.19-14-05782.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peng XM, Tehranian R, Dietrich P, Stefanis L, Perez RG. Alpha-synuclein activation of protein phosphatase 2A reduces tyrosine hydroxylase phosphorylation in dopaminergic cells. J Cell Sci. 2005;118:3523–3530. doi: 10.1242/jcs.02481. [DOI] [PubMed] [Google Scholar]
- 32.Perez RG, Hastings TG. Could a loss of alpha-synuclein function put dopaminergic neurons at risk? J Neurochem. 2004;89:1318–24. doi: 10.1111/j.1471-4159.2004.02423.x. [DOI] [PubMed] [Google Scholar]
- 33.Perez RG, Waymire JC, Lin E, Liu JJ, Guo F, Zigmond MJ. A role for alpha-synuclein in the regulation of dopamine biosynthesis. J Neurosci. 2002;22:3090–9. doi: 10.1523/JNEUROSCI.22-08-03090.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramsey AJ, Fitzpatrick PF. Effects of phosphorylation of serine 40 of tyrosine hydroxylase on binding of catecholamines: evidence for a novel regulatory mechanism. Biochemistry. 1998;37:8980–6. doi: 10.1021/bi980582l. [DOI] [PubMed] [Google Scholar]
- 35.Rideout HJ, Dietrich P, Wang Q, Dauer WT, Stefanis L. alpha-Synuclein is required for the fibrillar nature of ubiquitinated inclusions induced by proteasomal inhibition in primary neurons. J Biol Chem. 2004;279:46915–20. doi: 10.1074/jbc.M405146200. [DOI] [PubMed] [Google Scholar]
- 36.Salvatore MF, Garcia-Espana A, Goldstein M, Deutch AY, Haycock JW. Stoichiometry of tyrosine hydroxylase phosphorylation in the nigrostriatal and mesolimbic systems in vivo: effects of acute haloperidol and related compounds. J Neurochem. 2000;75:225–32. doi: 10.1046/j.1471-4159.2000.0750225.x. [DOI] [PubMed] [Google Scholar]
- 37.Salvatore MF, Waymire JC, Haycock JW. Depolarization-stimulated catecholamine biosynthesis: involvement of protein kinases and tyrosine hydroxylase phosphorylation sites in situ. J Neurochem. 2001;79:349–60. doi: 10.1046/j.1471-4159.2001.00593.x. [DOI] [PubMed] [Google Scholar]
- 38.Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc Natl Acad Sci U S A. 1998;95:6469–73. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tehranian R, Montoya SE, Van Laar AD, Hastings TG, Perez RG. Alpha-synuclein inhibits aromatic amino acid decarboxylase activity in dopaminergic cells. J Neurochem. 2006;99:1188. doi: 10.1111/j.1471-4159.2006.04146.x. [DOI] [PubMed] [Google Scholar]
- 40.Toska K, Kleppe R, Armstrong CG, Morrice NA, Cohen P, Haavik J. Regulation of tyrosine hydroxylase by stress-activated protein kinases. J Neurochem. 2002;83:775–83. doi: 10.1046/j.1471-4159.2002.01172.x. [DOI] [PubMed] [Google Scholar]
- 41.Ugarte SD, Lin E, Klann E, Zigmond MJ, Perez RG. Effects of GDNF on 6-OHDA-induced death in a dopaminergic cell line: Modulation by inhibitors of PI3 kinase and MEK. J Neurosci Res. 2003;73:105–112. doi: 10.1002/jnr.10632. [DOI] [PubMed] [Google Scholar]


