Abstract
Lipid peroxidation is implicated in the pathogenesis of various autoimmune diseases. Lipid peroxidation-derived aldehydes (LPDAs) such as malondialdehyde (MDA) and 4-hydroxynonenal (HNE) are highly reactive and bind to proteins, but their role in eliciting an autoimmune response and contribution to disease pathogenesis remains unclear. To investigate the role of lipid peroxidation in the induction and/or exacerbation of autoimmune response, 6-week-old autoimmune-prone female MRL +/+ mice were treated for 4 weeks with trichloroethene (TCE; 10 mmol/kg, i.p., once a week), an environmental contaminant known to induce lipid peroxidation. Sera from TCE-treated mice showed significant levels of antibodies against MDA- and HNE-adducted proteins along with anti-nuclear antibodies. This suggested that TCE exposure not only caused increased lipid peroxidation, but also accelerated autoimmune responses. Furthermore, stimulation of cultured splenic lymphocytes from both control and TCE-treated mice with MDA-adducted mouse serum albumin (MDA-MSA) or HNE-MSA for 72 h showed significant proliferation of CD4+ T cells in TCE-treated mice as analyzed by flow cytometry. Also, splenic lymphocytes from TCE-treated mice released more IL-2 and IFN-γ into cultures when stimulated with MDA-MSA or HNE-MSA, suggesting a Th1 cell activation. Thus, our data suggest a role for lipid peroxidation-derived aldehydes in TCE-mediated autoimmune responses and involvement of Th1 cell activation.
Keywords: Trichloroethene, autoimmunity, lipid peroxidation, anti-MDA antibodies, anti-HNE antibodies, IFN-γ, IL-2, Th1 cells
Introduction
Autoimmune diseases (ADs), such as systemic lupus erythematosus (SLE) and rheumatoid arthritis, are among the leading causes of death in young and middle-aged women [1]. ADs are of unknown etiology, but are believed to be multifactorial. In recent years, reactive oxygen species (ROS) have been implicated in the pathogenesis of ADs [2–5]. ROS can be produced exogenously or from a variety of intracellular processes collectively linked to the generation of superoxide anions, hydroxyl radicals, and hydrogen peroxide [6]. Such reactive oxidants can modify a variety of biological molecules, including polyunsaturated fatty acid-containing lipids generating lipid peroxides, which on decomposition lead to reactive aldehydes such as malondialdehyde (MDA) and 4-hydroxynonenal (HNE). These lipid peroxidation-derived aldehydes (LPDAs) can bind covalently with a variety of amino acids of proteins to form MDA- and HNE-modified protein adducts [7–10]. Increased lipid peroxidation [4,7,11–13] and higher levels of MDA- and HNE-modified proteins are reported in patients with ADs [4,7,14,15]. However, their involvement in the pathogenesis of ADs remains unknown.
Environmental factors, including chemicals, not only play a role in the development of SLE, but also contribute to increased prevalence of many other ADs [16–19]. Trichloroethene (TCE), a widely used volatile organic solvent, is a ubiquitous environmental contaminant [20–22]. A series of reports have implicated TCE in the development of various ADs including SLE, systemic sclerosis and fascitis, both from occupational [23–26] and environmental exposures [17,27–29]. Our laboratory was the first to demonstrate that TCE induces/exacerbates an autoimmune response in female MRL +/+ mice [30]. These findings were further substantiated by others [31,32] and our follow-up studies [2,33–36]. TCE is known to generate free radicals and induces lipid peroxidation both in vivo and in vitro [2,35–39]. Our recent studies show an association between TCE-induced lipid peroxidation and induction/exacerbation of autoimmune response in MRL+/+ mice, and thus suggest that lipid peroxidation and/or LPDA-protein adducts may play an important role in the disease pathogenesis [2,35,36]. Investigating a potential role for LPDA-protein adducts in the autoimmune response, with TCE as an inducer of lipid peroxidation, may thus provide mechanistic clues to their etiologic role in ADs.
T cells, in particular CD4+ T cells, have been implicated in mediating many aspects of autoimmune inflammation. Of fundamental importance in initiating, controlling, and driving these specific immune responses is the activation of CD4+ T cells [40–42]. Once activated, CD4+ T cells differentiate into specialized effector cells and become the central regulators of specific immune responses. Based on distinctive cytokine secretion patterns and concomitant effector functions, CD4+ T cells can be divided into at least two major subsets [43,44]. Th1 cells, upon activation, secrete the proinflammatory cytokines IL-2, IFN-γ and TNF-α, which activate macrophages to produce ROS and nitric oxide, stimulate their phagocytic functions, and enhance their ability for antigen presentation by upregulating MHC class II molecules. Th1 cells thus are involved in cell-mediated immunity [43,44]. On the other hand, Th2 cells produce anti-inflammatory cytokines IL-4, IL-5 and IL-10 and provide potent help for B-cell activation and immunoglobulin class switching to IgE and subtypes of IgG that do not fix complement [43,44]. However, there is little direct evidence to link increased lipid peroxidation with immune responses, especially the efficacy of MDA- and HNE-protein adducts in inducing lymphocyte proliferation and activation, leading to ADs.
To support our hypothesis that covalent binding of LPDAs causes structural alterations to endogenous proteins, resulting in the formation of neoantigens that elicit autoimmune responses by stimulating T and/or B lymphocytes, we have conducted studies in MRL+/+ mice by treating them with TCE. Specifically, we have measured serum levels of anti-MDA- and anti-HNE-protein adduct antibodies and anti-nuclear antibodies (ANA). Furthermore, splenic lymphocyte proliferation following the stimulation with MDA-adducted mouse serum albumin (MDA-MSA) or HNE-MSA adducts and release of Th1 and Th2 cytokines into the cultures were also measured. Our results not only show that TCE exposure induces/accelerates both oxidative stress and autoimmune responses, but also suggest that LPDA-protein adducts play a role in the TCE-mediated autoimmune response by inducing Th1 T cell activation.
Materials and methods
Animals and treatments
Five-week old female MRL+/+ mice (23–26 g) were purchased from Jackson Laboratory (Bar Harbor, ME) and housed in plastic cages on a bedding of wood chips at the UTMB animal house facility maintained at ~ 22°C, 50–60% relative humidity, and a 12 h light/dark cycle. The animals were provided standard lab chow and drinking water ad libitum and were acclimated for 1 week prior to the treatment. TCE (purity 99+%) was purchased from Sigma-Aldrich Inc.(St. Louis, MO). The mice were divided into two groups of eight animals each. The TCE exposure group received intraperitoneal injections of 10 mmol/kg TCE in 100 µl of corn oil [2,30,36]. The control mice received an equal volume of corn oil only. The TCE treatments were given once a week for 4 weeks, and animals were weighed on a weekly basis. After 4 weeks of TCE treatment, the animals were euthanized under nembutal (sodium pentobarbital) anesthesia, and blood was withdrawn from the inferior vena cava. Individual sera, obtained following blood clotting and centrifugation, were stored in small aliquots at −80°C till further analysis. At the same time, spleens were removed immediately and splenocytes were isolated and suspended in RPMI 1640 medium supplemented with 2 mM glutamine, 50 µg/ml gentamycin and 10% FBS [45].
Preparation of MDA-and HNE-protein adducts
MDA and HNE adducts of ovalbumin or mouse serum albumin (MSA) were prepared as described earlier in our laboratory [2,8,9,35,46]. Free amino groups of ovalbumin or MSA, which did not react with MDA or HNE, were determined by 2,4,6-trinitrobenzene-1-sulfonic acid assay [8,35,46,47]. Our results show that treatment of ovalbumin or MSA (5 mg/ml) with 50 mM MDA or 8.7 mM HNE modified ~88% and ~66% of the amino groups, respectively.
ELISAs for anti-MDA- and anti-HNE-protein adduct specific antibodies in the serum
ELISAs to analyze anti-MDA- and anti-HNE-protein adduct-specific antibodies in the mouse serum were performed as described earlier [2,35,36]. Briefly, flat-bottomed 96-well microtiter plates were coated with MDA-/HNE-ovalbumin adducts or ovalbumin (0.5 µg/well) overnight at 4 °C. The plates were washed with Tris-buffered saline-Tween 20 (TBST) and the non-specific binding sites were blocked with Tris-buffered saline (TBS) containing 1% BSA (Sigma) at room temperature (RT) for 1 h. After washing extensively with TBST, 50 µl of 1:100 diluted mouse serum samples were added to duplicate wells of the coated plates and incubated at RT for 2 h. The plates were washed five times with TBST and then 50 µl rabbit anti-mouse IgG-horseradish peroxidase (HRP, 1:2000 in TBS; Chemicon, Temecula, CA) was added and incubated at RT for 1 h. After washing, 100 µl of TMB peroxidase substrate (KPL, Gaithersburg, MD) was added to each well. The reaction was stopped after 10 min by adding 100 µl 2 M H2SO4 and the OD was read at 450 nm on a Bio-Rad Benchmark plus microplate spectrophotometer.
Serum anti-nuclear antibodies
Serum levels of anti-nuclear antibodies (ANA) were determined by using mouse-specific ELISA kits (Alpha Diagnostic Int’l, San Antonio, TX) as described earlier [30,33,35].
Determination of serum immunoglobulins
Immunoglobulin isotypes and IgG subtypes (IgG1, IgG2a and IgG2b) were quantified by specific ELISAs (Bethyl, Montgomery, TX) following the manufacturer’s instructions.
Flow cytometry analysis for splenocyte proliferation
Splenocytes isolated from spleens of control and TCE-treated mice were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) using CellTrace CFSE Cell Proliferation kit for Flow Cytometry (Molecular Probes, Eugene, OR), and then plated into 24-well flat-bottom plates at 2 × 106/well in a total volume of 1ml. Native mouse serum albumin (MSA, 20 µg/ml; Sigma), MDA-MSA (20 µg/ml) or HNE-MSA (20 µg/ml) were added to culture plates, respectively, to stimulate lymphocytes and incubated at 37°C with 5% CO2. After 72 h, the splenocytes from each well were blocked with anti-mouse CD16/CD32 (FcγIII/II Receptor, BD Biosciences, San Jose, CA), and then stained with anti-mouse CD45R/B220-PE, anti-mouse CD4-PE, anti-mouse CD8-PE (BD Biosciences), respectively, and analyzed using a Becton-Dickinson FacsCanto (BD Biosciences).
Determination of IL-2, IFN-γ, IL-4 and IL-10 in splenocyte cultures
Splenocytes isolated from spleens of control and TCE-treated MRL+/+ mice were plated into 24-well flat-bottom plates at 2 × 106/well in a total volume of 1ml. MSA (20 µg/ml), MDA-MSA (1, 5, 20 µg/ml), HNE-MSA (1, 5, 20 µg/ml) or anti- mouse CD3 (2.5 µg/ml, BD Biosciences) were added, respectively, to stimulate lymphocytes in the culture and incubated at 37°C with 5% CO2. After 72 h, culture supernatants from each well were harvested and the release of IL-2, IFN-γ, IL-4 and IL-10 into the cultures was quantitated using specific ELISA kits (Biosource, Camarillo, CA).
Statistical analyses
All data are expressed as means ± SD. Statistical comparisons were done by p value determinations using Student’s t test. When comparisons involved multiple groups, analysis of variance (ANOVA) followed by Student–Newman–Keuls test was performed. A p value less than 0.05 was considered as statistically significant.
Results
Anti-MDA- and anti-HNE-protein antibodies in the serum
In an earlier study, we have shown that TCE exposure in MRL+/+ mice leads to increased production of MDA- and HNE-proteins [36]. To assess the immunogenic potential of LPDA-protein adducts in TCE-induced autoimmune response, we determined the serum levels of anti-MDA- and anti-HNE-protein antibodies in control and TCE-treated mice. TCE treatment led to significant increases in the serum levels of both anti-MDA- and anti-HNE-protein antibodies (Fig. 1). As shown in Fig. 1, not only the levels of anti-MDA-protein antibodies were significantly higher, but also the number of samples positive for anti-MDA-protein antibodies were greater in TCE-treated mice compared to untreated controls (4/8 and 0/8, respectively). Similarly, serum levels of anti-HNE-protein antibodies and the number of samples positive for anti-HNE-protein antibodies (3/8 vs. 0/8 for TCE-treated vs. control mice) also increased following TCE treatment.
Fig. 1.
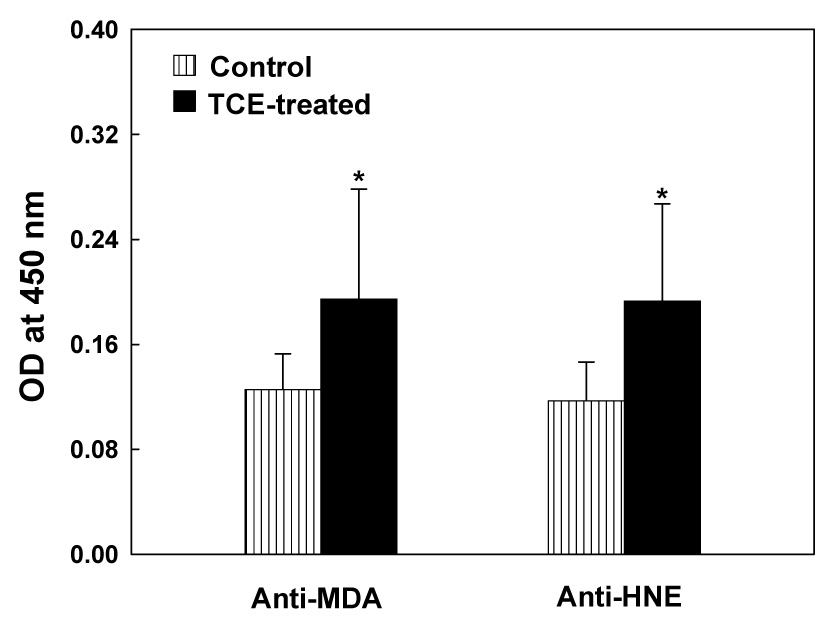
Anti-MDA- and anti-HNE-protein adduct antibodies in the sera of MRL+/+ mice treated with TCE for 4 weeks. The values are means ± SD of eight animals in each group. Net OD values exceeding the negative control values by more than 0.2 were considered positive. The numbers of mice positive for anti-MDA-protein adduct antibodies were 0/8 and 4/8 for control and TCE-treated mice, respectively, whereas numbers of mice positive for anti-HNE-protein adduct antibodies were 0/8 and 3/8 for control and TCE-treated mice, respectively. * p < 0.05 vs. controls.
TCE accelerates induction of ANA
To establish the association between TCE exposure and autoimmune response, we analyzed ANA, an initial laboratory evaluation index and biomarker of ADs [48–50], in the serum. Significant increase in serum ANA level was observed in mice treated with TCE compared to the controls (Fig. 2). More importantly, while only one of the eight mice was positive for ANA in the controls, five of the eight mice were positive in the TCE-treated group.
Fig. 2.
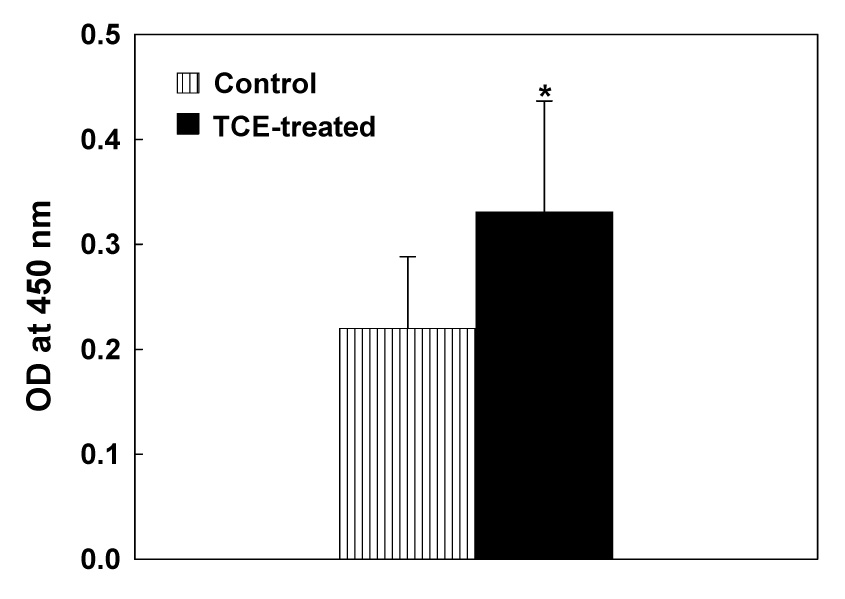
Serum ANA levels in MRL+/+ mice treated with TCE for 4 weeks. The values are means ± SD of eight animals in each group. Net OD values exceeding the negative control values by more than 0.2 were considered positive. The numbers of mice positive for ANA were 0/8 and 5/8 for control and TCE-treated mice, respectively. * p < 0.05 vs. controls.
Serum immunoglobulins in TCE-treated mice
To further evaluate the exacerbation of immune response following TCE exposure, we quantitated the immunoglobulin (Ig) isotypes and IgG subtypes in the serum of MRL+/+ mice treated with TCE. As shown in Fig. 3, TCE treatment led to significant increases in serum IgM and IgG, and also in IgG1 and IgG2a subtypes, compared to the controls. However, no significant change in serum IgG2b levels was observed following TCE exposure. Increases in Ig isotypes and IgG subtypes suggest that TCE treatment leads to an overall immunostimulatory response in MRL+/+ mice.
Fig. 3.
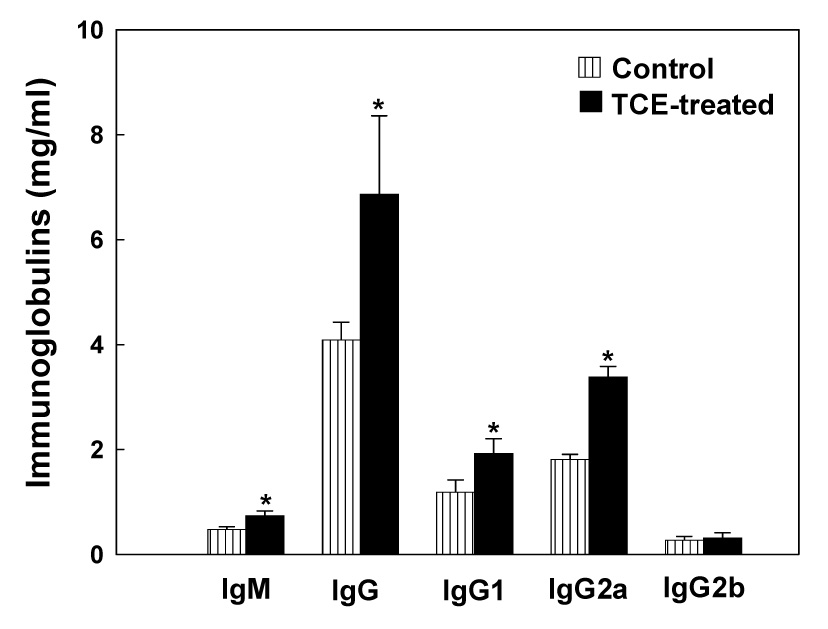
Serum levels of IgM, IgG and IgG subclasses in MRL+/+ mice treated with TCE for 4 weeks. The values are means ± SD of eight animals in each group. * p < 0.05 vs. controls.
Effect of stimulation with MDA-MSA or HNE-MSA on splenocyte proliferation
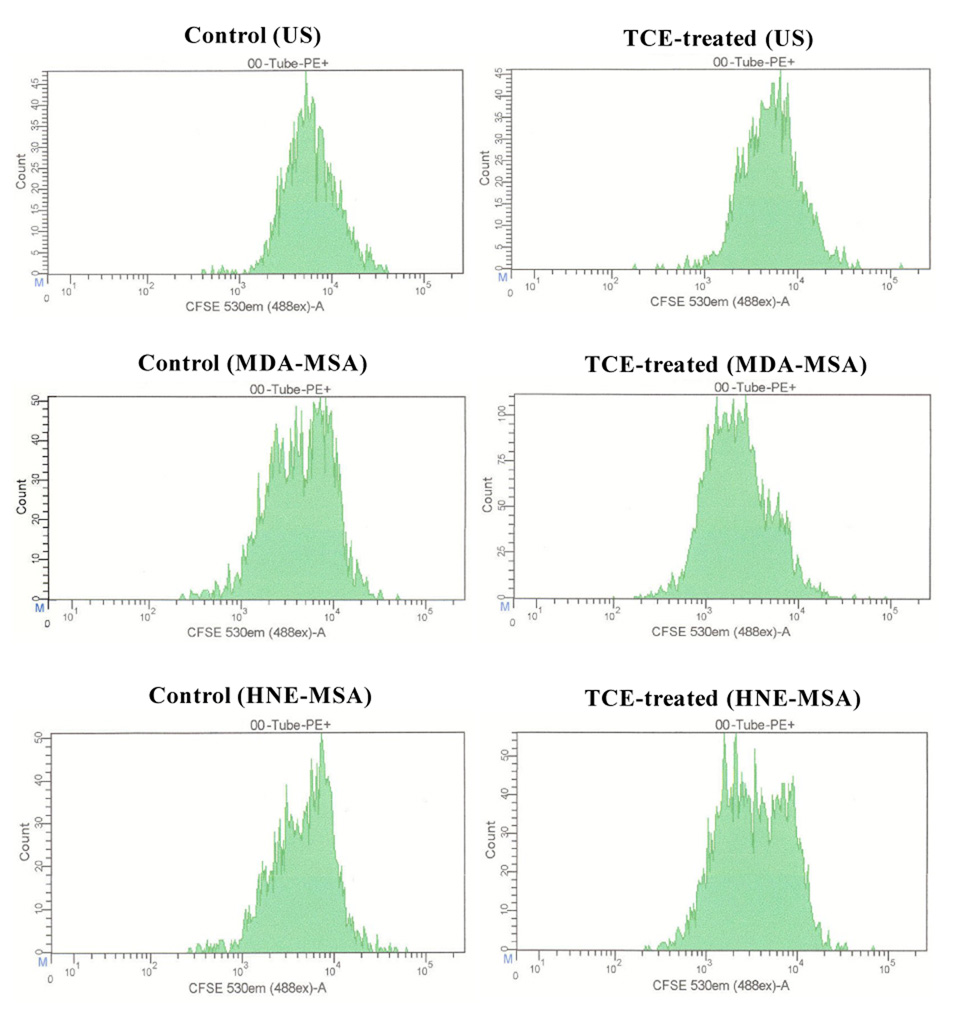
To determine the effects of LPDA-protein adducts on adaptive immune responses, splenocytes isolated from TCE-treated and control mice were labeled with CFSE and cultured with or without MDA-MSA or HNE-MSA. Seventy two hours later, T and B lymphocyte proliferation was analyzed using flow cytometry by staining with anti-mouse CD4-PE, anti-mouse CD8-PE or anti-mouse CD45R/B220-PE. Culture of splenocytes from TCE-treated mice with MDA-MSA or HNE-MSA resulted in significant stimulation in the proliferation of CD4+ T lymphocytes compared to those from control animals (Fig. 4 and Fig. 5). Incubation of splenocytes from TCE-treated and control mice with MSA alone did not have any effect on the proliferation of CD4+ T cells compared to respective unstimulated cells (data not shown). Interestingly, incubation of splenocytes from untreated control mice with MDA-MSA or HNE-MSA also resulted in significant increases in the proliferation of CD4+ cells compared to unstimulated cells (Fig. 4 and Fig. 5). Furthermore, compared to HNE-MSA, MDA-MSA led to greater increases in the CD4+ T cell proliferation. Stimulation with MDA-MSA or HNE-MSA did not result in any significant difference in the proliferation of B lymphocytes and CD8+ T lymphocytes (data not shown).
Fig. 4.
Proliferation of splenic CD4+ T cells from control and TCE-treated MRL+/+ mice. Splenocytes isolated from control and TCE-treated mice were labeled with CFSE, cultured with MDA-MSA or HNE-MSA for 72h, then stained with anti-mouse CD4-PE and analyzed by flow cytometry.
Fig. 5.
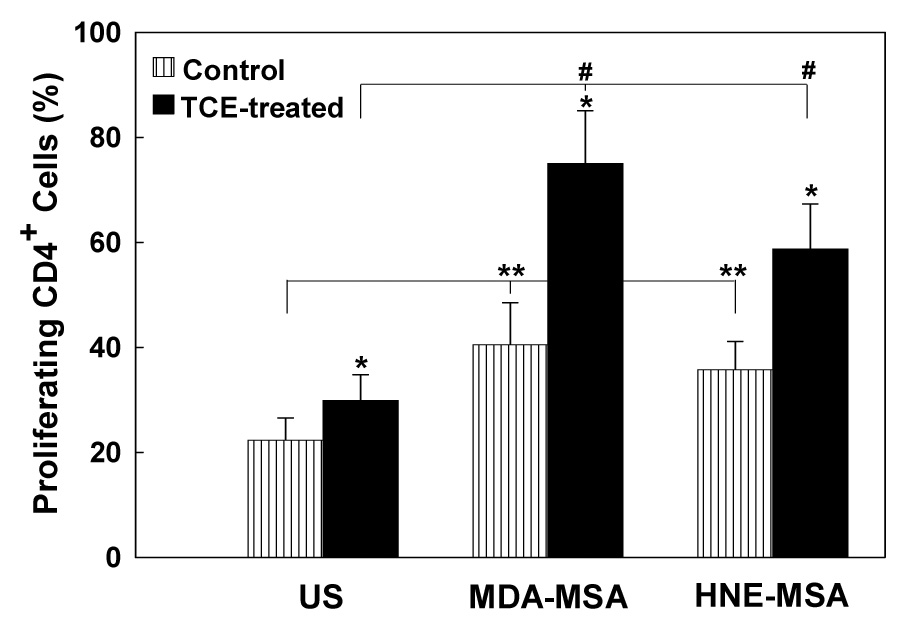
Quantitation of splenic CD4+ T cell proliferation from control and TCE-treated mice. Spenocytes were labeled with CFSE, cultured with MDA-MSA or HNE-MSA, then stained with anti-mouse CD4-PE and analyzed by flow cytometry. Proliferation is expressed as percent of proliferating CD4+ cells in the total CD4+ T cell population (PE+ cells). The values are means ± SD of eight animals in each group. * p < 0.05 vs. controls; # p < 0.05 vs. un-stimulated cells; ** p < 0.05 vs. un-stimulated control cells.
Release of IL-2, IFN-γ, IL-4 and IL-10 into splenocyte cultures following treatment with MDA-MSA or HNE-MSA
Cytokines produced by T helper cells have been suggested to play an important role in the immune dysregulation observed in SLE and rheumatoid arthritis patients, and in murine models [51–54]. To assess the effect of LPDA-protein adducts on cytokine release and further evaluate T cell response to LPDA-protein adducts, splenocytes isolated from TCE-treated and control mice were cultured with or without MDA-MSA, HNE-MSA or anti-mouse CD3 and the release of IL-2, IFN-γ, IL-4 and IL-10 in the culture supernatants was determined. Splenocytes from TCE-treated mice secreted significantly higher levels of IL-2 (Fig. 6A) than did splenocytes from untreated control mice following 72h stimulation with MDA-MSA or HNE-MSA, and the response was dose-dependent. The increases in IL-2 release into cultures were 1.8-, 2.0- and 3.4-fold for 1, 5 and 20 µg/ml of MDA-MSA, and 1.8-, 1.7- and 2.2-fold for 1, 5 and 20 µg/ml of HNE-MSA for TCE-treated mice compared to control mice. Similar patterns and greater increases in IFN-γ release into the splenocyte cultures with the MDA-MSA or HNE-MSA stimulation were observed (Fig. 6B). Even the splenocytes from control mice secreted more IL-2 and IFN-γ when stimulated with MDA-MSA or HNE-MSA, compared to unstimulated control cells. However, neither MDA-MSA not HNE-MSA led to significant differences in IL-4 or IL-10 release by cultured splenocytes of TCE-treated and control mice (data not shown).
Fig. 6.
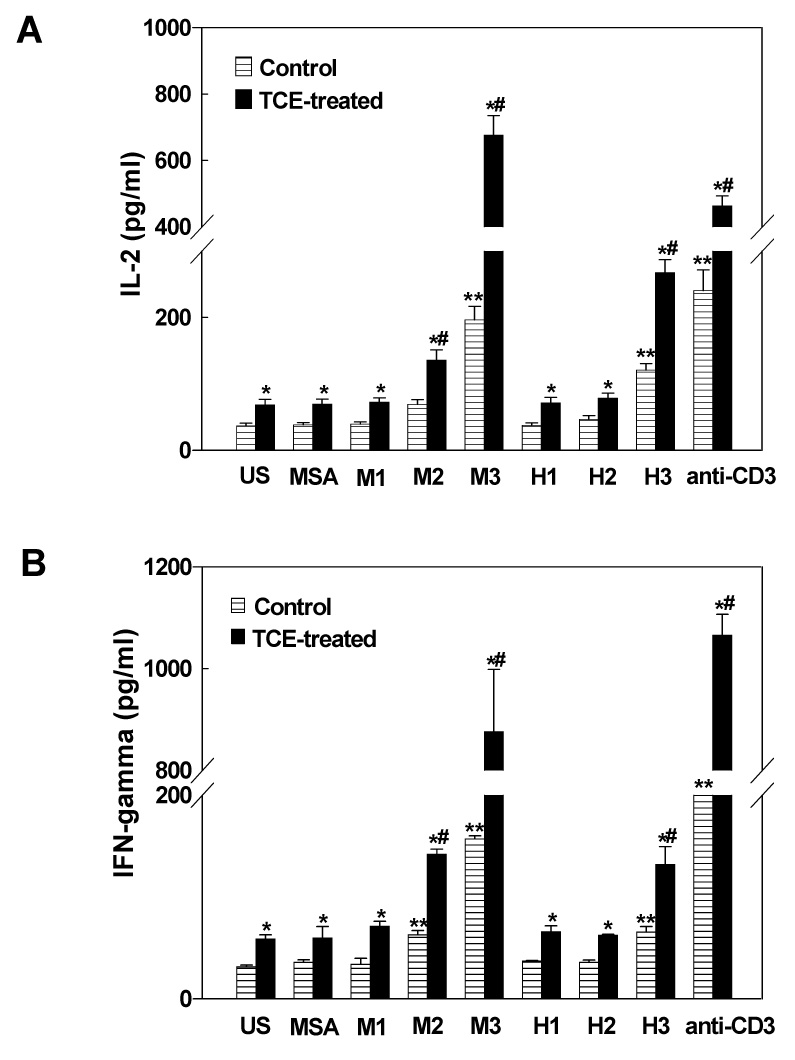
Release of IL-2 (A) and IFN-γ (B) into splenocyte cultures of control and TCE-treated MRL+/+ mice. Splenocytes were incubated with MSA alone, increasing concentrations of MDA-MSA (M1–M3), HNE-MSA (H1–H3), or anti-mouse CD3 antibody for 72h and the release of IL-2 and IFN-γ into cultures were measured by ELISA. The values are means ± SD of eight animals in each group. US: unstimulated cells. * p < 0.05 vs. respective controls; # p < 0.05 vs. MSA; ** p < 0.05 vs. MSA controls.
Discussion
Lipid peroxidation is implicated in the pathogenesis of various ADs [2–5,35,55,56]. LPDAs such as MDA and HNE are highly reactive and bind proteins to form LPDA-protein adducts, which may elicit an autoimmune response and contribute to disease pathogenesis. To investigate the role of LPDA-protein adducts in the induction and/or exacerbation of autoimmune responses, we conducted studies in female MRL+/+ mice, an animal model established earlier in our laboratory to demonstrate that TCE could induce both lipid peroxidation and autoimmune response [2, 30, 35, 36]. Our findings suggest that LPDA-protein adducts elicit an immune response that involves activation of Th1 cells and, thus, may contribute to the pathogenesis of ADs.
Non-enzymatic oxidative modification of proteins, including adduction with aldehydes, renders proteins immunogenic [15,36,57–59]. Antibodies against oxidatively modified proteins have been found in various ADs and other diseases, and have generally been suggested as biomarkers of increased oxidative damage [2,4,15,35,36,60,61]. Evidence has accumulated for the presence of significantly higher antibody responses to LPDA-modified proteins in patients with various ADs [4,62,63]. To evaluate our central hypothesis that exacerbation of an autoimmune response may be mediated through increased formation of LPDA-modified protein adducts following TCE exposure, we first determined anti-MDA- and HNE-protein specific antibodies in the sera of control and TCE-treated mice. Our data show significant induction of both anti-MDA-and anti-HNE-protein specific antibodies in the sera of mice treated with TCE. More importantly, the number of mice positive for anti-MDA or anti-HNE-protein antibodies was also higher in the TCE-treated group (Fig. 1). These findings in response to a short-term exposure to TCE along with results from chronic exposure studies [35] indicate that TCE is not only capable of inducing/accelerating lipid peroxidation in vivo, but also support the concept that LPDA-modified proteins are immunogenic and could contribute to the induction of an autoimmune response.
To demonstrate that TCE exposure leads to exacerbation of autoimmune response, we determined the levels of serum ANA, which serves as one of the initial laboratory evaluation indices for ADs such as SLE [48,50,64,65]. Human and experimental studies have documented that TCE exposure is associated with the induction of various autoantibodies, including ANA [24,29,30,32]. Our data showed that TCE treatment did not only increase serum ANA levels, but also led to increased ANA levels in more mice, suggesting that four weeks of TCE treatment was sufficient to induce an autoimmune response in MRL+/+ mice. Interestingly, the pattern of serum ANA appears to be similar to the patterns of anti-LPDA-protein antibodies, suggesting a close relationship between them, thus further supporting our hypothesis that LPDA-proteins could contribute to TCE-mediated autoimmune responses in MRL +/+ mice [2,35].
It has been suggested that covalent binding of LPDAs to endogenous proteins not only alters their function, but also results in structural modifications to generate neoantigens. It is thus not surprising that higher levels of anti-LPDA-protein adduct antibodies are observed in patients with ADs and in experimental studies [2,14,15,35,36,59], further supporting an association between covalent modification of LPDAs and ADs. However, the mechanism by which LPDA-protein adducts affect the adaptive autoimmune response remains unknown. It is well known that T cells, in particular activated CD4+ T cells, are the key mediators of autoimmune disorders [40–42,56,66]. Even though studies suggest that lipid peroxidation could play an important role in TCE-induced autoimmune response [2,35] and TCE accelerates an autoimmune response by CD4+ T cell activation [31,32], the potential role of LPDAs in the activation of CD4+ T cells is not known. In this study, we showed that both MDA-MSA and HNE-MSA were able to significantly promote CD4+ T cell proliferation, providing direct evidence for a link between LPDA-modified endogenous proteins and immune responses. The findings led to the hypothesis that post-translational modification of proteins with LPDAs, such as MDA and HNE, could serve as an immunological trigger in the activation of CD4+ T cells, potentially leading to autoimmune responses.
To further determine whether LPDA-protein adducts preferentially promote a specific T helper cell phenotype and to substantiate the role of T cells, we stimulated splenocytes obtained from TCE-treated and control mice in culture with LPDA-protein adducts, MDA-MSA or HNE-MSA, and quantitated the release of IL-2, IFN-γ, IL-4 and IL-10. Cytokines such as IL-2, IFN-γ and IL-4 are known to be important regulators of the immune system and their roles have been widely studied in the pathogenesis of SLE and other ADs [51–54]. However, the data regarding the levels of these cytokines are often not consistent due to variation in methods, disease stages and contribution of other cells, like mast and dendritic cells [52,67,68]. Following a 72 h-stimulation with MDA-MSA or HNE-MSA, the release of both IL-2 and IFN-γ from splenocytes isolated from TCE-treated mice showed a dose-dependent response and was significantly higher than from splenocytes from control mice (Figs. 6A and 6B). Even the cells from untreated control mice secreted greater IL-2 and IFN-γ when stimulated with MDA-MSA or HNE-MSA, compared with unstimulated controls. Furthermore, the serum IgG, IgG2a levels were significantly increased in TCE-treated mice compared to controls (Fig. 3). Since Th1 cells secrete IL-2 and IFN-γ and are tightly linked to IgG2a isotype switching [43,44], above findings clearly suggest that TCE treatment favored Th1 differentiation following activation by the LPDA-protein adducts. Hence, the data strongly imply that activated Th1 cells are involved in TCE-induced autoimmune responses. Other studies suggest a link between lipid peroxidation and increased secretion of Th1 cytokines (69–71), and our data provide further support to the idea that LPDAs preferentially promote the production of Th1 cytokines.
Together, this study provides evidence for the existence of an association between oxidative stress and induction of autoimmunity, and strongly supports the concept that LPDA-modified proteins act as an immunologic trigger by activating T lymphocytes and promoting Th1 differentiation, thus contributing to an autoimmune response. Further studies are needed to establish a clear link between oxidative stress and autoimmunity, especially by depleting distinct lymphocyte subsets, knocking out specific genes, and dissecting the role of redox status.
Acknowledgements
This work was supported by Grants ES013510 and ES016302 from the National Institute of Environmental Health Sciences (NIEHS), NIH, and it contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Walsh SJ, Rau LM. Autoimmune diseases: a leading cause of death among young and middle-aged women in the United States. Am. J. Pub. Health. 2000;90:1463–1466. doi: 10.2105/ajph.90.9.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan MF, Wu X, Ansari GAS. Anti-malondialdehyde antibodies in MRL+/+ mice treated with trichloroethene and dichloroacetyl chloride: possible role of lipid peroxidation in autoimmunity. Toxicol. Appl. Pharmacol. 2001;170:88–92. doi: 10.1006/taap.2000.9086. [DOI] [PubMed] [Google Scholar]
- 3.Hadjigogos K. The role of free radicals in the pathogenesis of rheumatoid arthritis. Panminerva Med. 2003;45:7–13. [PubMed] [Google Scholar]
- 4.Frostegard J, Svenungsson E, Wu R, Gunnarsson I, Lundberg IE, Klareskog L, Horkko S, Witztum JL. Lipid peroxidation is enhanced in patients with systemic lupus erythematosus and is associated with arterial and renal disease manifestations. Arthritis Rheum. 2005;52:192–200. doi: 10.1002/art.20780. [DOI] [PubMed] [Google Scholar]
- 5.Cuzzocrea S. Role of nitric oxide and reactive oxygen species in arthritis. Curr. Pharm. Des. 2006;12:3551–3570. doi: 10.2174/138161206778343082. [DOI] [PubMed] [Google Scholar]
- 6.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 7.Grune T, Michel P, Sitte N, Eggert W, Albrecht-Nebe H, Esterbauer H, Siems WG. Increased levels of 4-hydroxynonenal modified proteins in plasma of children with autoimmune diseases. Free Radic. Biol. Med. 1997;23:357–360. doi: 10.1016/s0891-5849(96)00586-2. [DOI] [PubMed] [Google Scholar]
- 8.Khan MF, Wu X, Kaphalia BS, Boor PJ, Ansari GA. Acute hematopoietic toxicity of aniline in rats. Toxicol. Lett. 1997;92:31–37. doi: 10.1016/s0378-4274(97)00032-5. [DOI] [PubMed] [Google Scholar]
- 9.Khan MF, Wu X, Boor PJ, Ansari GAS. Oxidative modification of lipids and proteins in aniline-induced splenic toxicity. Toxicol. Sci. 1999;48:134–140. doi: 10.1093/toxsci/48.1.134. [DOI] [PubMed] [Google Scholar]
- 10.Januszewski AS, Alderson NL, Jenkins AJ, Thorpe SR, Baynes JW. Chemical modification of proteins during peroxidation of phospholipids. J. Lipid Res. 2005;46:1440–1449. doi: 10.1194/jlr.M400442-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Serban MG, Negru T. Antioxidant protection in collagen-vascular diseases. Rom. J. Intern. Med. 1998;36:245–250. [PubMed] [Google Scholar]
- 12.Tam LS, Li EK, Leung VY, Griffith JF, Benzie IF, Lim PL, Whitney B, Lee VW, Lee KK, Thomas GN, Tomlinson B. Effects of vitamins C and E on oxidative stress markers and endothelial function in patients with systemic lupus erythematosus: a double blind, placebo controlled pilot study. J. Rheumatol. 2005;32:275–282. [PubMed] [Google Scholar]
- 13.Koch M, Mostert J, Arutjunyan AV, Stepanov M, Teelken A, Heersema D, De Keyser J. Plasma lipid peroxidation and progression of disability in multiple sclerosis. Eur. J. Neurol. 2007;14:529–533. doi: 10.1111/j.1468-1331.2007.01739.x. [DOI] [PubMed] [Google Scholar]
- 14.Kurien BT, Scofield RH. Free radical mediated peroxidative damage in systemic lupus rythematosus. Life Sci. 2003;73:1655–1666. doi: 10.1016/s0024-3205(03)00475-2. [DOI] [PubMed] [Google Scholar]
- 15.Kurien BT, Hensley K, Bachmann M, Scofield RH. Oxidatively modified autoantigens in autoimmune diseases. Free Radic. Biol. Med. 2006;41:549–556. doi: 10.1016/j.freeradbiomed.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 16.Fessel WJ. Systemic lupus erythematosus in the community. Incidence, prevalence, outcome, and first symptoms; the high prevalence in black women. Arch. Intern. Med. 1994;34:1027–1035. [PubMed] [Google Scholar]
- 17.Haustein UF, Ziegler V. Environmentally induced systemic sclerosis-like disorders. Int. J. Dermatol. 1985;24:147–151. doi: 10.1111/j.1365-4362.1985.tb05745.x. [DOI] [PubMed] [Google Scholar]
- 18.Parks CG, Conrad K, Cooper GS. Occupational exposure to crystalline silica and autoimmune disease. Environ. Health Perspect. 1999;107 Suppl 5:793–802. doi: 10.1289/ehp.99107s5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dahlgren J, Takhar H, Anderson-Mahoney P, Kotlerman J, Tarr J, Warshaw R. Cluster of systemic lupus erythematosus (SLE) associated with an oil field waste site: a cross sectional study. Environ. Health. 2007;6:8–23. doi: 10.1186/1476-069X-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ATSDR (Agency for Toxic Substances and Disease Registry, Division of Toxicology) Toxicological profile for trichloroethylene. Atlanta, GA: ATSDR; 1995. Update draft for public comments. [PubMed] [Google Scholar]
- 21.IARC. Some chlorinated solvents and other industrial chemicals. Lyon: International Agency for Research on Cancer; 1995. IARC monographs on the evaluation of carcinogenic risks to humans. Vol 63, DryCleaning. [PMC free article] [PubMed] [Google Scholar]
- 22.Hardin BD, Kelman BJ, Brent RL. Trichloroethylene and dichloroethylene: a critical review of teratogenicity. Birth Defects Res. Ann. Clin. Mol. Teratol. 2005;73:931–955. doi: 10.1002/bdra.20192. [DOI] [PubMed] [Google Scholar]
- 23.Phoon WH, Chan MO, Rajan VS, Tan KJ, Thirumoorthy T, Goh CL. Stevens-Johnson syndrome associated with occupational exposure to trichloroethylene. Contact Derm. 1984;10:270–276. doi: 10.1111/j.1600-0536.1984.tb00145.x. [DOI] [PubMed] [Google Scholar]
- 24.Flindt-Hansen H, Isager H. Scleroderma after occupational exposure to trichloroethylene and trichlorethane. Acta Derm. Venereol. 1987;67:263–264. [PubMed] [Google Scholar]
- 25.Lockey JE, Kelly CR, Cannon GW, Colby TV, Aldrich V, Livingston GK. Progressive systemic sclerosis associated with exposure to trichloroethylene. J. Occup. Med. 1987;29:493–496. [PubMed] [Google Scholar]
- 26.Waller PA, Clauw D, Cupps T, Metcalf JS, Silver RM, Leroy EC. Fasciitis (not scleroderma) following prolonged exposure to an organic solvent (trichloroethylene) J. Rheumatol. 1994;21:1567–1570. [PubMed] [Google Scholar]
- 27.Reinl W. Scleroderma caused by trichloroethylene? Bull. Hygiene. 1957;32:678–679. [PubMed] [Google Scholar]
- 28.Byers VS, Levin AS, Ozonoff DM, Baldwin RW. Association between clinical symptoms and lymphocyte abnormalities in a population with chronic domestic exposure to industrial solvent-contaminated domestic water supply and a high incidence of leukaemia. Cancer Immunol. Immunother. 1988;27:77–81. doi: 10.1007/BF00205762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kilburn KH, Warshaw RH. Prevalence of symptoms of systemic lupus erythematosus (SLE) and of fluorescent antinuclear antibodies associated with chronic exposure to trichloroethylene and other chemicals in well water. Environ. Res. 1992;57:1–9. doi: 10.1016/s0013-9351(05)80014-3. [DOI] [PubMed] [Google Scholar]
- 30.Khan MF, Kaphalia BS, Prabhakar BS, Kanz MF, Ansari GAS. Trichloroethene-induced autoimmune response in female MRL +/+ mice. Toxicol. Appl. Pharmacol. 1995;134:155–160. doi: 10.1006/taap.1995.1179. [DOI] [PubMed] [Google Scholar]
- 31.Gilbert KM, Griffin JM, Pumford NR. Trichloroethylene activates CD4+ T cells: potential role in an autoimmune response. Drug Metab. Rev. 1999;31:901–916. doi: 10.1081/dmr-100101945. [DOI] [PubMed] [Google Scholar]
- 32.Griffin JM, Blossom SJ, Jackson SK, Gilbert KM, Pumford NR. Trichloroethylene accelerates an autoimmune response by Th1 T cell activation in MRL+/+ mice. Immunopharmacology. 2000;46:123–137. doi: 10.1016/s0162-3109(99)00164-2. [DOI] [PubMed] [Google Scholar]
- 33.Khan MF, Kaphalia BS, Ansari GAS. Time-dependent autoimmune response of dichloroacetyl chloride in female MRL +/+ mice. Immunopharmacol. Immunotoxicol. 1997;19:265–277. doi: 10.3109/08923979709007662. [DOI] [PubMed] [Google Scholar]
- 34.Cai P, Konig R, Khan MF, Qiu S, Kaphalia BS, Ansari GAS. Autoimmune response in MRL+/+ mice following treatment with dichloroacetyl chloride or dichloroacetic anhydride. Toxicol. Appl. Pharmacol. 2006;216:248–255. doi: 10.1016/j.taap.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 35.Wang G, Cai P, Ansari GA, Khan MF. Oxidative and nitrosative stress in trichloroethene-mediated autoimmune response. Toxicology. 2007;229:186–193. doi: 10.1016/j.tox.2006.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang G, Ansari GA, Khan MF. Involvement of lipid peroxidative-derived aldehyde-protein adducts in autoimmunity mediated by trichloroethene. J. Toxicol. Environ. Health, Part A. 2007;70:1977–1985. doi: 10.1080/15287390701550888. [DOI] [PubMed] [Google Scholar]
- 37.Ogino K, Hobara T, Kobayashi H, Ishiyama H, Gotoh M, Imamura A, Egami N. Lipid peroxidation induced by trichloroethylene in rat liver. Bull Environ. Contam. Toxicol. 1991;46:417–421. doi: 10.1007/BF01688941. [DOI] [PubMed] [Google Scholar]
- 38.Channel SR, Latendresse JR, Kidneym JK, Grabaum JH, Lanem JW, Steel-Goodwinm L, Gothausm MC. A subchronic exposure to trichloroethylene causes lipid peroxidation and hepatocellular proliferation in male B6C3F1 mouse liver. Toxicol. Sci. 1998;43:145–154. doi: 10.1006/toxs.1998.2456. [DOI] [PubMed] [Google Scholar]
- 39.Zhu QX, Shen T, Ding R, Liang ZZ, Zhang XJ. Cytotoxicity of trichloroethylene and perchloroethylene on normal human epidermal keratinocytes and protective role of vitamin E. Toxicology. 2005;209:55–67. doi: 10.1016/j.tox.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 40.Breedveld FC, Dynesius-Trentham R, de Sousa M, Trentham DE. Collagen arthritis in the rat is initiated by CD4+ T cells and can be amplified by iron. Cell. Immunol. 1998;121:1–12. doi: 10.1016/0008-8749(89)90001-4. [DOI] [PubMed] [Google Scholar]
- 41.Voll RE, Roth EA, Girkontaite I, Fehr H, Herrmann M, Lorenz HM, Kalden JR. Histone-specific Th0 and Th1 clones derived from systemic lupus erythematosus patients induce double-stranded DNA antibody production. Arthritis Rheum. 1997;40:2162–2171. doi: 10.1002/art.1780401210. [DOI] [PubMed] [Google Scholar]
- 42.Lawson BR, Koundouris SI, Barnhouse M, Dummer W, Baccala R, Kono DH, Theofilopoulos AN. The role of alpha beta+ T cells and homeostatic T cell proliferation in Y-chromosome-associated murine lupus. J. Immunol. 2001;167:2354–2360. doi: 10.4049/jimmunol.167.4.2354. [DOI] [PubMed] [Google Scholar]
- 43.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 44.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 45.Khan MF, Kannan S, Wang J. Activation of transcription factor AP-1 and mitogen-activated protein kinases in aniline-induced splenic toxicity. Toxicol. Appl. Pharmacol. 2006;210:86–93. doi: 10.1016/j.taap.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 46.Khan MF, Wu X, Tipnis UR, Ansari GA, Boor PJ. Protein adducts of malondialdehyde and 4-hydroxynonenal in livers of iron loaded rats: quantitation and localization. Toxicology. 2002;173:193–201. [PubMed] [Google Scholar]
- 47.Habeeb AF. Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal. Biochem. 1966;14:328–336. doi: 10.1016/0003-2697(66)90275-2. [DOI] [PubMed] [Google Scholar]
- 48.Egner W. The use of laboratory tests in the diagnosis of SLE. J. Clin. Pathol. 2000;53:424–432. doi: 10.1136/jcp.53.6.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, Harley JB. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N. Engl. J. Med. 2003;349:1526–1533. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 50.Reveille JD. Predictive value of autoantibodies for activity of systemic lupus erythematosus. Lupus. 2004;13:290–297. doi: 10.1191/0961203303lu1015oa. [DOI] [PubMed] [Google Scholar]
- 51.Takahashi S, Fossati L, Iwamoto M, Merino R, Motta R, Kobayakawa T, Izui S. Imbalance towards Th1 predominance is associated with acceleration of lupus-like autoimmune syndrome in MRL mice. J. Clin. Invest. 1996;97:1597–1604. doi: 10.1172/JCI118584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Segal R, Bermas BL, Dayan M, Kalush F, Shearer GM, Mozes E. Kinetics of cytokine production in experimental systemic lupus erythematosus: involvement of T helper cell 1/T helper cell 2-type cytokines in disease. J. Immunol. 1997;158:3009–3016. [PubMed] [Google Scholar]
- 53.Rachmiel M, Bloch O, Bistritzer T, Weintrob N, Ofan R, Koren-Morag N, Rapoport MJ. TH1/TH2 cytokine balance in patients with both type 1 diabetes mellitus and asthma. Cytokine. 2006;34:170–176. doi: 10.1016/j.cyto.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 54.Lit LC, Wong CK, Li EK, Tam LS, Lam C, Lo YM. Elevated gene expression of Th1/Th2 associated transcription factors is correlated with disease activity in patients with systemic lupus erythematosus. J. Rheumatol. 2007;34:89–96. [PubMed] [Google Scholar]
- 55.Michel P, Eggert W, Albrecht-Nebe H, Grune T. Increased lipid peroxidation in children with autoimmune diseases. Acta Paediatr. 1997;86:609–612. doi: 10.1111/j.1651-2227.1997.tb08943.x. [DOI] [PubMed] [Google Scholar]
- 56.Gelderman KA, Hultqvist M, Olsson LM, Bauer K, Pizzolla A, Olofsson P, Holmdahl R. Rheumatoid arthritis: the role of reactive oxygen species in disease development and therapeutic strategies. Antioxid. Redox. Signal. 2007;9:1541–1568. doi: 10.1089/ars.2007.1569. [DOI] [PubMed] [Google Scholar]
- 57.Palinski W, Horkko S, Miller E, Steinbrecher UP, Powell HC, Curtiss LK, Witztum JL. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J. Clin. Invest. 1996;98:800–814. doi: 10.1172/JCI118853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wuttge DM, Bruzelius M, Stemme S. T-cell recognition of lipid peroxidation products breaks tolerance to self proteins. Immunology. 1999;98:273–279. doi: 10.1046/j.1365-2567.1999.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mottaran E, Stewart SF, Rolla R, Vay D, Cipriani V, Moretti M, Vidali M, Sartori M, Rigamonti C, Day CP, Albano E. Lipid peroxidation contributes to immune reactions associated with alcoholic liver disease. Free Radic. Biol. Med. 2002;32:38–45. doi: 10.1016/s0891-5849(01)00757-2. [DOI] [PubMed] [Google Scholar]
- 60.Wu JT, Wu LL. Autoantibodies against oxidized LDL. A potential marker for atherosclerosis. Clin. Lab. Med. 1997;17:595–604. [PubMed] [Google Scholar]
- 61.Steinerova A, Racek J, Stozicky F, Zima T, Fialova L, Lapin A. Antibodies against oxidized LDL-theory and clinical use. Physiol. Res. 2001;50:131–141. [PubMed] [Google Scholar]
- 62.Slatter DA, Bolton CH, Bailey AJ. The importance of lipid-derived malondialdehyde in diabetes mellitus. Diabetologia. 2000;43:550–557. doi: 10.1007/s001250051342. [DOI] [PubMed] [Google Scholar]
- 63.Palacio JR, Iborra A, Ulcova-Gallova Z, Badia R, Martinez P. The presence of antibodies to oxidative modified proteins in serum from polycystic ovary syndrome patients. Clin. Exp. Immunol. 2006;144:217–222. doi: 10.1111/j.1365-2249.2006.03061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tan EM. Antinuclear antibodies: diagnostic markers of autoimmune diseases and probes for cell biology. Adv. Immunol. 1989;44:93–151. doi: 10.1016/s0065-2776(08)60641-0. [DOI] [PubMed] [Google Scholar]
- 65.Treadwell EL, Cohen P, Williams D, O'Brien K, Volkman A, Eisenberg R. MRL mice produce anti-Su autoantibody, a specificity associated with systemic lupus erythematosus. J. Immunol. 1993;150:695–699. [PubMed] [Google Scholar]
- 66.Paulus HE, Machleder HI, Levine S, Yu DT, MacDonald NS. Lymphocyte involvement in rheumatoid arthritis. Studies during thoracic duct drainage. Arthritis Rheum. 1977;20:1249–1262. doi: 10.1002/art.1780200614. [DOI] [PubMed] [Google Scholar]
- 67.Nagy G, Pallinger E, Antal-Szalmas P, Aleksza M, Marschalko M, Brozik M, Falus A, Gergely P. Measurement of intracellular interferon-gamma and interleukin-4 in whole blood T lymphocytes from patients with systemic lupus erythematosus. Immunol. Lett. 2000;74:207–210. doi: 10.1016/s0165-2478(00)00265-0. [DOI] [PubMed] [Google Scholar]
- 68.Havarinasab S, Bjorn E, Ekstrand J, Hultman P. Dose and Hg species determine the T-helper cell activation in murine autoimmunity. Toxicology. 2007;229:23–32. doi: 10.1016/j.tox.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 69.Iuliano L, Pratico D, Ferro D, Pittoni V, Valesini G, Lawson J, FitzGerald GA, Violi F. Enhanced lipid peroxidation in patients positive for antiphospholipid antibodies. Blood. 1997;90:3931–3935. [PubMed] [Google Scholar]
- 70.Ronis MJJ, Hakkak R, Korourian S, Albano E, Yoon S, Ingelman-Sundberg M, Lindros KO, Badger TM. Alcoholic liver disease in rats fed ethanol as part of oral or intragastric low-carbohydrate liquid diets. Exp. Biol. Med. 2004;229:351–360. doi: 10.1177/153537020422900410. [DOI] [PubMed] [Google Scholar]
- 71.Ukil A, Maity S, Karmakar S, Datta N, Vedasiromoni JR, Das PK. Curcumin, the major component of food flavour turmeric, reduces mucosal injury in trinitrobenzene sulphonic acid-induced colitis. Br. J. Pharmacol. 2003;139:209–218. doi: 10.1038/sj.bjp.0705241. [DOI] [PMC free article] [PubMed] [Google Scholar]