Abstract
Background & Aims
Serologic tests are frequently used in celiac disease diagnosis. Gliadin antibodies generally lack the accuracy required for proper diagnosis. We evaluated the value of deamidated gliadin antibody measurements in the diagnosis and follow-up of celiac disease and compared their potential usefulness with that of gliadin and tissue-transglutaminase antibodies.
Methods
We tested deamidated gliadin, gliadin, and tissue-transglutaminase-IgA and -IgG in 216 biopsy-selected subjects including 92 biopsy-proven untreated celiac patients (46% with total villous atrophy and 54% with partial villous atrophy) and 124 biopsy-proven non-celiac controls. Fifty-nine of celiac patients were also tested after treatment with gluten-free diet. Antibodies were measured by commercial enzyme-linked immunosorbent assays. Deamidated gliadin-IgA+G was detected using a conjugate reactive to both isotypes which gives a positive if either isotype is present.
Results
The sensitivity, specificity, and accuracy of deamidated gliadin-IgA (74%, 95%, and 86%), deamidated gliadin-IgG (65%, 98%, and 84%) and deamidated gliadin-IgA+G (75%, 94%, and 86%) were superior to gliadin-IgA (63%, 90%, and 79%) (P < 0.05) and gliadin-IgG (42%, 90%, and 69%) (P < 0.01) and were similar to tissue-transglutaminase-IgA (78%, 98%, and 90%) before treatment. The sensitivity of IgA isotype for all tests was significantly greater in celiac patients with total villous atrophy compared to those with partial villous atrophy (P < 0.05). The proportion of positive test results for all tests decreased significantly after treatment (P < 0.0001).
Conclusions
Deamidated gliadin antibody is a better diagnostic test for celiac disease than the conventional gliadin antibody testing; although histopathology remains the gold standard test for diagnosis of celiac patients.
Introduction
Celiac disease (CD) is a gluten-sensitive enteropathy with an estimated prevalence of 1%.1,2 The early diagnosis of CD and treatment with gluten-free diet (GFD) prevents the risk of developing malnutrition complications (e.g. anemia and osteoporosis), autoimmune disorders, and malignancies.3,4 The gold standard for diagnosis of CD is histopathologic analysis of small intestinal biopsy, wherein the presence of enteropathy can be detected.
Serologic detection of antibodies and autoantibodies is frequently used as a diagnostic aid to detect those likely to have celiac disease and to avoid unnecessary intestinal biopsy in suspected celiac patients. Endomysial antibody (EMA), tissue transglutaminase antibody (TTG), and gliadin antibody (AGA) are commonly used serologic tests for the diagnosis and follow-up of CD patients in the clinical settings. Among these, EMA is considered to be a highly sensitive and specific test for the diagnosis of CD,5 but is not easily applied for screening and follow-up of CD patients because of its limitations (expensive, qualitative, and subjective). AGA and TTG avoid these limitations of EMA; however the poor sensitivity and specificity of AGA (52%–100% and 71%–100% for IgA, 57%–100% and 47%–94% for IgG) have limited its use in clinical practice.6 Thus, TTG-IgA has been recommended as the first step in celiac screening because it is less costly than EMA and its sensitivity is thought to be better than AGA.7–9
Recent studies have shown that deamidation of gliadin increases binding of AGA to the gliadin in the sera of CD patients, but not controls.10–12 Based on these findings an enzyme-linked immunosorbent assay (ELISA) was developed which detects antibodies against synthetic deamidated gliadin peptides (AGA II) in the sera of CD patients.
The main aim of this study was to determine the sensitivity, specificity, and accuracy of AGA II for the diagnosis of CD in subjects who were selected based on histopathologic results of small intestinal biopsy and to compare the diagnostic accuracy of this new assay with that of AGA and TTG in the same population of patients. We also aimed to explore the serologic response to gluten exclusion for each antibody in a subgroup of celiac patients who were followed after treatment with GFD.
Methods and Materials
Study design
Serum samples were collected from patients referred to the division of Gastroenterology and Hepatology at the Mayo Clinic, Rochester, MN, for the assessment of gastrointestinal symptoms, unexplained weight loss/anemia, or to rule out CD. All patients underwent small intestinal biopsy between January 1999 and December 2006. All serum samples were stored at or below −20ºC. The study was approved by the Institutional Review Boards of Mayo Clinic, Rochester, MN.
Subjects
Patients
Subjects whose serum samples were collected within 6 months before and 3 months after the date of CD diagnosis (made by histopathology) were included in the study (N=116). Diagnosis of CD was based on presence of villous atrophy (enteropathy type IIIa or greater based on currently accepted diagnostic criteria).9,13 Patients with Marsh 0, I, and II, as well as patients who had started a GFD for more than 2 weeks prior to the serum sample collection, were excluded (all patients were completely untreated except one who was on GFD for only 2 weeks before serum sample collection).14,15 The remaining patients comprised the biopsy-proven CD group. Based on these criteria 92 untreated CD patients were identified. Fifty-nine of these 92 patients had a second serum sample after treatment with GFD.
Controls
Controls were identified as subjects who had a saved serum sample and had undergone small intestinal biopsy but did not have any degree of enteropathy in histopathological examination. To select potential controls randomly from these subjects a frequency matching was performed for age and gender and 135 controls were included. Patients with enteropathy greater than Marsh 0 or high clinical suspicion of CD despite normal biopsy, and patients who did not authorize research use of their information were excluded. Based on these criteria 124 subjects were categorized into biopsy-proven non-celiac controls.
Serology
Serum samples were kept at or below −20°C until the time of the assays. All antibodies were measured by ELISA methods. Samples were tested in accordance with each manufacturer’s specifications. Each run was checked against stated quality control requirements.
Table 1 summarizes the tests characteristics. AGAII-IgA and AGAII-IgG were measured by ELISA using kits provided for in vitro diagnostic use (QUANTA Lite Gliadin-IgA II and Gliadin-IgG II, INOVA Diagnostics Inc., San Diego, CA). AGA II-IgA+G was measured by ELISA using a kit provided for research use only (QUANTA Lite Celiac DGP Screen, INOVA Diagnostics Inc., San Diego, CA). AGA-IgA and AGA-IgG were measured by ELISA using kits provided for in vitro diagnostic use (Scanlisa Anti-Gliadin-IgA Antibody and Anti-Gliadin-IgG Antibody, Scimedx Corporation, Denville, NJ). TTG-IgA and TTG-IgG were measured by ELISA using a kit for in vitro diagnostic use (BINDAZYME human IgA and IgG Anti-Tissue Transglutaminase EIA Kit, The Binding Site, Ltd., Birmingham, UK).
Table 1.
Tests characteristics
| Test | Manufacturer | Cutoff
|
||
|---|---|---|---|---|
| IgA | IgG | IgA + G | ||
| AGA II | INOVA Diagnostics | 20 U | 20 U | 20 U |
| AGA | SCIMEDX | 30 EU | 30 EU | - |
| TTG | The Binding Site | 4 U/mL | 6 U/mL | - |
To test for AGA II antibodies, a combination of deamidated gliadin peptides was used as antigen in coated plates. Serum samples were pre-diluted, added into separate wells, and incubated for 30 minutes. After washing away the unbound sera, horseradish peroxidase-labeled goat anti-human IgA or IgG conjugate was added to each well. In order to detect the AGA II-IgA+G, enzyme-labeled conjugate was used that reacts with both IgA and IgG isotype. After the second incubation, the unbound enzyme-labeled anti-human antibody was washed away and tetramethylbenzidine was added to each well. By measuring the intensity of the color that developed, serum concentration of antibodies was determined. Intra-assay variability was determined by running five replicates of each specimen within a single run. Inter-assay variability was determined by running a single replicate of each specimen in five separate runs over the course of several days. Variability was expressed as a coefficient of variance (CV).
Histopathology
Subjects underwent upper endoscopy and small intestinal biopsy as part of their clinical workup. The duodenal biopsy specimens were reviewed by expert pathologists. Patients who did not have any evidence of enteropathy (Marsh 0) comprised the control group. Patients with increased number of intraepithelial lymphocytes, crypt hyperplasia, inflammation, and some degrees of villous atrophy comprised the celiac group.
Statistics
Statistical analysis was conducted using JMP ™ version 6.0.0 software and FREQ procedure, SAS version 8 (SAS Institute Inc.; Cary, NC). The student’s T test assuming equal variances and chi square test were employed to compare continuous (age) and binary (gender, under-18 years of age indicator) variables between celiac and control group, respectively. The sensitivity, specificity, and accuracy with 95% confidence interval were calculated for each test using conventional formulas. Our primary aim was to compare the accuracy of AGA II-IgA and -IgG with that of AGA-IgA and -IgG, respectively. In a separate analysis we compared sensitivity and specificity of each AGA II antibody isotype with those of the same AGA and TTG isotypes. We also compared AGA II-IgA+G with IgA and IgG AGA and TTG. The significance of difference in sensitivity, specificity and accuracy between serologic tests was tested using McNemar’s or McNemar’s exact test (as appropriate). The agreement between the tests was measured using Kappa statistics. Good agreement and excellent agreement were defined as a Kappa coefficient≥0.6 and ≥0.8, respectively. To define the optimum sensitivity, specificity, and cut-off value for AGA II, receiver-operating characteristic (ROC) analysis was performed. The chi square test was used to compare the proportion of positive test results between celiac patients with PVA versus those with TVA. McNemar’s test or its exact version was also employed to detect the significance of the difference in the proportion of positive test results before and after treatment with GFD and to compare the sensitivity of AGA II-IgA and TTG-IgA in gluten-free treated CD patients. Statistical significance was inferred at P values <0.05 for all comparisons.
Results
Subjects characteristics
We evaluated serum antibodies from 92 biopsy-proven celiac patients and 124 biopsy-proven non-celiac controls. Patients and controls were similar regarding age and gender. Of 92 celiac patients 50 had partial villous atrophy, 4 had subtotal villous atrophy and 38 had total villous atrophy. For simplicity, patients with mild or partial degree of villous atrophy were categorized into a partial villous atrophy group (PVA) (54%) and patients with subtotal or total villous atrophy were categorized into a total villous atrophy group (TVA) (46%). The demographic characteristics of subjects are shown in table 2.
Table 2.
Demographic characteristics of subjects
| Biopsy-proven celiac patients (n = 92)
|
Biopsy-proven non-celiac controls (n = 124)
|
||
|---|---|---|---|
| Mean ± SD or N (%) | Mean ± SD or N (%) | P value | |
| Age (y), Mean ± SD | 46.7 ± 18.7 | 43.7 ± 20.0 | 0.279 |
| Children (<18y), n (%) | 8 (9) | 13 (10) | 0.661 |
| Female, n (%) | 65 (71) | 90 (73) | 0.756 |
| PVA, n (%) | 50 (54) | - | - |
| TVA, n (%) | 42 (46) | - | - |
Diagnostic values of AGA II
The sensitivity, specificity, and accuracy of the new antibody assays (AGA II) were determined using the manufacturer’s cutoff value for definition of positive and negative results. Intra-assay variability CVs were 6.6%–14.7% for AGA II-IgA and 2.2%–11.3% for AGA II-IgG. Inter-assay variability CVs were 11.0%–18.1% for AGA II-IgA and for 2.2%–6.5% for AGA II-IgG. The sensitivity, specificity and accuracy were, respectively, 74%, 95% and 86% for AGA II-IgA, 65%, 98% and 84% for AGA II-IgG, and 75%, 94% and 86% for AGA II-IgA+G.
Comparison of AGA II with AGA and TTG
The diagnostic values AGA II were compared to those of AGA and TTG. The sensitivity, specificity, and accuracy with 95% confidence interval for each analyte are shown in table 3. AGA II-IgA was significantly more sensitive and more accurate than AGA-IgA (P < 0.05 and P < 0.01, respectively). The sensitivity and accuracy of AGA II-IgA was not significantly different from TTG-IgA (P = 0.344). AGA II-IgA was similar to AGA-IgA and TTG-IgA in specificity. AGA II-IgA had excellent agreement with TTG-IgA (kappa = 0.85).
Table 3.
Comparison of the diagnostic values
| AGA II
|
AGA
|
TTG
|
|||||
|---|---|---|---|---|---|---|---|
| IgA | IgG | IgA+G | IgA | IgG | IgA | IgG | |
| Sensitivity (%) (95% CI) | 74* (64–82) | 65†† (55–74) | 75*†† (65–83) | 63 (53–72) | 42 (33–53) | 78 (68–85) | 28 (20–38) |
| Specificity (%) (95% CI) | 95 (90–98) | 98*† (94–100) | 94 (89–97) | 90 (84–94) | 90 (83–94) | 98 (94–100) | 97 (92–99) |
| Accuracy (%) (95% CI) | 86** (81–90) | 84†† (79–89) | 86**†† (81–90) | 79 (73–84) | 69 (63–75) | 90 (85–93) | 68 (61–73) |
P < 0.05 vs. AGA IgA,
P < 0.01 vs. AGA IgA,
P < 0.01 vs. AGA IgG,
P < 0.0001 vs. AGA IgG and TTG IgG (P values are obtained by McNemar’s or McNemar’s exact test as appropriate).
The sensitivity and the accuracy of AGA II-IgG were significantly greater than those of AGA-IgG and TTG-IgG (P < 0.0001). The specificity of AGA II-IgG was significantly higher than AGA-IgG (P < 0.01) and AGA-IgA (P = 0.01) and was similar to TTG-IgG (P = 0.625). AGA II-IgG had good agreement with both AGAII-IgA and TTG-IgA (kappa = 0.76). AGA II-IgA+G was very similar to AGA II-IgA in terms of sensitivity, specificity, and accuracy. AGA II-IgA+G also had excellent agreement with TTG-IgA (kappa = 0.84), AGA II-IgA (kappa = 0.86) and AGA II-IgG (kappa = 0.83), as expected. The AGA-IgA had good agreement with TTG-IgA, AGA II-IgA, and AGA II-IgA+G.
Diagnostic values of AGA II by ROC curve
The ROC curve analysis of the data determined the optimal cutoff value for each test at which the greatest sum of sensitivity and specificity was achieved. Figure 1 shows the ROC curve for AGA II-IgA, -IgG, and -IgA+G. The optimal cutoff values in our sample were lower than the manufacturer’s cutoff value for all tests. Table 4 illustrates the area under the ROC curve (AUC) as well as the sensitivity, specificity, and accuracy for all tests calculated based on optimal cutoff values. Using the new cutoff values, the sensitivity increased significantly for AGA II-IgA, AGA II-IgG and AGA II-IgA+G but at the expense of decreased specificity. The accuracy remained the same for AGA II-IgA (86%) and increased for AGA II-IgG (89%) and AGA II-IgA+G (88%), although the differences were not statistically significant.
Figure 1.
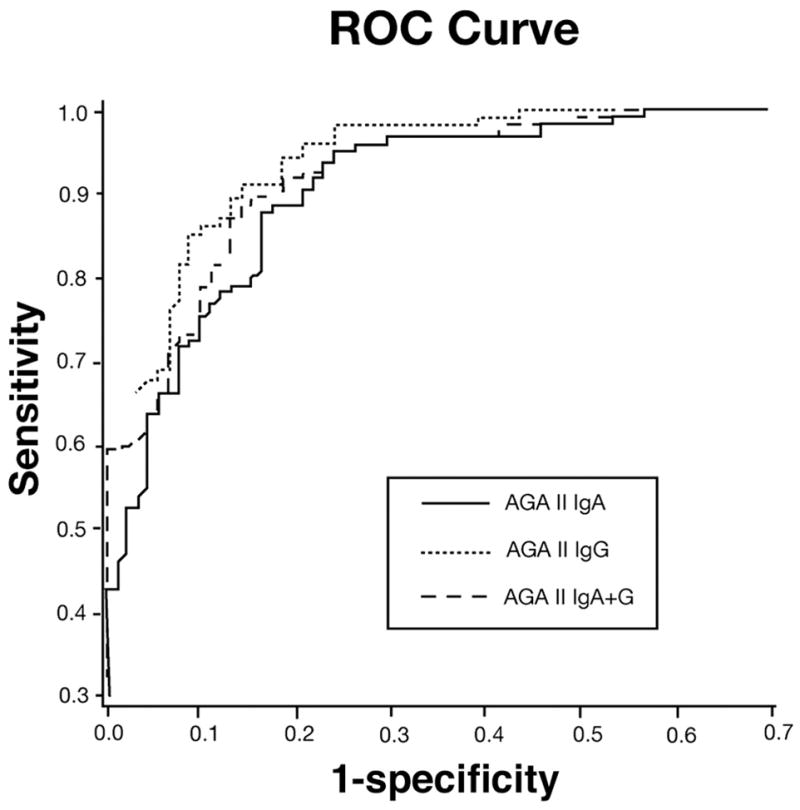
Receiver operating characteristic curves for AGA II-IgA, -IgG, and -IgA + G based on 92 celiac patients and 124 controls. True-positive (Y axis) and false-positive (X axis) rates are plotted together using each point as indicated on the semiquantitative scale as the cut point. There was no significant difference between the ROC curves for all three AGA II antibodies.
Table 4.
Optimal cutoff values and sensitivity, specificity and accuracy provided by ROC curve
| AGA II
|
AGA
|
TTG
|
|||||
|---|---|---|---|---|---|---|---|
| IgA | IgG | IgA + G | IgA | IgG | IgA | IgG | |
| Manufacturer Cutoff | 20 | 20 | 20 | 30 | 30 | 4 | 6 |
| ROC Cutoff | 11.4 | 3.7 | 9.6 | 24.6 | 10.3 | 1.6 | 2.8 |
| Sensitivity (%) | 84 | 86 | 86 | 71 | 78 | 84 | 46 |
| Specificity (%) | 88 | 91 | 89 | 89 | 72 | 98 | 90 |
| Accuracy (%) | 86 | 89 | 88 | 81 | 75 | 92 | 71 |
| AUC | 0.93 | 0.95 | 0.94 | 0.82 | 0.78 | 0.91 | 0.71 |
Comparison of sensitivity in PVA and TVA group
In order to assess whether the sensitivity of serologic tests would differ by the severity of mucosal damage, we stratified CD patients into two groups based on the degree of villous atrophy in histopathology (Figure 2). All of the tests that incorporated the IgA isotype (AGA II-IgA, AGA II-IgA+G, TTG-IgA, and AGA-IgA) were significantly more sensitive among patients with TVA as compared to those with PVA (P < 0.05 for AGA II-IgA and AGA II-IgA+G and P < 0.01 for TTG-IgA and AGA-IgA).
Figure 2. Difference in sensitivity by degree of villous atrophy.
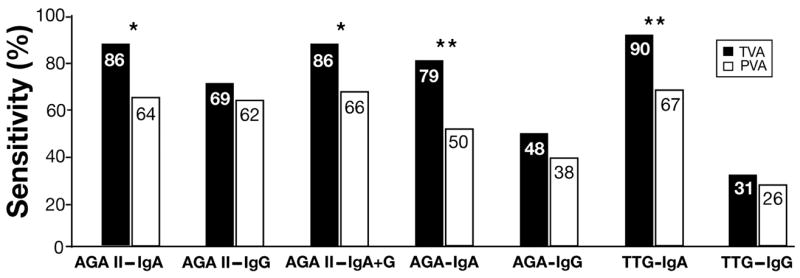
Percentage of positive results for each test in biopsy-proven untreated celiac patients with TVA (N = 42) vs. PVA (N = 50). *P<0.05, **P<0.01 (P values are obtained by chi square test).
TTG-IgA negative patients
After the serologic tests were performed, 20 TTG-IgA negative CD patients were identified (Table 5). Since the diagnosis of CD in our sample was only based on histopathology, we reevaluated TTG-IgA negative CD patients to provide more evidence for their CD diagnosis. In order to make sure that there was no error in the method of testing, we repeated TTG-IgA in these individuals. The sensitivity was the same with repeated measurements. Ten of these TTG-IgA negative patients were positive for other serologic tests. These included one patient with IgA deficiency that had a positive serology for IgG isotype of all serologic tests. Three patients who were negative for all the tests had had a positive EMA performed on the same sample in their medical record. Five of the remaining patients had clinical and/or histopathologic responses to GFD and the other two patients who had been lost to follow-up had a celiac predisposing HLA.
Table 5.
Characteristics of TTG IgA negative CD patients
| Patient # | AGA II | AGA | TTG | EMA | Response to GFD | HLA DQ2/DQ8 | IgA deficiency | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IgA | IgG | IgA+G | IgA | IgG | IgG | clinical | Histology | ||||
| 1 | − | − | − | − | − | − | + | + | NF | NF | NF |
| 2 | − | − | + | − | − | − | + | + | NF | NF | − |
| 3 | − | − | − | − | − | − | + | + | + | NF | − |
| 4 | − | − | − | − | − | − | + | + | + | NF | NF |
| 5 | + | − | − | − | − | − | − | ± | + | + | − |
| 6 | − | − | − | − | − | − | − | + | + | NF | NF |
| 7 | − | − | − | − | − | − | − | + | + | NF | − |
| 8 | − | − | − | − | + | − | − | + | NF | − | − |
| 9 | + | + | + | + | + | − | − | + | + | + | − |
| 10 | + | + | − | + | − | − | − | + | + | + | NF |
| 11 | − | − | − | − | − | − | − | − | + | NF | NF |
| 12 | − | − | − | − | − | − | NF | NF | NF | + | NF |
| 13 | − | − | − | + | − | − | − | + | + | NF | − |
| 14 | − | + | + | − | + | + | NF | + | − | NF | NF |
| 15 | − | − | − | − | − | − | − | NF | NF | + | NF |
| 16 | − | + | + | − | + | + | ± | + | NF | NF | + |
| 17 | − | − | − | − | − | − | NF | + | + | − | − |
| 18 | − | − | − | − | − | − | − | + | + | − | − |
| 19 | − | − | − | − | + | − | − | − | + | + | NF |
| 20 | − | − | − | + | − | − | − | NF | NF | + | NF |
NF, No follow-up
Effect of treatment with gluten-free diet on serologic tests
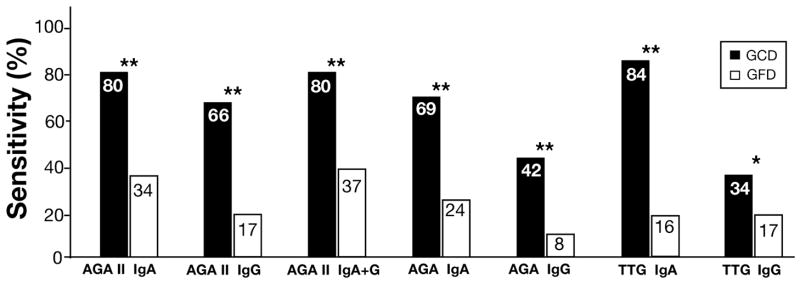
Fifty-nine celiac patients had paired serum samples before and after treatment with GFD. The median (range) time of treatment was 11 (3–43) months. The percentage of positive results dropped significantly after gluten exclusion for all tests (P < 0.01 for TTG-IgG and P < 0.0001 for all other tests) (Figure 3). In CD patients who were treated with GFD for 3 to 6 months (N=17), the sensitivity of AGA II-IgA was 47%, whereas only 18% of these patients were positive for TTG-IgA (P = 0.06). In patients with treatment length of greater than 6 months (N=42) the proportion of positive test results was 29% for AGA II-IgA and 14% for TTG-IgA (P = 0.14).
Figure 3. Decrease in Sensitivity after Treatment with GFD.
Percent of positive results for each test in 59 biopsy-proven celiac patients before and after treatment for median (range) of 11 (3 to 43) months; *P<0.05, **P<0.0001 for comparison of sensitivity before and after treatment with GFD (P values are obtained by McNemar’s exact test).
Discussion
The present study reports the diagnostic accuracy of a new deamidated gliadin antibody assay in a population of mainly adult patients who were selected only on the basis of histopathology. This represents a major difference from previous studies, wherein a positive serologic test was the inclusion criterion for patient recruitment.16,17 Our results demonstrating the superiority of AGA II over AGA were consistent with previous studies,16–18 however the sensitivity of AGA II antibodies in our study was not as high as previously reported. Initial reports suggested that the sensitivity of AGA II-IgA was greater than 90%, whereas a significantly lower sensitivity was found in this study.16,17,19 There may be several reasons for this discrepancy. First, a positive TTG and/or EMA were not the inclusion criteria for recruiting patients in our study. When we evaluated the results of AGA II-IgA among TTG-IgA positive CD patients, the sensitivity of AGA II-IgA improved to 90% in this group which is close to the prior reports (analysis not shown). A second likely reason relates to the difference in severity of disease between patient populations. In our sample, 54% of patients had mild degree of mucosal damage, while in a study that reported a sensitivity of 95% for AGA II-IgA, only 4% of patients had partial villous atrophy.17
Our results clearly showed that ELISA-based deamidated gliadin antibody assay is a more accurate diagnostic test for CD than conventional gliadin antibody testing. Based on present findings, the diagnostic values of AGA II-IgA were better than AGA-IgA and comparable to TTG-IgA which is the primary recommended serologic test in CD diagnosis.8,9 These findings were consistent with previous studies that have reported an at least similar accuracy for AGA II-IgA as compared to TTG-IgA.17,18,20 AGA II-IgG was superior to both AGA-IgG and TTG-IgG. Although based on manufacturer’s cutoff value the sensitivity of AGA II-IgG was lower than the IgA isotype, the ROC curve analysis of our data showed that the two tests were almost identical. Several other studies have also confirmed these findings.11,17,19 The assessment of the diagnostic values for the combination test (AGA II-IgA+G) demonstrated that this test did not improve over the sensitivity, specificity, or accuracy of AGA II-IgA.
In the present study, the overall sensitivity of TTG-IgA was also lower than previously reported values,5,21–23 however these results were consistent with those few studies that reported less than completely satisfactory sensitivity of TTG-IgA in the clinical settings.24–26 All of our TTG negative CD patients had either another positive serology test, a positive response to GFD, or a predisposing HLA for CD which confirmed their histologic findings of CD. We found that the sensitivity of TTG-IgA as well as AGA II-IgA, AGA II-IgA+G and AGA-IgA was significantly greater in patients with total villous atrophy as compared to those with partial villous atrophy. These findings confirmed that the degree of tissue injury was an important determinant of seropositivity.14,25,27–29
Our results demonstrated a significant reduction in the proportion of positive test results for all tests after treatment with GFD. However, there was a more rapid and more significant drop in the percentage of positive TTG-IgA in treated CD patients compared to AGA II-IgA. Almost half of patients who were treated with GFD for less than 6 months were still positive for AGA II-IgA. The less significant decrease in percentage of positive AGA II-IgA after short term treatment may suggest a use for AGA II-IgA in detection of CD patients who have already started the GFD before presenting for CD diagnosis; of course a negative test does not rule out CD in this circumstance. Nonetheless, the dynamics of antibody levels in response to GFD may differ in children.30 Several studies have reported that normalization of TTG-IgA is not a good indicator of intestinal recovery after treatment with GFD31,32 and one study has suggested that AGA II-IgA performs better than TTG-IgA in predicting villous atrophy despite treatment with GFD.18 Since many of our CD patients did not have a second intestinal biopsy after treatment, we were not able to analyze the correlation between AGA II-IgA and degree of recovery after treatment. The degree of adherence to the diet is another important factor in the rate of seroconversion after treatment. In our study both AGAII-IgA and TTG-IgA were measured in the same serum samples and therefore the degree of compliance was the same for both tests. Further studies on relative responsiveness of AGA II and TTG levels to treatment are needed.
Strengths of the present study are biopsy-based selection of individuals and inclusion of large number of patients with both mild and severe degree of intestinal damage, representing a wide spectrum of disease. In addition, by providing the statistical comparison of diagnostic values of the new antibody assay with both IgA and IgG isotypes of the currently used serologic tests and evaluation of the newer IgA+G AGA II, the present study has extended the previous knowledge about this new antibody assay. However, like other studies, our study also has a few limitations. Although the inclusion of subjects was based on histopathology, we can not totally exclude the possibility of previous serologic testing performed by outside physicians to identify whom to refer for biopsy. However, given that the serology and biopsy are typically ordered at the same time in patients suspected for CD at our center, we expect probably only a few patients were biopsied on the basis of their serology results. Also, since the study was retrospective and many patients did not have uniform information regarding the degree of adherence to the diet in their medical record, we were not able to assess the correlation between seronegativity after treatment and the degree of compliance to the GFD.
In conclusion, ELISA-based deamidated gliadin antibody assay is a more useful test in CD diagnosis than the native gliadin antibody assay and therefore should replace conventional gliadin antibody testing. Based on our results AGA II seems to be equivalent to, but not better than TTG-IgA; however, it may have additive benefits in celiac screening as the combination of the two tests can increase the sensitivity without really lowering the specificity.18 AGA II test may also be beneficial in circumstances when the TTG results are indeterminate. The other application of the new antibody assay is perhaps in screening and monitoring CD among particularly young children as it seems to appear before TTG and resolve faster in the context of gluten withdrawal in this group.30 It is necessary to study the value of this new antibody assay for detection of CD in high risk populations such as, family members of celiac patients, patients with type I diabetes, or patients with gluten sensitive ataxia and to evaluate its potential usefulness in prediction of latent CD. Nonetheless, our data suggest that neither TTG-IgA nor AGA II-IgA is reliable enough to substitute for small intestinal biopsy when confirming CD. Using these antibody assays as the only diagnostic test for CD may lead to missing a substantial number of CD patients, particularly those with lesser degree of histologic damage. Therefore, small intestinal biopsy should be considered when there is a high suspicion for CD despite a negative serology. Further improvement in serologic testing will be needed to maximize the accuracy of CD diagnosis.
Acknowledgments
We thank Brian D. Lahr MS. for statistical help and Tricia L. Shugart for running the TTG-IgA test. This work was supported by NIH grant (DK-057892) and the Mayo Foundation.
Grant support: This work was supported by NIH grant (DK-057892) and the Mayo Foundation.
Abbreviations
- AGA
gliadin antibody
- AGA II
deamidated gliadin antibody
- AUC
area under the curve
- CD
celiac disease
- CV
coefficient of variance
- ELISA
enzyme linked immunosorbent assay
- EMA
endomysial antibody
- Ig
immunoglobulin
- GCD
gluten containing diet
- GFD
gluten free diet
- HLA
human leukocyte antigen
- NF
no follow-up
- PVA
partial villous atrophy
- ROC
receiver-operating characteristic
- SD
standard deviation
- TTG
tissue transglutaminase antibody
- TVA
total villous atrophy
Footnotes
No Conflicts of interest exist
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tommasini A, Not T, Kiren V, Baldas V, Santon D, Trevisiol C, Berti I, Neri E, Gerarduzzi T, Bruno I, Lenhardt A, Zamuner E, Spano A, Crovella S, Martellossi S, Torre G, Sblattero D, Marzari R, Bradbury A, Tamburlini G, Ventura A. Mass screening for coeliac disease using antihuman transglutaminase antibody assay. Arch Dis Child. 2004;89:512–515. doi: 10.1136/adc.2003.029603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schuppan D, Hahn EG. IgA anti-tissue transglutaminase: setting the stage for coeliac disease screening. Eur J Gastroenterol Hepatol. 2001;13:635–637. doi: 10.1097/00042737-200106000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Ventura A, Magazzu G, Greco L. Duration of exposure to gluten and risk for autoimmune disorders in patients with celiac disease. SIGEP Study Group for Autoimmune Disorders in Celiac Disease. Gastroenterology. 1999;117:297–303. doi: 10.1053/gast.1999.0029900297. [DOI] [PubMed] [Google Scholar]
- 4.Corrao G, Corazza GR, Bagnardi V, Brusco G, Ciacci C, Cottone M, Sategna Guidetti C, Usai P, Cesari P, Pelli MA, Loperfido S, Volta U, Calabro A, Certo M. Mortality in patients with coeliac disease and their relatives: a cohort study. Lancet. 2001;358:356–361. doi: 10.1016/s0140-6736(01)05554-4. [DOI] [PubMed] [Google Scholar]
- 5.Rostom A, Dube C, Cranney A, Saloojee N, Sy R, Garritty C, Sampson M, Zhang L, Yazdi F, Mamaladze V, Pan I, MacNeil J, Mack D, Patel D, Moher D. The diagnostic accuracy of serologic tests for celiac disease: a systematic review. Gastroenterology. 2005;128:S38–46. doi: 10.1053/j.gastro.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 6.Hill ID. What are the sensitivity and specificity of serologic tests for celiac disease? Do sensitivity and specificity vary in different populations? Gastroenterology. 2005;128:S25–32. doi: 10.1053/j.gastro.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 7.Johnston SD, McMillan SA, Collins JS, Tham TC, McDougall NI, Murphy P. A comparison of antibodies to tissue transglutaminase with conventional serological tests in the diagnosis of coeliac disease. Eur J Gastroenterol Hepatol. 2003;15:1001–1004. doi: 10.1097/00042737-200309000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Rostom A, Murray JA, Kagnoff MF. American Gastroenterological Association (AGA) Institute technical review on the diagnosis and management of celiac disease. Gastroenterology. 2006;131:1981–2002. doi: 10.1053/j.gastro.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Hill ID, Dirks MH, Liptak GS, Colletti RB, Fasano A, Guandalini S, Hoffenberg EJ, Horvath K, Murray JA, Pivor M, Seidman EG. Guideline for the diagnosis and treatment of celiac disease in children: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2005;40:1–19. doi: 10.1097/00005176-200501000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Osman AA, Gunnel T, Dietl A, Uhlig HH, Amin M, Fleckenstein B, Richter T, Mothes T. B cell epitopes of gliadin. Clin Exp Immunol. 2000;121:248–254. doi: 10.1046/j.1365-2249.2000.01312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aleanzi M, Demonte AM, Esper C, Garcilazo S, Waggener M. Celiac disease: antibody recognition against native and selectively deamidated gliadin peptides. Clin Chem. 2001;47:2023–2028. [PubMed] [Google Scholar]
- 12.Schwertz E, Kahlenberg F, Sack U, Richter T, Stern M, Conrad K, Zimmer KP, Mothes T. Serologic assay based on gliadin-related nonapeptides as a highly sensitive and specific diagnostic aid in celiac disease. Clin Chem. 2004;50:2370–2375. doi: 10.1373/clinchem.2004.036111. [DOI] [PubMed] [Google Scholar]
- 13.Revised criteria for diagnosis of coeliac disease. Report of Working Group of European Society of Paediatric Gastroenterology and Nutrition. Arch Dis Child. 1990;65:909–911. doi: 10.1136/adc.65.8.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rostami K, Kerckhaert J, von Blomberg BM, Meijer JW, Wahab P, Mulder CJ. SAT and serology in adult coeliacs, seronegative coeliac disease seems a reality. Neth J Med. 1998;53:15–19. doi: 10.1016/s0300-2977(98)00050-3. [DOI] [PubMed] [Google Scholar]
- 15.Marsh MN. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue’) Gastroenterology. 1992;102:330–354. [PubMed] [Google Scholar]
- 16.Prince HE. Evaluation of the INOVA diagnostics enzyme-linked immunosorbent assay kits for measuring serum immunoglobulin G (IgG) and IgA to deamidated gliadin peptides. Clin Vaccine Immunol. 2006;13:150–151. doi: 10.1128/CVI.13.1.150-151.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sugai E, Vazquez H, Nachman F, Moreno ML, Mazure R, Smecuol E, Niveloni S, Cabanne A, Kogan Z, Gomez JC, Maurino E, Bai JC. Accuracy of testing for antibodies to synthetic gliadin-related peptides in celiac disease. Clin Gastroenterol Hepatol. 2006;4:1112–1117. doi: 10.1016/j.cgh.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Kaukinen K, Collin P, Laurila K, Kaartinen T, Partanen J, Maki M. Resurrection of gliadin antibodies in coeliac disease. Deamidated gliadin peptide antibody test provides additional diagnostic benefit. Scand J Gastroenterol. 2007:1–6. doi: 10.1080/00365520701452217. [DOI] [PubMed] [Google Scholar]
- 19.Agardh D. Antibodies Against Synthetic Deamidated Gliadin Peptides and Tissue Transglutaminase for the Identification of Childhood Celiac Disease. Clin Gastroenterol Hepatol. 2007;5:1276–1281. doi: 10.1016/j.cgh.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 20.Ankelo M, Kleimola V, Simell S, Simell O, Knip M, Jokisalo E, Tarkia M, Westerlund A, He Q, Viander M, Ilonen J, Hinkkanen AE. Antibody responses to deamidated gliadin peptide show high specificity and parallel antibodies to tissue transglutaminase in developing coeliac disease. Clin Exp Immunol. 2007;150:285–293. doi: 10.1111/j.1365-2249.2007.03487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dieterich W, Laag E, Schopper H, Volta U, Ferguson A, Gillett H, Riecken EO, Schuppan D. Autoantibodies to tissue transglutaminase as predictors of celiac disease. Gastroenterology. 1998;115:1317–1321. doi: 10.1016/s0016-5085(98)70007-1. [DOI] [PubMed] [Google Scholar]
- 22.Carroccio A, Vitale G, Di Prima L, Chifari N, Napoli S, La Russa C, Gulotta G, Averna MR, Montalto G, Mansueto S, Notarbartolo A. Comparison of anti-transglutaminase ELISAs and an anti-endomysial antibody assay in the diagnosis of celiac disease: a prospective study. Clin Chem. 2002;48:1546–1550. [PubMed] [Google Scholar]
- 23.Gillett HR, Freeman HJ. Comparison of IgA endomysium antibody and IgA tissue transglutaminase antibody in celiac disease. Can J Gastroenterol. 2000;14:668–671. doi: 10.1155/2000/598906. [DOI] [PubMed] [Google Scholar]
- 24.Dickey W, McMillan SA, Hughes DF. Sensitivity of serum tissue transglutaminase antibodies for endomysial antibody positive and negative coeliac disease. Scand J Gastroenterol. 2001;36:511–514. doi: 10.1080/003655201750153359. [DOI] [PubMed] [Google Scholar]
- 25.Abrams JA, Diamond B, Rotterdam H, Green PH. Seronegative celiac disease: increased prevalence with lesser degrees of villous atrophy. Dig Dis Sci. 2004;49:546–550. doi: 10.1023/b:ddas.0000026296.02308.00. [DOI] [PubMed] [Google Scholar]
- 26.Martini S, Mengozzi G, Aimo G, Giorda L, Pagni R, Guidetti CS. Comparative evaluation of serologic tests for celiac disease diagnosis and follow-up. Clin Chem. 2002;48:960–963. [PubMed] [Google Scholar]
- 27.Tursi A, Brandimarte G, Giorgetti GM. Prevalence of antitissue transglutaminase antibodies in different degrees of intestinal damage in celiac disease. J Clin Gastroenterol. 2003;36:219–221. doi: 10.1097/00004836-200303000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Rostami K, Kerckhaert J, Tiemessen R, von Blomberg BM, Meijer JW, Mulder CJ. Sensitivity of antiendomysium and antigliadin antibodies in untreated celiac disease: disappointing in clinical practice. Am J Gastroenterol. 1999;94:888–894. doi: 10.1111/j.1572-0241.1999.983_f.x. [DOI] [PubMed] [Google Scholar]
- 29.Rostami K, Mulder CJ, Stapel S, von Blomberg BM, Kerckhaert J, Meijer JW, Pena SA, Heymans HS. Autoantibodies and histogenesis of celiac disease. Rom J Gastroenterol. 2003;12:101–106. [PubMed] [Google Scholar]
- 30.Liu E, Li M, Emery L, Taki I, Barriga K, Tiberti C, Eisenbarth GS, Rewers MJ, Hoffenberg EJ. Natural history of antibodies to deamidated gliadin peptides and transglutaminase in early childhood celiac disease. J Pediatr Gastroenterol Nutr. 2007;45:293–300. doi: 10.1097/MPG.0b013e31806c7b34. [DOI] [PubMed] [Google Scholar]
- 31.Tursi A, Brandimarte G, Giorgetti GM. Lack of usefulness of anti-transglutaminase antibodies in assessing histologic recovery after gluten-free diet in celiac disease. J Clin Gastroenterol. 2003;37:387–391. doi: 10.1097/00004836-200311000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Kaukinen K, Sulkanen S, Maki M, Collin P. IgA-class transglutaminase antibodies in evaluating the efficacy of gluten-free diet in coeliac disease. Eur J Gastroenterol Hepatol. 2002;14:311–315. doi: 10.1097/00042737-200203000-00017. [DOI] [PubMed] [Google Scholar]