Abstract
Heart rate (HR) profile during exercise predicts all-cause mortality. However, less is known about its relation with sudden (versus non-sudden) death in asymptomatic individuals. We assessed the relation of exercise HR parameters (resting HR, target HR achievement, HR increase and HR recovery) with sudden death, coronary heart disease (CHD) death, myocardial infarction and all-cause mortality in 12,555 men who participated in the Multiple Risk Factor Intervention Trial. Participants were 35–57 years-old, without any clinical CHD but with above average Framingham risk. Trial follow-up was 7 years; post-trial extended follow-up for all-cause mortality was 25 years. After adjusting for cardiac risk factors, having to stop exercise before achieving 85% of age-specific maximal HR was associated with an increased risk of sudden death (hazard ratio 1.8, 95% CI 1.3–2.5; p=0.001), CHD death (hazard ratio 1.4, 95% CI 1.2–1.5; p<0.001) and all-cause mortality (hazard ratio 1.3, 95% CI 1.2–1.4; p<0.001). Elevated resting HR (p=0.001), attenuated HR increase (p=0.02), delayed HR recovery (p=0.04) and exercise duration (p<0.0001) were independent predictors of all-cause death in the overall study population and also among the subgroup that achieved target HR. In conclusion, middle age men without clinical CHD who stopped exercise prior to reaching 85% of maximal HR had a higher risk of sudden death. Other exercise HR parameters and exercise duration predicted all-cause mortality.
Non-electrocardiographic exercise parameters such as poor exercise capacity (1,2), chronotropic incompetence (3–6) and delayed heart rate (HR) recovery (7–13) have individually been associated with all-cause death independent of traditional coronary risk factors in asymptomatic individuals and patients with coronary heart disease (CHD) (14,15). However, the relation between >1 exercise HR parameters and sudden cardiac death has not been comprehensively examined in asymptomatic populations without clinical CHD. Therefore, the objective of the present investigation is to determine whether abnormal exercise HR profile is associated with sudden death, CHD death, myocardial infarction (MI) and all-cause mortality in a large, asymptomatic cohort of individuals free of clinical CHD -but with above average Framingham risk scores- who participated in the Multiple Risk Factor Intervention Trial (MRFIT).
METHODS
The MRFIT was a randomized, multicenter, clinical trial that enrolled 12,866 men from 1973 to 1976. Participants were 35–57 years-old and without baseline evidence of clinical CHD. Study design and results have previously been published (16,17). Briefly, 366,662 men were screened at 3 visits. At the first screen participants were judged eligible if they had increased risk for future CHD based on 3 risk factors – cigarette smoking, serum cholesterol and diastolic blood pressure- which placed them in the upper 10%–15% of Framingham risk. At the second and third screens participants with evidence of CHD on medical history, physical examination and resting electrocardiogram were excluded. Also, those taking digitalis, hydralazine, lipid-lowering agents or any medications for CHD were excluded (16–18). The MRFIT participants were randomly assigned to one of two management strategies: those assigned to the Special Intervention group were enrolled in a program which consisted of smoking cessation, diet, and stepped-care drug treatment of hypertension with thiazide diuretics, reserpine, hydralazine, and guanethidine. Those assigned to the Usual Care group were referred to their customary sources of health care. There was no difference in CHD death or all-cause mortality between the Special Intervention and the Usual Care groups in the MRFIT (16). The MRFIT complies with the Declaration of Helsinki and was approved by the local ethics committees at all collaborating centers. Written consent was obtained from all participants.
Upon study entry, all participants were asked to perform a graded treadmill exercise test according to standard Bruce protocol (16,19,20). Of the 12,866 men, 311 (2.4%) could not exercise due to locomotor problems or new ST-T segment changes on resting electrocardiogram. Of the 12,555 men who performed the test, 10,194 (81%) were able to exercise until they reached age-specific target HR (Figure 1). The remaining 2,361 participants (19%) stopped the exercise test early because of symptoms including dyspnea (n=922), ST segment abnormalities (n=389), arrhythmias (n=240), systolic blood pressure ≥250 mmHg (n=200), technical or other problems (n=681) or a combination of these factors. Target HR was 160 beats/min for ages 35–39 years; 157 beats/min for 40–44 years; 155 beats/min for 45–49 years; 153 beats/min for 50–54 years and 151 beats/min for 55–57 years. These rates were based on the predicted 85% of maximal HR for men of these ages (21). At the end of exercise participants were immediately helped to a chair. Blood pressure and HR were recorded at each stage of the exercise protocol and at peak exercise, immediate recovery and 3 minutes after exercise.
Figure 1.
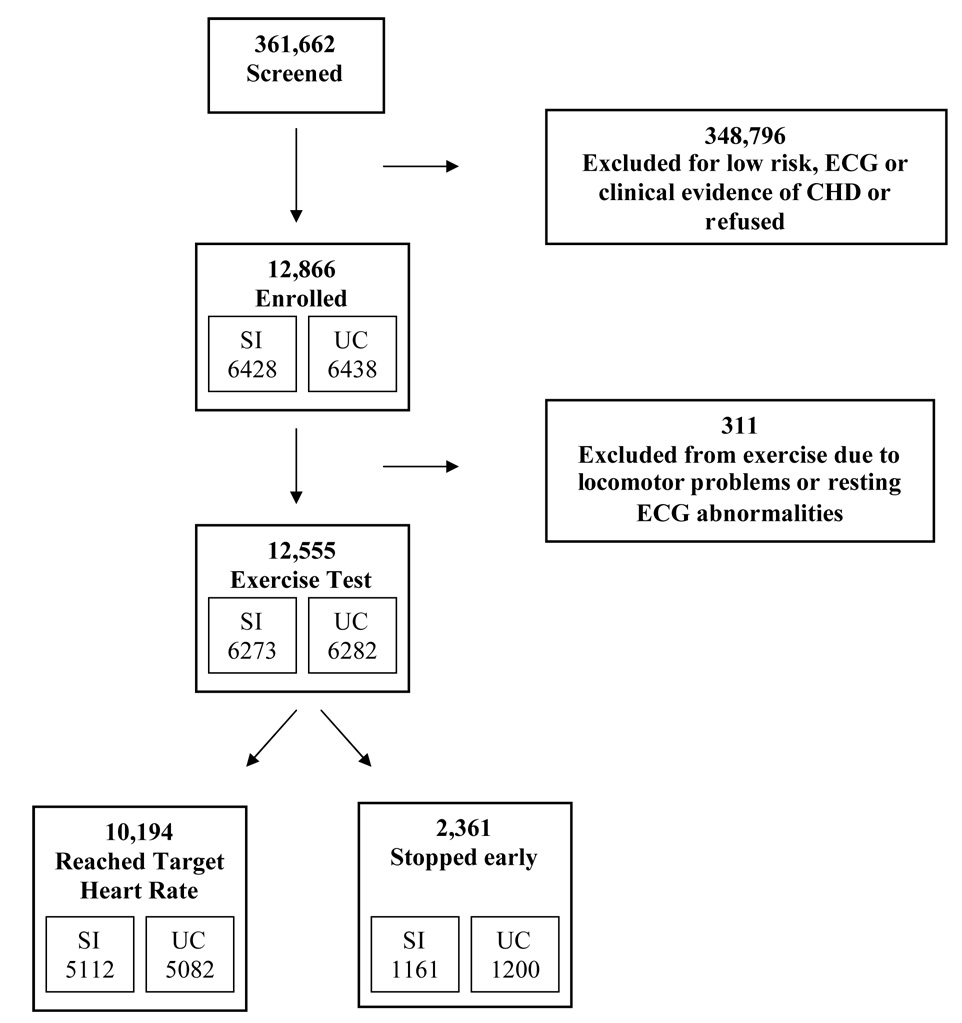
Flow diagram showing the selection of participants for exercise test in the Multiple Risk Factor Intervention Trial. SI=special intervention; UC=usual care
Chronotropic incompetence was assessed by 2 criteria 1) failure to achieve 85% of the age-predicted maximal HR and 2) the proportion of HR reserve used (i.e. chronotropic index), which was calculated by the equation [(Peak HR - resting HR) / (220 - age - resting HR)] (3,5). Chronotropic index <0.8 is an independent predictor of all-cause mortality (3,5). Also, estimated VO2max during Bruce exercise protocol was calculated using the equation VO2max = 14.8 – 1.379 (time in minutes) + 0.451 (time)2 − 0.012 (time)3 (22).
All participants were closely followed for fatal and non-fatal events through 1982, for an average 7 years (trial follow-up). After that mortality data were obtained from the Social Security Administration and the National Death Index (post-trial follow-up). These extended mortality data are available through 1999, for an average 25 years. The survival status of 99.8% of the MRFIT cohort was known at study closure (16). During the trial (through 1982) cause of death was assigned by a Mortality Review Committee blinded to interim results and treatment allocation. Causes of death ascribed to CHD were subclassifed as 1) MI; 2) sudden death (within 24 hours of symptom onset); 3) congestive heart failure; or 4) death due to cardiac revascularization surgery. From 1982 through 1999 causes of death were determined through death certificates using the International Classification of Diseases, 9th or 10th revision (ICD-9 or ICD-10). CHD was defined as ICD-9 codes 410 to 414 or 429.2 and by ICD-10 codes I-20 to I- 25. The diagnostic criteria for non-fatal MI have been previously described (23).
Statistical analyses were performed using SAS (Version 8.2). Quartiles of the following exercise HR parameters were determined: 1) resting supine HR, 2) the difference between peak exercise HR and resting HR (i.e. maximal HR increase), 3) the difference between peak exercise HR and HR 3 minutes after cessation of exercise (i.e. HR recovery), 4) exercise duration. Event rates per 1,000 person years of follow-up are cited. The Cox proportional hazards model with strata corresponding to 5-year age groups (35–39, 40–44, 45–49, 50–54, and 55–57 years) was used to compare the upper 3 quartiles of each HR measure to the lowest quartile. Linear trends were examined by including HR predictors as continuous variables in the Cox models. The p-values showing the relation between HR predictors and outcomes are specified as ptrend throughout the manuscript. The relative hazard of reaching 85% of age-adjusted maximal HR versus stopping early was also computed using an indicator variable in a Cox model. Univariate (age stratification only) and multivariable (age stratification and also adjustment for baseline measures of cigarettes smoked per day, systolic blood pressure, low density lipoprotein, high density lipoprotein, triglycerides, body mass index, fasting glucose, race, parental history of MI and exercise capacity) estimates of hazard ratios and 95% confidence intervals are cited for the following outcomes: 1) sudden death, 2) fatal or non-fatal MI, 3) fatal CHD through 1999, and 4) all-cause mortality through 1999. Analyses are shown for the Special Intervention and Usual Care groups combined since associations did not vary by treatment group. P-values cited are 2-sided and p<0.05 was considered statistically significant.
RESULTS
Baseline clinical characteristics of the participants are shown in Table 1. Participants who achieved target HR (n=10,194) were younger and had more favorable cardiac risk factor profile (Table 1) as well as greater exercise capacity, larger HR increase and more brisk HR recovery than participants who stopped early (Table 2).
Table 1.
Baseline characteristics of the 12,555 men who performed exercise test in the Multiple Risk Factor Intervention Trial
| Status of Target Heart Rate Reached |
||||
|---|---|---|---|---|
| Variable | All Participants (n=12,555) | Reached Target Heart Rate (n=10,194) | Stopped exercise test prematurely (n=2,361) | p-value |
| Age at screening (years) | 46.2 | 45.7 | 48.1 | <0.0001 |
| Black Race | 7.1% | 6.6% | 9.2% | <0.0001 |
| Cigarette smoker | 63.8% | 62.4% | 69.7% | <0.0001 |
| Hypertension | 65.7% | 64.8% | 69.6% | <0.0001 |
| Systolic blood pressure (mmHg) | 135.2 | 134.5 | 138.1 | <0.0001 |
| Diastolic blood pressure (mmHg) | 90.6 | 90.5 | 91.0 | 0.02 |
| Body Mass Index (kg/m2) | 27.7 | 27.6 | 28.2 | <0.0001 |
| Low Density Lipoprotein (mg/dl) | 160.0 | 160.0 | 160.3 | 0.65 |
| High Density Lipoprotein (mg/dl) | 42.1 | 42.3 | 40.9 | <0.0001 |
| Triglycerides (mg/dl) | 194.4 | 194.1 | 195.5 | 0.7 |
| Fasting glucose > 120 mg/dl | 5.5% | 5.2% | 6.8% | 0.002 |
| Parent had myocardial infarction | 39.6% | 39.3% | 40.9% | 0.1 |
The p-values reflect the comparison of the participants who reached target heart rate vs. those who stopped exercise test prematurely
Table 2.
Exercise test characteristics in the Multiple Risk Factor Intervention Trial
| Status of Target Heart Rate Reached |
||||
|---|---|---|---|---|
| Variable | All patients (n=12,555) | Reached Target Heart Rate (n=10,194) | Stopped exercise test prematurely (n=2,361) | p-value |
| Exercise duration (minutes) | 7.0 ± 1.7 | 7.1 ± 1.6 | 6.3 ± 1.9 | <0.001 |
| Estimated maximal oxygen uptake (mL.kg−1.min−1) | 23.7 ± 5.0 | 24.0 ± 4.9 | 21.8 ± 5.2 | <0.001 |
| Peak heart rate (beats/min) | 156 ± 12 | 160 ± 7 | 138 ± 13 | <0.001 |
| Increase in heart rate (beats/min) | 84 ± 16 | 87 ± 14 | 69 ± 16 | <0.001 |
| Peak systolic blood pressure (mmHg) | 193 ± 25 | 191 ± 24 | 196 ± 30 | <.0001 |
| Heart rate recovery (beats/min) | 65±13 | 57+12 | 48+13 | <.0001 |
The p-values reflect the comparison of the participants who reached target heart rate vs. those who stopped exercise test prematurely
After an average 7 years of follow-up, 153 (1.2%) of the 12,555 study participants died suddenly (64% of the CHD deaths over 7 years) and 824 (7%) had fatal/non-fatal MI. After 25 years, 1,586 participants (13%) died of CHD and 4,642 (37%) died of all causes.
Not being able to achieve 85% of maximal HR during exercise test predicted an increased risk of death and MI (Table 3). After adjusting for baseline risk factors, men who stopped the exercise test early had an 80% higher risk of sudden death (p=0.001), a 40% higher risk of CHD death (p<0.001) and a 30% higher risk of all-cause death (p<0.001) than those who reached target HR (Table 3). Participants with chronotropic index < 0.8 also had an increased risk of all-cause death (hazard ratio 1.1, 95% CI 1.0 to 1.2; p<0.01). Of note, the risk of sudden death (hazard ratio 5.0, 95% CI 3.0 to 8.4) and all-cause mortality (hazard ratio 1.9, 95% CI 1.7 to 2.2) were particularly high in the 389 participants who stopped exercise test due to ST segment abnormalities.
Table 3.
Risk of death and myocardial infarction in relation to achieving target heart rate during exercise test
| 7-year Follow-up | 25-year follow-up | ||||
|---|---|---|---|---|---|
| Exercise Test Status | Sudden Death (n= 153) | Fatal/Non-fatal MI (n=824) | CHD Death (n=1,586) | All-cause Mortality (n= 4,642) | |
| Reached Target Heart Rate | 1.00 | 1.00 | 1.00 | 1.00 | |
| (n=10,194) | |||||
| Stopped prematurely | Unadjusted | 2.1 (1.5 – 3.0) | 1.4 (1.2 – 1.7) | 1.6 (1.4 – 1.8) | 1.5 (1.4 – 1.6) |
| (n=2,361) | Adjusted* | 1.8 (1.3 – 2.6) | 1.3 (1.1 – 1.5) | 1.4 (1.2 – 1.5) | 1.3 (1.2 – 1.4) |
Abbreviations: CHD: coronary heart disease; MI: myocardial infarction
Adjusted for age, cigarettes smoked per day, systolic blood pressure, low density lipoprotein, high density lipoprotein, triglycerides, body mass index, fasting glucose, race and parental history of myocardial infarction.
All analyses were stratified by age categories; 35–39 years, 40–44 years, 45–49 years, 50–54 years and 55–57 years.
Higher resting HR predicted an increased risk of sudden death and all-cause mortality (Table 4). After adjustment for age, men with resting HR ≥80 beats/minute had 70% higher risk of sudden death (ptrend=0.02) and 25% higher risk of all-cause death (ptrend<0.0001) than men with resting HR <65 beats/minute. After adjusting for other cardiac risk factors, resting HR was independently associated with all-cause mortality (ptrend=0.001) (Table 4).
Table 4.
Risk of death and myocardial infarction in relation to the quartiles of resting heart rate
| 7-year Follow-up | |||
|---|---|---|---|
| Events, n (%) | HR (95% CI)1 | HR (95% CI) 2 | |
| Sudden Death | |||
| I (< 65) | 27 (0.9%) | 1.00 (reference) | 1.00 (reference) |
| II (65–71) | 41 (1.2%) | 1.42 (0.87 – 2.3) | 1.32 (0.81 – 2.14) |
| III (72–79) | 38 (1.1%) | 1.31 (0.80 – 2.14) | 1.22 (0.74 – 2.01) |
| IV (≥80) | 47 (1.5%) | 1.72 (1.07 – 2.77) | 1.50 (0.93 – 2.42) |
| Beta (P-value)3 | 0.015 (0.02) | 0.011 (0.09) | |
| Fatal/Non-Fatal MI | |||
| I (< 65) | 170 (5.7%) | 1.00 (reference) | 1.00 (reference) |
| II (65–71) | 239 (7.2%) | 1.30 (1.07 – 1.59) | 1.22 (1.00 – 1.48) |
| III (72–79) | 205 (6.1%) | 1.11 (0.90 – 1.36) | 1.04 (0.84 – 1.27) |
| IV (≥80) | 210 (6.7%) | 1.21 (0.99 – 1.48) | 1.11 (0.91 – 1.37) |
| Beta (P-value)3 | 0.005 (0.10) | 0.003 (0.37) | |
| 25-year Follow-up | |||
| CHD Death | |||
| I (< 65) | 376 (12.6%) | 1.00 (reference) | 1.00 (reference) |
| II (65–71) | 404 (12.2%) | 0.01 (0.88 – 1.17) | 0.94 (0.82 – 1.09) |
| III (72–79) | 397 (11.8%) | 0.99 (0.86 – 1.14) | 0.92 (0.80 – 1.07) |
| IV (≥80) | 409 (13.0%) | 1.13 (0.98 – 1.30) | 1.01 (0.88 – 1.17) |
| Beta (P-value)3 | 0.004 (0.04) | 0.001 (0.60) | |
| Death All-causes | |||
| I (< 65) | 1041 (34.8%) | 1.00 (reference) | 1.00 (reference) |
| II (65–71) | 1170 (35.4%) | 1.06 (0.98 – 1.16) | 1.02 (0.94 – 1.11) |
| III (72–79) | 1187 (35.2%) | 1.08 (0.99 – 1.17) | 1.02 (0.94 – 1.11) |
| IV (≥80) | 1244 (39.5%) | 1.25 (1.16 – 1.36) | 1.17 (1.07 – 1.27) |
| Beta (P-value)3 | 0.007 (0.000) | 0.005 (0.001) | |
Abbreviations: CHD: coronary heart disease; HR: Hazard ratio estimated from Cox-regression MI: myocardial infarction
Univariate analyses (age stratification only)
Multivariate analyses adjusted for age, cigarettes smoked per day, systolic blood pressure, low density lipoprotein, high density lipoprotein, triglycerides, body mass index fasting glucose, race, and parental history of CHD.
From model using heart rate as a continuous variable
All analyses were stratified by age categories
Delayed HR recovery predicted an increased risk of sudden death, MI, CHD death and all-cause death, after age adjustment only (ptrend<0.01 for all) (Table 5). Following adjustments for cardiac risk factors and exercise capacity, participants with HR recovery >65 beats (3 minutes after exercise) had a 10% lower risk of all-cause death than those with HR recovery <50 beats (ptrend=0.04) (Table 5). This association did not vary between age groups.
Table 5.
Risk of death and myocardial infarction in relation to the quartiles of heart rate recovery 3 minutes after exercise
| 7-year Follow-up | |||
|---|---|---|---|
| Events, n (%) | HR (95% CI)1 | HR (95% CI) 2 | |
| Sudden Death | |||
| I (< 50) | 43 (1.4%) | 1.00 (reference) | 1.00 (reference) |
| II (50–57) | 47 (1.4%) | 1.02 (0.67 – 1.54) | 1.24 (0.81 – 1.90) |
| III (58–65) | 20 (0.7%) | 0.50 (0.29 – 0.85) | 0.65 (0.37 – 1.14) |
| IV (>65) | 28 (0.9%) | 0.73 (0.45 – 1.18) | 1.10 (0.65 – 1.85) |
| Beta (P-value)3 | −.017 (0.009) | −.006 (0.41) | |
| Fatal/NF MI | |||
| I (< 50) | 223 (7.5%) | 1.00 (reference) | 1.00 (reference) |
| II (50–57) | 219 (6.7%) | 0.90 (0.75 – 1.09) | 0.96 (0.79 – 1.16) |
| III (58–65) | 177 (5.9%) | 0.83 (0.68 – 1.01) | 0.94 (0.77 – 1.16) |
| IV (>65) | 150 (5.1%) | 0.73 (0.59 – 0.89) | 0.90 (0.72 – 1.13) |
| Beta (P-value)3 | −.008 (0.003) | −.002 (0.57) | |
| 25-year Follow-up | |||
| CHD Death | |||
| I (< 50) | 396 (13.3%) | 1.00 (reference) | 1.00 (reference) |
| II (50–57) | 439 (13.4%) | 1.00 (0.88 – 1.15) | 1.11 (0.96 – 1.27) |
| III (58–65) | 341 (11.4%) | 0.88 (0.76 – 1.01) | 1.05 (0.90 – 1.22) |
| IV (>65) | 297 (10.0%) | 0.79 (0.68 – 0.92) | 1.03 (0.87 – 1.21) |
| Beta (P-value)3 | −.007 (0.001) | 0.000 (0.87) | |
| Death All-causes | |||
| I (< 50) | 1272 (42.8%) | 1.00 (reference) | 1.00 (reference) |
| II (50–57) | 1238 (37.7%) | 0.88 (0.81 – 0.95) | 0.95 (0.88 – 1.03) |
| III (58–65) | 969 (32.5%) | 0.78 (0.71 – 0.84) | 0.91 (0.83 – 0.99) |
| IV (>65) | 889 (30.1%) | 0.73 (0.67 – 0.80) | 0.90 (0.82 – 0.99) |
| Beta (P-value)3 | −.008 (0.000) | −.003 (0.04) | |
Abbreviations: CHD: coronary heart disease; HR: Hazard ratio estimated from Cox-regression MI: myocardial infarction
Univariate analyses (age stratification only)
Multivariate analyses adjusted for age, cigarettes smoked per day, systolic blood pressure, low density lipoprotein, high density lipoprotein, triglycerides, body mass index, fasting glucose, race, parental history of CHD and exercise duration.
From model using heart rate as a continuous variable
All analyses were stratified by age categories
Attenuated HR increase with exercise predicted an elevated risk of sudden death, CHD death, MI and all-cause death after age adjustment only (ptrend<0.01 for all). In multivariate analysis risk of all-cause death was lower (hazard ratio 0.87, 95% CI 0.79 to 0.96; ptrend=0.02) in those with HR increase ≥99 beats/min vs. <82 beats/min.
Exercise duration was inversely associated with sudden death, CHD death, fatal/nonfatal MI and all-cause mortality after adjustment for age. Each additional minute of exercise was associated with a 12% reduction in the risk of sudden death (ptrend=0.009) and a 5% reduction in the risks of CHD death, MI and all-cause mortality (ptrend<0.0001; ptrend=0.03 and ptrend<0.0001 respectively). After adjusting for other cardiac risk factors, men who exercised >8 minutes on standard Bruce protocol had a 15% lower risk of all-cause death than men who exercised <6 minutes (hazard ratio 0.85, 95% CI 0.7 to 0.9; ptrend<0.0001).
The relation of HR parameters with sudden death and all-cause mortality was examined in the subgroup (n=10,194) that exercised until reaching target HR (Figure 2). In this subgroup, sudden death risk was higher (adjusted hazard ratio 2.0, 95% CI 1.0 to 3.8; ptrend=0.015) in men with resting HR ≥80 beats/minute vs. <65 beats/minute. Also, all-cause mortality was significantly different for those in the 4th quartile vs. the 1st quartile of resting HR (adjusted hazard ratio 1.15, 95% CI 1.0 to 1.3; ptrend=0.008), HR increase (adjusted hazard ratio 0.92, 95% CI 0.83 to 1.02; ptrend=0.03) and exercise duration (adjusted hazard ratio 0.86, 95% CI 0.78 to 0.94; ptrend<0.0001); whereas a trend of association was present for HR recovery (adjusted hazard ratio 0.92, 95% CI 0.8 to 1.0; ptrend=0.13) (Figure 2).
Figure 2.
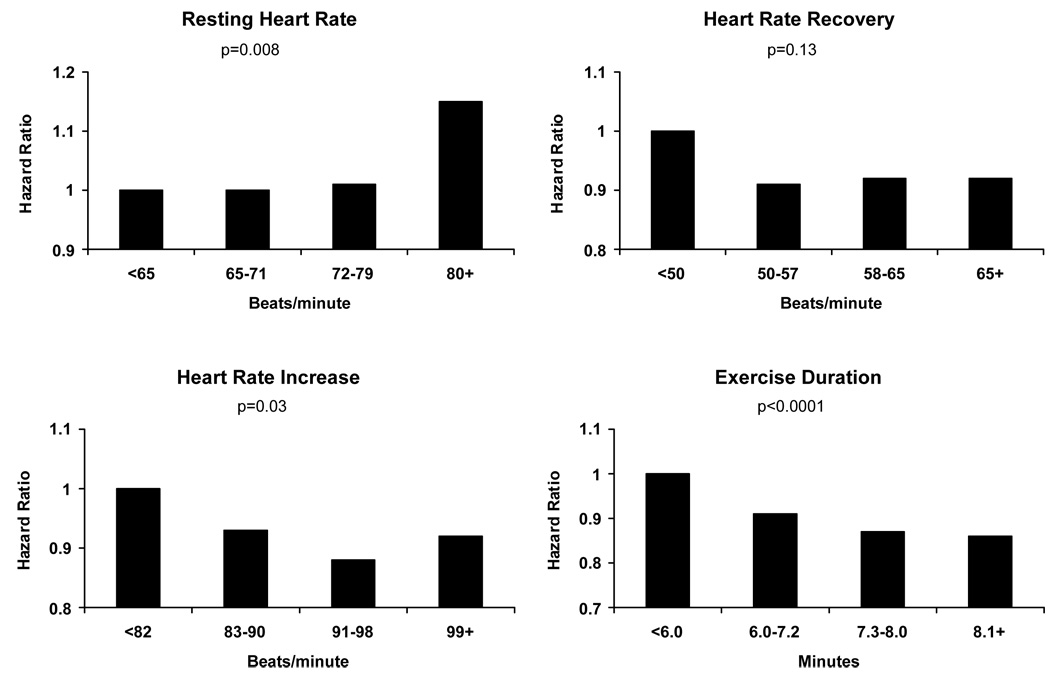
Predictors of all-cause death among the 10,194 men who reached target heart rate during exercise test. Adjusted HR (95% CI) for the 4th quartile vs. the 1st quartile and regression coefficients were as follows: Resting HR hazard ratio 1.15, 95% CI 1.0 to 1.3, Beta: 0.004; HR recovery hazard ratio 0.92, 95% CI 0.8 to 1.0, Beta: −0.002; HR increase hazard ratio 0.92, 95% CI 0.8 to 1.0, Beta: −0.003; Exercise duration hazard ratio 0.86, 95% CI 0.8 to 0.9, Beta −0.04
DISCUSSION
This investigation demonstrates that in a large cohort of men with no clinical CHD, having to stop exercise treadmill test before achieving 85% of age-specific maximal HR is associated with a significantly increased risk of sudden death, MI and all-cause mortality. To our knowledge, the relation between chronotropic incompetence and sudden death among asymptomatic adults had not been reported before. We also found that other exercise HR parameters (resting HR, HR recovery and HR increase) and exercise duration were independently predictive of all-cause death in the overall study cohort and in the subgroup of men who reached age-specific target HR.
Cardiac arrhythmias and sudden death are more common with an increase in sympathetic and a decrease in parasympathetic activity (autonomic imbalance) (24–27). HR response to exercise, a result of the interplay between the sympathetic and parasympathetic activities, is a marker of autonomic balance (14,28) and has the advantage of easy applicability over other autonomic markers because of widespread use of exercise testing in clinical practice.
Elevated resting HR (29), attenuated HR increase with exercise (3–5,15), and delayed HR recovery (7–14) have been shown to predict all-cause mortality independently of ischemia and traditional cardiac risk factors. Our results also show that exercise HR parameters were independent predictors of all-cause mortality, supporting these previous observations. However, the magnitude of this association, particularly in the case of HR recovery, was somewhat more modest than what was observed in previous studies. The reason for this difference may be because of our young and asymptomatic study population or due to variations in the termination protocol of the exercise treadmill test. While the exercise test was symptom-limited in most previous studies (9–11), it was stopped upon achieving age-specific target HR in the MRFIT, which may have led to reduced peak HR and HR recovery. It has been suggested that the associations with cardiac outcomes are stronger if patients are exercised to a symptom-limited endpoint rather than a pre-specified HR (4). Indeed, in a previous report from the Framingham Offspring Study, in which exercise test was also stopped upon achieving target HR (but prior to exhaustion), no association between HR recovery and death was found (10).
Recently, Jouven et al. reported that abnormal exercise HR profile using cycle ergometry was associated with sudden death from MI among Frenchmen employed by the Paris Civil Service (30). In the present study, target HR achievement was the only HR parameter independently associated with sudden death. The other HR parameters were predictors in univariate analysis (age adjusted) but not after adjusting for baseline risk factors. It should be noted that these 2 studies have dissimilarities in the study population, design and end-points, which may account for the differences in results. The participants in the present study were selected from the general population based on increased Framingham risk and may be at a somewhat higher risk than those in the Paris Prospective Study. Consequently, the sudden death rate in the present study (0.17%/year), is higher than the sudden death rate in the Paris Prospective Study (30). Also, treadmill exercise testing was used in the MRFIT versus cycle ergometry in the French study. Finally, as noted above, exercise tests in the MRFIT were terminated upon achievement of 85% of age-specific maximal HR but were symptom limited in the Paris Prospective Study.
We also examined the relation of exercise HR parameters with sudden death and all-cause mortality among participants who reached target HR (Figure 2). This subgroup of relatively healthy participants had not been comprehensively studied before. The association between short exercise duration and increased mortality in this subgroup suggests that a rapid (vs. slow) rise in HR during exercise predicts increased risk.
This investigation has many strengths. First, we performed a comprehensive assessment of the relation between several HR parameters and cardiac outcomes, including sudden death. Most previous reports in this area had examined one HR parameter and all-cause mortality. Second, the participants of the present study were community-based and were subjected to a rigorous screening process to ensure presence of major cardiovascular risk factors but exclude clinical CHD. Therefore, the possibility of a selection bias in this study is minimal. In contrast, the majority of previous investigations that have studied patients referred to cardiac exercise laboratories of tertiary care centers. Third, follow-up in the MRFIT has been long and ascertainment of death has been complete. Finally, the HR parameters were not influenced by the use of beta adrenergic blockers, calcium channel blockers or digitalis as these medications were not used at the time of the study or were excluded.
On the other hand the following limitations are worth noting. The participants in the MRFIT were all male. Therefore, the results may not be directly applicable to females. However, in previous studies exercise HR parameters were associated with all-cause mortality in both men and women. Also, in this investigation exercise tests were not continued until exhaustion in those who achieved age-specific target HR, which may have reduced the strength of the associations with cardiac outcomes. Finally, the timing of HR recovery measurement in this investigation differs from some of the previous reports (3 minutes vs. 1 minute after exercise). However, this difference is unlikely to affect our results substantially since previously HR recovery at 1, 2, 3, 4 or 5 minutes after exercise have all been found to be inversely associated with death (9, 30).
ACKNOWLEDGEMENTS
The authors acknowledge the efforts of the many MRFIT investigators who collected these data and the MRFIT Editorial Committee (Jerome D Cohen, MD, Jeffrey A Cutler, MD, Lynn Eberly, Ph.D., Greg Grandits, MS, Richard Grimm, Jr., M.D., Ph.D., Lewis H Kuller, MD, DPH, James D Neaton, PhD, Judith K Ockene, PhD, Ronald Prineas, M.D., Ph.D., Jeremiah Stamler, MD, Kenneth Svendsen, MS, Avis Thomas, MS) who provided valuable editorial assistance. For a complete list of all MRFIT investigators, see JAMA 1982;248:1465–1477
This work was funded by National Heart Lung and Blood Institute (R01-HL-43232, R01-HL-68140). Dr. Adabag is funded, in part, by Veterans Administration Clinical Science Research and Development Service Washington, DC (04S-CRCOE 001).
Footnotes
Conflict of Interest: None declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 2.Gulati M, Pandey DK, Arnsdorf MF, Lauderdale DS, Thisted RA, Wicklund RH, Al-Hani AJ, Black HR. Exercise capacity and the risk of death in women: the St James Women Take Heart Project. Circulation. 2003;108:1554–1559. doi: 10.1161/01.CIR.0000091080.57509.E9. [DOI] [PubMed] [Google Scholar]
- 3.Lauer MS, Okin PM, Larson MG, Evans JC, Levy D. Impaired heart rate response to graded exercise. Prognostic implications of chronotropic incompetence in the Framingham Heart Study. Circulation. 1996;93:1520–1526. doi: 10.1161/01.cir.93.8.1520. [DOI] [PubMed] [Google Scholar]
- 4.Ellestad MH. Chronotropic incompetence. The implications of heart rate response to exercise (compensatory parasympathetic hyperactivity?) Circulation. 1996;93:1485–1487. doi: 10.1161/01.cir.93.8.1485. [DOI] [PubMed] [Google Scholar]
- 5.Lauer MS, Francis GS, Okin PM, Pashkow FJ, Snader CE, Marwick TH. Impaired chronotropic response to exercise stress testing as a predictor of mortality. JAMA. 1999;281:524–529. doi: 10.1001/jama.281.6.524. [DOI] [PubMed] [Google Scholar]
- 6.Balady GJ, Larson MG, Vasan RS, Leip EP, O'Donnell CJ, Levy D. Usefulness of exercise testing in the prediction of coronary disease risk among asymptomatic persons as a function of the Framingham risk score. Circulation. 2004;110:1920–1925. doi: 10.1161/01.CIR.0000143226.40607.71. [DOI] [PubMed] [Google Scholar]
- 7.Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341:1351–1357. doi: 10.1056/NEJM199910283411804. [DOI] [PubMed] [Google Scholar]
- 8.Nishime EO, Cole CR, Blackstone EH, Pashkow FJ, Lauer MS. Heart rate recovery and treadmill exercise score as predictors of mortality in patients referred for exercise ECG. JAMA. 2000;284:1392–1398. doi: 10.1001/jama.284.11.1392. [DOI] [PubMed] [Google Scholar]
- 9.Shetler K, Marcus R, Froelicher VF, Vora S, Kalisetti D, Prakash M, Do D, Myers J. Heart rate recovery: validation and methodologic issues. J Am Coll Cardiol. 2001;38:1980–1987. doi: 10.1016/s0735-1097(01)01652-7. [DOI] [PubMed] [Google Scholar]
- 10.Morshedi-Meibodi A, Larson MG, Levy D, O'Donnell CJ, Vasan RS. Heart rate recovery after treadmill exercise testing and risk of cardiovascular disease events (The Framingham Heart Study) Am J Cardiol. 2002;90:848–852. doi: 10.1016/s0002-9149(02)02706-6. [DOI] [PubMed] [Google Scholar]
- 11.Vivekananthan DP, Blackstone EH, Pothier CE, Lauer MS. Heart rate recovery after exercise is a predictor of mortality, independent of the angiographic severity of coronary disease. J Am Coll Cardiol. 2003;42:831–838. doi: 10.1016/s0735-1097(03)00833-7. [DOI] [PubMed] [Google Scholar]
- 12.Mora S, Redberg RF, Cui Y, Whiteman MK, Flaws JA, Sharrett AR, Blumenthal RS. Ability of exercise testing to predict cardiovascular and all-cause death in asymptomatic women: a 20-year follow-up of the lipid research clinics prevalence study. JAMA. 2003;290:1600–1607. doi: 10.1001/jama.290.12.1600. [DOI] [PubMed] [Google Scholar]
- 13.Aktas MK, Ozduran V, Pothier CE, Lang R, Lauer MS. Global risk scores and exercise testing for predicting all-cause mortality in a preventive medicine program. JAMA. 2004;292:1462–1468. doi: 10.1001/jama.292.12.1462. [DOI] [PubMed] [Google Scholar]
- 14.Kligfield P, Lauer MS. Exercise electrocardiogram testing: beyond the ST segment. Circulation. 2006;114:2070–2082. doi: 10.1161/CIRCULATIONAHA.105.561944. [DOI] [PubMed] [Google Scholar]
- 15.Lauer M, Froelicher ES, Williams M, Kligfield P. Exercise testing in asymptomatic adults: a statement for professionals from the American Heart Association Council on Clinical Cardiology, Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention. Circulation. 2005;112:771–776. doi: 10.1161/CIRCULATIONAHA.105.166543. [DOI] [PubMed] [Google Scholar]
- 16.Multiple Risk Factor Intervention Trial Research Group. Multiple risk factor intervention trial. Risk factor changes and mortality results. JAMA. 1982;248:1465–1477. [PubMed] [Google Scholar]
- 17.Sherwin R, Kaelber CT, Kezdi P, Kjelsberg MO, Thomas HE., Jr The multiple risk factor intervention trial (MRFIT) II. The development of the protocol. Prev Med. 1981;10:402–425. doi: 10.1016/0091-7435(81)90058-x. [DOI] [PubMed] [Google Scholar]
- 18.Eberly LE, Neaton JD, Thomas AJ, Yu D Multiple Risk Factor Intervention Trial Research Group. Multiple-stage screening and mortality in the Multiple Risk Factor Intervention Trial. Clin Trials. 2004;1:148–161. doi: 10.1191/1740774504cn018oa. [DOI] [PubMed] [Google Scholar]
- 19.Multiple Risk Factor Intervention Trial Research Group. Exercise electrocardiogram and coronary heart disease mortality in the Multiple Risk Factor Intervention Trial. Am J Cardiol. 1985;55:16–24. doi: 10.1016/0002-9149(85)90291-7. [DOI] [PubMed] [Google Scholar]
- 20.Rautaharju PM, Prineas RJ, Eifler WJ, Furberg CD, Neaton JD, Crow RS, Stamler J, Cutler JA. Prognostic value of exercise electrocardiogram in men at high risk of future coronary heart disease: Multiple Risk Factor Intervention Trial experience. J Am Coll Cardiol. 1986;8:1–10. doi: 10.1016/s0735-1097(86)80084-5. [DOI] [PubMed] [Google Scholar]
- 21.Sheffield LT, Roitman D. Stress testing methodology. Prog Cardiovasc Dis. 1976;19:33–49. doi: 10.1016/0033-0620(76)90007-4. [DOI] [PubMed] [Google Scholar]
- 22.Whaley MH, Brubaker PH, Otto RM, editors. American College of Sports Medicine’s Guidelines for Exercise Testing and Prescription. 7th edition. Philadelphia, PA: Lippincott Williams & Wilkins; 2006. Metabolic Calculations; pp. 286–299. [Google Scholar]
- 23.Multiple Risk Factor Intervention Trial Research Group. Coronary heart disease death, nonfatal acute myocardial infarction and other clinical outcomes in the Multiple Risk Factor Intervention Trial. Am J Cardiol. 1986;58:1–13. doi: 10.1016/0002-9149(86)90232-8. [DOI] [PubMed] [Google Scholar]
- 24.Lown B, Verrier RL. Neural activity and ventricular fibrillation. N Engl J Med. 1976;294:1165–1170. doi: 10.1056/NEJM197605202942107. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz PJ, Vanoli E, Stramba-Badiale M, De Ferrari GM, Billman GE, Foreman RD. Autonomic mechanisms and sudden death. New insights from analysis of baroreceptor reflexes in conscious dogs with and without a myocardial infarction. Circulation. 1988;78:969–979. doi: 10.1161/01.cir.78.4.969. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz PJ, La Rovere MT, Vanoli E. Autonomic nervous system and sudden cardiac death. Experimental basis and clinical observations for post-myocardial infarction risk stratification. Circulation. 1992;85(1 Suppl):177–191. [PubMed] [Google Scholar]
- 27.La Rovere MT, Bigger JT, Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998;351:478–484. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 28.Chaitman BR. Exercise stress testing. In: Libby P, Zipes D, Bonow R, Braunwald E, editors. Braunwald’s 7th Edition of Heart Disease (A Textbook of Cardiovascular Medicine) Philadelphia, Pa: WB Saunders Co; 2005. pp. 153–177. [Google Scholar]
- 29.Gillum RF, Makuc DM, Feldman JJ. Pulse rate, coronary heart disease, and death: the NHANES I Epidemiologic Follow-up Study. Am Heart J. 1991;121:172–177. doi: 10.1016/0002-8703(91)90970-s. [DOI] [PubMed] [Google Scholar]
- 30.Jouven X, Empana JP, Schwartz PJ, Desnos M, Courbon D, Ducimetiere P. Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med. 2005;352:1951–1958. doi: 10.1056/NEJMoa043012. [DOI] [PubMed] [Google Scholar]


