Abstract
A minimal hypothesis is proposed concerning the brain processes underlying effortful tasks. It distinguishes two main computational spaces: a unique global workspace composed of distributed and heavily interconnected neurons with long-range axons, and a set of specialized and modular perceptual, motor, memory, evaluative, and attentional processors. Workspace neurons are mobilized in effortful tasks for which the specialized processors do not suffice. They selectively mobilize or suppress, through descending connections, the contribution of specific processor neurons. In the course of task performance, workspace neurons become spontaneously coactivated, forming discrete though variable spatio-temporal patterns subject to modulation by vigilance signals and to selection by reward signals. A computer simulation of the Stroop task shows workspace activation to increase during acquisition of a novel task, effortful execution, and after errors. We outline predictions for spatio-temporal activation patterns during brain imaging, particularly about the contribution of dorsolateral prefrontal cortex and anterior cingulate to the workspace.
We propose a simple hypothesis concerning the neural basis of “making a conscious mental effort.” Why are some cognitive tasks performed effortlessly, whereas others require focused attention and conscious control? Mental effort is clearly unrelated to objective measures of computational difficulty: we routinely perform vision and motor control tasks without awareness of the complex underlying information processing, whereas elementary tasks such as solving 37 − 9 call for our attention and conscious effort.
Neurophysiological, anatomical, and brain-imaging studies have revealed that tasks that can be performed effortlessly mobilize well-defined modular cerebral systems specialized for various aspects of sensory-motor processing (1, 2). On the other hand, humans exhibit the capacity to go beyond modularity and flexibly, though effortfully, recombine these specialized cerebral processes in novel ways (3, 4). Once we are conscious of an item, we can readily perform a large variety of operations on it, including evaluation, memorization, action guidance, and verbal report. This impressive ability must be reconciled with the neurobiological fact that there is no single “cardinal area” to which all areas project (5–8).
Here, we propose a formal architecture of distributed neurons with long-distance connectivity that provides a “global workspace” that can potentially interconnect multiple distributed and specialized brain areas in a coordinated, though variable manner, and whose intense mobilization might be associated with a subjective feeling of conscious effort. This minimal scheme extends former attempts to modelize effortful tasks of delayed response (9), card sorting (10), number-processing (11), and planning (12) on the basis of plausible molecular, anatomical, and functional features of the brain. Here, we present simulations of another task, the Stroop task, to explicitly specify a common architectural principle underlying the effortful character of all these tasks, thus providing empirically testable predictions.
THEORETICAL PREMISES
Two Main Computational Spaces.
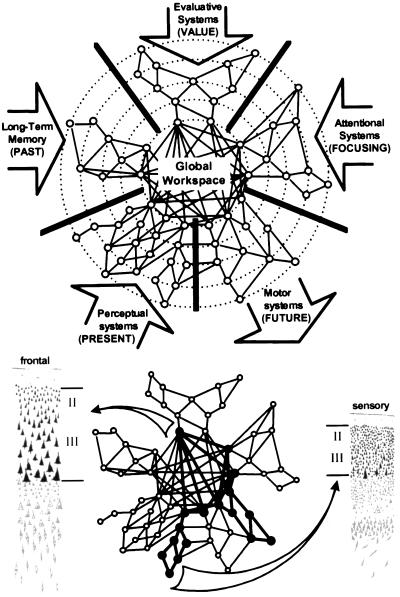
We distinguish two main computational spaces within the brain (Fig. 1). The first is a processing network, composed of a set of parallel, distributed and functionally specialized processors (5) or modular subsystems (6) ranging from primary sensory processors (such as area V1) or unimodal processors (such as area V4), which combine multiple inputs within a given sensory modality, up to heteromodal processors (such as the visuo-tactile neurons in area LIP or the “mirror” neurons in area F5) that extract highly processed categorical or semantic information. Each processor is subsumed by topologically distinct cortical domains with highly specific local or medium-range connections that “encapsulate” information relevant to its function (13).
Figure 1.
(Upper) Schematic representation of the five main types of processors connected to the global workspace (inspired from ref. 13). (Lower) Sample activation during effortful processing; a coherent link between two informationally encapsulated processors is established through the activation of distributed workspace neurons. The long-range workspace connectivity, supported by layer II/III neurons, is more prominent in Von Economo’s frontal-type cortex (left) than in sensory-type cortex (right) (14).
The second computational space is a global workspace, consisting of a distributed set of cortical neurons characterized by their ability to receive from and send back to homologous neurons in other cortical areas horizontal projections through long-range excitatory axons (which may impinge on either excitatory or inhibitory neurons). Our view is that this population of neurons does not belong to a distinct set of “cardinal” brain areas but, rather, is distributed among brain areas in variable proportions. It is known that long-range cortico-cortical tangential connections, including callosal connections, mostly originate from the pyramidal cells of layers 2 and 3, which give or receive the so-called “association” efferents and afferents. We therefore propose that the extent to which a given brain area contributes to the global workspace would be simply related to the fraction of its pyramidal neurons contributing to layers 2 and 3, which is particularly elevated in von Economo’s type 2 (dorsolateral prefrontal) and type 3 (inferior parietal) cortical structures (14). In addition, these cortical neurons establish strong vertical and reciprocal connections, via layer 5 neurons, with corresponding thalamic nuclei, thus contributing both to the stability of workspace activity, for instance via self-sustained circuits and to the direct access to the processing networks (15, 16).
Selective Gating of Workspace Inputs and Outputs.
Although a variety of processor areas project to the interconnected set of neurons composing the global workspace, at any given time only a subset of inputs effectively accesses it. We postulate that this gating is implemented by descending modulatory projections from workspace neurons to more peripheral processor neurons. These projections may selectively amplify or extinguish the ascending inputs from processing neurons, thus mobilizing, at a given time, a specific set of processors in the workspace while suppressing the contribution of others.
Spatio-Temporal Dynamics of Workspace Activity.
The global workspace is the seat of a particular kind of “brain-scale” activity states characterized by the spontaneous activation, in a sudden, coherent and exclusive manner, of a subset of workspace neurons, the rest of workspace neurons being inhibited. The entire workspace is globally interconnected in such a way that only one such “workspace representation” can be active at any given time. This all-or-none invasive property distinguishes it from peripheral processors in which, due to local patterns of connections, several representations with different formats may coexist.
A representation that has invaded the workspace may remain active in an autonomous manner and resist changes in peripheral activity. If it is negatively evaluated, or if attention fails, it may however be spontaneously and randomly replaced by another discrete combination of workspace neurons. Functionally, this neural property implements an active “generator of diversity,” which constantly projects and tests hypotheses (or prerepresentations) on the outside world (9–12). The dynamics of workspace neuron activity is thus characterized be a constant flow of individual coherent episodes of variable duration.
Content of the Global Workspace.
Through their mutual projection to and from workspace neurons, five major categories of processors can be dynamically mobilized and multiply reconfigured (Fig. 1).
Perceptual circuits give the workspace access to the present state of the external world. In humans, perceptual circuits include the object-oriented ventral and lateral areas of the temporal lobes as well as the temporal and inferior parietal areas involved in language comprehension (including Wernicke’s area) (13). Thus, the content of any attended object or discourse can access the global workspace.
Motor programming circuits allow the content of the workspace to be used to guide future intentional behavior. A hierarchy of nested circuits implements motor intentions, from the highest level of abstract plans to individual actions, themselves composed of gestures (12, 17). In humans, these circuits include premotor cortex, posterior parietal cortex, supplementary motor area, basal ganglia (notably the caudate nucleus), and cerebellum, as well as the high-level speech production circuits of the left inferior frontal lobe, including Broca’s area. Connections of the workspace to motor and language circuits at the higher levels of this hierarchy endow any active representation in the workspace with the property of reportability (18), namely the fact that it can be described or commented upon using words or gestures.
Long-term memory circuits provide the workspace with an access to past percepts and events. Hippocampal and parahippocampal areas play a special role in mediating the storage in and retrieval from long-term memory stores, which are presumably distributed throughout the cortex according to their original content and modality (13).
Evaluation circuits (9, 10, 19, 20) allow representations in the workspace to be associated with a positive or negative value. The main anatomical systems in this respect include the orbitofrontal cortex, anterior cingulate (AC), hypothalamus, amygdala, and ventral striatum as well as the mesocortical catecholaminergic and cholinergic projections to prefrontal cortex. Reciprocal projections allow evaluation circuits to be internally activated by the current workspace content [auto-evaluation (10)] and, conversely, to selectively maintain or change workspace activity according to whether its value is predicted to be positive or negative (9–12, 20).
Attention circuits allow the workspace to mobilize its own circuits independently from the external world. Changes in workspace contents need not necessarily lead to changes in overt behavior but may result in covert attention switches to selectively amplify or attenuate the signals from a subset of processor neurons. Although all descending projections from workspace neurons to peripheral modular processors are important in this selective amplification process, a particular role is played by areas of the parietal lobe in visuo-spatial attention (7, 8, 13).
Global Modulation of Workspace Activation.
The state of activation of workspace neurons is assumed to be under the control of global vigilance signals, for instance from mesencephalic reticular neurons. Some of these signals are powerful enough to control major transitions between the awake state (workspace active) and slow-wave sleep (workspace inactive). Others provide graded inputs that modulate the amplitude of workspace activation, which is enhanced whenever novel, unpredicted, or emotionally relevant signals occur, and conversely, drops when the organism is involved in a routine activity.
COMPUTER SIMULATION
To specify the above hypotheses in a computationally explicit manner, a minimal computer simulation of the workspace architecture and dynamics is presented. We are aware that it is necessarily partial and incomplete. We restrict it to the learning and execution of the well known Stroop task (21), which includes both an easy, automatic component and an effortful, attention-demanding component.
Network Architecture and Dynamics.
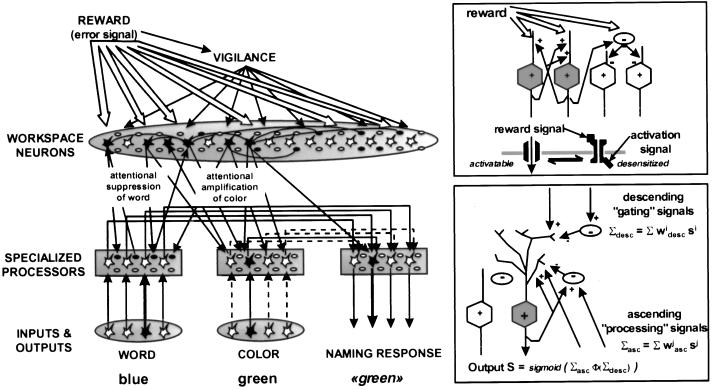
Fig. 2 schematizes the proposed neuronal architecture, composed of excitatory and inhibitory units grouped into different assemblies: input systems, specialized processors, workspace neurons, vigilance, and reward systems. Each assembly is composed of multiple replicas of a basic element comprising an excitatory unit, a gating inhibitory unit, and a processing inhibitory unit. Gating and processing inhibitory units are classical McCulloch–Pitts units whose activity level SINH, ranging from 0 to 1, obeys the update rule SiINH = sigmoid(Σ wi,j Sj), where the sigmoid function is defined as sigmoid(x) = 1/(1 + e−x), and the wi,j are the synaptic weights of neurons contacting inhibitory unit i. For simplicity only excitatory units (both local and long-distance) are assumed to make synaptic contact onto inhibitory units.
Figure 2.
Architecture of the simulated network. (Insets) The proposed mechanisms for reward-dependent changes in workspace unit activity (Upper) and for the interaction of ascending and descending connections to a given area (Lower). Although each unit in the simulation presumably represents ≈100 neurons in an actual brain, our scheme epitomizes the basic organization of a cortical column, with intra-columnar recurrent excitation, intra-areal and mid-range excitatory connections providing excitation or inhibition (via intermediate processing inhibitory interneurons), and descending excitatory connections providing upward or downward modulation of activity (via intermediate gating inhibitory interneurons). The network is depicted in a state of activity typical of a correct trial in the effortful Stroop task. Attentional amplification reverses the relation between conflicting word and color inputs by amplifying the weaker color unit activity and suppressing the stronger word unit activity.
The activity of excitatory units, SEXC, obeys a modified update rule:
SiEXC = sigmoid(Σiasc Φ(Σidesc)), where Σiasc = Σwi,jasc Sj and Σidesc = Σ wi,jdesc Sj.
The weights wi,jasc and wi,jdesc can be positive or negative, because inputs to excitatory units may come from excitatory as well as inhibitory units. The equation separates these inputs into two types: descending connections from hierarchically higher assemblies (subscript desc) and ascending or processing inputs (subscript asc), which represent all the other (nondescending) connections that give the neuron its specific functionality (Fig. 2, Lower Inset). The monotonic modulating function Φ is chosen as a sigmoid with Φ(x) → 0 when x → −∞, Φ(0) = 1 and Φ(x) → 2 when x → +∞. This equation implies that descending signals have a gating effect on lower-level neuronal activity, with attentional amplification if Σdesc > 0, normal unattended processing if Σdesc = 0, and attentional suppression if Σdesc < 0.
For simplicity, only the synaptic weights between two excitatory units are assumed to be modifiable according to a reward-modulated Hebbian rule Δwpost,pre = ɛ R Spre (2 Spost −1), where R is a reward signal provided after each network response (R = +1, correct; R = −1, incorrect), pre is the presynaptic unit and post the postsynaptic unit (9). Weights are bounded to remain between 0 and a maximum value (here arbitrarily fixed at 7).
Finally, workspace neuron activity is under the influence of both vigilance and reward signals. The vigilance signal V is treated as having a descending modulatory influence on all workspace neurons according to the above-described gating mechanism. It is updated after each response: if R > 0, then ΔV= −0.1 V, otherwise ΔV = 0.5 (1 − V). This rule has the effect of a slowly decreasing vigilance with sharp increases on error trials. The reward signal R influences the stability of workspace activity through a short-term depression or potentiation of synaptic weights (9, 10, 12): if R < 0, Spre > 0.5 and Spost > 0.5, then Δw′post,pre = −0.5 w′post,pre, otherwise Δw′post,pre = 0.2 (1 − w′post,pre), where w′ is a short-term multiplier on the excitatory synaptic weight from unit pre to unit post. A plausible molecular implementation of this rule has been proposed in terms of allosteric receptors (9, 10) (Fig. 2, Upper Inset). It postulates that the time coincidence of a diffuse reward signal and of a postsynaptic marker of recent neuronal activity transiently shifts the allosteric equilibrium either toward, or from, a desensitized refractory conformation. Through this “chemical Hebb rule,” negative reward destabilizes the self-sustaining excitatory connections between currently active workspace neurons, thus initiating a change in workspace activity.
Implementation of the Stroop Tasks.
We submitted the network to several versions of the word-color Stroop tasks (21). For this purpose, four input units were dedicated to encoding four color words, four other input units encoded the color of the ink used to print the word, and four internal units corresponded to the four naming responses (Fig. 2). Routine task 1 (color naming) consisted in turning a single color unit on and rewarding the network for turning the corresponding naming unit on. Direct one-to-one connections between color and naming units implemented a minimal version of the color naming process. Routine task 2 (word naming with color interference) consisted in turning a word unit on together with another incompatible ink color unit and rewarding the network for turning on the naming unit appropriate to the word, not the ink color. Again, word naming was implemented by direct one-to-one connections from word to naming units. As in previous models of the Stroop test (22, 23), stronger connections were used in the word-to-name pathway than in the color-to-name pathway, corresponding to the greater frequency of word naming in everyday use (21). Finally, the effortful task (color naming with word interference) consisted in providing conflicting word and color inputs, as in task 2, but rewarding the network for turning on the naming unit appropriate to the ink color, not the word.
Connections to and from workspace units were critical for the latter task. A random, patchy connection scheme was used, so that each processor had a Gaussian probability of contacting units in any given region of the workspace, and a similar Gaussian probability of receiving projections from units in the same region (with random initial weights). Note that ascending and descending connections in the model are reciprocal only in a statistical sense: any two processor and workspace units are generally not connected bidirectionally, but any region of the workspace that receives ascending projections from multiple processor units is highly likely to send back descending projections to the same units.
Simulation Results.
When placed in routine task 1 (color naming, no interfering word) the network performs correctly with only processor unit activation, using the direct one-to-one connections from color units to name units. Although workspace activity is occasionally observed if vigilance is initially set high, it is clearly not needed. Hence, vigilance quickly drops without impacting on performance (Fig. 3).
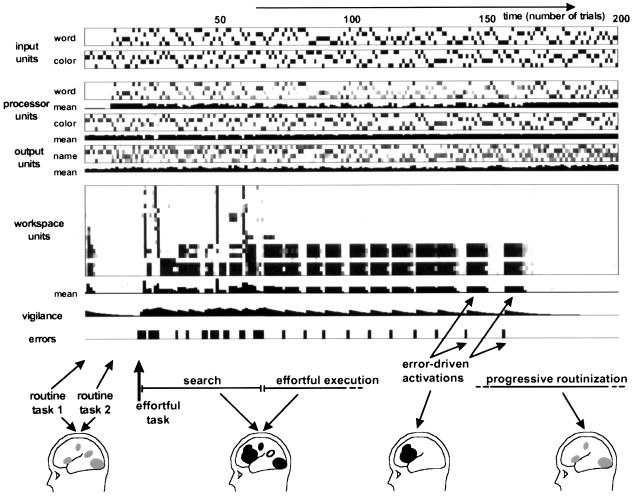
Figure 3.
Temporal dynamics of the simulation in the course of learning the effortful Stroop task; 200 trials were simulated. The Stroop task was introduced without warning after trial 20. Note the selective activation of workspace units with a simultaneous amplification of color processors and a suppression of word processors. Workspace activity is seen in the initial phase of searching for the appropriate response rule (with considerably inter-trial variability), during the effortful execution of the task and following each erroneous response. For illustration purposes, putative brain-imaging correlates of routine and workspace activation are shown (see refs. 24 and 25).
Similar results are obtained when the network is submitted to routine task 2 (word naming with color interference). Even though there are now two conflicting inputs, word-to-name connections are stronger than color-to-name connections. Hence, the naming response appropriate to the word is activated faster and more strongly than the one appropriate to the color, which is quickly extinguished by lateral inhibition. Thus, workspace unit activity is not needed for this task either.
When the naive network is then switched to the effortful task (color naming with word interference), an initial series of errors takes place as the network perseverates in applying the routine task 2. The delivery of negative reward leads to an increase in vigilance and to the sudden activation of variable patterns amongst workspace units. The next ≈30 trials can be described as a “search phase.” Workspace activation varies in a partly random manner as various response rules are explored. Workspace activation patterns that lead to activating the incorrect response unit are negatively rewarded and tend not to be repeated in subsequent trials. Eventually, the network settles into a stable activation pattern, with a fringe of variability that slowly disappears in subsequent trials. This stable pattern, which leads to correct performance, is characterized by (i) preferential descending projections to the excitatory units of the color processing network, thus causing an amplification of the color input, and its transmission to response units; (ii) preferential descending projections to the inhibitory gating units of the word processing network, thus causing a suppression of the word input; and (iii) strong long-distance excitatory connections amongst active workspace units maintaining the pattern active in the intertrial interval. Across multiple simulations with different initial connectivity, an activation pattern with these characteristics was invariably found (after ≈5–50 trials), although its detailed composition varied. The crucial factor here is the patchy distribution of initial connections to and from the workspace, which ensures that sectors of the workspace with the appropriate preexisting connections do indeed exist in the initial state and can be selectively stabilized.
Following the search phase, the network goes through a phase of “effortful task execution” in which workspace activation remains indispensable to correct performance. During this phase, workspace activity remains high even on occasional trials in which the word and ink color information do not conflict. When performance is correct for a series of consecutive trials, vigilance tends to drop. However, any lapse in workspace activation is immediately sanctioned by an error. Each error is immediately followed by an intense reactivation of the workspace. Progressively though, the task becomes routinized as the Hebbian rule applied to processor units tended to increase the color-to-name connections and to decrease the word-to-name connections. Routinization is characterized by increasingly longer periods of correct performance without accompanying workspace activation. Eventually, workspace activation disappears, as the processor network now handles the routinized task by itself.
An interesting property of the network is its ability to maintain an active, sustained state of workspace and processor unit activity for some delay. This is due to the mutually reinforcing excitatory ascending and descending connections between processor and workspace units, together with the excitatory connections within the workspace itself. Once this self-sustained state of activity is established, the descending attentional amplification is often sufficient to maintain processor units active for some duration even when input units are turned off. Hence, the network architecture is adequate to pass delayed-response versions of the routine and effortful tasks in which the response must be postponed after the stimulus has been turned off. It is noteworthy that given this additional delayed-response requirement, even the routine task of color naming now requires workspace activity.
EMPIRICAL TESTS AND PREDICTIONS
Brain Imaging.
The key empirical prediction of our hypothesis in the domain of brain imaging is the existence of a strong correlation between cortical areas that are found active in conscious effortful tasks, and areas that possess a strong long-distance cortico-cortical connectivity, presumably associated with dense cortical layers 2 and 3. Brain imaging techniques, once they resolve the transverse laminar distribution of brain activation, might show a differential laminar pattern of activity as a function of whether a given area is recruited for an automatic task or for an effortful task. The global activation of neurons dispersed in multiple cortical areas also might be visualized as a temporary increase in the long-distance coherence of brain activity in electro- and magneto-encephalography (26) or in studies of functional connectivity with functional MRI (27).
We also predict the conditions under which areas rich in workspace neurons should be seen as “active” by using brain-imaging techniques. In our simulation, workspace unit activation exhibits the following properties: (i) it is absent during routine tasks; (ii) it appears suddenly when a novel, nonroutine task is introduced; (iii) it varies semi-randomly during the initial learning of a novel task; (iv) it is high and stable during execution of a known but not yet routinized effortful task; (v) it decreases during routinization; (vi) it resumes sharply following an error; (vii) it is present during the delay period of a delayed-response task; and (viii) it temporarily mobilizes, in a descending manner, other units involved in specific task components.
Brain-imaging experiments indicate that dorsolateral prefrontal cortex (dlPFC) and AC possess these properties. Both are active in effortful cognitive tasks, including the Stroop test, with a graded level of activation as a function of task difficulty (28–30). With automatization, activation decreases in dlPFC and AC, but it immediately recovers if a novel, nonroutine situation occurs (31). AC activates in tight synchrony with subjects’ errors (25, 32). In the Wisconsin card sorting test, dlPFC activates when subjects have to search for a new sorting rule (33). dlPFC and AC possess the ability to remain active in the absence of external stimulation, such as during the delay period of a delayed-response task (28), or during internally driven activities such as mental calculation (34). dlPFC and AC activity also has been found to correlate with subjective conscious perception in various situations in which carefully matched conscious and unconscious conditions were contrasted (35, 36). Finally, concomittent to dlPFC and AC activation, a selective attentional amplification is seen in relevant posterior areas during focused-attention tasks (7, 37).
Workspace activity in our model is concentrated in distinct, localized subsets of neurons that vary with the peripheral processors that must be amplified or suppressed. This is compatible with the evidence for specialization within subregions of AC and dlPFC (30, 38). Our model also posits that effortless or automatic processing should activate specialized processors throughout the cortex without requiring coordination by global workspace neurons. Recent images of brain activity during unconscious processing support this hypothesis (35, 39, 40). In particular, subliminal word stimuli have been shown to cause an entire stream of perceptual, semantic, and motor processes ending up in primary motor cortex (41).
Anatomy and Physiology.
Consistent with a privileged contribution of horizontal, long-distance connections in establishing a coherent workspace, a dense network of connections linking dorso-lateral prefrontal and inferior parietal areas to anterior and posterior cingulate, temporal cortices, and parahippocampal cortices has been identified in the monkey (38). It may support the interconnection of the workspace to high-level perceptual, motor, memory, attentional, and evaluation circuits.
The model emphasizes the top-down mobilization of processor neurons by workspace neurons via excitatory descending connections. Such selective amplification or reduction of peripheral neuronal activity has been observed experimentally (42, 43). Because the descending projections are excitatory, they exert their modulatory effect in our model via intermediate connections to a special class of “gating” inhibitory interneurons that have a multiplicative effect on postsynaptic neuronal firing during effortful attentional suppression. These neurons differ from standard “processing inhibitory interneurons,” which are the main targets of ascending and horizontal connections, have additive effects on postsynaptic firing, and are active during any type of processing in a given area, automatic as well as effortful. The differential behavior of these two categories of neurons could be established by electrophysiological recordings.
Pharmacology and Molecular Biology.
Our theory predicts that workspace neurons are the specific targets of projections from neuronal structures that provide reward and vigilance inputs, presumably via specialize neurotransmitter pathways. Mesocortical dopaminergic neurons and cholinergic pathways, in particular, are known to differentially target prefrontal cortex (13, 44). The decoding of such signals by workspace neurons may be effected by specific subtypes of neurotransmitter receptors (45). Pathological mutations in humans and in genetically modified animals, in which the expression or the physiological properties of a specific subtype of receptor is altered, may thus help decipher the cerebral circuits involved in effortful tasks (46).
CONCLUSIONS
At variance with previous models (9, 10, 22, 23), the proposed neuronal architecture successfully learns the Stroop test without postulating prewired rule-coding units adequate for the task and on the basis of realistic neuronal processes. Our implementation of a global computational workspace operating under conditions of selection by reward does not aim at an exhaustive description of a “conscious workspace” (5). It is limited in scope to features characteristic of effortful tasks, for which it leads to a number of critical predictions, which can be experimentally tested, in particular, with brain-imaging techniques.
The model suffers from shortcomings that should be dealt with in future developments. Although workspace neurons are assumed to be heavily interconnected, they need not be functionally equivalent but rather may be organized in multiple hierarchically nested specialized circuits. An attempt at simulating these nested levels of internal planning was presented in a previous model of the Tower of London task (12). Other important issues include characterization of the variability in the initial connectivity needed to learn multiple tasks (47, 48); the inclusion of novelty detection mechanisms, presumably implemented in the hippocampus, which may serve as input to workspace units (49); and the connection to the workspace of self-representations that might allow the simulated organism to reflect on its own internal processes.
Acknowledgments
We thank M. Posner and M. Zoli for their comments and the Fondation pour la Recherche Médicale, the College de France, the Association Contre la Myopathie, and the European Union Biotech program for support.
ABBREVIATIONS
- dlPFC
dorsolateral prefrontal cortex
- AC
anterior cingulate
References
- 1.Felleman D J, Van Essen D C. Cereb Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- 2.Cheng K, Gallistel C R. Cognition. 1986;23:149–178. doi: 10.1016/0010-0277(86)90041-7. [DOI] [PubMed] [Google Scholar]
- 3.Hermer L, Spelke E S. Nature (London) 1994;370:57–59. doi: 10.1038/370057a0. [DOI] [PubMed] [Google Scholar]
- 4.Fodor J A. The Modularity of Mind. Cambridge, MA: MIT Press; 1983. [Google Scholar]
- 5.Baars B J. A Cognitive Theory of Consciousness. Cambridge, MA: Cambridge Univ. Press; 1989. [Google Scholar]
- 6.Shallice T. From Neuropsychology to Mental Structure. Cambridge, MA: Cambridge Univ. Press; 1988. [Google Scholar]
- 7.Posner M I, Dehaene S. Trends Neurosci. 1994;17:75–79. doi: 10.1016/0166-2236(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 8.Posner M I. Proc Natl Acad Sci USA. 1994;91:7398–7403. doi: 10.1073/pnas.91.16.7398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dehaene S, Changeux J P. J Cognit Neurosci. 1989;1:244–261. doi: 10.1162/jocn.1989.1.3.244. [DOI] [PubMed] [Google Scholar]
- 10.Dehaene S, Changeux J P. Cereb Cortex. 1991;1:62–79. doi: 10.1093/cercor/1.1.62. [DOI] [PubMed] [Google Scholar]
- 11.Dehaene S, Changeux J P. J Cognit Neurosci. 1993;5:390–407. doi: 10.1162/jocn.1993.5.4.390. [DOI] [PubMed] [Google Scholar]
- 12.Dehaene S, Changeux J P. Proc Natl Acad Sci USA. 1997;94:13293–13298. doi: 10.1073/pnas.94.24.13293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mesulam M M. Brain. 1998;121:1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- 14.Von Economo C. The Cytoarchitectonics of the Human Cerebral Cortex. London: Oxford Univ. Press; 1929. [Google Scholar]
- 15.Llinas R R, Paré D. Neuroscience. 1991;44:521–535. doi: 10.1016/0306-4522(91)90075-y. [DOI] [PubMed] [Google Scholar]
- 16.Munk M H, Roelfsema P R, Konig P, Engel A K, Singer W. Science. 1996;272:271–274. doi: 10.1126/science.272.5259.271. [DOI] [PubMed] [Google Scholar]
- 17.Jeannerod M. The Cognitive Neuroscience of Action. Oxford: Blackwell; 1997. [Google Scholar]
- 18.Weiskrantz L. Consciousness Lost and Found: A Neuropsychological Exploration. New York: Oxford Univ. Press; 1997. [Google Scholar]
- 19.Friston K J, Tononi G, Reeke G N, Sporns O, Edelman G M. Neuroscience. 1994;59:229–243. doi: 10.1016/0306-4522(94)90592-4. [DOI] [PubMed] [Google Scholar]
- 20.Schultz W, Dayan P, Montague P R. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 21.MacLeod C M. Psychol Bull. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- 22.Cohen J D, Dunbar K, McClelland J. Psychol Rev. 1990;97:332–361. doi: 10.1037/0033-295x.97.3.332. [DOI] [PubMed] [Google Scholar]
- 23.Kimberg D Y, Farah M J. J Exp Psychol Gen. 1993;122:411–428. doi: 10.1037//0096-3445.122.4.411. [DOI] [PubMed] [Google Scholar]
- 24.Raichle M E, Fiesz J A, Videen T O, MacLeod A K. Cereb Cortex. 1994;4:8–26. doi: 10.1093/cercor/4.1.8. [DOI] [PubMed] [Google Scholar]
- 25.Dehaene S, Posner M I, Tucker D M. Psychol Sci. 1994;5:303–305. [Google Scholar]
- 26.Tononi G, Srinivasan R, Russell D P, Edelman G M. Proc Natl Acad Sci USA. 1998;95:3198–3203. doi: 10.1073/pnas.95.6.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friston K J, Frith C D, Fletcher P, Liddle P F, Frackowiak R S. Cereb Cortex. 1996;6:156–164. doi: 10.1093/cercor/6.2.156. [DOI] [PubMed] [Google Scholar]
- 28.Cohen J D, Perlstein W M, Braver T S, Nystrom L E, Noll D C, Jonides J, Smith E E. Nature (London) 1997;386:604–608. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- 29.Pardo J V, Pardo P J, Janer K W, Raichle M E. Proc Natl Acad Sci USA. 1990;87:256–259. doi: 10.1073/pnas.87.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paus T, Koski L, Caramanos Z, Westbury C. NeuroReport. 1998;9:R37–R47. doi: 10.1097/00001756-199806220-00001. [DOI] [PubMed] [Google Scholar]
- 31.Raichle M E, Fiez J A, Videen T O, MacLeod A K, Pardo J V, Fox P T, Petersen S E. Cereb Cortex. 1994;4:8–26. doi: 10.1093/cercor/4.1.8. [DOI] [PubMed] [Google Scholar]
- 32.Carter C S, Braver T S, Barch D, Botvinick M M, Noll D, Cohen J D. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- 33.Konishi S, Nakajima K, Uchida I, Kameyama M, Nakahara K, Sekihara K, Miyashita Y. Nat Neurosci. 1998;1:80–84. doi: 10.1038/283. [DOI] [PubMed] [Google Scholar]
- 34.Roland P E, Friberg L. J Neurophysiol. 1985;53:1219–1243. doi: 10.1152/jn.1985.53.5.1219. [DOI] [PubMed] [Google Scholar]
- 35.Sahraie A, Weiskrantz L, Barbur J L, Simmons A, Williams S C R, Brammer M J. Proc Natl Acad Sci USA. 1997;94:9406–9411. doi: 10.1073/pnas.94.17.9406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grafton S T, Hazeltine E, Ivry R. J Cognit Neurosci. 1995;7:497–510. doi: 10.1162/jocn.1995.7.4.497. [DOI] [PubMed] [Google Scholar]
- 37.Corbetta M, Miezin F M, Dobmeyer S, Smulman G L, Petersen S E. J Neurosci. 1991;11:2383–2402. doi: 10.1523/JNEUROSCI.11-08-02383.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldman-Rakic P S. Annu Rev Neurosci. 1988;11:137–156. doi: 10.1146/annurev.ne.11.030188.001033. [DOI] [PubMed] [Google Scholar]
- 39.Morris J S, Öhman A, Dolan R J. Nature (London) 1998;393:467–470. doi: 10.1038/30976. [DOI] [PubMed] [Google Scholar]
- 40.Whalen P J, Rauch S L, Etcoff N L, McInerney S C, Lee M B, Jenike M A. J Neurosci. 1998;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dehaene S, Naccache L, Le Clec’H G, Koechlin E, Mueller M, Dehaene-Lambertz G, van de Moortele P F, Le Bihan D. Nature (London) 1998;395:597–600. doi: 10.1038/26967. [DOI] [PubMed] [Google Scholar]
- 42.Moran J, Desimone R. Science. 1985;229:782–784. doi: 10.1126/science.4023713. [DOI] [PubMed] [Google Scholar]
- 43.Motter B C. J Neurosci. 1994;14:2178–2189. doi: 10.1523/JNEUROSCI.14-04-02178.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Descarries L, Gisiger V, Steriade M. Prog Neurobiol. 1997;53:603–625. doi: 10.1016/s0301-0082(97)00050-6. [DOI] [PubMed] [Google Scholar]
- 45.Granon S, Poucet B, Thinus-Blanc C, Changeux J P, Vidal C. Psychopharmacology. 1995;119:139–144. doi: 10.1007/BF02246154. [DOI] [PubMed] [Google Scholar]
- 46.Picciotto M R, Zoli M, Rimondini R, Lena C, Marubio L M, Pich E M, Fuxe K, Changeux J P. Nature (London) 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- 47.Changeux J P, Courrège P, Danchin A. Proc Natl Acad Sci USA. 1973;70:2974–2978. doi: 10.1073/pnas.70.10.2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Edelman G. Neural Darwinism. New York: Basic Books; 1987. [Google Scholar]
- 49.Gray J A. Behav Brain Sci. 1994;18:659–722. [Google Scholar]