Abstract
Honeybee colonies are highly integrated functional units characterized by a pronounced division of labor. Division of labor among workers is mainly age-based, with younger individuals focusing on in-hive tasks and older workers performing the more hazardous foraging activities. Thus, experimental disruption of the age composition of the worker hive population is expected to have profound consequences for colony function. Adaptive demography theory predicts that the natural hive age composition represents a colony-level adaptation and thus results in optimal hive performance. Alternatively, the hive age composition may be an epiphenomenon, resulting from individual life history optimization. We addressed these predictions by comparing individual worker longevity and brood production in hives that were composed of a single age cohort, two distinct age cohorts, and hives that had a continuous, natural age distribution. Four experimental replicates showed that colonies with a natural age composition did not consistently have a higher life expectancy and/or brood production than the single cohort or double cohort hives. Instead, a complex interplay of age structure, environmental conditions, colony size, brood production, and individual mortality emerged. A general trade-off between worker life expectancy and colony productivity was apparent, and the transition from in-hive tasks to foraging was the most significant predictor of worker lifespan irrespective of the colony age structure. We conclude that the natural age structure of honeybee hives is not a colony-level adaptation. Furthermore, our results show that honeybees exhibit pronounced demographic plasticity in addition to behavioral plasticity to react to demographic disturbances of their societies.
Keywords: Aging, Mortality, Social Insects, Division of Labor, Homeostasis, Colony Productivity, Biodemography
Introduction
Honey bees live in complex, highly integrated societies that consist of one reproductive queen and thousands of non-reproductive female workers. These workers perform all non-reproductive tasks in the hive, including nest construction, maintenance and defense, brood care, food processing, and foraging. Despite genetic influences, the division of labor among workers is driven to a large extent by age (age-polyethism) (Beshers and Fewell 2001). Newly emerged workers clean cells and go on through an age-based progression of other, overlapping in-hive tasks (Lindauer 1953). This progression is accompanied by continuous physiological changes (Winston 1987), but the most severe changes occur during the transition from in-hive tasks to foraging: The titer of systemic juvenile hormone increases, while the titer of the major hemolymph protein vitellogenin drops and the negative feedback control between the two has been suggested as a main underlying cause for the behavioral maturation from in-hive to forager bee (Amdam and Omholt 2003; Amdam et al. 2007). After foraging initiation, the worker does not revert to in-hive activities under normal circumstances but continues foraging until its death (Huang and Robinson 1992).
To guarantee sufficient flexibility of the hive social organization, the intrinsic maturation of each worker is susceptible to social and other environmental factors. Colony food shortage decreases the age at which in-hive workers transition to become foragers (Schulz et al. 1998). Conversely, this transition also depends on the availability of food sources and recruitment stimuli (Rueppell et al. 2007). Brood pheromone has been shown to inhibit the behavioral development to foragers because in-hive workers are required for brood care (Le Conte et al. 2001). Queen pheromone also delays the behavioral maturation (Pankiw et al. 1998) for less obvious reasons. Most importantly, it has been shown that older workers delay the maturation of younger workers to foragers (Huang and Robinson 1992; Huang and Robinson 1996), an effect that is also mediated by pheromones (Leoncini et al. 2004). In spite of highly plastic responses to these environmental influences, the age of first foraging (AFF) is significantly different among honey bee races (Brillet et al. 2002; Pankiw 2003) and responds indirectly to artificial selection of pollen hoarding, resulting in a genetic differentiation that accounts for 46% of the phenotypic variance among individuals from selection lines (Rueppell et al. 2004).
The age at first foraging (AFF) is a major life history variable at the colony level because it determines the food influx into the colony. Available protein and nectar resources in turn determine colony growth and survival. On the other hand, the AFF is also the most important variable of individual worker life history and mortality (Rueppell et al. 2007). The process is accompanied by profound physiological adaptations to the new behavioral profile (Amdam et al. 2007). The transition also dramatically shifts the environment of the individual: Foragers are exposed to a variety of stresses, such as temperature, desiccation, etc. and their external mortality pressure is high. Combined, the regulatory changes and the environmental hazard cause a high mortality of foragers (Sakagami and Fukuda 1968; Rueppell et al. 2007).
The societies of social insects have often been compared to super-organisms with selection on group-level traits, such as the efficiency of the division of labor and overall colony productivity (Wilson 1971; Oster and Wilson 1978; Wilson and Sober 1989; Reeve and Hölldobler 2007). In that light, it has been postulated that the demography of social insect colonies represents a colony-level adaptive trait (Oster and Wilson 1978). The concept of adaptive demography of social insect colonies has been supported by studies of the caste composition of ant colonies with distinct morphological castes (Hölldobler and Wilson 1990; Schmid-Hempel 1992). However, the argument can be extended to include age-polyethism, the occurrence of castes based on division of labor by age (Schmid-Hempel 1992; Tofilski 2006). Whereas population demography in solitary organisms is an epiphenomenon of individual life history optimization, the age-structure of colonies is predicted to be under selection (Schmid-Hempel 1992), given that an age-polyethism exists and aging rates are plastic due to resource allocation to individuals. Specifically, in honey bees the centrifugal age-polyethism (Beshers and Fewell 2001) and nutritional transfers from older to younger individuals (Amdam and Page 2005) contribute to a rectangularization of the survival curve (Sakagami and Fukuda 1968), which is characterized by low early mortality and high mortality at older ages. Distortions of the colony age demography affect the age-polyethism (Huang and Robinson 1992; Huang and Robinson 1996) and are a widely used experimental tool to decouple chronological age from behavior. However, the consequences for individual survival and colony productivity of the colony age demography have never been investigated. The behavioral adjustments of honey bee workers to colony needs (Huang and Robinson 1996) represent a special form of phenotypic plasticity where individuals permanently alter their ontogenetic trajectory based on environmental circumstances and strong plasticity may bear a physiological or performance cost (West-Eberhard 2003) that influences worker longevity.
The question of how group age structure and the associated resource transfers influence individual longevity is also interesting from a general gerontological point of view (Lee 2003). Group age structure is a universal characteristic of social species and is intimately linked to social evolution and the evolution of longevity (Carey 2001). However, social gerontology remains a mainly descriptive science because in most social species, including humans and primates, group age structure cannot be manipulated experimentally, thus systematic studies on its effect on intra-group birth and mortality rates are scarce. The honeybee offers an excellent experimental system for investigating the consequences of group age structure on individual lifespan (Amdam and Rueppell 2006). This study is the first to explore this potential, comparing the patterns of worker mortality and colony productivity in colonies of different age composition: single-cohort, double-cohort, and natural control.
Materials and Methods
To observe the effects of colony age-composition on individual life expectancy and colony productivity, these variables were compared between three demographically manipulated hives of different age composition. One hive contained bees of only one age, the second consisted of two age cohorts that were one week apart, and the third colony served as a control with workers of a “normal” age distribution. A mixture of western honey bees (Apis mellifera L) from 8 – 12 different source hives were used for each of four independent experiments, as described below. The first experiment was conducted in May and June 2004, the second in July and August 2004, the third in May and June 2005, and the fourth in June and July 2007.
Twenty-seven and 21 days prior to the actual experiments, queens in the source hives were induced to lay eggs into empty combs. These combs were brought into a humidity- and temperature-controlled incubator (34°C/60% rel. humid.) one day before the emergence of the two focal cohorts of bees. Three other hives were placed in front of the entrance holes of the respective future experimental observation hives to serve as a basis for the set up of the experimental hives. These hives were of similar size and contained queens of the same age and the same commercial source (Wilbanks Apiaries, GA).
The first cohort of emerging workers were color-marked and introduced to one basis hive (#2) six days before the start of the actual experiment. On the first day of the experiment, we set up the first experimental hive (#1) with only one age-class of 3000 newly emerged bees and the queen from the basis hive (single cohort colony: (Huang and Robinson 1992)). A random sample of this cohort was individually marked as the focal cohort (approximate sample sizes: repeat 1: 800 workers, repeat 2: 200, repeat 3: 200, repeat 4: 800) with individually colored and numbered plastic tags (BeeWorks, Canada). We used little dots of Testors™ paint to increase the number of unique tags. This tagging did not require any special precaution (anesthesia or chilling of the bees) because newly-emerged bees are unable to fly or sting.
On the second day, we set up the experimental hive #2 with 1500 newly emerged bees and 1500 one-week old workers that were collected from basis hive #2. This dual cohort hive received the queen from basis hive #2. Focal cohorts of newly-emerged workers were individually tagged as above (approximate sample sizes: repeat 1: 400, repeat 2: 100 repeat 3:200, repeat 4: 800). The one-week old workers were collected in small mesh-wire cages and briefly chilled before tagging and subsequent introduction to the hive (approximate sample sizes: repeat 1: 400, repeat 2: 200, repeat 3: 200, repeat 4: none). On the third day, we set up the control hive (#3) with the queen and approximately 2000 – 2800 random workers from the third basis colony, excluding newly-emerged workers that could be visually distinguished from older workers. In addition, a focal cohort of newly-emerged, individually tagged workers was introduced (approximate sample sizes: repeat 1: 800 workers, repeat 2: 200 repeat 3:200, repeat 4: 800).
All experimental hives were maintained in four-frame observation hives with immediate access to the outside, in a dark, temperature-controlled room. Initially, these hives contained approximately one frame of honey reserves, half a frame of pollen stores, and two empty frames. Resource and brood levels were not manipulated during the experiment, except for one brood removal after 20 days to prevent any young bee emergence and thus to maintain the experimental age structure over the first 40 days of the experiment. After its removal, the amount of brood was quantified by counting eggs, small larvae (< L4), large larvae (L4 and L5), and capped cells to measure hive productivity. In the first experiment, we determined the average weight of eggs, small larvae, large larvae, and pupae from ten individuals of each class in two colonies. In the fourth experiment, only large brood and capped cells were counted because the contribution of eggs and small larvae to total brood weight proved negligible. The total number of workers in each hive was estimated during this first brood cycle to obtain an estimate of individual productivity (brood produced/average worker hive population) in the different hives.
Worker survival was estimated using regular censuses of all hives. These censuses were conducted between sunset and sunrise when all bees were present in the hives. For the first experiment, we conducted daily censuses, which was reduced in the remaining experiments to three censuses per week. The individual tagging allowed us to follow individuals and extrapolate missing data for individuals that had not been seen during a particular census but were seen afterwards. Individuals were excluded from the analysis if they were only recorded once, or they had a lifespan smaller than six days, or that had drifted between colonies during the experiment. In addition to the nightly censuses, entrance observations were performed for one hour per colony to determine foraging variables. These observations were performed daily during the first repeat and three times per week in the subsequent experiments. All returning foragers were recorded and classified as nectar or pollen foragers, except for the last experiment in which only pollen foragers were recorded.
Experiments were performed until less than 5% of the original cohorts were alive in any of the three experimental colonies. Lifespan was computed from the age when a bee was last seen during a foraging observation or nightly census. For each bee that was observed foraging, the age of first foraging (AFF), and the foraging lifespan (flightspan), and the proportion of foraging observations that involved pollen collection (pollen specialization) were computed, except for experiment #4, in which we lacked sufficient foraging data. Furthermore, bee-specific hive productivity was computed as the amount of brood produced in the hive [g] divided by the average total numbers of bees present in the hive. Lifespan, AFF and flightspan were multiplied by this bee-specific hive productivity to yield new, relative measures. Subsequent analyses were performed on both, absolute and relative values of lifespan, AFF, and flightspan. Kaplan-Meier survival analyses were used to estimate the average values and 95% confidence intervals. The main treatment effects in each experiment were assessed by Mantel-Cox log rank tests and Cox regressions were used to simultaneously assess treatment and covariate effects on AFF, flightspan, and lifespan for bees that were observed foraging. For the analyses of flightspan and lifespan, we used AFF and pollen specialization as covariates, for the analysis of AFF only pollen specialization.
In addition, we investigated the mortality dynamics with the computer program WinModest (Pletcher, 1999) conducting maximum likelihood searches for the best fit of the data to Gompertz, Gompertz-Makeham, logistic, and logistic-Makeham mortality models. Parameters were determined for the best model and a sensitivity analysis performed.
Results
Overall, data from 1688 (experiment 1), 404 (experiment 2), 498 (experiment 3), 1849 (experiment 4) worker bees with a lifespan > 5 days were included in the subsequent analyses. Respectively, 1205, 305, 338, and 46 workers were observed foraging during the experiments and included in forager-specific analyses. The frequency of censored cases in all 15 experimental cohorts ranged from 0 – 4%.
Lifespan and its components
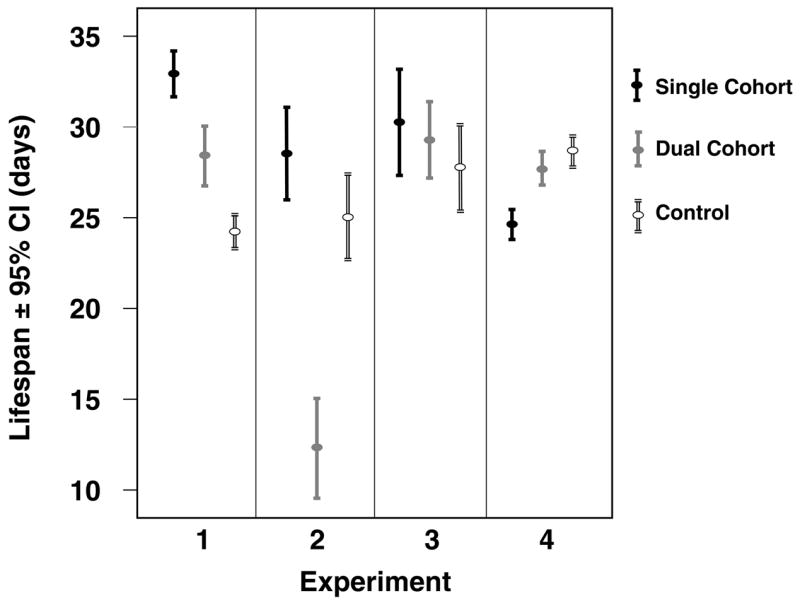
The overall life expectancy of workers across the four experiments and four experimental treatment groups (single cohort, young dual cohort, old dual cohort, control) was 27.9 (95% CI: 27.5 – 28.2) days with a mean age at first foraging (AFF) of 19.2 (18.7 – 19.6) and 12.9 (12.5 –13.4) days of foraging lifespan. The experimental treatment significantly affected the lifespan in the focal cohorts of young bees in all experiments, except in the third (Log rank test, experiment 1: χ2 = 158.7, df = 2, p < 0.001; experiment 2: χ2 = 63.8, df = 2, p < 0.001; experiment 3: χ2 = 1.7, df = 2, p = 0.435; experiment 4: χ2.= 27.6, df = 2, p < 0.001). The direction of these effects varies between experiments (Figure 1). While in experiments one and three, workers in the single cohort colony lived longest, followed by the dual cohort colony and lastly the control, this trend was reversed in experiment four. In the second experiment, the dual cohort colony workers had the shortest life expectancy and the single cohort colony workers had the longest, with the control intermediate (Table 1). The older cohort in the dual cohort colony was monitored in experiments #1-3 and showed significantly longer lifespans than the younger cohort in the second and third experiment but not in the first (Table 1).
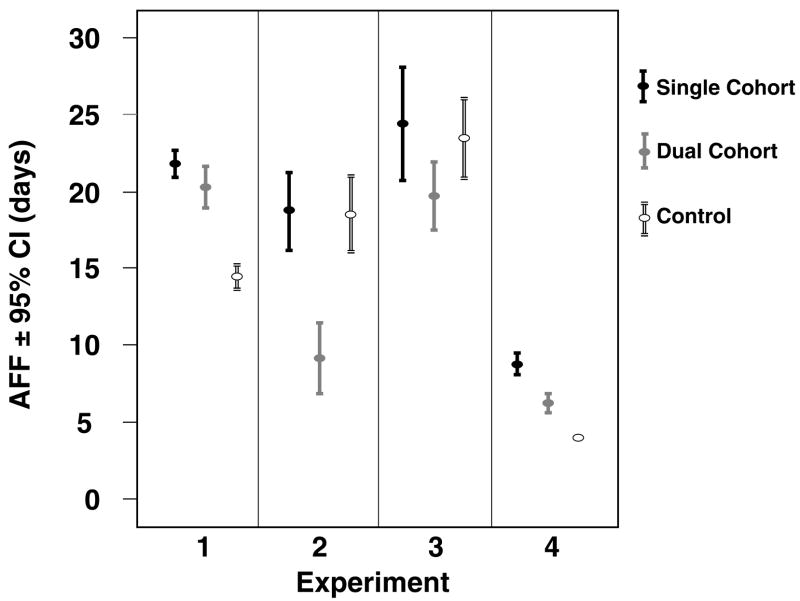
Figure 1.
Comparison of the average lifespan of all worker bees in the young experimental cohorts in all four experimental replicates. The hive descriptions refer to the three experimental demographic hive designs.
Table 1.
Comparison of mean lifespan and its components in all experimental groups
| Lifespan | AFF | Flightspan | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Single- cohort | Young dual cohort |
Control | Old dual cohort |
Single-cohort | Young dual cohort |
Control | Old dual cohort |
Single-cohort | Young dual cohort |
Control | Old dual cohort |
| Exp.1 | 32.9 (31.6 – 34.1) | 28.4 (26.7 – 30.0) | 24.2 (23.3 – 25.1) | 27.5 (26.2 – 28.9) | 22.1 (21.2 – 23.0) | 20.4 (19.0 – 21.7) | 14.4 (13.7 – 15.2) | 19.6 (18.7 – 20.4) | 17.1 (15.9 – 18.3) | 13.4 (11.9 – 14.8) | 12.5 (11.7 – 13.4) | 12.7 (11.6 – 13.7) |
| Exp.2 | 28.5 (26.0 – 31.0) | 12.3 (9.7 – 14.9) | 25.0 (22.7 – 27.3) | 28.2 (26.5 – 29.9) | 18.7 (16.3 – 21.2) | 9.2 (7.0 – 11.3) | 18.9 (16.5 – 21.4) | 21.2 (19.7 – 22.6) | 8.9 (7.2 – 10.5) | 4.8 (3.0 – 6.6) | 11.1 (9.0 – 13.3) | 10.0 (8.8 – 11.1) |
| Exp.3 | 30.2 (27.4 – 33.0) | 29.3 (27.2 – 31.4) | 27.7 (25.4 – 30.0) | 34.8 (33.2 – 36.4) | 24.6 (20.9 – 28.2) | 19.7 (17.5 – 21.9) | 23.6 (21.1 – 26.2) | 23.1 (21.4 – 24.9) | 11.0 (8.0 – 14.0) | 12.8 (10.8 – 14.8) | 9.2 (7.4 – 11.0) | 13.2 (11.4 – 14.9) |
| Exp.4 | 24.6 (23.8 – 25.4) | 27.7 (26.7 – 28.6) | 28.6 (27.8 – 29.4) | N/A | 8.8 (8.1 – 9.5) | 6.2 (5.7 – 6.8) | 4 (single value) | N/A | 9.6 (6.0 – 13.3) | 10.8 (8.2 – 13.5) | 5 (single value) | N/A |
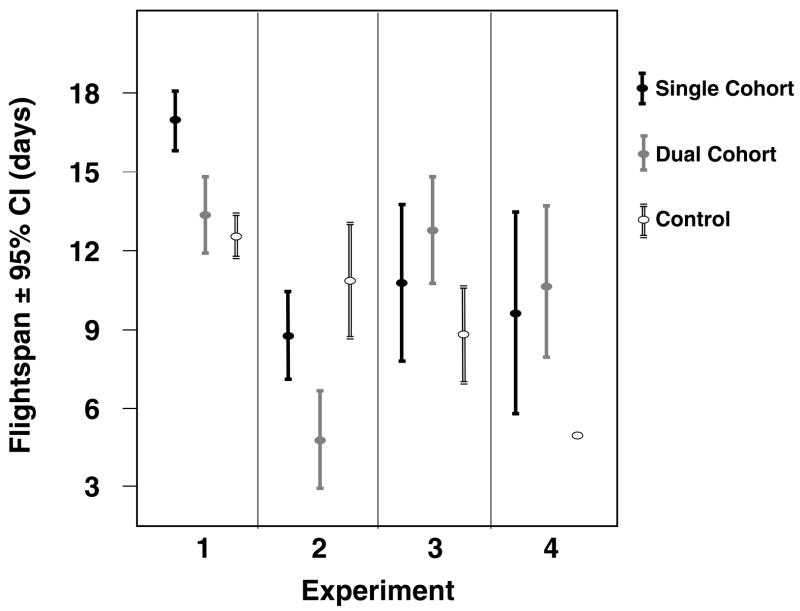
The treatment groups showed a significantly different AFF in all but the third experiment (Log rank test, experiment 1: χ2.= 125.7, df = 2, p < 0.001; experiment 2: χ2.= 55.5, df = 2, p < 0.001; experiment 3: χ2.= 5.7, df = 2, p = 0.058; experiment 4: χ2.= 71.0, df = 2, p < 0.001). The single cohort colony consistently had the highest AFF. This was closely followed by the control in experiments 2 and 3, while in the first experiment the dual cohort treatment showed the second highest AFF (Figure 2). The workers’ flightspan was also significantly different among groups, except for in the last experiment which had a very small sample size (Log rank test, experiment 1: χ2.= 44.7, df = 2, p < 0.001; experiment 2: χ2.= 15.5, df = 2, p < 0.001; experiment 3: χ2.= 6.1, df = 2, p = 0.048; experiment 4: χ2.= 2.1, df = 2, p = 0.357). In the first experiment, the single cohort hive showed a higher average flightspan than the other two colonies. In the second experiment, workers in the dual cohort colony lived shortest while foraging and the control lived longest, while the opposite was true in experiments #3 and #4 (Figure 3).
Figure 2.
Comparison of the age of first foraging (AFF) in the compared cohorts in all 4 experimental replicates.
Figure 3.
Comparison of the flightspan of foragers in the compared cohorts in all 4 experimental replicates.
Cox regression analyses showed that a high propensity to specialize on pollen foraging affected lifespan only in the first experiment, but not in the second or third (Table 2). Colony treatment and AFF showed significant effects on lifespan in all experiments that they were evaluated (Table 2). The single cohort colony was associated with significantly higher mortality in the second and forth experiment, but with lower mortality in the first and third. The dual cohort colony showed the same pattern, but results were significant in experiments #1–3 and not in #4. Most consistently, AFF had a significant positive effect on longevity (Table 2). AFF itself was influenced by treatment in experiment #1 and #2, but was not influenced by the propensity to collect pollen in any of the experiments. The single and dual cohort treatments significantly increased the AFF in the first experiment, but in the second experiment the dual cohort condition was associated with earlier foraging (Table 2). The flightspan was significantly decreased by AFF in all experiments. It was also influenced by treatment in the first two experiments: Single and dual cohort bees had significantly longer flightspans in experiment #1 and the same trend was apparent in experiment #3. In the second experiment these effects were reversed, although the effect of the single cohort treatment was not significant. The propensity to collect pollen was significantly associated with a decrease in flightspan in the first experiment. A similar but non-significant effect was found in experiment #3, while the reverse was true for experiment #2 (Table 2).
Table 2.
Cox regression results (hazard ratio and Wald statistics) of potentially influential factors for lifespan, AFF* and flightspan*
| Dependent variable | Independent factor | Experiment 1 | Experiment 2 | Experiment 3 | Experiment 4 |
|---|---|---|---|---|---|
| Lifespan | Single cohort | HR = 0.48 (0.41 – 0.57) z2 = 77.8 p < 0.001 | HR = 1.46 (1.04 – 2.06) z2 = 4.8 p = 0.028 | HR = 071 (0.47 – 1.06) z2 = 2.8 p = 0.094 | HR = 1.31 (1.17 – 1.46) z2 = 22.6 p < 0.001 |
| Dual cohort | HR = 0.68 (0.57 – 0.82) z2 = 17.1 p < 0.001 | HR = 4.96 (3.01 – 8.19) z2 = 39.4 p < 0.001 | HR = 0.69 (0.49 – 0.96) z2 = 4.7 p = 0.030 | HR = 1.05 (0.94 – 1.17) z2 = 0.7 p = 0.414 | |
| AFF | HR = 0.94 (0.9 4 – 0.95) z2 = 174.4 p < 0.001 | HR = 0.94 (0.93 – 0.95) z2 = 66.1 p < 0.001 | HR = 0.93 (0.92 – 0.95) z2 = 62.0 p < 0.001 | N/A | |
| Pollen proportion | HR = 1.38 (1.06 – 1.81) z2 = 5.6 p = 0.018 | HR = 0.84 (0.54 – 1.31) z2 = 0.6 p = 0.438 | HR = 1.32 (0.79 – 2.22) z2 = 1.1 p = 0.293 | N/A | |
| AFF | Single cohort | HR = 0.49 (0.42 – 0.57) z2 = 90.2 p < 0.001 | HR = 1.03 (0.75 – 1.42) z2 = 0.04 p = 0.852 | HR = 0.86 (0.58 – 1.30) z2 = 0.5 p = 0.478 | N/A |
| Dual cohort | HR = 0.54 (0.46 – 0.64) z2 = 49.6 p < 0.001 | HR = 4.00 (2.47 – 6.46) z2 = 32.0 p < 0.001 | HR = 1.28 (0.92 – 1.78) z2 = 2.1 p = 0.143 | N/A | |
| Pollen proportion | HR = 1.20 (0.96 – 1.51) z2 = 2.4 p = 0.118 | HR = 1.30 (0.89 – 1.91) z2 = 1.8 p = 0.176 | HR = 0.84 (0.54 – 1.32) z2 = 0.6 p = 0.449 | N/A | |
| Flightspan | Single cohort | HR = 0.48 (0.41 – 0.57) z2 = 75.8 p < 0.001 | HR = 1.40 (0.99 – 1.96) z2 = 3.7 p = 0.055 | HR = 0.77 (0.51 – 1.15) z2 = 1.6 p = 0.203 | N/A |
| Dual cohort | HR = 0.68 (0.57 – 0.82) z2 = 17.3 p < 0.001 | HR = 2.98 (1.82 – 4.86) z2 = 19.0 p < 0.001 | HR = 0.75 (0.53 – 1.05) z2 = 2.8 p = 0.096 | N/A | |
| AFF | HR = 1.03 (1.03 – 1.04) z2 = 56.8 p < 0.001 | HR = 1.03 (1.01 – 1.04) z2 = 10.2 p = 0.001 | HR = 1.03 (1.01 – 1.05) z2 = 10.7 p = 0.001 | N/A | |
| Pollen proportion | HR = 1.41 (1.08 – 1.85) z2 = 6.2 p = 0.013 | HR = 0.77 (0.49 – 1.22) ) z2 = 1.2 p = 0.270 | HR = 1.39 (0.82 – 2.36) z2 = 1.5 p = 0.220 | N/A |
limited sampling in experiment 4 precluded meaningful calculations
Across all experiments, a mortality cost to precocious foraging in the single cohort colonies was indicated by the following quadratic function of flightspan on the AFF: flightspan = −0.02 * AFF2 + 0.74 * AFF + 8.8 (R2 = 0.08, F(2,505)=21.9, p < 0.001). Based on a difference in Akaike’s Information Criterion (δAIC) of 27.2, this function fits the data better than the best linear fit (flightspan = −0.18 * AFF + 18.6 (R2 = 0.03, F(1,506)=13.2, p < 0.001).
Mortality Dynamics
Maximum-likelihood estimates show that honey bee worker mortality under the experimental conditions best fits a Gompertz model in seven cases and a logistic model in eight cases. Makeham extensions did not significantly improve the model fitting probability. All results were consistent over a wide range of parameter values. There was no consistent treatment effect on the mortality dynamics: each treatment resulted in half of the cases in a Gompertz fit, the other half in a logistic fit (Table 3). There was also no systematic influence on the mortality dynamics by the experimental replicate. The estimated initial mortality rate varied from negligible levels to 1.3% and the mortality rate doubling times ranged from 2.8 to 13.3 days with the exception of one abnormal fit that was based on a small sample size (experiment 2, dual cohort).
Table 3.
Mortality models based on the experimental data (λ: initial mortality, γ: rate of exponential mortality increase, s: mortality deceleration)
| Treatment | Model | λ • 10−3 | Γ | s | |
|---|---|---|---|---|---|
| 1. Experiment | Single Cohort | Logistic | 5.3 | 0.076 | 0.38 |
| Dual Cohort | Gompertz | 10.8 | 0.052 | N/A | |
| Control | Gompertz | 8.4 | 0.083 | N/A | |
| Dual Cohort old | Gompertz | 8.0 | 0.069 | N/A | |
| 2. Experiment | Single Cohort | Gompertz | 9.3 | 0.058 | N/A |
| Dual Cohort | Logistic | 0.0 | 1.42 | 7.39 | |
| Control | Gompertz | 13.1 | 0.055 | N/A | |
| Dual Cohort old | Logistic | 0.5 | 0.247 | 2.17 | |
| 3. Experiment | Single Cohort | Gompertz | 6.9 | 0.066 | N/A |
| Dual Cohort | Gompertz | 5.5 | 0.079 | N/A | |
| Control | Logistic | 5.6 | 0.108 | 0.86 | |
| Dual Cohort old | Logistic | 0.3 | 0.202 | 1.82 | |
| 4. Experiment | Single Cohort | Logistic | 1.8 | 0.228 | 2.08 |
| Dual Cohort | Logistic | 1.5 | 0.199 | 1.85 | |
| Control | Logistic | 3.7 | 0.108 | 0.32 |
Relative Lifespan
The average weight of eggs was 0.11mg, of small larvae 4.0mg, of large larvae 109.0 mg, and of pupae 95.5mg. For subsequent results, the large larvae and pupae were averaged to a weight of 100mg. Productivity was dependent on the colony type and the experiment, with a small interaction between the two (table 4). In general, productivity co-varied among colonies: Experiment 3 was characterized by the highest productivity (average of 0.24g/bee), followed by experiment 1 (0.15g/bee) and 2 (0.14g/bee), and the lowest productivity in experiment 4 (0.09g/bee). The single cohort colony raised the least and the dual cohort colony raised the most amount of brood per adult worker bee, except for the fourth experiment when all three colonies showed similar productivity. A simultaneous assessment of the effects of average colony size and the experimental treatment group on bee-specific productivity (Figure 5) showed a significant positive effect of average colony size (F(1,8) = 13.1, p = 0.007) but no significant independent effect of treatment (F(2,8) = 2.0, p = 0.195).
Table 4.
Brood production (relative brood production) during the initial three experimental weeks
| Single Cohort | Dual Cohort | Control | |
|---|---|---|---|
| Experiment 1 | 189g (0.10g/bee) | 447g (0.22g/bee) | 224g (0.12g/bee) |
| Experiment 2 | 86g (0.11g/bee) | 287g (0.20g/bee) | 151g (0.12g/bee) |
| Experiment 3 | 318g (0.17g/bee) | 706g (0.29g/bee) | 510g (0.25g/bee) |
| Experiment 4 | 87g (0.09g/bee) | 65g (0.09g/bee) | 120g (0.10g/bee) |
Figure 5.
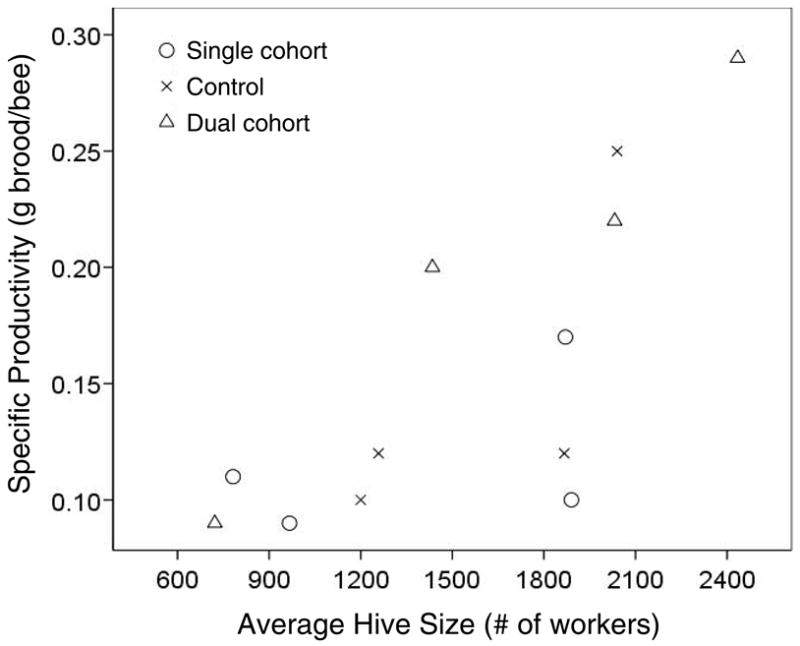
Hive size is correlated with the specific brood production per bee, indicating more efficient homeostasis or division of labor in larger societies. This effect may reinforce a positive association of individual worker longevity and colony growth.
Adjusting the lifespan, AFF, and flightspan by the different productivities lead to the following results (Table 5). The older half of the dual cohort colony had the highest relative lifespan in all three experiments that it was recorded and it also reached the highest values in the relative preforaging lifespan and relative foraging lifespan. Among the same-aged cohorts, the younger half of the dual cohort colony had the highest relative lifespan in the first and third experiment (Table 5). In the first experiment, the relative AFF and flightspan of foragers mirrored the relative lifespan result from all workers but in the third experiment, relative AFF in the control colony was highest. The single cohort colony showed the highest relative lifespan in the second experiment but the relative AFF and flightspan were highest in the control colony. Conversely, the workers in the control colony had the highest relative lifespan in the fourth experiment but their relative AFF and flightspan were lower than that of the workers from the single and double cohort colony (Table 5).
Table 5.
Comparison of mean lifespan, AFF, and flightspan relative to brood production in all experimental groups
| Relative lifespan | Relative AFF | Relative flightspan | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Single-cohort | Young dual cohort |
Control | Old dual cohort |
Single-cohort | Young dual cohort |
Control | Old dual cohort |
Single-cohort | Young dual cohort |
Control | Old dual cohort |
| 1 | 3.2 (3.1 – 3.3) | 6.2 (5.8 – 6.6) | 2.8 (2.7 – 2.9) | 6.0 (5.7 6.3) | 2.2 (2.1 – 2.3) | 4.5 (4.2 – 4.8) | 1.7 (1.6 – 1.8) | 4.3 (4.1 4.5) | 1.7 (1.6 – 1.8) | 2.9 (2.6 – 3.2) | 1.5 (1.4 – 1.6) | 2.8 (2.5 – 3.0) |
| 2 | 3.2 (3.0 – 3.5) | 2.5 (1.9 – 3.0) | 2.9 (2.6 – 3.2) | 5.7 (5.3 – 6.0) | 2.1 (1.9 – 2.4) | 1.8 (1.4 – 2.3) | 2.2 (1.9 – 2.5) | 4.3 (4.0 – 4.5) | 1.0 (0.8 – 1.2) | 1.0 (0.6 – 1.3) | 1.3 (1.0 – 1.5) | 2.0 (1.8 – 2.2) |
| 3 | 5.0 (4.5 – 5.5) | 8.6 (8.0 – 9.2) | 7.0 (6.4 – 7.6) | 10.3 (9.8 – 10.7) | 4.1 (3.5 – 4.7) | 5.8 (5.2 – 6.5) | 6.0 (5.3 – 6.6) | 6.8 (6.3 – 7.3) | 1.8 (1.3 – 2.3) | 3.8 (3.2 – 4.4) | 2.3 (1.9 – 2.8) | 3.9 (3.4 – 4.4) |
| 4 | 2.3 (2.2 – 2.3) | 2.4 (2.3 – 2.5) | 2.8 (2.8 – 2.9) | N/A | 0.8 (0.7 – 0.9) | 0.5 (0.5 – 0.6) | 0.4 (single value) | N/A | 0.9 (0.6 – 1.2) | 0.9 (0.7 – 1.2) | 0.5 (single value) | N/A |
Discussion
This study shows that profound manipulations of the age composition of honeybee hives can cause significant but variable demographic outcomes, with effects on both, worker life expectancy and brood production. The data suggest no consistent advantage of any of the three experimental demographic conditions, when considering worker life expectancy relative to colony brood production. This suggests that the natural age structure of the hive is not adaptive per se but rather an epiphenomenon of individual worker life history. In most cases, the single cohort condition decreased brood production but increased life expectancy and dual cohort hive had the highest brood production. The similarity between the two experiments conducted early in the season (#1 and #3) and between the two conducted later (#2 and #4) indicate an interaction between season and the experimental manipulations.
Based on the pronounced, presumably adaptive, age-polyethism among honeybee workers (Oster and Wilson 1978, Tofilski, 2002 #114), we had predicted a severe reduction of worker life expectancy and productivity in the single cohort hives. While these cohorts usually experienced a higher initial mortality, overall life expectancy was increased in three out of the four experimental replicates. However, in all but the fourth experiment the single cohort hive produced the least amount of brood. This effect was not due to the absence of food. Instead, brood production may be down-regulated as an adaptive response to the social stress caused by single cohort demographics. Reduced brood rearing and lack of recruitment from older foragers (Rueppell et al. 2007) explain the delayed AFF and the increased life expectancy of workers in the single cohort hives (Maurizio 1950; Winston and Fergusson 1985).
A survival advantage under single cohort conditions was only indicated in experiments #1 and #3, while in experiment #2 the high life expectancy was statistically explained by the other cofactors (AFF, pollen specialization), and in the fourth experiment the single cohort workers actually had the lowest life expectancy. The difference between the experiments could be attributed to a seasonal affect: Under favorable conditions early in the season (Neukirch 1982), the single cohort colony may reduce brood production and increase worker maintenance until a balanced age-composition would be restored. At the colony level, this would amount to an inactive, somatic maintenance state, instead of growth and reproduction (Perrin and Sibly 1993). Towards the end of the season, the colony is under more constraint to reach a critical size and collect resources for successful hibernation (Mattila et al. 2001). Consequently, down-regulation of the workload to preserve the current worker population may be not a viable option. This interpretation is supported by the earlier AFF in the single cohort hive later in the season.
In addition, we predicted that the single cohort condition would increase the variability of worker life histories within the colony due to the forced division of labor, independent of age (Huang and Robinson 1996; Beshers and Fewell 2001). However, neither lifespan, nor its components (AFF and flightspan) showed a consistent increase in variability compared to the control and dual colony cohorts. The AFF was highest under single cohort conditions which suggests that the workers reduced brood production instead of initiating precocious foraging when given the choice. Without initial brood, demographic plasticity by maintaining a reduced level of brood production in the single cohort hives may be preferable over behavioral plasticity (Schulz et al. 1998). This is consistent with our result that early foraging in the single cohort hives bears an elevated mortality cost and the finding that premature foragers are less efficient foragers (Tofilski 2000).
In contrast, the dual cohort colonies displayed the highest productivity, except for in the fourth experiment when productivity was low and similar across all colonies. This indicates that a dual cohort social environment suffices for an effective division of labor and actually sustains more brood production than the more diverse worker age distribution in the control colonies. In part, this effect may be explained by the larger hive size of the dual cohort colonies because productivity and hive size were positively correlated (Figure 5), in accordance with earlier data (Harbo 1986). Despite high productivity, these workers did not suffer from a higher mortality, except for the second experiment, in which survival of this particular cohort was so dramatically different from all other experimental cohorts that an experimental error (e.g. mishandling during tagging) seems likely. In the multi-factorial model the hazard rate was also decreased in the first and third experiment but not in the second and fourth experiment, suggesting that other, seasonal factors are potentially influential (Fukuda and Sekiguchi 1966; Neukirch 1982).
When brood production was taken into account, the relative life expectancy in the dual cohort colony was significantly higher than in the other two treatment groups in experiments #1 and #3 but statistically indistinguishable later in the season (experiments #2 and 4). While this was equally due to a longer relative hivespan and flightspan in the first experiment, in the third experiment the effect was mainly due to a longer relative flightspan. Only in the fourth experiment did the control colony show a significantly higher relative lifespan than the other two treatment groups. This result could be an indication that the natural hive age composition is beneficial under stressful conditions, but the result could also be explained by the larger size of the control hive relative to the other two hives (Harbo 1986). Across the four experiments, the control hive did not consistently outperform the single or the double cohort hives, and thus natural age demography seems not to be adaptive per se, as has been suggested for physical caste ratios (Oster and Wilson 1978; Schmid-Hempel 1992).
The overall estimates of life expectancy, the age of first foraging and foraging lifespan agree with previously published records (Free and Spencer-Booth 1959; Neukirch 1982). The data also corresponds well to the seasonal trends observed by Neukirch (1982), because worker life expectancy was generally higher earlier (experiments #1 and 3) in the season than later (experiments #2 and 4). The sum of average AFF and flightspan is slightly larger than the overall life expectancy, indicating that bees that were observed foraging lived longer than bees that were not observed foraging. Bees that died without foraging record could have died during their initial foraging trip, which is characterized by relatively high forager mortality (Rueppell et al. 2007). The only significant effects of foraging specialization (pollen collection) in the multi-factorial Cox-regression analyses were its negative effect on lifespan and flightspan in the first experiment. This is to be expected based on the phenotypic associations of the pollen hoarding syndrome in honeybees (Amdam et al. 2007). However, resource availability may break up the phenotypic linkage between pollen foraging, earlier foraging, and short lifespan; particularly if the AFF is statistically accounted for (Rueppell et al. 2007). Accordingly, pollen specialization shows an opposite trend in experiment #2.
The AFF showed the most significant effect in determining worker lifespan. In accordance with other studies (Becerra-Guzman et al. 2005; Rueppell et al. 2007) the AFF is positively related to the length of life but decreases the remaining foraging lifespan. Worker foraging lifespan in our study was reduced by up to 1/3 day per day of AFF delay. This trade-off reflects the relative longevity cost of the in-hive tasks and foraging, and its strength may drive the evolution of the rate of worker behavioral ontogeny (Rueppell et al. 2004, 2007). The relative early onset of flight activity under dual cohort conditions may be due to a combination of more recruitment from older foragers (Rueppell et al. 2007) and rapidly declining internal vitellogenin levels (Amdam et al. 2007) due to the high amount of brood rearing. The rate of worker behavioral ontogeny, as measured by the AFF, is significantly affected by the hive demography but also feeds back to the age composition because of the higher mortality of foragers, relative to in-hive workers.
In conclusion, the demographic age-composition of social insect colonies may be an epiphenomenon of individual mortality schedules (Wilson 1971) and not a group-selected trait (Oster and Wilson 1978; Reeve and Hölldobler 2007). Age-composition strongly influences colony productivity and social resilience, even though it may not affect individual life expectancy as much as predicted. Natural swarming or colony fission events, as well as honeybee husbandry may have profound effects on colony age structure and our results predict the preservation of multiple age classes to be beneficial. Societal age-structure is beneficial in other social species, including humans, with resource transfers between individuals shaping mortality dynamics (Lee 2003). Our study shows experimentally that the social context affects individual aging and suggests that honeybees are a compelling model for social gerontology.
Figure 4.
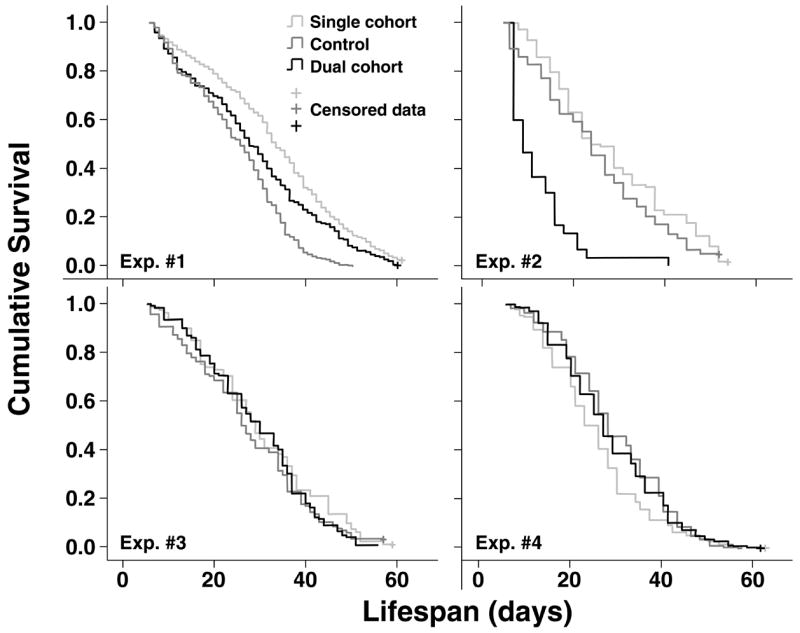
Overall survival dynamics in the experimental cohorts in the four experimental replicates.
Acknowledgments
We thank Kristen Ward for collecting the foraging data for the fourth experiment and two anonymous reviewers for helpful suggestions to improve the manuscript. Financial support was provided by NIA (grant #PO1 AG22500), the American Federation for Aging Research (AFAR) and the National Science Foundation (grant #0615502).
References
- Amdam GV, Nilsen KA, Norberg K, Fondrk MK, Hartfelder K. Variation in endocrine signaling underlies variation in social life history. Am Nat. 2007;170:37–46. doi: 10.1086/518183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdam GV, Omholt SW. The hive bee to forager transition in honeybee colonies: the double repressor hypothesis. J Theor Biol. 2003;223:451–464. doi: 10.1016/s0022-5193(03)00121-8. [DOI] [PubMed] [Google Scholar]
- Amdam GV, Page RE. Intergenerational transfers may have decoupled physiological and chronological age in a eusocial insect. Aging Res Rev. 2005;4:398–408. doi: 10.1016/j.arr.2005.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdam GV, Rueppell O. Models of aging in honeybee workers. In: Conn PM, editor. Handbook of Models for Human Aging. Academic Press; San Diego, CA: 2006. pp. 267–276. [Google Scholar]
- Becerra-Guzman F, Guzman-Novoa E, Correa-Benitez A, Zozaya-Rubio A. Length of life, age at first foraging and foraging life of Africanized and European honey bee (Apis mellifera) workers, during conditions of resource abundance. J Apicultural Res. 2005;44:151–156. [Google Scholar]
- Beshers SN, Fewell JH. Models of division of labor in social insects. Ann Rev Entomol. 2001;46:413–440. doi: 10.1146/annurev.ento.46.1.413. [DOI] [PubMed] [Google Scholar]
- Brillet C, Robinson GE, Bues R, LeConte Y. Racial differences in division of labor in colonies of the honey bee (Apis mellifera) Ethology. 2002;108:115–126. [Google Scholar]
- Carey JR. Demographic mechanisms for the evolution of long life in social insects. Exper Gerontol. 2001;36:713–722. doi: 10.1016/s0531-5565(00)00237-0. [DOI] [PubMed] [Google Scholar]
- Free JB, Spencer-Booth Y. The longevity of worker honey bees (Apis mellifera) Proc Royal Entomol Soc London A. 1959;34:141–150. [Google Scholar]
- Fukuda H, Sekiguchi K. Seasonal change of the honeybee worker longevity in Sapporo, north Japan, with notes on some factors affecting the life-span. Japan J Ecol. 1966;16:206–212. [Google Scholar]
- Harbo JR. Effect of population size on brood production, worker survival and honey gain in colonies of honeybees. J Apicultural Res. 1986;25:22–29. [Google Scholar]
- Hölldobler B, Wilson EO. The Ants. The Belknap Press of Harvard University Press; Cambridge, Mass: 1990. [Google Scholar]
- Huang ZY, Robinson GE. Honeybee colony integration – worker - worker interactions mediate hormonally regulated plasticity in division of labor. Proc Nat Acad Sci USA. 1992;89:11726–11729. doi: 10.1073/pnas.89.24.11726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZY, Robinson GE. Regulation of honey bee division of labor by colony age demography. Behavioral Ecology and Sociobiology. 1996;39:147–158. [Google Scholar]
- Le Conte Y, Mohammedi A, Robinson GE. Primer effects of a brood pheromone on honeybee behavioural development. Proc Royal Soc London B. 2001;268:163–168. doi: 10.1098/rspb.2000.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RD. Rethinking the evolutionary theory of aging: Transfers, not births, shape social species. Proc Nat Acad Sci USA. 2003;100:9637–9642. doi: 10.1073/pnas.1530303100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leoncini I, Le Conte Y, Costagliola G, Plettner E, Toth AL, Wang MW, Huang Z, Becard JM, Crauser D, Slessor KN, Robinson GE. Regulation of behavioral maturation by a primer pheromone produced by adult worker honey bees. Proc Nat Acad Sci USA. 2004;101:17559–17564. doi: 10.1073/pnas.0407652101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindauer M. Division of labour in the honeybee colony. Bee World. 1953;34:63–73. [Google Scholar]
- Mattila HR, Harris JL, Otis GW. Timing of production of winter bees in honey bee (Apis mellifera) colonies. Ins Soc. 2001;48:88–93. [Google Scholar]
- Maurizio A. The influence of pollen feeding and brood rearing on the length of life and physiological condition of the honeybee. Bee World. 1950;31:9–12. [Google Scholar]
- Neukirch A. Dependence of the life span of the honeybee (Apis mellifica) upon flight performance and energy consumption. J Comp Physiol. 1982;146:35–40. [Google Scholar]
- Oster GF, Wilson EO. Caste and Ecology in the Social Insects. Princeton University Press; Princeton: 1978. [PubMed] [Google Scholar]
- Pankiw T. Directional change in a suite of foraging behaviors in tropical and temperate evolved honey bees (Apis mellifera L.) Behav Ecol Sociobiol. 2003;54:458–464. [Google Scholar]
- Pankiw T, Huang ZY, Winston ML, Robinson GE. Queen mandibular gland pheromone influences worker honey bee (Apis mellifera L.) foraging ontogeny and juvenile hormone titers. J Insect Physiol. 1998;44:685–692. doi: 10.1016/s0022-1910(98)00040-7. [DOI] [PubMed] [Google Scholar]
- Perrin N, Sibly RM. Dynamic models of energy allocation and investment. Ann Rev Ecol Systematics. 1993;24:379–410. [Google Scholar]
- Reeve HK, Hölldobler B. The emergence of a superorganism through intergroup competition. Proc Nat Acad Sci USA. 2007;104:9736–9740. doi: 10.1073/pnas.0703466104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueppell O, Bachelier C, Fondrk MK, Page RE., Jr Regulation of life history determines lifespan of worker honey bees (Apis mellifera L.) Exp Gerontol. 2007;42:1020–1032. doi: 10.1016/j.exger.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueppell O, Pankiw T, Nielson DI, Fondrk MK, Beye M, Page RE., Jr The genetic architecture of the behavioral ontogeny of foraging in honey bee workers. Genetics. 2004;167:1767–1779. doi: 10.1534/genetics.103.021949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakagami SF, Fukuda H. Life tables for worker honeybees. Res Popul Ecol. 1968;10:127–139. [Google Scholar]
- Schmid-Hempel P. Worker castes and adaptive demography. J Evol Biol. 1992;5:1–12. [Google Scholar]
- Schulz DJ, Huang ZY, Robinson GE. Effects of colony food shortage on behavioral development in honey bees. Behav Ecol Sociobiol. 1998;42:295–303. [Google Scholar]
- Tofilski A. Senescence and learning in honeybee (Apis mellifera) workers. Acta Neurobiol Experim. 2000;60:35–39. doi: 10.55782/ane-2000-1323. [DOI] [PubMed] [Google Scholar]
- Tofilski A. Influence of caste polyethism on longevity social insect colonies. J Theor Biol. 2006;238:527–531. doi: 10.1016/j.jtbi.2005.06.008. [DOI] [PubMed] [Google Scholar]
- West-Eberhard MJ. Developmental Plasticity and Evolution. Oxford University Press; New York, NY: 2003. [Google Scholar]
- Wilson DS, Sober E. Reviving the superorganism. J Theor Biol. 1989;136:337–356. doi: 10.1016/s0022-5193(89)80169-9. [DOI] [PubMed] [Google Scholar]
- Wilson EO. The Insect Societies. The Belknap Press of Harvard University Press; Cambridge, Mass: 1971. [Google Scholar]
- Winston ML. The Biology of the Honey Bee. Harvard University Press; Cambridge, Massachusetts: 1987. [Google Scholar]
- Winston ML, Fergusson LA. The effect of worker loss on temporal caste structure in colonies of the honeybee (Apis mellifera L) Canad J Zoology. 1985;63:777–780. [Google Scholar]